Abstract
Psychiatric disorders such as anxiety, depression and addiction are often comorbid brain pathologies thought to share common mechanistic biology. As part of the cortico-limbic circuit, the nucleus accumbens shell (NAcSh) plays a fundamental role in integrating information in the circuit, such that modulation of NAcSh circuitry alters anxiety, depression, and addiction-related behaviors. Intracellular kinase cascades in the NAcSh have proven important mediators of behavior. To investigate glycogen-synthase kinase 3 (GSK3) beta signaling in the NAcSh in vivo we knocked down GSK3beta expression with a novel adeno-associated viral vector (AAV2) and assessed changes in anxiety- and depression-like behavior and cocaine self-administration in GSK3beta knockdown rats. GSK3beta knockdown reduced anxiety-like behavior while increasing depression-like behavior and cocaine self-administration. Correlative electrophysiological recordings in acute brain slices were used to assess the effect of AAV-shGSK3beta on spontaneous firing and intrinsic excitability of tonically active interneurons (TANs), cells required for input and output signal integration in the NAcSh and for processing reward-related behaviors. Loose-patch recordings showed that TANs transduced by AAV-shGSK3beta exhibited reduction in tonic firing and increased spike half width. When assessed by whole-cell patch clamp recordings these changes were mirrored by reduction in action potential firing and accompanied by decreased hyperpolarization-induced depolarizing sag potentials, increased action potential current threshold, and decreased maximum rise time. These results suggest that silencing of GSK3beta in the NAcSh increases depression- and addiction-related behavior, possibly by decreasing intrinsic excitability of TANs. However, this study does not rule out contributions from other neuronal sub-types.
Keywords: cocaine, self-administration, drug addiction, depression, nucleus accumbens, tonically active neurons
1. Introduction
Mood disorders such as anxiety and depression have high comorbidity with drug addiction in humans (Pettinati et al., 2013). Dysregulation of the reward system may constitute a common molecular mechanism of these neuropsychiatric disorders and therefore understanding complex neuroadaptations common to neuropsychiatric disorders constitutes a promising avenue for therapeutics.
The nucleus accumbens is heavily implicated in the control of emotional behavior and reward (Pontieri et al., 1995; Pliakas et al., 2001; Green et al., 2006; Nestler and Carlezon, 2006; Larson et al., 2011). As part of the ventral striatum, the nucleus accumbens has as its sole output two major populations of medium spiny neurons (MSNs) whose activity is modulated by a population of tonically active interneurons (TANs), which are mostly cholinergic (Lenz and Lobo, 2013). Despite comprising not more than 5% of the total population of neurons in the NAc, TANs play important roles in reward prediction, task attention, memory, addiction, and aversive behaviors (Aosaki et al., 1994; Apicella, 2002; Anagnostaras et al., 2003; Furey et al., 2008; Williams and Adinoff, 2008; Lenz and Lobo, 2013). TANs control MSN activity and are particularly responsive to salient reward-related stimuli (Morris et al., 2004). Early studies have provided evidence for a role of TANs in cocaine addiction with immunotoxin mediated cell ablation resulting in increased sensitivity to cocaine (Hikida et al., 2001) and preventing behavioral abnormalities associated with cocaine induced by centrally active acetylcholinesterase inhibitors in the NAc (Hikida et al., 2003). More recently, studies using optogenetics confirmed that selective inhibition of TANs results in suppression of cocaine induced behaviors (Witten et al., 2010), further confirming the pivotal role of these cells in reward behavior and addiction. At the circuit level, activation of TANs has been shown to elicit both fast glutamatergic transmission (Higley et al., 2011) and GABAergic inhibition of MSNs, the latter coincident with synchronous cholinergic activation and sufficient to pause MSNs firing (English et al., 2012). These studies suggest a complex role of these cells in the NAc circuit that deserves further investigation.
Intracellular kinase signaling cascades, activated through a variety of mechanisms, have proven important mediators of NAcSh function, and by extension, the etiology of neuropsychiatric disorders. Specifically, the ERK/MAPK, PKA, and PKC signaling cascades have been studied in the NAcSh with success (Self et al., 1998; Schroeder et al., 2008; Ortinski et al., 2015). The AKT/GSK3β pathway has also garnered particular attention for its role in dopamine signaling, the actions of antipsychotic drugs, and even responses to addictive drugs, especially in the nucleus accumbens (Perrine et al., 2008; Beaulieu et al., 2009; Nwaneshiudu and Unterwald, 2010; Beaulieu et al., 2011; Wilkinson et al., 2011; Miller et al., 2014).
GSK3β was originally discovered for its role in glycogen synthesis but has since been implicated in a variety of cellular processes (Wildburger and Laezza, 2012), and dysregulation of this kinase has been implicated in bipolar disorder and neurodegenerative disorders (Grimes and Jope, 2001; Jope, 2011). One of the mechanisms of action of lithium, the commonly prescribed mood stabilizer, is inhibition of GSK3β (Klein and Melton, 1996; Stambolic et al., 1996). Heterozygous GSK3β knockout mice show reductions in depression-like behavior similar to the effects of lithium (O’Brien et al., 2004). Drugs of abuse, especially cocaine, can modulate levels of GSK3β in the NAc (Perrine et al., 2008) and GSK3β is involved in cocaine-induced hyperactivity, cocaine sensitization, cocaine reward memory, and cocaine conditioned place preference (Miller et al., 2009; Miller et al., 2014; Shi et al., 2014). Previous studies indicate that the role of GSK3β is highly dependent on brain region and even cell type as global knockdown may not have the same effects as regional or even cell-type specific knockdown (Latapy et al., 2012; Urs et al., 2012; Zhou et al., 2012). Thus, GSK3β has therapeutic potential for comorbid depression and addiction, but knowledge gaps exist on its brain region specific mechanism of action.
The environmental enrichment manipulation combines novelty, exercise, and social contact to produce robust protective depression and addiction phenotypes (Green et al., 2002; Green et al., 2010). Enrichment increases the ratio of phosphorylated (inactive) to total GSK3β in the hippocampus and cortex (Hu et al., 2013) and environmental enrichment is able to reverse cognitive deficits caused by constitutively active expression of GSK3 in mice (Pardo et al., 2015). Therefore GSK3 may be involved in protecting against depression and addiction phenotypes.
The role of GSK3β in cocaine self-administration, the addiction paradigm with the most face validity, is so far lacking. Additionally, few studies have examined anxiety and depression behaviors along with addiction-related behaviors in the same animals. The current study therefore explores anxiety-like, depression-like, and addiction-related behaviors in the same animals following knockdown of GSK3β in the NAcSh of rats.
In order to analyze the role of GSK3β specifically in the NAcSh in behavior relevant to affective disorders and drug addiction, we designed and constructed a novel adeno-associated viral vector (AAV2) which uses RNA interference to knockdown GSK3β in adult rats and allows for prolonged knockdown of GSK3β in the adult brain. The AAV2 serotype infects neuronal cells in vivo but is not specific to any one neuronal cell type. To provide correlative functional outcomes to the behavioral studies we investigated the role of GSK3β on spontaneous firing and intrinsic excitability of tonically active neurons (TANs), comparing electrophysiological properties of these neurons between GSK3β knockdown vs. control.
2. Materials and Methods
2.1. Animals
Male Sprague-Dawley rats (Harlan, Houston, TX) were obtained at 21-days-old (electrophysiology) or 225–250 g (behavior) and maintained in a controlled environment (temperature, 22°C; relative humidity; 50%; 12h light/dark cycle, lights on 0600h) in an Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC) approved colony in standard polycarbonate cages with ad libitum access to food and water except during surgery and behavioral experiments. All surgical procedures and experiments conformed to the NIH Guide for the Care and Use of Laboratory Animals and approved by The University of Texas Medical Branch Institutional Animal Care and Use Committee.
2.1.1. Timeline of behavior following GSK3β knockdown
Rats in the behavioral cohort underwent stereotaxic vector injection one week after arrival. Three weeks later, rats underwent a battery of behavioral tests beginning with spontaneous behaviors (order of tests: elevated plus maze, sucrose neophobia, locomotor activity, social contact, sucrose preference, cold stress defecation) for two weeks, followed by food regulation to 85% body weight over one week and sucrose pellet responding for three weeks. Rats were subjected to one behavioral test at a time and anxiety and appetitive tests occurred prior to drug tests. Rats were then implanted with an indwelling jugular vein catheter and after one week of recovery, behavioral experiments resumed with drug self-administration (acquisition, maintenance, dose response, progressive ratio, and reinstatement). Rats were between 225–250g on arrival and the average weight on the day of the stereotaxic vector injections was 295g (average 293g controls, 296g for shGSK3β). Average weight after spontaneous behaviors and before food restriction prior to sucrose pellet responding was 425g (avg. 424g for controls, 425g for shGSK3β). Average rat weight on the day of catheter implantation was 445g (447g for controls, 445g for shGSK3β). Following drug-self administration, animals were anesthetized, decapitated, and the placement of the vector was verified.
2.1.2. In vivo knockdown of GSK3β
In order to knockdown GSK3β in vivo, rats were anesthetized with isoflurane (VetEquip, Pleasanton, CA) and injected bilaterally with control vector (AAV-shCTRL) or a novel vector designed to knockdown GSK3β (AAV-shGSK3β) into the NAcSh (1 μl/side over 10 min) using stereotaxic coordinates (21-day-old: AP=1.5, L=1.8, V=−5.9; behavior: AP=1.6, L=2.2, V= −6.7, 10° lateral angle). After injection of 1 μl bilaterally, the needles remained in place for 10 additional minutes in order to allow for spread of the vector and to prevent the vector from spreading up the needle track. AAV-shCTRL expresses a non-targeted hairpin, differing only in hairpin sequence (Benzon et al., 2014). Accurate placements were confirmed post-behavioral experiments by anesthesia and decapitation followed by extraction of the brain and visualization of the native enhanced green fluorescent protein (eGFP) fluorescence with a Dual Fluorescent Protein Flashlight and VG2 barrier filter glasses (Nightsea, Bedford, MA) (Anastasio et al., 2014). No animals were excluded based on vector placement. Electrophysiological recordings were made from cells expressing GFP only in the NAc shell region by visual identification with an upright fluorescent microscope.
2.2. Construction of Viral Vector Knocking Down GSK3β
The novel AAV2 vector was constructed to decrease GSK3β in vivo using methods previously described (Hommel et al., 2003; Benzon et al., 2014). First, target sequences (24nt) were chosen from the coding region of the rat GSK3β mRNA sequence. Optimal sequences contained ~50% cytosine/guanine, little overlap with other mRNAs, and low potential for unwanted secondary structure. Five target sequences were chosen and designed so that the antisense and sense sequences were linked by an miR23 loop. When expressed, the antisense and sense sequences duplex, forming a hairpin structure. Five hairpins were synthesized with XbaI and SapI restriction endonuclease sites, inserted into an AAV2 plasmid expressing GFP, and verified by sequencing. The mouse U6 promoter expresses the hairpins while the cytomegalovirus promoter expresses eGFP.
2.2.1. In vitro validation of hairpin
In order to determine which of the five hairpins was most effective in vitro, we co-transfected HEK293 cells with a plasmid that overexpresses rat GSK3β and either an shRNA plasmid or a control shRNA plasmid using Lipofectamine 2000 (LifeTechnologies, Grand Island, NY). Forty-eight hours later, the cells were harvested, RNA extracted (RNeasy Minikit, Qiagen) and reverse transcribed to cDNA (SuperScript III First Strand Synthesis: Invitrogen 18080051). Relative knockdown was determined with quantitative real-time PCR (Applied Biosystems, Foster City, CA) on an Applied Biosystems 7500 fast thermocycler with validated primers designed to detect rat GSK3β (forward: TGGCAGCAAGGTAACCACAG; reverse: CGGTTCTTAAATCGCTTGTCCTG). The hairpin plasmid with the highest knockdown in vitro was packaged and used for all experiments (5′.CAACTTTACCACTCAAGAACTGTC.3′). AAV was packaged by The University of North Carolina Gene Therapy Core facility. Viral titer was determined using dot blot analysis and ranged from 1 × 1010.2 to 1 × 1013 DRP/ml.
2.2.2. Ex vivo validation of packaged vector
In order to validate the knockdown of the AAV-GSK3β vector, the nucleus accumbens from AAV-shCTRL and AAV-shGSK3β injected rats were analyzed via Western blot with previous methods (Zhang et al., 2014). Briefly, the nucleus accumbens were homogenized in a buffer with sucrose, Hepes buffer, sodium fluoride, 10% SDS, and protease and phosphatase inhibitors (Sigma: P-8340, P-2850, P-5726) and the protein concentration was determined with the Pierce BCA Protein Assay Kit (Thermo Scientific, IL, USA). Samples were denatured at 95° for 5 min and run on a 10% polyacrylamide gel (Criterion TGX, Bio-Rad Laboratories, CA, USA) then transferred to a polyvinylidene fluoride (PVDF) membrane (Millipore, MA, USA). The membrane was blocked by nonfat dry milk, incubated with mouse anti-GSK3β (1:1000, Cell Signaling, cat. 9832S) and rabbit anti-GAPDH (1:2000, Cell Sig, cat. 2118S) primary antibodies, then washed with TBST and incubated with fluorescent secondary antibodies (donkey anti-rabbit (680 nm, 1:15000, Li-Cor Biosciences, NE, USA, cat. 926-32212) and donkey anti-mouse (800 nm, 1:15000, Li-Cor, cat. 926-32212). Western blots were imaged and protein levels quantified and normalized to GAPDH (Odyssey, Li-Cor Biosciences).
2.3. Anxiety-like behavior following GSK3β knockdown
2.3.1. Sucrose neophobia
Three weeks after vector injection, neophobia to a novel taste (sucrose) was assessed in shGSK3β and control rats using methods described previously (Zhang et al., 2014). Rats were separated into new cages at 1600h without access to water, and at 1800h, a 1% w/v sucrose solution in water was placed on each cage for 30 minutes. Sucrose consumption was calculated by the difference in bottle weight from pre-drinking. Rats were returned to their home cages and given a single water bottle with the same 1% w/v sucrose solution in water for 72hrs, ending 48hrs prior to the sucrose preference test.
2.3.2. Cold Stress-Induced Defecation
Anxiety-like behavior was also assessed by measuring the amount of defecation in response to a mild stressor (cold). Polycarbonate mouse cages (33×17×13cm) were pre-chilled on ice for 10 min or until the temperature was approximately 12°C. Rats were placed into the cages on ice (one rat per cage) and the number of fecal boli produced were recorded every 5 min for 30 min. After 30 min rats were returned to their home cages.
2.3.3. Elevated plus maze
Anxiety-like behavior was examined by placing animals on an elevated maze with two open arms and two closed arms measuring 12 × 50 cm, 75 cm above the floor for 5 min (~60 lux open arms, ~20 lux closed arms; Med Associates Inc., VT, USA). The amount of time spent on the open arms and closed arms along with the number of open arm entrances and closed arm entrances were analyzed by photobeam breaks using Med-PC software (Zhang et al., 2014).
2.4. Depression-like behavior following GSK3β knockdown
2.4.1. Sucrose preference
Depression-like behavior was assessed by determining preference for a 1% w/v sucrose solution over water. Rats were separated and placed into new cages without access to water at 1600h for 2 hrs. At 1800h, a bottle of water was placed in the normal water position and a bottle with sucrose (1% w/v) was placed ~10cm away. Rats were allowed to drink from either bottle for 30 minutes then returned to their home cages and the bottles were weighed. Percent sucrose consumed was determined by dividing the change in weight of the sucrose bottle divided by the change in weight of both the sucrose and the water bottles.
2.4.2. Social contact
Differences in social behavior were assessed by measuring grooming behavior after 24hrs of separation. Rats were separated in new cages for the 24hrs immediately prior to the social contact test. Control and shGSK3β rats were placed in a novel environment (plastic container, 45×40×45cm) with a 2-inch layer of regular corncob bedding with their original cagemate (controls with controls and knockdown with knockdown). Behavior was video recorded for 30 min. Rats were returned to their home cages after 30 min. The time spent grooming each other was measured for each pair by a researcher blinded to the conditions (Zhang et al., 2014).
2.5. Cocaine Self-Administration following GSK3β knockdown
2.5.1. Intravenous catheter implantation
Rats were anesthetized with ketamine (100 mg/kg IP) and xylazine (10 mg/kg IP) and a Silastic catheter was inserted into the jugular vein exiting the animal’s back (Green et al., 2008; Zhang et al., 2014). To promote catheter patency and prevent infection, catheters were infused daily with 0.1 ml of sterile saline solution with heparin (30.0U/ml), penicillin G potassium (250,000U/ml) and streptokinase (800U/ml). Animals recovered for 7 days before beginning cocaine self-administration (SA). One animal lost catheter patency during SA and did not complete all SA tests.
2.5.2. Acquisition
Prior to catheter implantation, rats were food restricted to 85% body weight over 7 days then placed in operant chambers (30×24×21cm, Med-Associates, St. Albans, VT) with one active lever, one inactive lever, two cue lights, a food hopper/dispenser, and a house light. Rats were trained to lever press for sucrose pellets on fixed ratio (FR) 1-FR5 for 15 min per day and trained on extinction and progressive ratio schedules of reinforcement (Zhang et al., 2014). After recovery from catheter surgery, rats were allowed to self-administer cocaine dissolved in saline (0.2 mg/kg/infusion, Research Triangle Institute, NC) on an FR1 schedule for 2hrs/day for 7 days. Infusions were signaled by illumination of two cue lights above the active and inactive levers for 20s, which also signaled a timeout period during which no further infusions could be obtained. Infusions were delivered intravenously in a volume of 0.1 ml over 5.8s upon depression of the active lever (left).
2.5.3. Maintenance
After animals acquired the task, maintenance responding was assessed at a higher dose of cocaine (0.5 mg/kg/inf) for 2hrs/day for 5 days on an FR1 schedule.
2.5.4. Fixed ratio dose response
Prior to dose response, rats underwent extinction training for 3 days where cocaine (0.5 mg/kg/inf) was available for 1hr followed by 3hrs where lever presses resulted in illumination of cue lights but no drug infusions. Rats then self-administered cocaine at 8 different doses (0.5, 0.25, 0.125, 0.06, 0.03, 0.015, 0.0075, 0.00325 mg/kg/inf) in descending order for 30 min per dose for a total session time of 4hrs on an FR1 schedule of reinforcement for five days.
2.5.5. Progressive ratio
Rats self-administered cocaine on a progressive ratio schedule of reinforcement (1, 2, 4, 6, 9, 12,15, 20, 25, 32, 40, 50, 62, 77, 95, 118, 145, 178, 219, 268, 328, 402, 492, 603, 737, 901) at a dose of 0.5 mg/kg/inf for 3 sessions and then 0.125 mg/kg/inf for 3 sessions. The program ended and rats were removed from the chamber when they did not receive an infusion for more than 1 hour or after 8hrs total.
2.5.6. Cocaine-induced reinstatement
Rats self-administered cocaine for 1 session (0.5 mg/kg/inf; 2 hrs; FR1) before a within-session reinstatement procedure began. Rats received 0.5 mg/kg/inf on an FR1 schedule for 1hr followed by 3hrs of extinction (with contingent cue light). Next they received an IV infusion of cocaine of one of five doses (0, 0.0625, 0.125, 0.25, 0.5 mg/kg) in a semi-random order for each rat across the 5 sessions of reinstatement (pattern of doses was randomly assigned for 10 animals and applied to both groups). Cocaine-induced reinstatement responding was assessed for 3h following the single IV infusion where the animals again received cocaine cues but no infusions as with extinction. Reinstatement sessions were followed by 2 intervening days of high dose (0.5 mg/kg/inf) cocaine on an FR1 schedule for 2h to maintain a high level of responding (Green et al., 2010).
2.6 Electrophysiology following GSK3β knockdown
2.6.1. Slice preparation
Acute coronal slices containing the NAc were prepared from control and shGSK3β rats 21–30 days following surgery. Rats were decapitated, brains were dissected and 300μm coronal slices containing the NAc were prepared with a vibratome (Leica Biosystems, Buffalo Grove, IL) in an iced sucrose-based artificial cerebral spinal fluid (aCSF), consisting of the following (in mM): 56 NaCl, 100 Sucrose, 2.5 KCl, 20 glucose, 5 MgCl2, 1 CaCl2, 30 NaHCO3, and 1.25 NaH2PO4, osmolarity 300–310 and continuously oxygenized and equilibrated to pH 7.4 with a mixture of 95% O2/5% CO2. Slices were transferred to an incubation chamber with standard aCSF consisting of the following (in mM): 130 NaCl, 3.5 KCl, 10 glucose, 1.5 MgCl2, 1.4 CaCl2, 23 NaHCO3, and 1.25 NaH2PO4, osmolarity 300–310, oxygenated and equilibrated to pH 7.4 with a mixture of 95% O2/5% CO2 at 31°C.
2.6.2. Loose-patch recording and data analysis
After 1–2hrs of recovery, acute brain slices were placed in a submerged recording chamber on the stage of an upright microscope (Axioskop2 FS plus; Zeiss). Slices were continuously perfused at room temperature with standard aCSF (~2ml/min) and equilibrated for 15–20 minutes prior to recordings. Loose-patch recordings were obtained from visually identified GFP positive neurons expressing AAV shRNA against GSK3β or scramble shRNA neurons in the NAcSh. Recording pipettes (3–4 MOhm) were fabricated from borosilicate glass (WPI) using a two-step vertical puller PC-10 (Narishige), and filled with standard aCSF. Loose-patch somatic recordings of spontaneously active neurons of relative large soma size corresponding to what has been previously characterized as tonically active neurons (Pisani et al., 2007) were performed using a MultiClamp 700B (Molecular Devices), low-pass filtered at 2.2 kHz, and sampled at 20 kHz using a Digidata 1322A analog-to-digital interface and pClamp9 acquisition software (Molecular Devices). The seal resistance for loose-patch recordings was typically 50–100 MΩ.
2.6.3. Whole cell patch clamp recording and data analysis
Whole-cell patch-clamp experiments were performed using Axopatch 200B and Multiclamp 700B amplifiers. Somatic recordings in standard aCSF from visually identified TANs were performed with pipettes (resistance of 3–5 MΩ) filled with an internal solution containing (in mM): 145 K-gluconate, 2 MgCl2, 0.1 EGTA, 2 Na2ATP, and 10 HEPES (pH 7.2 with KOH; 290 mOsm). After seal formation and cell membrane rupture, TANs were held in voltage-clamp mode for 1–2 minutes with subsequent switch to current clamp mode to assess neuronal activity. Data acquisition and stimulation were performed with a Digidata 1322A Series interface and pClamp 9 software (Molecular Devices, Sunnyvale, CA). Data were filtered at 2 kHz, digitized at 20 kHz. Neuronal intrinsic excitability was assessed by measuring evoked action potentials with a range of current injections of constant increment (800 msec current square pulses with 10 pA increment). Action potential (AP) current threshold (Itrh) was defined as the current step at which at least one spike was induced. AP voltage threshold (Vtrh) was defined as the voltage at which the first-order derivative of the rising phase of the AP exceeded 10 mV/ms (Nenov et al., 2015). Maximum rise and decay of APs were defined as maximal derivate value (dV/dt) of the depolarizing and repolarizing phases of the AP, respectively. Spontaneous action potential firing from TANs was assessed in current clamp mode at resting membrane potential. To analyze the sag of TANs, cells were set to membrane potential of −70 mV with current injection. The range of current steps from −120 pA to +20 pA with 20 pA increment (500 ms duration) was applied to estimate sag amplitude. All electrophysiology data were analyzed with pCLAMP 10 and GraphPad Prism 6 software.
2.7 Statistical analysis
Statistical significance was set at p<0.05 and all data are expressed as mean±SEM. Students t-test was used to compare means for single-factor analyses. The Welch-Satterthwaite method was used to adjust df in cases where there was a violation of the homogeneity of variance. The Mann-Whitney test was used for the cold stress-induced defecation, as these data are not normally distributed. Two-way repeated measures ANOVAs were used to compare differences among conditions for remaining experiments using SPSS and GraphPad Prism 6 software.
3. Results
3.1. Vector knockdown validation
The hairpin sequence was first validated in vitro by co-transfecting HEK293 cells with a plasmid expressing GSK3β and either the control hairpin plasmid or each of the GSK3β hairpin plasmids and comparing the amount of GSK3β mRNA in the cells. The chosen shRNA construct reduced the amount of GSK3β mRNA by > 90% (Figure 1A). AAV-shGSK3β was further validated in vivo by injecting AAV-shGSK3β or AAV-shCTRL into the NAcSh and comparing the amount of GSK3β protein expression via Western blot analysis. AAV-shGSK3β decreased the GSK3β protein level in the NAc by 35% (Figure 1B, normalized to GAPDH). This in vivo knockdown level is a similar level as a previous AAV RNA interference study (Hommel et al., 2003). The nature of gross dissection is that the majority of the cells present are non-neuronal and not transduced by the vector, so 35% knockdown is expected and represents in vivo validation of the vector.
Figure 1. GSK3β shRNA vector validation.
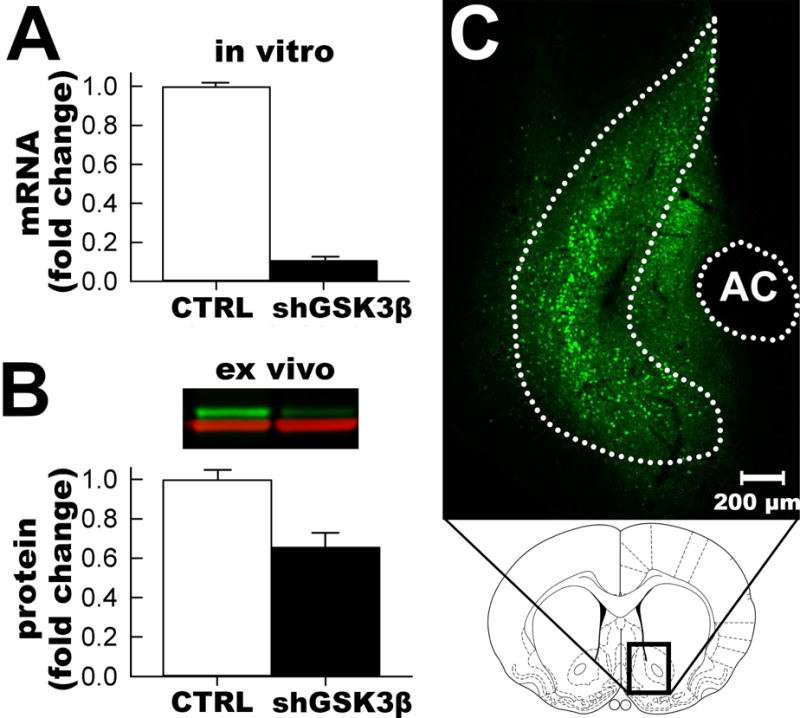
A. In vitro validation of mRNA knockdown in HEK293 cells. B. Quantification of in vivo validation of knockdown of GSK3β protein (green channel) normalized to GAPDH (red channel) in the NAcSh via Western blot (inset). C. Representative placement of viral vector (eGFP immunofluorescence) in the NAcSh (NAcSh outlined and AC=anterior commissure).
3.2. Vector placement validation
Accurate placement of the vectors was confirmed in all rats after the behavioral tests by extracting the brains and visualizing GFP fluorescence with a DFP flashlight while wearing VG2 barrier filter glasses. All rats had GFP expression in the left and right accumbens shell regions and spread of the vector was roughly spherical with a diameter of 1mm and generally between 2.2mm to 0.7mm anterior from bregma, which is expected with our stereotaxic coordinates. Although some GFP could be seen outside the NAcSh, the majority of GFP positive cells were in the accumbens shell, consistent with our previous vector studies (Green et al., 2006; Green et al., 2008; Wallace et al., 2008; Green et al., 2010). No rats were excluded from analysis based on vector placement (Figure 1C). Electrophysiological recordings were confined to GFP positive cells in the nucleus accumbens shell region via visual identification.
3.3. Knockdown of GSK3β in the NAcSh produces anxiolytic-like behavior
The effect of reduced GSK3β in the NAcSh on anxiety-like behavior was tested with three assays. We found that knockdown animals consumed more sucrose than controls (t(18)=−2.1, p=0.048) when unfamiliar with the taste of sucrose (Figure 2B). In addition, knockdown rats defecated less compared to controls in a mild-stress environment (i.e. cold; Figure 2C, U=29, p=0.049). We found no significant differences in open/closed arm exploration in the elevated plus maze due to high variance and no differences in open/closed arm entrances (Figure S1 A–B). Although we found no effect in the EPM test, the sucrose neophobia and cold stress defecation tests both indicate that knockdown of GSK3β in the NAcSh produces anxiolytic-like behavior.
Figure 2. Effect of GSK3β knockdown in the NAcSh on anxiety- and depression-like behavior.
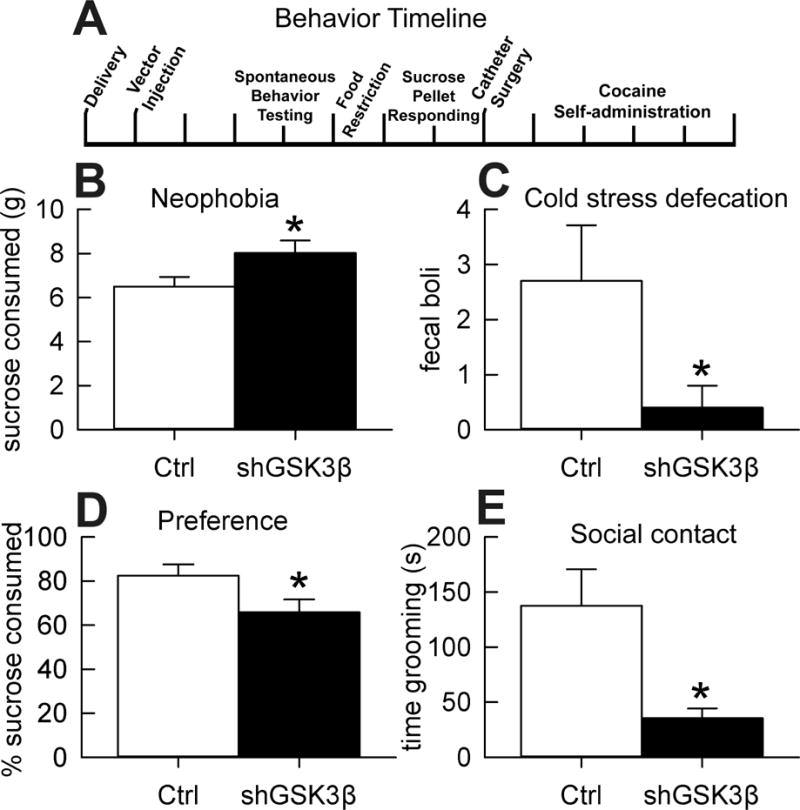
A. Timeline of behavioral experiments. B. Sucrose neophobia. Mean (±SEM) difference in 1% sucrose solution consumed for 30 min (n=10). C. Cold stress-induced defecation test. Mean (±SEM) fecal boli produced after 15 minutes of cold stress (n=10). D. Sucrose preference. Mean (±SEM) percent sucrose consumed (n=10–11). E. Social grooming behavior. Mean (±SEM) seconds spent grooming cage-mate across 30 min session (n=5). *Significant difference from controls, p<0.05.
3.4. Knockdown of GSK3β in the NAcSh increases depression-like behavior
Although GSK3β knockdown rats consumed more sucrose in the neophobia test (i.e. anxiety-related), we found that these same rats preferred the same sucrose concentration less than controls when familiar with the taste (i.e. depression-related; Figure 2D, t(19)=2.1, p=0.046). Modeling social withdrawal, we found that shGSK3β rats groomed each other less than controls after a brief separation (Figure 2E, t(4.5)=3, p=0.035). The assumption of homogeneity of variance in the social contact test was violated so the t-test degrees of freedom were adjusted to correct for this. It is important to note that this difference in grooming behavior was not due to differences in general locomotor activity, as spontaneous locomotor activity did not differ between knockdown and control rats (Figure S2). Sucrose preference is a model of anhedonia and social contact is a model of social withdrawal, therefore these results suggest knockdown of GSK3β in rat NAcSh increases depression-like behavior.
3.5. Knockdown of GSK3β in the NAcSh increases addiction-related behavior
Cocaine-taking and seeking behavior was assessed in GSK3β knockdown animals and controls using the intravenous cocaine self-administration paradigm. Prior to cocaine SA, shGSK3β and control rats were assessed for their ability to learn an operant task and we found no differences in operant responding for sucrose pellets (data not shown). However, we did find differences in cocaine-taking and seeking behavior. For acquisition, Mauchly’s Test of Sphericity indicated that the assumption of sphericity had been violated, χ2(20)=78.4, p<0.001, therefore degrees of freedom were corrected using Greenhouse-Geisser estimates of sphericity (ε=0.48). The results show a main effect of session during acquisition of cocaine self-administration (F(1,2.9)= 7.05, p<0.001) and a trend toward increased cocaine-taking in shGSK3β rats during acquisition (Figure 3A, F(1,19)=3.4, p=0.082). There was no significant interaction for acquisition of cocaine self-administration (Figure 3A, F(1,2.9)=1.15, p=0.33). For maintenance responding Mauchly’s Test of Sphericity indicated the assumption of sphericity had been violated, χ2(9)=21.7, p<0.01, therefore degrees of freedom were corrected using Greenhouse-Geisser estimates of sphericity (ε=0.66). There was a significant increase in maintenance responding at 0.5 mg/kg/inf in shGSK3β rats compared to control rats (Figure 3B, F(1,18)=6.3, p=0.021) with a main effect of session (F(1,2.62)=2.95, p=0.049). There was no significant interaction effect for maintenance responding (F(1,2.62)=0.48, p=0.672). We found no significant differences between shGSK3β and control rats in extinction responding or reinstatement responding (data not shown, p>0.05). For cocaine dose response, again Mauchly’s Test of Sphericity indicated the assumption of sphericity had been violated χ2(27)=83.2, p<0.001, therefore degrees of freedom were corrected using Greenhouse-Geisser estimates of sphericity (ε=0.42). There was no main effect of group (F(1,18)=2.06, p=0.168), however controls and shGSK3β rats both produced dose response functions for cocaine with a significant main effect of dose (F(1,2.96)=340, p<0.001) and there was a significant dose × group interaction (F(1,2.96)=3.7, p<0.018). The nature of this interaction was shGSK3β rats show increased intake at the highest dose. Overall, our cocaine self-administration results show that knockdown of GSK3β in the NAcSh altered addiction-related behavior, increasing maintenance responding, a trend toward increased acquisition, and an interaction of dose and group for dose response.
Figure 3. Knockdown of GSK3β in the NAcSh increases cocaine self-administration.
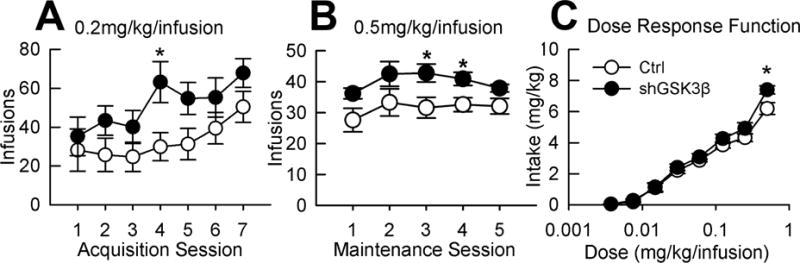
A. Effect on acquisition of cocaine self-administration. Mean (±SEM) number of infusions of cocaine in control or shGSK3β rats self-administering 0.2mg/kg/inf cocaine. B. Effect on maintenance of cocaine self-administration. Mean (±SEM) number of infusions of cocaine in control or shGSK3β rats of 0.5mg/kg/inf cocaine for 2 hr/session. C. Dose response function of self-administration. Mean (±SEM) intake (mg/kg) of cocaine at each dose (30 min ea). (n=10–11) *Significant difference from controls, p<0.05.
3.6. Knockdown of GSK3β in the NAcSh reduces spontaneous neuronal firing
To determine whether GSK3β knockdown correlated with functional outcomes of neuronal activity, AAV-shGSK3β or control vector was stereotaxically injected into the NAcSh and acute brain slices prepared 3–4 weeks later. Loose-patch recordings of visually identified, eGFP expressing neurons revealed that GSK3β knockdown led to a significant decrease in spontaneous firing rate compared to TANs from control animals (Figure 4A–B, t(36)=2.3, p=0.025) and an increase in the spike half-width (Figure 4C–D; t(35)=3.022, p=0.0047).
Figure 4. GSK3β knockdown in the NAcSh reduces tonic neuronal activity and increases action potential half width in NAcSh neurons.
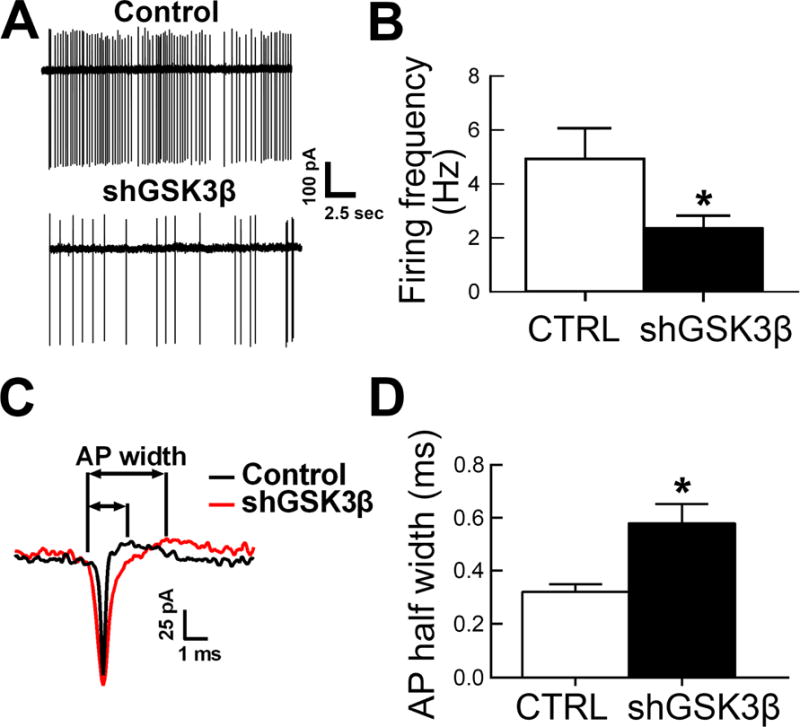
A. Representative traces of tonic activity in GSK3β knockdown compared to control NAcSh neurons. B. Summary bar graph of loose-patch recorded tonic activity, control n=16 cells, shGSK3β n=22 cells (mean±SEM); control n=2 rats, shGSK3β n=2. C. Representative traces of action potential width in GSK3β knockdown compared to control NAcSh neurons. D. Summary bar graph of AP half widths, control n=16 cells, shGSK3β n=21 cells (mean±SEM); control n=2 rats, shGSK3β n=2. *p<0.05.
3.7. Knockdown of GSK3β in the NAcSh altered intrinsic excitability of TANs
To determine whether reduced spontaneous firing in loose-patch correlated with changes in intrinsic excitability, whole-cell patch clamp recordings of TANs in both control vector and GSK3β knockdown vector conditions were performed. Representative traces of spontaneous firing recorded at resting membrane potential are shown for control vector in Figure 5A and GSK3β knockdown vector in Figure 5B. The spontaneous firing frequency was significantly lower in the cells transduced with the knockdown vector (t(26)=2.357, p=0.0262, Figure 5C). When TANs are hyperpolarized to negative voltages, a typical sag potential is observed as a result of activation of HCN-mediated Ih current (Bennett and Wilson, 1999; Wilson, 2005; Deng et al., 2007; Pisani et al., 2007). Accordingly, upon membrane hyperpolarization, typical depolarizing sag potentials in response to different current injections were observed in TANs from both control and knockdown groups (inset, 500 msec, 20 pA/step Figure 5D–E). Notably, though, GSK3β knockdown vector-transduced neurons exhibited a significant reduction in the amplitude of this hyperpolarization-induced sag at −80 pA (t(29.99)=2.704, p=0.011), −100 pA (t(26.5)=2.670, p=0.013), and −120 pA (t(26.66)=2.710, p=0.012). Intrinsic excitability of GSK3β knockdown transduced TANs and controls was further characterized by analyzing the input-output curve corresponding to the neuron firing frequency in response to incremental steps of depolarizing current injections (Figure 5G–I). As illustrated in Figure 5I, no changes in firing frequency were found in the two groups across all tested current steps. However, additional analysis revealed significant changes in the action potential (AP) kinetics and threshold induced by GSK3β knockdown (Figure 5J–N). In GSK3β knockdown transduced TANs, the AP maximum rise was reduced compared to the control vector (t(31)=2.167, p=0.038; Figure 5K) while no significant changes were found in the AP maximum decay (Figure 5L). Furthermore, AP current threshold was significantly increased in the GSK3β knockdown group (U(220,341)=84, p=0.0423; Figure 5N), while no changes were found in AP voltage threshold (Figure 5M). These results indicate that knockdown of GSK3β in the NAcSh alters intrinsic excitability of TANs, likely contributing to reduced spontaneous firing.
Figure 5. Whole cell patch clamp recordings of neurons with spontaneous activity in both control vector and GSK3β knockdown vector show tonically active interneuron (TAN) phenotype and GSK3β knockdown in the NAcSh reduces spontaneous neuronal activity in identified tonically active interneurons (TANs).
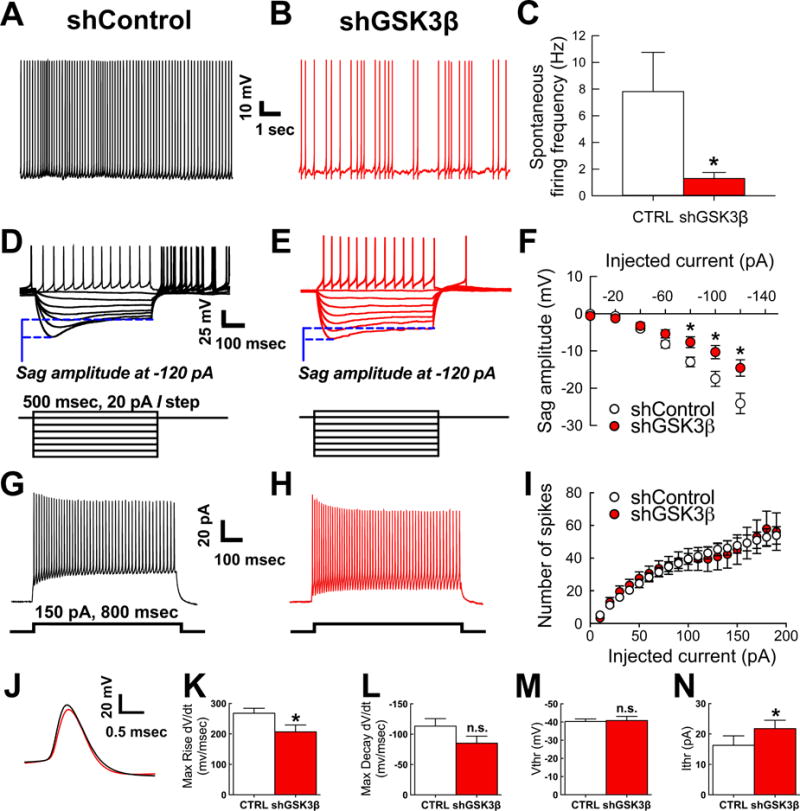
A–B. Representative traces of spontaneous firing at resting membrane potential recorded from shControl (A) and shGSK3β (B) neurons in whole-cell patch clamp. C. Summary bar graph of action potential firing frequency recorded in whole cell, control n=13 cells and shGSK3β n=15 cells (mean±SEM). Control n=6 rats, shGSK3β n=8 rats. D–E. Passive properties of same neurons as A–B showing hyperpolarizing sag and action potential rebound in control (D) and shGSK3β knockdown (E) with injected current (500 msec, 20 pA/step). F. Summary graph of sag amplitude (mV) at different injected current (pA) showing reduced sag amplitude in GSK3β knockdown (n=18 cells) versus control (n=13 cells) (mean±SEM). Control n=6 rats, shGSK3β n=8 rats. G. and H. Representative traces from control vector (G) and shGSK3β vector (H) transduced TANs showing trains of evoked action potentials at 800 msec and 150 pA current step injection. I. Input-output curve of evoked AP trains in response to repetitive current steps of constant increment. J. Action potential waveforms evoked at the current threshold are illustrated for control (black line) or shGSK3β vector (red line) transduced TANs. K. Bar graph showing reduced maximum rise (dV/dt, mV/msec) in GSK3β knockdown (n=17 cells) versus control (n=16 cells) (mean±SEM); control n=6 rats, shGSK3β n=8 rats. L. Bar graph showing no significant difference in action potential maximum decay (dV/dt, mV/msec) in GSK3β knockdown (n=17 cells) versus control (n=16 cells) (mean±SEM); control n=6 rats, shGSK3β n=8 rats. M. Bar graph showing no difference in the action potential voltage threshold (Vtrh, mV) in GSK3β knockdown (n=17 cells) versus control (n=16 cells) (mean±SEM); control n=6 rats, shGSK3β n=8 rats. N. Bar graph showing increased current threshold (Itrh, pA) in GSK3β knockdown (n=17 cells) versus control (n=16 cells) (mean±SEM); control n=6 rats, shGSK3β n=8 rats. *p<0.05.
4. Discussion
This study found that GSK3β in the NAcSh of rats modulates addiction-, depression-, and anxiety-related behavior and also causes a reduction in spontaneous activity of TANs attributable to changes in intrinsic excitability. However, it is important to note that with the current state of technology, it is impossible to state for certain that the observed electrophysiological results in TANs directly produced the observed behavioral effects. Regardless, given that GSK3β knockdown increases rather than decreases cocaine self-administration, these behavioral results are more consistent with decreased intrinsic excitability of TANs than that of medium spiny neurons (see discussion below).
Knockdown of GSK3β in the NAcSh specifically results in decreased anxiety-like behavior in the sucrose neophobia and cold-stress induced defecation tests but no change in behavior in the elevated plus maze, likely due to the high within-group variance inherent in this behavior. Additionally, knockdown of GSK3β increased depression-like behavior in the sucrose preference and social contact tests and increased drug taking behavior in cocaine self-administration maintenance responding, with a trend for an increase in acquisition. However, there were no differences in drug seeking behavior in extinction, progressive ratio, or cocaine-induced reinstatement tests.
Although depression and anxiety are often (but certainly not always) comorbid in humans (Kessler et al., 2005; Pettinati et al., 2013), the opposite modulation of depression and anxiety in the current studies is perfectly consistent with previous manipulation of the NAcSh, (Green et al., 2006; Green et al., 2008; Green et al., 2010) as is the congruent behavioral phenotypes for depression and addiction (Hikida et al., 2001; Green et al., 2010; Larson et al., 2011; Warner-Schmidt et al., 2012). This could be idiosyncratic to the NAcSh, or to rats in general; regardless, the current behavioral phenotypes (decreased anxiety-like, but increased depression- and addiction-like behavior) are perfectly consistent with what we understand about the rat NAcSh.
In behaving animals, tonically active cholinergic interneurons modulate release of dopamine in the accumbens and have been shown to play an important role in drug-related behaviors (Green et al., 2001; Berlanga et al., 2003; Pisani et al., 2007; Witten et al., 2010; Cachope et al., 2012). Consistent with our current behavioral and electrophysiological results, it has been reported that increased depression-like behavior and addiction-related behavior in the NAc can be induced by ablation of tonically active cholinergic interneurons (Hikida et al., 2001; Warner-Schmidt et al., 2012).
Tonically active neurons (TANs) are critical interneurons in the NAcSh previously shown to be important for reward processing (Hikida et al., 2001; Berlanga et al., 2003; Apicella, 2007; Witten et al., 2010; Cachope et al., 2012). They receive inputs from the cortex (glutamatergic), substantia nigra and VTA (dopaminergic), and from medium spiny neurons (GABAergic) and synapse mainly onto MSNs and other tonically active neurons (Lenz and Lobo, 2013). Although TANs are a relatively small percentage of the total neuronal population in the accumbens, they exert a powerful modulation of the NAc circuit activity through highly divergent and dense axonal projections (Lim et al., 2014) and are capable of altering cocaine conditioned place preference behavior in freely moving animals (Witten et al., 2010). In addition, TANs in the NAcSh are activated by cocaine self-administration (Berlanga et al., 2003). The regulation of TANs appears to be brain sub-region specific, for optogenetic activation of dopamine neurons causes TANs in the NAcSh to fire a specific burst-pause pattern, but other regions like the nucleus accumbens core and the dorsal striatum do not consistently show this pattern in response to dopaminergic activation (Chuhma et al., 2014). Additionally, optogenetic activation of TANs in the accumbens stimulates release of dopamine (Cachope et al., 2012) and reduced number of TANs causes hyperresponsiveness of the dopamine system and increased sensitivity to cocaine (Hikida et al., 2001; Laplante et al., 2011). This suggests that reduced TAN firing in the NAcSh is integral to depression-like behavior and cocaine self-administration, but because the GSK3β shRNA vector also transduced MSNs, further work is needed to determine a direct causal link between GSK3β in TANs and the behavioral results.
GSK3β has not yet been implicated in the reward processing of TANs, however, GSK3β has been suggested to be downstream of dopamine receptors, specifically the dopamine D2 receptor DRD2 (Beaulieu et al., 2011; Sutton and Rushlow, 2012; Urs et al., 2012). Dopamine receptors typically signal through G proteins, however they also have G protein independent signaling through β-arrestin, which is upstream of AKT and GSK3. There is some evidence that GSK3β even forms a protein complex with the dopamine D2 receptor (Sutton and Rushlow, 2012). Previous work has shown that D2 receptors in the NAcSh are important for the reinstatement of cocaine seeking (Anderson et al., 2006). D2 receptors are localized on cholinergic interneurons in the NAcSh (Alcantara et al., 2003) and D2 receptor agonists and antagonists but not D1 receptor compounds alter the activity of TANs (Deng et al., 2007). Therefore, knockdown of GSK3β may be influencing dopamine signals, contributing to TAN firing reduction.
The previous paragraph addresses mechanisms upstream of GSK3β, but the question remains how GSK3β can alter neuronal activity of TANs. The electrophysiological phenotypes reported in this study are consistent with a role of GSK3β in modulating spontaneous neuronal firing in TANs through effects on voltage-gated ion channels that are independent from network activity.
TANs recorded in vivo respond to sensory stimuli associated with learned behavior with a transient depression of tonic firing (Wilson et al., 1990; Reynolds et al., 2004; Apicella, 2007). Unlike in other neurons, firing in TANs is largely controlled by a combination of intrinsic voltage-gated conductances including Ih, Na+ persistent currents, and hyperpolarization-activated K+ channels that drive oscillations of the membrane potential (Wilson, 2005). Our whole-cell patch clamp analysis indicates that knockdown of GSK3β suppresses hyperpolarization-induced sag potentials and decreases action potential max rise while increasing AP threshold, suggesting a role of the Ih pacemaker current in establishing these phenotypes (Ko et al., 2016). TANs firing is controlled by the neuron intrinsic properties that usually dominate over stimuli arising from synaptic inputs (Wilson, 2005). Thus, it is conceivable that the suppression of spontaneous firing induced by GSK3β knockdown in the intact NAcSh circuit (Figure 4 and Figure 5A–C) is driven by autonomous changes in the intrinsic properties of the neurons rather than being network-driven.
Previous results from the hippocampus indicate that GSK3β regulates protein-protein interactions within the voltage-gated sodium channel (Nav) complex (Shavkunov et al., 2013) and investigation of Nav1.2 specifically found that GSK3β phosphorylates this channel, altering its functional properties (James et al., 2015). The lack of changes in maximum firing frequency of evoked action potentials in TANs expressing AAV-shGSK3β (Figure 5I) argues against a major involvement of Nav channels in mediating the observed phenotypes. However, which specific channels are GSK3β substrates in TANs should be addressed with future voltage-clamp studies.
The AKT/GSK3 intracellular signaling cascade is generally implicated in the etiology of several psychiatric disorders with relevance to the nucleus accumbens (Beaulieu et al., 2009). Dysregulation of GSK3β is thought to play a role in susceptibility to depression and bipolar disorder (Jope, 2011) and is associated with other diseases such as schizophrenia, and even Alzheimer’s disease (Kozlovsky et al., 2001; Emamian et al., 2004; Balaraman et al., 2006; Hooper et al., 2008). Efforts to globally knock out GSK3β in mice have proven embryonically lethal (Hoeflich et al., 2000), but heterozygous knockout mice survive and provide insights into the importance of GSK3β. These mice show reductions in depression-like behavior similar to the behavioral results seen with lithium, an inhibitor of GSK3β (O’Brien et al., 2004). However, lithium itself is not just an inhibitor of GSK3β but has other mechanisms of action and it is likely that the behavioral effects of the heterozygous knockout mice are a result of GSK3β action during development or in a different brain region, underscoring the utility of region-specific knockdown of GSK3β in adult animals.
Wilkinson et al., 2011 found that after social defeat stress, mice injected with a herpes simplex virus (HSV) overexpressing GSK3β in the nucleus accumbens showed a depression-like phenotype. Whereas the current study found that with little prior stress exposure, rats with decreased GSK3β in the nucleus accumbens with an AAV vector also show a depression-like phenotype, indicating modulatory effects of GSK3β under various levels of stress exposure. These seemingly disparate findings show GSK3β has important modulatory roles that can be adaptive or maladaptive even in the same brain region depending on stress exposure. We have previously hypothesized that the robust behavioral phenotypes resulting from environmental enrichment (i.e. decreases in depression-like behavior and decreases in cocaine self-administration (Green et al., 2010)) result from repeated mild stress exposure in the enriched animals and a lack of stress exposure in isolated animals (Crofton et al., 2015). Chronic mild stress in the case of environmental enrichment is adaptive whereas a severe stressor, such as social defeat, is maladaptive (Crofton et al., 2015). Therefore, our behavioral findings with knockdown of GSK3β shows similar depression-like behavioral effects as overexpression of GSK3β by Wilkinson et al., 2011 perhaps because of vast differences in stress exposure.
Previous evidence has suggested a role for GSK3β in addictive behaviors, particularly cocaine behaviors such as involvement in conditioned place preference, cocaine reward memory, cocaine hyperactivity, and cocaine behavioral sensitization (Miller et al., 2009; Xu et al., 2009; Miller et al., 2010; Miller et al., 2014; Shi et al., 2014). The current study assessed operant self-administration rather than locomotor activity in response to stimulants or place preference behavior and is the first to directly implicate GSK3β function in the NAcSh as a mediator of cocaine self-administration. We found that GSK3β knockdown in the NAcSh of rats caused an increase in cocaine self-administration, especially at high unit doses of cocaine.
These results show that GSK3β in the NAcSh modulates behaviors related to addiction, depression, and anxiety. Further, loss of GSK3β specifically decreases spontaneous neuronal activity in TANs of the NAcSh, a neuronal population that plays an important role in these behaviors. Future understanding of the role of GSK3β signaling in the NAcSh could be informative for the development of novel pharmacotherapeutics.
Supplementary Material
Highlights.
Specific knockdown of GSK3 beta in the NAc shell induces an anxiolytic-like effect
Knockdown of GSK3 beta in the NAcSh induces depression-like behavior
Viral-mediated knockdown of GSK3 beta increases cocaine self-administration
Knockdown also reduces spontaneous firing and alters intrinsic excitability of TANs
Acknowledgments
Funding: This work was supported by the National Institute on Drug Abuse at the National Institutes of Health (grant numbers: R01 DA029091 (TAG), T32 DA007287 (EJC)) and the National Institute of Mental Health (R01 MH095995 (FL)).
Abbreviations
- NAcSh
Nucleus accumbens shell
- GSK3β
glycogen synthase kinase 3 beta
- AAV-shCTRL
control vector
- AAV-shGSK3β
GSK3β knockdown vector
- TANs
tonically active interneurons
- EC
enriched condition
- IC
isolated condition
- MSN
medium spiny neuron
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest: The authors declare no financial conflicts of interest.
References
- Alcantara AA, Chen V, Herring BE, Mendenhall JM, Berlanga ML. Localization of dopamine D2 receptors on cholinergic interneurons of the dorsal striatum and nucleus accumbens of the rat. Brain research. 2003;986:22–29. doi: 10.1016/s0006-8993(03)03165-2. [DOI] [PubMed] [Google Scholar]
- Anagnostaras SG, Murphy GG, Hamilton SE, Mitchell SL, Rahnama NP, Nathanson NM, Silva AJ. Selective cognitive dysfunction in acetylcholine M1 muscarinic receptor mutant mice. Nature neuroscience. 2003;6:51–58. doi: 10.1038/nn992. [DOI] [PubMed] [Google Scholar]
- Anastasio NC, Stutz SJ, Fox RG, Sears RM, Emeson RB, DiLeone RJ, O’Neil RT, Fink LH, Li D, Green TA, Moeller FG, Cunningham KA. Functional status of the serotonin 5-HT2C receptor (5-HT2CR) drives interlocked phenotypes that precipitate relapse-like behaviors in cocaine dependence. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2014;39:370–382. doi: 10.1038/npp.2013.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson SM, Schmidt HD, Pierce RC. Administration of the D2 dopamine receptor antagonist sulpiride into the shell, but not the core, of the nucleus accumbens attenuates cocaine priming-induced reinstatement of drug seeking. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2006;31:1452–1461. doi: 10.1038/sj.npp.1300922. [DOI] [PubMed] [Google Scholar]
- Aosaki T, Tsubokawa H, Ishida A, Watanabe K, Graybiel AM, Kimura M. Responses of tonically active neurons in the primate’s striatum undergo systematic changes during behavioral sensorimotor conditioning. The Journal of neuroscience. 1994;14:3969–3984. doi: 10.1523/JNEUROSCI.14-06-03969.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apicella P. Tonically active neurons in the primate striatum and their role in the processing of information about motivationally relevant events. European Journal of Neuroscience. 2002;16:2017–2026. doi: 10.1046/j.1460-9568.2002.02262.x. [DOI] [PubMed] [Google Scholar]
- Apicella P. Leading tonically active neurons of the striatum from reward detection to context recognition. Trends in neurosciences. 2007;30:299–306. doi: 10.1016/j.tins.2007.03.011. [DOI] [PubMed] [Google Scholar]
- Balaraman Y, Limaye AR, Levey AI, Srinivasan S. Glycogen synthase kinase 3beta and Alzheimer’s disease: pathophysiological and therapeutic significance. Cellular and molecular life sciences : CMLS. 2006;63:1226–1235. doi: 10.1007/s00018-005-5597-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaulieu JM, Gainetdinov RR, Caron MG. Akt/GSK3 signaling in the action of psychotropic drugs. Annual review of pharmacology and toxicology. 2009;49:327–347. doi: 10.1146/annurev.pharmtox.011008.145634. [DOI] [PubMed] [Google Scholar]
- Beaulieu JM, Del’guidice T, Sotnikova TD, Lemasson M, Gainetdinov RR. Beyond cAMP: The Regulation of Akt and GSK3 by Dopamine Receptors. Front Mol Neurosci. 2011;4:38. doi: 10.3389/fnmol.2011.00038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett BD, Wilson CJ. Spontaneous activity of neostriatal cholinergic interneurons in vitro. The Journal of neuroscience. 1999;19:5586–5596. doi: 10.1523/JNEUROSCI.19-13-05586.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benzon C, Johnson S, McCue D, Li D, Green T, Hommel J. Neuromedin U receptor 2 knockdown in the paraventricular nucleus modifies behavioral responses to obesogenic high-fat food and leads to increased body weight. Neuroscience. 2014;258:270–279. doi: 10.1016/j.neuroscience.2013.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berlanga M, Olsen C, Chen V, Ikegami A, Herring B, Duvauchelle C, Alcantara A. Cholinergic interneurons of the nucleus accumbens and dorsal striatum are activated by the self-administration of cocaine. Neuroscience. 2003;120:1149–1156. doi: 10.1016/s0306-4522(03)00378-6. [DOI] [PubMed] [Google Scholar]
- Cachope R, Mateo Y, Mathur BN, Irving J, Wang H-L, Morales M, Lovinger DM, Cheer JF. Selective activation of cholinergic interneurons enhances accumbal phasic dopamine release: setting the tone for reward processing. Cell reports. 2012;2:33–41. doi: 10.1016/j.celrep.2012.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuhma N, Mingote S, Moore H, Rayport S. Dopamine neurons control striatal cholinergic neurons via regionally heterogeneous dopamine and glutamate signaling. Neuron. 2014;81:901–912. doi: 10.1016/j.neuron.2013.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crofton EJ, Zhang Y, Green TA. Inoculation stress hypothesis of environmental enrichment. Neuroscience and biobehavioral reviews. 2015;49c:19–31. doi: 10.1016/j.neubiorev.2014.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng P, Zhang Y, Xu ZC. Involvement of Ih in dopamine modulation of tonic firing in striatal cholinergic interneurons. The Journal of neuroscience. 2007;27:3148–3156. doi: 10.1523/JNEUROSCI.5535-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emamian ES, Hall D, Birnbaum MJ, Karayiorgou M, Gogos JA. Convergent evidence for impaired AKT1-GSK3βeta signaling in schizophrenia. Nature genetics. 2004;36:131–137. doi: 10.1038/ng1296. [DOI] [PubMed] [Google Scholar]
- English DF, Ibanez-Sandoval O, Stark E, Tecuapetla F, Buzsaki G, Deisseroth K, Tepper JM, Koos T. GABAergic circuits mediate the reinforcement-related signals of striatal cholinergic interneurons. Nat Neurosci. 2012;15:123–130. doi: 10.1038/nn.2984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furey ML, Pietrini P, Haxby JV, Drevets WC. Selective effects of cholinergic modulation on task performance during selective attention. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2008;33:913–923. doi: 10.1038/sj.npp.1301461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green TA, Gehrke BJ, Bardo MT. Environmental enrichment decreases intravenous amphetamine self-administration in rats: dose-response functions for fixed- and progressive-ratio schedules. Psychopharmacology. 2002;162:373–378. doi: 10.1007/s00213-002-1134-y. [DOI] [PubMed] [Google Scholar]
- Green TA, Crooks PA, Bardo MT, Dwoskin LP. Contributory role for nornicotine in nicotine neuropharmacology: nornicotine-evoked [3 H] dopamine overflow from rat nucleus accumbens slices. Biochemical pharmacology. 2001;62:1597–1603. doi: 10.1016/s0006-2952(01)00838-3. [DOI] [PubMed] [Google Scholar]
- Green TA, Alibhai IN, Unterberg S, Neve RL, Ghose S, Tamminga CA, Nestler EJ. Induction of activating transcription factors (ATFs) ATF2, ATF3, and ATF4 in the nucleus accumbens and their regulation of emotional behavior. The Journal of Neuroscience. 2008;28:2025–2032. doi: 10.1523/JNEUROSCI.5273-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green TA, Alibhai IN, Hommel JD, DiLeone RJ, Kumar A, Theobald DE, Neve RL, Nestler EJ. Induction of inducible cAMP early repressor expression in nucleus accumbens by stress or amphetamine increases behavioral responses to emotional stimuli. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2006;26:8235–8242. doi: 10.1523/JNEUROSCI.0880-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green TA, Alibhai IN, Roybal CN, Winstanley CA, Theobald DE, Birnbaum SG, Graham AR, Unterberg S, Graham DL, Vialou V, Bass CE, Terwilliger EF, Bardo MT, Nestler EJ. Environmental enrichment produces a behavioral phenotype mediated by low cyclic adenosine monophosphate response element binding (CREB) activity in the nucleus accumbens. Biological psychiatry. 2010;67:28–35. doi: 10.1016/j.biopsych.2009.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimes CA, Jope RS. The multifaceted roles of glycogen synthase kinase 3beta in cellular signaling. Prog Neurobiol. 2001;65:391–426. doi: 10.1016/s0301-0082(01)00011-9. [DOI] [PubMed] [Google Scholar]
- Higley MJ, Gittis AH, Oldenburg IA, Balthasar N, Seal RP, Edwards RH, Lowell BB, Kreitzer AC, Sabatini BL. Cholinergic interneurons mediate fast VGluT3-dependent glutamatergic transmission in the striatum. PLoS One. 2011;6:e19155. doi: 10.1371/journal.pone.0019155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hikida T, Kitabatake Y, Pastan I, Nakanishi S. Acetylcholine enhancement in the nucleus accumbens prevents addictive behaviors of cocaine and morphine. Proceedings of the National Academy of Sciences. 2003;100:6169–6173. doi: 10.1073/pnas.0631749100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hikida T, Kaneko S, Isobe T, Kitabatake Y, Watanabe D, Pastan I, Nakanishi S. Increased sensitivity to cocaine by cholinergic cell ablation in nucleus accumbens. Proc Natl Acad Sci U S A. 2001;98:13351–13354. doi: 10.1073/pnas.231488998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoeflich KP, Luo J, Rubie EA, Tsao M-S, Jin O, Woodgett JR. Requirement for glycogen synthase kinase-3β in cell survival and NF-κB activation. Nature. 2000;406:86–90. doi: 10.1038/35017574. [DOI] [PubMed] [Google Scholar]
- Hommel JD, Sears RM, Georgescu D, Simmons DL, DiLeone RJ. Local gene knockdown in the brain using viral-mediated RNA interference. Nature medicine. 2003;9:1539–1544. doi: 10.1038/nm964. [DOI] [PubMed] [Google Scholar]
- Hooper C, Killick R, Lovestone S. The GSK3 hypothesis of Alzheimer’s disease. J Neurochem. 2008;104:1433–1439. doi: 10.1111/j.1471-4159.2007.05194.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu YS, Long N, Pigino G, Brady ST, Lazarov O. Molecular mechanisms of environmental enrichment: impairments in Akt/GSK3βeta, neurotrophin-3 and CREB signaling. PloS one. 2013;8:e64460. doi: 10.1371/journal.pone.0064460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James TF, Nenov MN, Wildburger NC, Lichti CF, Luisi J, Vergara F, Panova-Electronova NI, Nilsson CL, Rudra JS, Green TA, Labate D, Laezza F. The Na1.2 channel is regulated by GSK3. Biochimica et biophysica acta. 2015;1850:832–844. doi: 10.1016/j.bbagen.2015.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jope RS. Glycogen synthase kinase-3 in the etiology and treatment of mood disorders. Front Mol Neurosci. 2011;4:16. doi: 10.3389/fnmol.2011.00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, Chiu WT, Demler O, Merikangas KR, Walters EE. Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication. Archives of general psychiatry. 2005;62:617–627. doi: 10.1001/archpsyc.62.6.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein PS, Melton DA. A molecular mechanism for the effect of lithium on development. Proc Natl Acad Sci U S A. 1996;93:8455–8459. doi: 10.1073/pnas.93.16.8455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko KW, Rasband MN, Meseguer V, Kramer RH, Golding NL. Serotonin modulates spike probability in the axon initial segment through HCN channels. Nature neuroscience. 2016 doi: 10.1038/nn.4293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozlovsky N, Belmaker RH, Agam G. Low GSK-3 activity in frontal cortex of schizophrenic patients. Schizophrenia research. 2001;52:101–105. doi: 10.1016/s0920-9964(00)00174-2. [DOI] [PubMed] [Google Scholar]
- Laplante F, Lappi DA, Sullivan RM. Cholinergic depletion in the nucleus accumbens: effects on amphetamine response and sensorimotor gating. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2011;35:501–509. doi: 10.1016/j.pnpbp.2010.12.005. [DOI] [PubMed] [Google Scholar]
- Larson EB, Graham DL, Arzaga RR, Buzin N, Webb J, Green TA, Bass CE, Neve RL, Terwilliger EF, Nestler EJ, Self DW. Overexpression of CREB in the nucleus accumbens shell increases cocaine reinforcement in self-administering rats. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2011;31:16447–16457. doi: 10.1523/JNEUROSCI.3070-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latapy C, Rioux V, Guitton MJ, Beaulieu J-M. Selective deletion of forebrain glycogen synthase kinase 3β reveals a central role in serotonin-sensitive anxiety and social behaviour. Philosophical Transactions of the Royal Society of London B: Biological Sciences. 2012;367:2460–2474. doi: 10.1098/rstb.2012.0094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenz JD, Lobo MK. Optogenetic insights into striatal function and behavior. Behavioural brain research. 2013;255:44–54. doi: 10.1016/j.bbr.2013.04.018. [DOI] [PubMed] [Google Scholar]
- Lim SAO, Kang UJ, McGehee DS. Striatal cholinergic interneuron regulation and circuit effects. Frontiers in synaptic neuroscience. 2014;6:22. doi: 10.3389/fnsyn.2014.00022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller JS, Tallarida RJ, Unterwald EM. Cocaine-induced hyperactivity and sensitization are dependent on GSK3. Neuropharmacology. 2009;56:1116–1123. doi: 10.1016/j.neuropharm.2009.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller JS, Tallarida RJ, Unterwald EM. Inhibition of GSK3 attenuates dopamine D1 receptor agonist-induced hyperactivity in mice. Brain Res Bull. 2010;82:184–187. doi: 10.1016/j.brainresbull.2010.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller JS, Barr JL, Harper LJ, Poole RL, Gould TJ, Unterwald EM. The GSK3 signaling pathway is activated by cocaine and is critical for cocaine conditioned reward in mice. PloS one. 2014;9:e88026. doi: 10.1371/journal.pone.0088026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris G, Arkadir D, Nevet A, Vaadia E, Bergman H. Coincident but distinct messages of midbrain dopamine and striatal tonically active neurons. Neuron. 2004;43:133–143. doi: 10.1016/j.neuron.2004.06.012. [DOI] [PubMed] [Google Scholar]
- Nenov MN, Tempia F, Denner L, Dineley KT, Laezza F. Impaired firing properties of dentate granule neurons in an Alzheimer’s disease animal model are rescued by PPARgamma agonism. Journal of neurophysiology. 2015;113:1712–1726. doi: 10.1152/jn.00419.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestler EJ, Carlezon WA., Jr The mesolimbic dopamine reward circuit in depression. Biological psychiatry. 2006;59:1151–1159. doi: 10.1016/j.biopsych.2005.09.018. [DOI] [PubMed] [Google Scholar]
- Nwaneshiudu CA, Unterwald EM. NK-3 receptor antagonism prevents behavioral sensitization to cocaine: a role of glycogen synthase kinase-3 in the nucleus accumbens. J Neurochem. 2010;115:635–642. doi: 10.1111/j.1471-4159.2010.06973.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Brien WT, Harper AD, Jove F, Woodgett JR, Maretto S, Piccolo S, Klein PS. Glycogen synthase kinase-3beta haploinsufficiency mimics the behavioral and molecular effects of lithium. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2004;24:6791–6798. doi: 10.1523/JNEUROSCI.4753-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortinski PI, Briand LA, Pierce RC, Schmidt HD. Cocaine-seeking is associated with PKC-dependent reduction of excitatory signaling in accumbens shell D2 dopamine receptor-expressing neurons. Neuropharmacology. 2015;92C:80–89. doi: 10.1016/j.neuropharm.2015.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardo M, King MK, Perez-Costas E, Melendez-Ferro M, Martinez A, Beurel E, Jope RS. Impairments in cognition and neural precursor cell proliferation in mice expressing constitutively active glycogen synthase kinase-3. Frontiers in Behavioral Neuroscience. 2015;9 doi: 10.3389/fnbeh.2015.00055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrine SA, Miller JS, Unterwald EM. Cocaine regulates protein kinase B and glycogen synthase kinase-3 activity in selective regions of rat brain. J Neurochem. 2008;107:570–577. doi: 10.1111/j.1471-4159.2008.05632.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettinati HM, O’Brien CP, Dundon WD. Current status of co-occurring mood and substance use disorders: a new therapeutic target. The American journal of psychiatry. 2013;170:23–30. doi: 10.1176/appi.ajp.2012.12010112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pisani A, Bernardi G, Ding J, Surmeier DJ. Re-emergence of striatal cholinergic interneurons in movement disorders. Trends in neurosciences. 2007;30:545–553. doi: 10.1016/j.tins.2007.07.008. [DOI] [PubMed] [Google Scholar]
- Pliakas AM, Carlson RR, Neve RL, Konradi C, Nestler EJ, Carlezon WA., Jr Altered responsiveness to cocaine and increased immobility in the forced swim test associated with elevated cAMP response element-binding protein expression in nucleus accumbens. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2001;21:7397–7403. doi: 10.1523/JNEUROSCI.21-18-07397.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pontieri FE, Tanda G, Di Chiara G. Intravenous cocaine, morphine, and amphetamine preferentially increase extracellular dopamine in the “shell” as compared with the “core” of the rat nucleus accumbens. Proc Natl Acad Sci U S A. 1995;92:12304–12308. doi: 10.1073/pnas.92.26.12304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds JN, Hyland BI, Wickens JR. Modulation of an afterhyperpolarization by the substantia nigra induces pauses in the tonic firing of striatal cholinergic interneurons. The Journal of neuroscience. 2004;24:9870–9877. doi: 10.1523/JNEUROSCI.3225-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder JP, Spanos M, Stevenson JR, Besheer J, Salling M, Hodge CW. Neuropharmacology. England: 2008. Cue-induced reinstatement of alcohol-seeking behavior is associated with increased ERK1/2 phosphorylation in specific limbic brain regions: blockade by the mGluR5 antagonist MPEP; pp. 546–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Self DW, Genova LM, Hope BT, Barnhart WJ, Spencer JJ, Nestler EJ. Involvement of cAMP-dependent protein kinase in the nucleus accumbens in cocaine self-administration and relapse of cocaine-seeking behavior. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1998;18:1848–1859. doi: 10.1523/JNEUROSCI.18-05-01848.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shavkunov AS, Wildburger NC, Nenov MN, James TF, Buzhdygan TP, Panova-Elektronova NI, Green TA, Veselenak RL, Bourne N, Laezza F. The fibroblast growth factor 14.voltage-gated sodium channel complex is a new target of glycogen synthase kinase 3 (GSK3) The Journal of biological chemistry. 2013;288:19370–19385. doi: 10.1074/jbc.M112.445924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi X, Miller JS, Harper LJ, Poole RL, Gould TJ, Unterwald EM. Reactivation of cocaine reward memory engages the Akt/GSK3/mTOR signaling pathway and can be disrupted by GSK3 inhibition. Psychopharmacology. 2014;231:3109–3118. doi: 10.1007/s00213-014-3491-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stambolic V, Ruel L, Woodgett JR. Lithium inhibits glycogen synthase kinase-3 activity and mimics wingless signalling in intact cells. Current biology: CB. 1996;6:1664–1668. doi: 10.1016/s0960-9822(02)70790-2. [DOI] [PubMed] [Google Scholar]
- Sutton LP, Rushlow WJ. The dopamine D2 receptor regulates Akt and GSK-3 via Dvl-3. International Journal of Neuropsychopharmacology. 2012;15:965–979. doi: 10.1017/S146114571100109X. [DOI] [PubMed] [Google Scholar]
- Urs NM, Snyder JC, Jacobsen JP, Peterson SM, Caron MG. Deletion of GSK3βeta in D2R-expressing neurons reveals distinct roles for beta-arrestin signaling in antipsychotic and lithium action. Proc Natl Acad Sci U S A. 2012;109:20732–20737. doi: 10.1073/pnas.1215489109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace DL, Vialou V, Rios L, Carle-Florence TL, Chakravarty S, Kumar A, Graham DL, Green TA, Kirk A, Iniguez SD. The influence of ΔFosB in the nucleus accumbens on natural reward-related behavior. The Journal of Neuroscience. 2008;28:10272–10277. doi: 10.1523/JNEUROSCI.1531-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warner-Schmidt JL, Schmidt EF, Marshall JJ, Rubin AJ, Arango-Lievano M, Kaplitt MG, Ibanez-Tallon I, Heintz N, Greengard P. Cholinergic interneurons in the nucleus accumbens regulate depression-like behavior. Proc Natl Acad Sci U S A. 2012;109:11360–11365. doi: 10.1073/pnas.1209293109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wildburger NC, Laezza F. Control of neuronal ion channel function by glycogen synthase kinase-3: new prospective for an old kinase. Front Mol Neurosci. 2012;5:80. doi: 10.3389/fnmol.2012.00080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson MB, Dias C, Magida J, Mazei-Robison M, Lobo M, Kennedy P, Dietz D, Covington H, 3rd, Russo S, Neve R, Ghose S, Tamminga C, Nestler EJ. A novel role of the WNT-dishevelled-GSK3βeta signaling cascade in the mouse nucleus accumbens in a social defeat model of depression. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2011;31:9084–9092. doi: 10.1523/JNEUROSCI.0039-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams MJ, Adinoff B. The role of acetylcholine in cocaine addiction. Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology. 2008;33:1779–1797. doi: 10.1038/sj.npp.1301585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson C, Chang H, Kitai S. Firing patterns and synaptic potentials of identified giant aspiny interneurons in the rat neostriatum. The Journal of neuroscience. 1990;10:508–519. doi: 10.1523/JNEUROSCI.10-02-00508.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson CJ. The mechanism of intrinsic amplification of hyperpolarizations and spontaneous bursting in striatal cholinergic interneurons. Neuron. 2005;45:575–585. doi: 10.1016/j.neuron.2004.12.053. [DOI] [PubMed] [Google Scholar]
- Witten IB, Lin S-C, Brodsky M, Prakash R, Diester I, Anikeeva P, Gradinaru V, Ramakrishnan C, Deisseroth K. Cholinergic interneurons control local circuit activity and cocaine conditioning. Science (New York, NY) 2010;330:1677–1681. doi: 10.1126/science.1193771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu CM, Wang J, Wu P, Zhu WL, Li QQ, Xue YX, Zhai HF, Shi J, Lu L. Glycogen synthase kinase 3beta in the nucleus accumbens core mediates cocaine-induced behavioral sensitization. J Neurochem. 2009;111:1357–1368. doi: 10.1111/j.1471-4159.2009.06414.x. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Crofton EJ, Li D, Lobo MK, Fan X, Nestler EJ, Green TA. Overexpression of DeltaFosB in nucleus accumbens mimics the protective addiction phenotype, but not the protective depression phenotype of environmental enrichment. Frontiers in Behavioral Neuroscience. 2014;8 doi: 10.3389/fnbeh.2014.00297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou W, Chen L, Paul J, Yang S, Li F, Sampson K, Woodgett JR, Beaulieu JM, Gamble KL, Li X. The effects of glycogen synthase kinase-3beta in serotonin neurons. PloS one. 2012;7:e43262. doi: 10.1371/journal.pone.0043262. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


