Abstract
Objective
Abnormal serum liver function tests are common in patients with untreated thyrotoxicosis, even prior to the initiation of antithyroidal medications that may worsen their severity. There is a wide range of the incidence of these abnormalities in the published literature. The aim of this study was to assess the risks factors and threshold of thyrotoxicosis severity for developing an abnormal liver biochemical test upon the diagnosis of new thyrotoxicosis.
Design
Single-institution retrospective cohort study.
Patients
Patients ≥18 years old receiving medical care at a large, academic, urban U.S. medical center between 2002–2016.
Measurements
Inclusion criteria were a serum thyroid stimulating hormone [TSH] concentration < 0.3 mIU/L or ICD-9 code for thyrotoxicosis, with thyrotoxicosis confirmed by either a concurrent elevated serum triiodothyronine (T3) and/or thyroxine (T4) concentration [total or free] within 3 months), and an available liver biochemical test(s) within 6 months of thyrotoxicosis. The biochemical liver tests assessed were serum aspartate transaminotransferase (AST), alanine aminotransferase (ALT), alkaline phosphatase (AP), gamma-glutamyltransferase (GGT), total bilirubin, and conjugated bilirubin concentrations.
Results
In this cohort of 1,514 subjects, the overall incidence of any biochemical liver test abnormality within 6 months of thyrotoxicosis was 39%. An initial serum TSH concentration <0.02 mIU/L, male gender, and African-American race were significant predictors of an abnormal serum liver biochemical test within 6 months of the diagnosis of new-onset untreated thyrotoxicosis.
Conclusions
This study identifies risk factors for patients who develop an abnormal serum liver biochemical test result within 6 months of a diagnosis of untreated thyrotoxicosis.
Keywords: liver function, hyperthyroidism, thyrotoxicosis
Introduction
The reported prevalence of liver biochemical abnormalities in patients with untreated thyrotoxicosis varies widely, ranging from 15–79% (1–6). Processes contributing to biochemical hepatic abnormalities include excess thyroid hormone, elevated thyroid receptor antibody (TRAb) titers in Graves’ disease, relative hepatic anoxia, apoptosis, and increased susceptibility to oxidative stress (2,5,7–12). Other contributing factors to hepatic abnormalities in individuals with untreated hyperthyroidism include heart failure, effects of anti-thyroid drugs, and concomitant liver disease (13).
Although the pattern of biochemical hepatic abnormalities and hepatic injury in the setting of hyperthyroidism is variable in character and severity (14), it appears to be predominantly hepatitis, (3,4,14–17) with a few case reports demonstrating a cholestatic pattern (18–20). The first report of hepatic necrosis in a patient with thyrotoxicosis occurred in 1874 (21). In 1967, liver biopsies of 23 hyperthyroid patients showed that 90% had fatty change (22). Other changes included megamitochondria with irregular volume and increased membrane volume (23). Further studies demonstrated that the degree of biochemical hyperthyroxinemia does not correlate with cellular and ultrastructural abnormalities (22,23). Liver abnormalities were mostly hepatitic with predominantly serum aspartate transaminotransferase (AST), alanine aminotransferase (ALT), and bilirubin elevations, with some increased serum alkaline phosphatase (AP) and gamma-glutamyltransferase (GGT) concentrations (3,4,14–16). However, AP elevations may not necessarily originate from the liver and can represent elevations in the bone isoenzyme (3). Other reports of untreated thyrotoxicosis also have reported a cholestatic pattern with a predominant AP elevation and/or biopsy-proven cholestasis (18–20).
There is limited understanding regarding the patterns of biochemical liver abnormality changes that occur upon treatment of hyperthyroidism. The relationships between use of anti-thyroid drugs and biochemical liver abnormalities has been described in a few small series(24–28), while the other therapies for certain cases of hyperthyroidism (radioactive iodine and surgery) have been rarely described (13,15,29–31). This retrospective study investigates the patterns of liver biochemical abnormalities in patients at our institution with a new diagnosis of thyrotoxicosis.
Materials and Methods
We conducted a retrospective chart analysis of all patients ≥18 years old with thyrotoxicosis (serum thyroid stimulating hormone [TSH] <0.3 mIU/L or ICD-9 code for thyrotoxicosis; either with a concurrent elevated serum triiodothyronine (T3) and/or thyroxine (T4) concentration [total or free] within 3 months) and first available liver biochemical test(s) within 6 months of thyrotoxicosis in the UCLA electronic medical record database from 2002–2016. The biochemical liver tests studied were serum AST, ALT, AP, GGT, total bilirubin, and conjugated bilirubin concentrations. Serum concentrations were considered abnormal according to the following criteria: AST > 47 U/L, ALT > 64 U/L, AP > 113 U/L, GGT > 68 U/L, total bilirubin > 1.2 mg/dl, conjugated bilirubin > 0.3 mg/dl. Patients using medications which may affect liver biochemistries or thyroid function tests (statins, azole antifungals, isoniazid, valproic acid, amiodarone) or patients with pre-existing liver conditions (fatty liver, alcoholic/nonalcoholic hepatitis, hepatitis B and C, cirrhosis, cholangitis) were excluded.
Differences in the frequencies of biochemical liver abnormalities were assessed by Chi-square tests. Cox regression models were used to determine significant predictors of a biochemical liver abnormality. P-values < 0.05 were considered significant. The study was approved by the UCLA Institutional Review Board (IRB).
Results
There were 1,514 patients (mean±SD age 60.0±19.1 years; 77% women; 60% White) with a new diagnosis of thyrotoxicosis and available biochemical liver test(s) within 6 months of the onset of thyrotoxicosis during the study period (Table 1). Age, gender, race, and ethnicity were self-reported, as recorded in the medical record.
Table 1.
Demographics of patients (n=1,514)
| Age (years) | |
| Mean ± SD | 60.0 ± 19.1 |
| Median (IQR) | 51.0 (35.6 – 65.5) |
| Gender, n (%) | |
| Female | 1,167 (77.1%) |
| Race, n (%) | |
| White or Caucasian | 907 (59.9%) |
| Asian | 211 (13.9%) |
| Black or African-American | 131 (8.6%) |
| Other | 265 (17.5%) |
| Ethnicity, n (%) | |
| Hispanic | 214 (14.13%) |
| Not Hispanic or Latino | 1164 (76.88%) |
| Other | 136 (8.98%) |
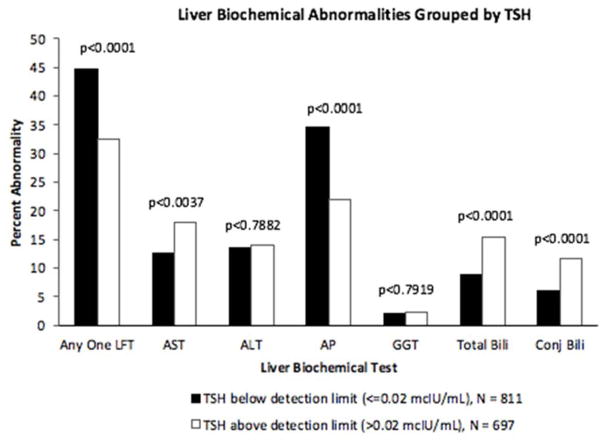
The overall incidence of any biochemical liver test abnormality within 6 months of thyrotoxicosis was 39%. Of the 811 patients whose serum TSH level was below the detection limit (≤ 0.02 mIU/L), 45% had an abnormal liver biochemical test (13% AST, 13% ALT, 35% AP, 2% GGT, 9% total bilirubin, 6% conjugated bilirubin). In comparison, of the 697 patients whose serum TSH level was above the detection limit (> 0.02 mcIU/mL), 33% had an abnormal liver biochemical test (18% AST, 14% ALT, 22% AP, 2% GGT, 15% total bilirubin, 12% conjugated bilirubin (Figure 1).
Figure 1.
Liver Biochemical Abnormalities Grouped by TSH
Serum TSH concentration ≤0.02 mIU/L, male gender, and African-American race were significant positive predictors for a biochemical liver abnormality (Table 2). Serum TSH concentration ≤0.02 mIU/L, male gender, and African-American race were significant positive predictors for abnormal alkaline phosphatase; non-Hispanic or Latino and other ethnicities were significant negative predictors for abnormal alkaline phosphatase (Table 3). There were no significant positive predictors for abnormal serum GGT concentrations.
Table 2.
Cox regression model predicting initial liver biochemical test abnormality
| Hazards Ratio | 95% CI | p-value | |
|---|---|---|---|
| TSH (≤0.02 vs. >0.02 mIU/L) | 1.42 | (1.19, 1.68) | <.0001 |
| Age (in 10 year increments) | 0.96 | (0.91, 1.00) | 0.0555 |
| Gender (women vs. men) | 0.48 | (0.40, 0.57) | <.0001 |
| Race | |||
| White or Caucasian | Reference | - | - |
| Asian | 0.89 | (0.69, 1.15) | 0.3602 |
| Black or African American | 1.63 | (1.25, 2.12) | 0.0003 |
| Other | 0.83 | (0.63, 1.09) | 0.1798 |
| Ethnicity | |||
| Hispanic | Reference | - | - |
| Not Hispanic or Latino | 0.59 | (0.47, 0.73) | <.0001 |
| Other | 0.62 | (0.43, 0.91) | 0.0136 |
Table 3.
Cox regression predicting abnormal serum alkaline phosphatase concentration
| Hazards Ratio | 95% CI | p-value | |
|---|---|---|---|
| TSH (≤0.02 vs. >0.02 mIU/L) | 1.58 | (1.29, 1.94) | <.0001 |
| Age (in 10 year increments) | 0.96 | (0.91, 1.02) | 0.1621 |
| Gender (women vs. men) | 0.49 | (0.40, 0.60) | <.0001 |
| Race | |||
| White or Caucasian | Reference | - | - |
| Asian | 1.10 | (0.83, 1.46) | 0.5264 |
| Black or African American | 1.73 | (1.28, 2.35) | 0.0004 |
| Other | 0.88 | (0.64, 1.20) | 0.4153 |
| Ethnicity | |||
| Hispanic | Reference | - | - |
| Not Hispanic or Latino | 0.57 | (0.44, 0.74) | <.0001 |
| Other | 0.63 | (0.40, 0.97) | 0.0362 |
Serum TSH concentration ≤0.02 mIU/L and non-Hispanic or Latino and other ethnicities were significant negative predictors for abnormal total bilirubin and abnormal conjugated bilirubin (Table 4, Table 5); male gender was a significant positive predictor for abnormal total conjugated bilirubin (Table 4); male gender and African American race were significant positive predictors for abnormal conjugated bilirubin (Table 5). Male gender and African-American race were significant positive predictors for abnormal serum ALT and AST concentrations (Table 6, Table 7); not Hispanic or Latino ethnicity was a significant negative predictor for abnormal serum AST concentration (Table 7).
Table 4.
Cox regression predicting abnormal total bilirubin concentration
| Hazards Ratio | 95% CI | p-value | |
|---|---|---|---|
| TSH (≤0.02 vs. >0.02 mIU/L) | 0.59 | (0.43, 0.80) | 0.0008 |
| Age (in 10 year increments) | 1.01 | (0.93, 1.09) | 0.8527 |
| Gender (women vs. men) | 0.38 | (0.28, 0.52) | <.0001 |
| Race | |||
| White or Caucasian | Reference- | - | - |
| Asian | 0.61 | (0.35, 1.07) | 0.0828 |
| Black or African American | 1.55 | (0.96, 2.51) | 0.0733 |
| Other | 0.71 | (0.42, 1.19) | 0.1910 |
| Ethnicity | |||
| Hispanic | Reference | - | - |
| Not Hispanic or Latino | 0.46 | (0.32, 0.66) | <.0001 |
| Other | 0.37 | (0.16, 0.84) | 0.0174 |
Table 5.
Cox regression predicting abnormal serum conjugated bilirubin concentration
| Hazards Ratio | 95% CI | p-value | |
|---|---|---|---|
| TSH (≤0.02 vs. >0.02 mIU/L) | 0.53 | (0.37, 0.77) | 0.0008 |
| Age (in 10 year increments) | 1.00 | (0.91, 1.09) | 0.9713 |
| Gender (women vs. men) | 0.27 | (0.19, 0.38) | <.0001 |
| Race | |||
| White or Caucasian | Reference- | - | - |
| Asian | 0.77 | (0.42, 1.43) | 0.4098 |
| Black or African American | 2.09 | (1.25, 3.50) | 0.0052 |
| Other | 0.58 | (0.3, 1.14) | 0.1127 |
| Ethnicity | |||
| Hispanic | Reference | - | - |
| Not Hispanic or Latino | 0.40 | (0.26, 0.60) | <.0001 |
| Other | 0.28 | (0.09, 0.86) | 0.0263 |
Table 6.
Cox regression predicting abnormal serum ALT concentration
| Hazards Ratio | 95% CI | p-value | |
|---|---|---|---|
| TSH (≤0.02 vs. >0.02 mIU/L) | 0.94 | (0.7, 1.24) | 0.6411 |
| Age (in 10 year increments) | 0.94 | (0.87, 1.01) | 0.0843 |
| Gender (women vs. men) | 0.46 | (0.34, 0.61) | <.0001 |
| Race | |||
| White or Caucasian | Reference- | - | - |
| Asian | 0.94 | (0.61, 1.45) | 0.7773 |
| Black or African American | 1.59 | (1.02, 2.48) | 0.0418 |
| Other | 0.82 | (0.51, 1.31) | 0.4006 |
| Ethnicity | |||
| Hispanic | Reference | - | - |
| Not Hispanic or Latino | 0.73 | (0.5, 1.07) | 0.1065 |
| Other | 0.86 | (0.45, 1.65) | 0.6508 |
Table 7.
Cox regression predicting abnormal serum AST concentration
| Hazards Ratio | 95% CI | p-value | |
|---|---|---|---|
| TSH (≤0.02 vs. >0.02 mIU/L) | 0.70 | (0.53, 0.91) | 0.0087 |
| Age (in 10 year increments) | 1.00 | (0.94, 1.08) | 0.9082 |
| Gender (women vs. men) | 0.47 | (0.36, 0.62) | <.0001 |
| Race | |||
| White or Caucasian | Reference- | - | - |
| Asian | 0.95 | (0.63, 1.45) | 0.8181 |
| Black or African American | 1.86 | (1.24, 2.79) | 0.0029 |
| Other | 0.66 | (0.41, 1.06) | 0.0842 |
| Ethnicity | |||
| Hispanic | Reference | - | - |
| Not Hispanic or Latino | 0.51 | (0.36, 0.71) | <.0001 |
| Other | 0.63 | (0.33, 1.21) | 0.1655 |
Discussion
In this retrospective cohort study of patients at a large, urban academic medical center, there was a 39% incidence of liver biochemical abnormalities within 6 months of a new diagnosis of thyrotoxicosis. Increased risks were observed in those with an initial serum TSH concentration of ≤0.02 mIU/L, in men, and in African-Americans.
Although there are many previous studies that have linked thyrotoxicosis with liver biochemical abnormalities(1–6), few have investigated whether the severity of thyrotoxicosis affects the presence of abnormal LFTs. The present findings indicate that amongst patients with the diagnosis of thyrotoxicosis, serum TSH concentration ≤0.02 mIU/L, as compared to those with a serum TSH > 0.02 mIU/L, is a significant distinction as a predictor of any LFT abnormality, as well as for specific LFT abnormalities, including alkaline phosphatase, total bilirubin, and conjugated bilirubin. However, this distinction does not seem to be significant in analyses of abnormal serum ALT, AST, or GGT concentrations.
Unlike previous studies in which the prevalence of liver biochemical abnormalities were not significantly affected by gender(7), this study consistently indicates that male gender is a significant positive predictor for LFT abnormalities, including serum alkaline phosphatase, total bilirubin, conjugated bilirubin, ALT, and AST concentrations. Additionally, few studies in the past have investigated race and/or ethnicity as predictors of abnormal biochemical tests in hyperthyroidism. The African-American race is not known to be a predictor for LFT abnormalities independent of hyperthyroidism. In this cohort, African-American race is found to be a significant positive predictor for abnormal serum alkaline phosphatase, conjugated bilirubin, ALT, and AST concentrations. This may be explained by increased severity of thyrotoxicosis compared to other racial groups, or the presence of comorbidities with a higher prevalence in African-Americans that affect thyroid and/or liver function.
Limitations to the study include the low frequency of positive serum thyroid autoantibodies in our cohort, which may be an important covariate for the development of liver function test abnormalities. Rather than investigating the effect of thyroid medications on liver biochemical patterns (24–28), we have excluded patients who were using propylthiouracil or methimazole, as well as any other drugs that may affect liver metabolism, from this patient cohort. The study focused on predictors of incident abnormal LFTs, rather than changes in abnormal LFTs after treatment for thyrotoxicosis. Although we excluded patients who used medications which may affect liver biochemistries or thyroid function tests, as well as patients with pre-existing liver conditions, other possible conditions that may affect liver and thyroid function, such as cardiac disease, remain as potential confounding variables in this study. Finally, the study did not assess for the time course of resolution of the abnormal liver biochemical tests.
The study utilizes a large cohort of patients seen at a large, academic, urban U.S. medical center to provide further understanding of the patients who were evaluated for liver biochemical abnormalities upon the onset of a new diagnosis of thyrotoxicosis. In the patient populations in which increased risks for abnormal liver biochemical tests were found (initial serum TSH concentration ≤0.02 mIU/L, men, African-Americans), clinicians may consider monitoring liver function at an earlier time (1–3 months immediately after initial diagnosis of thyrotoxicosis), to prevent progression to liver disease. Further research is needed to explore the reasons for increased risk of liver biochemical abnormalities in certain populations, the time course to resolution, and guidance for use of antithyroidal therapy in such patients.
Acknowledgments
Acknowledgements/Funding: Supported by NIH K23HD068552 (AML)
References
- 1.Ashkar FS, Miller R, Smoak WM, Gilson AJ. Liver disease in hyperthyroidism. South Med J [Internet] 1971;64(4):462–5. doi: 10.1097/00007611-197104000-00016. Available from: http://www.ncbi.nlm.nih.gov/pubmed/5552019. [DOI] [PubMed] [Google Scholar]
- 2.He K, Hu Y, Xu XH, Mao XM. Hepatic dysfunction related to thyrotropin receptor antibody in patients with Graves’ disease. Exp Clin Endocrinol Diabetes [Internet] 2014;122(6):368–72. doi: 10.1055/s-0034-1375667. Available from: http://www.ncbi.nlm.nih.gov/pubmed/24941434. [DOI] [PubMed] [Google Scholar]
- 3.Huang MJ, Liaw YF. Clinical associations between thyroid and liver diseases. J Gastroenterol Hepatol [Internet] 1995;10(3):344–50. doi: 10.1111/j.1440-1746.1995.tb01106.x. Available from: http://www.ncbi.nlm.nih.gov/pubmed/7548816. [DOI] [PubMed] [Google Scholar]
- 4.Biscoveanu M, Hasinski S. Abnormal results of liver function tests in patients with Graves’ disease. Endocr Pr [Internet] 2000;6(5):367–9. doi: 10.4158/EP.6.5.367. Available from: http://www.ncbi.nlm.nih.gov/pubmed/11141587. [DOI] [PubMed] [Google Scholar]
- 5.Gürlek A, Cobankara V, Bayraktar M. Liver tests in hyperthyroidism: effect of antithyroid therapy. J Clin Gastroenterol [Internet] 1997;24(3):180–3. doi: 10.1097/00004836-199704000-00013. Available from: http://www.ncbi.nlm.nih.gov/pubmed/9179740. [DOI] [PubMed] [Google Scholar]
- 6.Kubota S, Amino N, Matsumoto Y, Ikeda N, Morita S, Kudo T, et al. Serial changes in liver function tests in patients with thyrotoxicosis induced by Graves’ disease and painless thyroiditis. Thyroid [Internet] 2008 Mar;18(3):283–7. doi: 10.1089/thy.2007.0189. [cited 2016 Jun 30] Available from: http://www.ncbi.nlm.nih.gov/pubmed/18001177. [DOI] [PubMed] [Google Scholar]
- 7.Zhang R, Tian X, Qin L, Wei X, Wang J, Shen J. Factors predicting abnormal liver function tests induced by Graves’ disease alone: a retrospective cohort study. Med [Internet] 2015;94(19):e839. doi: 10.1097/MD.0000000000000839. Available from: http://www.ncbi.nlm.nih.gov/pubmed/25984670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang W, Tian L, Han Y, Ma H, Wang L, Guo J, et al. Presence of thyrotropin receptor in hepatocytes: not a case of illegitimate transcription. J Cell Mol Med [Internet] 13(11–12):4636–42. doi: 10.1111/j.1582-4934.2008.00670.x. [cited 2016 Jun 30] Available from: http://www.ncbi.nlm.nih.gov/pubmed/19187127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Upadhyay G, Singh R, Kumar A, Kumar S, Kapoor A, Godbole MM. Severe hyperthyroidism induces mitochondria-mediated apoptosis in rat liver. Hepatology [Internet] 2004 Apr;39(4):1120–30. doi: 10.1002/hep.20085. [cited 2016 Jun 30] Available from: http://www.ncbi.nlm.nih.gov/pubmed/15057916. [DOI] [PubMed] [Google Scholar]
- 10.MYERS JD, BRANNON ES, HOLLAND BC. A correlative study of the cardiac output and the hepatic circulation in hyperthyroidism. J Clin Invest [Internet] 1950 Aug;29(8):1069–77. doi: 10.1172/JCI102338. [cited 2016 Jun 30] Available from: http://www.ncbi.nlm.nih.gov/pubmed/15436876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Seven A, Seymen O, Hatemi S, Hatemi H, Yiğit G, Candan G. Antioxidant status in experimental hyperthyrodism: effect of vitamin E supplementation. Clin Chim Acta [Internet] 1996 Dec;256(1):65–74. doi: 10.1016/s0009-8981(96)06415-7. [cited 2016 Jun 30] Available from: http://linkinghub.elsevier.com/retrieve/pii/S0009898196064157. [DOI] [PubMed] [Google Scholar]
- 12.Venditti P, Di Meo S. Thyroid hormone-induced oxidative stress. Cell Mol Life Sci [Internet] 2006 Feb;63(4):414–34. doi: 10.1007/s00018-005-5457-9. [cited 2016 Jun 30] Available from: http://www.ncbi.nlm.nih.gov/pubmed/16389448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.de Campos Mazo DF, de Vasconcelos GB, Pereira MA, de Mello ES, Bacchella T, Carrilho FJ, et al. Clinical spectrum and therapeutic approach to hepatocellular injury in patients with hyperthyroidism. Clin Exp Gastroenterol [Internet] 2013;6:9–17. doi: 10.2147/CEG.S39358. Available from: http://www.ncbi.nlm.nih.gov/pubmed/23550044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Elias RM, Dean DS, Barsness GW. Hepatic dysfunction in hospitalized patients with acute thyrotoxicosis: a decade of experience. ISRN Endocrinol [Internet] 2012;2012:325092. doi: 10.5402/2012/325092. Available from: http://www.ncbi.nlm.nih.gov/pubmed/23251814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Akande TO, Balogun WO. A report of three cases of jaundice with thyrotoxicosis. Afr Heal Sci [Internet] 2013;13(3):853–6. doi: 10.4314/ahs.v13i3.48. Available from: http://www.ncbi.nlm.nih.gov/pubmed/24250332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Babb RR. Associations between diseases of the thyroid and the liver. Am J Gastroenterol [Internet] 1984;79(5):421–3. Available from: http://www.ncbi.nlm.nih.gov/pubmed/6720663. [PubMed] [Google Scholar]
- 17.Khemichian S, Fong TL. Hepatic dysfunction in hyperthyroidism. Gastroenterol Hepatol (N Y) [Internet] 2011;7(5):337–9. Available from: http://www.ncbi.nlm.nih.gov/pubmed/21857837. [PMC free article] [PubMed] [Google Scholar]
- 18.Hull K, Horenstein R, Naglieri R, Munir K, Ghany M, Celi FS. Two cases of thyroid storm-associated cholestatic jaundice. Endocr Pr [Internet] 2007;13(5):476–80. doi: 10.4158/EP.13.5.476. Available from: http://www.ncbi.nlm.nih.gov/pubmed/17872349. [DOI] [PubMed] [Google Scholar]
- 19.Kibirige D, Kiggundu DS, Sanya R, Mutebi E. Cholestatic hepatic injury due to a thyroid storm: a case report from a resource limited setting. Thyroid Res [Internet] 2012;5(1):6. doi: 10.1186/1756-6614-5-6. [cited 2016 Jun 30] Available from: http://www.ncbi.nlm.nih.gov/pubmed/22839423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sola J, Pardo-Mindán FJ, Zozaya J, Quiroga J, Sangro B, Prieto J. Liver changes in patients with hyperthyroidism. Liver [Internet] 1991;11(4):193–7. doi: 10.1111/j.1600-0676.1991.tb00516.x. Available from: http://www.ncbi.nlm.nih.gov/pubmed/1943501. [DOI] [PubMed] [Google Scholar]
- 21.Habershon S. Exophthalmic goiter, heart disease, jaundice: death. Lancet. 1874;1:510–2. [Google Scholar]
- 22.Dooner HP, Parada J, Aliaga C, Hoyl C. The liver in thyrotoxicosis. Arch Intern Med [Internet] 1967;120(1):25–32. Available from: http://www.ncbi.nlm.nih.gov/pubmed/6028694. [PubMed] [Google Scholar]
- 23.Klion FM, Segal R, Schaffner F. The effect of altered thyroid function on the ultrastructure of the human liver. Am J Med [Internet] 1971;50(3):317–24. doi: 10.1016/0002-9343(71)90220-8. Available from: http://www.ncbi.nlm.nih.gov/pubmed/5553951. [DOI] [PubMed] [Google Scholar]
- 24.Bahn Chair RS, Burch HB, Cooper DS, Garber JR, Greenlee MC, Klein I, et al. Hyperthyroidism and other causes of thyrotoxicosis: management guidelines of the American Thyroid Association and American Association of Clinical Endocrinologists. Thyroid [Internet] 2011;21(6):593–646. doi: 10.1089/thy.2010.0417. Available from: http://www.ncbi.nlm.nih.gov/pubmed/21510801. [DOI] [PubMed] [Google Scholar]
- 25.Heidari R, Niknahad H, Jamshidzadeh A, Abdoli N. Factors affecting drug-induced liver injury: antithyroid drugs as instances. Clin Mol Hepatol [Internet] 2014;20(3):237–48. doi: 10.3350/cmh.2014.20.3.237. Available from: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=4197171&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nakamura H, Noh JY, Itoh K, Fukata S, Miyauchi A, Hamada N, et al. Comparison of methimazole and propylthiouracil in patients with hyperthyroidism caused by Graves’ disease. J Clin Endocrinol Metab. 2007;92(6):2157–62. doi: 10.1210/jc.2006-2135. [DOI] [PubMed] [Google Scholar]
- 27.Yang J, Li L-F, Xu Q, Zhang J, Weng W-W, Zhu Y-J, et al. Analysis of 90 cases of antithyroid drug-induced severe hepatotoxicity over 13 years in China. Thyroid. 2015;25(3):278–83. doi: 10.1089/thy.2014.0350. [DOI] [PubMed] [Google Scholar]
- 28.Niculescu DA, Dusceac R, Galoiu SA, Capatina CA-M, Poiana C. Serial Changes of Liver Function Tests Before and During Methimazole Treatment in Thyrotoxic Patients. Endocr Pract [Internet] 2016;22(8):974–9. doi: 10.4158/EP161222.OR. Available from: http://journals.aace.com/doi/10.4158/EP161222.OR. [DOI] [PubMed] [Google Scholar]
- 29.Leeuwenburgh I, Stijnen PJ, Verburg GP. Recovery of chronic hepatitis by treatment of concomitant hyperthyroidism. Eur J Gastroenterol Hepatol [Internet] 2001 Nov;13(11):1389–92. doi: 10.1097/00042737-200111000-00021. [cited 2016 Jun 30] Available from: http://www.ncbi.nlm.nih.gov/pubmed/11692069. [DOI] [PubMed] [Google Scholar]
- 30.Regelmann MO, Miloh T, Arnon R, Morotti R, Kerkar N, Rapaport R. Graves’ disease presenting with severe cholestasis. Thyroid [Internet] 2012 Apr;22(4):437–9. doi: 10.1089/thy.2011.0267. [cited 2016 Jun 30] Available from: http://www.ncbi.nlm.nih.gov/pubmed/22458973. [DOI] [PubMed] [Google Scholar]
- 31.Liaw YF, Huang MJ, Fan KD, Li KL, Wu SS, Chen TJ. Hepatic injury during propylthiouracil therapy in patients with hyperthyroidism. A cohort study. Ann Intern Med [Internet] 1993;118(6):424–8. doi: 10.7326/0003-4819-118-6-199303150-00005. Available from: http://www.ncbi.nlm.nih.gov/pubmed/8439116. [DOI] [PubMed] [Google Scholar]