Abstract
Anxiety disorders are one of the most common and debilitating mental illnesses worldwide. Growing evidence indicates an age-dependent rise in the incidence of anxiety disorders from adolescence through adulthood, suggestive of underlying neurodevelopmental mechanisms. Kappa opioid receptors (KORs) are known to contribute to the development and expression of anxiety; however, the functional role of KORs in the basolateral amygdala (BLA), a brain structure critical in mediating anxiety, particularly across ontogeny, are unknown. Using whole-cell patch-clamp electrophysiology in acute brain slices from adolescent (postnatal day (P) 30–45) and adult (P60+) male Sprague-Dawley rats, we found that KOR activation increased the frequency of GABAA-mediated spontaneous inhibitory postsynaptic currents (sIPSCs) in the adolescent BLA, without an effect in the adult BLA or on sIPSC amplitude at either age. The KOR effect was blocked by the KOR antagonist, nor-BNI, which alone did not alter GABA transmission at either age, and the effect of the KOR agonist was TTX-sensitive. Additionally, KOR activation did not alter glutamatergic transmission in the BLA at either age. In contrast, U69593 inhibited sIPSC frequency in the central amygdala (CeA) at both ages, without altering sIPSC amplitude. Western blot analysis of KOR expression indicated that KOR levels were not different between the two ages in either the BLA or CeA. This is the first study to provide compelling evidence for a novel and unique neuromodulatory switch in one of the primary brain regions involved in initiating and mediating anxiety that may contribute to the ontogenic rise in anxiety disorders.
Keywords: Anxiety, kappa opioid receptor, basolateral amygdala, GABA, development, ontogeny
Introduction
Anxiety disorders are among the most common mental disorders and can manifest in various manners, such as generalized anxiety, obsessive-compulsion, panic, post-traumatic stress, and social anxiety. In the United States, the estimated lifetime prevalence of anxiety disorders in adolescents averages 25.1% (age 13–18) with a staggering increase of ~15% at age 6 to >30% by 18 years of age (Merikangas et al., 2010), whereas the average prevalence in adults is 35.1% (ages 30–44) (Kessler et al., 2005). Although our understanding of age-dependent changes in behaviors is growing, the neurobiological contributions that influence age disparities in anxiety disorders are not well understood. Adolescence is a critical developmental period when numerous neurobiological systems are changing, including, but not limited to, neuronal maturation (i.e. synaptic pruning, axonal myelination) and refinement of synaptic connections and neurotransmitter systems (Spear, 2014). Additionally, fine-tuning and activity of brain networks involved in processing rewarding, aversive, arousing, and emotionally-provoking stimuli have been shown to be greater in adolescents compared to adults, albeit some studies have shown reduced activity of these reward/emotion networks [see review by (Spear, 2011)]. It is clear that ontogenic differences in neural functioning and emotional processing exist, yet the underlying neurobiological substrates that regulate these differences are unknown.
The amygdala is one of the primary brain structures in the anxiety and reward circuit that undergoes robust remodeling throughout development. Specifically, the basolateral amygdala (BLA), primarily comprised of glutamatergic pyramidal neurons and various GABAergic interneuron populations, has been shown to be critical in initiating and regulating anxiety-like behaviors (Janak and Tye, 2015; LeDoux, 2000). While development of the BLA glutamate and GABA systems appear to fully mature by early adolescence in rodents (~ postnatal day (P) 28) (Ehrlich et al., 2013; Ehrlich et al., 2012), studies have shown that the number of neurons decrease in the BLA after adolescence (Rubinow and Juraska, 2009), consistent with age-dependent reductions in cell proliferation in this structure (Saul et al., 2014). BLA excitability, primarily driven by glutamatergic pyramidal neuron activity, is positively associated with increased anxiety-like responses (Wang et al., 2011) and elevations in BLA GABAergic activity can robustly suppress anxiety-like behaviors (Bueno et al., 2005; Lack et al., 2007). While the balance of glutamate and GABA transmission within the BLA orchestrates anxiety states, these two neurotransmitter systems are heavily regulated by numerous neuromodulatory systems that fine-tune the expression and/or suppression of anxiety-like behaviors; however, the developmental trajectories of most of these modulatory systems are unknown.
The kappa opioid system has received much attention due to its influence on emotional and rewarding behaviors, particularly following stress and induction of drug dependence (Bruchas et al., 2010; Chavkin and Ehrich, 2014; Crowley and Kash, 2015; Knoll and Carlezon, 2010; Van't Veer and Carlezon, 2013; Wee and Koob, 2010). Alterations in kappa opioid receptor (KOR) expression and function associated with changes in emotional states have been found in many brain regions implicated in anxiety and reward, including the central amygdala (CeA) (Kissler et al., 2014; Kissler and Walker, 2016), the nucleus accumbens (Karkhanis et al., 2016; Rose et al., 2015; Siciliano et al., 2016), and the bed nucleus of the stria terminalis (BNST) (Crowley et al., 2016). However, the role of KORs in the BLA is not well understood despite it being a brain structure with high levels of KOR expression (Gackenheimer et al., 2005; Slowe et al., 1999). Interestingly, activation of BLA KORs was shown to decrease field excitatory postsynaptic potentials (fEPSPs) and blocked long-term potentiation (LTP) in younger animals (Huge et al., 2009), suggestive of an anxiolytic role of KORs in adolescents. Conversely, blockade of BLA KORs with the selective KOR antagonist, nor-Binaltorphimine (nor-BNI), reduced anxiety-like responses following restraint stress (Bruchas et al., 2009) and fear conditioning (Knoll et al., 2011) in older animals, suggestive of an anxiogenic role of KORs in adults. Consistent with developmental regulation of KOR function, systemic KOR-induced aversion seen in adults was completely absent in adolescents (Anderson et al., 2014), and anxiolytic effects of a systemic KOR agonist have also been reported in younger animals (Privette and Terrian, 1995). Conversely, anxiogenic effects were shown following systemic activation of KORs in adult mice (Bruchas et al., 2009). While the mechanisms underlying these age-dependent changes in behavior are not clear, studies have consistently shown that KOR activation can alter GABA transmission in various structures of the anxiety circuit (Crowley et al., 2016; Gilpin et al., 2014; Kang-Park et al., 2013; Li et al., 2012). Taken together, these studies support the idea that KOR-modulation of GABA transmission in the BLA may be developmentally regulated, providing a novel mechanism that may contribute to our understanding of the etiology of anxiety disorders across ontogeny.
Given the apparent behavioral and physiological age-dependent differences in BLA KOR function, the objective of this study was to examine age-dependent differences in KOR-modulation of synaptic transmission in the BLA of naïve adolescent and adult Sprague-Dawley rats. We report that in adolescents, KOR activation increases GABA transmission in the BLA in an action potential-dependent manner, while having no effect on glutamate transmission. Interestingly, KOR activation has no effect on BLA GABA or glutamate transmission in adults. Furthermore, this ontogenic difference in KOR function is unique to the BLA, as KOR activation similarly reduces GABA transmission in the CeA of adolescents and adults.
Methods
Animals
Adolescent (postnatal day (P) 30–45) and adult (P60+) male Sprague-Dawley rats were obtained from Envigo/Harlan (Indianapolis, IN). Animals were group-housed and received food and water ad libitum. All animal procedures were approved by the Binghamton University Institutional Animal Care and Use Committee.
Drugs and chemicals
All chemicals were purchased form Sigma-Aldrich (St. Louis, MO, USA). Kynurenic acid, dynorphin A, APV, QX314-Cl, and tetrodotoxin (TTX) were purchased from Tocris/R&D Systems (Bristol, UK).
Slice Preparation
Rats were anesthetized with a lethal dose of ketamine and quickly decapitated. Brains were rapidly removed and immersed in ice-cold oxygenated (95% O2-5% CO2) sucrose artificial cerebrospinal fluid (ACSF) containing (in mM): sucrose (220), KCl (2), NaH2PO4 (1.25), NaHCO3 (26), glucose (10), MgSO4 (12), CaCl2 (0.2), and ketamine (0.43). BLA-containing coronal slices (300 μm) were made using a Vibratome (Leica Microsystems. Bannocknurn, IL, USA). Slices were incubated in normal ACSF containing (in mM): NaCl (1250), KCl (20), NaH2PO4 (13), NaHCO3 (260), glucose (100), CaCl2 (1), MgSO4 (1), ascorbic acid (0.4), continuously bubbled at 95% O2-5% CO2 for at least 40 minutes at 34°C before recording, and all experiments were performed ~1–4 hr after slice preparation.
Whole-cell Patch-Clamp Electrophysiology
Following incubation, slices were transferred to a recording chamber in which oxygenated ACSF was warmed to 32°C and superfused over the submerged slice at 3 ml/min. Recordings were collected from either pyramidal neurons in the BLA or neurons in the CeA with patch pipettes filled with a KCl-based internal solution containing (in mM): KCl (135), HEPES (10), MgCl2 (2), EGTA (0.5), Mg-ATP (5), Na-GTP (1), and QX314-Cl (1). Data were acquired with a MultiClamp 700B (Molecular Devices, Sunnyvale, CA) at 10 kHz, filtered at 1 kHz, and stored for later analysis using pClamp software (Molecular Devices). BLA pyramidal neurons were visualized using infrared-differential interference contrast microscopy (Olympus America, Center Valley, PA) and identified based on morphology and capacitance (≥150 pF), as previously described (Baculis et al., 2015). For experiments in the CeA, recordings were made in the medial subdivision of the CeA as previously described (Gilpin et al., 2014; Kang-Park et al., 2013). For recordings of GABAA receptor-mediated spontaneous inhibitory postsynaptic currents (sIPSCs), we pharmacologically blocked AMPA and NMDA glutamate receptors using 1 mM kynurenic acid and 50 μM APV, respectively. For recordings of glutamate-mediated spontaneous excitatory postsynaptic currents (sEPSCs), we pharmacologically blocked GABAA receptors with 10 μM gabazine. To examine action potential-independent miniature IPSCs (mIPSCs), recordings were done in the presence of the Na+ channel blocker, TTX (1 μM). Neurons were allowed to equilibrate for at least 5 min before a baseline was recorded. We recorded a baseline period of 2 minutes, followed by at least 3 minutes of continuous drug application. In initial experiments, drugs were applied for ~10 minutes and we found that with drugs that produced an effect, the effects were maximal and stable by 2 minutes of continuous drug application (data not shown). Therefore, to minimize potential changes in access resistance, our analysis of postsynaptic currents focused on 30 seconds of baseline (60–90 seconds into the recording) and at least 30 seconds in the presence of a given drug following 3 minutes of drug application (300–330 seconds into the recording). Only recordings where access resistance changed <20% were kept for analysis.
Western Blotting
BLA, CeA and cerebellum (negative control due to low/nonexistent KOR expression) tissue was micro-dissected from coronal slices of adult and adolescent rats immediately after slicing using methods described above and immediately frozen at −80°C. Tissue was weighed and homogenized in whole-cell lysate buffer (1% sodium dodecyl sulfate (SDS)), 1 mM EDTA, 10 mM Tris as we have done elsewhere (Santerre et al., 2014), and protein concentrations were determined using a bicinchoninic acid method. Samples were denatured, separated using SDS polyacrylamide gel electrophoresis, and transferred to polyvinylidine difluoride membranes (Life Technologies, Carlsbad, CA). Membranes were blocked with BSA and probed with an anti-kappa opioid receptor primary antibody (ABN456; Millipore, Bilerica, MA). Bands were detected using enhanced chemiluminescence (GE Healthcare, Piscataway, NJ), exposed to x-ray films under non-saturating conditions, and analyzed using NIH Image J. Membranes were subsequently exposed to antibody directed against β-actin (Millipore) to verify protein loading. All samples were run in duplicate and averaged.
Statistics
Data were analyzed using MiniAnalysis (SynaptoSoft Inc.) and statistically analyzed with Prizm 6 (GraphPad, San Diego, CA). Data were first analyzed with the Pearson omnibus and K-S normality tests. If data followed a normal distribution, parametric tests were used; otherwise, nonparametric tests were used. All data are presented as mean ± SEM, with p < 0.05 considered statistically significant. For all statistical analyses, the experimental unit is an animal; in cases where the same experiment was conducted in multiple slices from one animal, data were averaged to yield a single unit of determination (n = 1).
Results
Membrane properties and basal synaptic transmission in the BLA
Assessment of membrane properties of pyramidal neurons from adolescent and adult BLA-containing brain slices did not reveal significant differences in membrane capacitance or resistance when using a KCl internal solution – this internal solution was used to record GABAA receptor-mediated synaptic transmission. Similarly, there were no significant differences in basal sIPSC frequency, amplitude, area, or decay time between adolescents and adults (Table 1). When recording glutamatergic transmission using a K-gluconate internal solution, we also did not find significant differences between adolescents and adults in membrane capacitance, nor in basal sEPSC frequency, amplitude, area, or decay time (Table 2). However, there was a strong trend (p = 0.055) toward a larger membrane resistance in adults relative to adolescents when using a K-gluconate internal solution.
Table 1.
BLA membrane properties (KCl) and basal GABAergic synaptic transmission
| Adolescent (n = 14) | Adult (n = 10) | |
|---|---|---|
| Capacitance (pF) | 206.1 ± 14.85 | 204.0 ± 11.49 |
| Membrane Resistance (MΩ) | 102.3 ± 33.21 | 97.62 ± 9.33 |
| sIPSC Frequency (Hz) | 6.11 ± 0.83 | 5.51 ± 0.63 |
| sIPSC Amplitude (pA) | 38.50 ± 10.09 | 28.53 ± 4.29 |
| sIPSC Area (pA*ms) | 1173.00 ± 272.50 | 745.50 ± 88.99 |
| sIPSC Decay Time (ms) | 25.20 ± 1.60 | 20.49 ± 1.71 |
Table 2.
BLA membrane properties (K-gluconate) and basal glutamatergic synaptic transmission
| Adolescent (n = 5) | Adult (n = 5) | |
|---|---|---|
| Capacitance (pF) | 168.3 ± 17.75 | 221.8 ± 24.23 |
| Membrane Resistance (MΩ) | 43.30 ± 8.40 | 125.5 ± 38.98 $ |
| sEPSC Frequency (Hz) | 5.57 ± 1.14 | 6.87 ± 1.00 |
| sEPSC Amplitude (pA) | 6.32 ± 0.75 | 6.24 ± 1.27 |
| sEPSC Area (pA*ms) | 85.15 ± 7.63 | 90.09 ± 7.21 |
| sEPSC Decay Time (ms) | 11.02 ± 0.44 | 11.89 1.94 |
p = 0.055 by Mann Whitney test (sum of ranks = 18,37; Mann-Whitney U = 3)
KOR activation age-dependently potentiates GABA transmission in the BLA
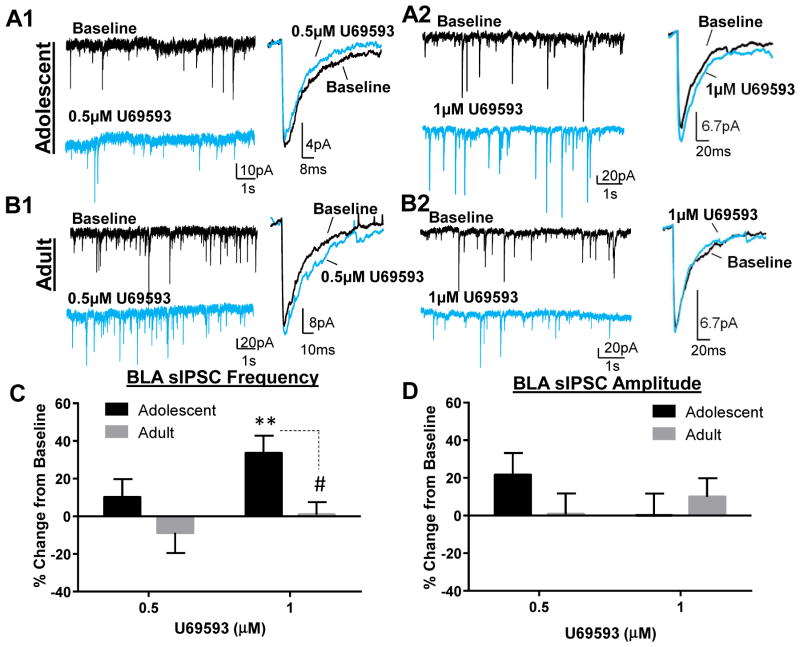
KOR activation has been shown to inhibit neurotransmitter release throughout the brain, and more specifically it can suppress GABA transmission in structures associated with anxiety (Crowley et al., 2016; Gilpin et al., 2014; Kang-Park et al., 2013; Li et al., 2012). Therefore, we first examined the effect of KOR activation on GABAA receptor-mediated sIPSCs in adolescent and adult BLA pyramidal neurons. Analysis of sIPSC frequency showed that while 0.5 μM U69593 did not significantly alter sIPSC frequency in adolescents (Fig. 1A1&C; t = 1.05, df = 7, n = 8, p > 0.05 compared to 0), 1 μM significantly increased sIPSC frequency in adolescents (Fig. 1A2&C; t = 3.64, df = 7, n = 8, p < 0.01 compared to 0). Surprisingly, the effect of 1 μM U69593 was completely absent in adult BLA slices (Fig. 1B2&C; t = 0.90, df = 7, n = 8, p > 0.05 compared to 0) and these effects were significantly different (t = 2.86, df = 14, p < 0.05; t-test). 0.5 μM also did not affect sIPSC frequency in adults (Fig. 1B1&C; 0.5 μM: t = 0.81, df = 5, n = 6, p > 0.05). Neither concentration altered sIPSC amplitude, regardless of age (Fig. 1A, B&D; adolescent: 0.5 μM: t = 1.82, df = 7, n = 8, p > 0.05; 1 μM: t = 0.01, df = 7, n = 8, p > 0.05; adult: 0.5 μM: t = 0.06, df = 5, n = 6, p > 0.05; 1 μM: t = 0.99, df = 7, n = 8, p > 0.05).
Figure 1. KOR activation age-dependently potentiates GABA transmission in the BLA.
Exemplar traces showing effect of 2 doses of U69593 on GABAA-mediated sIPSC frequency and amplitude in (A) adolescents and (B) adult. Effect of U69593 on sIPSC (C) frequency and (D) amplitude in adolescents and adults. ** - p < 0.01 compared to 0; # - p < 0.05 compared to adolescent.
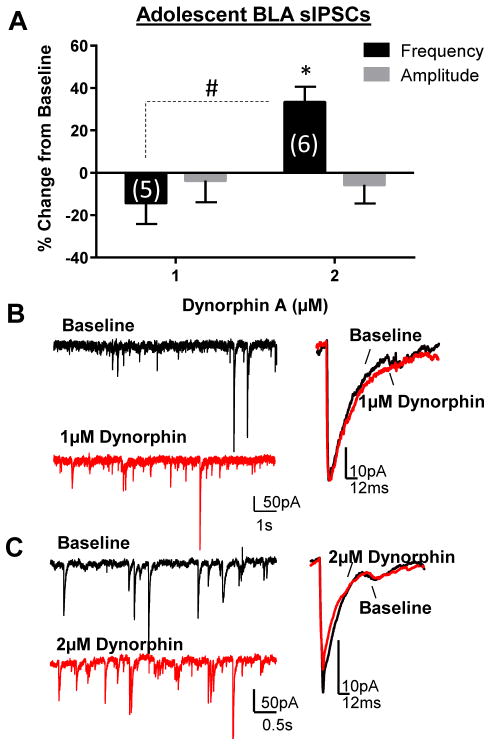
To determine if the effect of U69593 in adolescents could be reproduced with the endogenous KOR agonist, we tested the effect of exogenous dynorphin A in slices from adolescents. Similar to U69593, we found a dose-dependent effect of dynorphin A in adolescents (Fig. 2), whereby 2 μM dynorphin A significantly increased sIPSC frequency (t = 4.49, df = 5, n = 6, p < 0.05 compared to 0), but 1 μM did not (t = 1.41, df = 4, n = 5, p > 0.05 compared to 0). As with U69593, dynorphin A did not affect sIPSC amplitude at either dose tested in adolescents (1 μM: t = 0.35, df = 5, n = 6, p > 0.05 compared to 0; 2 μM: t = 0.63, df = 4, n = 5, p > 0.05 compared to 0).
Figure 2. Dynorphin also potentiates BLA GABA transmission in adolescents.
(A) Dynorphin A dose-dependently potentiates sIPSC frequency, but not amplitude in adolescents. * - p < 0.05 compared to 0; # - p < 0.05 compared to 1 μM dynorphin. Exemplar traces demonstrate effect of (B) 1 μM and (C) 2μM dynorphin on sIPSC frequency and amplitude in the adolescent BLA.
KORs are not tonically activated in the adolescent or adult BLA
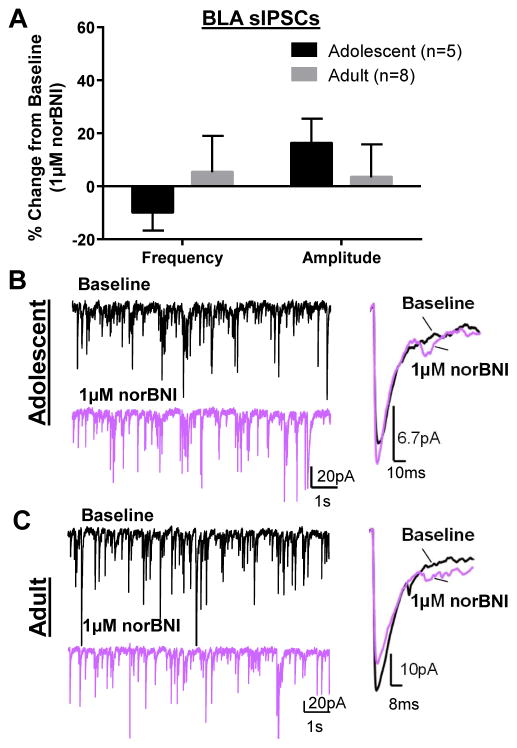
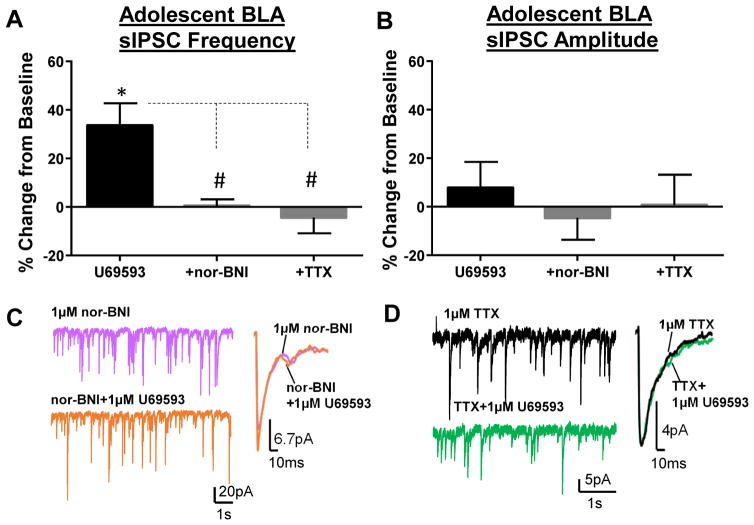
A lack of effect of the KOR activation on GABA transmission in adults could suggest that KORs are tonically activated, as has been shown in the CeA (Gilpin et al., 2014; Kang-Park et al., 2013). To test this we used the selective KOR antagonist (Fig. 3), nor-BNI, and found that application of nor-BNI (1 μM) did not significantly alter sIPSC frequency in either adolescents (t = 1.407, df = 4, n = 5, p > 0.05 compared to 0) or adults (t = 0.39, df = 7, n = 8, p > 0.05 compared to 0). Similarly, nor-BNI did not affect sIPSC amplitude in either adolescents (t = 01.76, df = 4, p > 0.05 compared to 0) or adults (t = 0.27, df = 7, n = 8, p > 0.05 compared to 0). These data suggest that KORs are not tonically activated in the BLA at either age. However, the 1 μM U69593-induced increase in sIPSC frequency in adolescents was completely blocked by pre-application of nor-BNI (1 μM) (Fig. 4A–C; n = 5, p < 0.05 by Bonferroni post-hoc).
Figure 3. KORs are not tonically activated in the BLA.
(A) norBNI (1 μM) does not affect sIPSC frequency or amplitude in adolescent or adult BLA. Exemplar traces demonstrate lack of effect norBNI on sIPSC frequency and amplitude in (B) adolescent and (C) adult BLA cells.
Figure 4. U69593-mediated potentiation of GABA transmission is blocked by nor-BNI and TTX in adolescents.
Effect of co-application of U69593 (1 μM) with nor-BNI (1μM) or TTX (1 μM) on sIPSC (A) frequency and (B) amplitude in adolescents. Exemplar traces of sIPSC frequency and amplitude of co-application of U69593 with (C) nor-BNI or (D) TTX. * - p < 0.05 compared to 0; # - p < 0.05 compared to U69593 alone.
KOR-mediated potentiation of GABA transmission is action potential-dependent in adolescents
sIPSCs include a mixture of action potential-dependent and –independent spontaneous GABAA receptor-mediated currents, and it was unknown through which mechanism KORs increased GABA release in adolescents. To test this we applied U69593 (1 μM) in the presence of TTX and found that the KOR-mediated potentiation of sIPSC frequency was completely abolished (Fig. 4A, B, and D; n = 6, p < 0.05 by Bonferroni post-hoc), suggesting that the KOR-mediated increase in GABA transmission is likely through actions on interneuron excitability.
KOR effects on glutamate transmission in the BLA
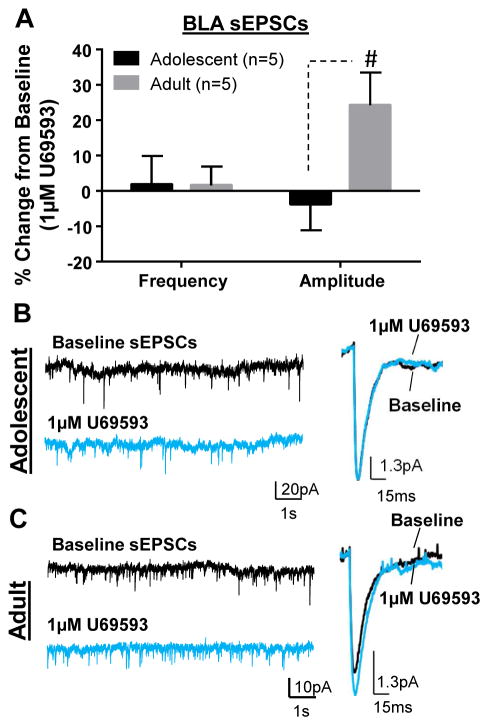
It was previously shown that KOR activation decreased fEPSPs in the BLA of younger mice, presumably by inhibiting glutamate transmission (Huge et al., 2009). However, given that GABA transmission was not blocked in that study, KOR-mediated decreases in fEPSPs could have resulted from increased GABAergic inhibition. Therefore, to determine if KOR activation altered glutamate transmission, we tested the effect of U69593 (1 μM) on glutamate-mediated transmission at both ages (Fig. 5). We did not find an effect of KOR activation on sEPSC frequency at either age (Fig. 5A; adolescent: t = 0.22, df = 4, n = 5, p > 0.05 compared to 0; adult: t = 0.29, df =4, n = 5, p > 0.05 compared to 0). Conversely, while there was no effect of U69593 on sEPSC amplitude in adolescents (Fig. 5A&B; t = 0.49, df = 4, n = 5, p > 0.05 compared to 0), we found a suggestive increase in amplitude in adults (Fig. 5A&C; t = 2.64, df = 4, n = 5, p = 0.058). Interestingly, the effect on sEPSC amplitude in adults was significantly different from adolescents (Fig. 5A; t = 2.36, df = 8, n = 5, p < 0.05 by unpaired t-test).
Figure 5. Effect of KOR activation on BLA glutamate transmission.
(A) Effect of U69593 (1 μM) on glutamate-mediated sEPSC frequency and amplitude in adolescents and adults. Exemplar traces showing effect of U69593 on sEPSCs in (B) adolescents and (D) adults. # - p < 0.05 compared to adolescent.
Membrane properties and basal synaptic transmission in the CeA
Although there are numerous types of neurons in the CeA with various electrophysiological properties (Herman et al., 2013), we could not distinguish them under our recording conditions. However, we did not find any significant differences in membrane capacitance, membrane resistance, sIPSC frequency, amplitude, area or decay time between adolescent and adult CeA neurons.
KOR function is not age-dependent in the CeA
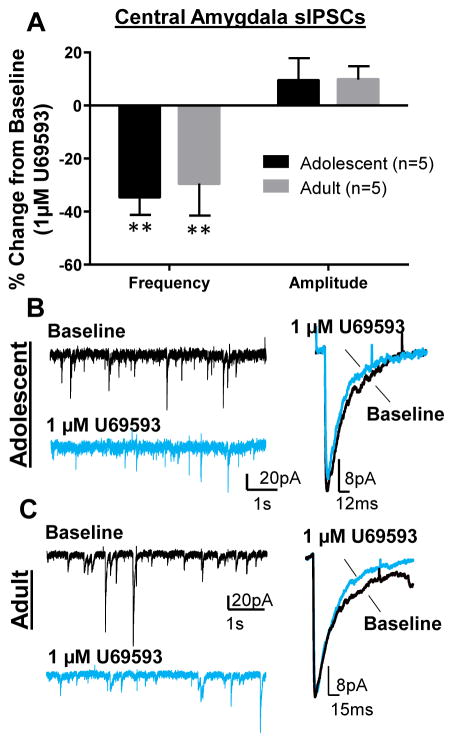
It has previously been reported that KOR activation suppresses GABA transmission in the CeA of younger rats (Gilpin et al., 2014) and adult mice (Kang-Park et al., 2013). However, whether KOR function in the CeA changes across ontogeny in the same species has not been directly examined. Given our findings in the BLA, we tested the effect of KOR activation on GABA transmission in the CeA of adolescents and adults. Similar to previous reports, but unlike the BLA, we found that U69593 (1 μM) significantly suppressed sIPSC frequency in both adolescents (Fig. 6A&B; t = 5.12, df = 4, n = 5, p < 0.01 compared to 0) and adults (Fig. 6A&C; t = 3.76, df = 4, n = 5, p < 0.05). We found one cell in adults that showed a subtle (~14%) potentiation of sIPSC frequency that was excluded from the analysis. Also consistent with previous reports, there was no effect of 1 μM U69593 on sIPSC amplitude in either adolescents (Fig. 6A&B; t = 1.12, df = 4, n = 5, p > 0.05 compared to 0) or adults (Fig. 6A&C; t = 2.04, df = 4, n =5, p > 0.05).
Figure 6. KOR activation suppressed GABA transmission in the CeA independent of age.
(A) Effect of U69593 (1 μM) on GABAA-mediated sIPSC frequency and amplitude in adolescents and adults. * - p < 0.05 compared to 0. Exemplar traces showing effect of U69593 on CeA sIPSCs in (B) adolescents and (D) adults.
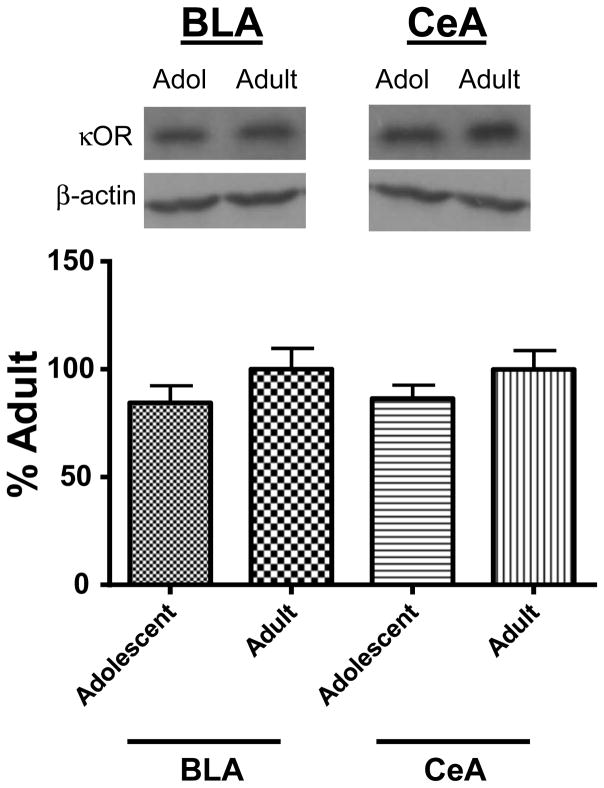
KOR expression is not developmentally-regulated in the BLA or CeA
A potential explanation for the age-dependent effect of KOR-modulation of BLA GABA transmission is a difference in KOR protein expression. Examination of KOR protein expression using western blotting techniques revealed that KOR levels did not differ in the BLA of adolescents compared to adults (Fig. 7; sum of ranks = 8,20; Mann-Whitney U = 2; n = 3–4; p > 0.05 by Mann Whitney test). Given that the magnitude of the KOR effect on GABA transmission in the CeA was not age-dependent, we also assessed KOR levels in the CeA and found that KOR expression did not significantly differ between adolescents and adults (Fig. 7; sum of ranks = 9,19; Mann-Whitney U = 3; n =3–4; p > 0.05 by Mann Whitney test). To verify the selectivity of the KOR antibody, as a negative control, cerebellum tissue was also analyzed (Nizhnikov et al., 2014). No bands were detected (not shown).
Figure 7. Western blot analysis of KOR expression in the BLA and CeA across ontogeny.
Representative bands of KOR protein in BLA and CeA of adolescents and adult. Averaged KOR protein levels normalized to β-actin. Data in graph are normalized to adult bands for each brain structure.
Discussion
KORs are known to modulate synaptic transmission throughout the central nervous system and are implicated in the negative affect associated with stress and addiction (Bruchas et al., 2010; Chavkin and Ehrich, 2014; Crowley and Kash, 2015; Knoll and Carlezon, 2010; Schwarzer, 2009; Van't Veer and Carlezon, 2013; Wee and Koob, 2010). However, little work has examined the role of KORs in adolescence, a critical developmental period vulnerable to numerous factors that increase the risk of mental disorders, particularly anxiety. This is the first study to systematically assess the functional role of KORs in modulating GABA transmission in the BLA, a structure critical in the initiation and maintenance of anxiety, across development. We provide compelling evidence that in drug- and stress-naïve adolescent males, KOR activation potentiates GABAergic transmission onto BLA pyramidal neurons, the main output cells of this amygdalar nucleus, and this effect is completely absent in adult males. Furthermore, we found that KORs are not tonically activated by endogenous dynorphin nor do they significantly alter glutamate transmission at either age. Strikingly, this developmental transition in KOR function is unique to the BLA, as KOR activation suppresses GABA transmission in the CeA of both adolescents and adults. Lastly, as BLA KOR expression did not differ between adolescents and adults, age-related functional differences in KOR modulation of GABAergic transmission is likely independent of transcriptional and translational mechanisms.
One of the most striking findings was that KOR activation increased GABA release in the adolescent BLA with both a synthetic KOR agonist and dynorphin A, the endogenous KOR agonist. While numerous studies have shown that KORs are Gi/o coupled [reviewed by (Bruchas and Chavkin, 2010)], which ultimately reduces neuronal excitability and/or neurotransmitter release in various brain structures (Chen et al., 2015; Crowley et al., 2016; Gilpin et al., 2014; Kang-Park et al., 2013; Karkhanis et al., 2016; Lemos et al., 2012; Li et al., 2012; Rose et al., 2015; Siciliano et al., 2016), studies have also shown that KORs can couple to Gs in various cellular models (Baraban et al., 1995; Hampson et al., 2000; Shen and Crain, 1990a, b, 1994) which can increase action potential duration (Shen and Crain, 1990a, b, 1994) and neuronal excitability in dentate granule cells (McDermott and Schrader, 2011). While we have not yet directly investigated these coupling mechanisms, our findings could be explained by a KOR-dependent increase in action potential duration of local GABAergic interneurons, resulting in the observed increase in sIPSC frequency. That the effect on sIPSC frequency was completely blocked by the action potential blocker, TTX, further supports an effect on action potential mechanisms, such as interneuron excitability, rather than an effect on release machinery. Another possible, but unlikely, explanation is that the observed increase in GABA transmission onto pyramidal neurons is a result of interneuron-interneuron disinhibition, which would be consistent with Gi coupled KORs. It is well known that BLA interneurons are both chemically- and electrically-coupled (Woodruff and Sah, 2007) and reduced inhibition of one interneuron onto another through a Gi coupled KOR would increase its excitability, ultimately resulting in the observed increase in GABA release onto pyramidal neurons. Follow-up studies examining these potential mechanisms in detail are ongoing in laboratory.
Another surprising and unexpected finding from this study was that the KOR-mediated increase in GABA transmission was completely absent in the adult BLA. Although there are no studies that have tested KOR function across development in the BLA, it was previously reported that KOR function is developmentally regulated in the paraventricular nucleus of the thalamus, with a peak effect at 4 weeks of age followed by a robust and stable drop at 8 weeks (Chen et al., 2015). However, Bruchas and colleagues have reported an interaction between CRF and KORs within the BLA of adults, specifically showing that only under stress (when BLA CRF levels are presumably elevated) do KORs become activated within the BLA, as BLA microinfusions of norBNI only altered anxiety-like behaviors in stressed adult mice, but not in unstressed mice (Bruchas et al., 2009). Therefore, it is possible that CRF must be present in order to effectively activate KORs in adults. It is worth noting that while our western data argue against differences in KOR expression in the adult BLA relative to adolescence as a primary factor, within the amygdala, this age-dependent regulation of KOR function is unique to the BLA as we found that KOR activation similarly suppresses GABA transmission in the CeA of adolescents and adults. Nonetheless, further studies are warranted to better understand this age-dependent switch in KOR function.
It was also interesting that norBNI alone did not affect GABA transmission in the BLA in either adolescents or adults. However, this is was not surprising given that norBNI did not alter fEPSPs in the adolescent BLA (Huge et al., 2009). Furthermore, microinjections of norBNI into the BLA did not alter anxiety-like behaviors in unstressed adult mice (Bruchas et al., 2009). While these previous studies have indicated that KORs are not tonically activated in unstressed animals, our data further supports that this is independent of age.
It is worth noting that basal neurotransmission (i.e. sIPSC/sEPSC frequency, amplitude, area, decay time) in BLA pyramidal neurons did not differ between adolescents and adults, consistent with the notion that both GABA and glutamate systems are stable after P28 (Ehrlich et al., 2013; Ehrlich et al., 2012) and previous comparisons of adolescents and adults (Zhang and Rosenkranz, 2016). However, we observed a very strong trend toward an increase in the membrane resistance of adult BLA pyramidal neurons in adolescents only when using a K-gluconate internal solution – a common solution used to record glutamate-mediated neurotransmission – but not with a KCl internal solution. It is well known that the composition of internal solutions can significantly impact numerous neuronal conductances that ultimately contribute to electrophysiological measures, including the membrane resistance (Kaczorowski et al., 2007; Lenz et al., 1997; Woehler et al., 2014). Furthermore, different internal solutions can also alter intracellular enzymatic activity, such as protein kinase A (Vargas et al., 1999). Another more likely possibility is that adult BLA pyramidal neurons have a more complex dendritic arborization and/or spine density (Bosch and Ehrlich, 2015), which could be better detected with the K-gluconate internal. While the reasons for this observation are not clear, these data suggest that differences in ion channels/conductances may exist in BLA pyramidal neurons across development.
KOR activation did not significantly impact glutamate transmission in the BLA. Only one other study has examined the functional role of KORs in the BLA and showed that KOR activation inhibited fEPSPs and blocked LTP in the BLA of late adolescent animals (Huge et al., 2009). While that study assumed that those effects were directly on glutamate transmission, our adolescent data suggest that KOR-mediated increases in GABA transmission, with no direct effect on glutamate transmission, could significantly reduce BLA excitability, resulting in the observed suppression of fEPSPs reported by Huge and colleagues. However, although we did not observe any significant changes in sEPSC frequency or amplitude in the presence of a KOR agonist in adults, there was a trend toward an increase in sEPSC amplitude. The significance of this subtle increase in sEPSC amplitude is unknown, but a KOR-mediated increase in glutamate activity in the BLA would ultimately result in increased anxiety-like behavior, consistent with the known anxiogenic effects of KOR agonists. However, as previously discussed, inhibition of BLA KORs has been shown to have no effect on the elevated-plus maze in stress- and drug-naïve adult mice (Bruchas et al., 2009). Conversely, in adult rats, BLA KOR blockade with the KOR antagonist, JDTic, reduced anxiety-like behaviors in the elevated-plus maze (Knoll et al., 2011). The complexity of the BLA KOR system is evident from these studies even within a single age group and future experiments are warranted.
An important consideration is, what the implications are of these functional processes on behavior? Numerous studies have shown that KOR modulation can significantly regulate anxiety-like behavior; however, the majority of these studies have been conducted in either adults or following procedures that elevate anxiety-like behaviors, such as chronic stress paradigms and models of drug dependence (i.e. alcohol, nicotine, cocaine) [reviewed by (Bruchas et al., 2010; Chavkin and Ehrich, 2014; Crowley and Kash, 2015; Knoll and Carlezon, 2010; Van't Veer and Carlezon, 2013; Wee and Koob, 2010)]. Recently it was also shown that in stress- and drug-naïve adults, systemic activation of KORs at low doses produces anxiogenic effects, while high doses are anxiolytic, and these effects appear to be driven by different brain structures (Wang et al., 2016). Moreover, social status also contribute to the behavioral responses observed following KOR activation in adults (Kudryavtseva et al., 2006). Although few studies have examined the behavioral effects of KOR activation in adolescents, all of them have shown that KOR agonists are anxiolytic in stress- and drug-naïve younger animals (Alexeeva et al., 2012; Braida et al., 2009; Privette and Terrian, 1995), particularly when infused in specific brain structures, such as the infralimbic cortex (Wall and Messier, 2000). Furthermore, adolescents display reduced aversion to systemic injections of KOR agonists (Anderson et al., 2014; Tejeda et al., 2012). Our study provides a potential mechanism for these observations; in stress- and drug-naïve adolescents, the BLA functions as the master regulator of the anxiety circuit, whereby BLA KOR-mediated potentiation of GABA transmission drives the anxiolytic properties of KOR agonists, shadowing the effects of CeA KORs. Conversely, in adults, a lack of BLA KOR regulation disengages the BLA from regulating the anxiety circuit, permitting the KOR-mediated decrease in CeA GABA transmission to drive the known anxiogenic effects of KOR agonists. Future studies should systematically test these hypotheses to determine the role of these individual amygdalar nuclei across ontogeny.
Taken together, we provide the first functional evidence for a developmental switch in BLA KOR function. Additionally, we demonstrate brain region-specific differences in the neuromodulatory effects of KORs that suggest differential regulation and integration of brain structures associated with anxiety. Importantly, our findings provide a novel neurobiological mechanism that may contribute to the known age-dependent rise in anxiety disorders. Bettering our understanding of this dynamic system will ultimately aid in the diagnosis and treatment of anxiety disorders across development.
Table 3.
CeA membrane properties and basal synaptic transmission
| Adolescent (n = 5) | Adult (n = 6) | |
|---|---|---|
| Capacitance (pF) | 60.97 ± 7.64 | 63.82 ± 31.45 |
| Membrane Resistance (MΩ) | 138.50 ± 62.60 | 201.00 ± 43.90 |
| sIPSC Frequency (Hz) | 2.46 ± 0.81 | 2.00 ± 0.63 |
| sIPSC Amplitude (pA) | 29.00 ± 11.52 | 27.42 ± 4.47 |
| sIPSC Area (pA*ms) | 1088.00 ± 226.20 | 964.00 ± 260.0 |
| sIPSC Decay Time (ms) | 28.72 ± 6.53 | 35.12 ± 6.64 |
Highlights.
Incidence of anxiety disorders increases from adolescence into adulthood
Kappa opioid receptors (KORs) are involved in anxiety
KORs increase GABA transmission in the BLA of adolescent, but not adults
KORs decrease GABA transmission in the adolescent and adult CeA
Age-dependent switch in BLA KOR function may contribute to anxiety disorders
Acknowledgments
The research reported here was funded by NIAAA (AA024890).
Abbreviations
- ACSF
artificial cerebral spinal fluid
- BNST
bed nucleus of the stria terminalis
- BLA
basolateral amygdala
- CeA
central amygdala
- fEPSP
field excitatory postsynaptic potential
- KOR
kappa opioid receptor
- LTP
long-term potentiation
- nor-BNI
nor-Binaltorphimine
- P
postnatal day
- sEPSC
spontaneous excitatory postsynaptic current
- sIPSC
spontaneous inhibitory postsynaptic current
- TTX
tetrodotoxin
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alexeeva EV, Nazarova GA, Sudakov SK. Effects of peripheral mu, delta, and Kappa-opioid receptor agonists on the levels of anxiety and motor activity of rats. Bull Exp Biol Med. 2012;153:720–721. doi: 10.1007/s10517-012-1809-2. [DOI] [PubMed] [Google Scholar]
- Anderson RI, Morales M, Spear LP, Varlinskaya EI. Pharmacological activation of kappa opioid receptors: aversive effects in adolescent and adult male rats. Psychopharmacology (Berl) 2014;231:1687–1693. doi: 10.1007/s00213-013-3095-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baculis BC, Diaz MR, Valenzuela CF. Third trimester-equivalent ethanol exposure increases anxiety-like behavior and glutamatergic transmission in the basolateral amygdala. Pharmacol Biochem Behav. 2015;137:78–85. doi: 10.1016/j.pbb.2015.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baraban SC, Lothman EW, Lee A, Guyenet PG. Kappa opioid receptor-mediated suppression of voltage-activated potassium current in a catecholaminergic neuronal cell line. J Pharmacol Exp Ther. 1995;273:927–933. [PubMed] [Google Scholar]
- Bosch D, Ehrlich I. Postnatal maturation of GABAergic modulation of sensory inputs onto lateral amygdala principal neurons. J Physiol. 2015;593:4387–4409. doi: 10.1113/JP270645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braida D, Capurro V, Zani A, Rubino T, Vigano D, Parolaro D, Sala M. Potential anxiolytic- and antidepressant-like effects of salvinorin A, the main active ingredient of Salvia divinorum, in rodents. Br J Pharmacol. 2009;157:844–853. doi: 10.1111/j.1476-5381.2009.00230.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruchas MR, Chavkin C. Kinase cascades and ligand-directed signaling at the kappa opioid receptor. Psychopharmacology (Berl) 2010;210:137–147. doi: 10.1007/s00213-010-1806-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruchas MR, Land BB, Chavkin C. The dynorphin/kappa opioid system as a modulator of stress-induced and pro-addictive behaviors. Brain Res. 2010;1314:44–55. doi: 10.1016/j.brainres.2009.08.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruchas MR, Land BB, Lemos JC, Chavkin C. CRF1-R activation of the dynorphin/kappa opioid system in the mouse basolateral amygdala mediates anxiety-like behavior. PLoS One. 2009;4:e8528. doi: 10.1371/journal.pone.0008528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bueno CH, Zangrossi H, Jr, Viana MB. The inactivation of the basolateral nucleus of the rat amygdala has an anxiolytic effect in the elevated T-maze and light/dark transition tests. Braz J Med Biol Res. 2005;38:1697–1701. doi: 10.1590/s0100-879x2005001100019. [DOI] [PubMed] [Google Scholar]
- Chavkin C, Ehrich JM. How does stress-induced activation of the kappa opioid system increase addiction risk? Biol Psychiatry. 2014;76:760–762. doi: 10.1016/j.biopsych.2014.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z, Tang Y, Tao H, Li C, Zhang X, Liu Y. Dynorphin activation of kappa opioid receptor reduces neuronal excitability in the paraventricular nucleus of mouse thalamus. Neuropharmacology. 2015;97:259–269. doi: 10.1016/j.neuropharm.2015.05.030. [DOI] [PubMed] [Google Scholar]
- Crowley NA, Bloodgood DW, Hardaway JA, Kendra AM, McCall JG, Al-Hasani R, McCall NM, Yu W, Schools ZL, Krashes MJ, Lowell BB, Whistler JL, Bruchas MR, Kash TL. Dynorphin Controls the Gain of an Amygdalar Anxiety Circuit. Cell Rep. 2016;14:2774–2783. doi: 10.1016/j.celrep.2016.02.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowley NA, Kash TL. Kappa opioid receptor signaling in the brain: Circuitry and implications for treatment. Prog Neuropsychopharmacol Biol Psychiatry. 2015 doi: 10.1016/j.pnpbp.2015.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrlich DE, Ryan SJ, Hazra R, Guo JD, Rainnie DG. Postnatal maturation of GABAergic transmission in the rat basolateral amygdala. J Neurophysiol. 2013;110:926–941. doi: 10.1152/jn.01105.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrlich DE, Ryan SJ, Rainnie DG. Postnatal development of electrophysiological properties of principal neurons in the rat basolateral amygdala. J Physiol. 2012;590:4819–4838. doi: 10.1113/jphysiol.2012.237453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gackenheimer SL, Suter TM, Pintar JE, Quimby SJ, Wheeler WJ, Mitch CH, Gehlert DR, Statnick MA. Localization of opioid receptor antagonist [3H]-LY255582 binding sites in mouse brain: comparison with the distribution of mu, delta and kappa binding sites. Neuropeptides. 2005;39:559–567. doi: 10.1016/j.npep.2005.09.006. [DOI] [PubMed] [Google Scholar]
- Gilpin NW, Roberto M, Koob GF, Schweitzer P. Kappa opioid receptor activation decreases inhibitory transmission and antagonizes alcohol effects in rat central amygdala. Neuropharmacology. 2014;77:294–302. doi: 10.1016/j.neuropharm.2013.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampson RE, Mu J, Deadwyler SA. Cannabinoid and kappa opioid receptors reduce potassium K current via activation of G(s) proteins in cultured hippocampal neurons. J Neurophysiol. 2000;84:2356–2364. doi: 10.1152/jn.2000.84.5.2356. [DOI] [PubMed] [Google Scholar]
- Herman MA, Contet C, Justice NJ, Vale W, Roberto M. Novel subunit-specific tonic GABA currents and differential effects of ethanol in the central amygdala of CRF receptor-1 reporter mice. J Neurosci. 2013;33:3284–3298. doi: 10.1523/JNEUROSCI.2490-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huge V, Rammes G, Beyer A, Zieglgansberger W, Azad SC. Activation of kappa opioid receptors decreases synaptic transmission and inhibits long-term potentiation in the basolateral amygdala of the mouse. Eur J Pain. 2009;13:124–129. doi: 10.1016/j.ejpain.2008.03.010. [DOI] [PubMed] [Google Scholar]
- Janak PH, Tye KM. From circuits to behaviour in the amygdala. Nature. 2015;517:284–292. doi: 10.1038/nature14188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaczorowski CC, Disterhoft J, Spruston N. Stability and plasticity of intrinsic membrane properties in hippocampal CA1 pyramidal neurons: effects of internal anions. J Physiol. 2007;578:799–818. doi: 10.1113/jphysiol.2006.124586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang-Park M, Kieffer BL, Roberts AJ, Siggins GR, Moore SD. kappa-Opioid receptors in the central amygdala regulate ethanol actions at presynaptic GABAergic sites. J Pharmacol Exp Ther. 2013;346:130–137. doi: 10.1124/jpet.112.202903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karkhanis AN, Rose JH, Weiner JL, Jones SR. Early-Life Social Isolation Stress Increases Kappa Opioid Receptor Responsiveness and Downregulates the Dopamine System. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2016 doi: 10.1038/npp.2016.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62:593–602. doi: 10.1001/archpsyc.62.6.593. [DOI] [PubMed] [Google Scholar]
- Kissler JL, Sirohi S, Reis DJ, Jansen HT, Quock RM, Smith DG, Walker BM. The one-two punch of alcoholism: role of central amygdala dynorphins/kappa-opioid receptors. Biol Psychiatry. 2014;75:774–782. doi: 10.1016/j.biopsych.2013.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kissler JL, Walker BM. Dissociating Motivational From Physiological Withdrawal in Alcohol Dependence: Role of Central Amygdala kappa-Opioid Receptors. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2016;41:560–567. doi: 10.1038/npp.2015.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knoll AT, Carlezon WA., Jr Dynorphin, stress, and depression. Brain Res. 2010;1314:56–73. doi: 10.1016/j.brainres.2009.09.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knoll AT, Muschamp JW, Sillivan SE, Ferguson D, Dietz DM, Meloni EG, Carroll FI, Nestler EJ, Konradi C, Carlezon WA., Jr Kappa opioid receptor signaling in the basolateral amygdala regulates conditioned fear and anxiety in rats. Biol Psychiatry. 2011;70:425–433. doi: 10.1016/j.biopsych.2011.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudryavtseva N, Gerrits MA, Avgustinovich DF, Tenditnik MV, Van Ree JM. Anxiety and ethanol consumption in victorious and defeated mice; effect of kappa-opioid receptor activation. Eur Neuropsychopharmacol. 2006;16:504–511. doi: 10.1016/j.euroneuro.2006.01.002. [DOI] [PubMed] [Google Scholar]
- Lack AK, Diaz MR, Chappell A, DuBois DW, McCool BA. Chronic ethanol and withdrawal differentially modulate pre- and postsynaptic function at glutamatergic synapses in rat basolateral amygdala. J Neurophysiol. 2007;98:3185–3196. doi: 10.1152/jn.00189.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeDoux JE. Emotion circuits in the brain. Annu Rev Neurosci. 2000;23:155–184. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- Lemos JC, Roth CA, Messinger DI, Gill HK, Phillips PE, Chavkin C. Repeated stress dysregulates kappa-opioid receptor signaling in the dorsal raphe through a p38alpha MAPK-dependent mechanism. J Neurosci. 2012;32:12325–12336. doi: 10.1523/JNEUROSCI.2053-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenz RA, Pitler TA, Alger BE. High intracellular Cl- concentrations depress G-protein-modulated ionic conductances. J Neurosci. 1997;17:6133–6141. doi: 10.1523/JNEUROSCI.17-16-06133.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Pleil KE, Stamatakis AM, Busan S, Vong L, Lowell BB, Stuber GD, Kash TL. Presynaptic inhibition of gamma-aminobutyric acid release in the bed nucleus of the stria terminalis by kappa opioid receptor signaling. Biol Psychiatry. 2012;71:725–732. doi: 10.1016/j.biopsych.2011.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDermott CM, Schrader LA. Activation of kappa opioid receptors increases intrinsic excitability of dentate gyrus granule cells. J Physiol. 2011;589:3517–3532. doi: 10.1113/jphysiol.2011.211623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merikangas KR, He JP, Burstein M, Swanson SA, Avenevoli S, Cui L, Benjet C, Georgiades K, Swendsen J. Lifetime prevalence of mental disorders in U.S. adolescents: results from the National Comorbidity Survey Replication--Adolescent Supplement (NCS-A) J Am Acad Child Adolesc Psychiatry. 2010;49:980–989. doi: 10.1016/j.jaac.2010.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nizhnikov ME, Pautassi RM, Carter JM, Landin JD, Varlinskaya EI, Bordner KA, Werner DF, Spear NE. Brief prenatal ethanol exposure alters behavioral sensitivity to the kappa opioid receptor agonist (U62,066E) and antagonist (Nor-BNI) and reduces kappa opioid receptor expression. Alcohol Clin Exp Res. 2014;38:1630–1638. doi: 10.1111/acer.12416. [DOI] [PubMed] [Google Scholar]
- Privette TH, Terrian DM. Kappa opioid agonists produce anxiolytic-like behavior on the elevated plus-maze. Psychopharmacology (Berl) 1995;118:444–450. doi: 10.1007/BF02245945. [DOI] [PubMed] [Google Scholar]
- Rose JH, Karkhanis AN, Chen R, Gioia D, Lopez MF, Becker HC, McCool BA, Jones SR. Supersensitive Kappa Opioid Receptors Promotes Ethanol Withdrawal-Related Behaviors and Reduce Dopamine Signaling in the Nucleus Accumbens. Int J Neuropsychopharmacol. 2015 doi: 10.1093/ijnp/pyv127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinow MJ, Juraska JM. Neuron and glia numbers in the basolateral nucleus of the amygdala from preweaning through old age in male and female rats: a stereological study. J Comp Neurol. 2009;512:717–725. doi: 10.1002/cne.21924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santerre JL, Rogow JA, Kolitz EB, Pal R, Landin JD, Gigante ED, Werner DF. Ethanol dose-dependently elicits opposing regulatory effects on hippocampal AMPA receptor GluA2 subunits through a zeta inhibitory peptide-sensitive kinase in adolescent and adult Sprague-Dawley rats. Neuroscience. 2014;280:50–59. doi: 10.1016/j.neuroscience.2014.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saul ML, Helmreich DL, Callahan LM, Fudge JL. Differences in amygdala cell proliferation between adolescent and young adult rats. Dev Psychobiol. 2014;56:517–528. doi: 10.1002/dev.21115. [DOI] [PubMed] [Google Scholar]
- Schwarzer C. 30 years of dynorphins--new insights on their functions in neuropsychiatric diseases. Pharmacol Ther. 2009;123:353–370. doi: 10.1016/j.pharmthera.2009.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen KF, Crain SM. Cholera toxin-A subunit blocks opioid excitatory effects on sensory neuron action potentials indicating mediation by Gs-linked opioid receptors. Brain Res. 1990a;525:225–231. doi: 10.1016/0006-8993(90)90868-c. [DOI] [PubMed] [Google Scholar]
- Shen KF, Crain SM. Cholera toxin-B subunit blocks excitatory effects of opioids on sensory neuron action potentials indicating that GM1 ganglioside may regulate Gs-linked opioid receptor functions. Brain Res. 1990b;531:1–7. doi: 10.1016/0006-8993(90)90751-v. [DOI] [PubMed] [Google Scholar]
- Shen KF, Crain SM. Nerve growth factor rapidly prolongs the action potential of mature sensory ganglion neurons in culture, and this effect requires activation of Gs-coupled excitatory kappa-opioid receptors on these cells. J Neurosci. 1994;14:5570–5579. doi: 10.1523/JNEUROSCI.14-09-05570.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siciliano CA, Calipari ES, Yorgason JT, Lovinger DM, Mateo Y, Jimenez VA, Helms CM, Grant KA, Jones SR. Increased presynaptic regulation of dopamine neurotransmission in the nucleus accumbens core following chronic ethanol self-administration in female macaques. Psychopharmacology (Berl) 2016;233:1435–1443. doi: 10.1007/s00213-016-4239-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slowe SJ, Simonin F, Kieffer B, Kitchen I. Quantitative autoradiography of mu-,delta- and kappa1 opioid receptors in kappa-opioid receptor knockout mice. Brain Res. 1999;818:335–345. doi: 10.1016/s0006-8993(98)01201-3. [DOI] [PubMed] [Google Scholar]
- Spear LP. Rewards, aversions and affect in adolescence: emerging convergences across laboratory animal and human data. Dev Cogn Neurosci. 2011;1:392–400. doi: 10.1016/j.dcn.2011.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear LP. Adolescents and alcohol: acute sensitivities, enhanced intake, and later consequences. Neurotoxicol Teratol. 2014;41:51–59. doi: 10.1016/j.ntt.2013.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tejeda HA, Natividad LA, Orfila JE, Torres OV, O'Dell LE. Dysregulation of kappa-opioid receptor systems by chronic nicotine modulate the nicotine withdrawal syndrome in an age-dependent manner. Psychopharmacology (Berl) 2012;224:289–301. doi: 10.1007/s00213-012-2752-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van't Veer A, Carlezon WA., Jr Role of kappa-opioid receptors in stress and anxiety-related behavior. Psychopharmacology (Berl) 2013;229:435–452. doi: 10.1007/s00213-013-3195-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vargas G, Yeh TY, Blumenthal DK, Lucero MT. Common components of patch-clamp internal recording solutions can significantly affect protein kinase A activity. Brain Res. 1999;828:169–173. doi: 10.1016/s0006-8993(99)01306-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wall PM, Messier C. U-69,593 microinjection in the infralimbic cortex reduces anxiety and enhances spontaneous alternation memory in mice. Brain Res. 2000;856:259–280. doi: 10.1016/s0006-8993(99)01990-3. [DOI] [PubMed] [Google Scholar]
- Wang DV, Wang F, Liu J, Zhang L, Wang Z, Lin L. Neurons in the amygdala with response-selectivity for anxiety in two ethologically based tests. PLoS One. 2011;6:e18739. doi: 10.1371/journal.pone.0018739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang YJ, Hang A, Lu YC, Long Y, Zan GY, Li XP, Wang Q, Zhao ZX, He L, Chi ZQ, Liu JG. kappa Opioid receptor activation in different brain regions differentially modulates anxiety-related behaviors in mice. Neuropharmacology. 2016 doi: 10.1016/j.neuropharm.2016.04.022. [DOI] [PubMed] [Google Scholar]
- Wee S, Koob GF. The role of the dynorphin-kappa opioid system in the reinforcing effects of drugs of abuse. Psychopharmacology (Berl) 2010;210:121–135. doi: 10.1007/s00213-010-1825-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woehler A, Lin KH, Neher E. Calcium-buffering effects of gluconate and nucleotides, as determined by a novel fluorimetric titration method. J Physiol. 2014;592:4863–4875. doi: 10.1113/jphysiol.2014.281097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodruff AR, Sah P. Networks of parvalbumin-positive interneurons in the basolateral amygdala. J Neurosci. 2007;27:553–563. doi: 10.1523/JNEUROSCI.3686-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Rosenkranz JA. Effects of Repeated Stress on Age-Dependent GABAergic Regulation of the Lateral Nucleus of the Amygdala. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2016 doi: 10.1038/npp.2016.33. [DOI] [PMC free article] [PubMed] [Google Scholar]