Abstract
While the etiology of depression is not fully understood, increasing evidence from animal models suggests a role for the ventral tegmental area (VTA) in pathogenesis. In this paper, we investigate the potential role of VTA mechanistic target of rapamycin 2 (TORC2) signaling in mediating susceptibility to chronic social defeat stress (CSDS), a well-established mouse model of depression. Utilizing genetic and viral knockout of Rictor (rapamycin-insensitive companion of target of rapamycin), a requisite component of TORC2, we demonstrate that decreasing Rictor-dependent TORC2 signaling in catecholaminergic neurons, or within the VTA specifically, does not alter susceptibility to CSDS. Opiate abuse and mood disorders are often comorbid, and previous data demonstrate a role for VTA TORC2 in mediating opiate reward. Thus, we also investigated its potential role in mediating changes in opiate reward following CSDS. Catecholaminergic deletion of Rictor increases water, sucrose, and morphine intake but not preference in a two-bottle choice assay in stress-naïve mice, and these effects are maintained after stress. VTA-specific knockout of Rictor increases water and sucrose intake after physical CSDS, but does not alter consummatory behavior in the absence of stress. These findings suggest a novel role for TORC2 in mediating stress-induced changes in consummatory behaviors that may contribute to some aspects of mood disorders.
1. Introduction
Depression is a serious mental illness that induces a significant societal burden as a leading cause of disability (Ferrari et al., 2013) and is highly co-morbid with other disorders such as drug addiction (Swendsen and Merikangas, 2000; Volkow, 2004). While the exact causes of depression remain elusive, a combination of factors, including stressful life events, are known to increase the likelihood of developing a major mood disorder (Shapero et al., 2014). Increasing evidence suggests a role for the dopamine reward circuit, and specifically activity of ventral tegmental area (VTA) dopamine neurons, in mediating susceptibility to chronic social defeat stress (CSDS), a rodent model of depression (Krishnan et al., 2007). Moreover, CSDS also induces biochemical changes in the VTA, including decreased phosphorylation of AKT at Ser473 (pAKT), and preventing or mimicking this biochemical event is sufficient to rescue or induce CSDS susceptibility, suggesting changes in VTA AKT activity are behaviorally relevant (Krishnan et al., 2008). Interestingly, VTA pAKT is also decreased in rats and mice treated chronically with morphine, and modulation of VTA AKT activity is sufficient to alter opiate reward, as measured by conditioned place preference (CPP) (Russo et al., 2007). Together, these data suggest that alteration of VTA AKT phosphorylation plays a critical role in both mood disorders and drug reward.
AKT is phosphorylated at Ser473 by the mechanistic target of rapamycin complex 2 (TORC2) (Sarbassov et al., 2005), and we have recently shown that altering TORC2 activity in the VTA is sufficient to induce changes in morphine reward (Mazei-Robison et al., 2011). Given the lack of a selective pharmacological inhibitor, we altered VTA TORC2 signaling via genetic deletion and viral-mediated overexpression of rapamycin-insensitive companion of TOR (Rictor), as this protein is a necessary component for TORC2 kinase activity. Global deletion of TORC2 is embryonically lethal (Shiota et al., 2006), and thus floxed-Rictor mice have been developed and used in combination with Cre driver lines (Dadalko et al., 2015a; Dadalko et al., 2015b; Siuta et al., 2010; Thomanetz et al., 2013) or stereotaxic infusion of AAV-Cre in adult mice (Mazei-Robison et al., 2011) to produce cell-type or brain-region specific Rictor KO mice to allow examination of the role of TORC2 signaling in vivo. Recently, floxed-Rictor mice have been crossed to the tyrosine hydroxylase (TH)-Cre reporter line to KO TORC2 signaling specifically from catecholaminergic neurons (TH-Rictor) (Dadalko et al., 2015a). TH-Rictor KO mice display an increase in novelty-induced locomotion compared to their wild-type controls, as well as an increase in lean and overall body mass, but with no reported difference in fat mass (Dadalko et al., 2015a).
Given our data that VTA KO of Rictor was sufficient to modulate morphine reward, we sought to determine whether KO of Rictor in the VTA or in TH neurons would increase susceptibility to CSDS, as predicted by pAKT results. Further, we sought to determine whether VTA- or TH-Rictor KO mice would have altered morphine reward following CSDS. While data on morphine reward following CSDS is limited (Covington and Miczek, 2001; Vivian and Miczek, 1999), susceptible mice have increased cocaine CPP following CSDS (Krishnan et al., 2007) and social defeat stress also increases cocaine self-administration in both rats (Covington and Miczek, 2001) and mice (Han et al., 2015). Thus, we assessed voluntary intake and preference for morphine using a two-bottle choice test. We found that while decreasing TORC2 signaling in either TH neurons or in the VTA does not increase susceptibility to CSDS, there were changes in consummatory behavior between Rictor KO mice and controls. Interestingly, whereas there were differences between TH- and VTA-Rictor KO prior to CSDS, all Rictor KO mice exhibited a similar phenotype post-CSDS. These data suggest that additional TORC2 substrates may exhibit competing effects to those of AKT in stress susceptibility and that behavioral outputs of TORC2 signaling may be dependent on subsets of catecholaminergic neurons.
2. Materials and Methods
2.1. Mice
All mice were housed at 22–25° C on a 12 hour light/dark cycle with food and water available ad libitum. Experiments utilized adult male and female mice (8–15 weeks). Homozygous floxed Rictor mice were generated as previously described (Mazei-Robison et al., 2011; Shiota et al., 2006; Siuta et al., 2010), and were also crossed with heterozygous tyrosine hydroxylase (TH)-Cre mice (Jackson Laboratories, 008601) to generate developmental Rictor knock-out (KO) mice (Dadalko et al., 2015a); all mice were fully backcrossed to the c57Bl/6 background. Mouse genotypes were verified at 21–28 days using standard procedures. Published primers to assess floxed-Rictor (5′-CCT GAG CAG TGC CCG ACT TCT CTA G-3′ and 5′-CCT TTC GCA TCG CCA CTG CA-3′) and TH-Cre (5′-GAT CTC CGG TAT TGA AAC TCC AGC-3′ and 5′-GCT AAA CAT GCT TCA TCG TCG G-3′) status were used. Of note, Cre-mediated deletion of Rictor using this floxed-Rictor line (via either AAV-Cre infusion or cross with Cre-driver line) has been shown to be sufficient to disrupt TORC2-mediated kinase activity as assessed by phosphorylation of AKT at Ser473 (Dadalko et al., 2015b; Mazei-Robison et al., 2011; Siuta et al., 2010). For social defeat stress studies, retired CD-1 male breeders (Charles River) were purchased and screened for aggressive behavior as described previously (Golden et al., 2011). All experiments were approved by the Institutional Animal Care and Use Committee at Michigan State University.
2.2. Drugs
Morphine sulfate (generously provided by the NIDA Drug Supply Program) and quinine sulfate (Sigma) were dissolved in water for drinking studies.
2.3. Viral-mediated gene transfer
Stereotaxic surgeries were completed as previously described (Mazei-Robison et al., 2011). Briefly, mice were anesthetized (100 mg/kg ketamine, 10 mg/kg xylazine) and bilateral infusions (0.5 μl) of AAV-GFP or AAV-Cre-GFP (UNC Vector Core) were targeted to the VTA (from bregma: −3.2 mm AP, +1.0 mm ML, and −4.6 mm DV, 7° angle). Mice were allowed to recover for ≥14 days prior to behavioral testing to allow for Cre-mediated gene silencing and the degradation of all remaining Rictor in target cells.
2.4. Validation of Rictor deletion
2.4.1. Viral targeting
Following experimental testing, mice were perfused with 4% paraformaldehyde-PBS and brains were cryo-preserved in 30% sucrose-PBS. Brains were sectioned (30 μm) and bilateral VTA targeting was confirmed by GFP expression. The representative VTA targeting and viral expression shown in Fig 4A was generated using standard immunohistochemistry techniques to label GFP- (Life Technologies A11122, 1:3000) and TH-positive (Sigma, T1299, 1:3000) cells in the VTA (Mazei-Robison et al., 2011). Mice with GFP expression outside the VTA were not included in analyses.
Figure 4.
Evaluation of baseline behaviors in stress-naïve AAV-Rictor-KO mice. AAV-Cre significantly decreased VTA Rictor expression (A). Knockdown of Rictor using AAV-Cre did not alter anxiety-like behavior in EPM (B: male: GFP=9, CRE=11; female: GFP=11, CRE=8) or OF (C: male: GFP=9, CRE=12; female: GFP=11, CRE=8) testing in either male (left) or female (right) mice. Locomotor activity in the OF test was also similar between AAV-Rictor-KO mice and controls (D: male: GFP=10, CRE= 12; female: GFP=11, CRE=8), **p<0.01, n=8–12 mice/group, individual data points shown.
2.4.2. Quantitative Real-Time (RT)-PCR
Rictor deletion was verified by RT-PCR using published procedures (Mazei-Robison et al., 2011). Briefly, VTA was microdissected from mice and stored at −80° C until processing. RN A was isolated and purified from VTA using Trizol and RNeasy microcolumns (Qiagen). Following reverse-transcription (Applied Biosystems), RNA levels were quantified by RT-PCR using the ΔΔCt method and GAPDH as a normalization control, and all analyses were performed in triplicate. All primers were previously validated: Rictor: 5′-ATG GCG GCG ATC GGC CGC G-3′ and 5′-GAT ACT CCT TGC AAT TTG GCC ACA-3′; GAPDH: 5′-AGG TCG GTG TGA ACG GAT TTG-3′ and 5′-TGT AGA CCA TGT AGT TGA GGT CA-3′; Cre: 5′-CCC GGC AAA ACA GGT AGT TA-3′ and 5′-GAA CGA AAA CGC TGG TTA GC-3′ (Berton et al., 2006; Mazei-Robison et al., 2011).
2.5. Chronic social defeat stress (CSDS)
CSDS was performed as previously described (Golden et al., 2011). Briefly, male control and Rictor KO mice were subjected to a brief daily physical encounter in the home-cage of an aggressive CD-1 retired breeder followed by sensory contact for the following 24 hours via a perforated plexiglass partition. Non-stress controls were handled and housed across from a novel c57Bl6 mouse daily. Following the 10th defeat episode, mice were singly housed. A variant of CSDS that utilizes a purely psychological stressor, witness or emotional stress, was also performed as previously described (Warren et al., 2013). Emotional CSDS was performed as described above, with the exception that a second experimental mouse was placed on the opposite side of the plexiglass partition during the physical encounter allowing this mouse to witness physical social defeat stress.
2.6. Behavioral overview
Behavioral characterization was completed ≥14 days following surgery to allow time for Cre-mediated gene silencing. Mice were 8–14 weeks of age and were weighed before, during, and following behavioral testing. All tests were completed during the light cycle under red light illumination and video-tracking software (Clever Systems Top Scan) was used for quantification. For cohorts of mice that underwent the entire battery of baseline behaviors, the order of testing was open field, elevated plus maze, then social interaction testing to minimize exposure effects; each completed on a different day.
2.6.1. Social interaction
Social interaction was assessed as previously described (Golden et al., 2011). Mice were placed in a 38 cm x 38 cm arena for two-2.5 min. test sessions, with a CD-1 target mouse absent, then present in a plexiglass and mesh cylinder. Time spent in the interaction zone (7.5 cm surrounding the cylinder) and corners was measured, along with distance traveled. The social interaction (SI) ratio was calculated as time spent in the interaction zone with target present/time spent with target absent *100. Susceptibility to CSDS was defined as an SI ratio <100 (Krishnan et al., 2007).
2.6.2. Open Field
Mice were placed in a 38cm x 38cm arena and distance traveled and time spent in the center and periphery in the 10 min. test session were assessed.
2.6.3. Elevated plus maze
Mice were placed in the center of a 5cm x 35cm plus maze. Distance traveled, time spent in open and closed arms, and entries into open and closed arms were assessed during the 5 min. test session.
2.6.4. Two-bottle choice voluntary intake
Mice were singly housed and had access to two 50 ml conical tubes with sipper tops in their home cage. Throughout the experiment, bottles were weighed at the same time each morning, bottle location was switched daily, and consumption was determined by the volume of fluid intake. Prior to sucrose or morphine preference, baseline water intake was measured for 2–3 days. For sucrose preference and consumption, mice had access to bottles containing water and 1% sucrose for 4 days. For morphine preference and consumption, morphine sulfate or quinine sulfate (taste control for morphine) were dissolved in 0.2% sucrose based on prior studies (Belknap et al., 1993; Ferraro et al., 2005; Forgie et al., 1988). Bottles for male mice contained 0.06 mg/ml quinine and 0.3 mg/ml morphine and bottles for female mice contained 0.01 mg/ml quinine and 0.05 mg/ml morphine.
2.7. Statistics
All values are reported as mean +/− SEM. Graph Pad Prism was used to perform all statistical analyses. Unpaired t-tests were used to compare groups of two. One way analysis of variance (ANOVA) was used to compare groups of three, followed by a Tukey post-hoc test, when appropriate. Two way ANOVA was used to compare groups with two independent variables (factors), followed by Tukey post-hoc test when appropriate. Differences were considered significant when p<0.05.
3.0 Results
3.1. TH-Rictor-KO mice do not exhibit baseline differences in anxiety- and depressive-like behavior
To determine whether developmental KO of Rictor in TH cells influences anxiety-like behavior, we first assessed performance in the elevated plus maze (EPM). Heterozygous or homozygous deletion of Rictor in male mice did not affect their time spent in the open arm compared to littermate controls (Fig 1A). Similarly, there was no significant difference in open arm time observed between female TH-Rictor-KO mice and littermate controls. We next completed open field (OF) testing and determined the time spent in the center as a second measure of anxiety-like behavior (Fig 1B). There were no significant differences in center time between male TH-Rictor KO mice and their littermate controls, nor were there differences in female mice. Together, these data suggest that deletion of Rictor in TH cells does not affect baseline anxiety-like behavior.
Figure 1.
Evaluation of anxiety-like baseline behaviors in stress naïve TH-Rictor-KO mice. There were no statistical differences in anxiety-like behaviors including time spent in the open arms of the EPM test (A: male: CON=6, HET=10, KO=10; female: CON=15, KO=11) or center time in the OF test (B: male: CON=18, HET=36, KO=22; female: CON=20, KO=12) between control, heterozygous, or homozygous male (left) or female (right) TH-Rictor-KO mice. Homozygous male TH-Rictor-KO mice exhibited increased locomotor activity in the OF test compared to controls (CON=19, HET=36, KO=23), but this difference was not observed in female Rictor-KO-mice (CON=28, KO=15) (C), *p<0.05, n=6–36 mice/group, individual data points shown.
During OF testing, we also assessed general locomotor activity (Fig 1C). We found that the total distance traveled was significantly increased in male homozygous TH-Rictor KO mice compared to heterozygous TH-Rictor KO mice and littermate controls (F(2,75)=5.1, p=0.008, Tukey’s post-hoc test, p<0.05), however this effect was not observed in female mice. This difference in activity may be partially explained by the fact that the total activity of female mice, regardless of genotype, was greater than that of males (Two-way ANOVA, Sex factor F(1,81)=17.9, p<0.0001). The increase in baseline locomotor activity in male homozygous Rictor-TH KO mice is consistent with published data (Dadalko et al., 2015a), however female mice were not examined in the previous study.
Given that modulation of VTA AKT activity, a TORC2/Rictor substrate, alters social interaction (SI) following CSDS, we next sought to determine whether TH-Rictor KO mice had any baseline differences in social interaction (Fig 2A). We found that SI scores did not differ between either male or female TH-Rictor KO mice and their control littermates. Additionally, we found no differences in locomotor activity during SI testing (males: Con: 6729 +/− 735, Het: 5842 +/− 352, Homo: 7397 +/− 731, p>0.05 and females Con: 9640 +/− 519, KO: 10,559 +/− 427, p>0.05), suggesting that the hyperlocomotion of male TH-Rictor mice may be abrogated in the presence of environmental stimuli, in this case an empty wire mesh enclosure.
Figure 2.
Evaluation of depressive-related behaviors in stress naïve TH-Rictor-KO mice. There were no statistical differences in social interaction (A: male: CON=9, HET=14, KO=12; female: CON=18, KO=10) or sucrose preference (B: male: CON=9, HET=14, KO=11; female: CON=11, KO=12) between control, heterozygous, or homozygous male (left) or female (right) TH-Rictor-KO mice. Homozygous male TH-Rictor-KO mice drank significantly more sucrose solution than heterozygous TH-Rictor-KO mice and controls (CON=9, HET=14, KO=11), with a similar non-significant trend observed in female TH-Rictor-KO mice (CON=10, KO=11) (C), *p<0.05, +p=0.08, n=9–18 mice/group, individual data points shown.
As another indicator of depressive-like behavior, we examined sucrose preference using a two-bottle choice test (Fig 2B). Neither male nor female TH-Rictor mice exhibited significant differences in preference for a 1% sucrose solution compared to control mice, suggesting TH-Rictor KO does not induce anhedonia in the absence of stress. However, while there were no significant differences in sucrose preference, we found that TH-Rictor KO mice drank a greater volume of the sucrose solution (Fig 2C). This effect was significant for homozygous male TH-Rictor KO mice compared to heterozygous and wild-type littermates (F(2,31)=5.96, p<0.01, Tukey’s post-hoc test, p<0.05) and a similar non-significant trend was observed in female TH-Rictor KO mice (t(19)=1.8, p=0.08). This drove a significant increase in the total volume of liquid consumed (sucrose + water) by TH-Rictor KO mice (Con: 7.27 +/− 0.23, Het: 7.83 +/− 0.19, Homo: 9.50 +/− 0.59; F(2,31)=8.94, p<0.001, Tukey’s post-hoc test, p<0.01), and it appears that sucrose preference was not altered because water intake was also significantly increased (Con: 1.08 +/− 0.09, Het: 1.60 +/− 0.15, Homo: 1.59 +/− 0.10; F(2,31)=4.83, p<0.05, Tukey’s post-hoc test, p<0.05 from Controls, Het not significantly different from Homo). Together, these data suggest that while TH-Rictor KO mice do not exhibit baseline depressive-like behavior, they do display sex-specific differences in general locomotor behavior and voluntary fluid intake.
3.2. TH-Rictor-KO mice have higher levels of voluntary fluid intake
To further explore this difference in voluntary fluid intake, we examined water intake in a two-bottle choice test where both bottles contain water. Consistent with our sucrose data, we found that male homozygous TH-Rictor KO mice drank significantly more water than heterozygous and wild-type littermates (Fig 3A, F(2,40)=4.725, p=0.0144, Tukey’s post-hoc test, p<0.05). Interestingly, when we examined female TH-Rictor KO, we did not observe any differences in water intake from controls (Fig. 3A, p>0.05), in contrast to increased water intake in male TH-Rictor-KO mice, and the trend for increased sucrose intake in female TH-Rictor KO mice.
Figure 3.
Evaluation of water and morphine consumption in stress naïve TH-Cre mice. Male TH-Rictor-KO mice drank more water (A: CON=11, HET=18, KO=14) and morphine solution (C: CON=11, HET=18, KO=14) compared to controls, with no difference in morphine preference (B: CON=11, HET=18, KO=14). Female TH-Rictor-KO mice did not drink more water than controls (A: CON=10, KO=11) but did drink more morphine solution (C: CON=10, KO=11), with no difference morphine preference (B; CON=10, KO=11), *p<0.05, n=10–18 mice/group, individual data points shown.
Given that Rictor KO in the VTA via AAV-Cre infusion is sufficient to decrease morphine reward as measured by CPP (Mazei-Robison et al., 2011), we next decided to measure voluntary morphine consumption and preference using the two-bottle choice test in TH-Rictor KO mice. Similar to the sucrose preference results, we found that both male and female TH-Rictor KO mice exhibit similar morphine preference to littermate controls (Fig 3B). However, when we examined morphine intake in these mice, we found that both male and female TH-Rictor-KO mice had significantly elevated intake compared to controls (Figure 3C, Male: F(2,40)=5.31, p=0.009; Female: t(19)=2.74, p=0.013, Tukey’s post-hoc test, p<0.05). Quinine solution intake was not significantly different between TH-Rictor KO and control mice (Male: Con: 0.89 +/− 0.10, Het: 0.95 +/− 0.10, Homo: 0.98 +/− 0.10; Female: Con: 1.69 +/− 0.24, KO: 1.91 +/− 0.18), which likely contributed to the lack of an overall effect on morphine preference. Together, these data suggest that TH-Rictor-KO mice have increased voluntary fluid intake, and that this effect is more pronounced in male mice, which exhibit significantly increased intake of water, sucrose, and morphine solutions.
3.3. VTA-Rictor-KO mice do not exhibit any differences in baseline behaviors
In order to determine whether the differences we observed in locomotor activity and fluid consumption in TH-Rictor-KO mice were consistent with changes in TORC2 signaling in the VTA, we generated VTA-specific KO mice (VTA-Rictor-KO) via AAV-Cre infusion into the VTA of floxed-Rictor mice. These mice have significantly decreased Rictor mRNA expression in VTA compared to AAV-GFP infused controls (Fig 4A, t(9)=3.65, p=0.005), however this effect is not genetically limited to TH-positive cells in the VTA, and Rictor KO may thus occur in any transduced VTA cells, including GABAergic, dopaminergic, or others.
We first examined VTA-Rictor-KO mice using the same measures of anxiety- and depressive-like behaviors used for TH-Rictor-KO mice. Similar to TH-Rictor-KO mice, we found no significant difference between VTA-Rictor-KO mice and their GFP controls during EPM testing (Fig 4B). Center time in the OF testing was also similar between VTA-Rictor-KO mice and controls (Fig 4C), suggesting that Rictor KO in VTA does not alter baseline anxiety. In contrast to male TH-Rictor-KO mice, we found that male VTA-Rictor-KO mice did not display increased locomotor activity compared to controls (Fig 4D). Similarly, there were no differences in locomotor activity between female VTA-Rictor-KO mice and their GFP controls (Fig 4D). These data suggest that changes in locomotor activity in male TH-Rictor-KO mice may be driven by brain regions other than VTA, such as substantia nigra, whose dopaminergic cells project to and modulate motor-oriented dorsal striatum neurons.
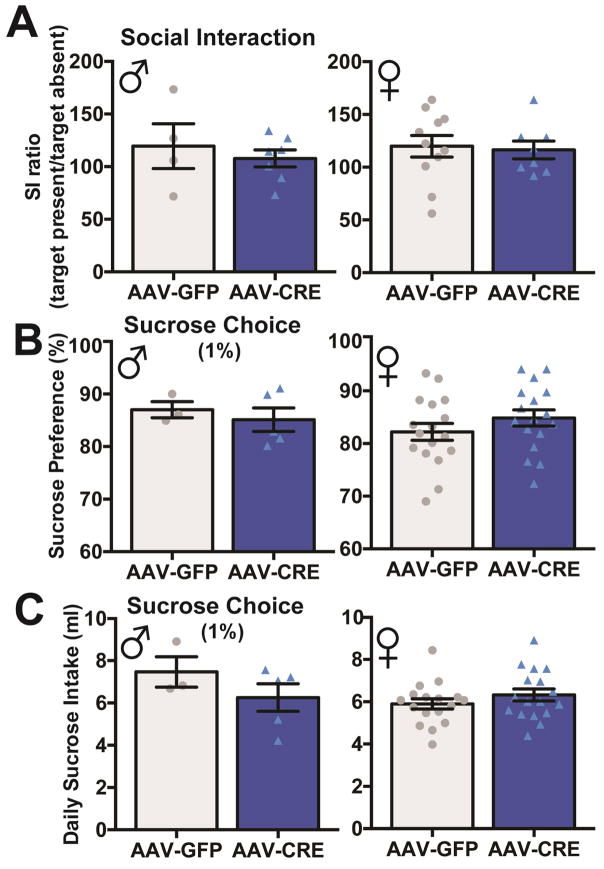
We next examined social interaction and found no significant differences between VTA-Rictor KO mice and GFP controls (Fig 5A). Sucrose preference was also similar between VTA-Rictor KO mice and GFP controls (Fig 5B), consistent with data from TH-Rictor-KO mice that baseline depressive-like behaviors are unaltered by Rictor KO.
Figure 5.
Evaluation of depressive-related behaviors in stress naïve AAV-Rictor-KO mice. There were no significant differences in social interaction (A: male: GFP=4, CRE=7; female: GFP=11, CRE=9), sucrose preference (B: male: GFP=3, CRE=5; female: GFP=17, CRE=17), or volume of sucrose intake (C: male: GFP=3, CRE=5; female: GFP=17, CRE=17) between male (left) or female (right) AAV-Rictor-KO mice and GFP controls, n=3–17 mice/group, individual data points shown.
3.4. VTA-Rictor-KO mice do not exhibit any differences in voluntary fluid intake
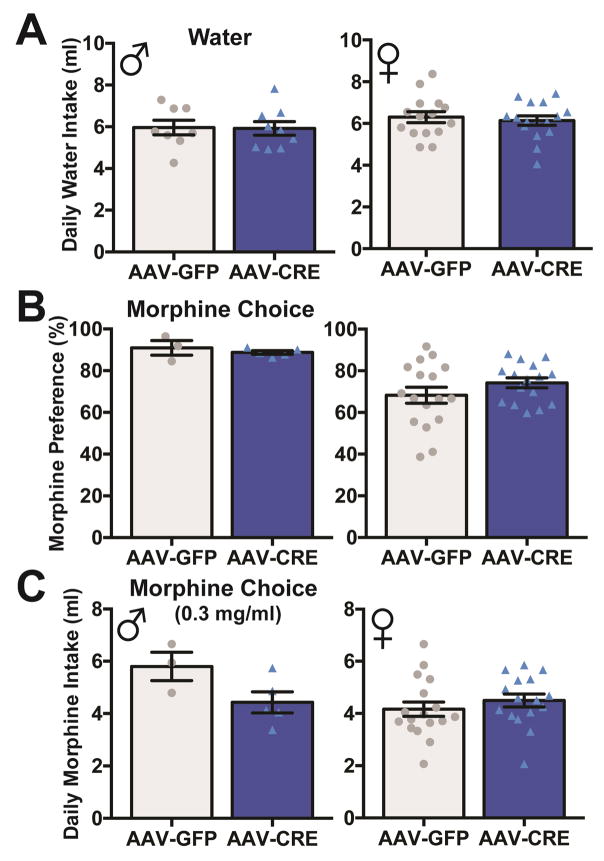
In contrast to TH-Rictor-KO mice, there was no significant difference in the volume of sucrose consumed for either male or female VTA-Rictor-KO mice compared to controls (Fig 5C). Additionally, VTA-Rictor-KO mice do not exhibit increased water intake from GFP controls (Fig 6A). Finally, we found no differences in morphine preference (Fig 6B), volume of morphine consumed (Fig 6C), or quinine consumed (data not shown), in male or female VTA-Rictor-KO mice. Together, these data suggest that VTA-Rictor-KO is not sufficient to alter voluntary fluid intake
Figure 6.
Evaluation of water and morphine consumption in stress naïve AAV-Rictor-KO mice. Knockdown of VTA TORC2 signaling did not alter water intake (A: male: GFP=8, CRE=9; female: GFP=17, CRE=17), morphine preference (B: male: GFP=3, CRE=5; female: GFP=17, CRE=16) or morphine intake (C: male: GFP=3, CRE=5; female: GFP=17, CRE=16) in either male or female mice compared to GFP controls, n=3–17 mice/group, individual data points shown.
3.5. TH-Rictor-KO mice are not more susceptible to physical or emotional CSDS
Given previous data that decreasing VTA AKT activity increased susceptibility to physical CSDS (Krishnan et al., 2008), we sought to determine whether decreasing TORC2 signaling, thereby decreasing AKT phosphorylation and activity, would also increase susceptibility to CSDS. We exposed male TH-Rictor-KO mice and littermate controls to standard 10-day physical and emotional CSDS (Golden et al., 2011; Sial et al., 2016). As expected, male mice that underwent physical CSDS had significantly reduced SI scores compared to controls (Fig 7A). However, while TH-Rictor-KO mice and their wild type controls subjected to physical CSDS had SI scores significantly lower than their non-stressed controls (Fig 7A: F(5,48)=7.18, p=0.0001), there was no significant difference in SI scores between physical CSDS groups, nor was there an increase in the number of mice susceptible to stress in the KO group (SI scores <100, wt = 5/7, KO = 7/12). We also assessed the impact of emotional CSDS, as this model of chronic stress induces a more subtle phenotype on day 11, allowing us to uncover even a weak effect of TH-Rictor-KO on susceptibility to stress. Mice exposed to emotional CSDS had SI ratios closer to 100, but there were no differences between TH-Rictor-KO mice and wild type controls (Fig 7A). These data suggest that decreasing TORC2 signaling in TH neurons does not increase susceptibility to physical and emotional CSDS.
Figure 7.
Evaluation of susceptibility of TH-Rictor-KO mice to physical and emotional CSDS and post-stress consumption of water, sucrose, and morphine solutions. Physical CSDS significantly decreased social interaction (SI) of both TH-Rictor-KO (n=12) and control mice (n=7), but there was no difference between genotypes (A; non-stressed controls: CON=12, KO=10). Exposure to emotional stress did not significantly alter SI (CON=7, KO=6). TH-Rictor-KO mice exposed to physical CSDS had significantly increased water (B: Non-stressed controls: CON=12, KO=10; Physical: CON=7, KO=12; Emotional: CON=6, KO=6), sucrose (C: Non-stressed controls: CON=12, KO=10; Physical: CON=7, KO=12; Emotional: CON=5, KO=5), and morphine (D: Non-stressed controls: CON=11, KO=10; Physical: CON=7, KO=11; Emotional: CON=5, KO=5) intake compared to control mice exposed to physical CSDS and non-stressed controls. No significant differences in consumption were observed in TH-Rictor-KO mice exposed to emotional CSDS, *p<0.05, n=6–12 mice/group, individual data points shown.
3.6. VTA-Rictor-KO mice are not more susceptible to physical CSDS
To allow direct comparison to the previous VTA AKT study (Krishnan et al., 2008), we also subjected VTA-Rictor-KO mice and GFP controls to physical CSDS. While mice that underwent physical CSDS had decreased SI scores compared to non-stressed controls, there was no difference between VTA-Rictor-KO mice and GFP controls (Fig 8A), again suggesting that decreasing TORC2 signaling in VTA neurons does not increase susceptibility to physical CSDS.
Figure 8.

Evaluation of susceptibility of AAV-Rictor-KO mice to physical CSDS and post-stress consumption of water, sucrose, and morphine solutions. Physical CSDS generally decreased SI score but did not result in a significant differences between AAV-Rictor-KO mice and their GFP controls (A: Non-stressed controls: CON=6, KO=7; Physical Stress: CON=6, KO=7). Exposure to physical CSDS increased water (B: Non-stressed controls: CON=6, KO=7; Physical Stress: CON=6, KO=5) and sucrose (C: Non-stressed controls: CON=4, KO=5; Physical Stress: CON=6, KO=5) intake in AAV-Rictor-KO mice compared to AAV-GFP controls, while morphine intake was not significantly increased (D: Non-stressed controls: CON=6, KO=7; Physical Stress: CON=6, KO=5), *p<0.05, n=4–7 mice/group, individual data points shown.
3.7. Physical CSDS increases voluntary fluid intake of TH-Rictor-KO and VTA-Rictor-KO mice
Following SI testing, two-bottle choice testing was completed to assess water, sucrose, and morphine intake. Male TH-Rictor-KO mice that underwent physical CSDS exhibited a significant increase in water consumption compared to both unstressed controls and physical CSDS wild type mice (Fig 7B, F(5,47)=5.09, p=0.0008, one-way ANOVA, Tukey’s post-hoc test, p<0.05). However, this effect was not evident in TH-Rictor-KO mice exposed to emotional CSDS (Fig 7B). Similarly, TH-Rictor-KO mice that underwent physical CSDS exhibited increased sucrose intake compared to wild-type controls (Fig 7C, F(5,45)=4.17, p=0.003, one-way ANOVA, Tukey’s post-hoc test, p<0.05). Finally, morphine consumption was significantly increased in TH-Rictor-KO mice exposed to physical, but not emotional CSDS (Fig 7D, F(5,45)=9.35, p=0.0001, one-way ANOVA, Tukey’s post-hoc test, p<0.05).
We also examined the drinking behavior of VTA-Rictor-KO mice after exposure to physical CSDS. While there was no difference in water intake between non-stressed VTA-Rictor-KO mice and GFP controls, VTA-Rictor-KO mice exposed to physical CSDS drank significantly more water than physically stressed GFP controls (Fig 8B, F(3,20)=4.46, p=0.01, one-way ANOVA, Tukey post-hoc test). Similarly, VTA-Rictor-KO mice exposed to physical stress consumed more sucrose than non-stressed mice (Fig 8C, F(3,16)=3.54, p=0.04, one-way ANOVA, Tukey’s post-hoc test, p<0.05). Finally, a similar trend for increased morphine consumption by VTA-Rictor-KO mice exposed to physical CSDS was observed (Fig 8D), but this did not reach statistical significance (p>0.05).
4.0 Discussion
Molecular and behavioral studies have identified VTA AKT activity as a mediator of susceptibility to stress, as overexpression of catalytically inactive AKT was shown to increase susceptibility to sub-chronic stress and increasing expression rescued CSDS-induced social avoidance (Krishnan et al., 2008). Since TORC2 modulates AKT via Ser473 phosphorylation, and abrogation of phosphorylation at this site is sufficient to decrease AKT’s ability to phosphorylate substrates such as Fox01/3a (Guertin et al., 2006; Jacinto et al., 2006), our goal was to determine if decreasing VTA TORC2 signaling was sufficient to increase susceptibility to CSDS. Because similar decreases in VTA AKT activity are observed following chronic opiate exposure and CSDS, we also investigated morphine intake after CSDS to determine the effect of decreasing TORC2 signaling on morphine reward and ingestive behavior.
We found that decreasing VTA TORC2 activity, either in catecholaminergic cells or specifically in the VTA, does not increase susceptibility to physical or emotional CSDS. One possible reason for the difference in the current findings from those that investigated AKT directly is that decreasing phosphorylation of AKT at Ser473 is not the same as overexpressing an AKT mutant form (K174M) that eliminates all catalytic activity. For example, in the current studies even though AKT is not phosphorylated at Ser473, it can be phosphorylated at other sites (such as Thr308, by PDK (Siuta et al., 2010)), as well as interact with binding partners. Although studies indicate that AKT phosphorylation at Ser473 is necessary for full catalytic activation (Sarbassov et al., 2005), it is possible that in our studies residual AKT activity is sufficient to mediate normal behavioral responses. For example, Rictor- or Sin1-deficient murine embryonic fibroblasts (MEFs) display a loss of phosphorylation of AKT Ser473 while retaining some phosphorylation of AKT Thr308, and while in vitro AKT kinase activity is decreased to 10–15% of control cells, phosphorylation of the AKT substrate FOXO3 is significantly decreased while phosphorylation of GSK3B and TSC2 are not, suggesting AKT Ser473 and Thr308 phosphorylation might differentially impact substrate phosphorylation (Guertin et al., 2006 and Jacinto et al., 2006). Moreover, such substrate-selective effects may underlie different neuropsychiatric disorders, as Ser473 phosphorylation is linked to schizophrenia-associated symptoms in mouse models and human lymphocytes (Keri et al., 2011; Sei et al., 2007; Siuta et al., 2010). Additionally, while TORC2 controls the phosphorylation of AKT Ser473, it also phosphorylates other substrates, including other AGC kinases (Foster and Fingar, 2010). Thus, KO of TORC2 activity could be influencing activity of other substrates whose actions normally oppose those of AKT.
While Rictor KO mice were not more susceptible to CSDS, they did exhibit some baseline differences in behavior and these differed between the two Rictor KO models we employed. In our developmental model, Rictor expression is eliminated from neurons that express tyrosine hydroxylase (TH), the rate-limiting enzyme in dopamine synthesis. While this does decrease TORC2 signaling in the VTA, a brain region with a high concentration of TH-positive dopamine neurons, one caveat of this model is that it also decreases TORC2 signaling in other brain regions with a high proportion of DA neurons such as the substantia nigra, as well as those containing noradrenergic neurons such as the locus coeruleus. Thus, we also used Cre-expressing viral vectors to specifically target the VTA, but in this model TORC2 signaling is likely decreased in all subtypes of VTA neurons (GABAergic, glutamatergic and dopaminergic). Additionally, while Rictor expression is significantly decreased in this model (~50%, Fig 4A) and is sufficient to induce morphological and behavioral effects in mice (Mazei-Robison et al., 2011), there still exists a population of cells with intact TORC2 signaling that could impact results.
We found that male developmental Rictor knockouts (TH-Rictor-KO) had increased locomotor activity compared to control littermates, consistent with previous data (Dadalko et al., 2015a). Interestingly, we did not observe any difference in locomotor activity in VTA-Rictor-KO mice. This difference could suggest that decreased TORC2 signaling in a catecholaminergic region outside the VTA, such as the substantia nigra, drives increased locomotor activity. However, a similar increase in novelty-induced locomotor activity has been observed in forebrain-specific Rictor KO mice (Nestin-Cre x flox-Rictor), which suggests TORC2 signaling in neurons that influence locomotor output centers such as the striatum is also sufficient to alter locomotor activity (Dadalko et al., 2015b). It is also possible that the altered locomotor response is developmentally regulated, as locomotor results have been consistently noted in crosses with various Cre lines, but Rictor KO in VTA of adult mice was not sufficient to alter morphine-induced locomotor activity (Mazei-Robison et al., 2011). However, local Rictor KO in striatum was sufficient to increase AMPH-induced locomotor activity (Dadalko et al., 2015b), suggesting that alteration of TORC2 activity in adult mice, and its associated changes in dopaminergic signaling, can be sufficient to change locomotor behavior. Interestingly, we found that the increase in locomotor activity was limited to male TH-Rictor-KO mice, as female TH-Rictor-KO mice did not differ from their controls. This was surprising, but given that all prior studies utilized only male mice, the possibility of sex differences in TORC2-related behaviors was unexplored. One possibility is that we failed to see an increase in locomotor activity because females had higher overall rates of activity than males (Fig. 1C). However, given that higher rates of locomotor activity than those we observed are possible for female mice, as in psychostimulant studies, it is unlikely that a ceiling effect is the sole cause of this difference. Our sex-specific difference in locomotor activity could inform other behaviors, as one method to investigate individual differences in neuropsychiatric-related behaviors is to categorize rodents as “high” or “low” responders based on locomotor activity in a novel environment. This novelty-induced locomotion has been found to correlate with a variety of traits including learning, anxiety, and drug reward (Carreira et al., 2017; Harro, 2010; Kabbaj et al., 2000). However, most of the studies to date have only examined the correlation of novelty-induced locomotor response with other behaviors in male mice, so little is known about whether differences in novelty-induced locomotor activity are similarly predictive in females. A recent study attempted to address this question in mice, and while they didn’t find any differences in the median locomotor activity or center time between males and females, they found by principal component analysis that male behavior on a battery of tests was explained by locomotion-related variables while anxiety- and depressive-like behaviors accounted for more of the variance in the females (Carreira et al., 2017). Thus, TORC2-dependent changes in locomotor activity may be expected to alter other behavioral phenotypes in a sex-dependent manner. We did not observe any sex-specific differences in the other baseline behaviors (EPM, FST), but future studies that examine differences in male vs. female TH-Rictor-KO responses to acute or chronic stress, or fear-related learning, may offer additional insights into whether changes in locomotor activity predict sex-specific phenotypes. Our findings suggest that there is a critical need to explore behavioral traits and the impact of altered neuronal signaling in both male and female mice, as the predictions made between assays may not correlate between the sexes. Future studies should delve further into this locomotor phenotype, including whether this difference is also apparent in the home cage or whether it is restricted to novel environments, and investigate the interplay between neuroendocrine and TORC2 signaling.
We also observed differences between male TH-Rictor-KO mice and VTA-Rictor KO mice in fluid consumption. At baseline, male TH-Rictor-KO mice consume more fluids (water, sucrose, or morphine) than their control littermates, while VTA-Rictor-KO mice are similar to GFP controls. The changes in ingestive behavior appeared more prominent when rewarding substances like sucrose and morphine were available, for example TH-Rictor KO mice exhibited 116% water intake of wild-type controls, but 127% and 122% of sucrose and morphine, respectively. However, there is not a concomitant increase in sucrose or morphine preference, suggesting that the increased consumption is not driven by a difference in reward or palatability. One possibility is that the increased fluid intake is a result of the increased locomotor activity, given that both the activity and intake increases are found in the male TH-Rictor-KO mice, but not the VTA-Rictor-KO mice. However, we also found that female TH-Rictor-KO mice consumed more fluid than controls in sucrose and morphine choice experiments, and these mice did not exhibit increased locomotor activity. Moreover, while it was not formally investigated in this study, we did not observe any differences in consumption of normal chow in either male or female TH-Rictor-KO mice (data not shown), consistent with prior data from male TH-Rictor-KO mice (Dadalko et al., 2015a), suggesting that there is not a global change in consummatory behavior. It is also possible that the mice are not actually ingesting the fluid, but exhibit increased interactions/manipulation of the sipper tops that results in increased fluid leaking from the bottles. We think this is unlikely based on our observations that the proportions of water and rewarding substance consumed are consistent from day to day, even as the bottle location is alternated, suggesting that animals are making a similar choice/activity at bottles throughout the experiment. Thus, the mechanism underlying increased fluid consumption in TH-Rictor-KO mice remains obscure. It is possible that the effect could be driven by signaling effects in noradrenergic cells (either central or peripheral), a hypothesis that could be tested by crossing the floxed-Rictor mice to norepinephrine transporter (NET)-Cre mice. However, given that we see a similar increase in fluid intake in VTA-Rictor-KO mice following stress, a region that lacks NE neurons, it suggests that there is also a role for VTA DA neuronal effects.
While neither TH-Rictor-KO nor VTA-Rictor-KO exhibited increased susceptibility to stress, exposure to physical stress increased fluid intake in both models. Since physical stress exposure induced increased intake in VTA-Rictor-KO mice that were unaffected at baseline, this suggests that Rictor KO mice do have altered responses to stress, albeit without changes in social avoidance. Similar to the baseline TH-Rictor-KO mice data, the stress-induced change in fluid intake was not accompanied by a concomitant increase in preference, suggesting changes in reward were not responsible. These data were somewhat surprising as we have previously found that VTA-Rictor-KO mice exhibited decreased morphine reward as assessed by conditioned place preference assay (Mazei-Robison et al., 2011). Thus, we predicted that in the baseline state, Rictor KO mice would exhibit a decrease in morphine preference in the two-bottle choice assay. Given the differences in the route of morphine administration between the two studies (voluntary vs. experimenter), it may be that KO of Rictor in VTA is sufficient to alter association of the relatively high dose of morphine in CPP studies to a context, but is not sufficient to alter the motivation to obtain a lower morphine dose in the two-bottle task. In contrast to exposure to physical stress, emotional stress did not alter fluid intake in Rictor KO mice. This difference might be due to the magnitude of the stress exposure, as the social avoidance phenotype incubates in emotional stress mice, with a small effect 1 day post-stress that then becomes indistinguishable from physical stress mice 28 days later (Warren et al., 2013).
Overall, these studies reveal that disruption of TORC2 signaling, either in catecholaminergic neurons or specifically within the VTA, does not influence susceptibility to CSDS-induced social avoidance. Instead, these experiments reveal a novel role for TORC2 signaling in mediating changes in activity and fluid intake that appear linked to stress responses. Further, given differences between the two models, our work demonstrates that it will be critical in future studies to evaluate the specific functions of TORC2 signaling within DA versus NE neurons, their potential developmental contributions, and the role of sex in both baseline and stress-induced behaviors.
Highlights.
Rictor knockout in TH neurons alters voluntary fluid intake and locomotor activity
Rictor knockout in VTA does not alter baseline activity or fluid intake
Rictor knockout in TH neurons or VTA alters fluid intake after chronic stress
Rictor knockout increases locomotor activity in males but not females
Acknowledgments
Funding: We would like to thank Ken Moon for excellent technical assistance and AJ Robison and Andrew Eagle for helpful discussion. This work was funded by the National Institute on Drug Abuse (R01 DA039895 and R03 DA037426, MMR) and graduate fellowships from the PhRMA foundation and the National Institute of General Medical Sciences (T32 GM092715, SK), with no direct input on the design, conduct, analysis or publication of the studies. We would also like to thank the NIDA Drug Supply Program for generously providing morphine.
Footnotes
Disclosure: The authors have no financial interests or conflicts of interest to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Belknap JK, Crabbe JC, Riggan J, O’Toole LA. Voluntary consumption of morphine in 15 inbred mouse strains. Psychopharmacology (Berl) 1993;112:352–358. doi: 10.1007/BF02244932. [DOI] [PubMed] [Google Scholar]
- Berton O, McClung CA, Dileone RJ, Krishnan V, Renthal W, Russo SJ, Graham D, Tsankova NM, Bolanos CA, Rios M, Monteggia LM, Self DW, Nestler EJ. Essential role of BDNF in the mesolimbic dopamine pathway in social defeat stress. Science. 2006;311:864–868. doi: 10.1126/science.1120972. [DOI] [PubMed] [Google Scholar]
- Carreira MB, Cossio R, Britton GB. Individual and sex differences in high and low responder phenotypes. Behav Processes. 2017;136:20–27. doi: 10.1016/j.beproc.2017.01.006. [DOI] [PubMed] [Google Scholar]
- Covington HE, 3rd, Miczek KA. Repeated social-defeat stress, cocaine or morphine. Effects on behavioral sensitization and intravenous cocaine self-administration “binges”. Psychopharmacology (Berl) 2001;158:388–398. doi: 10.1007/s002130100858. [DOI] [PubMed] [Google Scholar]
- Dadalko OI, Niswender K, Galli A. Impaired mTORC2 signaling in catecholaminergic neurons exaggerates high fat diet-induced hyperphagia. Heliyon. 2015a;1:e00025. doi: 10.1016/j.heliyon.2015.e00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dadalko OI, Siuta M, Poe A, Erreger K, Matthies HJ, Niswender K, Galli A. mTORC2/rictor signaling disrupts dopamine-dependent behaviors via defects in striatal dopamine neurotransmission. J Neurosci. 2015b;35:8843–8854. doi: 10.1523/JNEUROSCI.0887-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrari AJ, Charlson FJ, Norman RE, Patten SB, Freedman G, Murray CJ, Vos T, Whiteford HA. Burden of depressive disorders by country, sex, age, and year: findings from the global burden of disease study 2010. PLoS Med. 2013;10:e1001547. doi: 10.1371/journal.pmed.1001547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferraro TN, Golden GT, Smith GG, Martin JF, Schwebel CL, Doyle GA, Buono RJ, Berrettini WH. Confirmation of a major QTL influencing oral morphine intake in C57 and DBA mice using reciprocal congenic strains. Neuropsychopharmacology. 2005;30:742–746. doi: 10.1038/sj.npp.1300592. [DOI] [PubMed] [Google Scholar]
- Forgie ML, Beyerstein BL, Alexander BK. Contributions of taste factors and gender to opioid preference in C57BL and DBA mice. Psychopharmacology (Berl) 1988;95:237–244. doi: 10.1007/BF00174516. [DOI] [PubMed] [Google Scholar]
- Foster KG, Fingar DC. Mammalian target of rapamycin (mTOR): conducting the cellular signaling symphony. J Biol Chem. 2010;285:14071–14077. doi: 10.1074/jbc.R109.094003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golden SA, Covington HE, 3rd, Berton O, Russo SJ. A standardized protocol for repeated social defeat stress in mice. Nat Protoc. 2011;6:1183–1191. doi: 10.1038/nprot.2011.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guertin DA, Stevens DM, Thoreen CC, Burds AA, Kalaany NY, Moffat J, Brown M, Fitzgerald KJ, Sabatini DM. Ablation in mice of the mTORC components raptor, rictor, or mLST8 reveals that mTORC2 is required for signaling to Akt-FOXO and PKCalpha, but not S6K1. Dev Cell. 2006;11:859–871. doi: 10.1016/j.devcel.2006.10.007. [DOI] [PubMed] [Google Scholar]
- Han X, Albrechet-Souza L, Doyle MR, Shimamoto A, DeBold JF, Miczek KA. Social stress and escalated drug self-administration in mice II. Cocaine and dopamine in the nucleus accumbens. Psychopharmacology (Berl) 2015;232:1003–1010. doi: 10.1007/s00213-014-3734-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harro J. Inter-individual differences in neurobiology as vulnerability factors for affective disorders: implications for psychopharmacology. Pharmacol Ther. 2010;125:402–422. doi: 10.1016/j.pharmthera.2009.11.006. [DOI] [PubMed] [Google Scholar]
- Jacinto E, Facchinetti V, Liu D, Soto N, Wei S, Jung SY, Huang Q, Qin J, Su B. SIN1/MIP1 maintains rictor-mTOR complex integrity and regulates Akt phosphorylation and substrate specificity. Cell. 2006;127:125–137. doi: 10.1016/j.cell.2006.08.033. [DOI] [PubMed] [Google Scholar]
- Kabbaj M, Devine DP, Savage VR, Akil H. Neurobiological correlates of individual differences in novelty-seeking behavior in the rat: differential expression of stress-related molecules. J Neurosci. 2000;20:6983–6988. doi: 10.1523/JNEUROSCI.20-18-06983.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keri S, Seres I, Kelemen O, Benedek G. The relationship among neuregulin 1-stimulated phosphorylation of AKT, psychosis proneness, and habituation of arousal in nonclinical individuals. Schizophr Bull. 2011;37:141–147. doi: 10.1093/schbul/sbp063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan V, Han MH, Graham DL, Berton O, Renthal W, Russo SJ, Laplant Q, Graham A, Lutter M, Lagace DC, Ghose S, Reister R, Tannous P, Green TA, Neve RL, Chakravarty S, Kumar A, Eisch AJ, Self DW, Lee FS, Tamminga CA, Cooper DC, Gershenfeld HK, Nestler EJ. Molecular adaptations underlying susceptibility and resistance to social defeat in brain reward regions. Cell. 2007;131:391–404. doi: 10.1016/j.cell.2007.09.018. [DOI] [PubMed] [Google Scholar]
- Krishnan V, Han MH, Mazei-Robison M, Iniguez SD, Ables JL, Vialou V, Berton O, Ghose S, Covington HE, 3rd, Wiley MD, Henderson RP, Neve RL, Eisch AJ, Tamminga CA, Russo SJ, Bolanos CA, Nestler EJ. AKT signaling within the ventral tegmental area regulates cellular and behavioral responses to stressful stimuli. Biol Psychiatry. 2008;64:691–700. doi: 10.1016/j.biopsych.2008.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazei-Robison MS, Koo JW, Friedman AK, Lansink CS, Robison AJ, Vinish M, Krishnan V, Kim S, Siuta MA, Galli A, Niswender KD, Appasani R, Horvath MC, Neve RL, Worley PF, Snyder SH, Hurd YL, Cheer JF, Han MH, Russo SJ, Nestler EJ. Role for mTOR signaling and neuronal activity in morphine-induced adaptations in ventral tegmental area dopamine neurons. Neuron. 2011;72:977–990. doi: 10.1016/j.neuron.2011.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo SJ, Bolanos CA, Theobald DE, DeCarolis NA, Renthal W, Kumar A, Winstanley CA, Renthal NE, Wiley MD, Self DW, Russell DS, Neve RL, Eisch AJ, Nestler EJ. IRS2-Akt pathway in midbrain dopamine neurons regulates behavioral and cellular responses to opiates. Nat Neurosci. 2007;10:93–99. doi: 10.1038/nn1812. [DOI] [PubMed] [Google Scholar]
- Sarbassov DD, Guertin DA, Ali SM, Sabatini DM. Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science. 2005;307:1098–1101. doi: 10.1126/science.1106148. [DOI] [PubMed] [Google Scholar]
- Sei Y, Ren-Patterson R, Li Z, Tunbridge EM, Egan MF, Kolachana BS, Weinberger DR. Neuregulin1-induced cell migration is impaired in schizophrenia: association with neuregulin1 and catechol-o-methyltransferase gene polymorphisms. Mol Psychiatry. 2007;12:946–957. doi: 10.1038/sj.mp.4001994. [DOI] [PubMed] [Google Scholar]
- Shapero BG, Black SK, Liu RT, Klugman J, Bender RE, Abramson LY, Alloy LB. Stressful life events and depression symptoms: the effect of childhood emotional abuse on stress reactivity. J Clin Psychol. 2014;70:209–223. doi: 10.1002/jclp.22011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiota C, Woo JT, Lindner J, Shelton KD, Magnuson MA. Multiallelic disruption of the rictor gene in mice reveals that mTOR complex 2 is essential for fetal growth and viability. Dev Cell. 2006;11:583–589. doi: 10.1016/j.devcel.2006.08.013. [DOI] [PubMed] [Google Scholar]
- Sial OK, Warren BL, Alcantara LF, Parise EM, Bolanos-Guzman CA. Vicarious social defeat stress: Bridging the gap between physical and emotional stress. J Neurosci Methods. 2016;258:94–103. doi: 10.1016/j.jneumeth.2015.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siuta MA, Robertson SD, Kocalis H, Saunders C, Gresch PJ, Khatri V, Shiota C, Kennedy JP, Lindsley CW, Daws LC, Polley DB, Veenstra-Vanderweele J, Stanwood GD, Magnuson MA, Niswender KD, Galli A. Dysregulation of the norepinephrine transporter sustains cortical hypodopaminergia and schizophrenia-like behaviors in neuronal rictor null mice. PLoS Biol. 2010;8:e1000393. doi: 10.1371/journal.pbio.1000393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swendsen JD, Merikangas KR. The comorbidity of depression and substance use disorders. Clin Psychol Rev. 2000;20:173–189. doi: 10.1016/s0272-7358(99)00026-4. [DOI] [PubMed] [Google Scholar]
- Thomanetz V, Angliker N, Cloetta D, Lustenberger RM, Schweighauser M, Oliveri F, Suzuki N, Ruegg MA. Ablation of the mTORC2 component rictor in brain or Purkinje cells affects size and neuron morphology. J Cell Biol. 2013;201:293–308. doi: 10.1083/jcb.201205030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vivian JA, Miczek KA. Interactions between social stress and morphine in the periaqueductal gray: effects on affective vocal and reflexive pain responses in rats. Psychopharmacology (Berl) 1999;146:153–161. doi: 10.1007/s002130051101. [DOI] [PubMed] [Google Scholar]
- Volkow ND. The reality of comorbidity: depression and drug abuse. Biol Psychiatry. 2004;56:714–717. doi: 10.1016/j.biopsych.2004.07.007. [DOI] [PubMed] [Google Scholar]
- Warren BL, Vialou VF, Iniguez SD, Alcantara LF, Wright KN, Feng J, Kennedy PJ, Laplant Q, Shen L, Nestler EJ, Bolanos-Guzman CA. Neurobiological sequelae of witnessing stressful events in adult mice. Biol Psychiatry. 2013;73:7–14. doi: 10.1016/j.biopsych.2012.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]