Abstract
Background
Antibiotics are widely and heavily used in the treatment of chronic sinusitis. Bactericidal antibiotics can stimulate reactive oxygen species (ROS) formation, a pro-inflammatory response and cell death in cultured human sinonasal epithelial cells(SNECs). Sulforaphane is a potent stimulator of the antioxidant Nrf-2 system and a suppressor of inflammation. In this study we utilized sulforaphane to further explore the relationship between levofloxacin treatment, ROS formation and the cell death response.
Methods
SNECs were collected from patients during endoscopic sinus surgery and grown in culture at the air-liquid interface. Differentiated SNECs were stimulated with levofloxacin with or without sulforaphane pretreatment. Reactive oxygen species were quantified. Apoptosis markers of Caspase-3 activity and DNA fragmentation were quantified.
Results
Cultured SNECs treated with levofloxacin resulted in a significant increase in activity of the pro-apoptotic Caspase-3 protease (5.9 fold, p = 0.01). The increase in activity was suppressed by pretreatment with sulforaphane (1.9 fold). ROS levels increased with levofloxacin treatment, (range 1.2-1.8 fold) but were not significantly suppressed by pretreatment with sulforaphane (range 1-1.3 fold).
Discussion
In this study, we demonstrate that treatment of cultured SNECs with levofloxacin leads to an increase in Caspase-3 activity. Sulforaphane pretreatment suppresses the increased apoptotic response possibly through its antioxidant stimulating properties. Our results suggest that levofloxacin treatment stimulates a potent pro-apoptotic possibly through an ROS-dependent mechanism. Future studies will explore if this antibiotic-induced response is harmful to recovery of function in those with sinusitis.
Keywords: Antibiotics, ROS, Rhinosinusitis, sulforaphane, apoptosis, Innate Immunity
Introduction
Chronic rhinosinusitis (CRS) is an inflammatory disease of the sinuses comprised of a spectrum of inflammatory states1. The role of bacterial infection as a causative agent for CRS remains unclear, yet antibiotics are widely used in the treatment of both acute and chronic rhinosinusitis2. Macrolides, quinolones and penicillins are the most common antibiotics used in the treatment of sinusitis3 and a diagnosis of acute or chronic rhinosinusitis accounts for 11% of all antibiotics prescribed in an ambulatory setting4. The quinolone, levofloxacin, is commonly utilized for bacterial upper respiratory infections5.
Quinolones are bactericidal antibiotics–those which kill greater than 99.9% of bacteria. Quinolones are highly potent antibiotics that target bacterial DNA gyrase as part of their primary mechanism of killing bacteria6. In addition to the classic mechanisms of action, bactericidal antibiotics also promote altered bacterial metabolism, respiration, and iron homeostasis that results in reactive oxygen species (ROS) formation that contributes to a portion of the cell death7-14. Expanding upon this common mechanism of cell death8, bactericidal antibiotics are also able to stimulate reactive oxygen species formation in mammalian cells through mitochondrial dysfunction15. This results in accumulation of damaged DNA, proteins, and lipids that may have long term consequences to human systems15. Recent evidence demonstrates that bactericidal antibiotics stimulate ROS formation in human sinonasal epithelial cells16. This resulted in increased expression of Nuclear factor erythroid 2-related factor 2 (Nrf-2)-mediated antioxidant genes, secretion of the pro-inflammatory cytokines Interleukin 1β (IL-1β) and Tumor Necrosis Factor (TNFα) 16.
Nuclear factor erythroid 2-related factor 2 (Nrf2) is a transcription factor that binds to antioxidant responsive elements and activates expression of systems that can mitigate the damage caused by ROS 17,18. Nrf2 also appears to have a role in preserving mitochondrial integrity, particularly during periods of stress19. Sulforaphane is a potent stimulator of the antioxidant Nrf-2 system that can suppress elements of the inflammatory response20.
In this study we sought to further explore the relationship between antibiotic-mediated ROS formation and cell death in human sinonasal epithelial cells. We demonstrate that treatment with levofloxacin leads to an increase in ROS formation and Caspase-3 activity. This response is blunted by addition of sulforaphane. Our results suggest that levofloxacin stimulates a potent pro-apoptotic response in human sinonasal epithelial cells.
Material and Methods
Human Subjects
Ten subjects were enrolled in the study. The research protocol was approved through our Institutional Review process, and all subjects gave signed informed consent. Mucosal tissue was collected from the ethmoid sinuses during endoscopic sinus surgery and grown in culture at the air-liquid interface (ALI) as previously described16,21. 7 of 10 samples were from control patients and the remainder from those with CRS. All control patients were defined as those without CRS who were undergoing endoscopic sinonasal surgery for dacrocystorhinostomy, cerebrospinal fluid leak repair or endoscopic skull base surgery.
The mucosal tissue was transferred to phosphate buffered saline (PBS) supplemented by penicillin (100 μg/mL, Gibco, Gaithersburg, MD), streptomycin (100 μg/mL, Gibco), amphotericin B (2.5 μg/mL, Gibco), and gentamicin (50μg/mL, Gibco). Samples were collected through a cell strainer (BD Falcon) and digested in 4°C overnight in Ham’s F12 media containing 0.01% protease Sigma Type XIV (Sigma, St. Louis, MO) supplemented with antibiotics as above. The cells were separated by straining into a conical tube to which fetal bovine serum (FBS, Sigma) was added to a final concentration of 10% to inactivate the protease. Cells were centrifuged at 1200 rpm for 10 minutes in 4°C, after which the supernatant was aspirated. The washed SNEC were re-suspended in Bronchial Epithelium Growth Medium (BEGM) and seeded at a density ≥ 1.5 × 104 cells/cm2 in collagen coated 100-mm culture dishes. The media was changed initially 24 hours after the cells were grown, and then every 48 hours until cells reached confluence.
SNEC Culture at the Air-Liquid Interface (ALI)
Confluent cells were washed with HBSS prior to trypsinization, then treated at 37°C for 2 minutes with a solution containing 0.2% Trypsin (Sigma), 1% polyvinylpyrrolidone (Sigma), and 0.02% EGTA (Sigma) in HBSS. The trypsin was then neutralized by the addition of an equal volume of cold soybean trypsin inhibitor at a concentration of 1 mg/mL in Ham’s F12 media. Dissociated cells were washed and re-suspended into BEGM media and plated into human type IV placental collagen (Sigma, Type VI) coated 12-well Falcon filter inserts (0.4-μm pore size; Becton Dickinson, Franklin Lakes, NJ). When confluent, media was removed from above the cultures and the media below the inserts was changed to Lechner and LaVeck LHC Basal Medium:DMEM-H (Gibco) (50:50) containing the same concentrations of additives as BEGM with the exception that the concentration of epidermal growth factor was reduced to 0.63 ng/mL, and penicillin, gentamicin, streptomycin and amphotericin B were omitted (ALI media). Each set of SNEC cultures came from a separate patient source and was maintained at the air-liquid interface with the apical surfaces remaining free of medium for at least 3 weeks prior to study. This differentiated cell culture model, with media in the basolateral compartment and air at the apical surface, is an established model for studying sinonasal epithelial cells that closely resembles nasal cavity mucosa21,22.
Treatment of SNECs with Levofloxacin and Sulforaphane and Measurement of Reactive Oxygen Species Formation
SNECs were pretreated for 72 hours with 10 μM sulforaphane (SFN) or sulforaphane diluent[control, (0.1% DMSO)] prior to levofloxacin treatment (0, 1μg/ml, 10μg/ml, 20 μg/ml, 40μg/ml or 80 μg/ml) for 24-,48- or 72- hours. Levofloxacin stock solution was made in ALI media and diluted into ALI media and applied to the basal chamber. Prior work by Kalghati et al.15, which examined antibiotic concentrations near peak serum concentrations (e.g. ampicillin at 20μg/ml), suggests that bactericidal antibiotics may interact directly with cytochrome complexes. This “off target” interaction would likely be a low affinity interaction and we therefore chose to use the higher concentrations of levofloxacin noted above to saturate the system to determine if potential downstream effects of this interaction, such as mitochondrial ROS generation and induction of apoptosis were stimulated. To quantify ROS formation7,10,16, SNECs were loaded with 20 μM 2’,7’-dichlorofluorescin diacetate (H2-DCFDA) (Abcam, Cambridge, MA) for 45 minutes, washed once with 1xPBS, and treated with levofloxacin at the concentrations noted above. Fluorescence readings were measured in duplicate using a plate reader set to an excitation wavelength of 485 nm and an emission wavelength of 528 nm. SNECs were re-dosed with H2-DCFDA and levofloxacin every 24 hours.
Cell Death Analysis
SNECs were pretreated for 24 hours with 10 μM sulforaphane (SFN) or sulforaphane diluent[control, (0.1% DMSO)] prior to levofloxacin treatment (0, 20 μg/ml, 40μg/ml or 80 μg/ml) for 48 hours. Apoptotic markers were analyzed by quantifying Caspase-3 activity and by terminal deoxynucleotidyl transferase (TdT) dUTP Nick-End Labeling (TUNEL)10,23,24. To quantify Caspase-3 activity, cell lysates were collected at 48 hours post stimulation, incubated on ice for 30 minutes, and centrifuged at 15,000xg for two minutes. Supernatants were assayed for Caspase-3 activity using the Caspase-3 colorimetric assay kit (R&D Systems, Minneapolis, MN). DNA fragmentation was analyzed in situ using the TACS 2 TdT fluorescein kit (Trevigen, Gaithersburg. MD) according to manufacturer’s instructions.
Statistical Analysis
Raw data was entered into a spreadsheet and statistical analysis was performed using a software program (GraphPad Prism; GraphPad Software, Inc, LaJolla, CA). Data are expressed as mean ± SEM. Statistical significance was determined by utilizing a paired one-way analysis of variance (ANOVA) and a Tukey post-hoc test. Differences were considered statistically significant at P<0.05.
Results
Levofloxacin Stimulates Reactive Oxygen Species Formation
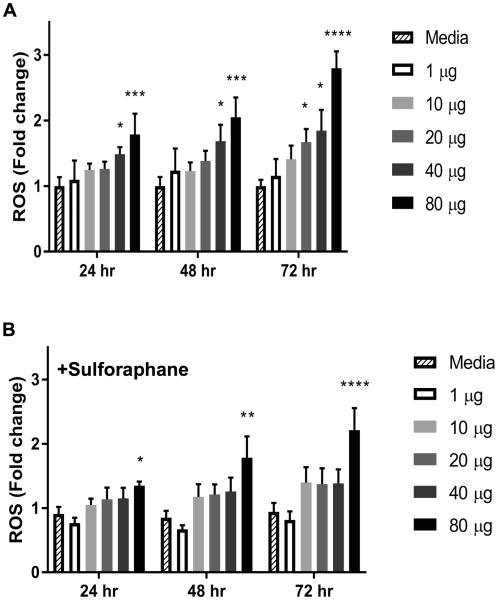
Cultured human sinonasal epithelial cells (SNECs) from patients were treated with a range of levofloxacin for 24-hrs, 48-hrs or 72-hrs and ROS formation was quantified. There was a significant (p<0.05), increase in ROS formation following treatment with ≥40μg/ml levofloxacin for 24-hrs, 48-hrs and 72-hrs, respectively (Figure 1A). At 24-hrs, there was a 1.2-fold increase in ROS formation with 10μg/ml levofloxacin and a 1.8-fold increase in ROS formation with 80μg/ml levofloxacin (p < 0.001). At 72-hrs, there was a 1.4-fold increase in ROS formation with 10μg/ml levofloxacin and a 2.8-fold increase (p < 0.0001) in ROS formation with 80μg/ml levofloxacin.
Figure 1.
Levofloxacin-stimulated ROS Formation in SNECs is blunted by pretreatment with sulforaphane. ROS formation was detected using the fluorescent probe CM-H2DCFDA in SNECs after treatment levofloxacin (0,1, 10, 20, 40, or 80μg/mL) alone (A), or pretreated for 72 hours with 10μM sulforaphane (B) for 24 hours, 48 hours or 72 hours. Shown are mean fluorescence +/− standard error of the mean (s.e.m.). * indicates *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001 relative to treatment with media alone.
SNECs were also pretreated with sulforaphane, a compound that can stimulate protective responses against oxidative damage. These SNECs were then treated with a range of levofloxacin as described above. There was a trend toward decreased ROS formation compared to no sulforaphane pretreatment at 24-hrs, 48-hrs and 72-hrs (Figure 1B). Sulforaphane reduced ROS formation to 1.04-fold at 24-hrs and 1.4-fold at 72-hrs when co-treated with 10μg/ml levofloxacin and to 1.3-fold at 24-hrs and 2.2-fold at 72-hrs when co-treated with 80μg/ml levofloxacin. These data suggest that sulforaphane pretreatment may reduce levofloxacin-mediated ROS formation.
Levofloxacin activates pro-apoptotic Caspase-3 System
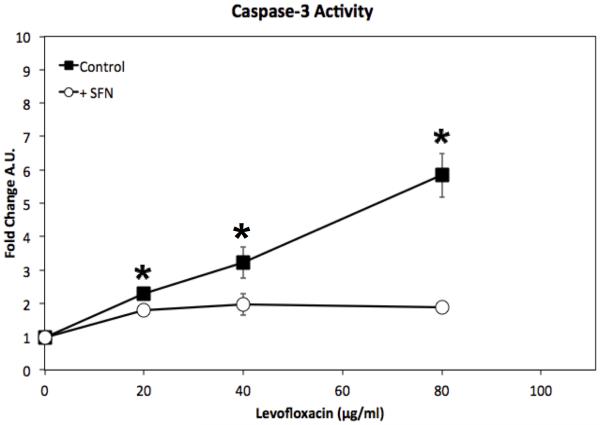
We previously demonstrated that bactericidal antibiotics cause cell death in human SNECs16. Cell death could occur through ROS-activated pathways that activate cellular necrosis, autophagy, or through apoptosis. Caspase-3 is one of the key effector caspases in the apoptotic cell death pathway25. We measured caspase-3 activity in SNECs treated with levofloxacin for 48-hrs. Treatment of SNECs with a range of levofloxacin concentrations led to a significant (p<0.05), increase in Caspase-3 activity (Figure 2). Caspase-3 activity increased from 0.98 to 2.3 A.U. (p = 0.002) with 20μg/ml levofloxacin and to 5.8 A.U. (p=0.01) with 80μg/ml levofloxacin. We also found that this increase in Caspase-3 activity is significantly (p<0.05) reduced when SNECs treated with levofloxacin were also pre-treated with sulforaphane (Figure 2), with a reduction in Caspase-3 activity to 1.78 (co-treatment with 20μg/ml levofloxacin) and 1.89 (co-treatment with 80μg/ml levofloxacin). This suggests that the ROS response generated by the bactericidal antibiotics is blunted to a physiologically significant level by sulforaphane.
Figure 2.
Levofloxacin treatment leads to increased Caspase-3 activity which is reduced by pretreatment with sulforaphane. Caspase-3 activity was detected by colorimetric assay after SNECs were treated for 48-hours levofloxacin (0,20,40, or 80μg/mL) alone (black squares), or pretreated for 24 hours with 10μM sulforaphane (open circles). Colorimetric output of the assay is shown as arbitrary units (A.U.) +/− the standard error of the mean (s.e.m). * indicates p< 0.05 +/− sulforaphane treatment.
Sulforaphane reduces Levofloxacin-induced DNA fragmentation
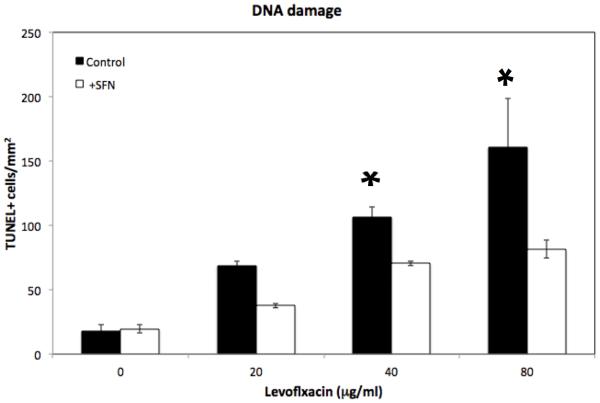
We then measured DNA fragmentation (TUNEL assay), one of the end-points of apoptosis, in SNECs treated with levofloxacin (0,20,40, 80μg/ml) for 48-hrs. We found that increasing the concentration of levofloxacin correlated significantly (p<0.05) with increased DNA fragmentation (Figure 3), with an increase in TUNEL positive cells of 65/mm2 with 20μg/ml levofloxacin to 198/mm2 with 80μg/ml levofloxacin. Blunting of the levofloxacin-mediated ROS response through pre-treatment with sulforaphane decreased DNA fragmentation (Figure 3), with a significant (p = 0.02) decrease in TUNEL positive cells (82/mm2) at 80μg/ml levofloxacin when compared to no sulforaphane pretreatment. These data demonstrate that levofloxacin-mediated cell death occurs in an ROS-dependent fashion through apoptotic pathways.
Figure 3.
Levofloxacin treatment leads to increased DNA fragmentation. DNA fragmentation was detected by TUNEL assay after SNECs were treated for 48-hours with levofloxacin (0,20,40,80μg/mL) alone (black squares), or pretreated for 24 hours with 10μM sulforaphane (open circles). Data are expressed as TUNEL positive cells/mm2 +/− the standard error of the mean (s.e.m). * indicates p< 0.05 relative to untreated cells.
Discussion
While the primary use of antibiotics is to reduce the burden of a bacterial infection, some classes of antibiotics have non-microbial functions. The study by Kalghatgi, et al. demonstrated that bactericidal antibiotics can induce mitochondrial dysfunction and oxidative tissue damage in mammary epithelial cells15 and recent evidence shows this to also be the case in SNECs16. In this study, we demonstrate for the first time that treatment of cultured human SNECs with levofloxacin triggers apoptosis through a pathway that appears to be ROS-dependent.
Previous work has demonstrated ROS generation and cell death with ampicillin in SNECs16. Furthermore, ROS generation in a mammary epithelial cell line by ciprofloxacin, ampicillin and kanamycin15 suggests that antibiotic-mediated stimulation of apoptosis may also be at play with additional classes of bactericidal antibiotics. While the focus of this study was on levofloxacin, additional studies examining this ROS and cell death phenomena across a range of quinolone, β-lactam and aminoglycoside antibiotics is warranted to determine if this mechanism is generalizable across the different classes of bactericidal antibiotics.
The exact mechanism of antibiotic-induced ROS formation is unknown, and current evidence suggests a link to mitochondrial function. Mitochondria are the major source of ROS formation in mammalian cells26, and there is evidence suggesting that bactericidal antibiotics have the ability to interact with mammalian cytochromes in an in-vitro system15. Mitochondria-derived ROS can activate the NLRP-3 inflammasome27 and increase the amount of IL-1β and IL-18. Increased production of active IL-1β was recently seen in bactericidal antibiotic-treated SNECs16 suggesting that antibiotic derived ROS are likely originating from the mitochondria. The cellular stress and damage inflicted by bactericidal antibiotic-mediated ROS could activate the pro-apoptotic caspase cascade, and in this paper, we demonstrate that the bactericidal antibiotic levofloxacin activates pro-apoptotic machinery through increased Caspase-3 activity (Figure 2) and DNA fragmentation (Figure 3).
The consequences of long-term, repetitive antibiotic exposure on the in-vivo sinus epithelium are unknown. The concentrations of drug used in this study are likely greater than what can be achieved in the sinus tissue through parenteral drug administration, however, with ROS formation and the chance for DNA, protein and lipid damage, there is the potential for chronic deleterious cellular changes over time. There is evidence that suggests there is a link between antibiotic associated tissue damage and mitochondrial function. Aminoglycoside-mediated ototoxicity is associated with mutations in mitochondrial DNA and changes in mitochondrial respiration,28-31 which could lead to ROS formation and apoptosis.
Sulforaphane is an effective stimulator of the antioxidant Nrf2-regulated antioxidant pathways. Nrf2-regulated systems can reduce the harmful impact of ROS 17,18 and are able to maintain mitochondrial integrity during periods of stress19. In this study, sulforaphane pretreatment reduced levofloxacin-mediated ROS, Caspase-3 activity and DNA fragmentation. It is interesting to note that Caspase-3 activity is more strongly inhibited than DNA damage by sulforaphane. This may be due to the fact that sulforaphane reduces but does not eliminate ROS formation, and ROS can directly damage DNA. Our results may reflect continued low-level DNA damage by a reduced level of ROS in the sulforaphane pre-treated condition. In addition to sulforaphane, the antioxidant, N-Acetyl-Cysteine, can also reduce bactericidal-antibiotic mediated ROS formation15, and further studies examining the effects of additional ROS inhibitors may provide additional insight into this mechanism of cell death. It is also possible that pretreatment with compounds like sulforaphane may allow for higher concentrations of antibiotics, possibly in topical form, to be utilized to manage difficult to treat infections while mitigating harmful side effects.
While the potential consequences of bactericidal antibiotic-mediated ROS accumulation have yet to be demonstrated in SNECs from those with CRS, the use of repetitive and extended courses of bactericidal antibiotics as part of maximal medical therapy may lead to long-term damage to the sinonasal mucosa in these patients. The chronic inflammatory state seen in CRS may increase the susceptibility of SNECs to antibiotic-mediated ROS and it will be interesting to see if this has deleterious consequences on the sinusitis disease phenotype. It may be possible to take advantage of levofloxacin-induced apoptosis through topical applications of antibiotics to chemically debride inflamed mucosa, which could allow for repair of injured mucosa. This will require additional studies examining tissue cultures in-vitro in conditions that stimulate inflammation prior to antibiotic treatment to determine if inflamed tissues are more susceptible to antibiotic-induced ROS and apoptosis. The clinical relevance of the antibiotic-induced pro-apoptotic model proposed here remains un-explored at this time, and further efforts in this area may add insight into the possible deleterious side effects to sinonasal epithelial tissue from the long-term or inappropriate use of antibiotics in the treatment of sinusitis.
Conclusions
Treatment of sinonasal epithelial cells with levofloxacin leads to an increase in ROS as well as Caspase-3 activity and DNA fragmentation. Sulforaphane pretreatment suppresses the antibiotic driven apoptotic response, possibly through its antioxidant-stimulating properties. The results from this tissue culture system provide additional evidence for a potentially clinically relevant model whereby long-term or inappropriate antibiotic use in the treatment of sinusitis, may result in oxidative tissue damage to the sinonasal epithelium. Future studies will explore if this is harmful to recovery of function in those with sinusitis.
Acknowledgments
Research supported by NIH AI072502 (A.P.L.). and NIH ES020859(M.R.)
Footnotes
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Presented at the Annual Meeting of the American Rhinologic Society, September 17, 2016, San Diego, CA.
References
- 1.Orlandi RR, Kingdom TT, Hwang PH, et al. International Consensus Statement on Allergy and Rhinology: Rhinosinusitis. Int Forum Allergy Rhinol. 2016;6(Suppl 1):S22–209. doi: 10.1002/alr.21695. [DOI] [PubMed] [Google Scholar]
- 2.Bhattacharyya N. Incremental health care utilization and expenditures for chronic rhinosinusitis in the United States. The Annals of otology, rhinology, and laryngology. 2011;120:423–427. doi: 10.1177/000348941112000701. [DOI] [PubMed] [Google Scholar]
- 3.Fairlie T, Shapiro DJ, Hersh AL, Hicks LA. National trends in visit rates and antibiotic prescribing for adults with acute sinusitis. Archives of internal medicine. 2012;172:1513–1514. doi: 10.1001/archinternmed.2012.4089. [DOI] [PubMed] [Google Scholar]
- 4.Smith SS, Evans CT, Tan BK, Chandra RK, Smith SB, Kern RC. National burden of antibiotic use for adult rhinosinusitis. J Allergy Clin Immunol. 2013;132:1230–1232. doi: 10.1016/j.jaci.2013.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Torres A, Liapikou A. Levofloxacin for the treatment of respiratory tract infections. Expert Opin Pharmacother. 2012;13:1203–1212. doi: 10.1517/14656566.2012.688952. [DOI] [PubMed] [Google Scholar]
- 6.Drlica K, Hiasa H, Kerns R, Malik M, Mustaev A, Zhao X. Quinolones: action and resistance updated. Curr Top Med Chem. 2009;9:981–998. doi: 10.2174/156802609789630947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dwyer DJ, Belenky PA, Yang JH, et al. Antibiotics induce redox-related physiological alterations as part of their lethality. Proceedings of the National Academy of Sciences of the United States of America. 2014;111:E2100–2109. doi: 10.1073/pnas.1401876111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kohanski MA, Dwyer DJ, Hayete B, Lawrence CA, Collins JJ. A common mechanism of cellular death induced by bactericidal antibiotics. Cell. 2007;130:797–810. doi: 10.1016/j.cell.2007.06.049. [DOI] [PubMed] [Google Scholar]
- 9.Brynildsen MP, Winkler JA, Spina CS, MacDonald IC, Collins JJ. Potentiating antibacterial activity by predictably enhancing endogenous microbial ROS production. Nature biotechnology. 2013;31:160–165. doi: 10.1038/nbt.2458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dwyer DJ, Camacho DM, Kohanski MA, Callura JM, Collins JJ. Antibiotic-induced bacterial cell death exhibits physiological and biochemical hallmarks of apoptosis. Molecular cell. 2012;46:561–572. doi: 10.1016/j.molcel.2012.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dwyer DJ, Kohanski MA, Hayete B, Collins JJ. Gyrase inhibitors induce an oxidative damage cellular death pathway in Escherichia coli. Molecular systems biology. 2007;3:91. doi: 10.1038/msb4100135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Foti JJ, Devadoss B, Winkler JA, Collins JJ, Walker GC. Oxidation of the guanine nucleotide pool underlies cell death by bactericidal antibiotics. Science. 2012;336:315–319. doi: 10.1126/science.1219192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kohanski MA, DePristo MA, Collins JJ. Sublethal antibiotic treatment leads to multidrug resistance via radical-induced mutagenesis. Molecular cell. 2010;37:311–320. doi: 10.1016/j.molcel.2010.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kohanski MA, Dwyer DJ, Wierzbowski J, Cottarel G, Collins JJ. Mistranslation of membrane proteins and two-component system activation trigger antibiotic-mediated cell death. Cell. 2008;135:679–690. doi: 10.1016/j.cell.2008.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kalghatgi S, Spina CS, Costello JC, et al. Bactericidal antibiotics induce mitochondrial dysfunction and oxidative damage in Mammalian cells. Science translational medicine. 2013;5:192ra185. doi: 10.1126/scitranslmed.3006055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kohanski MA, Tharakan A, Lane AP, Ramanathan M., Jr Bactericidal antibiotics promote reactive oxygen species formation and inflammation in human sinonasal epithelial cells. Int Forum Allergy Rhinol. 2016;6:191–200. doi: 10.1002/alr.21646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ma Q. Role of nrf2 in oxidative stress and toxicity. Annual review of pharmacology and toxicology. 2013;53:401–426. doi: 10.1146/annurev-pharmtox-011112-140320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jain A, Lamark T, Sjottem E, et al. p62/SQSTM1 is a target gene for transcription factor NRF2 and creates a positive feedback loop by inducing antioxidant response element-driven gene transcription. The Journal of biological chemistry. 2010;285:22576–22591. doi: 10.1074/jbc.M110.118976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dinkova-Kostova AT, Abramov AY. The emerging role of Nrf2 in mitochondrial function. Free Radic Biol Med. 2015;88:179–188. doi: 10.1016/j.freeradbiomed.2015.04.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee J, Ahn H, Hong EJ, An BS, Jeung EB, Lee GS. Sulforaphane attenuates activation of NLRP3 and NLRC4 inflammasomes but not AIM2 inflammasome. Cell Immunol. 2016 doi: 10.1016/j.cellimm.2016.07.007. [DOI] [PubMed] [Google Scholar]
- 21.Ramanathan M, Jr., Lane AP. A comparison of experimental methods in molecular chronic rhinosinusitis research. American journal of rhinology. 2007;21:373–377. doi: 10.2500/ajr.2007.21.3034. [DOI] [PubMed] [Google Scholar]
- 22.Ramanathan M, Jr., Turner JH, Lane AP. Technical advances in rhinologic basic science research. Otolaryngol Clin North Am. 2009;42:867–881. doi: 10.1016/j.otc.2009.07.008. x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Muppidi J, Porter M, Siegel RM. Measurement of apoptosis and other forms of cell death. Curr Protoc Immunol. 2004 doi: 10.1002/0471142735.im0317s59. Chapter 3:Unit 3 17. [DOI] [PubMed] [Google Scholar]
- 24.Mazumder S, Plesca D, Almasan A. Caspase-3 activation is a critical determinant of genotoxic stress-induced apoptosis. Methods Mol Biol. 2008;414:13–21. doi: 10.1007/978-1-59745-339-4_2. [DOI] [PubMed] [Google Scholar]
- 25.Kumar S. Caspase function in programmed cell death. Cell Death Differ. 2007;14:32–43. doi: 10.1038/sj.cdd.4402060. [DOI] [PubMed] [Google Scholar]
- 26.Turrens JF. Mitochondrial formation of reactive oxygen species. The Journal of physiology. 2003;552:335–344. doi: 10.1113/jphysiol.2003.049478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhou R, Yazdi AS, Menu P, Tschopp J. A role for mitochondria in NLRP3 inflammasome activation. Nature. 2011;469:221–225. doi: 10.1038/nature09663. [DOI] [PubMed] [Google Scholar]
- 28.Dong Y, Liu D, Hu Y, Ma X. NaHS Protects Cochlear Hair Cells from Gentamicin-Induced Ototoxicity by Inhibiting the Mitochondrial Apoptosis Pathway. PLoS One. 2015;10:e0136051. doi: 10.1371/journal.pone.0136051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jadidian A, Antonelli PJ, Ojano-Dirain CP. Evaluation of apoptotic markers in HEI-OC1 cells treated with gentamicin with and without the mitochondria-targeted antioxidant mitoquinone. Otol Neurotol. 2015;36:526–530. doi: 10.1097/MAO.0000000000000517. [DOI] [PubMed] [Google Scholar]
- 30.Jensen-Smith HC, Hallworth R, Nichols MG. Gentamicin rapidly inhibits mitochondrial metabolism in high-frequency cochlear outer hair cells. PLoS One. 2012;7:e38471. doi: 10.1371/journal.pone.0038471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Guan MX. Mitochondrial 12S rRNA mutations associated with aminoglycoside ototoxicity. Mitochondrion. 2011;11:237–245. doi: 10.1016/j.mito.2010.10.006. [DOI] [PubMed] [Google Scholar]