Abstract
Objective
Clinicians currently use different low-weight cut-offs both to diagnose anorexia nervosa (AN) and to determine AN severity in adolescent girls. The purpose of this study was to evaluate the clinical utility of existing cut-offs and severity criteria by determining which are most strongly associated with risk for low bone mineral density (BMD).
Methods
Height adjusted BMD Z-scores were calculated for 352 females: 262 with AN and 90 healthy controls (controls) (12–20.5 years), using data from the BMD in Childhood Study, for the lumbar spine, whole body less head, and total hip.
Results
For most cut-offs used to define low weight (5th or 10th BMI percentile, BMI of 17.5 or 18.5, and 85% or 90% of median BMI), AN had lower BMD Z-scores than controls. AN at >85 or >90% expected body weight for height (EBW-Ht) did not differ in BMD Z-scores from controls, but differed significantly from AN at ≤85 or ≤90% EBW-Ht. Among AN, any amenorrhea was associated with lower BMD. AN had lower BMD than controls across DSM-5 and The Society for Adolescent Health and Medicine (SAHM) severity categories. The SAHM moderate severity classification was differentiated from the mildly malnourished classification by lower BMD at hip and spine sites.
Discussion
Amenorrhea and %EBW-Ht ≤ 85 or ≤ 90% are markers of severity of bone loss within AN. Among severity categories, BMI Z-scores (SAHM) may have the greatest utility in assessing the degree of malnutrition in adolescent girls that corresponds to lower BMD.
Anorexia nervosa (AN) is a serious illness that frequently develops in adolescence and can be complicated by significant medical sequelae [1]. For example, during adolescent growth and development, starvation can result in irreversible bone loss [2, 3]. The Diagnostic and Statistical Manual of Mental Disorders, 5th edition (DSM-5) [4]’s revised definition of low weight requires clinical judgment and removes the amenorrhea criterion (absence of 3 consecutive menstrual cycles) for the diagnosis of AN. Broadening the criteria was supported by research demonstrating that underweight individuals who narrowly miss DSM-IV-TR [5] criteria for AN do not differ from those with full syndrome AN in eating pathology or recovery rates [6, 7] Following implementation of the revised criteria, lifetime prevalence of AN among female adolescents increased by ~50%, ranging from 0.8–1.7% [6, 8, 9]. Although the broader definition allows for detection of cases that may have been previously missed, operationalizing “significantly low weight” in order to confer a diagnosis of AN continues to be challenging in clinical practice [1, 10–13]. Importantly, the impact of revised weight and menstrual criteria on clinical outcomes, such as bone mineral density (BMD), has not been assessed in adolescent girls. As adolescence is a critical time for bone accrual [2, 14], determining effects of revised AN criteria on bone endpoints is key to (i) better understanding associated medical sequelae, and (ii) developing clear and empirically informed treatment guidelines designating when to refer low-weight patients for dual energy x-ray absorptiometry (DXA) scanning for BMD.
To guide treatment planning [4], DSM-5 includes new severity specifiers for adults with AN that categorize the disorder as “mild,” “moderate,” “severe,” or “extreme” based on World Health Organization (WHO) BMI classifications. No study to date has evaluated their relationship to severity of medical sequelae, such as BMD. Furthermore, it is unclear whether these categories are relevant for adolescents, whose expectations for absolute BMI increase with age. The Society for Adolescent Health and Medicine (SAHM) recommends calculating both the percent median BMI (%mBMI)—a comparison of the individual’s BMI to a reference population—and the BMI z-score (degree of deviation between an individual’s BMI and the mean) to evaluate severity of malnutrition [1]. Therefore, the SAHM’s alternative low-weight severity ratings, based on %mBMI and BMI z-scores, may be more appropriate for youth AN.
To examine the clinical utility of various definitions for determining low-weight status in adolescent girls with AN, we evaluated common low-weight definitions and severity categories as suggested by the DSM-IV, DSM-5, WHO, and the SAHM (Table 1), in relation to BMD. We also investigated amenorrhea as a moderator of BMD across low-weight parameters, as disruptions in estrogen status are known determinants of BMD [15]. Reduced BMD is a major morbidity of AN and early detection and weight gain may help reduce fracture risk [2, 16–18]. As such, our overarching aim was to allow for risk analysis for low BMD in adolescent girls with AN, by combining diagnostic and severity classifications with objective clinical data.
Table 1.
| Table 1a. Classifications of weight parameters and their cut-offs used to diagnose low weight in patients with AN
| ||||||||
|---|---|---|---|---|---|---|---|---|
| Absolute BMI kg/m2 Reference | BMI percentile Reference | %mBMI | %EBW-Ht | |||||
| Low-weight cut-offs | ≤17.5 | >17.5 | ≤5 | >5 | ≤85 | >85 | ≤85 | >85 |
| DSM-IV-TR | DSM-5 | |||||||
|
| ||||||||
| ≤18.5 | >18.5 | ≤10 | >10 | ≤90 | >90 | ≤90 | >90 | |
| WHO | WHO | |||||||
| 1b. Classifications of severity measures for low weight
| ||||||
|---|---|---|---|---|---|---|
| Absolute BMI (WHO and DSM-5) | %mBMI* (SAHM) | BMI z-score (SAHM) | ||||
| Low-weight severity categories | <15 | Extreme | <70% | Severe | ≤−3 | Severe |
| 15–15.9 | Severe | 70%–79% | Moderate | −2.9 to −2 | Moderate | |
| 16–16.9 | Moderate | 80%–90% | Mild | −1.9 to −1 | Mild | |
| ≥17 | Mild | >90% | - | >−1 | - | |
%mBMI: Percent median BMI for age
%EBW-Ht: Percent expected body weight for height
WHO: World Health Organization
SAHM: Society of Adolescent Health and Medicine
Because so few patients were classified as severe using %mBMI (n=8), we grouped those identified as moderate and severe together for analyses. We chose not to eliminate these eight participants as this would have led to a reduction in the number of participants in the severe/extreme category for other severity systems, and resulted in an underrepresentation of the severity of low BMD using these other systems.
METHODS
Participants
Participants in the current study (262 with AN; 90 healthy controls) were taken from a sample of participants 12–20.5 years old screened for past or current AN studies conducted by the Neuroendocrine and Pediatric Endocrine units at Massachusetts General Hospital between 2002 and 2016 [17, 19–21]. All those who met criteria for AN and had a DXA scan were included in the analyses in the AN group. All those who met criteria for normal-weight controls and had a DXA scan were similarly included in the analyses as controls. Comparisons of BMD in a subset of AN vs. controls have been previously reported, but not in relation to diagnostic and severity classifications [17, 19–21]. Participants were recruited through advertisements and referrals from eating disorder centers and regional practitioners; 98.8% of AN participants and 93.4% controls were Caucasian. A study psychologist/psychiatrist confirmed the AN diagnosis per DSM-IV-TR or DSM-5 (depending on whether assessments were done before or after the 2013 publication of DSM-5) by reviewing clinical data and conducting a clinical interview including items from the Structured Clinical Interview for DSM-IV [22]. To establish significantly low weight, participants were required to meet ≥2 of 3 criteria (%mBMI, percent expected body weight (%EBW) for height, or %EBW for age <90%). Controls were defined as having a BMI between the 10th-90th percentiles, eumenorrheic (when post-menarchal), with no lifetime history of eating disorders. Exclusion criteria for both groups included use of medications and concurrent diseases that may affect bone within three months of study participation [1].
Clinical Protocol
The Partners HealthCare Institutional Review Board approved this study. Participants were weighed on a calibrated scale wearing a hospital gown, and height measured in triplicate on a stadiometer. Tanner stage, menarchal age, presence of amenorrhea (absence of menarche in those ≥15 years or absence of menstruation for three consecutive months preceding the study in post-menarchal girls), and bone age (from an X-ray of the left hand and wrist) were evaluated by a study endocrinologist [23]. We obtained information regarding duration since AN diagnosis at the time of the study visit, but could not reliably determine the total illness duration due to the limitations of self-reported information about AN onset. Whole body less head (WBLH), lumbar spine (L1–L4), and total hip areal BMD were determined using DXA (Hologic 4500 A, Waltham, MA). Age, sex, and race specific Z-scores were calculated using the Longitudinal Bone Density in Childhood Study [24]. WBLH, spine and hip BMD assessments were available for 238, 258 and 234 AN participants, and 87, 90 and 68 controls respectively. Whole body DXA data are absent for the earliest enrollees because this scan was not available at the time of their participation. Similarly, hip BMD scans are not available for the group of AN participants who participated in an early study that did not include these measurements. Further, for each site, four to five participants had DXA scans that were either unusable or could not be completed at the study visit.
Data Preparation
We used CDC tables to calculate the height Z-score, BMI Z-score, BMI percentile (BMI%ile), percent median BMI (%mBMI [current BMI/50th percentile BMI for age and sex]*100), and percent expected body weight corresponding to height percentile (%EBW-Ht) [(Weight of participant/Weight corresponding to the weight percentile that is the same as the height percentile of the participant)*100] [25]. Post-menarchal participants were further classified based on duration of amenorrhea: no amenorrhea, < 6 months, and ≥6 months without menstruation, and also no amenorrhea, <1 year, and ≥1 year without menstruation, (referring to consecutive months of amenorrhea). Further, we examined outcomes based on presence of primary or secondary amenorrhea. While numbers of controls remained the same at any site, numbers of AN participants in each group changed (as expected) depending on the low weight parameter (Table 1).
Statistical Analyses
We conducted analyses using JMP Statistical Discovery Software, v11PRO and SPSS v23. We used analysis of variance or the Kruskal-Wallis test to compare differences across groups (depending on data distribution), followed by the Tukey-Kramer or Steel-Dwass test respectively to adjust for multiple comparisons. The Fisher’s-Exact test with Bonferroni’s correction was used to compare proportions across groups. We conducted an ANOVA with contrast weights for each severity classification scheme (i.e., BMI, %mBMI, and BMI Z-scores) to determine if BMD decreased linearly corresponding to increased AN severity. We followed up these ANOVAs with planned pairwise comparisons of BMD between individual severity groups within each classification scheme. Finally, we ran regression models to determine whether amenorrhea status and menarchal age modified the impact of weight criteria on BMD in AN. We first ran separate regression models for each low-weight parameter dichotomized per the cut-off (e.g., BMI of ≤ or >17.5) and included the duration of amenorrhea as a moderator (dichotomized as amenorrhea > or ≤6 months or 1 year). In an expanded model, we also included menarchal age (a known determinant of BMD) in this analysis to determine whether effects of low weight or duration of amenorrhea on BMD were independent of menarchal age.
RESULTS
Table 2 displays the clinical characteristics for the AN and control groups. Among AN, 21.7% were premenarchal, and 81.9% of postmenarchal patients had amenorrhea. In those with amenorrhea, 67.8% and 33.9% had amenorrhea lasting >6 months and >1year respectively. Eleven percent of AN participants had primary amenorrhea (absence of menarche at age ≥15).
Table 2.
Clinical characteristics of adolescent girls with anorexia nervosa and healthy controls
| Anorexia Nervosa (n=262) | Controls (n=90) | P value | |
|---|---|---|---|
| Age (years) | 17.5 ± 0.1 | 16.2 ± 0.2 | <0.0001* |
| Height (cm) | 164.8 ± 0.4 | 162.3 ± 0.7 | 0.0051 |
| Weight (kg) | 46.8 ± 0.4 | 55.5 ± 0.6 | <0.0001 |
| Height Z-score | 0.3 ± 0.06 | 0.1 ± 0.1 | NS |
| Bone Age (years) | 16.3 ± 0.1 | 16.2 ± 0.2 | NS |
| BMI (kg/m2) | 17.2 ± 0.09 | 21.01 ± 0.2 | <0.0001* |
| BMI percentile | 8.5 ± 0.8 | 54.1 ± 1.3 | <0.0001* |
| BMI Z-score | −1.7 ± 0.05 | 0.1 ± 0.08 | <0.0001 |
| % Median BMI | 82.2 ± 0.4 | 103.2 ± 0.7 | <0.0001* |
| %Expected body weight for height | 79.1 ± 0.6 | 99.9 ± 1.0 | <0.0001* |
| Age at menarche (years) | 12.8 ± 0.1 | 12.2 ± 0.2 | 0.0026* |
| Age at diagnosis of anorexia nervosa (years) | 15.9 ± 2.2 | - | - |
| Duration since diagnosis (months) | 17.8 ± 20.4 | - | - |
| Duration of amenorrhea (for those with amenorrhea (months) | 9.1 ± 0.5 | - | - |
| Whole body less head BMD Z-score | −1.03 ± 0.06 | −0.4 ± 0.1 | <0.0001 |
| Spine BMD Z-score | −1.0 ± 0.06 | −0.04 ± 0.1 | <0.0001 |
| Hip BMD Z-score | −0.8 ± 0.06 | 0.1 ± 0.1 | <0.0001 |
Mean ±SEM *Wilcoxon test is used for nonparametric samples
BMD across weight cut-offs for AN
BMD Z-scores based on absolute BMI
Regardless of the absolute BMI cut-off used for diagnosis, compared with controls, AN participants had lower BMD Z-scores, and a larger proportion of AN participants had BMD Z-scores <−1 for all sites (with no difference across AN groups). We found no differences in BMD Z-scores between AN participants grouped as ≤ or >17.5 (per DSM-IV-TR criteria), and ≤ or >18.5 (WHO criteria); however, all AN groups had lower BMD at all sites than controls (Figure 1, Suppl. Table 1). Similarly, for both BMI cut-offs, a larger proportion of participants in both AN groups had BMD Z-scores <−1 compared with controls, but the proportion did not differ across AN groups (Table 3).
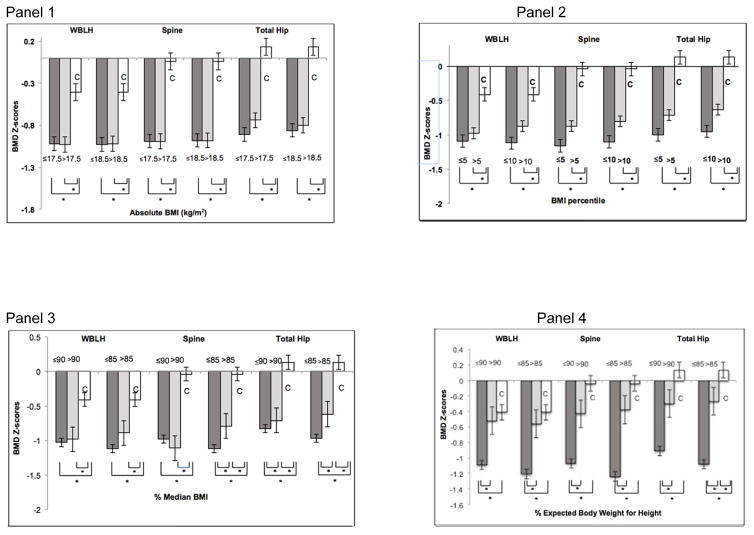
Figure 1.
Bone mineral density (BMD) Z-scores of whole body less head (WBLH), lumbar spine (spine), and total hip skeletal sites across tested low-weight parameters in participants with Anorexia Nervosa (AN) and healthy controls (C).
Panel 1: BMD Z-scores of AN participants based on body mass index (BMI) cut-offs 17.5 and 18.5 (gray bars), and controls (white bars). BMD Z-scores at all sites are lower in both groups of AN participants compared with controls. However, within participants with AN, having a BMI less than or greater than 17.5 or 18.5 does not predict lower BMD Z-scores. *p<0.05.
Panel 2: BMD Z-scores of AN participants based on BMI percentile cut-offs; 5th and 10th (gray bars) and controls (white bars). BMD Z-scores at all sites are lower in both groups of AN participants compared with controls. However, within participants with AN, those with a BMI percentile less than the 10th percentile have significantly lower total hip BMD Z-scores than those with a BMI percentile above the 10th percentile. *p<0.05.
Panel 3: BMD Z-scores of AN participants based on median BMI (mBMI) cut-offs 85% and 90% (gray bars) and controls (white bars). BMD Z-scores at all sites are lower in both groups of AN participants compared with controls. Within AN participants, those with mBMI >85% have higher total hip and lumbar spine BMD Z-scores than those with mBMI ≤85%, and AN participants with mBMI >90% have higher total hip BMD-Z scores than those with mBMI ≤90%. *p<0.05.
Panel 4: BMD Z-scores of anorexia nervosa (AN) participants based on % EBW-Ht cut-offs 85% and 90% (gray bars) and controls (white bars). BMD Z-scores at all sites are lower in AN participants with % EBW-Ht ≤85% and ≤90% compared with controls. At all sites, BMD Z-scores are lower in AN participants with % EBW-Ht ≤85% and ≤90% than AN participants with >85% and >90% respectively. Patients with %EBW-Ht ≤85% have significantly lower total hip BMD-Z scores compared with controls. * p<0.05.
Table 3.
Percentage of participants with anorexia nervosa and controls with BMD Z-scores less than −1 in groups with different weight parameters and cut-offs
| BMD Z | A | B | C | Overall | A vs B | A vs C | B vs C |
|---|---|---|---|---|---|---|---|
| <−1 | Anorexia nervosa | Controls | p | p | p | p | |
| BMI ≤17.5 | BMI >17.5 | ||||||
|
| |||||||
| WBLH | 52.0% | 49.0% | 24.0% | <0.0001 | 0.61 | <0.0001* | <0.0004* |
| Spine | 51.0% | 50.4% | 15.5% | <0.0001 | 0.91 | <0.0001* | <0.0001* |
| Hip | 48.3% | 39.1% | 10.3% | <0.0001 | 0.16 | <0.0001* | <0.0001* |
|
| |||||||
| BMI ≤18.5 | BMI >18.5 | ||||||
|
| |||||||
| WBLH | 49.7% | 53.5% | 24.0% | <0.0001 | 0.65 | <0.0001* | <0.001* |
| Spine | 49.5% | 56.8% | 15.5% | <0.0001 | 0.37 | <0.0001* | <0.0001* |
| Hip | 43.2% | 45.4% | 10.3% | <0.0001 | 0.79 | <0.0001* | <0.0001* |
|
| |||||||
| BMI ≤10th percentile | BMI >10th percentile | ||||||
|
| |||||||
| WBLH | 56.0% | 41.1% | 24.0% | <0.0001 | 0.024 | <0.0001* | 0.015* |
| Spine | 54.5% | 44.0% | 15.5% | <0.0001 | 0.10 | <0.0001* | 0.0001* |
| Hip | 48.2% | 36.0% | 10.3% | <0.0001 | 0.07 | <0.0001* | 0.0002* |
|
| |||||||
| BMI ≤5th percentile | BMI >5th percentile | ||||||
|
| |||||||
| WBLH | 59.0% | 44.2% | 24.0% | <0.0001 | 0.023 | <0.0001* | <0.002* |
| Spine | 56.8% | 46.3% | 15.5% | <0.0001 | 0.093 | <0.0001* | <0.0001* |
| Hip | 48.9% | 40.2% | 10.3% | <0.0001 | 0.19 | <0.0001* | <0.0001* |
|
| |||||||
| ≤85% mBMI | >85% mBMI | ||||||
|
| |||||||
| WBLH | 55.3% | 43.3% | 24.0% | <0.0001 | 0.068 | <0.0001* | 0.0059* |
| Spine | 55.06% | 44.0% | 15.5% | <0.0001 | 0.083 | <0.0001* | <0.0001* |
| Hip | 48.5% | 36.8% | 10.3% | <0.0001 | 0.077 | <0.0001* | <0.0001* |
|
| |||||||
| ≤90% mBMI | >90% mBMI | ||||||
|
| |||||||
| WBLH | 50.22% | 53.8% | 24.0% | <0.0001 | 0.79 | <0.0001* | 0.034 |
| Spine | 50.2% | 61.4% | 15.5% | <0.0001 | 0.42 | <0.0001* | 0.0006* |
| Hip | 44.04% | 38.% | 10.3% | <0.0001 | 0.69 | <0.0001* | 0.0185 |
|
| |||||||
| EBW-Ht ≤85% | EBW-Ht >85% | ||||||
|
| |||||||
| WBLH | 58.5 % | 30.9% | 24.0% | <0.0001 | <0.0001* | <0.0001* | 0.34 |
| Spine | 59%% | 30.3% | 15.5% | <0.0001 | <0.0001* | <0.0001* | 0.02 |
| Hip | 52.8% | 23.9% | 10.3% | <0.0001 | <0.0001* | <0.0001* | 0.03 |
|
| |||||||
| EBW-Ht ≤90% | EBW-Ht>90% | ||||||
|
| |||||||
| WBLH | 53.5% | 28.5% | 24.0% | <0.0001 | 0.0118* | <0.0001* | 0.64 |
| Spine | 53.5% | 29.0% | 15.5% | <0.0001 | 0.0096* | <0.0001* | 0.11 |
| Hip | 47.3% | 22.6% | 10.3% | <0.0001 | 0.0078* | <0.0001* | 0.11 |
WBLH: Whole body less head; mBMI: median BMI; EBW-Ht: expected body weight for height
Significant after adjusting for multiple comparisons (Bonferroni’s correction; p value for significance 0.0167)
BMD Z-scores based on BMI Percentiles
Regardless of the BMI percentile cut-off used for diagnosis, compared with controls, AN participants had lower BMD Z-scores and a larger proportion of AN participants had BMD Z-scores <−1 for all sites (with no difference across AN groups). AN groups defined according to BMI percentile (≤ or >5th percentile per DSM-IV-TR criteria and ≤ or >10th percentile per WHO criteria) had lower BMD Z-scores at all sites than controls (Figure 1, Suppl Table 1). Additionally, AN participants with BMI percentiles above the respective cut-off had WBLH, spine, and hip BMD Z-scores intermediate between those below that cut-off and controls; however this difference was only significant for hip BMD Z-scores using the 10th percentile for BMI cut-off criterion (Figure 1, Suppl. Table 1). For the 5th and 10th BMI percentile cut-offs, a larger proportion of participants in both AN groups had BMD Z-scores <−1 compared with controls, and the proportion did not differ across AN groups (Table 3).
BMD Z-scores based on Percent Median BMI
Regardless of the percent median BMI cut-off used for diagnosis, compared with controls, AN participants had lower BMD Z-scores and a larger proportion of AN participants had BMD Z-scores <−1 for all sites (with no difference across AN groups). AN groups defined according to %mBMI (≤85% or >85%, and ≤ 90% vs. >90%) had lower BMD Z-scores at all sites than controls. AN participants with %mBMI above the respective cutoff had BMD Z-scores intermediate between those with %mBMI below the cut-off and controls. The difference between AN groups was only significant for spine and hip BMD Z scores using 85%mBMI as the cut-off criterion (Figure 1, Suppl. Table 1). Data were similar overall for the proportion of participants with BMD Z-scores <−1 (Table 3).
BMD Z-scores based on Percent Expected Body Weight for Height
AN participants at ≤85% or ≤90% EBW-Ht had lower BMD Z-scores overall and a larger proportion of these participants had a Z-score of <−1 compared with AN participants at >85% or >90% EBW-Ht respectively, as well as controls. Importantly, AN participants with EBW-Ht >85% or >90% did not differ from controls for BMD Z-scores. BMD Z-scores for WBLH and spine did not differ between AN participants with %EBW-Ht >85% and controls, although hip BMD Z-scores were lower in those with %EBW-Ht > 85% compared to controls (Suppl. Table 1). AN participants with %EBW-Ht ≤85% had lower BMD Z-scores at the WBLH, spine, and hip than those with %EBW-Ht >85% and controls (Figure 1, Suppl. Table 1). Similarly, AN participants with %EBW-Ht >90% did not differ from controls for BMD Z-scores at all sites, whereas AN participants with %EBW-Ht ≤90% had lower BMD Z-scores at the WBLH, spine, and hip than those with %EBW-Ht >90% and controls (Figure 1, Suppl. Table 1). Data were similar for differences across groups for the proportion with BMD Z-scores <−1 (Table 3).
Summary of results for BMD across low-weight weight cut-offs for AN
EBW-Ht was the only low-weight parameter to differentiate AN participants based on BMD Z-scores, such that AN participants below 85% or 90% EBW-Ht had significantly lower BMD Z-scores than AN above these cut-offs and controls. Further AN groups above these cut-offs were the only AN participants who did not differ in BMD Z-scores from controls. For all other parameters (absolute BMI, BMI percentiles, and %mBMI median cut-offs), there was no difference in BMD Z-scores between AN participants above and below tested parameters, and all AN groups (regardless of whether they were above or below the tested cut-off) had lower BMD Z-scores than controls.
BMD across AN severity categories
AN participants across all severity schemes demonstrated lower BMD Z-scores than controls (Figure 2) (except AN at >90% mBMI or with BMI Z-scores >−1, who did not differ from controls for WBLH Z-scores) (Figure 2, Suppl. Tables 3 and 4).
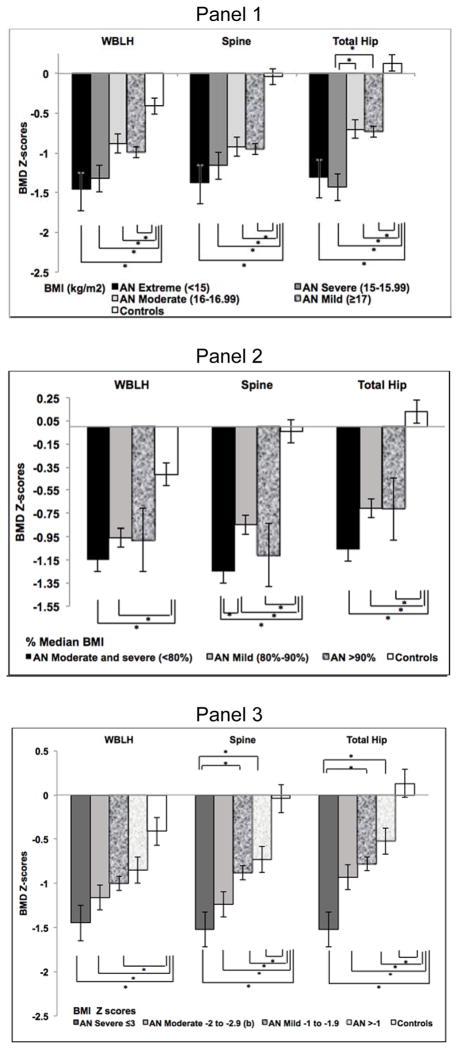
Figure 2.
Bone mineral density (BMD) Z-scores of whole body less head (WBLH), lumbar spine (spine), and total hip skeletal sites across tested low-weight severity schemes in participants with Anorexia Nervosa (AN) and healthy controls (C).
Panel 1: BMD Z-scores based on severity of malnutrition classified according to absolute body mass index (BMI: kg/m2) (DSM-5/WHO BMI severity categories). BMD Z-scores at all sites are lower in AN participants with absolute BMI <15 (extreme), 15–15.99 (severe), 16–16.99 (moderate) and ≥17 (mild) compared to controls. AN participants with severe malnutrition (BMI 15–15.99) have lower total hip BMD Z-scores than those with moderate (BMI 16–16.99) and mild (BMI ≥17) malnutrition. *p<0.05.
Panel 2: BMD Z-scores, based on severity of malnutrition classified according to percent median BMI (%mBMI) as suggested by the Society for Adolescent Health and Medicine (SAHM). BMD Z-scores at the lumbar spine and total hip are lower in anorexia nervosa (AN) participants at <80% (moderate and severe), 80–90% (mild) and >90% mBMI than controls, and at the WBLH in AN participants <80% (moderate and severe) and 80–90% (mild) than controls. AN participants with moderate/severe malnutrition (<80% mBMI) have lower lumbar spine BMD Z-scores than participants with mild malnutrition (>80–90% mBMI). *p<0.05.
Panel 3: BMD Z-scores, based on severity of malnutrition classified according to BMI Z-scores (SAHM). BMD Z-scores at all sites are lower in AN participants with BMI Z-scores ≤ −3 (severe), between −2 to −2.9 (moderate) or between −1 to −1.9 (mild) malnutrition compared to controls, and at the lumbar spine and total hip in AN participants with BMI Z-scores >−1 compared to controls. AN participants with severe malnutrition (BMI Z-scores ≤ −3) have lower lumbar spine and total hip BMD Z-scores than those with mild malnutrition (BMI Z-scores between −1 to −1.9) and AN participants with BMI Z-scores >−1. *p<0.05.
Absolute BMI (DSM-5/WHO BMI severity categories)
By ANOVA contrast, BMD Z-scores demonstrated a monotonic decrease across all three sites with increasing severity of absolute BMI category (p≤0.002 for all). Follow-up pairwise comparisons indicated that those with severe AN had lower hip BMD than mild or moderate AN (Figure 2, Suppl. Table 2). However, no between-group differences emerged at WBLH or spine.
Percentage of median BMI (SAHM severity categories)
ANOVA contrast demonstrated a linear decrease in BMD Z-scores at the spine (p=0.018), but not the WBLH or hip, with increasing %mBMI severity. Follow-up pairwise comparisons demonstrated lower spine BMD in the combined moderate/severe group vs. the mild group (Figure 2, Suppl. Table 3).
BMI-Z scores (SAHM severity categories)
ANOVA contrast demonstrated a monotonic decrease in BMD Z-scores across all three sites concomitant with increasing severity of BMI Z-scores (p<0.0001 for all). In follow-up pairwise comparisons, severe AN had lower spine and hip BMD Z-scores than mild AN (Figure 2, Suppl. Table 4), with no differences in WBLH.
Moderating effect of duration since diagnosis
In a regression model that included (i) the low weight parameter of interest dichotomized per the cut-off used to describe low weight, and (ii) duration since diagnosis, we found that in each of these models, duration since diagnosis inversely predicted BMD Z-scores at all sites (p≤0.03). Adding duration since diagnosis to these models did not change our results for the effect of the low weight parameter of interest on BMD Z-scores. EBW-Ht was consistently the only low-weight parameter to differentiate AN participants based on BMD Z-scores, such that AN participants ≤85% or 90% EBW-Ht had significantly lower BMD Z-scores than AN above these cut-offs and controls (p<0.0001). The only change was that spine BMD Z-scores were lower in those with %mBMI ≤85% vs. those with %mBMI>85% (p=0.04).
Moderating effect of amenorrhea
Regardless of duration of amenorrhea (≤6 months vs. > 6 months, and ≤12 months vs. > 12 months), AN participants had lower BMD Z-scores at all sites than controls. Similarly, regardless of menstrual status (amenorrheic or eumenorrheic), all AN participants had lower BMD Z-scores at the spine and hip than controls. However, AN with > 6 months or >1 year of amenorrhea had lower WBLH BMD Z-scores than AN without amenorrhea (Table 4). We also compared bone density measures in those with primary vs. secondary amenorrhea. Girls with primary and secondary amenorrhea did not differ for spine and WBLH BMD Z-scores; total hip BMD Z-scores trended lower in those with secondary amenorrhea (p=0.08).
Table 4.
| Table 4a. Comparison of BMD measurements of anorexia nervosa (AN) patients based on menstrual status and controls
| |||||
|---|---|---|---|---|---|
| BMD Z- scores | AN with amenorrhea
|
AN with eumenorrhea | Controls | ANOVA | |
| >6 months | ≤6 months | ||||
| n=114 | n=54 | n=37 | n=90 | P value | |
|
| |||||
| WBLH | −1.11 ± 0.13 | −1.07 ± 0.09 | −0.54 ± 0.16 | −0.41 ± 0.09 | <0.0001a,b,d,e |
| Spine | −1.04 ± 0.09 | −0.97 ± 0.14 | −0.66 ± 0.16 | −0.04 ± 0.10 | <0.0001a,b,c |
| Hip | −0.90 ± 0.09 | −0.73 ± 0.13 | −0.57 ± 0.16 | 0.13 ± 0.11 | <0.0001a,b,c |
| 4b.
| |||||
|---|---|---|---|---|---|
| BMD Z- scores | AN with amenorrhea
|
AN with eumenorrhea | Controls | ANOVA | |
| >1 year | ≤ 1 year | ||||
| n=57 | n=111 | n=37 | n=90 | P value | |
|
| |||||
| WBLH | −1.23 ± 0.12 | −1.00 ± 0.09 | −0.54 ± 0.16 | −0.41 ± 0.09 | <0.0001a,b,d |
| Spine | −1.19 ± 0.13 | −0.92 ± 0.10 | −0.66 ± 0.16 | −0.04 ± 0.10 | <0.0001a,b,c |
| Hip | −1.09 ± 0.13 | −0.73 ± 0.09 | −0.57 ± 0.16 | 0.13 ± 0.11 | <0.0001a,b,c |
WBLH: whole body less head
p≤ 0.0001 for AN with amenorrhea >6 months vs. controls
p≤ 0.001 for AN with amenorrhea ≤6 months vs. controls
p<0.005 for AN with eumenorrhea vs controls
p<0.05 for AN with amenorrhea >6 months vs. eumenorrhea
p<0.05 for AN with amenorrhea ≤6 months vs. eumenorrhea
WBLH: whole body less head
p≤ 0.0001 for AN with amenorrhea >1 year vs. controls
p≤ 0.0002 for AN with amenorrhea <1 year vs. controls
p≤0.01 for AN with eumenorrhea vs. controls
p<0.05 for AN with amenorrhea >1 year vs. AN with eumenorrhea
In a regression model that included (i) the low weight parameter of interest dichotomized per the cut-off used to describe low weight, and (ii) duration of amenorrhea (categorized as amenorrhea >6 months or ≤6 months, eumenorrhea, and controls), amenorrhea duration did not predict lower BMD measurements. However, in this model, both groups of amenorrheic AN participants consistently had lower BMD of the WBLH (p<0.05) than the AN girls with eumenorrhea and controls, independent of the weight cut-off assessed. Our results were similar when we used amenorrhea > or ≤ 1 year as the variable of interest.
When we included both amenorrhea duration and menarchal age in this regression model that also included the low weight parameter of interest, older age of menarche emerged as a consistent inverse predictor of lower BMD Z-scores (p<0.05) (details not shown).
DISCUSSION
We evaluated the clinical utility of commonly applied cut-offs for significantly low weight used to diagnose and determine the severity of AN by establishing their relation to BMD. Female adolescents were classified according to seven schemes, including parameters suggested by DSM-IV, DSM-5, the WHO, and SAHM. Our findings support existing literature [15, 16, 26] showing that for all low-weight cutoffs, girls with AN have lower BMD across all skeletal sites compared to controls, increasing their risk for future fractures. However, our results are unique because they demonstrate that low BMD is a complication even for girls above commonly utilized low-weight cut-offs, lending support to the relaxed criteria introduced in DSM-5 [4] and WHO [10]. Thus, our data demonstrate the very significant impact of even mild severity AN on BMD. Because early detection and treatment of low BMD can lead to better prognosis for bone [21], these data encourage clinicians to consider bone assessments for all adolescent girls with AN and those with atypical AN. This information may be acutely relevant when engaging patients/families in eating-disorder treatment. Explaining that bone health may already be compromised may mobilize family urgency and enhance motivation for recovery [27].
Bone Density by Weight Cut-offs for AN
Notably, for most comparisons we found no difference between those with AN classified below versus above suggested low weight parameters. In most instances, AN participants, regardless of whether they were below or above low-weight parameters, differed significantly in BMD Z-scores from controls. It may be that the cut-offs for defining low weight currently in use are still too strict for adolescent girls, particularly for early identification of risk to bone health. This is consistent with research showing that adolescents with atypical AN (i.e., meet all other criteria for AN without reaching low-weight cut-offs) have many of the same medical complications as those with AN [1, 28].
%EBW-Ht was the only parameter to show differences between those with AN falling below the cut-offs of 85% or 90% versus participants with AN above these cut-offs at all three skeletal sites. In most comparisons using %EBW-Ht, AN >85% and 90% EBW-Ht cut-offs did not differ from controls. However, those below the cut-offs differed for BMD Z-scores across all sites from controls and AN above the cut-offs. Of note, weight-for-stature and BMI methods for determining EBW yield distinct results, with EBW based on BMI being consistently lower and possibly underestimating the degree of malnutrition, particularly in very tall girls [29]. This may reduce the capability of cut-offs based on %mBMI to pick up medical morbidity associated with AN, and that %EBW-Ht may better estimate nutritional status in tall girls. Overall, our data suggest that EBW-Ht ≤ 90% may be a uniquely important low-weight parameter in identifying those AN patients most at risk for low BMD and associated complications, and our results did not change after adjusting for duration since diagnosis. Therefore, we recommend practitioners calculate this parameter in adolescent girls with AN or atypical AN, and limit BMD assessments to those with EBW-Ht ≤90%.
Bone Density across AN Severity Categories
Girls with AN had lower BMD across all 4 newly proposed DSM-5 AN severity categories, however, we did not find differences between these classifications except that those with a BMI <17.0 (moderately or severely malnourished) showed lower hip BMD scores than those with a BMI ≥17. Similarly, participants with AN organized by the SAHM severity classification scheme also had lower BMD Z-scores across all skeletal sites than controls regardless of whether %mBMI or BMI Z-scores were applied.
However, the SAHM severity scheme had greater utility in stratifying BMD risk, particularly with BMI Z-scores. Participants with BMI Z-scores ≤−3 (severe) had lower spine and hip BMD Z-scores than those with BMI Z-scores between −1.0 and −1.99 (mild) and those with BMI Z-scores >−1 (but not between −2 and −2.99, i.e. moderate malnutrition), suggesting that patients in this range may be at higher risk for extreme bone loss. Further, ANOVA analyses revealed that BMD decreases in an expected linear fashion corresponding to increased severity defined by BMI Z-scores. SAHM low-weight recommendations also advise that clinicians assess the extent and rate of weight loss. Although we could not assess this with our data, inclusion of such analyses may reveal further distinctions between severity classifications. Given that the SAHM BMI Z-score severity scheme showed more distinctions between severity classifications using follow-up pairwise comparisons, we recommend practitioners employ the SAHM classifications based on BMI Z-scores when determining AN severity for adolescent girls, at least in the United States. This is particularly important for younger adolescents who are still growing, given that BMI is expected to change with increasing age.
Effect of Menstrual Status
Amenorrhea moderated BMD scores in AN such that those with amenorrhea of any duration had lower WBLH BMD Z-scores than eumenorrheic AN. However, regardless of duration of amenorrhea or menstrual status, all groups of AN participants had lower BMD Z-scores at the spine and hip than controls. Consistent with results from a recent meta-analysis [15, 30], these findings indicate that duration of amenorrhea is a severity marker for low weight and bone health for the WBLH, and emphasize the importance of early intervention to minimize bone loss. However, the spine and hip may be impacted regardless of menstrual status. Further, higher menarchal age was a negative determinant of BMD independent of low weight and amenorrhea duration, consistent with previous reports [16].
Limitations
Power may have been limited for certain comparisons, as we did not have data across all skeletal sites for all participants. Further, AN participants were slightly older than controls; however, this should have made comparisons more conservative, because older adolescents would have accrued more bone than younger participants. BMD and bone mineral content vary across age, race, and sex [24, 31, 32], and our sample was entirely female adolescents and mostly Caucasian. Thus, replication of findings in different racial/ethnic groups and males is required, and our data may not apply to adults. Although we obtained information about duration since AN diagnosis, the age of AN onset was based on self-report. Thus, we did not have a reliable way to calculate total illness duration, especially for younger participants. Further, we do not have information regarding participant smoking status, level of physical activity, or use of psychotropic medication, all of which could influence results. Finally, our study was cross-sectional, preventing examination of long-term effects of low weight on bone.
Conclusion
Individuals with AN have lower WBLH, spine, and hip BMD than controls across common low-weight parameters, and even when the condition is less severe. In fact, this difference is observed also for participants above tested low-weight cut-offs, indicating that BMD is quickly compromised in adolescent girls with AN. Only %EBW-Ht showed differences between AN above versus those below low-weight cut-offs at every site, suggesting that it should be calculated as a severity marker of low BMD in patients presenting with AN. Overall, these data indicate that all girls with AN ≤ 90% EBW-Ht, should get a DXA scan. AN participants regardless of menstrual status have lower spine and hip BMD measures than controls, indicating that BMD assessment should be performed regardless of menstrual status. Of relevance, the onset of amenorrhea indicates risk for lower WBLH BMD, indicating that those with AN and amenorrhea, even if they are >90% EBW-Ht, should be referred for a DXA scan. Further, categorizing adolescent girls with AN using the SAHM BMI Z-score severity ratings has the greatest utility for identifying risk for low BMD. This is particularly so because absolute BMI (used for DSM-5 severity ratings) continues to increase with increasing age during adolescence. Future studies should examine percent and rate of weight loss in relation to bone health and take a longitudinal approach to examining bone health in AN classified per low-weight parameters.
Supplementary Material
Acknowledgments
Funding Source: Supported by grants 1UL1TR001102-01, 8 UL1 TR000170-05, 1 UL1 RR025758-04, M01-RR-01066, 2 R01 DK062249-07, 5R01 MH083657-04, 2R01 DK052625-11, 1R01MH105515-01, 5R01HD060827-05, 1 K24HD071843 from NIH.
Footnotes
Financial Disclosures: The authors have no financial relationships relevant to this article to disclose
Conflict of interest: The authors have no conflicts of interest to disclose
References
- 1.Golden NH, Katzman DK, Sawyer SM, et al. Position Paper of the Society for Adolescent Health and Medicine: medical management of restrictive eating disorders in adolescents and young adults. J Adolesc Health. 2015;56:121–5. doi: 10.1016/j.jadohealth.2014.10.259. [DOI] [PubMed] [Google Scholar]
- 2.Katzman DK. Medical complications in adolescents with anorexia nervosa: a review of the literature. Int J Eat Disord. 2005;37(Suppl):S52–9. doi: 10.1002/eat.20118. discussion S87–9. [DOI] [PubMed] [Google Scholar]
- 3.Mitchell JE, Crow S. Medical complications of anorexia nervosa and bulimia nervosa. Curr Opin Psychiatry. 2006;19:438–43. doi: 10.1097/01.yco.0000228768.79097.3e. [DOI] [PubMed] [Google Scholar]
- 4.Diagnostic and Statistical Manual Of Mental Disorders. Washington, DC: American Psychiatric Assoc; 2013. [Google Scholar]
- 5.American Psychiatric Association. Diagnostic and statistical manual of mental disorders: DSM-IV-TR. Washington: DAPA; 2000. [Google Scholar]
- 6.Smink FR, van Hoeken D, Oldehinkel AJ, Hoek HW. Prevalence and severity of DSM-5 eating disorders in a community cohort of adolescents. Int J Eat Disord. 2014;47:610–9. doi: 10.1002/eat.22316. [DOI] [PubMed] [Google Scholar]
- 7.Eddy KT, Celio Doyle A, Hoste RR, et al. Eating disorder not otherwise specified in adolescents. J Am Acad Child Adolesc Psychiatry. 2008;47:156–64. doi: 10.1097/chi.0b013e31815cd9cf. [DOI] [PubMed] [Google Scholar]
- 8.Mustelin L, Silen Y, Raevuori A, et al. The DSM-5 diagnostic criteria for anorexia nervosa may change its population prevalence and prognostic value. J Psychiatr Res. 2016;77:85–91. doi: 10.1016/j.jpsychires.2016.03.003. [DOI] [PubMed] [Google Scholar]
- 9.Nagl M, Jacobi C, Paul M, et al. Prevalence, incidence, and natural course of anorexia and bulimia nervosa among adolescents and young adults. Eur Child Adolesc Psychiatry. 2016 doi: 10.1007/s00787-015-0808-z. [DOI] [PubMed] [Google Scholar]
- 10.Knoll S, Bulik CM, Hebebrand J. Do the currently proposed DSM-5 criteria for anorexia nervosa adequately consider developmental aspects in children and adolescents? Eur Child Adolesc Psychiatry. 2011;20:95–101. doi: 10.1007/s00787-010-0141-5. [DOI] [PubMed] [Google Scholar]
- 11.Sawyer SM, Whitelaw M, Le Grange D, et al. Physical and Psychological Morbidity in Adolescents With Atypical Anorexia Nervosa. Pediatrics. 2016:137. doi: 10.1542/peds.2015-4080. [DOI] [PubMed] [Google Scholar]
- 12.Thomas JJ, Eddy KT, Murray HB, et al. The impact of revised DSM-5 criteria on the relative distribution and inter-rater reliability of eating disorder diagnoses in a residential treatment setting. Psychiatry Res. 2015;229:517–23. doi: 10.1016/j.psychres.2015.06.017. [DOI] [PubMed] [Google Scholar]
- 13.Thomas JJ, Roberto CA, Brownell KD. Eighty-five per cent of what? Discrepancies in the weight cut-off for anorexia nervosa substantially affect the prevalence of underweight. Psychol Med. 2009;39:833–43. doi: 10.1017/S0033291708004327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Halmi KA, Casper RC, Eckert ED, et al. Unique features associated with age of onset of anorexia nervosa. Psychiatry Res. 1979;1:209–15. doi: 10.1016/0165-1781(79)90063-5. [DOI] [PubMed] [Google Scholar]
- 15.Solmi M, Veronese N, Correll CU, et al. Bone mineral density, osteoporosis, and fractures among people with eating disorders: a systematic review and meta-analysis. Acta Psychiatr Scand. 2016;133:341–51. doi: 10.1111/acps.12556. [DOI] [PubMed] [Google Scholar]
- 16.Misra M, Aggarwal A, Miller KK, et al. Effects of anorexia nervosa on clinical, hematologic, biochemical, and bone density parameters in community-dwelling adolescent girls. Pediatrics. 2004;114:1574–83. doi: 10.1542/peds.2004-0540. [DOI] [PubMed] [Google Scholar]
- 17.Faje AT, Fazeli PK, Miller KK, et al. Fracture risk and areal bone mineral density in adolescent females with anorexia nervosa. Int J Eat Disord. 2014;47:458–66. doi: 10.1002/eat.22248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Robinson L, Aldridge V, Clark EM, et al. A systematic review and meta-analysis of the association between eating disorders and bone density. Osteoporos Int. 2016;27:1953–66. doi: 10.1007/s00198-015-3468-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Misra M, Katzman D, Miller KK, et al. Physiologic estrogen replacement increases bone density in adolescent girls with anorexia nervosa. J Bone Miner Res. 2011;26:2430–8. doi: 10.1002/jbmr.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Misra M, McGrane J, Miller KK, et al. Effects of rhIGF-1 administration on surrogate markers of bone turnover in adolescents with anorexia nervosa. Bone. 2009;45:493–8. doi: 10.1016/j.bone.2009.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Misra M, Prabhakaran R, Miller KK, et al. Weight gain and restoration of menses as predictors of bone mineral density change in adolescent girls with anorexia nervosa-1. J Clin Endocrinol Metab. 2008;93:1231–7. doi: 10.1210/jc.2007-1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.First M, Spitzer R, Gibbon M, Williams J. The structured clinical interview for DSM-IV Axis I Disorders, Research Version, Patient Edition, with Psychotic Screen New York. State Psychiatric Institute; New York: 1997. [Google Scholar]
- 23.Greulich W, Pyle S. Radiographic atlas of skeletal development of the hand and wrist. 2. Stanford University Press; Stanford: 1959. [Google Scholar]
- 24.Zemel BS, Kalkwarf HJ, Gilsanz V, et al. Revised reference curves for bone mineral content and areal bone mineral density according to age and sex for black and non-black children: results of the bone mineral density in childhood study. J Clin Endocrinol Metab. 2011;96:3160–9. doi: 10.1210/jc.2011-1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kuczmarski RJ, Ogden CL, Grummer-Strawn LM, et al. CDC growth charts: United States. Adv Data. 2000:1–27. [PubMed] [Google Scholar]
- 26.Grinspoon S, Thomas E, Pitts S, et al. Prevalence and predictive factors for regional osteopenia in women with anorexia nervosa. Ann Intern Med. 2000;133:790–4. doi: 10.7326/0003-4819-133-10-200011210-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lock J, Le Grange D. Treatment Manual For Anorexia Nervosa. 2. Guilford Press; 2012. [Google Scholar]
- 28.Whitelaw M, Gilbertson H, Lee KJ, Sawyer SM. Restrictive eating disorders among adolescent inpatients. Pediatrics. 2014;134:e758–64. doi: 10.1542/peds.2014-0070. [DOI] [PubMed] [Google Scholar]
- 29.Golden NH, Yang W, Jacobson MS, et al. Expected body weight in adolescents: comparison between weight-for-stature and BMI methods. Pediatrics. 2012;130:e1607–13. doi: 10.1542/peds.2012-0897. [DOI] [PubMed] [Google Scholar]
- 30.Mehler PS, MacKenzie TD. Treatment of osteopenia and osteoporosis in anorexia nervosa: a systematic review of the literature. Int J Eat Disord. 2009;42:195–201. doi: 10.1002/eat.20593. [DOI] [PubMed] [Google Scholar]
- 31.Kalkwarf HJ, Zemel BS, Gilsanz V, et al. The bone mineral density in childhood study: bone mineral content and density according to age, sex, and race. J Clin Endocrinol Metab. 2007;92:2087–99. doi: 10.1210/jc.2006-2553. [DOI] [PubMed] [Google Scholar]
- 32.Misra M, Ackerman KE, Bredella MA, et al. Racial Differences in Bone Microarchitecture and Estimated Strength at the Distal Radius and Distal Tibia in Older Adolescent Girls: a Cross-Sectional Study. J Racial Ethn Health Disparities. 2016 doi: 10.1007/s40615-016-0262-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.