Summary
Objective
Recent evidence suggests a metabolic contribution of cytochrome P450 enzymes (CYPs) to the drug‐resistant phenotype in human epilepsy. However, the upstream molecular regulators of CYP in the epileptic brain remain understudied. We therefore investigated the expression and function of pregnane xenobiotic (PXR) and glucocorticoid (GR) nuclear receptors in endothelial cells established from post‐epilepsy surgery brain samples.
Methods
PXR/GR localization was evaluated by immunohistochemistry in specimens from subjects who underwent temporal lobe resections to relieve drug‐resistant seizures. We used primary cultures of endothelial cells obtained from epileptic brain tissues (EPI‐ECs; n = 8), commercially available human brain microvascular endothelial cells (HBMECs; n = 8), and human hepatocytes (n = 3). PXR/GR messenger RNA (mRNA) levels in brain ECs was initially determined by complementary DNA (cDNA) microarrays. The expression of PXR/GR proteins was quantified by Western blot. PXR and GR silencing was performed in EPI‐ECs (n = 4), and the impact on downstream CYP expression was determined.
Results
PXR/GR expression was detected by immunofluorescence in ECs and neurons in the human temporal lobe samples analyzed. Elevated mRNA and protein levels of PXR and GR were found in EPI‐ECs versus control HBMECs. Hepatocytes, used as a positive control, displayed the highest levels of PXR/GR expression. We confirmed expression of PXR/GR in cytoplasmic‐nuclear subcellular fractions, with a significant increase of PXR/GR in EPI‐ECs versus controls. CYP3A4, CYP2C9, and CYP2E1 were overexpressed in EPI‐ECs versus control, whereas CYP2D6 and CYP2C19 were downregulated or absent in EPI‐ECs. GR silencing in EPI‐ECs led to decreased CYP3A4, CYP2C9, and PXR expression. PXR silencing in EPI‐ECs resulted in the specific downregulation of CYP3A4 expression.
Significance
Our results indicate increased PXR and GR in primary ECs derived from human epileptic brains. PXR or GR may be responsible for a local drug brain metabolism sustained by abnormal CYP regulation.
Keywords: Drug resistance, Epilepsy, Nuclear Receptors, Neurovascular unit
Key Points.
Neurovascular PXR‐GR and CYPs are coexpressed in human epileptic brain
Increased PXR‐GR expression is found in EPI‐ECs derived from drug‐resistant epileptic brain resections
EPI‐ECs show elevated PXR‐GR levels in both cytoplasmic and nuclear fractions
PXR‐GR regulation of CYP in EPI‐ECs may affect drug metabolism at the neurovascular interface
Nuclear receptors (NRs) directly control the cytochrome P450 (CYP) response to xenobiotics and endogenous toxins.1, 2 In particular, two types of NRs—pregnane X (PXR) and glucocorticoids (GRs)—are key players in modifying drug metabolism and toxicity in the liver. GR and PXR functions are intertwined, because GR activation promotes the expression of PXR and its downstream target genes.3, 4, 5 GR and PXR respond to stress hormones and xenobiotics by inducing the expression of phase I and phase II metabolic enzymes, the same mechanism is involved in the expression of efflux transporters associated with cellular detoxification.5, 6, 7, 8
A number of studies have suggested an extrahepatic role for GR/PXR and CYP enzymes, including a possible contribution to drug biotransformation by CYP at neurovascular structures.6, 9, 10, 11, 12, 13 For instance, specific drug‐metabolizing enzymes overexpressed in human epileptic endothelial cells (EPI‐ECs) are involved in the metabolism of antiepileptic drugs, which may form reactive and/or inactive metabolites.12, 14, 15 In addition, recently published data have shown xenobiotic NR expression in ECs, also controlling downstream targets such as P‐glycoprotein.6, 16, 17
Despite this evidence, it remains to be elucidated whether the expression of specific NRs in the human drug‐resistant epileptic brain is modified or contributes to a drug‐resistant phenotype. We have focused on two specific NRs—PXR and GR—and studied their expression in human brain ECs derived from brain resections of patients with temporal lobe epilepsy. We further explored the effect of GR and PXR silencing and the subsequent impact on the downstream regulation of CYP enzymes.
Methods
Human epileptic brain tissues, endothelial cells, and hepatocytes
Brain specimens were obtained from patients with medically intractable epilepsy. The collection of such samples conforms to the principles outlined in the Declaration of Helsinki and the Institutional Review Board–approved protocol (IRB 07‐322). Patients’ information is summarized in Table S1.
We used primary ECs derived from brain specimens resected from patients with drug‐resistant epilepsy (EPI‐ECs), as described earlier.18 Briefly, surgical specimens were incubated in collagenase type II (2 mg/ml, Worthington Biochemical Corp., Lakewood, NJ, U.S.A.) at 37°C for 20 min to dissociate the ECs. The collagenase was then washed off with medium (1.5 g/100 ml, MCDB 105 supplemented with endothelial cell growth supplement 15 mg/100 ml, heparin 800 U/100 ml, 10% fetal bovine serum, and penicillin/streptomycin 1%). Cells were stained positive for von Willebrand factor and negative for glial fibrillary acidic protein (GFAP). EPI‐ECs were initially expanded in 75 cm2 flasks precoated with fibronectin (3 μg/cm2).11, 12 Control human brain microvascular endothelial cells (HBMECs) were purchased from Cell Systems (Kirkland, WA, U.S.A.; catalog number ACBRI 376, used for Western blotting) and ScienCell (Carlsbad, CA, U.S.A.; catalog number 1000, for cDNA arrays used in past). According to the information provided by the company, the HBMECs were dissociated from normal human brain cortex tissue (obtained from healthy donors), isolated with a Beckman elutriation system, and characterized by staining for von Willebrand factor. Other specific details are available on the company websites (Cell Systems, http://www.cell-systems.com/cert/22/137/Products/Products/ACBRI-Site-S and ScienCell, https://www.sciencellonline.com/human-brain-microvascular-endothelial-cells.html). Human hepatocytes were purchased from ATCC (American Type Culture Collection, Manassas, VA, U.S.A.; catalog number CRL‐11233), and the suggested media components were used.11
NR mRNA derived from previous cDNA microarray data.18, 19 CYPs (CYP3A4, CYP2C9, CYP2C19, CYP2D6, and CYP2E1) involved in hepatic metabolism of antiepileptic drugs were studied along with PXR and GR. Expression levels of the CYPs and of PXR and GR proteins were determined in EPI‐ECs and compared to HBMECs. The experimental outline is depicted in Figure S1.
Immunohistochemistry
Immunohistochemical staining was performed on tissues obtained during surgery on patients with temporal lobe epilepsy (Table S1). Samples resected from the temporal cortex of human drug‐resistant epileptic brain (30–35 μm) were used for histologic screening.11, 12 Immunofluorescence staining was performed on free‐floating sections using PXR, GR, and CYP3A4 antibodies (overnight at 4°C). Astrocytic (GFAP) and neuronal (NeuN) markers12, 14 were used to confirm cellular localization. The specific dilutions and sources of the primary antibodies are listed in Table S2A. Respective secondary antibodies were applied for 2 h at room temperature (Table S2B). Auto‐fluorescence was blocked with Sudan black B. Sections were analyzed by fluorescence microscopy. All sections were scanned in a 1600 × 1200‐pixel format in the x–y direction, and the acquired images were processed using Q‐Capture Pro 7 software (QImaging, Inc., Surrey, BC, Canada) and Photoshop CS2 (Adobe Systems Inc., San Jose, CA, U.S.A.).
cDNA arrays
Gene analysis derives from previous reports (Gene Filter, Research Genetics Inc., Huntsville, AL, U.S.A.).18, 19 Transcription changes were analyzed using Ingenuity Pathway Analysis (QIAGEN, Redwood City, CA, U.S.A.). Identification numbers from GenBank and its subset UniGene (both from the National Center for Biotechnology Information, Bethesda, MD, U.S.A.) were used. Among the genes screened, we extracted the mRNA levels of NRs involved in drug metabolism.
Protein isolation, subcellular fractionation, and Western blotting
EPI‐ECs used for the present study were passaged no more than two times after isolation.11, 12 Total proteins were extracted from EPI‐ECs, HBMECs, and hepatocytes as described previously.11, 14 Subcellular fractions (cytoplasmic and nuclear) from EPI‐ECs and HBMECs were fractionated using a Subcellular Protein Fractionation Kit (Pierce Biotechnology, Thermo Scientific, Rockford, IL, U.S.A.; catalog numbers 78833/78835) according to the manufacturer's guidelines.
Western blot analysis
Proteins were separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis and transferred onto polyvinylidene fluoride membranes (EMD Millipore Corp., Billerica, MA, U.S.A.). The membranes were probed overnight at 4°C with the primary antibody (see Table S2A) and the appropriate secondary antibody (Table S2B). The specificity of the antibodies for PXR, GR, CYP3A4, CYP2E1, CYP2D6, CYP2C9, and CYP2C19 is provided (Figures S2 and S3). Polyvinylidene fluoride membranes were incubated for 30 min at 50°C in stripping buffer and later normalized with β‐actin or proliferating cell nuclear antigen (PCNA; for nuclear fractions) where used as loading controls.20, 21 Protein expression was quantified by ImageJ software (National Institutes of Health, Bethesda, MD, U.S.A.).
siRNA gene silencing of PXR and GR
Gene‐specific human NR1/2 PXR (catalog number E‐003415‐00‐0005) and human NR3C1 GR (catalog number E‐003424‐00‐0005) small interfering RNA (siRNA) oligonucleotides were purchased from GE Dharmacon Inc. (Lafayette, CO, U.S.A.) and used at a final concentration of 1 μm for EPI‐ECs in the respective experiments.
Silencing of PXR (human NR1/2) was performed using Accell SMART pool siRNAs containing four siRNAs (5′‐CCCUCAUGCAGGAGUUGUU‐3′, 5′‐GCCCUGGGUUUAAUGUCAA‐3′, 5′‐GCAUUGACUCAGAUAUAGA‐3′, and 5′‐CCAUUUGAACACAUUAUUA‐3′). Silencing of GR (human NR3C1) was performed using Accell SMARTpool siRNAs containing four siRNAs designed for the GR gene: (5′‐GGAGCAAAUAUAAUUGGUA‐3′, 5′‐GCAUGUACGACCAAUGUAA‐3′, 5′‐GGGUGGAGUUUCGUAAUUU‐3′, and 5′‐CUAACAUGAUUUGUGUCUA‐3′). Cells were cultured on 60‐mm‐diameter plates and transfected at 80% confluence using Accell delivery medium according to the manufacturer's instructions. Cell morphology was monitored for 96 h, after which the cells were harvested. Positive controls, Accell glyceraldehyde 3‐phosphate dehydrogenase siRNA, and a nontargeting Accell siRNA pool were used in the respective experiments. The effects of siRNA on EPI‐EC gene expression were confirmed by Western blot using an anti‐PXR or anti‐GR antibody, each compared with their respective nontransfected EPI‐ECs.
Statistical analysis
All data are expressed as mean ± standard error of the mean (SEM). Student's t‐test was used for direct comparison of two populations of data. One‐way analysis of variance (ANOVA) followed by a Bonferroni test was used as required. A p‐value of <0.05 was considered statistically significant. Origin 9.0 software (OriginLab, Northampton, MA, U.S.A.) was used for statistical analyses.
Results
PXR and GR expression in human drug‐resistant epileptic brain cells
Immunofluorescence staining (Fig. 1) indicated PXR and GR expression at the interface of brain capillaries and neurons (Fig. 1A,B), as confirmed using neuronal (NEUN) and GFAP co‐localizations. Examples of gliotic epileptic brain regions (Fig. 1C,C1) are provided, showing GR staining at the microvascular interface (C1). The co‐localization of PXR or GR with CYP3A4 (Fig. 1A2,B2) is further illustrated in these regions.
Figure 1.
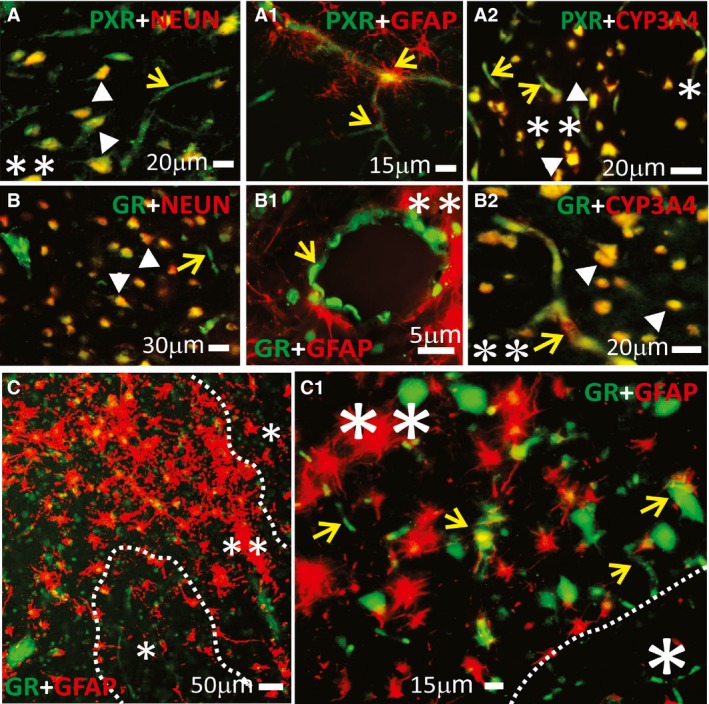
Expression of PXR, GR, and CYP3A4 in human epileptic brains. (A–C1) NRs were found at the blood–brain barrier interface and in neurons in epileptic brains (n = 5). Magnification of gliotic regions (**) and GFAP+ staining on a resected human epileptic brain showed increased expression of NRs (PXRs, GRs), and CYP3A4. This staining pattern was less evident in nongliotic regions (*). Note: The indicators for neurons are depicted as arrowheads and those for capillaries as yellow arrows. Co‐localization was evaluated with neuronal markers (NeuN, A,B) and glial markers (GFAP, A1–B1 and C–C1). (A–A2) PXR expression was observed at the vascular interface (A–A1, yellow arrows) and in neurons (A and A2). (B–B2) A similar pattern was found for GR expression across the epileptic brain slices. (A2,B2) Co‐localization of GR or PXR staining with CYP3A4 was found in regions of reactive gliosis (**). (C,C1) Representative images of brain samples from patients with temporal lobe epilepsy show GR staining in regions of reactive gliosis (**).
The levels of PXR and GR expression were determined in human brain ECs. Results are provided according to the following categories: mRNA levels of orphan receptors (e.g., PXRs), aryl hydrocarbon receptors (AHRs), retinoid X receptors (RXRs, a downstream PXR target), and GRs. mRNA levels of all the above‐mentioned genes were found to be elevated in EPI‐ECs (Fig. 2A) compared to controls. Although variability existed among EPI‐ECs, the average PXR and GR protein expression was significantly elevated (Fig. 2B,B2). Hepatocytes showed the highest levels of PXR and GR proteins compared with brain EC (EPI‐ECs or HBMECs; Fig. 2B1,B2). We also found elevated levels of PXR and GR in cytoplasmic and nuclear EPI‐EC fractions (Fig. 2C,C2).
Figure 2.
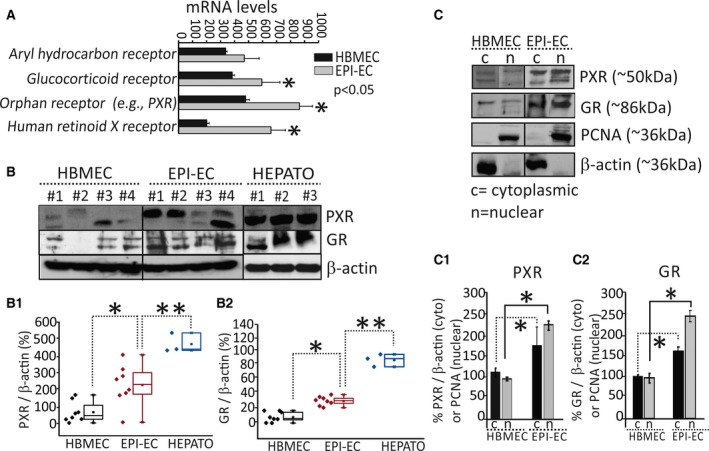
PXR and GR expression patterns in human EPI‐ECs. (A) The mRNA levels of NRs (human retinoid x receptor, RXR; aryl hydrocarbon receptors, AHRs; glucocorticoid receptors, GRs); and orphan receptors [e.g., pregnane X receptors, PXRs]) in EPI‐ECs were significantly increased (*p < 0.05, n = 4) compared to controls (HBMECs). (B–B2) PXR and GR protein expression evaluated by Western blot showed variability among the samples analyzed; increased levels of PXRs (*p < 0.05) and GR (*p < 0.05) were found in EPI‐ECs compared with HBMECs (B1–B2) (n = 8). Representative Western blots are shown in B for PXR/GR (n = 4/each, HBMEC or EPI‐ECs). The hepatocytes (HEPATO) consistently showed an increased level (**p < 0.01, n = 3) of PXRs and GRs as compared to EPI‐ECs. Glyceraldehyde 3‐phosphate dehydrogenase and β‐actin were used as loading controls for mRNA and protein, respectively. Results are expressed as mean ± standard error of the mean (SEM) (analysis of variance, ANOVA). (C–C2) Western blot results increased levels of PXR and GR in the cytoplasmic and nuclear (*p < 0.05, n = 4) EPI‐ECs fractions. β‐Actin was used as a loading control for cytoplasmic fractions; proliferating cell nuclear antigen (PCNA) was used for nuclear fractions. Results are expressed as mean ± SEM (ANOVA).
Consistent with increased PXR/GR expression, we also found CYP3A4 (4/4) and CYP2C9 (3/4) overexpression in EPI‐ECs compared with controls. Conversely, expression of CYP2D6 (4/4) and CYP2C19 (4/4) were down‐regulated or absent in EPI‐ECs (Fig. 3A). Significant correlations were found in the expression levels of PXR or GR and CYP in brain ECs (Fig. 3B–E). The observed CYP2E1‐PXR regulation in EPI‐ECs may be different from the hepatic one, where a non–receptor‐mediated induction of CYP2E1 has been reported.22
Figure 3.
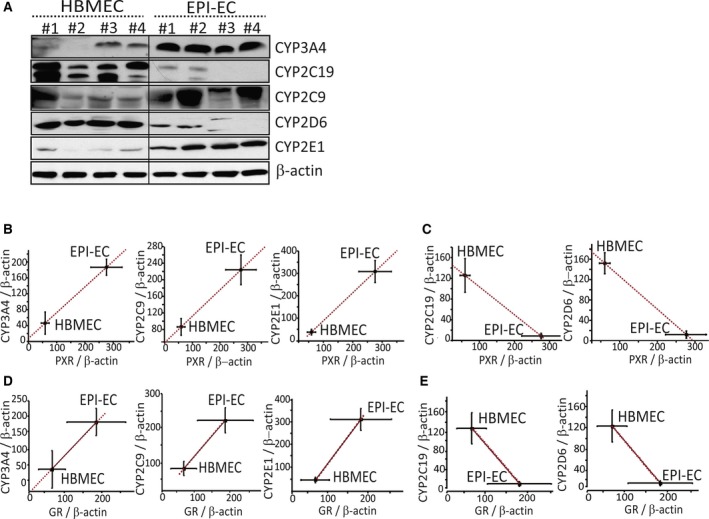
Overexpression of specific CYPs and correlation with PXR and GR protein levels in EPI‐ECs. (A) Western blot showing elevated expression of CYP3A4, CYP2C9, and CYP2E1 in EPI‐ECs (1 to 4), compared to HBMECs (1 to 4). In contrast, CYP2D6 and CYP2C19 were down‐regulated or absent. Quantitative analysis of protein expression and correlation of PXR/GR with CYP levels showed that CYP3A4, CYP2E1, and CYP2C9 levels increased significantly in accordance with increases in PXR (B) and GR (D) expression in EPI‐ECs. CYP2C19 and CYP2D6 showed decreased levels in correlation with decreased levels of PXR (C) or GR (E) expression in EPI‐ECs compared with HBMECs (n = 4/each for HBMECs or EPI‐ECs, in triplicate). Results are expressed as mean ± SEM (ANOVA) and were normalized with β‐actin as loading control.
GR in human EPI‐ECs modulates expression of CYP3A4, CYP2C9, and PXR
GR silencing on EPI‐ECs (n = 4) was successfully performed (Fig. 4), as demonstrated by decreased GR protein levels in siRNA (p < 0.001), compared with non‐siRNA ECs. GR silencing decreased the expression of the target proteins CYP3A4 (4/4 EPI‐ECs, p < 0.001) and CYP2C9 (4/4 EPI‐ECs, p < 0.001) versus non‐siRNA EPI‐ECs. No significant change was observed for CYP2E1 expression, an enzyme not controlled by GR (Fig. 4A,B). GR silencing in EPI‐ECs (4/4 EPI‐ECs) also showed corresponding decrease in PXR expression (Fig. 4A). GR levels were correlated to CYP3A4 and CYP2C9 levels (Fig. 4C,C2, respectively). The results show GR regulation of CYP3A4 and CYP2C9 in EPI‐ECs.
Figure 4.
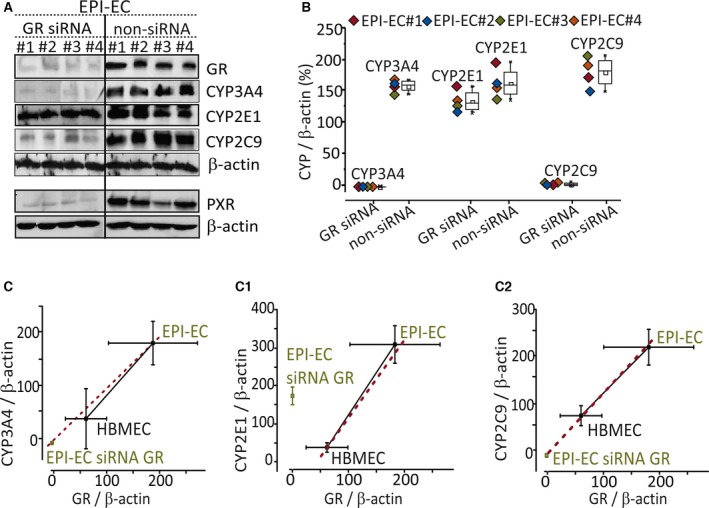
GR in human EPI‐ECs modulates expression of CYP3A4, CYP2C9, and PXR. (A) GR silencing of genes in EPI‐ECs was evaluated by Western blot, also showing a corresponding decrease in CYP3A4 and CYP2C9 expression. CYP2E1 expression did not change. GR silencing in EPI‐ECs also showed a corresponding decrease in PXR expression. (B) CYP3A4, CYP2E1, and CYP2C9 levels in GR‐silenced (siRNA) EPI‐ECs are compared to nontransfected EPI‐ECs (non‐siRNA), in which GR silencing influenced CYP3A4 and CYP2C9 expression. (C) The quantitative plots of the CYP3A4, CYP2E1, and CYP2C9 levels in GR siRNA and non‐siRNA EPI‐ECs showed that the levels of CYP3A4‐GR and CYP2C9‐GR are correlated, and siRNA GR/CYPs in EPI‐ECs levels are comparable to those of control brain endothelial cells (HBMECs). Such correlation was not applicable for CYP2E1‐GR (n = 4 for each EPI‐EC siRNA or non‐siRNA or HBMECs, in triplicate). β‐Actin was used as a loading control and normalization. Results are expressed as mean ± SEM (ANOVA).
We subsequently performed PXR silencing (Fig. 5). PXR silencing significantly (p < 0.001) decreased levels of the target gene CYP3A4 (4/4 EPI‐ECs), whereas those of CYP2C9 were unchanged (4/4 EPI‐ECs). The latter result reflects the upstream control of GR on CYP2C9, possibly overriding the effect of PXR loss.3, 4, 5 Surprisingly, the levels of CYP2E1 were decreased upon PXR silencing, perhaps suggesting the existence of a yet‐unknown brain‐specific regulatory mechanism that arises under pathologic conditions. PXR silencing in EPI‐ECs has a negligible effect on GR expression (Fig. 5A). The results (Fig. 5B) indicated sample variability, before and after PXR silencing. A comparable pattern of CYP3A4/PXR (Fig. 5C) and CYP2E1/PXR (Fig. 5C1) levels in the EPI‐EC siRNA group and HBMEC (control) levels was observed.
Figure 5.
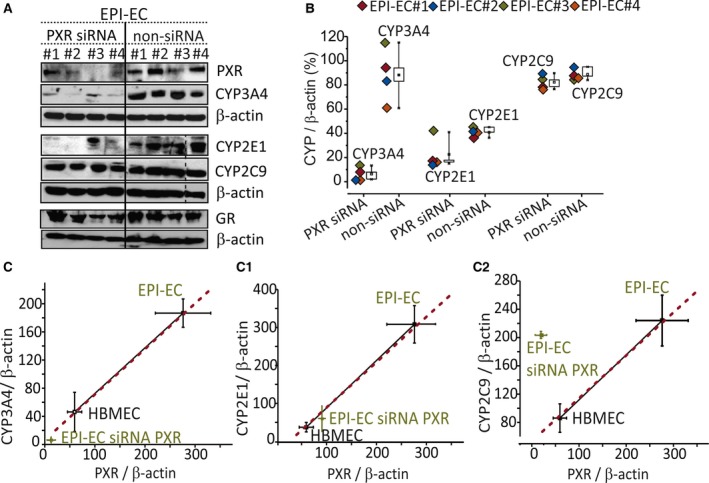
Differential involvement of PXR and CYPs in EPI‐ECs. (A) Western blot showing PXR silencing and resulting CYP3A4, CYP2C9, CYP2E1, and GR protein expression in EPI‐ECs. The PXR siRNA (denoted by EPI‐ECs 1 to 4) and non‐siRNA counterparts in EPI‐ECs were compared individually. PXR silencing showed negligible alternation in CYP2C9 and GR expression. The dotted line shows blots that were performed separately. (B) CYP3A4, CYP2E1, and CYP2C9 levels in PXR silenced (siRNA) EPI‐ECs and nontransfected EPI‐ECs (non‐siRNA EPI‐ECs) were quantified. (C–C2) CYP3A4, CYP2E1, and CYP2C9 levels corresponding to PXR silencing (or not) in EPI‐ECs and compared to control ECs (n = 4/EPI‐EC siRNA or non‐siRNA or HBMECs). β‐Actin was used as a loading control and normalization. Results are expressed as mean ± SEM (ANOVA).
Discussion
The present study shows that ECs derived from human drug‐resistant epileptic brain overexpress PXR/GR, thus affecting specific CYP regulation.23, 24 Although the pathophysiologic causes of PXR/GR overexpression remain to be elucidated, abnormal activity of specific NRs in the epileptic brain could affect drug biotransformation by altering CYP expression levels. Of interest, genetic modulation of PXRs and GRs in EPI‐ECs reduced CYP3A4 and CYP2C9 overexpression, suggesting a CYP‐PXR and CYP‐GR regulatory interplay occurring in the brain.
PXR, GR, and CYPs in the drug‐resistant epileptic brain
In the liver and gut, ligand‐activated NRs regulate a network of genes encoding enzymes responsible for xenobiotic oxidation and conjugation, as well as active transport mechanisms.2, 5, 8, 25 Of interest, brain microcapillaries and ECs derived from epileptic patients overexpress a panel of drug‐metabolizing enzymes and transporters.10, 12, 26, 27 In addition, antiepileptic drug biotransformation is significant in EPI‐ECs.14, 15, 28 The efflux transporter system may work in concert with several drug metabolizing enzymes (including monoamine oxidases and CYPs) via activation of steroid and xenobiotic sensing NRs.5, 10, 29 We found mRNA levels of AHRs, GRs, orphan receptors (e.g., PXRs), and human RXRs in EPI‐ECs. Other studies suggest activity of AHR, constitutive androstane receptor (CAR), and PXR in isolated brain capillaries30, 31 and a role of CAR in brain functions in vivo.32 Our results also indicate interpatient variability affecting individual expression of PXR/GR and selected CYPs. However, the overall trend was an increased expression in EPI‐ECs versus controls. Factors such as gender, age, history of drugs, and seizures could be responsible for the variability observed in EPI‐ECs. Such variability could also be due to polymorphic variants of CYPs and NRs.33 The selection of a “personalized medicine” approach based on optimizing therapies according to patient's genetic content such as genetic profiling or risk factor would be important in future.34, 35, 36
PXR and GR regulation of CYPs in EPI‐ECs
Our results indicate that in EPI‐ECs, CYP3A4, and CYP2C9 are regulated by GRs. Our findings are in accord with the upstream GR regulation of PXRs and a direct induction of CYP3A4 and CYP2C9 by GRs. Conversely, PXRs control CYP3A4 directly and CYP2C9 in part.3, 4, 5, 33
The possibility exists that, in the brain, unexpected drug–drug interactions involving CYP could be a consequence of abnormal PXR or GR activity.37, 38, 39 For instance, CYP3A4 participates in the cytotoxic interaction between the antiepileptic drug carbamazepine and the antidepressant drug sertraline.40 In general, increased transcription of the PXR and CAR initiates a cascade in which ligand‐binding to the receptor is followed by nuclear translocation.41 The expression of both PXR and CARs is controlled, at least in part by GR as reported earlier in hepatocytes, by displaying interdependency and possibly complicating the CYP regulation pattern during drug therapy. 8, 24, 42
The PXR‐GR regulatory mechanism is intertwined, as reports found for human hepatocytes.4, 5 This notion is similar to our results obtained using EPI‐ECs. In addition, studies have shown that co‐treatment of PXR ligands with dexamethasone resulted in enhanced basal and ligand‐dependent CYP3A4 promoter activity.43 The induction was attenuated by treatment with a GR antagonist and by introduction of GR siRNA.43 Ketoconazole and miconazole are known antagonists of GR. Treatment of hepatocytes with these azole compounds down‐regulated the expression of PXR and PXR‐target genes.44 Additional studies have shown that activated GR is involved synergistically in the xenobiotic‐responsive regulation of PXR‐target genes, including CYPs.4, 5, 45
Potential clinical relevance of CYP regulation and PXR‐GR in predicting drug resistance
The mechanisms underlying drug resistance in epilepsy are multifactorial and include altered drug biotransformation, overexpression of drug transporters, and reduced target sensitivity in the epileptogenic brain tissue.15, 27, 46, 47 It is noteworthy that several of these antiepileptic drugs are not only substrates, but can inhibit or induce genes implicated in CYP drug metabolism. Our previous studies further suggested that CYP enzymes convert carbamazepine (an antiepileptic drug) to a proconvulsive agent (quinolinic acid) in ECs from patients with drug‐resistant epilepsy.15 CYP‐regulated medication also encounters drug–drug interaction and cytotoxicity.40 Therefore, understanding the molecular events leading to CYP induction and determining whether these genes share identical regulatory mechanisms should ultimately lead to better models for the screening of drug interactions and to predict drug resistance.
Concluding Remarks
Our findings suggest a novel mechanism that may contribute to drug resistance in epilepsy, based on NR‐CYP signaling in human brain EPI‐ECs (Fig. 6). This pathway could also play a major role in predicting drug bioavailability in the diseased brain. The ligand‐specific effect of PXRs and GRs in human brain ECs shares similarities with activity seen in hepatocytes. Nevertheless, a unique expression pattern prevails, based on disease pathophysiology. PXR and GR activity and regulation with CYPs in human EPI‐ECs could be a potential target to further decipher drug modulation at the neurovascular unit.
Figure 6.
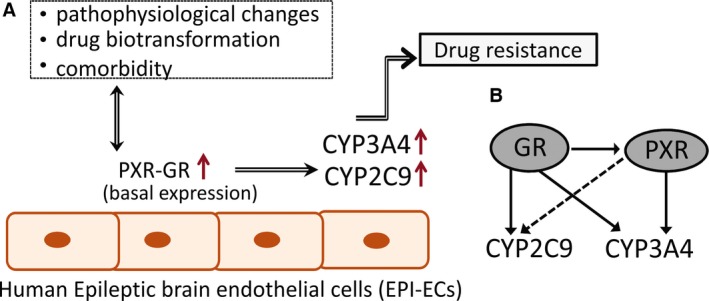
Schematic representation of potential PXR‐GR and CYP regulation in human EPI‐ECs. (A) Pathophysiologic factors, drugs, and disease etiology may affect the basal expression levels of PXR and GR in EPI‐ECs. These, in turn, could regulate specific CYPs levels (CYP3A4, CYP2E1, and CYP2C9), possibly partaking to drug resistance in epilepsy. (B) The PXR/GR expression pattern and CYP regulation in EPI‐ECs suggests that GRs could possibly be a master controller of PXRs (Fig. 4 and as described earlier in hepatocytes5). GRs directly regulate CYP3A4, CYP2C9, and PXR expression in EPI‐ECs.
Disclosure
None of the authors has any other potential conflict of interest to disclose. We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Supporting information
Figure S1. Experimental approach and study design. Epileptic brain resections (from patients with temporal lobe epilepsy) were used. The localization of NR (PXR and GR) expression and CYP expression was studied in human epileptic brains. Primary epileptic brain endothelial cells were isolated from resected human brain samples (EPI‐ECs) and compared with commercial primary control human brain microvascular endothelial cells (HBMECs). Standard procedures for immunohistochemistry, Western blot, cDNA microarray, transfection on primary human epileptic brain cells, and subcellular characterization were performed.
Figure S2. Western blot of PXR and GR showing the specificity of the antibodies used. Comparative Western blots showing expression of PXR (A) and GR (B) in HBMECs (1 to 4) and EPI‐ECs (1 to 4). Representative blots are presented.
Figure S3. Western blot of CYP3A4, CYP2E1, CYP2D6, CYP2C9, and CYP2C19 showing antibody specificity. Comparative Western blots showing expression of CYP3A4 (A), CYP2D6 (B), CYP2C19 (C), CYP2E1 (D), and CYP2C9 (E) in HBMECs (1 to 4) and EPI‐ECs (1 to 4).
Table S1. Demographic details.
Table S2. List of antibodies used for immunohistochemistry (IHC) and Western blot (WB).
Acknowledgments
This work was supported by National Institutes of Health grants R01NS078307 (to NM, DJ, and CG); R42MH093302 and R21HD057256 (to DJ); and UH4TR000491 (to DJ and CG). Grants were awarded from Brain and Behavior Research Foundation (formerly NARSAD); the American Heart Association (13SDG13950015); and Alternatives Research & Development Foundation (to CG). We would like to thank Dr. Jean‐Marc Pascussi who critically assisted us during the performance of this work.
Biography
Dr. Chaitali Ghosh is an Assistant Professor of Molecular Medicine, Biomedical Engineering, Cleveland Clinic.

Contributor Information
Chaitali Ghosh, Email: ghoshc@ccf.org.
Damir Janigro, Email: djanigro@flocel.com.
References
- 1. Gronemeyer H, Gustafsson JA, Laudet V. Principles for modulation of the nuclear receptor superfamily. Nat Rev Drug Discov 2004;3:950–964. [DOI] [PubMed] [Google Scholar]
- 2. Tirona RG, Kim RB. Nuclear receptors and drug disposition gene regulation. J Pharm Sci 2005;94:1169–1186. [DOI] [PubMed] [Google Scholar]
- 3. Gerbal‐Chaloin S, Pascussi JM, Pichard‐Garcia L, et al. Induction of CYP2C genes in human hepatocytes in primary culture. Drug Metab Dispos 2001;29:242–251. [PubMed] [Google Scholar]
- 4. Pascussi JM, Gerbal‐Chaloin S, Drocourt L, et al. The expression of CYP2B6, CYP2C9 and CYP3A4 genes: a tangle of networks of nuclear and steroid receptors. Biochim Biophys Acta 2003;1619:243–253. [DOI] [PubMed] [Google Scholar]
- 5. Pascussi JM, Gerbal‐Chaloin S, Duret C, et al. The tangle of nuclear receptors that controls xenobiotic metabolism and transport: crosstalk and consequences. Annu Rev Pharmacol Toxicol 2008;48:1–32. [DOI] [PubMed] [Google Scholar]
- 6. Bauer B, Hartz AM, Fricker G, et al. Pregnane X receptor up‐regulation of P‐glycoprotein expression and transport function at the blood‐brain barrier. Mol Pharmacol 2004;66:413–419. [DOI] [PubMed] [Google Scholar]
- 7. Moreau A, Vilarem MJ, Maurel P, et al. Xenoreceptors CAR and PXR activation and consequences on lipid metabolism, glucose homeostasis, and inflammatory response. Mol Pharm 2008;5:35–41. [DOI] [PubMed] [Google Scholar]
- 8. Pascussi JM, Drocourt L, Gerbal‐Chaloin S, et al. Dual effect of dexamethasone on CYP3A4 gene expression in human hepatocytes – sequential role of glucocorticoid receptor and pregnane X receptor. Eur J Biochem 2001;268:6346–6357. [DOI] [PubMed] [Google Scholar]
- 9. Ghersi‐Egea JF, Leininger‐Muller B, Cecchelli R, et al. Blood‐brain interfaces: relevance to cerebral drug metabolism. Toxicol Lett 1995;82–83:645–653. [DOI] [PubMed] [Google Scholar]
- 10. Dauchy S, Dutheil F, Weaver RJ, et al. ABC transporters, cytochromes P450 and their main transcription factors: expression at the human blood‐brain barrier. J Neurochem 2008;107:1518–1528. [DOI] [PubMed] [Google Scholar]
- 11. Ghosh C, Gonzalez‐Martinez J, Hossain M, et al. Pattern of P450 expression at the human blood‐brain barrier: roles of epileptic condition and laminar flow. Epilepsia 2010;51:1408–1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ghosh C, Marchi N, Desai NK, et al. Cellular localization and functional significance of CYP3A4 in the human epileptic brain. Epilepsia 2011;52:562–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Miksys SL, Tyndale RF. Drug‐metabolizing cytochrome P450s in the brain. J Psychiatry Neurosci 2002;27:406–415. [PMC free article] [PubMed] [Google Scholar]
- 14. Ghosh C, Hossain M, Puvenna V, et al. Expression and functional relevance of UGT1A4 in a cohort of human drug‐resistant epileptic brains. Epilepsia 2013;54:1562–1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ghosh C, Marchi N, Hossain M, et al. A pro‐convulsive carbamazepine metabolite: quinolinic acid in drug resistant epileptic human brain. Neurobiol Dis 2012;46:692–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bauer B, Yang X, Hartz AM, et al. In vivo activation of human pregnane X receptor tightens the blood‐brain barrier to methadone through P‐glycoprotein up‐regulation. Mol Pharmacol 2006;70:1212–1219. [DOI] [PubMed] [Google Scholar]
- 17. Chan GNY, Hoque T, Cummins CL, et al. Regulation of P‐glycoprotein by orphan nuclear receptors in human brain microvessel endothelial cells. J Neurochem 2011;118:163–175. [DOI] [PubMed] [Google Scholar]
- 18. Dombrowski SM, Desai SY, Marroni M, et al. Overexpression of multiple drug resistance genes in endothelial cells from patients with refractory epilepsy. Epilepsia 2001;42:1501–1506. [DOI] [PubMed] [Google Scholar]
- 19. Desai SY, Marroni M, Cucullo L, et al. Mechanisms of endothelial survival under shear stress. Endothelium 2002;9:89–102. [DOI] [PubMed] [Google Scholar]
- 20. Jang SY, Jang EH, Jeong SY, et al. Shikonin inhibits the growth of human prostate cancer cells via modulation of the androgen receptor. Int J Oncol 2014;44:1455–1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Yang WW, Zheng YH, Xia Y, et al. ERK1/2‐dependent phosphorylation and nuclear translocation of PKM2 promotes the Warburg effect. Nat Cell Biol 2012;14:1295–1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Tompkins LM, Wallace AD. Mechanisms of cytochrome P450 induction. J Biochem Mol Toxicol 2007;21:176–181. [DOI] [PubMed] [Google Scholar]
- 23. Dvorak Z, Modriansky M, Pichard‐Garcia L, et al. Colchicine down‐regulates cytochrome P4502B6, 2C8, 2C9, and 3A4 in human hepatocytes by affecting their glucocorticoid receptor‐mediated regulation. Mol Pharmacol 2003;64:160–169. [DOI] [PubMed] [Google Scholar]
- 24. Pascussi JM, Gerbal‐Chaloin S, Fabre JM, et al. Dexamethasone enhances constitutive androstane receptor expression in human hepatocytes: consequences on cytochrome P450 gene regulation. Mol Pharmacol 2000;58:1441–1450. [DOI] [PubMed] [Google Scholar]
- 25. Handschin C, Meyer UA. Induction of drug metabolism: the role of nuclear receptors. Pharmacol Rev 2003;55:649–673. [DOI] [PubMed] [Google Scholar]
- 26. Hartz AMS, Bauer B. Regulation of ABC transporters at the blood‐brain barrier: new targets for CNS therapy. Mol Interv 2010;10:293–304. [DOI] [PubMed] [Google Scholar]
- 27. Marchi N, Hallene KL, Kight KM, et al. Significance of MDR1 and multiple drug resistance in refractory human epileptic brain. BMC Med 2004;2:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ghosh C, Hossain M, Solanki J, et al. Pathophysiological implications of neurovascular P450 in brain disorders. Drug Discov Today 2016;21:1609–1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Marchi N, Gonzalez‐Martinez J, Nguyen MT, et al. Transporters in drug‐refractory epilepsy: clinical significance. Clin Pharmacol Ther 2010;87:13–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wang X, Hawkins BT, Miller DS. Aryl hydrocarbon receptor‐mediated upregulation of ATP‐driven xenobiotic efflux transporters at the blood‐brain barrier. FASEB J 2011;25:644–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wang XQ, Sykes DB, Miller DS. Constitutive androstane receptor‐mediated up‐regulation of ATP‐driven xenobiotic efflux transporters at the blood‐brain barrier. Mol Pharmacol 2010;78:376–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Boussadia B, Gangarossa G, Mselli‐Lakhal L, et al. Lack of CAR impacts neuronal function and cerebrovascular integrity in vivo. Exp Neurol 2016;283:39–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Bozina N, Bradamante V, Lovric M. Genetic polymorphism of metabolic enzymes P450 (Cyp) as a susceptibility factor for drug response, toxicity, and cancer risk. Arh Hig Rada Toksikol 2009;60:217–242. [DOI] [PubMed] [Google Scholar]
- 34. Preissner SC, Hoffmann MF, Preissner R, et al. Polymorphic cytochrome P450 enzymes (CYPs) and their role in personalized therapy. PLoS One 2013;8:e82562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Zanger UM, Schwab M. Cytochrome P450 enzymes in drug metabolism: regulation of gene expression, enzyme activities, and impact of genetic variation. Pharmacol Ther 2013;138:103–141. [DOI] [PubMed] [Google Scholar]
- 36. Zhou SF, Liu JP, Chowbay B. Polymorphism of human cytochrome P450 enzymes and its clinical impact. Drug Metab Rev 2009;41:89–295. [DOI] [PubMed] [Google Scholar]
- 37. Hasegawa M, Kapelyukh Y, Tahara H, et al. Quantitative prediction of human pregnane X receptor and cytochrome P450 3A4 mediated drug‐drug interaction in a novel multiple humanized mouse line. Mol Pharmacol 2011;80:518–528. [DOI] [PubMed] [Google Scholar]
- 38. Koutsounas I, Theocharis S, Patsouris E, et al. Pregnane X receptor (PXR) at the crossroads of human metabolism and disease. Curr Drug Metab 2013;14:341–350. [DOI] [PubMed] [Google Scholar]
- 39. Ma X, Shah Y, Cheung C, et al. The pregnane X receptor gene‐humanized mouse: a model for investigating drug‐drug interactions mediated by cytochromes P450 3A. Drug Metab Dispos 2007;35:194–200. [DOI] [PubMed] [Google Scholar]
- 40. Ghosh C, Hossain M, Spriggs A, et al. Sertraline‐induced potentiation of the CYP3A4‐dependent neurotoxicity of carbamazepine: an in vitro study. Epilepsia 2015;56:439–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Squires EJ, Sueyoshi T, Negishi M. Cytoplasmic localization of pregnane X receptor and ligand‐dependent nuclear translocation in mouse liver. J Biol Chem 2004;279:49307–49314. [DOI] [PubMed] [Google Scholar]
- 42. Pascussi JM, Drocourt L, Fabre JM, et al. Dexamethasone induces pregnane X receptor and retinoid X receptor‐alpha expression in human hepatocytes: synergistic increase of CYP3A4 induction by pregnane X receptor activators. Mol Pharmacol 2000;58:361–372. [DOI] [PubMed] [Google Scholar]
- 43. Cooper BW, Cho TM, Thompson PM, et al. Phthalate induction of CYP3A4 is dependent on glucocorticoid regulation of PXR expression. Toxicol Sci 2008;103:268–277. [DOI] [PubMed] [Google Scholar]
- 44. Duret C, Daujat‐Chavanieu M, Pascussi JM, et al. Ketoconazole and miconazole are antagonists of the human glucocorticoid receptor: consequences on the expression and function of the constitutive androstane receptor and the pregnane X receptor. Mol Pharmacol 2006;70:329–339. [DOI] [PubMed] [Google Scholar]
- 45. Ferguson SS, Chen Y, LeCluyse EL, et al. Human CYP2C8 is transcriptionally regulated by the nuclear receptors constitutive androstane receptor, pregnane X receptor, glucocorticoid receptor, and hepatic nuclear factor 4alpha. Mol Pharmacol 2005;68:747–757. [DOI] [PubMed] [Google Scholar]
- 46. Loscher W, Potschka H. Role of multidrug transporters in pharmacoresistance to antiepileptic drugs. J Pharmacol Exp Ther 2002;301:7–14. [DOI] [PubMed] [Google Scholar]
- 47. Granata T, Marchi N, Carlton E, et al. Management of the patient with medically refractory epilepsy. Expert Rev Neurother 2009;9:1791–1802. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Experimental approach and study design. Epileptic brain resections (from patients with temporal lobe epilepsy) were used. The localization of NR (PXR and GR) expression and CYP expression was studied in human epileptic brains. Primary epileptic brain endothelial cells were isolated from resected human brain samples (EPI‐ECs) and compared with commercial primary control human brain microvascular endothelial cells (HBMECs). Standard procedures for immunohistochemistry, Western blot, cDNA microarray, transfection on primary human epileptic brain cells, and subcellular characterization were performed.
Figure S2. Western blot of PXR and GR showing the specificity of the antibodies used. Comparative Western blots showing expression of PXR (A) and GR (B) in HBMECs (1 to 4) and EPI‐ECs (1 to 4). Representative blots are presented.
Figure S3. Western blot of CYP3A4, CYP2E1, CYP2D6, CYP2C9, and CYP2C19 showing antibody specificity. Comparative Western blots showing expression of CYP3A4 (A), CYP2D6 (B), CYP2C19 (C), CYP2E1 (D), and CYP2C9 (E) in HBMECs (1 to 4) and EPI‐ECs (1 to 4).
Table S1. Demographic details.
Table S2. List of antibodies used for immunohistochemistry (IHC) and Western blot (WB).


