Abstract
Aims
NUT midline carcinoma (NMC) is a rare undifferentiated and aggressive carcinoma that characteristically locates to the midline of the head and neck, and mediastinum. NMC is characterised by chromosomal rearrangements of the gene encoding nuclear protein in testis, NUT, at 15q14. The BRD4 gene on 19q13 is the most common translocation partner forming a fusion oncogene, BRD4-NUT. By the end of 2014, the International NUT Midline Carcinoma Registry had 48 patients treated for NMC. Laryngeal NMC are exceedingly rare and we report a case series of seven cases.
Material and Methods
We searched for cases in files of different hospitals as well as a thorough search of the English literature. The diagnosis of NMC is made by demonstration of NUT rearrangement either by immunohistochemistry, FISH or RT-PCR. We found three previously published cases and add in this series four cases of our own.
Conclusions
NMC consists of monomorphic, often discohesive, cells with an epithelioid appearance and distinct nucleoli. The tumours typically show abrupt squamous differentiation. The mean age of the patients was 34 years, hence significantly lower than that for conventional laryngeal carcinoma. All tumours were located in the supraglottis and five patients died of the disease after 3, 7, 8, 9 and 11 months. Laryngeal NMC may be underdiagnosed and an increased awareness amongst pathologists is warranted. NMC has characteristic morphological features and positive immunostaining with the NUT antibody is diagnostic. Its aggressive behaviour demands a very intense treatment strategy and the need for its recognition is further emphasised by new promising treatment strategies.
Keywords: NUT, larynx, NUT midline carcinoma, t(15;19), undifferentiated carcinoma, BRD4
Introduction
NUT midline carcinoma (NMC) is a rare aggressive cancer of which exceedingly few laryngeal cases have been reported. Albeit NMC is an undifferentiated carcinoma it almost invariably shows features of squamous cell differentiation and is by many regarded as a subtype of squamous cell carcinoma (1–6). NMC is characterised by chromosomal rearrangements of the gene encoding nuclear protein in testis, NUT, at 15q14, also known as NUTM1 or Chr15orf55 (1,7). The BRD4 (bromodomain containing 4) gene on 19q13 is the most common (approximately 70% of cases) translocation partner gene to NUT resulting in the t(15;19)(q14;p13) karyotype (8). The fusion forms a 6.4-kb fusion oncogene, BRD4-NUT. The encoded oncoprotein BRD4-NUT contributes to carcinogenesis by blocking epithelial cell differentiation and drives growth of the NMC cells (6–9). Although only recently recognised, a series of publications since 2001 have established the concept of NUT midline carcinoma as a distinct entity (1–25).
NUT midline carcinoma is a poorly differentiated carcinoma that can arise in different organs but characteristically locates to the midline of head and neck, and mediastinum. Initially NMC was thought to be a childhood cancer but later studies have shown that it affects people of all ages and there is a slight female predominance of 1.5:1 (23). The histology ranges from almost entirely undifferentiated carcinomas to carcinomas with focal or even prominent squamous differentiation and NMC is likely best regarded as distinctly aggressive subtype of SCC that has a unique and defined genetic abnormality. By 2011 at least 28 cases of NUT midline carcinomas had been reported. Fourteen of the 28 cases had originated in the head and neck but only one of these in the larynx (18). A recent study, however, reported a 5-fold increase in the diagnosis of head and neck NMC from 2011 to 2014, and as of December 31st 2014, the International NUT Midline Carcinoma Registry had 48 patients treated for NMC (23). Part of this increase is very likely due to the availability of the new antibody specific for the NUT protein (16), facilitating more widespread laboratory diagnoses. NUT protein expression is normally exclusive to the testes, and its expression outside the testes is diagnostic of NMC (16). The diagnosis of NMC is made by demonstration of NUT rearrangement either by NUT immunohistochemistry, FISH or RT-PCR (or cytogenetic analysis). We here describe the clinicopathological features of 7 cases of laryngeal NMC, the largest series to date, and emphasise the importance of its recognition by the pathologist, particularly as the patients need very intensive treatment and also because new promising treatment strategies are at the horizon.
Material and methods
Due to its rarity, we made a thorough search of the English literature (Medline, Pubmed) for any documented laryngeal NMC. We were aware of three unpublished cases from the different hospitals of the collaborating authors but we also searched for cases in files of three other different hospital departments by Snomed code T24000 and M codes 80203 (undifferentiated), 80103 (Not Otherwise Specified) and 80003 (malignancy NOS). Furthermore, in an attempt to obtain a very crude idea about the prevalence of laryngeal NMC amongst laryngeal SCCs, the database (1996–2016) of yet another hospital was searched. Here all cases of diagnosed laryngeal SCC (M80703) was recorded (out of which 100 random cases were histologically reviewed), and during the same time period all laryngeal cases M coded 80203, 80103 and 80003 were collected (all of which were reviewed histologically).
Immunohistochemistry was performed in five cases using the NUT (C52B1) Rabbit mAb #3625 (Cell Signaling Technology, Inc., Boston, MA, USA). The immunohistochemical procedure was performed strictly according the manufacturer’s recommendation for paraffin embedded tissue. This antibody detects endogenous levels of total NUT protein and endogenous levels of the BRD4-NUT fusion protein found in NUT midline carcinoma. In all four laboratories human testis was used as positive control (post-meiotic spermatids express NUT at approximately the same level as NMC does). In Case 7 we also used a case of thymic NUT midline carcinoma, previously established by FISH and C52B1. The C52B1 antibody has been shown to be 100% specific and 87% sensitive for the diagnosis of NMC, and thus a positive NUT immunostaining by itself is diagnostic of NMC (16, 26).
Fluorescence in situ hybridization (FISH) analysis was performed in four cases. Dual-colour bring-together FISH assays for BRD4 and NUT gene loci using homebrew probes were performed on formalin-fixed, paraffin-embedded, 4 μm tissue sections as described previously (7).
Results
We found only three cases published in the English literature (13,15,25) and have added four new cases of our own. There were 4 males and 3 females and the age varied from 5 to 78 years with a mean of 34 years. All seven cases were located in supraglottis and hence none in the glottic or subglottic region. Five patients died of the disease after 3, 7, 8, 9 and 11 months. One patient is currently undergoing treatment and survival data was not given in one of the previously published cases. The initial histological diagnosis was in two cases poorly differentiated carcinoma, and the remaining cases undifferentiated carcinoma, invasive basaloid squamous cell carcinoma, high grade malignant neoplasm, and in two cases NUT carcinoma (Table 1). The diagnosis of NUT midline carcinoma was in the three previously published cases based on FISH in two cases and IHC in one. In the three new cases known to us and initiating this study (cases 4–6) the diagnosis was based on IHC (and supplemented by FISH in two of the cases). The search for tumours M coded 80203, 80103 and 80003 in three other hospitals yielded 38 cases out which 7 had a morphology that could be compatible with NUT midline carcinoma. All 38 cases were stained with the C52B1 antibody and in one of the cases (one of the 7 with NUT compatible morphology) the immunostaining was unequivocally positive (Case 7; Table 1). Immunohistochemistry with the NUT antibody was thus positive with diffuse nuclear staining in 5 of the cases (Fig. 1B). In two cases (Cases 2 and 5), FISH showed a reciprocal fusion at both NUT and BRD4 (Fig. 1C). The tumour cells were pancytokeratin, p63, and p40 positive but negative for neuroendocrine, lymphocytic and melanocytic markers.
Table 1.
Clinicopathological characteristics of laryngeal NUT carcinomas.
| No cases | Author (Ref) | Year | Age | Sex | Location | Initial diagnosis | Basis for diagnosis and genetics | Immunohistochemistry | Treatment | Died of Disease (months) |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Vargas et al (13) | 2001 | 13 | F | Epiglottis | PDC |
FISH BDR4-NUT Trisomy 8, t(15;19)(q13;p13.1) |
(+):CK | QRT+Surgery (Radical neck dissection). Palliative QT. |
9 |
| (−): AFP, CD3, CD20, CD30, CEA, Chromogranin A, Desmin, EMA, HMB-45, LCA, MSA, PLAP, SMA, S-100, EBER-ISH | ||||||||||
| 2 | Stelow et al (15) | 2008 | 78 | F | Supraglottis | UC |
FISH BRD4-NUT |
*(+): panCK, p63. | QRT+Surgery (Laryngectomy+ neck dissection) | 8 |
| *(−): synaptophysin, chromogranin, LCA, CD19, CD30, CD15, S100, HMB45, tyrosinase, ALK, desmin, SMA, EMA, lysozyme, CD43. | ||||||||||
| 3 | Kundra et al (25) | 2016 | 39 | M | Supraglottis | HGMN | NUT IHC | (+ focal):CK5/6, p40, CAM 5.2, and AE-1/AE-3. | NA | NA |
| (−):CD10, CD20, CD43, CD3, CD23, BCL-2, BCL-6, MUM-1, S100, MELAN-1, MART-1, HMB-45, actin, desmin, myogenin, FLI-1, chromogranin, synaptophysin | ||||||||||
| 4 | Hospital for Sick Children, Toronto, Canada | 2012 | 5 | F | Larynx/ base of tongue | NUT Midline Carcinoma | NUT IHC, fusion unknown | (+) p63, p53 (high expression) 60% loss p27 80–90% loss p21 Increased Mib-1 Uniformly NUT protein positive (−) p16 ISH for EBV and EBER Negative Both T and B cells present with no clear evidence of significant antigen loss in T cells, orlight-chain restricted B cells |
Tracheotomy and induction chemotherapy with HDAC inhibitor followed by bilateral neck dissections and postoperative radiationtherapy. Eventually required total laryngectomy for recurrence. | 7 |
| 5 | University of Chicago, Chicago, IL | 2014 | 41 | M | Supraglottis | Invasive basaloid SCC |
FISH NUT IHC BRD4-NUT |
NUT | Partial laryngectomy & neck dissection; 6.5 cycles adjuvant QRT | 11 |
| 6 | Auckland City Hospital, Auckland, New Zealand | 2016 | 17 | M | Supraglottis | NUT midline carcinoma |
FISH NUT IHC BRD4-NUT Karyotype: 47,XY,+8,i(8)(p10),t(15;19)(q14;p13.1)[16]/46,XY[4] |
(+) p63, p40, Ki-67 (high) (+ in 50% of cells) p16 (+ focal) CKAE1/AE3, High Molecular Weight CK, CK56, CD30 (−) - CD56, Synaptophysin, Chromogranin, Calcitonin – S100, MelanA, Sox10 – CD20, CD3, LCA(CD45) – PLAP, CD117, SALL4, OCT ¾, HCG, AFP – TTF1, CK7, CK20, PAX8 – Desmin, myogenin, CD34 EBER ISH was negative. |
Transoral CO2 Laser Microsurgical resection; with staged Bilateral neck dissections (5 days later) – ipsilateral extended radical Neck Dissection and contralateral modified radical neck dissection with pec major flap to ipsilateral neck | AWD |
| 7 | Portuguese Institute of Oncology Porto, Portugal | 2015 | 47 | M | Larynx/ base of tongue | Squamous cell carcinoma, poorly differentiated | NUT IHC fusion unknown | NUT | Palliative chemotherapy with taxol and carboplatin | 3 months |
PDC: Poorly differentiated carcinoma, UC: Undifferentiated carcinoma, HGMN: High-Grade malignant neoplasm, IHC: Immunohistochemistry, NA: Not available, AWD: Alive with disease, QT: Chemotherapy, QRT: Chemoradiotherapy.
Figure 1. Pathological and molecular features of laryngeal NMC.
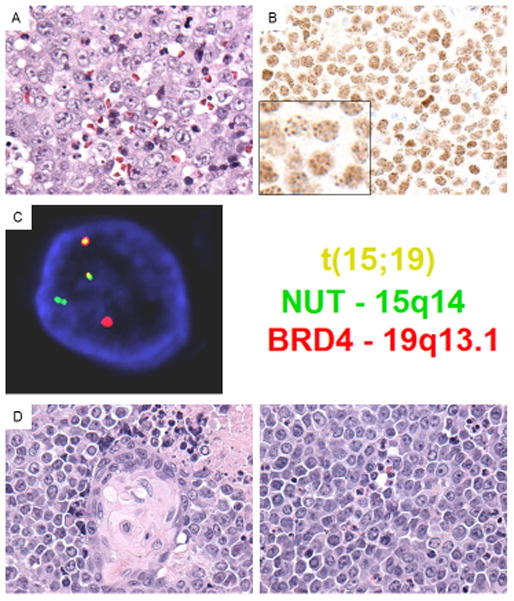
(A) Case 1 reveals typical undifferentiated morphology of NMC, exhibiting sheets of moderate sized, cells with monomorphic round nuclei and distinct nucleoli. The neutrophilic infiltrate observed in this case is unusual in carcinoma, but frequently seen in NMC (H&E, 400x). (B) Diagnostic NUT immunohistochemistry in case 5 revealing diffuse nuclear staining in a speckled pattern (inset). (C) BRD4-NUT fusion fluorescent in situ hybridization (FISH) using probes covering NUT (green) and BRD4 (red) genes reveals a reciprocal fusion at both NUT (ch. 15q14) and BRD4 (ch. 19p13.1) loci (yellow, overlapping probes). The findings are consistent with a BRD4-NUT fusion in this tumor. (D) Case 5 reveals classic NMC morphology with abrupt squamous differentiation (left), and sheets of monomorphic rounds cells separated by clear spaces (right). The frequent single necrotic cells and mitoses are indicative of the aggressive biology of this cancer.
The retrospective search in one hospital (1996–2016) yielded seven cases of undifferentiated laryngeal carcinoma compared to the approximately 400 cases of conventional SCC (none of the random 100 cases reviewed histologically showed any undifferentiated areas). All seven patients with undifferentiated carcinoma were deceased (at an age between 35 and 80 years) but we were unable to trace the cause of death. For different reasons no suitable tissue was available in for immunohistochemistry in any of the seven cases. Thus we could not demonstrate the existence of a single case of laryngeal NUT midline carcinoma during the same time as we saw 400 cases of conventional laryngeal squamous cell carcinoma. This in accordance with previous observations where none of 438 head and neck squamous cell carcinomas stained positively with the C52B1 antibody (16), and emphasises the rarity of NUT midline carcinoma, both in the larynx as well as in the head and neck as a whole.
The histology showed in all cases features of an undifferentiated carcinoma consisting of monomorphic cells with round to oval nuclei and distinct nucleoli. The cells were primarily medium-sized and arranged in sheets of different sizes, and cells slightly separated from each other by clear spaces. Many of these relatively discohesive cells had an epithelioid appearance. Typically, the tumours showed abrupt squamous differentiation (Figs. 1A & 1D). Necrosis and high mitotic rate were other features of these tumours.
Discussion
NMC is defined by rearrangement of the NUT gene. The t(15:19) translocation is the most common rearrangement that places NUT in frame with BRD4, the latter being a ubiquitously expressed transcriptional activator that drives expression of the BRD4-NUT fusion gene. The BRD4-NUT fusion was first described in 2003 by French and co-workers (7). It has been hypothesised that NMC utilises a genetic shortcut to the development of SCC, which normally takes years and the accumulation of multiple mutational hits to develop (1,27,28). BRD4-NUT forms enormous contiguous stretches of active chromatin (0.1 – to 1.5-Mb) that have been termed “megadomains”, enriched with BRD4-NUT and p300. Expression of at least a subset of megadomain-containing genes is critical to the growth of the host NMC cell. For example, the ability of BRD4-NUT to fill whole regulatory compartments for genes like MYC is thought to be one explanation of the extremely aggressive nature of NMC (6,29). In support of this idea, it has been shown that MYC is a key downstream target of BRD4-NUT, and is necessary and sufficient for blockade of differentiation in NUT carcinomas (3). It is of further interest that TP63, a p53 family member whose oncogenic ΔN isoform is expressed in the majority of NMCs, maps to a megadomain in all NMC cells tested (6) and is required for NMC cell viability in vitro (19,30)
In approximately 75% of NMCs NUT is fused to BRD4, BRD3 or NSD3 (2,5,7,9) but in the remaining cases to an unknown partner gene; these latter tumours are termed NUT-variants. The commercially available monoclonal antibody C52B1 will detect all forms of NMC, including BRD4-NUT, BRD3-NUT, NSD3-NUT, and NUT-variants (17). Recently a case of hypopharyngeal NMC were reported and that had a novel three-way translocation t(9;15;19;q34;q13;p13.1) (20). Five of the six cases presented here were positive for the NUT antibody whilst the BRD4-NUT fusion was demonstrated by FISH in one case. Our extensive review shows that primary laryngeal NMC is a rarity and has been underdiagnosed. It shares the same morphology of NMC located elsewhere and in any case of undifferentiated laryngeal carcinoma immunohistochemistry with the NUT antibody should be considered. Morphological clues to NMC are features of an undifferentiated carcinoma admixed with squamous differentiation, and where the undifferentiated cells tend to be round and may mimic a small round blue cell tumour. The important differential diagnosis of a small round cell tumour has been pointed out by Bishop and associates (30,31). Necrosis, including frequent single cell necrosis, and high mitotic rate, are present in NMCs. Neutrophilic infiltrates are often present, a feature not commonly seen in other laryngeal carcinomas. A young age of the patient should also alert the pathologist. In our review the mean age was 34 years which is considerably younger and atypical for conventional laryngeal carcinomas. The patients also tend to present at an advanced clinical stage, often Stage IV, illustrated e.g. by case 7 where the patient at diagnosis was staged T4aN3M1.
The clinical outcome for patients with laryngeal NMC, and for NMC located elsewhere, is in general dismal. Chemotherapy or radiation therapy alone for head and neck NMC has shown to be inadequate whilst aggressive initial resection with or without postoperative chemotherapy or radiation has been reported to carry a significantly enhanced survival in a series of 48 patients with NMC (23). Still, head and neck NMC in general has a poor prognosis, and is the case with laryngeal NMC. In our series of 7 patients, clinical outcome data was available in 6 patients, and here the survival ranged from 3 to 11 months. A very recent report has shown very promising clinical response by the use of bromodomain and extra-terminal (BET) bromodomain (BRD) inhibitors. The antitumor activity of novel oral BET inhibitor (OTX015/MK-8628) was evaluated in four patients with advanced-stage NMC and showed impressive and rapid antitumour activity in NMC. Two of the patients responded rapidly with tumour regression and a third had meaningful disease stabilisation (32).
We conclude that laryngeal NMC is a rarity but may be underdiagnosed. It has characteristic morphological features and a positive immunohistochemical staining with the NUT antibody is diagnostic. An increased awareness amongst pathologists is warranted due to its aggressive behaviour demanding a different treatment strategy than conventional poorly differentiated laryngeal carcinomas.
Acknowledgments
This work was supported by a grant from the National Institutes of Health, number 2R01CA124633 to C.A.F.
Footnotes
No conflict of interest
This article was written by members and invitees of the International Head and Neck Scientific Group (www.IHNSG.com). Two of the authors (H.H. and C.A.F) have done the main part of the article, and for the rest the other authors contributed equally.
References
- 1.French CA. Pathogenesis of NUT midline carcinoma. Annu Rev Pathol. 2012;7:247–265. doi: 10.1146/annurev-pathol-011811-132438. [DOI] [PubMed] [Google Scholar]
- 2.Bauer DE, Mitchell CM, Strait KM, et al. Clinicopathologic features and long-term outcomes of NUT midline carcinoma. Clin Cancer Res. 2012;18:5773–5779. doi: 10.1158/1078-0432.CCR-12-1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grayson AR, Walsh EM, Cameron MJ, et al. MYC, a downstream target of BRD-NUT, is necessary and sufficient for the blockade of differentiation in NUT midline carcinoma. Oncogene. 2014;33:1736–1742. doi: 10.1038/onc.2013.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.French CA. The importance of diagnosing NUT midline carcinoma. Head Neck Pathol. 2013;7:11–16. doi: 10.1007/s12105-013-0428-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.French CA, Rahman S, Walsh EM, et al. NSD3.-NUT fusion oncoprotein in NUT midline carcinoma: implications for a novel oncogenic mechanism. Cancer Discov. 2014;4:928–241. doi: 10.1158/2159-8290.CD-14-0014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alekseyenko AA, Walsh EM, Wang X, et al. The oncogenic BRD4-NUT chromatin regulator drives aberrant transcription within large topological domains. Genes Dev. 2015;29:1507–1523. doi: 10.1101/gad.267583.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.French CA, Kutok JL, Faquin WC, et al. Midline carcinoma of children and young adults with NT rearrangement. J Clin Oncol. 2004;22:4135–4139. doi: 10.1200/JCO.2004.02.107. [DOI] [PubMed] [Google Scholar]
- 8.French CA, Miyoshi I, Kubonishi I, et al. BRD4-NUT fusion oncogene: a novel mechanism in aggressive carcinoma. Cancer Res. 2003;63:304–307. [PubMed] [Google Scholar]
- 9.French CA, Ramirez CL, Kolmakova J, et al. BRD-NUT oncoproteins: a family of closely related nuclear proteins that block epithelial differentiation and maintain the growth of carcinoma cells. Oncogene. 2008;27:2237–2242. doi: 10.1038/sj.onc.1210852. [DOI] [PubMed] [Google Scholar]
- 10.Kubonishi I, Takehara N, Iwata J, et al. Novel t(15;19)(q15;p13) chromosome abnormality in a thymic carcinoma. Cancer Res. 1991;51:3327–3328. [PubMed] [Google Scholar]
- 11.Kees UR, Mulcahy MT, Willoughby ML. Intrathoracic carcinoma in an 11-year-old girl showing a translocation t(15;19) Am J Pediatr Hematol Oncol. 1991;13:469–464. doi: 10.1097/00043426-199124000-00011. [DOI] [PubMed] [Google Scholar]
- 12.Lee AC, Kwong YI, Fu KH, et al. Disseminated mediastinal carcinoma with chromosomal translocation (15;19) A distinctive clinicopathologic syndrome Cancer. 1993;72:2273–2276. doi: 10.1002/1097-0142(19931001)72:7<2273::aid-cncr2820720735>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 13.Vargas SO, French CA, Faul PN, et al. Upper respiratory tract carcinoma with chromosomal translocation 15;19. Evidence for a distinct disease entity of young patients with a rapidly fatal course. Cancer. 2001;92:1195–1203. doi: 10.1002/1097-0142(20010901)92:5<1195::aid-cncr1438>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 14.Dang TP, Gazdar AF, Virmani AK, et al. Chromosome 19 translocation, overexpression of Notch3, and human lung cancer. J Natl Cancer Inst. 2000;92:1355–1357. doi: 10.1093/jnci/92.16.1355. [DOI] [PubMed] [Google Scholar]
- 15.Stelow EB, Bellizzi AM, Taneja K, et al. NUT rearrangement in undifferentiated carcinomas of the upper aerodigestive tract. Am J Surg Pathol. 2008;32:828–834. doi: 10.1097/PAS.0b013e31815a3900. [DOI] [PubMed] [Google Scholar]
- 16.Haack H, Johnson LA, Fry CJ, et al. Diagnosis of NUT midline carcinoma using a NUT-specific monoclonal antibody. Am J Surg Pathol. 2009;33:984–991. doi: 10.1097/PAS.0b013e318198d666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.French CA. NUT midline carcinoma. Cancer Genet Cytogenet. 2010;203:16–20. doi: 10.1016/j.cancergencyto.2010.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stelow EB. A review of NUT midline carcinoma. Head Neck Pathol. 2011;5:31–35. doi: 10.1007/s12105-010-0235-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bishop JA, Westra WH. NUT midline carcinomas of the sinonasal tract. Am J Surg Pathol. 2012;36:1216–1221. doi: 10.1097/PAS.0b013e318254ce54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mills AF, Lanfranchi M, Wein RO, et al. NUT midline carcinoma. A case report with a novel translocation and review of the literature. Head Neck Pathol. 2014;8:182–186. doi: 10.1007/s12105-013-0479-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sholl LM, Nishino M, Pokharel S, et al. Primary pulmonary NUT midline carcinoma: Clinical, radiographic, and pathologic characterizations. J Thorac Oncol. 2015;10:951–959. doi: 10.1097/JTO.0000000000000545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Andreasen S, French CA, Josiassen M, et al. NUT carcinoma of the sublingual gland. Head Neck Pathol. 2016;10:362–366. doi: 10.1007/s12105-015-0672-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chau NG, Hurwitz S, Mitchell CM, et al. Intensive treatment and survival outcomes in NUT midline carcinoma of the head and neck. Cancer. 2016 Aug 10; doi: 10.1002/cncr.30242. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bellizzi AM, Bruzzi C, French CA, et al. The cytologic features of NUT midline carcinoma. Cancer. 2009;117:508–515. doi: 10.1002/cncy.20044. [DOI] [PubMed] [Google Scholar]
- 25.Kundra A, Andrei M, Westra W, et al. Nuclear protein in testis midline carcinoma of larynx: An underdiagnosed entity. Head Neck. 2016;38:E2471–2474. doi: 10.1002/hed.24418. [DOI] [PubMed] [Google Scholar]
- 26.Chirieac LR, French CA, Sholl L, et al. WHO. Lung: Other and unclassified carcinomas. 4. 2015. classification of tumours of the lung, pleura, thymus and heart; pp. 97–98. [Google Scholar]
- 27.Stransky N, Egloff AM, Tward AD, et al. The mutational landscape of head and neck squamous cell carcinoma. Science. 2011;333:1157–1160. doi: 10.1126/science.1208130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pickering CR, Zhou JH, Lee JJ, et al. Mutational landscape of aggressive cutaneous squamous cell carcinoma. Clin Cancer Res. 2014;20:6582–6592. doi: 10.1158/1078-0432.CCR-14-1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reynoird N, Schwartz BE, Delvecchio M, et al. Oncogenesis by sequestration of CBP/p300 in transcriptionally inactive hyperacetylated chromatin domains. EMBO J. 2010;29:2943–2952. doi: 10.1038/emboj.2010.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tilson MP, Bishop JA. Utility of p40 in the differential diagnosis of small round blue cell tumors of the sinonasal tract. Head Neck Pathol. 2014;8:141–145. doi: 10.1007/s12105-013-0496-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bishop JA, French CA, Ali SZ. Cytopathological features of NUT midline carcinoma: A series of 26 specimens from 13 patients. Cancer Cytopathol. 2016 Jul; doi: 10.1002/cncy.21761. [Epub ahead of Print] [DOI] [PubMed] [Google Scholar]
- 32.Stathis A, Zucca E, Bekradda M, et al. Clinical response of carcinomas harbouring the BRD4-NUT oncoprotein to the targeted Bromodomain inhibitor OTX015/MK-8628. Cancer Discov. 2016;6:492–500. doi: 10.1158/2159-8290.CD-15-1335. [DOI] [PMC free article] [PubMed] [Google Scholar]


