Abstract
Retrovirus entry into cells is mediated by specific interactions between virus envelope glycoproteins and cell surface receptors. Many of these receptors contain multiple membrane-spanning regions, making their purification and study difficult. The jaagsiekte sheep retrovirus (JSRV) receptor, hyaluronidase 2 (Hyal2), is a glycosylphosphatidylinositol (GPI)-anchored molecule containing no peptide transmembrane regions, making it an attractive candidate for study of retrovirus entry. Further, the hyaluronidase activity reported for human Hyal2, combined with its broad expression pattern, may point to a critical function of Hyal2 in the turnover of hyaluronan, a major extracellular matrix component. Here we describe the properties of a soluble form of human Hyal2 (sHyal2) purified from a baculoviral expression system. sHyal2 is a 54-kDa monomer with weak hyaluronidase activity compared to that of the known hyaluronidase Spam1. In contrast to a previous report indicating that Hyal2 cleaved hyaluronan to a limit product of 20 kDa and was active only at acidic pH, we find that sHyal2 is capable of further degradation of hyaluronan and is active over a broad pH range, consistent with Hyal2 being active at the cell surface where it is normally localized. Interaction of sHyal2 with the JSRV envelope glycoprotein was analyzed by viral inhibition assays, showing >90% inhibition of transduction at 28 nM sHyal2, and by surface plasmon resonance, revealing a remarkably tight specific interaction with a dissociation constant (KD) of 32 ± 1 pM. In contrast to results obtained with avian retroviruses, purified receptor was not capable of promoting transduction of cells that do not express the virus receptor.
Retroviruses enter host cells by executing a complex molecular program resulting in the fusion of viral envelope and the host cell plasma membrane. Key players in this process are the retroviral envelope glycoprotein (Env) and the corresponding target cell receptor proteins. Although protocols for production and purification of solubilized recombinant Env fragments have been developed for several retroviruses, their receptor counterparts have generally proven difficult to produce and purify because they are class IV (multi-pass) integral membrane proteins. For example, the gammaretrovirus receptors Pit1, Pit2, Xpr, Flvcr, and Cat-1 function or are predicted to function as transporters for small molecules and contain from 8 to 13 predicted membrane-spanning regions (44).
The current model for fusogenic virus entry is based on studies done with the influenza virus (8). Endocytosis, followed by the acidification of the endosomal compartment, causes the envelope glycoprotein to undergo profound structural reorganization, bringing the viral membrane into very close proximity to the endolysosomal membrane (reviewed in references 10 and 21). Although some retroviruses require this acidification step for productive infection (42, 49), many are able to infect cells directly at the plasma membrane at neutral pH (25, 42, 60). In these cases, it has been proposed that the interaction between the retroviral envelope glycoprotein and its entry receptor, displayed on the target cell surface, results in a conformational change thereby serving as an initial step in the fusogenic program (4). Receptor-induced conformational changes in retroviral envelope glycoproteins have been observed in a number of systems (4, 24, 28, 51, 57), and in each case the conformational change is believed to lead to activation of envelope glycoprotein fusogenicity. Thus, understanding the nature of the interaction between retroviral envelope glycoproteins and their respective receptors is essential to understanding retroviral entry.
Most previous work aimed at the characterization of Env-receptor interactions has been carried out by studying binding of envelope glycoprotein fragments to receptor-expressing cells. Env/receptor complex formation has typically been detected by either direct labeling of the envelope construct with 125I (5, 16, 31, 32) or by using fluorescently labeled secondary antibodies against Env (18, 36). While these strategies circumvent the difficulties associated with receptor protein purification, the binding measurements are likely complicated by the presence of other proteins and macromolecules on the cell surface. A major advance in the field was recently made by Hoffman et al. with their report of direct kinetic measurements of the human immunodeficiency virus type 1 (HIV-1) gp120 interaction with chemokine receptors by surface plasmon resonance (SPR) (27). However, even in this case, CCR5 and CXCR4 had to be incorporated into a cell-derived membrane containing other surface molecules that could potentially complicate the analysis.
Ideally, detailed analysis of Env-receptor binding would be carried out in a system containing only the two interacting species, and there are a few characterized retroviral systems that involve receptors with one or no membrane-spanning regions that can be made in soluble form. For instance, studies aimed at examining the fusogenic properties of subgroup A avian sarcoma and leukosis virus (ASLV-A) Env (13, 20, 26, 54) have become possible since the development of a functional soluble Tva receptor (3). The Tvb receptor, used by ASLV groups B, D, and E (7), as well as the recently identified mouse mammary tumor virus receptor (49), each possess a single membrane-spanning region. Lastly, the jaagsiekte sheep retrovirus (JSRV) receptor hyaluronidase 2 (Hyal2) is linked to the cell surface solely through a glycosylphosphatidylinositol (GPI) anchor (47).
JSRV is a simple retrovirus that causes lung cancer in sheep. Several lines of evidence indicate that Hyal2 is the JSRV receptor, including radiation hybrid mapping results (46), the fact that resistant cells can be rendered susceptible to transduction by expression of human or ovine Hyal2 orthologs (19, 47), and the detection of specific binding of a hybrid JSRV Env SU subunit/human immunoglobulin G (IgG) constant region (JSU-IgG) to cells expressing Hyal2 receptors (36).
JSRV can cause acute multifocal cancer in infected sheep (53), but unlike most other acutely transforming retroviruses, it does not contain a host-derived oncogene. Expression of JSRV Env can transform cells in culture (2, 37, 45, 47), indicating that Env is the oncogene responsible for JSRV pathogenesis in vivo. In the epithelium-derived BEAS cell line, expression of JSRV Env causes transformation that is dependent on its interaction with Hyal2 and the subsequent degradation of the JSRV Env/Hyal2 complex (15). It is interesting to note that the Hyal2 gene is contained within the 3p21.3 locus, which is deleted in the majority of small-cell lung cancers and has been proposed to be a tumor suppressor (35). In contrast, fibroblast-derived cell lines are transformed in a Hyal2-independent manner, which involves the activation of the phosphatidylinositol 3-kinase (PI3K)/Akt signaling pathway by the cytoplasmic tail of JSRV Env (1, 45). It is not known which mechanism predominates in diseased animals.
The normal function of Hyal2 is unclear. Hyal2 is a member of the hyaluronidase family of proteins, which includes the sperm hyaluronidase Spam1 and the serum hyaluronidase Hyal1 (12). Hyaluronidase-catalyzed degradation of hyaluronan, a linear repeating disaccharide, is thought to be important for the remodeling of the extracellular matrix as well as for maintaining homeostasis in various connective tissues in vertebrates. In addition, multiple roles for hyaluronan and its degradation products in cancer biology have been proposed (for review, see reference 56). Hyaluronidase activity associated with expression of human Hyal2 in cultured cells has been reported (34). Unlike other hyaluronidases, it appeared that Hyal2 was unable to digest hyaluronan to completion and left a 20-kDa-limit product, which might have unique biological roles (34). However, we were unable to detect hyaluronidase activity in cells following expression of human Hyal2 using a retrovirus expression system, while we were easily able to detect the activity of Hyal1 using the same expression system, and we estimated that the hyaluronidase activity of Hyal2 was at least 50-fold lower than that of human Hyal1 (47). The difference in results from these previous studies may be due to a higher level of Hyal2 production from the vaccinia virus expression system used in the first study, but it is puzzling that Hyal2 has such low activity if its normal function is cleavage of hyaluronan.
Here we describe the production and purification of a soluble form of human Hyal2 (sHyal2), characterize its hyaluronidase activity, and examine its interaction with JSRV Env. We find that sHyal2 is a weak hyaluronidase, active over a wide pH range and capable of complete degradation of hyaluronan. Purified sHyal2 specifically interacts with retroviral particles pseudotyped with JSRV Env, as evidenced by its ability to inhibit transduction of susceptible cells. However, purified sHyal2 was not capable of facilitating transduction of non-receptor-expressing cells. SPR measurements of the binding of sHyal2 to the JSU-IgG show an unusually tight interaction due primarily to an extremely slow dissociation rate.
MATERIALS AND METHODS
Cell culture.
Mammalian cell lines were maintained in Dulbecco's modified Eagle medium with high glucose (4.5 g per liter) (Gibco) and 10% fetal bovine serum (HyClone) at 37°C in a 10% CO2-air atmosphere unless otherwise noted. Insect cell lines were maintained at 27°C in air. Sf9 cells were grown in SF-900 II serum-free medium (SFM), and High5 cells were grown in Express Five SFM (Gibco).
Generation of recombinant baculovirus encoding sHyal2.
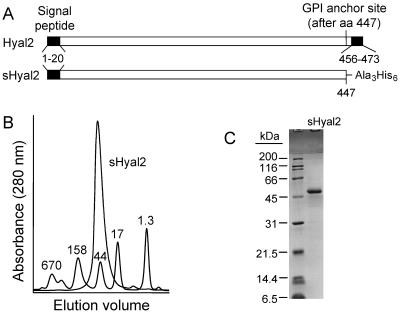
Mutagenic PCR was used to modify a cDNA encoding human Hyal2 to contain an SphI restriction site upstream of the start codon and a NotI site in place of the codon just after the GPI addition site in the protein. The resulting fragment was ligated to a NotI/HindIII linker encoding His6 and was then cloned into the pFastbac1 (Gibco) transfer vector. In this procedure, the Hyal2 leader peptide was preserved, while the C-terminal hydrophobic region, predicted to be removed in the mature polypeptide, was replaced by a His6 tag (Fig. 1A). The resulting cDNA encoded residues 1 to 447 of human Hyal2, separated from the His6 tag by Ala3 (encoded by the NotI restriction site). Subsequent steps for generation of the starting stock of recombinant baculovirus in Sf9 cells were carried out according to the manufacturer's instructions. Expression of recombinant protein in infected cultures was monitored by immunoblotting with anti-Penta-His antibody (QIAGEN). Final virus stock expansion was carried out by Custom Baculovirus Services (BD Biosciences).
FIG. 1.
Structure and properties of purified sHyal2. (A) The structures of native Hyal2 and sHyal2 are shown (both drawn to scale). Amino acids 1 to 21 constitute the endoplasmic reticulum signal peptide for both proteins. A GPI anchor is predicted to replace all residues following amino acid 447 in Hyal2, which also contains a C-terminal hydrophobic tail at residues 456 to 473 that localizes the protein in the membrane prior to GPI anchor addition. In sHyal2, a C-terminal His6 tag (attached with an Ala3 linker) replaces the GPI anchor attachment site and the hydrophobic tail of the native sequence. (B) Size exclusion chromatography using a Superdex 200 HR 10/30 column shows that sHyal2 is monomeric in solution and migrates with an apparent molecular mass of ∼40 kDa. Overlaid onto the trace of sHyal2 is a trace of Bio-Rad gel filtration chromatography standards with sizes in kilodaltons indicated. (C) SDS-PAGE of sHyal2 and protein standards under reducing conditions indicates a molecular mass for sHyal2 of ∼50 kDa.
Expression and purification of recombinant sHyal2.
Recombinant baculovirus stock (∼108 PFU/ml) was used to inoculate spinner cultures of High5 cells for protein production at the optimal multiplicity of infection of 5. The cultures were infected by resuspending log-phase cells in virus stock, incubating the suspension at room temperature for 10 to 15 min, and diluting in fresh medium to a density of 106 cells/ml. The infected cultures were grown in ≤500 ml per 1-liter spinner flask.
When cell viability in infected cultures dropped to ∼50% (approximately 48 to 72 h postinfection), cells and cell debris were removed by centrifugation at 3,000 × g and the supernatant was further clarified by centrifugation at 7,000 × g. The resulting material either was dialyzed immediately against equilibration buffer (150 mM NaCl, 50 mM Tris-HCl, pH 8) or was first precipitated by addition of ammonium sulfate to 30% (wt/vol). The precipitate was dissolved in phosphate-buffered saline (PBS), and the solution was then dialyzed. The treated baculovirus supernatant was next passed over a column containing Talon resin (Clontech), the column was washed with 10 column volumes of wash buffer (300 mM NaCl, 50 mM Tris-HCl, pH 7.4, 10% glycerol, 20 mM imidazole) and the bound protein was eluted with 3 column volumes of elution buffer (wash buffer with 200 mM imidazole). The eluted protein was concentrated by ultrafiltration (Amicon Ultra-15 modules; Millipore) and fractionated on a Superdex 200 HR 10/30 sizing column (Amersham Biosciences AB) using PBS or PNEA, which is composed of 20 mM PIPES [piperazine-N,N′-bis(2-ethanesulfonic acid), pH 7.0], 150 mM NaCl, 1 mM EDTA, and 0.02% NaN3.
Expression and purification of the JSU-IgG.
The generation of a plasmid encoding JSU-IgG has been described (36). This plasmid encodes a hybrid protein consisting of the JSRV Env SU fused to a human IgG Fc domain. The plasmid was transfected into 293 human embryonic kidney cells, and the cells were grown for 24 to 36 h in Dulbecco's modified Eagle's medium with 10% Ultra-Low IgG fetal bovine serum (FBS) (Gibco). Conditioned medium was harvested and added to a column containing protein A Sepharose resin (Amersham Biosciences AB), the column was washed with 10 column volumes of 100 mM sodium citrate-0.02% NaN3 buffer, pH 4.5, and the JSU-IgG protein was eluted with 2 column volumes of the same buffer adjusted to pH 3.5. The eluate was washed into PNEA by ultrafiltration (Amicon Ultra-15 modules; Millipore).
Hyaluronidase assay.
Hyaluronidase activity was assayed by incubation of protein samples with hyaluronan and analysis of digestion products by agarose gel electrophoresis, as previously described (47). Briefly, 50-μg samples of hyaluronan derived from human umbilical cord (Calbiochem) were treated with either purified sHyal2 or Spam1 (bovine testes; Calbiochem) for 12 to 16 h at 37°C in a reaction buffer containing 100 mM formate (for pH 3 to 5.1), 100 mM MES (morpholineethanesulfonic acid) (for pH 5.5 to 7.3), or 100 mM Tris (for pH 7.8 to 11). The samples were mixed with 1/6 volume of 7× loading buffer (7× TAE [40 mM Tris-acetate, 1 mM EDTA, pH 8.0], 85% glycerol) and electrophoresed in 0.5% TAE-agarose gels at 50 V for 8 to 10 h. A 1-kb DNA ladder (Invitrogen) was used as a molecular weight standard. The gels were stained in 0.005% Stains-All (Sigma) in 50% ethanol overnight and then photographed on a fluorescent light transilluminator.
Biosensor analysis of the interaction between JSU-IgG and sHyal2.
SPR measurements were carried out in HBS-EP buffer (10 mM HEPES [pH 7.4], 150 mM NaCl, 3 mM EDTA, 0.005% P-20 surfactant [Biacore AB, Uppsala, Sweden]) using a Biacore 3000 system (Biacore AB). JSU-IgG was immobilized on a CM5 research-grade sensor chip (Biacore AB) by amine coupling chemistry by using the manufacturer's protocols. Immobilization of 3,700 response units (RU) resulted in optimal responses for subsequent analyses.
The kinetics of the interaction between JSU-IgG and sHyal2 were measured by injecting five concentrations of sHyal2 (5 to 74 nM) in randomized duplicate runs. Optimal regeneration was achieved by injection of 3 M KSCN at a flow rate of 100 μl/min for 30 s over the biosensor surface followed by injection of HBS-EP buffer at 20 μl/min for 3 h. Injections of sHyal2 and control solutions were carried out at 20 μl/min for a period of 200 s followed by an hour-long dissociation phase.
The data were analyzed by the method described by Myszka (43). First, the sHyal2 binding signal measured on the biosensor reference surface (capped by amine coupling reagents) was subtracted from the sHyal2 binding signal obtained on the surface derivatized by the JSU-IgG. Next, a data set was constructed by averaging data obtained from several HBS-EP injections over the biosensor surface. This averaged data set was then subtracted from the referenced signal, resulting in doubly referenced data which were then analyzed with BIAevaluation 3.0 software (Biacore AB) to globally fit data and derive kinetic and equilibrium values describing the intermolecular interactions.
RESULTS
Expression and purification of sHyal2.
During maturation, the carboxy end of Hyal2 is removed and replaced with a GPI anchor that tethers Hyal2 to the cell surface. To make a soluble form of the protein (sHyal2), we made an expression vector that encodes Hyal2 with a His6 tag replacing the GPI anchor (Fig. 1A). The native signal peptide was retained in the construct to allow proper posttranslational processing and secretion from cells. sHyal2 was expressed in insect cells by using a recombinant baculovirus expression system and was purified with a combination of immobilized metal affinity and size exclusion chromatography. The identity of the protein was verified by N-terminal sequencing (data not shown). sHyal2 is a monomeric protein in solution (Fig. 1B). A size consistent with the predicted molecular mass of 50 kDa was apparent by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) analysis (Fig. 1C), indicating low utilization of the four predicted N-linked glycosylation sites in this construct. Matrix-assisted laser desorption ionization-time-of-flight (MALDI-TOF) mass spectrometry analysis showed a single 54-kDa species in the purified protein samples. This value was used for subsequent concentration calculations.
Hyaluronidase activity of sHyal2.
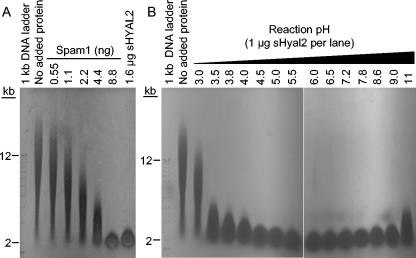
We found that sHyal2 and the known hyaluronidase Spam1 could convert a mixed high-molecular-mass population of hyaluronan to a population with a narrow size range comigrating with the 2-kb DNA marker (Fig. 2A), previously determined to be around 20 kDa (33, 34). Comparison of hyaluronan degradation by 1.6 and 1.0 μg of sHyal2 (Fig. 2A and B, lane corresponding to pH 3.8, respectively) to that by known amounts of Spam1 led to the conclusion that approximately 200-fold more sHyal2 than Spam1 is required to digest 50 μg of hyaluronan to the 20-kDa stage in the same period of time. Therefore sHyal2 is ∼200-fold less active than Spam1 at this pH (pH 3.8). The pH optimum of the sHyal2 hyaluronidase activity was broad, with an optimum centered around pH 5.5 (Fig. 2B). In a control reaction, incubation of hyaluronan at pH 3.8 or 11.0 without added protein did not result in degradation of the polymer (Fig. 2B and data not shown, respectively).
FIG. 2.
sHyal2 is a weak neutral-pH-active hyaluronidase. (A) Fifty-microgram samples of HA were incubated at 37°C and pH 3.8 for 14 h in a total volume of 40 μl with no added protein, with various amounts of sperm hyaluronidase Spam1, or with 1.6 μg of sHyal2. The samples were separated in 0.5% agarose and visualized with Stains-All. (B) Fifty-microgram samples of HA were incubated at 37°C for 14 h in a total volume of 40 μl with no added protein at pH 3.8 or with sHyal2 (1.0 μg per sample) at the indicated pH values. Samples were analyzed as in panel A.
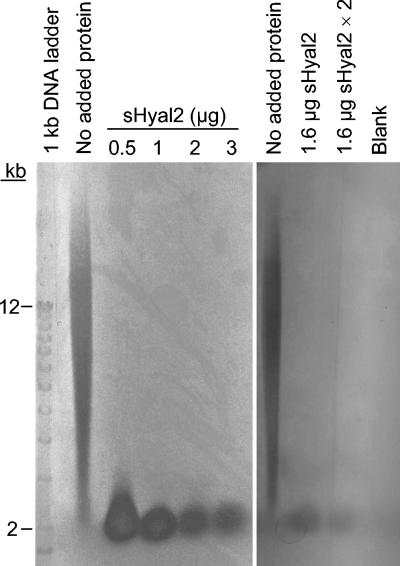
In contrast to previous results which indicated that Hyal2 cleaved hyaluronan to a limit product of 20 kDa (34), we find that degradation of hyaluronan by sHyal2 can proceed beyond this intermediate. Digestion of hyaluronan with increasing concentrations of sHyal2 at the optimal pH of 5.5 resulted in increasing degradation of the 20-kDa intermediate (Fig. 3, left panel), similar to results obtained with Spam1 (47; data not shown). Further degradation of the 20-kDa hyaluronan intermediate was also observed after an additional treatment with sHyal2 after the initial incubation period (Fig. 3, right panel). Degradation of this 20-kDa population was a much slower reaction step than the initial cleavage of high-molecular-mass hyaluronan to the 20-kDa form for both sHyal2 (Fig. 3) and Spam1 (47).
FIG. 3.
sHyal2 is capable of complete digestion of hyaluronan. (Left panel) Fifty-microgram samples of HA were incubated with no added protein or with various amounts of sHyal2 at 37°C and pH 5.5 for 14 h in a total volume of 40 μl. Samples were analyzed by agarose gel electrophoresis as in Fig. 2. (Right panel) To examine the effect of repeated sHyal2 treatment on HA degradation, a 50-μg sample of HA was incubated with 1.6 μg of sHyal2 for 12 h, 1.6 μg of sHyal2 in PNEA buffer was added to the sample, and the sample was incubated for an additional 12 h (lane labeled “1.6 μg sHYAL2 × 2”). In parallel, 50-μg samples of HA were incubated with or without 1.6 μg of sHYAL2 for 12 h, PNEA buffer without sHyal2 was added, and the samples were incubated for an additional 12 h (lanes labeled “1.6 μg sHyal2” and “No added protein”, respectively). Samples were analyzed by agarose gel electrophoresis as in Fig. 2.
Inhibition of JSRV vector transduction by sHyal2.
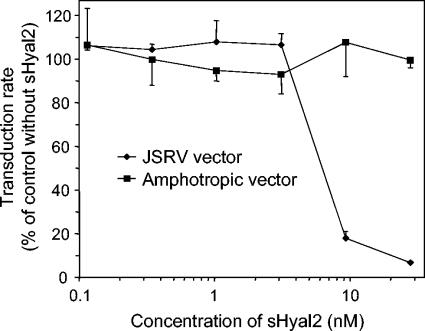
To test whether purified sHyal2 could specifically interact with JSRV-pseudotype retroviral particles, JSRV-pseudotype and amphotropic murine leukemia virus (MLV)-pseudotype vectors were used to infect NIH 3T3/LL2SN cells that express receptors for both viruses, in the presence or absence of sHyal2 (Fig. 4). The presence of 9 nM or more sHyal2 caused a dramatic decrease in titer for the JSRV-pseudotype vector, with 28 nM sHyal2 inhibiting transduction by 93%. In comparison, sHyal2 had no effect on the titer of the control amphotropic MLV-pseudotype vector at any concentration tested. These results demonstrate a specific interaction of sHyal2 with the JSRV Env protein on virions that results in inhibition of virus entry into cells.
FIG. 4.
sHyal2 specifically inhibits transduction by a JSRV-pseudotype retroviral vector. PJ4/LAPSN (similar to PJ14/LAPSN) (46) and PA317/LAPSN (39) vector-producing cell lines were used to generate JSRV-pseudotype and amphotropic MLV-pseudotype vectors. The LAPSN vector encodes human placental alkaline phosphatase (AP) and bacterial neomycin phosphotransferase. These vector stocks were used to transduce NIH 3T3 TK− cells expressing human Hyal2 and that also express the endogenous mouse amphotropic retrovirus receptor Pit2. Experiments were carried out in the presence of various concentrations of purified sHyal2 that was added to the cells just prior to virus addition. Two days after virus exposure, the cells were stained for AP and AP+ foci were counted. Results are expressed as a percentage of the transduction rate observed without sHyal2 addition for each vector. Each data point represents the average of three experiments, and standard deviations are shown.
sHyal2 does not stimulate JSRV vector transduction of receptor-deficient cells.
Others have shown that soluble receptor protein can induce infection of receptor-deficient cells by avian retroviral vectors (13, 30), and we tested whether sHyal2 could induce JSRV vector infection of receptor-deficient NIH 3T3 cells in a similar manner. A JSRV-pseudotype vector was incubated with or without sHyal2 at concentrations from 2 to 1,000 nM for 30 min on ice and cells were exposed to the vector in the presence of Polybrene with or without centrifugation as described previously (13). While the titer of the untreated virus on NIH 3T3 cells expressing human Hyal2 was 7 ×104 per ml, the titer of treated or untreated vector on receptor-deficient NIH 3T3 cells was ≤50 per ml, indicating that sHyal2 cannot efficiently induce infection of receptor-deficient cells.
Analysis of purified JSU-IgG.
JSU-IgG prepared as described in Materials and Methods was analyzed by size exclusion chromatography on a Superdex 200 HR 10/30 column and was found to be highly multimeric (apparent molecular mass of ∼400 kDa) (data not shown). However, nonreducing SDS-PAGE analyses showed that the multimers were likely “daisy chains” of interchain disulfide-bonded protein (data not shown), consistent with the large number of cysteines (15 residues) per JSU-IgG monomer that could provide free thiols for multimerization. However, the purified JSU-IgG multimers appeared to be properly folded and not the result of nonspecific aggregation, because the material was stable in solution and did not precipitate. Flow cytometry experiments also show specific binding of JSU-IgG to cells (36; data not shown), and control SPR experiments demonstrated that JSU-IgG did not interact with other proteins nonspecifically (data not shown).
Binding kinetics of recombinant Hyal2 to the JSU-IgG.
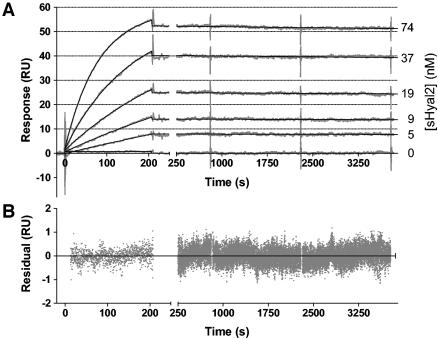
The interaction between Hyal2 and the JSRV Env was further studied by SPR using JSU-IgG covalently bound to the sensor surface (ligand) and sHyal2 in solution flowing over the surface (analyte) (Fig. 5, top panel). The analysis of the SPR responses with a 1:1 Langmuir binding model (Fig. 5) resulted in a good global fit (χ2 = 0.097), with a dissociation constant (KD) value of 32 ± 1 pM. Such tight binding is explained by the extremely slow dissociation of the complex (koff = [5.33 ± 0.07] × 10−6 s−1; kon = [1.68 ± 0.01] × 105 M−1 s−1), corresponding to a complex half-life of ∼36 h. Binding of either Spam1 or soluble JSU-IgG to JSU-IgG coupled to the sensor surface was undetectable, demonstrating the specificity of the interaction of Hyal2 with bound JSU-IgG (data not shown).
FIG. 5.
Measurement of the kinetics of the interaction between sHyal2 and JSU-IgG. (A) Reference-subtracted data (gray) illustrating the interaction between the immobilized JSU-IgG and sHyal2 are shown. Following the initial phase of buffer flow, solutions of indicated concentrations of sHyal2 in buffer were injected during the time interval of 0 to 200 s at a rate of 20 μl/min. This was followed by a return to initial buffer flow to allow for measurements of dissociation of the complexes formed. BIAevaluation software was used to build a curve fit (black line) to the data. (B) Deviations of data from the fit in panel A are shown.
DISCUSSION
In this report we describe the production of a soluble form of the JSRV receptor Hyal2 using a baculovirus expression system. Since Hyal2 is normally tethered to the cell membrane by a GPI anchor, we generated sHyal2 by replacing the same amino acids deleted during GPI anchor addition with a His6 tag. The resulting sHyal2 protein was purified as a 54-kDa monomer with perhaps one N-linked glycosylation.
We used SwissModel (52) to generate a model of the structure of Hyal2 (data not shown), based on the bee venom hyaluronidase structure (38; PDB accession code 1FCQ), encompassing human Hyal2 residues 32 to 355. This region includes two of the four potential N-linked glycosylation sites, one of which is predicted to be fully buried in the model, while the other is partially buried. Thus, a likely explanation for the underutilization of N-linked glycosylation sites observed for sHyal2 is that these sites are insufficiently exposed on the molecular surface for proper modification.
Our modeling efforts also revealed that the pattern of primary sequence conservation between Hyal2 and bee venom hyaluronidase is consistent with conservation of the overall fold. In particular, the predicted catalytic residues of Hyal2 were appropriately mapped to the active site of the bee venom hyaluronidase structure. Residues 355 to 447 fall outside of the model, due to the absence of corresponding sequences in bee venom hyaluronidase, and may represent a separate, cysteine-rich C-terminal domain.
Hyal2 was previously reported to be a functional lysosomal hyaluronidase with an acidic pH optimum (34). A subsequent study found that Hyal2 was actually a cell surface protein, but hyaluronidase activity could not be detected in cells expressing Hyal2 following retrovirus-mediated gene transduction (47). Here we find that sHyal2 is indeed able to degrade hyaluronan, but the rate of degradation is about 200-fold lower that that of Spam1. This result is consistent with the previous finding that hyaluronidase activity is below the limit of detection in lysates of cells transduced with a Hyal2-expressing retroviral vector. Further, we found that sHyal2 is active over the pH range of 4.5 to 8.6 (Fig. 2B), but unlike the neutral-pH-active Spam1 (23; data not shown), it shows no significant activity minimum at around pH 5.5. The broad pH range of the hyaluronidase activity of sHyal2 including normal physiological conditions is consistent with Hyal2 being active at the cell surface where it is normally localized.
sHyal2-mediated hyaluronan degradation proceeds in a manner like that previously observed for Spam1 and Hyal1 (47; unpublished data). The reaction first transforms a population of hyaluronan of highly heterogeneous molecular mass to a population previously estimated to be ∼20 kDa (33, 34). This initial rapid degradation is followed by a slower degradation step in which the 20-kDa polysaccharide population is further digested (Fig. 3), likely producing end-stage tetramer and hexamer products, as is the case for Spam1 (11, 50). Although it was previously reported that Hyal2-mediated hyaluronan degradation stops at the 20-kDa polysaccharide fragment size (34), it is likely that the amount of enzyme or time of digestion was insufficient to detect digestion of hemagglutinin (HA) past the 20-kDa HA intermediate.
We tested the ability of sHyal2 to specifically inhibit transduction by JSRV-pseudotype MLV particles as a measure of sHyal2 biological activity. sHyal2 is capable of significantly inhibiting JSRV Env-mediated transduction events, while having no effect on an amphotropic vector. This result indicates that sHyal2 can specifically interact with JSRV Env and gives further confirmation that sHyal2 is a biologically active, properly folded protein. Inhibition of viral entry by soluble receptor-derived Env ligands has been demonstrated for at least two other retroviral systems. Soluble CD4 has been shown to inhibit infection by certain strains of HIV, in part by causing shedding of the gp120 surface subunit of HIV Env (40, 41). Similarly, a soluble version of the ASLV-A receptor Tva is capable of inhibiting ASLV-A infection (3), and in this case the mechanism appears to involve triggering of the Env-mediated fusion (14, 26).
Although sHyal2 inhibits transduction of receptor-expressing cells, it does not facilitate transduction of receptor-deficient cells, as has been observed for soluble receptor-like molecules in the ASLV-A and ASLV-B systems (13, 30). It has recently been reported that initiation of the fusogenic program of ASLV can occur at neutral pH (20). It is possible that, unlike ASLV, JSRV requires acidification of its environment for initiation of fusion, which might explain its inability to transduce receptor-deficient cells even in the presence of soluble receptor. Alternatively, the binding of the sHyal2 to the JSRV-pseudotype particles may destabilize the metastable Env glycoprotein to an extent greater than is observed for the ASLV systems, resulting in rapid JSRV Env inactivation.
Using SPR, we found very tight binding of sHyal2 to JSU-IgG, with a 32-pM KD, primarily due to an extremely slow dissociation rate. This KD is significantly different from the previously reported value for binding of JSU-IgG to cells expressing human Hyal2 of 9 ± 6 nM (36), as determined by flow cytometry. This discrepancy may be explained by several factors. Since the state of the JSU-IgG, as discussed in this report, was multimeric, the cytometry experiments formally examined non-1:1 interactions, which were not accounted for in the earlier analysis. The prior analysis also did not take into account the amount of inactive JSU-IgG in such preparations, which would lead to an overestimate of the actual KD. Furthermore, the macroscopic recognition events described in that report took place in the context of the complex environment of the cell surface (glycocalyx), perhaps leading to reduced affinity between JSU-IgG and the cell surface-bound Hyal2. In addition, since Hyal2 is a GPI-anchored protein (47), it is likely to be associated with lipid rafts (6), which would affect the context in which it is presented on the cell surface and may bias its ability to bind ligands. The SPR analysis performed here directly measured the association between two biochemically pure interactors, giving the best estimate of the microscopic equilibrium and rate constants describing the interaction of JSRV Env and Hyal2 in the absence of other factors.
Previously reported affinities for other retroviral Env/receptor pairs span a wide range of values and include 500 nM for the HIV-1 Env/CXCR4 (determined by SPR) (27), a range of 11.6 to 0.83 nM for the HIV-1-CD4 interaction (for citations, see reference 3), values of 17 and 1.5 nM (determined by flow cytometry; references 17 and 62, respectively) and 0.3 nM (determined by enzyme-linked immunosorbent assay [ELISA]) (3) for the ASLV-A Env/soluble recombinant Tva, and 92 pM for the amphotropic MLV Env immunoadhesin/Pit2 (determined by cell-associated radiolabel detection) (32). Thus, the affinity value we are reporting is the strongest Env-receptor interaction observed to date, although it is within an order of magnitude of its closest neighbor.
It has been previously noted that viral interactions with target cell receptors typically display higher affinities than the normal biological interactions in which these cell surface molecules are implicated (59). In our case, the extreme stability of the complex suggests that its formation is essentially irreversible, particularly considering the additional effects of avidity and rebinding due to the higher local concentrations of two membrane-anchored interactors. Such slow dissociation kinetics have been described for multiple types of interactions for which complex dissociation is undesirable. For the interaction between major histocompatibility complex molecules and bound antigenic peptides, complex stability is essential for proper antigen presentation at the cell surface, where complex Koff rates have been estimated at 10−4 s−1 and slower (29, 48). High-affinity antibodies with similar Koff rates (10−5 to 10−4 s−1) have also been described (61), and additional examples exist among the more classical protein-protein interactions such as the VEGF/VEGFR-1 complex (10−5 s−1) (58) and the pepsin/rSQAPI complex (10−4 s−1) (22).
The high affinity of JSU-IgG for sHyal2 and the resultant long half-life of the complex indicates that JSRV Env is highly proficient in its ability to anchor incoming virus particles onto target cells expressing human Hyal2, and presumably onto cells expressing sheep Hyal2 as well. One reason this might be important for the biology of JSRV is that this enhanced attachment function may reduce the impact of environmental stresses that JSRV normally has to confront during infection of the lung. In fact, it has been reported that JSRV-pseudotype particles demonstrate greater resistance to the antimicrobial properties of lung fluid (9), which contains compounds with surfactant- and detergent-like activities. If the exposure of virus to lung fluid causes damage to most of the exposed Env glycoprotein molecules, the remainder may still successfully anchor the particle to the host cell by virtue of their strong interaction with the Hyal2 receptor and allow fusion to occur.
Another way in which the high-affinity interaction between JSRV Env and Hyal2 may be important for JSRV is the ability to utilize low levels of available surface Hyal2. The relative abundance of surface Hyal2 in cells of the lung epithelium or established cell lines has not been reported to date. However, it has been recently demonstrated that overexpression of endogenous JSRV-derived envelope glycoprotein can interfere with exogenous JSRV infection in sheep cells (55). Since this study also found that endogenous JSRV is expressed in ovine tissues, it was argued that this may offer a degree of resistance to natural exogenous JSRV infection by lowering the surface levels of Hyal2. Thus, the stability of the JSU-IgG/sHyal2 complex may offer JSRV the ability to compete for surface receptor “occupied” by the endogenous JSRV Env or may suggest that few interactions with its receptor are required for stable JSRV adhesion to host cell surface.
Finally, the extremely high stability of the JSU-IgG/sHyal2 complex may help explain the oncogenic properties that JSRV Env displays in epithelial cells. It has been reported that JSRV Env expression can disrupt the repressed state of the RON tyrosine kinase by causing the degradation of its natural repressor, Hyal2 (15). In its active state, RON is capable of initiating a ligand-independent signaling cascade which leads to cell transformation. It is proposed that the degradation of Hyal2 results from the formation of a stable complex between it and JSRV Env. Data presented here show that this complex is indeed very stable.
Acknowledgments
We thank Deb McMillen (Genomics & Proteomics Facility, University of Oregon) for performing the N-terminal sequencing of sHyal2 and Phil Gafken (Proteomics, Fred Hutchinson Cancer Research Center) for performing the MALDI-TOF analysis.
This work was supported by National Cancer Institute training grant CA77116 (V.V.), grant AI48675 (R.K.S.), and grants DK47754 and HL66947 (A.D.M.) from the National Institutes of Health.
REFERENCES
- 1.Alberti, A., C. Murgia, S.-L. Liu, M. Mura, C. Cousens, M. Sharp, A. D. Miller, and M. Palmarini. 2002. Envelope-induced cell transformation by ovine betaretroviruses. J. Virol. 76:5387-5394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Allen, T. E., K. J. Sherrill, S. M. Crispell, M. R. Perrott, J. O. Carlson, and J. C. DeMartini. 2002. The jaagsiekte sheep retrovirus envelope gene induces transformation of the avian fibroblast cell line DF-1 but does not require a conserved SH2 binding domain. J. Gen. Virol. 83:2733-2742. [DOI] [PubMed] [Google Scholar]
- 3.Balliet, J. W., J. Berson, C. M. D'Cruz, J. Huang, J. Crane, J. M. Gilbert, and P. Bates. 1999. Production and characterization of a soluble, active form of Tva, the subgroup A avian sarcoma and leukosis virus receptor. J. Virol. 73:3054-3061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barnett, A. L., and J. M. Cunningham. 2001. Receptor binding transforms the surface subunit of the mammalian C-type retrovirus envelope protein from an inhibitor to an activator of fusion. J. Virol. 75:9096-9105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Battini, J.-L., P. Rodrigues, R. Muller, O. Danos, and J. M. Heard. 1996. Receptor-binding properties of a purified fragment of the 4070A amphotropic murine leukemia virus envelope glycoprotein. J. Virol. 70:4387-4393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Benting, J. H., A. G. Rietveld, and K. Simons. 1999. N-glycans mediate the apical sorting of a GPI-anchored, raft-associated protein in Madin-Darby canine kidney cells. J. Cell Biol. 146:313-320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brojatsch, J., J. Naughton, M. M. Rolls, K. Zingler, and J. A. Young. 1996. CAR1, a TNFR-related protein, is a cellular receptor for cytopathic avian leukosis-sarcoma viruses and mediates apoptosis. Cell 87:845-855. [DOI] [PubMed] [Google Scholar]
- 8.Bullough, P. A., F. M. Hughson, J. J. Skehel, and D. C. Wiley. 1994. Structure of influenza haemagglutinin at the pH of membrane fusion. Nature 371:37-43. [DOI] [PubMed] [Google Scholar]
- 9.Coil, D. A., J. H. Strickler, S. K. Rai, and A. D. Miller. 2001. Jaagsiekte sheep retrovirus Env protein stabilizes retrovirus vectors against inactivation by lung surfactant, centrifugation, and freeze-thaw cycling. J. Virol. 75:8864-8867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Colman, P. M., and M. C. Lawrence. 2003. The structural biology of type I viral membrane fusion. Nat. Rev. Mol. Cell Biol. 4:309-319. [DOI] [PubMed] [Google Scholar]
- 11.Cramer, J. A., L. C. Bailey, C. A. Bailey, and R. T. Miller. 1994. Kinetic and mechanistic studies with bovine testicular hyaluronidase. Biochim. Biophys. Acta 1200:315-321. [DOI] [PubMed] [Google Scholar]
- 12.Csoka, A. B., G. I. Frost, and R. Stern. 2001. The six hyaluronidase-like genes in the human and mouse genomes. Matrix Biol. 20:499-508. [DOI] [PubMed] [Google Scholar]
- 13.Damico, R., and P. Bates. 2000. Soluble receptor-induced retroviral infection of receptor-deficient cells. J. Virol. 74:6469-6475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Damico, R. L., J. Crane, and P. Bates. 1998. Receptor-triggered membrane association of a model retroviral glycoprotein. Proc. Natl. Acad. Sci. USA 95:2580-2585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Danilkovitch-Miagkova, A., F. M. Duh, I. Kuzmin, D. Angeloni, S. L. Liu, A. D. Miller, and M. I. Lerman. 2003. Hyaluronidase 2 negatively regulates RON receptor tyrosine kinase and mediates transformation of epithelial cells by jaagsiekte sheep retrovirus. Proc. Natl. Acad. Sci. USA 100:4580-4585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Davey, R. A., C. A. Hamson, J. J. Healey, and J. M. Cunningham. 1997. In vitro binding of purified murine ecotropic retrovirus envelope surface protein to its receptor, MCAT-1. J. Virol. 71:8096-8102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Delos, S. E., and J. M. White. 2000. Critical role for the cysteines flanking the internal fusion peptide of avian sarcoma/leukosis virus envelope glycoprotein. J. Virol. 74:9738-9741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dimitrov, D. S., K. Hillman, J. Manischewitz, R. Blumenthal, and H. Golding. 1992. Kinetics of soluble CD4 binding to cells expressing human immunodeficiency virus type 1 envelope glycoprotein. J. Virol. 66:132-138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dirks, C., F.-M. Duh, S. K. Rai, M. I. Lerman, and A. D. Miller. 2002. Mechanism of cell entry and transformation by enzootic nasal tumor virus. J. Virol. 76:2141-2149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Earp, L. J., S. E. Delos, R. C. Netter, P. Bates, and J. M. White. 2003. The avian retrovirus avian sarcoma/leukosis virus subtype A reaches the lipid mixing stage of fusion at neutral pH. J. Virol. 77:3058-3066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Eckert, D. M., and P. S. Kim. 2001. Mechanisms of viral membrane fusion and its inhibition. Annu. Rev. Biochem. 70:777-810. [DOI] [PubMed] [Google Scholar]
- 22.Farley, P. C., J. T. Christeller, M. E. Sullivan, P. A. Sullivan, and W. A. Laing. 2002. Analysis of the interaction between the aspartic peptidase inhibitor SQAPI and aspartic peptidases using surface plasmon resonance. J. Mol. Recognit. 15:135-144. [DOI] [PubMed] [Google Scholar]
- 23.Frost, G. I., T. B. Csoka, T. Wong, and R. Stern. 1997. Purification, cloning, and expression of human plasma hyaluronidase. Biochem. Biophys. Res. Commun. 236:10-15. [DOI] [PubMed] [Google Scholar]
- 24.Gilbert, J. M., L. D. Hernandez, J. W. Balliet, P. Bates, and J. M. White. 1995. Receptor-induced conformational changes in the subgroup A avian leukosis and sarcoma virus envelope glycoprotein. J. Virol. 69:7410-7415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hernandez, L. D., L. R. Hoffman, T. G. Wolfsberg, and J. M. White. 1996. Virus-cell and cell-cell fusion. Annu. Rev. Cell Dev. Biol. 12:627-661. [DOI] [PubMed] [Google Scholar]
- 26.Hernandez, L. D., R. J. Peters, S. E. Delos, J. A. Young, D. A. Agard, and J. M. White. 1997. Activation of a retroviral membrane fusion protein: soluble receptor-induced liposome binding of the ALSV envelope glycoprotein. J. Cell Biol. 139:1455-1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hoffman, T. L., G. Canziani, L. Jia, J. Rucker, and R. W. Doms. 2000. A biosensor assay for studying ligand-membrane receptor interactions: binding of antibodies and HIV-1 Env to chemokine receptors. Proc. Natl. Acad. Sci. USA 97:11215-11220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jones, P. L., T. Korte, and R. Blumenthal. 1998. Conformational changes in cell surface HIV-1 envelope glycoproteins are triggered by cooperation between cell surface CD4 and co-receptors. J. Biol. Chem. 273:404-409. [DOI] [PubMed] [Google Scholar]
- 29.Khilko, S. N., M. T. Jelonek, M. Corr, L. F. Boyd, A. L. Bothwell, and D. H. Margulies. 1995. Measuring interactions of MHC class I molecules using surface plasmon resonance. J. Immunol. Methods 183:77-94. [DOI] [PubMed] [Google Scholar]
- 30.Knauss, D. J., and J. A. T. Young. 2002. A fifteen-amino-acid TVB peptide serves as a minimal soluble receptor for subgroup B avian leukosis and sarcoma viruses. J. Virol. 76:5404-5410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kozak, S. L., D. C. Siess, M. P. Kavanaugh, A. D. Miller, and D. Kabat. 1995. The envelope glycoprotein of an amphotropic murine retrovirus binds specifically to the cellular receptor/phosphate transporter of susceptible species. J. Virol. 69:3433-3440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kurre, P., H.-P. Kiem, J. Morris, S. Heyward, J.-L. Battini, and A. D. Miller. 1999. Efficient transduction by an amphotropic retrovirus vector is dependent on high-level expression of the cell surface virus receptor. J. Virol. 73:495-500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee, H. G., and M. K. Cowman. 1994. An agarose gel electrophoretic method for analysis of hyaluronan molecular weight distribution. Anal. Biochem. 219:278-287. [DOI] [PubMed] [Google Scholar]
- 34.Lepperdinger, G., B. Strobl, and G. Kreil. 1998. HYAL2, a human gene expressed in many cells, encodes a lysosomal hyaluronidase with a novel type of specificity. J. Biol. Chem. 273:22466-22470. [DOI] [PubMed] [Google Scholar]
- 35.Lerman, M. I., J. D. Minna et al. 2000. The 630-kb lung cancer homozygous deletion region on human chromosome 3p21.3: identification and evaluation of the resident candidate tumor suppressor genes. Cancer Res. 60:6116-6133. [PubMed] [Google Scholar]
- 36.Liu, S.-L., F.-M. Duh, M. I. Lerman, and A. D. Miller. 2003. Role of virus receptor Hyal2 in oncogenic transformation of rodent fibroblasts by sheep betaretrovirus Env proteins. J. Virol. 77:2850-2858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu, S.-L., and A. D. Miller. Transformation of Madin-Darby canine kidney epithelial cells by sheep retrovirus envelope proteins. J. Virol., in press. [DOI] [PMC free article] [PubMed]
- 38.Markovic-Housley, Z., G. Miglierini, L. Soldatova, P. J. Rizkallah, U. Muller, and T. Schirmer. 2000. Crystal structure of hyaluronidase, a major allergen of bee venom. Structure Fold Des. 8:1025-1035. [DOI] [PubMed] [Google Scholar]
- 39.Miller, A. D., and C. Buttimore. 1986. Redesign of retrovirus packaging cell lines to avoid recombination leading to helper virus production. Mol. Cell. Biol. 6:2895-2902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Moore, J. P., J. A. McKeating, W. A. Norton, and Q. J. Sattentau. 1991. Direct measurement of soluble CD4 binding to human immunodeficiency virus type 1 virions: gp120 dissociation and its implications for virus-cell binding and fusion reactions and their neutralization by soluble CD4. J. Virol. 65:1133-1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Moore, J. P., J. A. McKeating, R. A. Weiss, and Q. J. Sattentau. 1990. Dissociation of gp120 from HIV-1 virions induced by soluble CD4. Science 250:1139-1142. [DOI] [PubMed] [Google Scholar]
- 42.Mothes, W., A. L. Boerger, S. Narayan, J. M. Cunningham, and J. A. Young. 2000. Retroviral entry mediated by receptor priming and low pH triggering of an envelope glycoprotein. Cell 103:679-689. [DOI] [PubMed] [Google Scholar]
- 43.Myszka, D. G. 1999. Improving biosensor analysis. J. Mol. Recognit. 12:279-284. [DOI] [PubMed] [Google Scholar]
- 44.Overbaugh, J., A. D. Miller, and M. V. Eiden. 2001. Receptors and entry cofactors for retroviruses include single and multiple transmembrane-spanning proteins as well as newly described glycosylphosphatidylinositol-anchored and secreted proteins. Microbiol. Mol. Biol. Rev. 65:371-389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Palmarini, M., N. Maeda, C. Murgia, C. De-Fraja, A. Hofacre, and H. Fan. 2001. A phosphatidylinositol 3-kinase docking site in the cytoplasmic tail of the Jaagsiekte sheep retrovirus transmembrane protein is essential for envelope-induced transformation of NIH 3T3 cells. J. Virol. 75:11002-11009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rai, S. K., J. C. DeMartini, and A. D. Miller. 2000. Retrovirus vectors bearing jaagsiekte sheep retrovirus Env transduce human cells by using a new receptor localized to chromosome 3p21.3. J. Virol. 74:4698-4704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rai, S. K., F.-M. Duh, V. Vigdorovich, A. Danilkovitch-Miagkova, M. I. Lerman, and A. D. Miller. 2001. Candidate tumor suppressor HYAL2 is a glycosylphosphatidylinositol (GPI)-anchored cell-surface receptor for jaagsiekte sheep retrovirus, the envelope protein of which mediates oncogenic transformation. Proc. Natl. Acad. Sci. USA 98:4443-4448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Roche, P. A., and P. Cresswell. 1990. High-affinity binding of an influenza hemagglutinin-derived peptide to purified HLA-DR. J. Immunol. 144:1849-1856. [PubMed] [Google Scholar]
- 49.Ross, S. R., J. J. Schofield, C. J. Farr, and M. Bucan. 2002. Mouse transferrin receptor 1 is the cell entry receptor for mouse mammary tumor virus. Proc. Natl. Acad. Sci. USA 99:12386-12390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Saitoh, H., K. Takagaki, M. Majima, T. Nakamura, A. Matsuki, M. Kasai, H. Narita, and M. Endo. 1995. Enzymic reconstruction of glycosaminoglycan oligosaccharide chains using the transglycosylation reaction of bovine testicular hyaluronidase. J. Biol. Chem. 270:3741-3747. [DOI] [PubMed] [Google Scholar]
- 51.Sattentau, Q. J., J. P. Moore, F. Vignaux, F. Traincard, and P. Poignard. 1993. Conformational changes induced in the envelope glycoproteins of the human and simian immunodeficiency viruses by soluble receptor binding. J. Virol. 67:7383-7393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schwede, T., J. Kopp, N. Guex, and M. C. Peitsch. 2003. SWISS-MODEL: an automated protein homology-modeling server. Nucleic Acids Res. 31:3381-3385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sharp, J. M., K. W. Angus, E. W. Gray, and F. M. Scott. 1983. Rapid transmission of sheep pulmonary adenomatosis (jaagsiekte) in young lambs. Brief report. Arch. Virol. 78:89-95. [DOI] [PubMed] [Google Scholar]
- 54.Smith, J. G., W. Mothes, S. C. Blacklow, and J. M. Cunningham. 2004. The mature avian leukosis virus subgroup A envelope glycoprotein is metastable, and refolding induced by the synergistic effects of receptor binding and low pH is coupled to infection. J. Virol. 78:1403-1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Spencer, T. E., M. Mura, C. A. Gray, P. J. Griebel, and M. Palmarini. 2003. Receptor usage and fetal expression of ovine endogenous betaretroviruses: implications for coevolution of endogenous and exogenous retroviruses. J. Virol. 77:749-753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Stern, R. 2003. Devising a pathway for hyaluronan catabolism: are we there yet? Glycobiology 13:105R-115R. [DOI] [PubMed] [Google Scholar]
- 57.Sullivan, N., Y. Sun, Q. Sattentau, M. Thali, D. Wu, G. Denisova, J. Gershoni, J. Robinson, J. Moore, and J. Sodroski. 1998. CD4-induced conformational changes in the human immunodeficiency virus type 1 gp120 glycoprotein: consequences for virus entry and neutralization. J. Virol. 72:4694-4703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.von Tiedemann, B., and U. Bilitewski. 2002. Characterization of the vascular endothelial growth factor-receptor interaction and determination of the recombinant protein by an optical receptor sensor. Biosens. Bioelectron. 17:983-991. [DOI] [PubMed] [Google Scholar]
- 59.Wang, J. 2002. Protein recognition by cell surface receptors: physiological receptors versus virus interactions. Trends Biochem. Sci. 27:122-126. [DOI] [PubMed] [Google Scholar]
- 60.Wilson, C. A., J. W. Marsh, and M. V. Eiden. 1992. The requirements for viral entry differ from those for virally induced syncytium formation in NIH 3T3/DTras cells exposed to Moloney murine leukemia virus. J. Virol. 66:7262-7269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Xavier, K. A., and R. C. Willson. 1998. Association and dissociation kinetics of anti-hen egg lysozyme monoclonal antibodies HyHEL-5 and HyHEL-10. Biophys. J. 74:2036-2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zingler, K., and J. A. Young. 1996. Residue Trp-48 of Tva is critical for viral entry but not for high-affinity binding to the SU glycoprotein of subgroup A avian leukosis and sarcoma viruses. J. Virol. 70:7510-7516. [DOI] [PMC free article] [PubMed] [Google Scholar]