Abstract
Objectives
We examined mechanisms that contribute to the rapid antidepressant effect of ketamine in mice that is dependent on glycogen synthase kinase-3 (GSK3) inhibition.
Methods
We measured serotonergic (5HT)-2C-receptor (5HTR2C) cluster microRNA (miRNA) levels in mouse hippocampus after administering an antidepressant dose of ketamine (10 mg/kg) in wild-type and GSK3 knockin mice, after GSK3 inhibition with L803-mts, and in learned helpless mice.
Results
Ketamine up-regulated cluster miRNAs 448-3p, 764-5p, 1264-3p, 1298-5p and 1912-3p (2- to 11-fold). This up-regulation was abolished in GSK3 knockin mice that express mutant constitutively active GSK3. The GSK3 specific inhibitor L803-mts was antidepressant in the learned helplessness and novelty suppressed feeding depression-like behaviours and up-regulated the 5HTR2C miRNA cluster in mouse hippocampus. After administration of the learned helplessness paradigm mice were divided into cohorts that were resilient (non-depressed) or were susceptible (depressed) to learned helplessness. The resilient, but not depressed, mice displayed increased hippocampal levels of miRNAs 448-3p and 1264-3p. Administration of an antagonist to miRNA 448-3p diminished the antidepressant effect of ketamine in the learned helplessness paradigm, indicating that up-regulation of miRNA 448-3p provides an antidepressant action.
Conclusions
These findings identify a new outcome of GSK3 inhibition by ketamine that may contribute to antidepressant effects.
Keywords: Ketamine, glycogen synthase kinase-3, microRNA, depression, hippocampus
Introduction
Ketamine produces a rapid antidepressant effect in many patients with major depressive disorder or bipolar disorder that occurs within a time-frame of hours, as opposed to several weeks required for conventional antidepressants (Niciu et al. 2014; Newport et al. 2015; Scheuing et al. 2015). The mechanism underlying this action of ketamine remains to be established, but it has been reported to depend on the inhibition of glycogen synthase kinase-3 (GSK3; Beurel et al. 2011). This is of interest because substantial evidence has implicated abnormally active GSK3 as a contributory factor for mood disorders, and inhibition of GSK3 as contributing to therapeutic actions of mood stabilisers and antidepressants (Jope 2011). GSK3 is primarily regulated by phosphorylation of an N-terminal serine in each of the two isoforms, serine-21 in GSK3α and serine-9 in GSK3β (Beurel et al. 2015). Phosphorylation on these sites inhibits the activity of GSK3, and diminished serine-phosphorylation is thought to contribute to hyperactive GSK3 associated with mood disorders. The functional importance of this mechanism of GSK3 inhibition can be studied using GSK3 knockin mice in which serine-9 of GSK3β and serine-21 of GSK3α are mutated to alanines, thus preventing inhibitory serine-phosphorylation. GSK3 knockin mice reproduce normally and do not display any overt behavioural or morphological phenotypes (McManus et al. 2005), and are physiologically relevant because they express GSK3 at levels that are identical to wild-type mice (Polter et al. 2010). Administration of the antidepressants fluoxetine or imipramine to mice increased the inhibitory serine-phosphorylation of GSK3 in mouse brain (Li et al. 2004). Inhibitory serine-phosphorylation of GSK3 in mouse brain also was found to occur rapidly after administration of ketamine, and the rapid anti-depressant effect of ketamine in mice is dependent on inhibition of GSK3 (Beurel et al. 2011). Also, GSK3 inhibitors enhanced the antidepressant effects of sub-therapeutic levels of ketamine in mice, suggesting that the agents cooperate via a common mechanism (Ghasemi et al. 2010; Liu et al. 2013; Chiu et al. 2014). Furthermore, in patients with depression, ketamine administration increased inhibitory serine-phosphorylation of GSK3 in lymphocytes (Yang et al. 2013). Taken together, these findings demonstrate that GSK3 is inhibited by ketamine and that this may contribute to the antidepressant action of ketamine.
Although inhibition of GSK3 by ketamine is required for the rapid antidepressant effect in mice, it is unclear how this relieves depression. We were interested in the possibility that it may involve regulation of the serotonin (5HT) 2C receptor (5HTR2C). The 5HTR2C has been linked to modulation of mood-relevant behaviours in rodents and suggested to be linked to depression, but this remains controversial and it is unclear what effect antidepressants have on 5HTR2C expression (Van Oekelen et al. 2003; Chagraoui et al. 2016). The 5HTR2C gene is located on the X-chromosome and the 5HTR2C gene locus hosts five intronic microRNAs (miRNAs), 448-3p, 764-5p, 1264-3p, 1298-5p and 1912-3p (Eacker et al. 2011; Hinske et al. 2014). These miRNAs share the same transcriptional direction as the 5HTR2C gene, raising the possibility that their expression may be coordinately regulated with the expression of 5HTR2C messenger RNA (mRNA). The five intronic miRNAs in the 5HTR2C gene are expressed in human brain (Ziats and Rennert 2014) and in mouse brain, where their expression correlates with 5HTR2C expression (Hinske et al. 2014).
Here we report that administration of an anti-depressant dose of ketamine leads to increased expression of the cluster of five intronic miRNAs within the 5HTR2C gene in mouse hippocampus, that this requires ketamine-induced inhibition of GSK3, and that administration of an antagonist to miRNA 448-3p diminished the antidepressant effect of ketamine in the learned helplessness paradigm.
Methods
Mice
Male, adult (8–10 weeks old) C57BL/6 wild-type and homozygous GSK3α/β21A/21A/9A/9A knockin mice (McManus et al. 2005) were used. GSK3 knockin mice develop and reproduce normally with no overt phenotype (McManus et al. 2005; Eom and Jope 2009; Polter et al. 2010). Mice were housed in groups of three to five in standard cages in light and temperature controlled rooms and were treated in accordance with National Institutes of Health and the University of Miami Institutional Animal Care and Use Committee regulations. Mice were treated intraperitoneally (i.p.) with vehicle, ketamine (10 mg/kg), 2,3-dihdroxyl-6-nitro-7-sulfamoylbenzo(f)quinoxaline-2, 3-dione (NBQX, 10 mg/kg; Tocris) or fluoxetine (20 mg/kg; National Institute of Mental Health Chemical Synthesis and Drug Supply Program). The specific GSK3 inhibitor L803-mts, a substrate-competitive peptide inhibitor (Plotkin et al. 2003), was dissolved in DDX1 vehicle (128 mM NaCl, 8 mM citric acid, 17 mM Na2HPO4, 0.0005% benzalkonium chloride), using a protocol (60 μg/mouse; intranasal, 24 hr pretreatment) that was effective in previous studies (Kaidanovich-Beilin et al. 2004; Beurel et al. 2013). For miRNA antagonism experiments, 2 and 4 h after ketamine was administered (10 mg/kg; i.p.), mice received a miR448-3p mirVANA inhibitor (5 mg/kg hsa-miR-448 in DDX1; intranasal; 5 μl/nostril in each nostril; Ambion catalogue number MH10520, Thermo Fisher Scientific), which was designed to specifically bind to and inhibit endogenous miRNA-448.
Quantitative real-time polymerase chain reaction
Total RNA from mouse hippocampus and prefrontal cortex was isolated by TRIzol extraction according to the manufacturer’s instructions (Invitrogen). RNA was converted to cDNA using ImProvII reverse transcriptase (Promega) according to the manufacturer’s instructions. Quantitative changes in the mRNA levels were determined by real-time PCR using TaqMan Gene Expression Assays for 5HTR2C (00434127) and 18S (4332641) according to the manufacturer’s instructions. Low-molecular weight RNA fractions were isolated from total RNA using the NanoSep 100K spin column (Pall). Low molecular weight RNA fractions were then concentrated using the RNeasy MinElute spin column (QIAGEN). Low molecular weight RNAs were converted to cDNA using the TaqMan miRNA reverse transcription kit. Quantitative changes in the miRNA levels were determined by real-time PCR using TaqMan miRNA expression assays for miRNAs 193a-3p (002250), 448-3p (001029), 764-5p (002031), 1264-3p (464426), 1298-5p (002861), 1912-3p (464370), 1941-3p (121130) and for the small nuclear RNA U6 (001973), according to the manufacturer’s instructions. Experiments were performed on a 7900 HT Fast (Applied Biosystems) instrument.
Learned helplessness
Inescapable foot shocks were used to induce learned helplessness depression-like behaviour in mice as described previously (Polter et al. 2010; Beurel et al. 2013). Mice were placed inside a Modular Shuttle Box (Med Associates, St. Albans, VT), with the gate between the two chambers closed. One hundred and eighty inescapable foot shocks were delivered at an amplitude of 0.3 mA, at random durations of 6–10 s, with a randomised inter-shock interval of 5–45 s. Twenty-four hours later, depression-like behaviour was tested using the shuttle box by exposing the mice to 30 escapable 0.3-mA foot shocks that lasted for a maximum of 24 s. Latency to escape from these foot shocks was recorded using the MED-PC Data Acquisition software. An escape failure was tallied if a mouse did not escape within the 24-s time limit. Mice with greater than 15 escape failures were defined as having acquired learned helplessness depression-like behaviour.
Novelty suppressed feeding
The novelty suppressed feeding (NSF) test was carried out as previously described (Beurel et al. 2013). Mice were weighed prior to and after a 24-h period of food deprivation to assure that body weight loss was equal between groups. During testing mice were allowed to explore a novel open field arena with a food pellet placed in the centre on a small platform for a maximum of 10 min. Latency to begin feeding on the pellet was recorded. Mice were then returned to their home cages, and latency to feed and total food consumed was monitored during a 5-min period in order to control for potential differences in feeding not due to the novel environment.
Statistical analysis
Statistical significance was analysed with a Student’s t-test or one- or two-way analysis of variance (ANOVA) with a Bonferroni post-hoc test using Prism software, and P <.05 was considered significant.
Results
Ketamine treatment up-regulates 5HTR2C mRNA and an associated cluster of five miRNAs
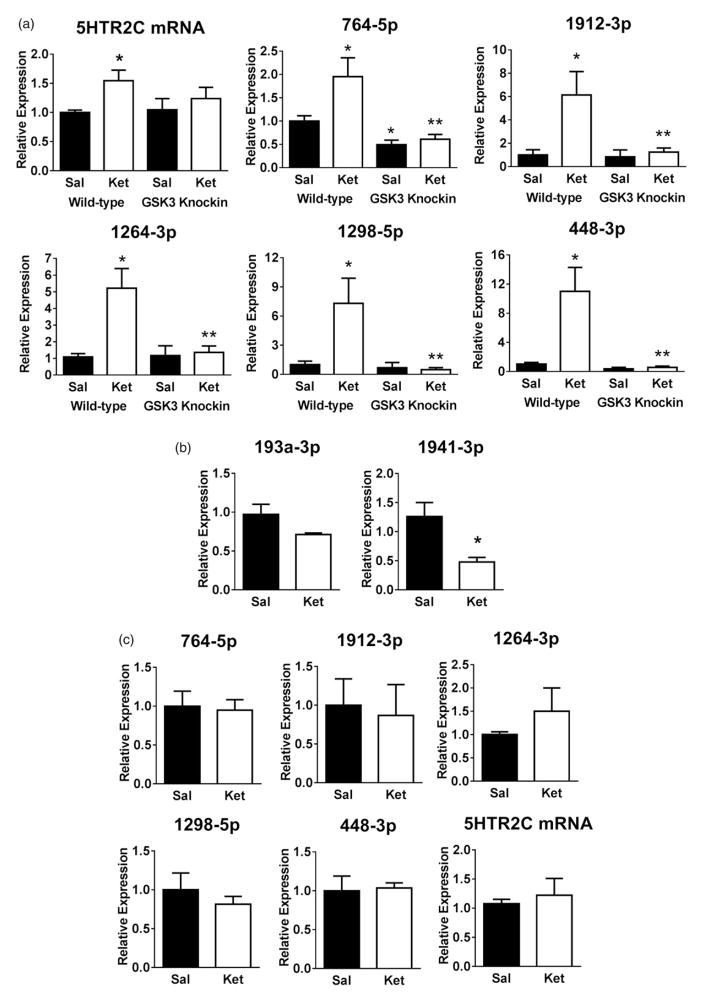
Examination of 5HTR2C mRNA expression 24 h after treatment with a sub-anaesthetic, antidepressant dose of ketamine (10 mg/kg; i.p.), revealed a modest, but significant, increase in 5HTR2C mRNA levels (1.5 ± 0.1-fold of control levels) in mouse hippocampus (Figure 1(a)). We used GSK3 knockin mice, in which the regulatory serines in both isoforms of GSK3 are mutated to alanine to abrogate inhibitory serine-phosphorylation, to test if the regulation of the 5HTR2C mRNA by ketamine requires inhibition of GSK3. This demonstrated that up-regulation of 5HTR2C mRNA induced by ketamine treatment was dependent on inhibition of GSK3 because ketamine treatment did not increase 5HTR2C mRNA levels in the hippocampus of GSK3 knockin mice.
Figure 1.
Ketamine treatment up-regulates 5HTR2C mRNA and the 5HTR2C cluster miRNAs in mouse hippocampus. Wild-type (n = 12–20) and GSK3 knockin mice (n = 6–8) were treated with ketamine (10 mg/kg; i.p.) and were sacrificed after 24 h. (a) Expression levels of 5HTR2C mRNA and 5HTR2C cluster miRNAs (764-5p, 1912-3p, 1264-3p, 1298-5p and 448-3p) in the hippocampus. Data represent means ± SEM (two-way ANOVA (genotype × treatment); 764-5p: Fgenotype(1,40) = 11.40; 1912-3p: Ftreatment(1,38) = 4.330; 1264-3p: Finteraction(1,38) = 6.048; 1298-5p: Finteraction(1,42) = 4.608; 448-3p: Finteraction(1,42) = 6.347; Bonferroni post-hoc test, *P <.05, compared to saline-treated wild-type mice, **P <.05, compared to ketamine-treated wild-type mice). (b) Expression levels of miRNAs 193a-3p and 1941-3p in the hippocampus of wild-type mice. Data represent means ± SEM, n = 3–4 (Student’s t-test; 1941-3p: t(4) = 4.901, *P <.05). (c) Expression levels of 5HTR2C mRNA and the 5HTR2C cluster miRNAs (764-5p, 1912-3p, 1264-3p, 1298-5p and 448-3p) in the prefrontal cortex of wild-type mice (means ± SEM).
Introns in the 5HTR2C gene code for a cluster of five miRNAs (Hinske et al. 2014), which we examined for changes in expression following administration of ketamine. Treatment with ketamine (10 mg/kg; 24 h) significantly increased the levels of all five miRNAs in mouse hippocampus, increasing miRNA 764-5p (2-fold), 1912-3p (6-fold), 1264-3p (5-fold), 1298-5p (7-fold) and 448-3p (11-fold) (Figure 1(a)). Two miRNAs not within the 5HTR2C cluster, 193a-3p and 1941-3p, were unaltered or down-regulated by ketamine treatment (Figure 1(b)), demonstrating selectivity of the response to ketamine. GSK3 knockin mice were used to test if the up-regulation of the 5HTR2C cluster miRNAs by ketamine requires inhibition of GSK3. Without drug treatment, levels of all five 5HTR2C cluster miRNAs were equivalent in the hippocampi of wild-type mice and GSK3 knockin mice except for a lower level of 764-5p in GSK3 knockin mice (Figure 1(a)). The ketamine treatment-induced increases in all five miRNAs were abolished in GSK3 knockin mice, demonstrating the requirement for ketamine-induced inhibition of GSK3 for the miRNAs to be up-regulated. In contrast to the hippocampus, ketamine treatment did not alter 5HTR2C mRNA expression or the levels of the 5HTR2C cluster miRNAs in the pre-frontal cortex (Figure 1(c)). Basal miRNA levels were not significantly different in the hippocampus and the prefrontal cortex (Supplemental Figure 1 available online). Thus, ketamine up-regulates the expression of 5HTR2C mRNA and the 5HTR2C cluster of five miRNAs in mouse hippocampus and these responses are dependent on ketamine-induced inhibition of GSK3.
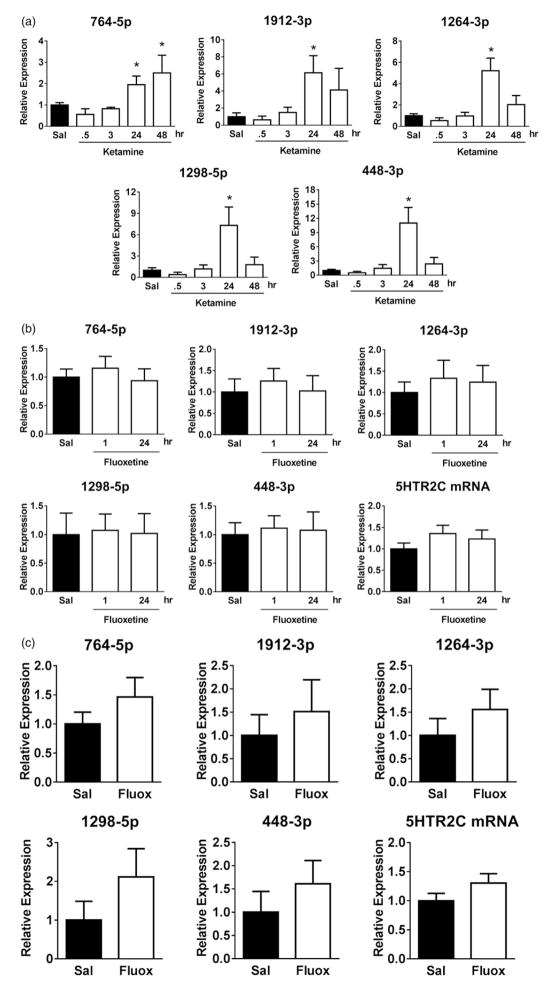
We examined the time-dependence of ketamine-induced up-regulation of the 5HTR2C cluster miRNAs. In the hippocampus, the levels of all five miRNAs did not change 30 min or 3 h after ketamine administration, but were significantly elevated after 24 h, and levels returned towards basal levels after 48 h except for 764-5p, which was still significantly up-regulated at this time (Figure 2(a)). These results demonstrated that miRNA up-regulation was maximal 24 hr after ketamine administration.
Figure 2.
Time-dependence of the effects of ketamine or fluoxetine treatment on the 5HTR2C cluster miRNA expression in mouse hippocampus. (a) Expression levels of 5HTR2C cluster miRNAs (764-5p, 1912-3p, 1264-3p, 1298-5p and 448-3p) in the hippocampus 30 min (n = 4), 3 h (n = 3), 24 h (n = 12) and 48 h (n = 5) after treatment with ketamine (open bars; 10 mg/kg; i.p.) in wild-type mice. Data represent means ± SEM (one-way ANOVA; 764-5p: F(4,38) = 3.856; 1912-3p: F(4,36) = 2.782; 1264-3p: F(4,38) = 6.873; 1298-5p: F(4,40) = 3.649; 448-3p: F(4,40) = 5.765; Bonferroni post-hoc test, *P <.05, compared to saline-treated control mice). (b, c) Expression levels of 5HTR2C mRNA and the 5HTR2C cluster miRNAs in wild-type mice hippocampi (b) 1 or 24 h after acute treatment with fluoxetine (Fluox; open bars; 20 mg/kg; i.p.) (n = 4) and (c) 24 h after 2 weeks of daily treatment with fluoxetine (20 mg/kg; i.p.) (n = 10) (means ± SEM).
In order to determine if the ketamine-induced up-regulation of the 5HTR2C cluster miRNAs was unique or also was a response to a classical antidepressant, we tested the effect of fluoxetine treatment on the 5HTR2C cluster miRNAs. Expression of the 5HTR2C cluster miRNAs did not significantly change either 1 or 24 h after acute fluoxetine treatment (Figure 2(b)) or after 2 weeks of chronic fluoxetine treatment (Figure 2(c)).
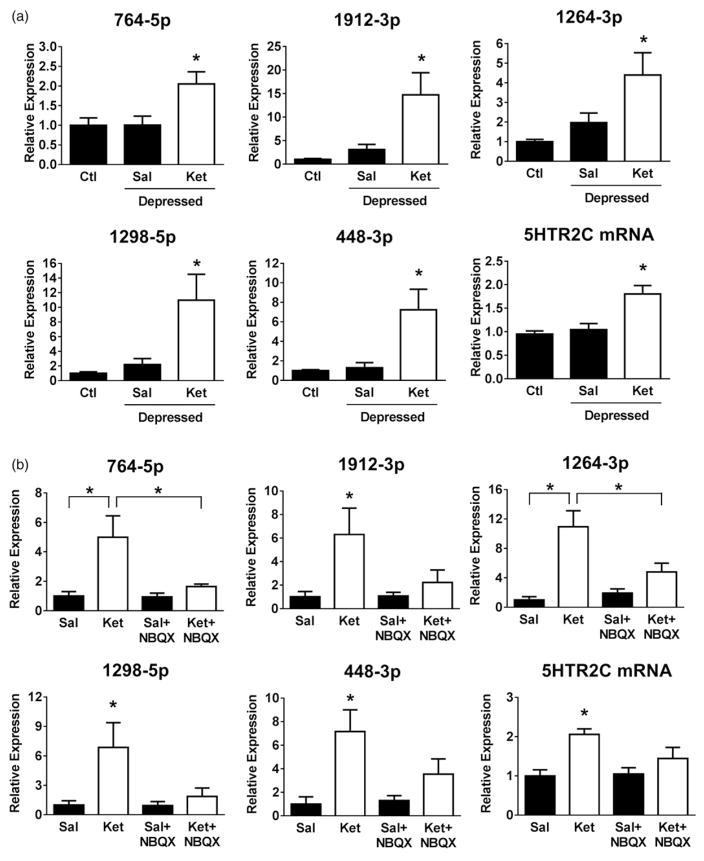
Ketamine is administered therapeutically to patients with depression. Therefore, we tested if ketamine administration up-regulated the cluster miRNAs in mice that were previously rendered depressed by induction of learned helplessness. Indeed, ketamine administration up-regulated expression levels of all five cluster miRNAs and 5HTR2C mRNA in learned helpless mice to a similar extent as occurred in untreated mice (Figure 3(a)).
Figure 3.
Ketamine up-regulates the 5HTR2C miRNA cluster expression. (a) Expression levels of the 5HTR2C cluster miRNAs (764-5p, 1912-3p, 1264-3p, 1298-5p and 448-3p) and 5HTR2C mRNA in the hippocampus 24 h after treatment with ketamine (Ket; 10 mg/kg; i.p.; white bars) or saline (Sal; black bars) that was administered to wild-type mice 48 h after completion of the learned helplessness protocol (Depressed). Data represent means ± SEM, n = 6–10 (one-way ANOVA; 764-5p: F(2,20) = 4.820; 1912-3p: F(2,20) = 4.601; 1264-3p: F(2,20) = 4.587; 1298-5p: F(2,22) = 4.343; 448-3p: F(2,20) = 5.137; Bonferroni post-hoc test, *P <.05, compared to saline-treated control (Ctl, mice). (b) Wild-type mice were pre-treated with NBQX (10 mg/kg; i.p.) or saline (Sal) for 1 h, and treated with saline or ketamine (Ket; 10 mg/kg; i.p.) for 24 hr. Expression levels of the 5HTR2C cluster miRNAs (764-5p, 1912-3p, 1264-3p, 1298-5p and 448-3p) and 5HTR2C mRNA were measured in the hippocampus 24 h after ketamine treatment. The four treatment groups are: saline (Sal), ketamine (Ket.), NBQX, or ketamine plus NBQX (Ket +NBQX). Data represent means ± SEM, n = 4–6 (two-way ANOVA (Treatment(NBQX) × Treatment(Ket)); 764-5p: Finteraction(1,13) = 5.292; 1912-3p: Ftreatment(Ket)(1,15) = 5.925; 1264-3p: Finteraction(1,13) = 7.315; 1298-5p: Ftreatment(Ket)(1,14) = 5.578; 448-3p: Ftreatment(Ket)(1,13) = 10.14; 5HTR2C: Ftreatment(Ket)(1,13) = 14.44; Bonferroni post-hoc test, *P <.05, compared to saline-treated or ketamine-treated mice as indicated).
To test if the miRNA responses to ketamine were mediated by AMPA receptors, mice were pre-treated with the AMPA receptor antagonist NBQX (10 mg/kg) for 1 h followed by administration of a sub-anaesthetic, antidepressant dose of ketamine (10 mg/kg), and measurements of miRNAs 24 h after ketamine treatment. Ketamine significantly up-regulated each of the miRNAs and the 5HTR2C mRNA level, but there was not a significant increase in any of these by ketamine after blockade of AMPA receptors by NBQX (Figure 3(b)), consistent with previous reports that the antidepressant effect of ketamine is mediated by signalling through AMPA receptors (Malinow and Malenka 2002; Maeng et al. 2008; Koike et al. 2011).
Inhibition of GSK3 up-regulates the 5HTR2C cluster miRNAs
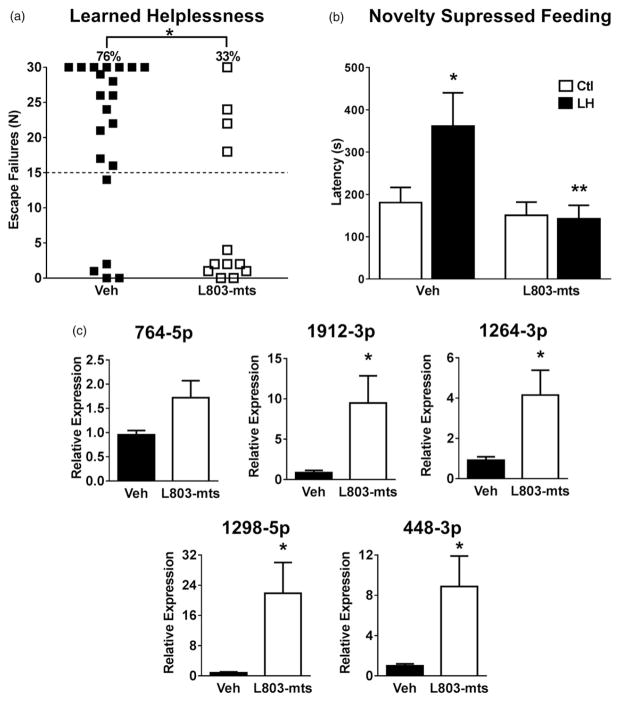
The lack of up-regulation of the 5HTR2C cluster of miRNAs by ketamine in GSK3 knockin mice indicated that inhibition of GSK3 is necessary for this response. To test if inhibition of GSK3 is sufficient to up-regulate the expression of this cluster of miRNAs, we evaluated the effects of selectively inhibiting GSK3 on susceptibility to the learned helplessness and NSF depression-like behaviours and on expression of the 5HTR2C miRNA cluster by administering the specific GSK3 inhibitor L803-mts using a protocol (60 μg/mouse; intranasal, 24 h pretreatment) that was effective in previous studies (Kaidanovich-Beilin et al. 2004; Beurel et al. 2013). In the learned helplessness paradigm, 76% of control mice treated with intranasal administration of vehicle developed learned helplessness, which was reduced to 33% by L803-mts administration (Figure 4(a)). After exposure to the learned helplessness paradigm, vehicle-treated mice had a significantly increased feeding latency in the NSF test when placed in a novel arena containing a food pellet after a 24-h period of food deprivation, whereas L803-mts-treated mice exposed to the learned helplessness paradigm did not (Figure 4(b)). Control measurements found that there were no differences in weight loss or appetite (feeding in the home cage) between the groups of mice (Supplemental Figure 2). Therefore administration of L803-mts confers mice with protection from these two depression-like behaviour models. Administration of L803-mts also increased the levels of the cluster miRNAs to a similar extent as that caused by ketamine administration (Figure 4(c)). Thus, in vivo inhibition of GSK3 is sufficient to recapitulate the ketamine-induced protection from two depression-like behaviours and up-regulation of expression of the 5HTR2C cluster of miRNAs.
Figure 4.
Inhibition of GSK3 attenuates depression-like behaviours and up-regulates expression of the 5HTR2C miRNA cluster. (a) Escape failures in the learned helplessness test for wild-type mice treated with intranasal administration of vehicle (n = 21) or L803-mts (n = 12). Each symbol represents results from an individual mouse. The dashed line at 15 escape failures indicates the criteria for learned helplessness (Student’s t-test; t(31) = 2.958; *P <.05). (b) Latency to feed in the NSF test with (closed bars) or without (open bars) induction of learned helplessness (LH) in wild-type mice treated intranasally with vehicle (Veh) or L803-mts. Data represent means ± SEM, n = 8–10 (two-way ANOVA (LH X treatment); Finteraction(1,32) = 4.181; Bonferroni post-hoc test, *P <.05, compared to the vehicle-treated control mice, **P <.05, compared to vehicle-treated learned helplessness mice). (c) Expression levels of the 5HTR2C cluster miRNAs (764-5p, 1912-3p, 1264-3p, 1298-5p and 448-3p) in the hippocampus from wild-type mice treated intranasally with vehicle (black bars) or L803-mts (white bars). Data represent means ± SEM, n = 4 (Student’s t-test; 1912-3p: t(6) = 2.547; 1264-3p: t(6) = 2.589; 1298-5p: t(6) = 2.578; 448-3p: t(6) = 2.608; *<0.05).
5HTR2C cluster miRNAs expression and learned helplessness
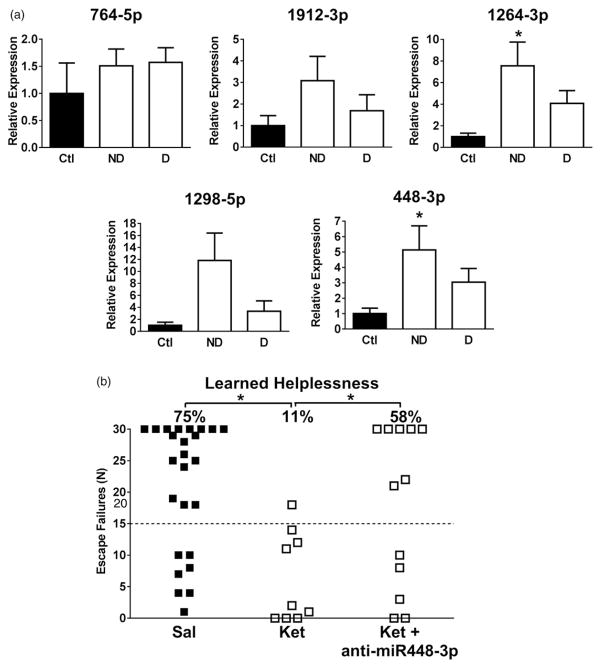
Since administration of an antidepressant dose of ketamine up-regulated the 5HTR2C cluster miRNAs, we tested if their expression levels were altered by the induction of the learned helplessness depression-like behaviour. Mice were exposed to inescapable foot shocks followed 24 h later by exposure to escapable foot shocks, and failure to escape from 15 out of 30 trials of escapable foot shocks is defined as learned helplessness behaviour. Mice were grouped into a resilient group that did not display learned helplessness and a learned helpless group, and mice that were not exposed to foot shocks were used as a control group. Twenty-four hours after the learned helplessness paradigm, mice that were resilient had significant increases in the hippocampal expression of miRNAs 1264-3p (7-fold) and 448-3p (5-fold), but not 764-5p, 1912-3p or 1298-5p compared to control mice (Figure 5(a)). Thus, stress-induced up-regulation of 1264-3p and 448-3p correlated with resistance to learned helplessness, suggesting that up-regulation of these two miRNAs may contribute to ketamine’s anti-depressant effect.
Figure 5.
The 5HTR2C miRNA cluster and susceptibility to learned helplessness. (a) Expression levels of the 5HTR2C cluster miRNAs (764-5p, 1912-3p, 1264-3p, 1298-5p and 448-3p) in the hippocampus 24 h after administration of the learned helplessness protocol in wild-type mice. Mice were divided into two groups, those that were resistant to learned helplessness (non-depressed, ND) and those that developed learned helplessness (depressed, D), and these were compared to control (Ctl) mice that were not subjected to learned helplessness. Data represent means ± SEM, n = 6–12 (one-way ANOVA; 1264-3p: F(2,17) = 7.311; 448-3p: F(2,22) = 5.261; Bonferroni post-hoc test, *P <.05, compared to control mice). (b) Escape failures in the learned helplessness test for wild-type mice treated with saline (Sal; n = 24), ketamine (Ket; n = 9), or ketamine plus a miRNA 448-3p antagonist (n = 12). Each symbol represents results from an individual mouse. The dashed line at 15 escape failures indicates the criteria for learned helplessness (one-way ANOVA; F(2,43) = 6.438; Bonferroni post-hoc test, *P <.05, compared to vehicle-treated or ketamine-treated mice).
To determine if the antidepressant effect of ketamine in mice is dependent upon up-regulation of one of the 5HTR2C miRNAs, we tested if miRNA interference of 448-3p reduced ketamine’s antidepressant effect in the learned helplessness paradigm because it was up-regulated to the greatest extent by ketamine and was up-regulated in resilient mice. Seventy-five percent of control mice treated with vehicle developed learned helplessness and this was reduced to only 11% of mice treated with ketamine. However, co-treatment of ketamine plus a miRNA 448-3p antagonist resulted in 58% of mice developing learned helplessness (Figure 5(b)). Therefore miRNA 448-3p antagonism blocked approximately 73% of ketamine’s antidepressant effect on this depression-like behaviour.
Discussion
Ketamine can induce a rapid antidepressant effect in patients with mood disorders and in mice with depression-like behaviours (Niciu et al. 2014; Newport et al 2015; Scheuing et al. 2015). Although the antidepressant mechanism of action of ketamine has yet to be fully established, several studies have linked it to inhibition of GSK3 (Ghasemi et al. 2010; Beurel et al. 2011; Liu et al. 2013; Yang et al. 2013; Chiu et al. 2014; Zhou et al. 2014). Following up that link, here we found that in mouse hippocampus an antidepressant dose of ketamine up-regulated the expression of a cluster of five miRNAs intronic within the 5HTR2C gene, that this was dependent on inhibition of GSK3 and was matched by administration of a specific GSK3 inhibitor, and that blocking the most highly up-regulated miRNA, 448-3p, diminished the anti-depressant action of ketamine in mice.
Selectivity in the up-regulation of the 5HTR2C cluster miRNAs by ketamine treatment was indicated for three aspects of the response: the miRNA cluster, the hippocampus, and the drug. Two miRNAs, 193a-3p and 1941-3p, outside of the 5HTR2C gene were not up-regulated by ketamine treatment, indicating that the effect of ketamine did not extend to all miRNAs. Brain region selectivity was indicated by the up-regulation of the 5HTR2C miRNA cluster in the hippocampus but not in the prefrontal cortex by ketamine treatment, which is in agreement with several previous studies that emphasised hippocampal responses to ketamine administration (Garcia et al. 2008; Maeng et al. 2008; Autry et al. 2011; Tizabi et al. 2012). Although our measurements were made primarily using hippocampal tissue, these results do not exclude similar effects in other brain regions that may contribute to the response to ketamine. In addition, the classical serotonin-specific re-uptake inhibitor antidepressant fluoxetine did not up-regulate the expression of the 5HTR2C cluster miRNAs, raising the possibility that this response to ketamine may contribute to its antidepressant capacity in patients non-responsive to classical antidepressants.
Expression of 5HTR2C mRNA was also up-regulated in conjunction with the miRNA cluster by treatment with ketamine. The 5HTR2C has been linked to depression in several studies, although its potential regulatory role remains to be firmly established (Chagraoui et al. 2016). Studies in 5HTR2C null mice (Tecott et al. 1995) and 5HTR2C knockin mice (Bombail et al. 2014) may be complicated if some of the phenotypes result in part by altered expression of intronic miRNAs in the 5HTR2C gene. However, since the ketamine-induced up-regulation of 5HTR2C mRNA was modest compared with the up-regulation of the miRNAs we did not pursue further studies to determine if it contributed to the antidepressant action of ketamine.
The up-regulated expression of the cluster of miRNAs induced by ketamine was linked to the inhibition of GSK3 induced by ketamine by two findings. First, up-regulation of the miRNA cluster did not occur in GSK3 knockin mice, in which GSK3 cannot be inhibited by ketamine (McManus et al. 2005). Second, inhibition of GSK3 by administration of the specific inhibitor L803-mts was sufficient to diminish susceptibility to learned helplessness, a reduction in susceptibility equivalent to that caused by acute administration of ketamine (Beurel et al. 2011). This extends several previous reports that administration of GSK3 inhibitors had antidepressant effects in rodent models of depression. Mouse immobility time in the forced swim test was reduced by several inhibitors of GSK3, including L803-mts (Kaidanovich-Beilin et al. 2004; Shapira et al. 2007), CHIR99021 (Pan et al. 2011), AR-A014418 (Gould et al. 2004; Rosa et al. 2008), NP031115 (Rosa et al. 2008), SB216763 (Li et al. 2014) and lithium (O’Brien et al. 2004; Shapira et al. 2007; Silva et al. 2008; Can et al. 2011; Pan et al. 2011), but not by SB216763 (Ma et al. 2013). Mouse immobility time in the tail suspension test was reduced by TDZD-8 (Beaulieu et al. 2008b) and lithium (Beaulieu et al. 2008a), but not by SB216763 (Ma et al. 2013), and lithium reduced mouse escape failures in the learned helplessness test (Beurel et al. 2011; Beurel et al. 2013). Molecular modifications of GSK3 also indicate its inhibition promotes antidepressant effects (reviewed in Jope 2011). L803-mts also was sufficient to up-regulate the expression of the 5HTR2C cluster miRNAs in the hippocampus to an extent similar to that of ketamine administration. Thus, inhibition of GSK3 is both necessary and sufficient to reduce susceptibility to learned helplessness and to increase the expression of the 5HTR2C cluster miRNAs.
The key question of whether the ketamine-induced up-regulation of this cluster of miRNAs contributes to the antidepressant response to ketamine was addressed by several approaches. Ketamine administration up-regulated expression of all five cluster miRNAs in mice that were previously rendered learned helpless, demonstrating that ketamine induces this response not only in untreated mice but also in learned helpless mice, which models the clinical situation better than testing responses in control mice. The peak increase in the expression of the 5HTR2C cluster miRNAs occurred 24 hr after ketamine treatment, which matches the time of the antidepressant effect of ketamine treatment in mice (Maeng et al. 2008; Li et al. 2010; Autry et al. 2011; Beurel et al. 2011; Zanos et al. 2016), raising the possibility that the miRNAs contribute to the antidepressant effect. However, in patients ketamine can produce anti-depressant effects at shorter times, suggesting that either the kinetics may differ between humans and mice, or that up-regulated miRNAs do not have a role in human antidepressant responses to ketamine. Although not addressed here, it is also possible that ketamine-induced miRNA up-regulation is related to the recently described prophylactic effect of ketamine against stress-induced depressive-like behaviours in mice (Brachman et al. 2016). Although ketamine treatment up-regulated all five 5HTR2C cluster miRNAs, there were several differences among their responses. Up-regulation of 764-5p by ketamine was lower than the other miRNAs in the cluster, 764-5p was the only miRNA in the 5HTR2C cluster that was significantly lower in GSK3 knockin mouse hippocampus compared with wild-type mice, and 764-5p was the only miRNA in the 5HTR2C cluster that did not return to basal levels 48 h after ketamine administration. miRNA 448-3p also differed from the other cluster miRNAs in that it displayed the most robust increase after ketamine administration, and is the only miRNA that is in an intronic and coding region of the 5HTR2C gene. Particularly interesting was the finding that after exposure to learned helplessness only miRNAs 448-3p and 1264-3p were up-regulated in resilient mice that exhibited resistance to learned helplessness but not in mice that developed learned helplessness, raising the possibility that one or both of these miRNAs may play a role in promoting resilience. An antidepressant role for miR448-3p was supported by the finding that antagonising 448-3p counteracted the antidepressant effect of ketamine in the learned helplessness model.
In summary, this study found that ketamine up-regulates a cluster of intronic miRNAs associated with the 5HTR2C gene locus in mouse hippocampus. This ketamine-induced up-regulation is dependent on GSK3 inhibition, is matched by administration of a GSK3 inhibitor, and is diminished by antagonism of miRNA 448-3p. Thus, in mice ketamine-induced up-regulation of miR448-3p appears to contribute to the antidepressant response.
Acknowledgments
Funding
National Institute of Mental Health, 10.13039/100000025 [MH038752,MH090236,MH104656] and a grant from National Alliance for Research on Schizophrenia and Depression (NARSAD).
Footnotes
Disclosure statement
The authors report no conflict of interest.
References
- Autry AE, Adachi M, Nosyreva E, Na ES, Los MF, Cheng PF, Kavalali ET, Monteggia LM. NMDA receptor blockade at rest triggers rapid behavioural antidepressant responses. Nature. 2011;475:91–95. doi: 10.1038/nature10130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaulieu JM, Marion S, Rodriguiz RM, Medvedev IO, Sotnikova TD, Ghisi V, Wetsel WC, Lefkowitz RJ, Gainetdinov RR, Caron MG. A beta-arrestin 2 signaling complex mediates lithium action on behavior. Cell. 2008a;132:125–136. doi: 10.1016/j.cell.2007.11.041. [DOI] [PubMed] [Google Scholar]
- Beaulieu JM, Zhang X, Rodriguiz RM, Sotnikova TD, Cools MJ, Wetsel WC, Gainetdinov RR, Caron MG. Role of GSK3 beta in behavioral abnormalities induced by serotonin deficiency. Proc Natl Acad Sci USA. 2008b;105:1333–1338. doi: 10.1073/pnas.0711496105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beurel E, Song L, Jope RS. Inhibition of glycogen synthase kinase-3 is necessary for the rapid antidepressant effect of ketamine in mice. Mol Psychiatry. 2011;16:1068–1070. [Google Scholar]
- Beurel E, Kaidanovich-Beilin O, Yeh WI, Song L, Palomo V, Michalek SM, Woodgett JR, Harrington LE, Eldar-Finkelman H, Martinez A, et al. Regulation of Th1 cells and experimental autoimmune encephalomyelitis by glycogen synthase kinase-3. J Immunol. 2013;190:5000–5011. doi: 10.4049/jimmunol.1203057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beurel E, Harrington LE, Jope RS. Inflammatory T helper 17 cells promote depression-like behavior in mice. Biol Psychiatry. 2013;73:622–630. doi: 10.1016/j.biopsych.2012.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beurel E, Grieco SF, Jope RS. Glycogen synthase kinase-3 (GSK3): regulation, actions, and diseases. Pharmacol Ther. 2015;148:114–131. doi: 10.1016/j.pharmthera.2014.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bombail V, Qing W, Chapman KE, Holmes MC. Prevention of 5-hydroxytryptamine2C receptor RNA editing and alternate splicing in C57BL/6 mice activates the hypothalamic-pituitary-adrenal axis and alters mood. Eur J Neurosci. 2014;40:3663–3673. doi: 10.1111/ejn.12727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brachman RA, McGowan JC, Perusini JN, Lim SC, Pham TH, Faye C, Gardier AM, Mendez-David I, David DJ, Hen R, et al. Ketamine as a prophylactic against stress-induced depressive-like behavior. Biol Psychiatry. 2016;79:776–786. doi: 10.1016/j.biopsych.2015.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Can A, Blackwell RA, Piantadosi SC, Dao DT, O’Donnell KC, Gould TD. Antidepressant-like responses to lithium in genetically diverse mouse strains. Genes Brain Behav. 2011;10:434–443. doi: 10.1111/j.1601-183X.2011.00682.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chagraoui A, Thibaut F, Skiba M, Thuillez C, Bourin M. 5-HT2C receptors in psychiatric disorders: a review. Prog Neuropsychopharmacol Biol Psychiatry. 2016;66:120–135. doi: 10.1016/j.pnpbp.2015.12.006. [DOI] [PubMed] [Google Scholar]
- Chiu CT, Scheuing L, Liu G, Liao HM, Linares GR, Lin D, Chuang DM. The mood stabilizer lithium potentiates the antidepressant-like effects and ameliorates oxidative stress induced by acute ketamine in a mouse model of stress. Int J Neuropsychopharmacol. 2014;18 doi: 10.1093/ijnp/pyu102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eacker SM, Keuss MJ, Berezikov E, Dawson VL, Dawson TM. Neuronal activity regulates hippocampal miRNA expression. PLoS One. 2011;6:e25068. doi: 10.1371/journal.pone.0025068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eom TY, Jope RS. Blocked inhibitory serine-phosphorylation of glycogen synthase kinase-3alpha/beta impairs in vivo neural precursor cell proliferation. Biol Psychiatry. 2009;66:494–502. doi: 10.1016/j.biopsych.2009.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia LS, Comim CM, Valvassori SS, Réus GZ, Barbosa LM, Andreazza AC, Stertz L, Fries GR, Gavioli EC, Kapczinski F, et al. Acute administration of ketamine induces anti-depressant-like effects in the forced swimming test and increases BDNF levels in the rat hippocampus. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32:140–144. doi: 10.1016/j.pnpbp.2007.07.027. [DOI] [PubMed] [Google Scholar]
- Ghasemi M, Raza M, Dehpour AR. NMDA receptor antagonists augment antidepressant-like effects of lithium in the mouse forced swimming test. J Psychopharmacol (Oxford) 2010;24:585–594. doi: 10.1177/0269881109104845. [DOI] [PubMed] [Google Scholar]
- Gould TD, Einat H, Bhat R, Manji HK. AR-A014418, a selective GSK-3 inhibitor, produces antidepressant-like effects in the forced swim test. Int J Neuropsychopharmacol. 2004;7:387–390. doi: 10.1017/S1461145704004535. [DOI] [PubMed] [Google Scholar]
- Hinske LC, França GS, Torres HA, Ohara DT, Lopes-Ramos CM, Heyn J, Reis LF, Ohno-Machado L, Kreth S, Galante PA. miRIAD-integrating microRNA inter- and intragenic data. Database (Oxford) 2014;2014:bau099. doi: 10.1093/database/bau099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jope RS. Glycogen synthase kinase-3 in the etiology and treatment of mood disorders. Front Mol Neurosci. 2011;4:16. doi: 10.3389/fnmol.2011.00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaidanovich-Beilin O, Milman A, Weizman A, Pick CG, Eldar-Finkelman H. Rapid antidepressive-like activity of specific glycogen synthase kinase-3 inhibitor and its effect on beta-catenin in mouse hippocampus. Biol Psychiatry. 2004;55:781–784. doi: 10.1016/j.biopsych.2004.01.008. [DOI] [PubMed] [Google Scholar]
- Koike H, Iijima M, Chaki S. Involvement of AMPA receptor in both the rapid and sustained antidepressant-like effects of ketamine in animal models of depression. Behav Brain Res. 2011;224:107–111. doi: 10.1016/j.bbr.2011.05.035. [DOI] [PubMed] [Google Scholar]
- Li G, Liu T, Kong X, Wang L, Jin X. Hippocampal glycogen synthase kinase 3β is critical for the antidepressant effect of cyclin-dependent kinase 5 inhibitor in rats. J Mol Neurosci. 2014;54:92–99. doi: 10.1007/s12031-014-0254-2. [DOI] [PubMed] [Google Scholar]
- Li X, Zhu W, Roh MS, Friedman AB, Rosborough K, Jope RS. In vivo regulation of glycogen synthase kinase-3beta (GSK3beta) by serotonergic activity in mouse brain. Neuropsychopharmacology. 2004;29:1426–1431. doi: 10.1038/sj.npp.1300439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li N, Lee B, Liu RJ, Banasr M, Dwyer JM, Iwata M, Li XY, Aghajanian G, Duman RS. mTOR-dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists. Science. 2010;329:959–964. doi: 10.1126/science.1190287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu RJ, Fuchikami M, Dwyer JM, Lepack AE, Duman RS, Aghajanian GK. GSK-3 inhibition potentiates the synaptogenic and antidepressant-like effects of subthreshold doses of ketamine. Neuropsychopharmacology. 2013;38:2268–2277. doi: 10.1038/npp.2013.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma XC, Dang YH, Jia M, Ma R, Wang F, Wu J, Gao CG, Hashimoto K. Long-lasting antidepressant action of ketamine, but not glycogen synthase kinase-3 inhibitor SB216763, in the chronic mild stress model of mice. PLoS One. 2013;8:e56053. doi: 10.1371/journal.pone.0056053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeng S, Zarate CA, Jr, Du J, Schloesser RJ, McCammon J, Chen G, Manji HK. Cellular mechanisms underlying the antidepressant effects of ketamine: role of alpha-amino-3-hydroxy-5-methylisoxazole-4-propionic acid receptors. Biol Psychiatry. 2008;63:349–352. doi: 10.1016/j.biopsych.2007.05.028. [DOI] [PubMed] [Google Scholar]
- Malinow R, Malenka RC. AMPA receptor trafficking and synaptic plasticity. Annu Rev Neurosci. 2002;25:103–126. doi: 10.1146/annurev.neuro.25.112701.142758. [DOI] [PubMed] [Google Scholar]
- McManus EJ, Sakamoto K, Armit LJ, Ronaldson L, Shpiro N, Marquez R, Alessi DR. Role that phosphorylation of GSK3 plays in insulin and Wnt signalling defined by knockin analysis. Embo J. 2005;24:1571–1583. doi: 10.1038/sj.emboj.7600633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newport DJ, Carpenter LL, McDonald WM, Potash JB, Tohen M, Nemeroff CB. Ketamine and other NMDA antagonists: early clinical trials and possible mechanisms in depression. Am J Psychiatry. 2015;172:950–966. doi: 10.1176/appi.ajp.2015.15040465. [DOI] [PubMed] [Google Scholar]
- Niciu MJ, Henter ID, Luckenbaugh DA, Zarate CA, Jr, Charney DS. Glutamate receptor antagonists as fast-acting therapeutic alternatives for the treatment of depression: ketamine and other compounds. Annu Rev Pharmacol Toxicol. 2014;54:119–139. doi: 10.1146/annurev-pharmtox-011613-135950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Brien WT, Harper AD, Jové F, Woodgett JR, Maretto S, Piccolo S, Klein PS. Glycogen synthase kinase-3beta haploinsufficiency mimics the behavioural and molecular effects of lithium. J Neurosci. 2004;24:6791–6798. doi: 10.1523/JNEUROSCI.4753-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan JQ, Lewis MC, Ketterman JK, Clore EL, Riley M, Richards KR, Berry-Scott E, Liu X, Wagner FF, Holson EB, et al. AKT kinase activity is required for lithium to modulate mood-related behaviors in mice. Neuropsychopharmacology. 2011;36:1397–1411. doi: 10.1038/npp.2011.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plotkin B, Kaidanovich O, Talior I, Eldar-Finkelman H. Insulin mimetic action of synthetic phosphorylated peptide inhibitors of glycogen synthase kinase-3. J Pharmacol Exp Ther. 2003;305:974–980. doi: 10.1124/jpet.102.047381. [DOI] [PubMed] [Google Scholar]
- Polter A, Beurel E, Yang S, Garner R, Song L, Miller CA, Sweatt JD, McMahon L, Bartolucci AA, Li X, et al. Deficiency in the inhibitory serine-phosphorylation of glycogen synthase kinase-3 increases sensitivity to mood disturbances. Neuropsychopharmacology. 2010;35:1761–1774. doi: 10.1038/npp.2010.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosa AO, Kaster MP, Binfaré RW, Morales S, Martín-Aparicio E, Navarro-Rico ML, Martinez A, Medina M, García AG, López MG, et al. Antidepressant-like effect of the novel thiadiazolidinone NP031115 in mice. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32:1549–1556. doi: 10.1016/j.pnpbp.2008.05.020. [DOI] [PubMed] [Google Scholar]
- Shapira M, Licht A, Milman A, Pick CG, Shohami E, Eldar-Finkelman H. Role of glycogen synthase kinase-3beta in early depressive behavior induced by mild traumatic brain injury. Mol Cell Neurosci. 2007;34:571–577. doi: 10.1016/j.mcn.2006.12.006. [DOI] [PubMed] [Google Scholar]
- Silva R, Mesquita AR, Bessa J, Sousa JC, Sotiropoulos I, Leão P, Almeida OF, Sousa N. Lithium blocks stress-induced changes in depressive-like behavior and hippocampal cell fate: the role of glycogen-synthase-kinase-3beta. Neuroscience. 2008;152:656–669. doi: 10.1016/j.neuroscience.2007.12.026. [DOI] [PubMed] [Google Scholar]
- Scheuing L, Chiu CT, Liao HM, Chuang DM. Antidepressant mechanism of ketamine: perspective from preclinical studies. Front Neurosci. 2015;9:249. doi: 10.3389/fnins.2015.00249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tecott LH, Sun LM, Akana SF, Strack AM, Lowenstein DH, Dallman MF, Julius D. Eating disorder and epilepsy in mice lacking 5-HT2c serotonin receptors. Nature. 1995;374:542–546. doi: 10.1038/374542a0. [DOI] [PubMed] [Google Scholar]
- Tizabi Y, Bhatti BH, Manaye KF, Das JR, Akinfiresoye L. Antidepressant-like effects of low ketamine dose is associated with increased hippocampal AMPA/NMDA receptor density ratio in female Wistar-Kyoto rats. Neuroscience. 2012;213:72–80. doi: 10.1016/j.neuroscience.2012.03.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Oekelen D, Luyten WH, Leysen JE. 5-HT2A and 5-HT2C receptors and their atypical regulation properties. Life Sci. 2003;72:2429–2449. doi: 10.1016/s0024-3205(03)00141-3. [DOI] [PubMed] [Google Scholar]
- Yang C, Zhou ZQ, Gao ZQ, Shi JY, Yang JJ. Acute increases in plasma mammalian target of rapamycin, glycogen synthase kinase-3β, and eukaryotic elongation factor 2 phosphorylation after ketamine treatment in three depressed patients. Biol Psychiatry. 2013;73:e35–e36. doi: 10.1016/j.biopsych.2012.07.022. [DOI] [PubMed] [Google Scholar]
- Zanos P, Moaddel R, Morris PJ, Georgiou P, Fischell J, Elmer GI, Alkondon M, Yuan P, Pribut HJ, Singh NS, et al. NMDAR inhibition-independent antidepressant actions of ketamine metabolites. Nature. 2016 doi: 10.1038/nature17998. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou W, Dong L, Wang N, Shi JY, Yang JJ, Zuo ZY, Zhou ZQ. Akt mediates GSK-3β phosphorylation in the rat pre-frontal cortex during the process of ketamine exerting rapid antidepressant actions. Neuroimmunomodulation. 2014;21:183–188. doi: 10.1159/000356517. [DOI] [PubMed] [Google Scholar]
- Ziats MN, Rennert OM. Identification of differentially expressed microRNAs across the developing human brain. Mol Psychiatry. 2014;19:848–852. doi: 10.1038/mp.2013.93. [DOI] [PMC free article] [PubMed] [Google Scholar]