Abstract
Purpose of review:
To review the available evidence for the detection and management of depression in Parkinson disease (PD) and dementia.
Recent findings:
Depression is a common comorbidity in those with PD or dementia, and leads to increased morbidity. There are several available and accurate tools for the detection of depression in PD (e.g., Geriatric Depression Scale) and dementia (e.g., Cornell Scale for Depression in Dementia). Treatment of depression depends on patient preference, severity of depression, comorbidities, and available resources. Despite variable evidence, the use of nonpharmacologic strategies to manage depression is suggested. Pharmacologic management is guided by modest evidence in PD and dementia, but also informed by the management of late-life depression (LLD).
Summary:
There is evidence to guide the diagnosis and management of depression in PD or dementia. However, more research is required in this field to better inform clinical decision-making.
Depression is common in those experiencing neurodegenerative diseases, such as Parkinson disease (PD) or dementia, and results in poorer patient outcomes.1,2 Depression is also underdiagnosed and undertreated in these patient populations.3,4 In PD, depression leads to poor quality of life, cognitive impairment, functional limitations, caregiver burden, less adherence to therapy, and mortality.5 In addition, depression in PD has been linked to exaggerated motor symptoms and higher disease severity.6 Thus, comorbid depression represents a target for improving care.7 Even when diagnosed with depression, just 20% of patients with PD and 18% of patients with dementia receive therapy.8,9 Assessment is further complicated by evidence suggesting that late-life depression (LLD) may have an overlapping cognitive profile with AD.10 Depressive symptoms in dementia are associated with institutionalization, cognitive decline, mortality, caregiver burden, and greater plaque and tangle burden.11,12 Similarly, depressive symptoms in mild cognitive impairment (MCI) are associated with greater cognitive impairment and progression to dementia.13 In addition, depression is commonly associated with other nonmotor or noncognitive symptoms such as anxiety.14,15 Thus, depression has considerable influence on the risk and course of dementia. While the public health significance of depression alone is profound, as a risk factor and modifying agent in dementia, this is amplified.2
Epidemiology
Patients with PD frequently experience symptoms of depression (35%), with 17% of patients experiencing major depression.16 A recent systematic review and meta-analysis on the prevalence of depression in Alzheimer disease (AD) found significant heterogeneity in estimated rates (5%–48%) based on patient sampling, dementia severity, and diagnostic criteria used.17 The odds ratio (OR) for depression in patients with dementia compared to those without dementia is 2.64 (95% confidence interval [CI] 2.43–2.86).18 Depression is even common in MCI, with an omnibus prevalence estimate of 32%,19 and may herald the progression to dementia.20
Detection and diagnosis
Depression is common in neurodegenerative disease and is a marker for greater disease burden and severity.2 In addition, depression is intimately linked with cognition, especially in older adults.21 However, being a heterogeneous disease, there is no clear biomarker or gold standard for diagnosis. Depression is a syndrome diagnosed clinically, including cardinal symptoms of depressed mood and anhedonia, and rating scales are often used to assess and screen for depression. Studies have attempted to determine the best tools for diagnosis of depression in neurodegenerative disease, but have generally found poor correlation between rating scales,21 low sensitivity,22 or decreased scale performance with greater cognitive impairment,23,24 in part due to several misfit items in the cognitively impaired.25 The best evidence in PD supports the use of the Geriatric Depression Scale (GDS-15), at a cutoff of 5 with pooled sensitivity at 0.91, with nonsignificant heterogeneity.26 In dementia, the Cornell Scale for Depression in Dementia (CSDD) and Hamilton Depression Rating Scale (HDRS) have higher pooled sensitivities than the GDS,27 with the CSDD having the highest pooled sensitivity at 0.91 at a cutoff of 6.27 However, it is unclear if depression in neurodegenerative disease is in fact the same thing as major depression as described in current psychiatric nosology.2 Thus clinical acumen is also required in differentiating and disentangling depressive symptoms from other neurologic symptoms that can manifest in neurodegenerative disease. Apathy or abulia is common in neurodegenerative disease and is often mistaken for depression, but is generally unresponsive to antidepressant treatment.28 Meta-analyses have shown a prevalence of apathy in PD of 39.8%, and associated with poorer motor function, greater disability, lower cognitive scores, and a 57.2% comorbidity with depression.29 Thus, special attention is required for comorbid psychiatric symptomatology, as well as the natural history of symptoms to help confirm the clinical significance and etiology of depressive symptoms when they present in neurodegenerative disease.13
Treatment
Both nonpharmacologic and pharmacologic therapies are available for depression in PD and dementia; however, there is a lack of consensus in the evidence for some therapies due to a lack of high-quality evidence.5,30,31
Nonpharmacologic therapy
A randomized control trial (RCT) of 80 patients with PD and depression examined cognitive-behavioral therapy (CBT) vs monitoring, and found a decrease in HDRS scores by 7.35 at 10 weeks (p < 0.0001) with a number needed to treat of 2.1.32 The results of a systematic review found this trial examining CBT, caregiver, and behavior intervention32 had a higher effect size at 1.57 vs pooled effect for 8 antidepressant trials at 0.69.30
A systematic review identified 12 studies looking at CBT or psychodynamic therapy for depression in PD.33 For the studies (n = 10) that used the HDRS, there was improvement with brief psychotherapy over controls (standard mean difference [SMD] −1.45 [95% CI −2.00 to −0.91]); however, there was high heterogeneity in this estimate (I2 = 91%, p < 0.00001).33 There were 2 studies that demonstrated an improvement in cognition on the Montreal Cognitive Assessment (SMD 0.52; 95% CI 0.15–0.88; I2 = 0%, p = 0.99).33 Psychodynamic therapy had a larger effect than CBT (SMD −2.02; 95% CI −1.66 to −2.99; I2 = 93%, p < 0.00001); however, this estimate was potentially skewed due to a small study with large effect.33
Other interventions such as group therapy, group psychodrama, education, and multidisciplinary rehabilitation have each demonstrated benefit in single studies.30,34 Exercise, although effective for other symptoms, has not consistently shown benefit for mood across 4 studies.35
Four guidelines review nonpharmacologic management for depression in dementia.36 These list several interventions such as caregiver involvement, CBT, exercise, stimulation-oriented therapy, reminiscence, and animal therapy.36 However, there is a concern that this evidence is based on small, low-quality studies.36
Psychosocial interventions (CBT, interpersonal therapy, and counseling) were pooled in a systematic review for depression in dementia, and had a positive effect across 6 RCTs compared to usual treatment (SMD −0.22; 95% CI −0.41 to −0.03; I2 = 21%, p = 0.28]).31 Despite this, there was no difference noted in cognition or quality of life.31
Several individual trials have found benefit with non-psychotherapy interventions such as pet therapy,37 light therapy,38 and group music therapy.39 A recent systematic review looked at music therapy for anxiety and depression in dementia, identifying 10 studies of low quality (n = 10–100 patients per study) with mixed results.40 Five studies reported a reduction, 3 found no difference, and 1 identified an increase in depression scores.40 Of the 4 RCTs that examined exercise therapy for depression in dementia, only one demonstrated a decrease in CSDD scores but not on the HDRS.41
It is important to note that these studies examining nonpharmacologic interventions are often small and non-randomized, and thus are usually classified as lower quality.31 In addition, nonpharmacologic interventions often vary considerably even when they are in the same category.31 For example, music therapy can encompass listening, playing, or singing; this can be done in varied doses by a multitude of practitioners.40 These fundamental study differences make comparison difficult and make their application at bedside more difficult. One of the main benefits of the psychological interventions is the minimal risk associated with their use.31 Generally, these therapies are often limited to patients with mild dementia, although some have been adapted for patients with severe dementia and caregivers.42,43
Pharmacologic therapy
Pharmacologic therapy is a common component of depression management; however, there is a lack of consensus about the approach. Eight placebo-controlled RCTs examined pharmacologic treatment for depression or anxiety in PD.30 When pooled across citalopram,44 sertraline, desipramine,44 nortriptyline, paroxetine,45 and venlafaxine,45 the SMD for antidepressants vs placebo is 0.69 (95% CI −1.51 to 2.93).30 However, there was significant heterogeneity associated with the estimate, which resolved when the authors performed subgroup analysis.46 When selective serotonin reuptake inhibitors (SSRIs) were examined separately, the effect size was moderate (SMD 0.44; 95% CI −1.37 to 2.26).46 Tricyclic antidepressants (TCAs), however, had a large effect (SMD 1.36; CI 95% 0.19–2.52).46 Thus, the authors concluded that more studies with larger sample sizes and greater methodologic rigor are needed to improve understanding of the effect of pharmacologic therapy.5,30 In addition, despite their improvement in depressive symptoms, there are major concerns with TCAs given the risk of side effects.46
An earlier systematic review found the risk ratio (RR) for response to antidepressants compared to placebo to be 1.36 (95% CI 0.98–1.87) across 5 studies for depression in PD.5 To demonstrate the effect of risk of bias, a sensitivity analysis found an increase in the RR to 1.41 (95% CI 1.01–1.96) when a study of low methodologic rigor was excluded.5 This further increased to 1.48 (95% CI 1.05–2.10) when only studies with low risk of bias were included.5 This points to the importance of methodologic rigor and reducing risk of bias in future studies to ensure their resulting estimates are reliable.5 Important aspects to improve study quality include larger sample sizes, inclusion of patients with different disease severity, placebo-controlled designs, longer follow-up periods, and comparisons of multiple agents.5
Although the evidence is mixed, practitioners are prescribing medications for 22% of patients with depression in PD living at home and 50% living in an institution.47 These patterns of prescribing show in recent data from a Swedish registry study that SSRIs were most commonly prescribed, followed by mirtazapine for those taking antiparkinsonian medications.47 However, we were unable to identify any trial looking at mirtazapine for depression in PD.
Outside of traditional pharmaceuticals, there have been few studies examining complementary alternative drugs.
Outside of traditional pharmaceuticals, there have been few studies examining complementary alternative drugs. In a systematic review discussed above, omega-3 supplements had a large effect, with SMD = 0.92 (95% CI 0.15–1.69); however, this was an open-label trial with few patients (n = 18).30 When pooled across 10 studies, traditional Chinese medicine (TCM) was found to improve scores on the HDRS (weighted mean difference −4.19 [95% CI −5.14 to −3.14]) when combined with pharmacologic therapy vs pharmacologic therapy alone.48 The majority of included studies were classified as high risk of bias.48
Guidelines recommend considering dopamine agonists and monoamine oxidase inhibitors for depression in PD.36 A systematic review of 7 studies examined pramipexole (dopamine agonist) for mood, including manufacturer-led RCTs and unpublished data.49 Pramipexole improved mood (as measured by the Unified Parkinson's Disease Rating Scale [UPDRS]) in 64.7% of patients compared to 43.4% in the placebo group (OR 2.41 [n = 480; 95% CI 1.62–3.58; p < 0.001]).49,50 When looking at those with advanced disease, there was a 2.71 odds of improvement on pramipexole vs placebo (OR 2.71 [95% CI 1.78–4.13; p < 0.001]).49 The concern with this estimate is that no diagnostic criterion was used and UPDRS is not accurate for depression alone nor is it designed to detect changes due to treatment.49,50 In addition, all trials excluded patients with major depressive disorder (DSM-IV-TR).49 The largest placebo-controlled RCT (n = 323) was a 12-week study of pramipexole for clinically relevant depressive symptoms (defined as a score ≥5 on GDS-15 and ≥2 on the depression item of the UPDRS) and found a difference in Beck Depression Inventory (BDI) scores of 1.9 (p < 0.001) favoring treatment over placebo.51 What is unclear is whether a change of approximately 2 points represents a clinically meaningful change.50,51 A recent study looking at rotigotine did not demonstrate improvement in depressive symptoms as measured by the HDRS.52
The other nonergot and ergot dopamine agonists have been studied in smaller, mostly non-placebo-controlled studies, demonstrating inconsistent evidence for mood.50 Along with these concerns, depression is often measured among many neuropsychiatric symptoms in these studies, and is not the primary outcome or measured by gold standard criteria.50 Conversely, levodopa has not been shown to be effective.53 Rasagiline, an MAO-B inhibitor, was studied in a double-blinded placebo-controlled RCT (n = 123), and demonstrated no benefit on the primary outcome (BDI-IA) at 12 weeks.54 Transcranial magnetic stimulation and electroconvulsive therapy (ECT) have also been demonstrated in a few studies to have an effect in PD with depression.30,55
In dementia, 6 trials of pharmacologic therapy (clomipramine, sertraline, fluoxetine, venlafaxine) show a 2.12 odds of response in those with depression on an antidepressant vs those on placebo (OR 2.12 [95% CI 0.95–4.70; p = 0.07]) (response rate was defined as either improvement on a global assessment or ≥50% improvement on the HDRS or Montgomery-Åsberg Depression Rating Scale [MÅDRS]).56 Similarly, there was an OR of 1.97 (95% CI 0.85–4.55; p = 0.10) for remission rates (as measured by a score ≤7 on the HDRS, a CSDD score of ≤6, or an “equivalent global rating”).56 The authors concluded that antidepressants had a low adverse event rate, but results were not significant for efficacy.56 This is in part due to variable methodology and underpowered studies.56 In fact, these same issues have plagued the study of LLD—older adults require longer trial duration to adequately elicit pharmacotherapeutic effects, there is often greater medical comorbidity, and cognitive impairment results in diagnostic uncertainty.57
The Health Technology Assessment Study of the Use of Antidepressants for Depression in Dementia evaluated patients with dementia treated with either sertraline or mirtazapine and found no significant difference in CSDD scores between treatment and placebo at 13 or 39 weeks follow-up (n = 326).58 There were some typical trial concerns with loss to follow-up, recruitment, and perhaps measurement bias.58 Safety of these interventions was also assessed with adverse reactions occurring in 46%, 41%, and 26% of the sertraline, mirtazapine, and placebo groups, respectively.58 The most common reaction with sertraline was gastrointestinal symptoms and drowsiness or sedation with mirtazapine.58 There was no significant difference between the number of serious adverse reactions between the antidepressant and placebo groups, although more were severe in the antidepressant group.58 Still, there was no difference in mortality.58 One key finding was that there were fewer reported neuropsychiatric symptoms, decreased depression scores (not significant), and improved quality of life on one drug (mirtazapine),58 consistent with emerging data on the role of antidepressants in treating neuropsychiatric symptoms in patients with AD without depression.59 A small (n = 31) placebo-controlled RCT examining venlafaxine over 6 weeks did not see any significant change in the MÅDRS scores or clinician global impression of change.60 Overall, there is some evidence regarding the pharmacologic treatment of depression in dementia; however, gaps remain and future studies are needed looking at new agents or approaches.61
Cholinesterase inhibitors are suggested as an option in the guidelines for dementia to address behavioral and psychological symptoms of dementia, which can include depression.36 There are few rigorous trials examining solely the effect of the cholinesterase inhibitors on depression in dementia. One non-placebo-controlled 2007 donepezil study (n = 135) found a significant improvement on the GDS-15 in the depressed group from baseline to 16 weeks by approximately 2 points compared to the nondepressed group.62 Of the studies that report depression, it is often reported as one of many behavioral outcomes or a change on a neuropsychiatric symptom subscale. These studies have, however, noted benefit of cholinesterase inhibitors for behaviors in dementia.63 Interestingly, a study looking at MCI found that the use of donepezil in those experiencing depression reduced progression to dementia significantly at 1.7 and 2.2 years compared to placebo and vitamin E; however, donepezil is not approved for use in MCI.64
There may be specific cases in which ECT is considered an option.36 Evidence in this area is largely case study and prospective cohort level evidence.65 One cohort study examined ECT in those with dementia (n = 31) with both unipolar and bipolar depression (mean of 9 sessions)65 and found there was a significant decrease in MÅDRS scores by 12.28 points.65 Along with this, the authors identified an increase in Mini-Mental State Examination (MMSE) score of 1.62 points; conversely, about half of patients developed delirium and 4 had major side effects (atrial fibrillation, ventricular tachycardia, TIA, or prolonged seizure).65 A major concern for the use of ECT in patients with dementia is the concern for delirium or worsened cognitive outcomes.65 This was seen in the most recent cohort, where the authors found that cognition prior to ECT predicted post ECT declines in MMSE scores.65
Overall approach
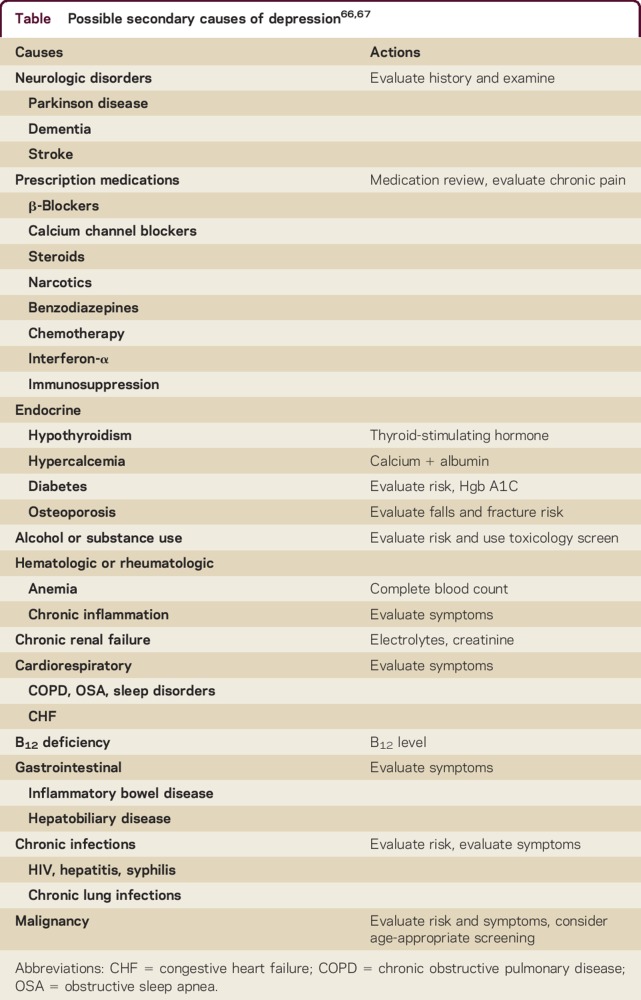
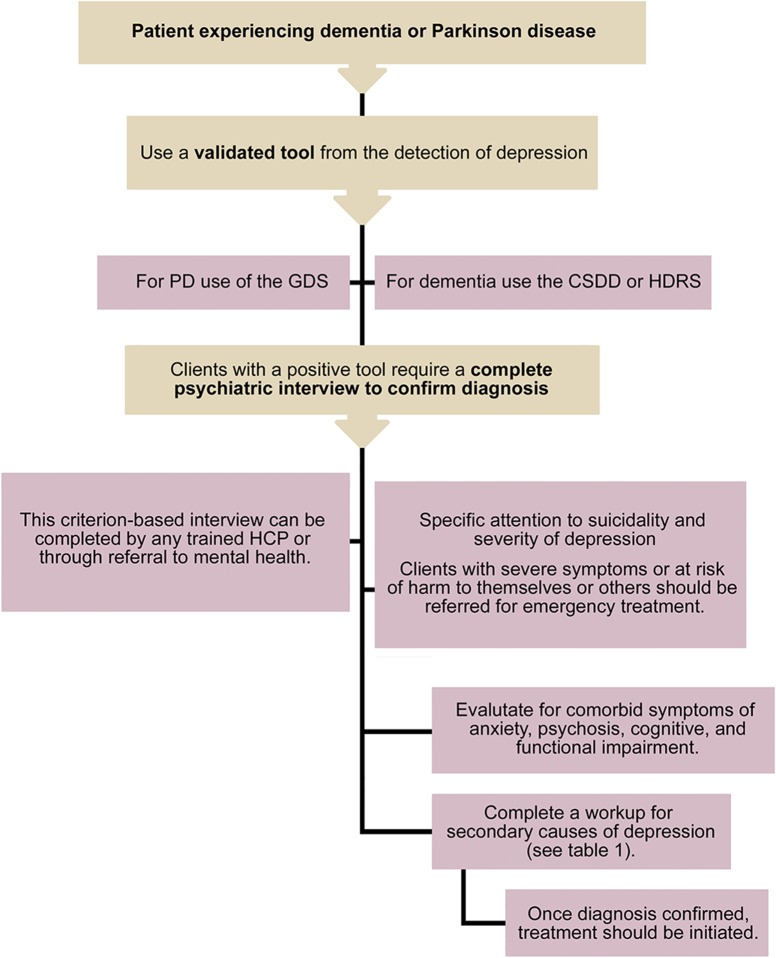
When evaluating patients with PD or dementia, screening for depression should incorporate a validated instrument.36 Positive screens should trigger a full diagnostic clinical interview based on criteria26,27 and secondary causes of depression should be investigated (table).36,66,67 Illness severity and suicide risk assessment help establish the acuity of the treatment plan, and inform the need for specialized mental health services or hospitalization.36,66 This approach is summarized in figure 1.
Table.
Figure 1. An approach to the initial screening, diagnosis, and workup of depression in Parkinson disease (PD) or dementia.
CSDD = Cornell Scale for Depression in Dementia; GDS = Geriatric Depression Scale; HCP = health care provider; HDRS = Hamilton Depression Rating Scale.
In most cases, tailored nonpharmacologic treatment strategies should be considered first-line.36,58 For patients with PD, the evidence supports using brief psychotherapy and CBT.31,33 For dementia, use of CBT, interpersonal therapy, and counseling may be useful in the earlier stages when the patient still has the ability to participate and retain the therapy.31 However, as the disease progresses, other techniques such as increasing pleasurable activities, caregiver involvement, light, music, animal, or stimulation-oriented therapy are recommended.36 Nonpharmacologic strategies can be used on their own or in conjunction with pharmacologic treatment.
While there is modest evidence for pharmacologic management of depression in PD, the evidence in dementia is scant. Nonetheless, patients with neurodegenerative diseases often experience depression and medication trials are common in order to ameliorate patient suffering. The American Psychiatric Association dementia treatment guidelines state the following: “clinical consensus still supports undertaking one or more trials of an antidepressant to treat clinically significant and persistent depressed mood in patients with dementia because of the increased rates of disability, impaired quality of life, and greater mortality associated with depression.”68 Until more data emerge, pharmacologic approaches are conservative, based on clinical experience, and informed by the above evidence as well as guidelines for the treatment of LLD,57 which incorporate systematic and algorithmic approaches to management.69 Consistent with guidelines, the adage of “start low and go slow” applies, with longer treatment trials and greater vigilance for adverse drug reactions, as these are more common in the elderly.57
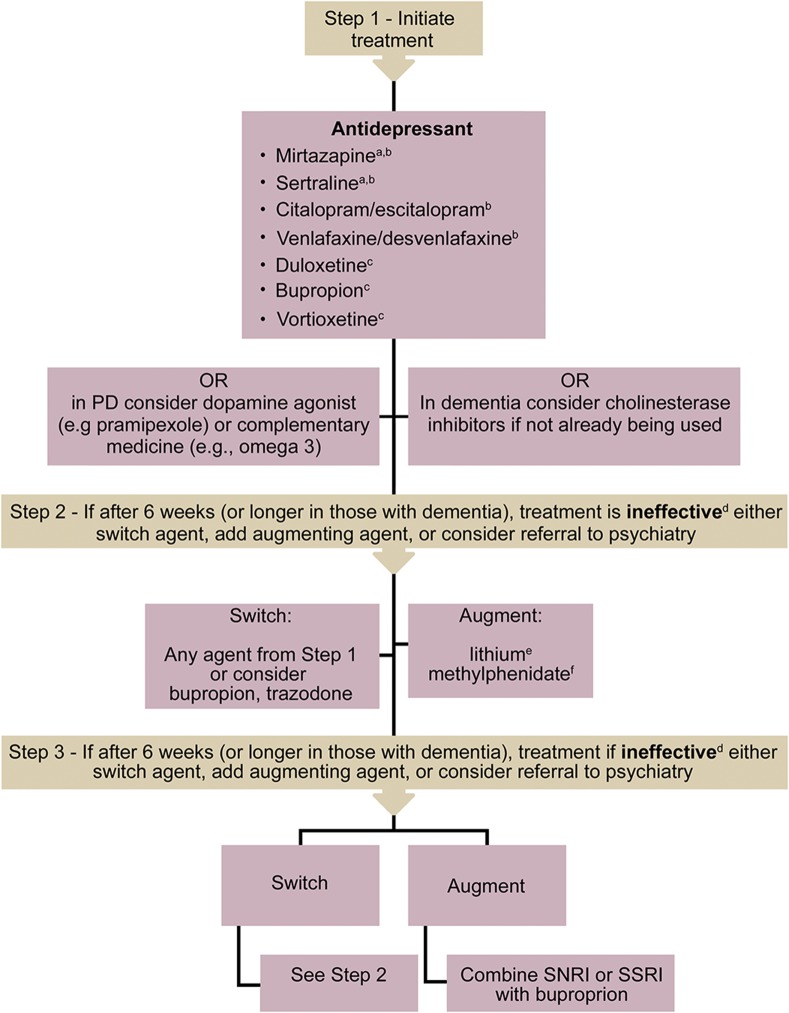
An adapted version of the treatment algorithm for LLD57 is presented in figure 2. In PD, we suggest the use of newer-generation antidepressants, specifically venlafaxine,45 citalopram,44 or mirtazapine47 first-line, due to the favorable side effect profile over TCAs.46 Although there is evidence for paroxetine,45 its use is not recommended due to tolerability issues.57 There are a few trials that demonstrate the benefit of alternative therapies such as omega-3 or TCM, but more study is needed.46,48 Dopamine agonists, especially pramipexole, may also be part of the treatment plan; however, the evidence is more limited.50 Despite evidence being limited in dementia, results show safety for the use of sertraline or mirtazapine monotherapy.58 Alternatively, other antidepressants used in LLD may be considered, such as citalopram/escitalopram, venlafaxine/desvenlafaxine, bupropion, duloxetine, or vortioxetine.57 Of note, the best evidence for improvement of cognitive symptoms of depression in older adults is with duloxetine and vortioxetine,57 whereas citalopram demonstrated a decline in MMSE scores and QTc prolongation in a dementia agitation trial.59 Alternatively, a cholinesterase inhibitor could be considered, which may also improve behaviors.62,63 While there is very good evidence for the utility of atypical antipsychotics (alone or as augmenting agents) in treatment of geriatric depression, these agents are not recommended due to the risk of all-cause mortality in dementia, worsened symptoms, and lack of evidence in PD.36,57 Again, overall conservative approaches should be used considering the best available safety and efficacy evidence, with vigilance for emerging data and newer trials.
Figure 2. Treatment algorithm.
This algorithm is a suggested approach looking at the available evidence in dementia and Parkinson disease (PD) and the evidence available for prescribing in late-life depression in the 2016 CANMAT guidelines.57 All of the above drugs have side effects, drug–drug interactions, and drug–disease interactions. When choosing an agent, it is important to be informed of these, and how they apply to the patient. All these concerns need to be discussed with the patient to allow the patient to make an informed decision and express his or her preference. Consider drug initiation at the lowest possible dose, with slow titration. aDue to limited evidence in dementia, one must consider agents that do not have robust evidence; however, the results demonstrate safety for the use of sertraline or mirtazapine.58 bIn PD, tricyclic antidepressants demonstrated more benefit to mood, but at the risk of worsening motor symptoms.46 As such, the current recommendation is to consider selective serotonin reuptake inhibitors (SSRIs) or mirtazapine first line.36,44,45,47 cNo explicit evidence in dementia or PD, but there is evidence in late-life depression. dPrior to changing medication, evaluate for medication adherence, tolerability, and side effects. eAugmenting agents should be used with caution; consider expert consultation. Lithium has been shown to have adverse effects and is not recommended in PD.66 fAugmenting agents should be used with caution; consider expert consultation. Methylphenidate is associated with adverse effects, such as agitation in dementia.70SNRI = serotonin and norepinephrine reuptake inhibitor.
DISCUSSION
Given the burden of depression, it is important that there are clear evidence-based strategies for management. Along with this, health care practitioners need to consider the available evidence and their local resources to plan appropriate interventions for these patients. It is clear from the evidence that there is a gap in our understanding of the treatment of depression in PD and dementia, and higher quality studies are needed. Until that evidence is presented, a conservative adaptation of approaches to manage LLD is recommended.
Take-home points
Detection of depression in neurodegenerative disease is facilitated by using both valid rating scales and clinical acumen.
Refer to psychiatry for assessment in atypical or treatment-resistant cases.
Management of depression requires clinicians to consider several factors including patient preferences, illness severity (including suicidality), and comorbidities (both medical and psychiatric).
First-line therapy should include nonpharmacologic treatment (e.g., CBT) if possible.
While additional PD and dementia-specific evidence on pharmacologic therapy of depression is needed, clinicians should inform their choice by the available evidence in LLD.
Related articles from AAN physician and patient resources
Neurology® Clinical Practice
Parkinson disease and cognitive impairment: Five new things
Neurology®
Natural course of mild cognitive impairment in Parkinson disease: A 5-year population-based study
Impulsive-compulsive behaviors in parkin-associated Parkinson disease
Continuum®
Lewy Body Dementias: Dementia With Lewy Bodies and Parkinson Disease Dementia
Neurology Today®
When Do Parkinson's Patients Progress to Dementia?
Prospective Study Identifies New Predictors of Dementia in Parkinson's Disease
Longitudinal Study Finds Neuropsychiatric Symptoms Emerge Early in Parkinson's Disease and Often Go Untreated
ACKNOWLEDGMENT
The authors thank Dr. Tamara Pringsheim for providing movement disorder expertise and in reviewing the final draft.
Footnotes
See editorial, page 96
AUTHOR CONTRIBUTIONS
Z.S. Goodarzi: drafting/revising the manuscript, study concept or design, analysis or interpretation of data. Z. Ismail: drafting/revising the manuscript, study concept or design, analysis or interpretation of data.
STUDY FUNDING
No targeted funding reported.
DISCLOSURES
Z.S. Goodarzi has received travel and/or research support from Alberta Innovates Health Solutions, Western Regional Training Centre, University of Calgary, Canadian Society for Clinical Investigation, Canadian Geriatric Society, and Knowledge Translation Summer Institute. Z. Ismail has received honoraria for ad hoc speaking or advising/consulting or received research funds from Canadian Biomarker Integration Network for Depression, Canadian Consortium for Neurodegeneration and Aging, Canadian Institutes of Health Research, Janssen, Joan and Clifford Hatch Foundation, Kathy Taylor Chair in Vascular Dementia, Lundbeck, National Institute of Aging, Ontario AFP Innovation Fund, Otsuka, Pfizer, and Sunovion. Full disclosure form information provided by the authors is available with the full text of this article at Neurology.org/cp.

REFERENCES
- 1.Djamshidian A, Friedman JH. Anxiety and depression in Parkinson's disease. Curr Treat Options Neurol 2014;16:285. [DOI] [PubMed] [Google Scholar]
- 2.Ismail Z, Malick A, Smith EE, Schweizer T, Fischer C. Depression versus dementia: is this construct still relevant? Neurodegener Dis Manag 2014;4:119–126. [DOI] [PubMed] [Google Scholar]
- 3.Knapskog AB, Barca ML, Engedal K. A comparison of the validity of the Cornell Scale and the MADRS in detecting depression among memory clinic patients. Dement Geriatr Cogn Disord 2011;32:287–294. [DOI] [PubMed] [Google Scholar]
- 4.Pachana NA, Egan SJ, Laidlaw K, et al. . Clinical issues in the treatment of anxiety and depression in older adults with Parkinson's disease. Mov Disord 2013;28:1930–1934. [DOI] [PubMed] [Google Scholar]
- 5.Rocha FL, Murad MG, Stumpf BP, Hara C, Fuzikawa C. Antidepressants for depression in Parkinson's disease: systematic review and meta-analysis. J Psychopharmacol 2013;27:417–423. [DOI] [PubMed] [Google Scholar]
- 6.Dissanayaka NN, Sellbach A, Matheson S, et al. . Anxiety disorders in Parkinson's disease: prevalence and risk factors. Mov Disord 2010;25:838–845. [DOI] [PubMed] [Google Scholar]
- 7.Sagna A, Gallo JJ, Pontone GM. Systematic review of factors associated with depression and anxiety disorders among older adults with Parkinson's disease. Parkinsonism Relat Disord 2014;20:708–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Frisina PG, Borod JC, Foldi NS, Tenenbaum HR. Depression in Parkinson's disease: health risks, etiology, and treatment options. Neuropsychiatr Dis Treat 2008;4:81–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Watson LC, Garrett JM, Sloane PD, Gruber-Baldini AL, Zimmerman S. Depression in assisted living: results from a four-state study. Am J Geriatr Psychiatry 2003;11:534–542. [PubMed] [Google Scholar]
- 10.Ting C, Rajji TK, Ismail Z, et al. . Differentiating the cognitive profile of schizophrenia from that of Alzheimer disease and depression in late life. PLoS One 2010;5:e10151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Olin JT, Katz IR, Meyers BS, Schneider LS, Lebowitz BD. Provisional diagnostic criteria for depression of Alzheimer disease: rationale and background. Am J Geriatr Psychiatry 2002;10:129. [PubMed] [Google Scholar]
- 12.Rapp MA, Schnaider-Beeri M, Wysocki M, et al. . Cognitive decline in patients with dementia as a function of depression. Am J Geriatr Psychiatry 2011;19:357–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ismail Z, Aguera-Ortiz L, Brodaty H, et al. . The Mild Behavioral Impairment Checklist (MBI-C): a rating scale for neuropsychiatric symptoms in pre-dementia populations. J Alzheimers Dis 2017;56:929–938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Prado-Jean A, Couratier P, Druet-Cabanac M, et al. . Specific psychological and behavioral symptoms of depression in patients with dementia. Int J Geriatr Psychiatry 2010;25:1065–1072. [DOI] [PubMed] [Google Scholar]
- 15.de la Riva P, Smith K, Xie SX, Weintraub D. Course of psychiatric symptoms and global cognition in early Parkinson disease. Neurology 2014;83:1096–1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reijnders JS, Ehrt U, Weber WE, Aarsland D, Leentjens AF. A systematic review of prevalence studies of depression in Parkinson's disease. Mov Disord 2008;23:183–189; quiz 313. [DOI] [PubMed] [Google Scholar]
- 17.Chi S, Wang C, Jiang T, Zhu XC, Yu JT, Tan L. The prevalence of depression in Alzheimer's disease: a systematic review and meta-analysis. Curr Alzheimer Res 2015;12:189–198. [DOI] [PubMed] [Google Scholar]
- 18.Snowden MB, Atkins DC, Steinman LE, et al. . Longitudinal association of dementia and depression. Am J Geriatr Psychiatry 2015;23:897–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ismail Z, Elbayoumi H, Smith EE, et al. . A systematic review and meta-analysis for the prevalence of depression in mild cognitive impairment. JAMA Psychiatry 2017;74:58–67. [DOI] [PubMed] [Google Scholar]
- 20.Ismail Z, Smith EE, Geda Y, et al. . Neuropsychiatric symptoms as early manifestations of emergent dementia: provisional diagnostic criteria for mild behavioral impairment. Alzheimer's Demen 2016;12:195–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Knapskog A-B, Barca ML, Engedal K. A comparison of the Cornell scale for depression in dementia and the Montgomery-Aasberg depression rating scale in a memory clinic population. Demen Geriatr Cogn Disord 2013;35:256–265. [DOI] [PubMed] [Google Scholar]
- 22.Samaras N, Herrmann FR, Samaras D, et al. . The hospital anxiety and depression scale: low sensitivity for depression screening in demented and non-demented hospitalized elderly. Int Psychogeriatr 2013;25:82–87. [DOI] [PubMed] [Google Scholar]
- 23.Müller-Thomsen T, Arlt S, Mann U, Maß R, Ganzer S. Detecting depression in Alzheimer's disease: evaluation of four different scales. Arch Clin Neuropsychol 2005;20:271–276. [DOI] [PubMed] [Google Scholar]
- 24.Debruyne H, Van Buggenhout M, Le Bastard N, et al. . Is the geriatric depression scale a reliable screening tool for depressive symptoms in elderly patients with cognitive impairment? Int J Geriatr Psychiatry 2009;24:556–562. [DOI] [PubMed] [Google Scholar]
- 25.Huang M, Koski L. The Geriatric Depression Scale (GDS-15): is cognitive ability associated with how geriatric patients respond to self-reported depressive symptoms? Presented at the National Initiative for the Care of the Elderly (NICE) Annual Knowledge Exchange; May 21, 2014; Winnipeg, MB.
- 26.Goodarzi Z, Mrklas KJ, Roberts DJ, Jette N, Pringsheim T, Holroyd-Leduc J. Detecting depression in Parkinson disease: a systematic review and meta-analysis. Neurology 2016;87:426–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Goodarzi ZS, Mele BS, Roberts DJ, Holroyd-Leduc J. Depression case finding in individuals with dementia: a systematic review and meta-analysis. J Am Geriatr Soc Epub 2017 Feb 2. [DOI] [PubMed]
- 28.Lanctôt KL, Agüera-Ortiz L, Brodaty H, et al. . Apathy associated with neurocognitive disorders: recent progress and future directions. Alzheimers Dement (in press 2017). [DOI] [PubMed]
- 29.den Brok MG, van Dalen JW, van Gool WA, Moll van Charante EP, de Bie R, Richard E. Apathy in Parkinson's disease: a systematic review and meta-analysis. Mov Disord 2015;30:759–769. [DOI] [PubMed] [Google Scholar]
- 30.Troeung L, Egan SJ, Gasson N. A waitlist-controlled trial of group cognitive behavioural therapy for depression and anxiety in Parkinson's disease. BMC Psychiatry 2014;14:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Orgeta V, Qazi A, Spector A, Orrell M. Psychological treatments for depression and anxiety in dementia and mild cognitive impairment: systematic review and meta-analysis. Br J Psychiatry 2015;207:293–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dobkin RD, Menza M, Allen LA, et al. . Cognitive-behavioral therapy for depression in Parkinson's disease: a randomized, controlled trial. Am J Psychiatry 2011;168:1066–1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xie CL, Wang XD, Chen J, et al. . A systematic review and meta-analysis of cognitive behavioral and psychodynamic therapy for depression in Parkinson's disease patients. Neurol Sci 2015;36:833–843. [DOI] [PubMed] [Google Scholar]
- 34.Yang S, Sajatovic M, Walter BL. Psychosocial interventions for depression and anxiety in Parkinson's disease. J Geriatr Psychiatry Neurol 2012;25:113–121. [DOI] [PubMed] [Google Scholar]
- 35.Goodwin VA, Richards SH, Taylor RS, Taylor AH, Campbell JL. The effectiveness of exercise interventions for people with Parkinson's disease: a systematic review and meta-analysis. Mov Disord 2008;23:631–640. [DOI] [PubMed] [Google Scholar]
- 36.Goodarzi Z, Mele B, Guo S, et al. . Guidelines for dementia or Parkinson's disease with depression or anxiety: a systematic review. BMC Neurol 2016;16:244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Majic T, Gutzmann H, Heinz A, Lang UE, Rapp MA. Animal-assisted therapy and agitation and depression in nursing home residents with dementia: a matched case-control trial. Am J Geriatr Psychiatry 2013;21:1052–1059. [DOI] [PubMed] [Google Scholar]
- 38.Figueiro MG, Plitnick BA, Lok A, et al. . Tailored lighting intervention improves measures of sleep, depression, and agitation in persons with Alzheimer's disease and related dementia living in long-term care facilities. Clin Interv Aging 2014;9:1527–1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chu H, Yang CY, Lin Y, et al. . The impact of group music therapy on depression and cognition in elderly persons with dementia: a randomized controlled study. Biol Res Nurs 2014;16:209–217. [DOI] [PubMed] [Google Scholar]
- 40.Petrovsky D, Cacchione PZ, George M. Review of the effect of music interventions on symptoms of anxiety and depression in older adults with mild dementia. Int Psychogeriatr 2015;27:1661–1670. [DOI] [PubMed] [Google Scholar]
- 41.Potter R, Ellard D, Rees K, Thorogood M. A systematic review of the effects of physical activity on physical functioning, quality of life and depression in older people with dementia. Int J Geriatr Psychiatry 2011;26:1000–1011. [DOI] [PubMed] [Google Scholar]
- 42.Orgeta V, Qazi A, Spector AE, Orrell M. Psychological treatments for depression and anxiety in dementia and mild cognitive impairment. Cochrane Database Syst Rev 2014;1:CD009125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Enache D, Winblad B, Aarsland D. Depression in dementia: epidemiology, mechanisms, and treatment. Curr Opin Psychiatry 2011;24:461–472. [DOI] [PubMed] [Google Scholar]
- 44.Devos D, Dujardin K, Poirot I, et al. . Comparison of desipramine and citalopram treatments for depression in Parkinson's disease: a double-blind, randomized, placebo-controlled study. Mov Disord 2008;23:850–857. [DOI] [PubMed] [Google Scholar]
- 45.Richard IH, McDermott MP, Kurlan R, et al. . A randomized, double-blind, placebo-controlled trial of antidepressants in Parkinson disease. Neurology 2012;78:1229–1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Troeung L, Egan SJ, Gasson N. A meta-analysis of randomised placebo-controlled treatment trials for depression and anxiety in Parkinson's disease. PLoS One 2013;8:e79510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Haasum Y, Fastbom J, Johnell K. Use of antidepressants in Parkinson's disease: a Swedish register-based study of over 1.5 million older people. Parkinsonism Relat Disord 2016;27:85–88. [DOI] [PubMed] [Google Scholar]
- 48.Zhang Y, Wang ZZ, Sun HM, Li P, Li YF, Chen NH. Systematic review of traditional Chinese medicine for depression in Parkinson's disease. Am J Chin Med 2014;42:1035–1051. [DOI] [PubMed] [Google Scholar]
- 49.Leentjens AF, Koester J, Fruh B, Shephard DT, Barone P, Houben JJ. The effect of pramipexole on mood and motivational symptoms in Parkinson's disease: a meta-analysis of placebo-controlled studies. Clin Ther 2009;31:89–98. [DOI] [PubMed] [Google Scholar]
- 50.Leentjens AF. The role of dopamine agonists in the treatment of depression in patients with Parkinson's disease: a systematic review. Drugs 2011;71:273–286. [DOI] [PubMed] [Google Scholar]
- 51.Barone P, Poewe W, Albrecht S, et al. . Pramipexole for the treatment of depressive symptoms in patients with Parkinson's disease: a randomised, double-blind, placebo-controlled trial. Lancet Neurol 2010;9:573–580. [DOI] [PubMed] [Google Scholar]
- 52.Chung SJ, Asgharnejad M, Bauer L, Ramirez F, Jeon B. Evaluation of rotigotine transdermal patch for the treatment of depressive symptoms in patients with Parkinson's disease. Expert Opin Pharmacother 2016;17:1453–1461. [DOI] [PubMed] [Google Scholar]
- 53.Choi C, Sohn YH, Lee JH, Kim J. The effect of long-term levodopa therapy on depression level in de novo patients with Parkinson's disease. J Neurol Sci 2000;172:12–16. [DOI] [PubMed] [Google Scholar]
- 54.Barone P, Santangelo G, Morgante L, et al. . A randomized clinical trial to evaluate the effects of rasagiline on depressive symptoms in non-demented Parkinson's disease patients. Eur J Neurol 2015;22:1184–1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Borisovskaya A, Bryson WC, Buchholz J, Samii A, Borson S. Electroconvulsive therapy for depression in Parkinson's disease: systematic review of evidence and recommendations. Neurodegener Dis Manag 2016;6:161–176. [DOI] [PubMed] [Google Scholar]
- 56.Nelson JC, Devanand DP. A systematic review and meta-analysis of placebo-controlled antidepressant studies in people with depression and dementia. J Am Geriatr Soc 2011;59:577–585. [DOI] [PubMed] [Google Scholar]
- 57.MacQueen GM, Frey BN, Ismail Z, et al. . Canadian Network for mood and anxiety treatments (CANMAT) 2016 clinical guidelines for the management of adults with major depressive disorder Section 6. Special populations: youth, women, and the elderly. Can J Psychiatry 2016;61:588–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Banerjee S, Hellier J, Dewey M, et al. . Sertraline or mirtazapine for depression in dementia (HTA-SADD): a randomised, multicentre, double-blind, placebo-controlled trial. Lancet 2011;378:403–411. [DOI] [PubMed] [Google Scholar]
- 59.Porsteinsson AP, Drye LT, Pollock BG, et al. . Effect of citalopram on agitation in Alzheimer disease: the CitAD randomized clinical trial. JAMA 2014;311:682–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.de Vasconcelos Cunha UG, Lopes Rocha F, Avila de Melo R, et al. . A placebo-controlled double-blind randomized study of venlafaxine in the treatment of depression in dementia. Dement Geriatr Cogn Disord 2007;24:36–41. [DOI] [PubMed] [Google Scholar]
- 61.Banerjee S, Hellier J, Romeo R, et al. . Study of the use of antidepressants for depression in dementia: the HTA-SADD trial: a multicentre, randomised, double-blind, placebo-controlled trial of the clinical effectiveness and cost-effectiveness of sertraline and mirtazapine. Health Technol Assess 2013;17:1–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rozzini L, Vicini Chilovi B, Bertoletti E, Trabucchi M, Padovani A. Acetylcholinesterase inhibitors and depressive symptoms in patients with mild to moderate Alzheimer's disease. Aging Clin Exp Res 2007;19:220–223. [DOI] [PubMed] [Google Scholar]
- 63.Cummings JL, McRae T, Zhang R, Donepezil-Sertraline Study G. Effects of donepezil on neuropsychiatric symptoms in patients with dementia and severe behavioral disorders. Am J Geriatr Psychiatry 2006;14:605–612. [DOI] [PubMed] [Google Scholar]
- 64.Lu PH, Edland SD, Teng E, et al. . Donepezil delays progression to AD in MCI subjects with depressive symptoms. Neurology 2009;72:2115–2121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Oudman E. Is electroconvulsive therapy (ECT) effective and safe for treatment of depression in dementia? A short review. J ECT 2012;28:34–38. [DOI] [PubMed] [Google Scholar]
- 66.Gelenberg AJ, Freeman MP, Markowitz JC, et al. . Practice Guideline for Treatment of Patients with Major Depressive Disorder, 3rd ed Arlington, VA: American Psychiatric Association; 2010. [Google Scholar]
- 67.Glover J, Srinivasan S. Assessment of the person with late-life depression. Psychiatr Clin North Am 2013;36:545–560. [DOI] [PubMed] [Google Scholar]
- 68.Rabins PV, Rovner BW, Rummans T, Schneider LS, Tariot PN. Guideline Watch (October 2014): Practice Guideline for the Treatment of Patients with Alzheimer's Disease and Other Dementias. Arlington, VA: American Psychiatric Association; 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mulsant BH, Blumberger DM, Ismail Z, Rabheru K, Rapoport MJ. A systematic approach to Pharmacotherapy for geriatric major depression. Clin Geriatr Med 2014;30:517–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rosenberg PB, Lanctot KL, Drye LT, et al. . Safety and efficacy of methylphenidate for apathy in Alzheimer's disease: a randomized, placebo-controlled trial. J Clin Psychiatry 2013;74:810–816. [DOI] [PMC free article] [PubMed] [Google Scholar]