Abstract
Human immunodeficiency virus (HIV) is a mucosally transmitted infection that rapidly targets and depletes CD4+ T cells in mucosal tissues and establishes a major reservoir for viral persistence in gut-associated lymphoid tissues. Therefore, vaccines designed to prevent HIV infections must induce potent and durable mucosal immune responses, especially in the genital tract. Here we investigated whether intranasal (i.n.) immunization with inactivated gp120-depleted HIV-1 antigen (Ag) plus CpG oligodeoxynucleotide (ODN) as an adjuvant induced local immune responses in the genital tract and cross-clade protection against intravaginal (IVAG) challenge. Lymphocytes isolated from the iliac lymph nodes (ILNs) and genital tracts of female mice i.n. immunized with HIV-1 Ag plus CpG showed significant HIV-specific proliferation and produced significantly higher levels of gamma interferon (IFN-γ) and β-chemokines than mice immunized with HIV-1 Ag alone or mixed with non-CpG ODN. CD8+ lymphocytes were dramatically increased in the genital tracts of mice immunized with HIV-1 Ag plus CpG, and protection following IVAG challenge with recombinant vaccinia viruses (rVVs) expressing HIV-1 gag was shown to be CD8 dependent. Finally, cross-clade protection was observed between clades A, C, and G but not B following IVAG challenge with rVVs expressing HIV-1 gag from different clades. These studies provide evidence that mucosal (i.n.) immunization induced strong local T-cell-mediated immune responses in the genital tract and cross-clade protection against IVAG challenge.
Infection with human immunodeficiency virus type 1 (HIV-1) has led to an expanding global health crisis, with over 42 million people currently infected. Women now constitute about 50% of infected individuals, and girls and young women are 2.5 times more likely to become infected with HIV than young men. The major route of HIV transmission is through exposure of mucosal surfaces to cell-free virus and HIV-infected cells, and 70 to 80% of all HIV infections result from heterosexual transmission (40). Further, during primary infection, HIV rapidly targets and dramatically depletes CD4+ T cells in mucosal-associated lymphoid tissues (MALTs) and establishes a major reservoir for viral persistence in the MALTs (9, 41). Thus, vaccine strategies designed to prevent or protect against HIV infections must induce potent and durable mucosal immune responses, especially in the genital tract. The importance of mucosal immunity is also supported by evidence from individuals who are highly exposed to HIV but remain persistently seronegative. In a number of different cohorts of individuals highly exposed to HIV but persistently seronegative, resistance to HIV infection was associated with HIV-specific antibodies in genital secretions and local HIV-specific T cells detected in cervical scrapings (8, 22, 23, 30).
Although an impediment to mucosal delivery of nonreplicating and subunit vaccines has been the lack of safe and effective adjuvants, recent advances in our understanding of innate immune recognition are ushering in a new era of mucosal vaccine adjuvants. We and others have shown that synthetic oligodeoxynucleotides (ODNs) containing unmethylated CpG motifs (CpG ODNs), which act through activation of Toll-like receptor 9 (TLR9), serve as an excellent mucosal adjuvant in murine models (2, 18, 20, 32, 33). Notably, mucosal immunization with CpG ODNs significantly enhanced the level and duration of immunoglobulin A (IgA) and IgG antibody-secreting cells (ASCs), as well as promoting a Th1 microenvironment and cytotoxic T-lymphocyte (CTL) responses in mucosal tissues, including the genital tract. The sustainability of ASCs and T cells appears to be due to an antiapoptotic effect of CpG (7, 42). Furthermore, mucosal immunization with CpG led to better protection against intravaginal (IVAG) challenge with herpes simplex virus type 2 (HSV-2) (18) and HIV-1 infection in mice (10). Most recently, we showed that CpG can serve as an effective adjuvant for IVAG delivery of nonreplicating viral subunit vaccines in female mice (28).
Another challenge confronting development of HIV vaccines is the great diversity of HIV-1 subtypes or clades. Thus, it is not unclear whether it will be necessary to formulate a different vaccine for each HIV-1 clade or whether there are sufficient numbers of conserved epitopes to provide cross-clade protection. It had been shown that anti-HIV-1 neutralizing monoclonal antibodies (nMAbs) directed against clade B were able to neutralize primary HIV-1 clade B isolates in vitro, as well as primary isolates of clades A, C, D, E, and F (12). More recently, it was shown that these nMAbs could also potently neutralize across groups of primary HIV-1 in vitro (11) and, importantly, that passive transfer of mixtures of high-titer nMAbs provided sterile protection against mucosal challenge of primates with pathogenic simian-HIV (3, 19, 29, 36). With respect to cellular immunity, although most CTL epitopes have been identified for clade B, a number of conserved T-cell epitopes shared among HIV-1 clades have been identified (13, 14, 31, 38). Therefore, it is likely that a successful vaccine will have to induce an immune response directed against conserved neutralizing and T-cell epitopes.
In this study, we used gp120-depleted whole-killed HIV-1 antigen (Ag) plus CpG ODNs as an adjuvant to evaluate mucosal (intranasal [i.n.]) delivery and protection against IVAG virus challenge in a mouse model. The HIV-1 Ag used here was an early isolate from an HIV-1-infected individual in Zaire in 1976 (HZ321) that contains a clade A envelope and clade G gag (5, 37). Our results show that i.n. immunization with HIV-1 Ag plus CpG elicited significant increases in production of Th1 cytokines and β-chemokines by lymphocytes isolated from genital tract and iliac lymph nodes (ILNs) and cross-protection against IVAG challenge with recombinant vaccinia viruses (rVVs) expressing HIV-1 gag from clades A, C, and G but not from clade B.
MATERIALS AND METHODS
Animals.
C57BL/6 female mice (Charles River Canada), 6 to 8 weeks old, were bred under standard pathogen-free conditions in the central animal facility of McMaster University. Mouse colonies were maintained on a 12-h light-dark cycle. All animal experiments were performed in accordance with institutional guidelines as approved by the Animal Care Review Board of McMaster University.
Vaccine preparations and cell culture.
Vero cells were grown in α-minimum essential medium (Life Technologies) supplemented with 10% fetal bovine serum (Life Technologies) and 1% penicillin-streptomycin and l-glutamine (Life Technologies). The HIV-1 Ag used in the present study consisted of inactivated gp120-depleted HIV-1 (HZ321; The Immune Response Corporation). The CpG ODN (5′-TCCATGACGTTCCTGACGTT-3′) (motif 1862) and non-CpG control ODN (5′-TCCAGGACTTCTCTAGGTT-3′) (motif 1982) (Coley Pharmaceutical) were used at 10 μg/immunization. Both ODNs had a nuclease-resistant phosphorothioate backbone.
Immunizations and IVAG rVV challenge.
All groups of mice were immunized with HIV-1 Ag (10 μg) alone or HIV-1 Ag plus CpG (10 μg) or non-CpG ODN (10 μg), respectively, using phosphate-buffered saline (PBS) as a control. Reagents were applied at 15 μl; each mouse was halothane anesthetized and held inverted with the nose down until droplets of vaccine that were applied to the external nares were completely inhaled. All mice were immunized twice with a 2-week interval between prime and boost. Three weeks following the second immunization, mice were given 2 mg of progesterone/mouse subcutaneously (Depo-Provera; Upjohn, Don Mills, Ontario, Canada). Five days later, mice were anesthetized with ketamine (150 mg/kg; MTC Pharmaceuticals) and xylazine (10 mg/kg; Bayer), placed on their backs, and infected IVAG for 1 h with 1 × 107 PFU of rVV expressing HIV-1 clade G gag (vT243) in 10 μl of PBS while being maintained under anesthetic. Six days after challenge, the mice were euthanized and ILNs, ovaries, and genital tracts were removed for further study. This immunization schedule was applied to each experiment, unless otherwise indicated. For cross-clade protection against mucosal viral challenge, mice were infected IVAG with 107 PFU of rVV expressing HIV-1 gag from clade G (vT243), A (vT135), B (vP1287), or C (vT196) (supplied by the AIDS Reference and Reagent Program) after immunization, as mentioned previously. Parental VV was also used for IVAG challenge as a control for specificity. After euthanasia, ovaries were removed from mice and homogenized and assayed for virus content by standard virus plaque assay on Vero cell monolayers and staining with 1% crystal violet. No protection was observed in ovaries after control VV challenge (data not shown).
Isolation and purification of lymphocytes from ILNs and genital tract.
ILN cells were isolated from individual mice. For genital tract lymphocyte isolation, the entire genital tract was removed and cut into 0.5-cm pieces that were then rinsed with Ca-Mg-free Hanks' balanced salt solution (HBSS). The tissue was incubated in a mixture of 5 mM EDTA and Ca-Mg-free HBSS at 37°C for two 15-min periods with gentle stirring. The tissue was then incubated with RPMI 1640 containing 2% bovine calf serum, antibiotics, 25 mM HEPES, and 1.5-mg/ml collagenase (Roche Diagnostics, Indianapolis, Ind.) and incubated at 37°C with stirring, for two periods of 1 h. The isolated cells were pooled together and separated on a 40/75% discontinuous Percoll gradient (Pharmacia, Piscataway, N.J.) centrifuged at 600 × g at 25°C for 20 min. Mononuclear cell pellets were resuspended in complete RPMI 1640 at 4°C until use.
Cytokine and β-chemokine measurement.
Production of cytokines and β-chemokines was measured in supernatants from cell cultures. Supernatants were stored at −70°C until analysis by enzyme-linked immunosorbent assays specific for IFN-γ and β-chemokine with MIP-1α, MIP-1β, and RANTES performed according to the manufacturer's specifications (R&D Systems, Minneapolis, Minn.). Calibrated standards were provided by the manufacturer.
ELISPOT assay.
Single-cell suspensions from genital tract and ILN were prepared as previously described (17). Ninety-six-well nitrocellulose plates (Millipore, Bedford, Mass.) were coated with 10-μg/ml anti-IFN-γ antibody in PBS and incubated overnight at 4°C. IFN-γ-producing cells were analyzed by enzyme-linked immunospot (ELISPOT) assay according to the manufacturer's instructions.
Lymphocyte proliferation assays.
Fresh lymphocytes were isolated from immunized mice and seeded in a round-bottom 96-well plate (Becton Dickinson) at 5 × 105 cells/well in complete RPMI 1640 medium that contained 10% FBS and 1% antibiotics. Cells were cultured with medium alone, phytohemagglutinin (PHA; 10 μl/ml), or inactivated gp120-depleted HIV-1 immunogen (10 μg/ml). All assays were done in triplicate. After incubation, cells were labeled with 1 μCi of [3H]thymidine in complete RPMI medium for 16 h. The next day, cells were harvested and the incorporated label was determined by scintillation counting in a β-counter. Geometric cpm were calculated from the triplicate wells with and without Ag. Results were calculated as a lymphocyte stimulation index, which is the geometric mean cpm of cells incubated with Ag divided by the geometric mean cpm of cells without Ag.
Flow cytometry.
Preparation for flow cytometry analysis involved suspending 5 × 105 lymphocytes isolated from the genital tract in PBS-0.1% (wt/vol) bovine serum albumin supplemented with 0.1% (wt/vol) sodium azide. Cells were then incubated with the relevant MAbs for 30 min at 4°C and washed. Three-color flow cytometry acquisition was performed on FACScan (BD Becton Dickson, San Jose, Calif.). The following reagents and MAbs were obtained from BD PharMingen (Mississauga, Ontario, Canada): fluorescein isothiocyanate (FITC)-conjugated hamster anti-CD3ɛ, Cy-chrome-conjugated anti-CD4 MAb, and phycoerythrin (PE)-conjugated anti-CD8. A total of 5 × 104 gated events were collected by FACScan using CellQuest software, and the data were analyzed by WinList version 5.0 (Verity Software House, Topsham, Maine).
In vivo depletion of CD8 T cells.
MAb TKG0624-2.43 was used to deplete CD8 T cells in vivo. The intraperitoneal injections were given with a total volume of 350 μl of hybridoma ascites diluted in PBS. The depletion protocol started at days 3 and 2 before IVAG challenge and on the day of challenge. A control antibody was also used. Mice were sacrificed on day 6 after challenge, and ovaries were taken for virus titration. The efficacy of the in vivo depletion of CD8 was 98%, as determined by fluorescence-activated cell sorter analysis.
Statistical analysis.
Data were expressed as the mean ± standard error. Statistical analysis was performed with the two-tailed Student's t test for independent samples. The differences between the means of two groups were considered significant when the P value was <0.05. The Mann-Whitney U test was used to analyze protection data.
RESULTS
Intranasal immunization with HIV-1 Ag plus CpG induces IFN-γ and β-chemokine production by lymphocytes isolated from ILNs and genital tract.
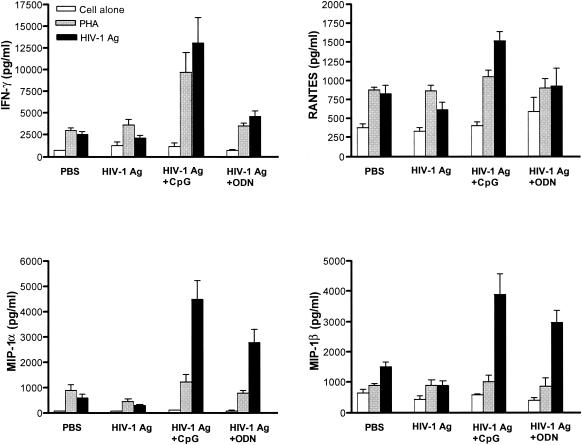
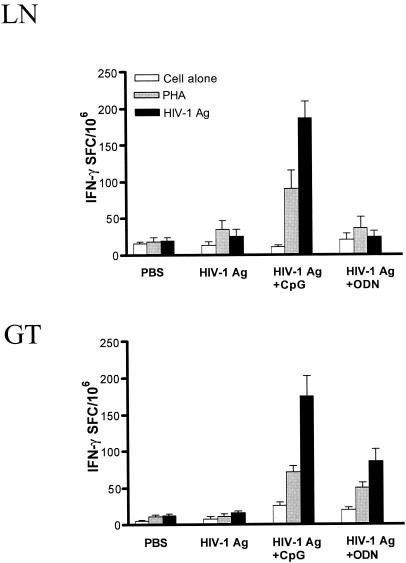
Lymphocytes isolated from ILNs and genital tracts of mucosally immunized mice were examined for production of IFN-γ and β-chemokines. The data presented in Fig. 1 and 2 show that following in vitro restimulation with HIV-1 Ag ILNs and genital tract lymphocytes obtained from mice immunized with HIV-1 Ag plus CpG had significantly increased IFN-γ production (P < 0.05) compared to that with lymphocytes from mice immunized with HIV-1 Ag alone or HIV-1 Ag plus control non-CpG ODN. Indeed, ELISPOT assays clearly showed higher frequencies of HIV-1 Ag-specific IFN-γ-producing lymphocytes in ILNs and genital tracts of mice immunized i.n. with HIV-1 Ag plus CpG compared to those in mice immunized with HIV-1 Ag alone or with control ODN (Fig. 3).
FIG. 1.
ILN lymphocytes from mice i.n. immunized with HIV-1 Ag plus CpG ODN produced high levels of IFN-γ and β-chemokines. ILN lymphocytes were isolated from immunized mice 6 days after IVAG challenge and restimulated in vitro with 10-μg/ml HIV-1 Ag or with 5-μg/ml PHA for 5 days. Subsequently, IFN-γ and β-chemokines in supernatants were tested by ELISA. Data represent the mean ± standard error of five independent experiments with five mice/group. P < 0.05 in IFN-γ, RANTES, MIP-1α, and MIP-1β, compared to PBS-treated controls.
FIG. 2.
Production of IFN-γ and β-chemokines by genital tract lymphocytes (GT) recovered from mice immunized with HIV-1 Ag plus CpG ODN. Lymphocytes were isolated from the genital tracts of immunized mice 6 days after IVAG challenge and in vitro cultured with medium, PHA (5 μg/ml), or HIV-1 Ag (10 μg/ml) for 5 days. IFN-γ, RANTES, MIP-1α, and MIP-1β were measured by ELISA. Data represent the mean ± standard error of five independent experiments with five mice/group. P < 0.05 in IFN-γ, RANTES, MIP-1α, and MIP-1β compared to controls. Error bars indicate standard error.
FIG. 3.
High levels of IFN-γ-secreting cells in ILNs and genital tract lymphocytes (GT) of mice immunized with HIV-1 Ag plus CpG. Immunized mice were challenged IVAG with 107 PFU of VV, and 5 days later, lymphocytes were isolated from individual ILNs and genital tracts. IFN-γ spot-forming cells (SFC) were enumerated in an IFN-γ ELISPOT assay with or without PHA or HIV-1 Ag stimulation. Data are the mean values of triplicate samples (P < 0.05 in the group immunized with HIV-1 Ag plus CpG compared with controls). Error bars indicate standard error.
Since RANTES, MIP-1α, and MIP-1β are natural ligands to the CCR5 coreceptor used by HIV-1 for mucosal infection and thus serve as soluble host antiviral factors, we assessed β-chemokine production by ILNs and genital tract lymphocytes following mucosal immunization. Both ILNs and genital tract lymphocytes from mice immunized with HIV-1 Ag plus CpG secreted significantly higher levels of RANTES, MIP-1α, and MIP-1β when restimulated with HIV-1 Ag in vitro compared with control mice (P < 0.05) (Fig. 1 and 2). Mice immunized with HIV-1 Ag alone did not have higher production than the control group, and although mice immunized with HIV-1 Ag plus the control ODN group had slightly higher production, the increase was not significant (Fig. 1 and 2).
In vitro proliferative response of lymphocytes from ILN and genital tract.
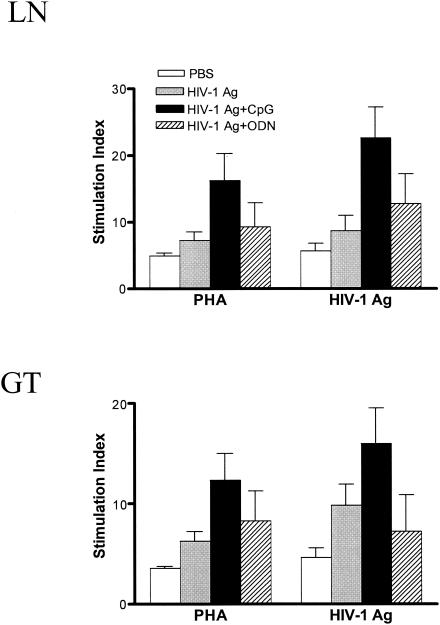
To further address mucosal immunity induced by gp120-depleted HIV-1 Ag plus CpG, in vitro lymphocyte proliferation assays were performed (Fig. 4). Lymphocytes from ILN and genital tract showed strong proliferation in response to viral Ag in mice i.n. immunized with HIV-1 Ag plus CpG (P < 0.01) compared with lymphocytes from mice immunized with PBS. Minimal proliferation was observed in lymphocytes from mice immunized with HIV-1 Ag alone or in combination with control ODN. Together, these data indicate that i.n. immunization with HIV-1 Ag plus CpG induced strong T-cell-mediated immune responses in genital-associated lymphoid tissues and the genital tract.
FIG. 4.
Proliferation response of ILN lymphocytes or genital tract lymphocytes (GT) to HIV-1 Ag and PHA in naïve mice (PBS) or mice immunized with HIV-1 Ag alone, HIV-1 Ag plus CpG, or control non-CpG ODN. Data are the mean values of triplicate samples, and each group was composed of five mice. Proliferation of lymphocytes from HIV-1 Ag plus CpG was significantly greater than that of lymphocytes from mice that were immunized with PBS (P < 0.01).
CD8 T cells are increased in the genital tract and mediate protection against IVAG challenge following i.n. administration of inactivated gp120-depleted HIV-1 Ag plus CpG.
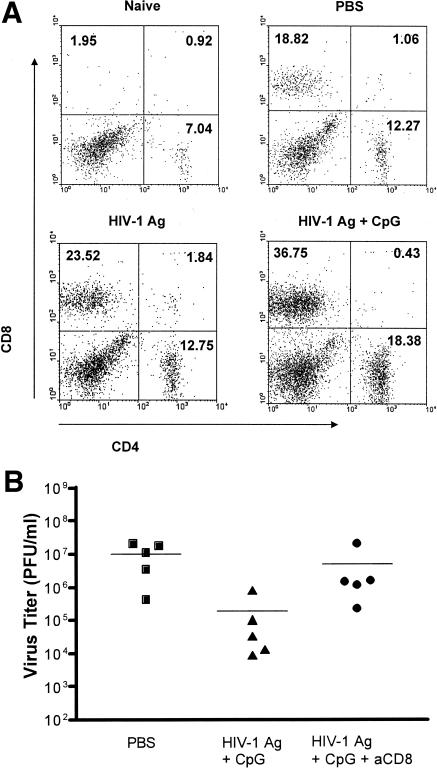
We previously demonstrated that mice immunized i.n. with HIV-1 Ag plus CpG were protected against IVAG challenge with rVV expressing HIV-1 gag (10). To further dissect the mechanism behind this protection, we examined the phenotype of lymphocytes in genital tracts of immunized mice following IVAG challenge with rVV expressing HIV-1 gag. Lymphocytes from naïve mice served as a baseline. Our results (Fig. 5A) revealed that IVAG challenge with rVV gag increased both CD4+ and CD8+ T cells in the genital tract. Mice immunized with HIV-1 plus CpG had greatly increased CD8+ T-cell population in the genital tract, with a twofold increase in comparison with PBS immunization. Similarly, the CD4+ profile in the genital tracts of mice immunized with HIV-1 Ag plus CpG showed a 50% increase compared with the PBS-immunized group. These data may suggest that CD8+ and/or CD4+ T cells play a major role in our model.
FIG. 5.
(A) Intranasal administration of inactivated gp120-depleted HIV-1 Ag plus CpG ODN increases CD4 and CD8 T cells in the genital tract. Lymphocytes were isolated from genital tracts (GT) of mice immunized with PBS as a control, HIV-1 Ag alone, or HIV-1 Ag plus CpG or control ODN and challenged with VV expressing HIV gag. Cells from untreated mice served as baseline. Cells were stained with PE-anti-CD8, FITC-anti-CD3, or Cy-chrome-conjugated anti-CD4 MAbs. Representative dot plots are shown. The quadrant data represent the percentage of gated lymphocytes. The dot plots are representative of three independent experiments. (B) Anti-CD8 MAb treatment abrogates protection against IVAG virus challenge. Mice were immunized twice with PBS or HIV-1 Ag plus CpG and were treated with anti-CD8 MAb on days 3 and 2 before challenge and the day of challenge. Five days after challenge, ovaries were removed for virus plaque assay. Data are titers of rVV in mouse ovaries. Each group consists of five mice.
Anti-CD8 MAb treatment blocks protection against IVAG virus challenge.
To further determine the role of CD8+ T cells in our model, immunized mice were treated with PBS, anti-CD8 MAb, or isotype control antibody before IVAG challenge. Data in Fig. 5B showed that anti-CD8 MAb treatment almost completely blocked protection from IVAG challenge since mice immunized with HIV-1 Ag plus CpG with anti-CD8 treatment had virus levels comparable to those of mice immunized with PBS alone. In contrast, mice immunized with HIV-1 Ag plus CpG without anti-CD8 treatment, as well as treated with antibody isotype control (data not shown), still showed substantial protection from immunization. Thus, these results strongly indicate that CD8+ T lymphocytes are actively involved in IVAG protection with this challenge model.
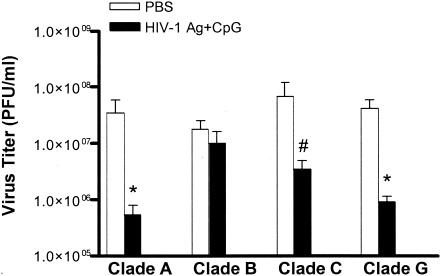
Cross-clade protection against mucosal viral challenge.
Previously we demonstrated that HIV-1 Ag plus CpG induced a protective immune response against IVAG rVV expressing clade G gag protein challenge (10). To determine whether the mucosal immunization could induce cross-clade protection, mice immunized with HIV-1 Ag plus CpG or PBS alone were challenged IVAG with rVVs expressing HIV gag from different clades, including HIV gag from clades G (cT247), A (vT135), B (vP1287), and C (vT196). As shown in Fig. 6, cross-clade protection was observed between clades A, C, and G but not clade B in mice immunized with HIV-1 Ag plus CpG.
FIG. 6.
Intranasal immunization with HIV-1 Ag plus CpG protects mice against IVAG challenge with an HIV-1 gag from clades G (vT247), A (vT135), and C (vT196). Mice were immunized and challenged IVAG with 107 PFU of rVV expressing gag of clades A, B, C, and G. Data are titers of rVV in mouse ovaries determined on Vero monolayers. The mean of each set of data is indicated by a bar with the standard error. * and #, P < 0.01 and P < 0.05, respectively, versus the virus titer in naïve mice by Mann-Whitney U test from clade G (vT243), A (vT135), B (vP1287), or C (vT196).
DISCUSSION
Nearly all HIV infections are acquired by sexual transmission across mucosal barriers (40), and MALTs are a major target for early virus replication and persistence (9). Consequently, HIV vaccine strategies that maximize mucosal immunity, especially in the genital tract, may be more effective at preventing or containing HIV infection. Further, in light of the extraordinary diversity of HIV-1 strains circulating in human populations, it will be important to evaluate the capacity of promising HIV vaccines to protect against heterologous challenge strains. Previously, we showed that i.n. immunization with inactivated gp120-depleted HIV-1 Ag plus CpG ODN induced enhanced levels of anti-HIV antibodies in serum and vaginal washes and protection against IVAG challenge with rVV expressing HIV-1 gag from the same clade as the immunogen (10). Here we extend these studies and show that i.n. immunization with HIV-1 Ag plus CpG induced local T-cell-mediated immune responses in the ILN and genital tract and CD8-mediated cross-clade protection against IVAG challenge.
The mucosal surfaces of the genital tract represent the primary site for sexual transmission of HIV. To prevent virus transmission across the mucosal epithelium and dissemination to the regional LNs, effective vaccines should ideally stimulate immune responses at the mucosal tissues and the associated LNs. Our results support the effectiveness of mucosal i.n. immunization for induction of immune response in the genital tract and associated ILNs, which is consistent with others' findings (4, 15, 24, 35). We found that i.n. prime-boost inoculation using inactivated gp120-depleted HIV-1 Ag plus CpG ODN as an adjuvant generated significant HIV-1 antigen-specific immune responses in the genital tract, which were stronger than those in mice immunized with HIV-1 Ag alone or HIV-1 Ag plus ODN. Similar responses were also detected in the local draining ILNs. An important question that still remains unresolved is the mechanism by which i.n. immunization elicits vaginal immune responses. The nasal route of immunization has demonstrated the greatest potential for simultaneously inducing humoral and cell-mediated immunity in both the rectal and genital tract mucosa, as well as the systemic compartment (26). It is likely that i.n. delivery results in uptake of HIV-1 Ag, which consists of inactivated gp120-depleted virus particles, by M cells that overlie nasal-associated lymphoid tissue and transport to the underlying lamina propria. Previously we showed that i.n. immunization results in induction of antiviral CTLs in mucosal compartments and is more effective than parenteral routes for long-term maintenance of CTLs in the female genital tract (16). Although genital tract homing receptors remain unidentified, it appears that i.n. immunization results in enhanced ability of antigen-specific lymphocytes to disseminate to many distal mucosal compartments (26). We recently demonstrated that i.n. immunization of mice with recombinant glycoprotein B of HSV-2 plus CpG ODN induced IgA in both vaginal and nasal washes, whereas IVAG immunization only resulted in glycoprotein B-specific IgA locally in the genital tract but not in nasal washes (28). In humans and primates, nasal immunization has produced specific IgA antibodies in secretions of the salivary glands, upper and lower respiratory tract, the large intestine, and male and female genital tracts (26).
Recent advances in our understanding of recognition by the innate immune system is revolutionizing our understanding of the importance of innate immunity for protection against infection, induction of adaptive immune responses, and the mechanisms underlying the activity of adjuvants (2). Bacterial DNA sequences containing unmethylated CpG motifs and synthetic CpG ODN activate the innate immune system via TLR9. A large number of studies have shown that CpG ODN is an effective adjuvant for both systemic as well as mucosal vaccination (27). The effectiveness of CpG ODN as an adjuvant may be due to its ability to directly activate dendritic cells, B cells, and macrophages to induce secretion of Th1 cytokines and chemokines. In our studies, immunization with HIV-1 Ag plus CpG ODN resulted in enhanced production of IFN-γ and β-chemokines in response to HIV-1 Ag. RANTES, MIP-1α, and MIP-1β are potent inhibitors of HIV infection (6), and stimulation of their production at mucosal surfaces may serve to prevent binding or transfer of virus to susceptible coreceptor-bearing cells. Accumulated evidence has shown that Th1 immunity is critical for control of HIV infection. Indeed, disease progression is characterized by a loss of Th1 activity, a shift to a more Th2-type response, and loss of cytotoxic T-cell activity against infected host cells. Since CpG serves as a strong Th1-promoting adjuvant, even in the absence of T-cell help, using it in prophylactic or therapeutic vaccines may be useful. Furthermore, CpG induces an antiapoptotic effect in both B and T cells, which may serve to maintain critical adaptive immune effector responses. Indeed, following i.n. immunization with recombinant viral protein plus CpG ODN led to significantly increased numbers and persistence of specific antiviral ASCs in the genital tract and maintenance of high levels of vaginal wash IgA and IgG antibodies throughout the estrous cycle (18).
It is widely believed that CD8+ T cells play the major role in controlling HIV replication. This is based on evidence showing that depletion of CD8+ lymphocytes from monkeys during chronic simian immunodeficiency virus (SIV) infection resulted in a rapid and marked increase in viremia, which in turn was suppressed coincident with the reconstitution of SIV-specific CD8+ T cells (21, 39) and studies showing that mutation of HIV epitopes is important for immune escape from CD8 T-cell recognition (25). Moreover, sex workers who are highly exposed to HIV without seroconversion generate anti-HIV CD8+ T-cell responses locally in the genital tract (38). This evidence has promoted efforts to develop CTL-based vaccine strategies against HIV-1. Amara et al. (1) have shown that vaccinating macaques to induce SIV-specific responses of CD8+ T cells enables the animals to control infection more effectively after challenge with an aggressive SIV.
In our study, female mice mucosally immunized with HIV-1 Ag plus CpG ODN significantly increased antigen-specific CD8+ T cells in the genital tract. After antigen stimulation, these cells produced high levels of IFN-γ, β-chemokines, and possibly other antiviral factors. Importantly, our data also showed that treatment of mucosally immunized mice with anti-CD8 MAb abolished the protection against IVAG challenge. Thus, our results show that i.n. immunization with HIV-1 Ag plus CpG ODN dramatically increased the percentage of CD8+ T cells in the genital tract, and these cells play a critical role for protection against IVAG virus challenge in our murine model.
A major challenge to development of effective HIV vaccines and treatments is the extraordinarily high genetic variability of HIV-1 and rapid turnover of virions. There are eight subtypes of HIV-1 circulating worldwide, and individual isolates may vary by 20 to 30% at the nucleotide and amino acid levels. Although mixtures of high concentrations of nMAbs have been shown to neutralize and protect against a variety of clades of HIV-1 (12), induction of these antibodies during natural infection is rare and methods to induce such broadly neutralizing antibodies through vaccination are an as yet unmet challenge. In this study, we demonstrated that immunization with HIV-1 Ag plus CpG induced cross-clade protection against IVAG challenge with rVV expressing HIV-1 gag from clades A, G, and C but not clade B. The HIV-1 Ag used here is composed of gp120-depleted HIV-1 (HZ321), which contains a clade G gag (34). Since the HIV-1 immunogen used here lacks gp120, which is important for induction of neutralizing antibodies, and since the protection in our model appears to be due to CD8+ T cells, the cross-clade protection observed was most likely due to sharing of an H-2b-restricted gag-specific CTL epitope shared between clades A, C, and G but lacking in clade B. Although we have not yet characterized the protection seen in our model, our results indicate that cross-reactive CD8+ T cells can be generated following mucosal immunization with nonreplicating HIV-1 Ag plus CpG ODN as an adjuvant. Furthermore, the IVAG challenge model used here provides a method to assess the induction of such cross-reactive T cells in the mucosa.
In conclusion, the present study provides new evidence that i.n. immunization with inactivated gp120-depleted HIV-1 Ag plus CpG elicits strong T-cell-mediated immune responses in both genital-associated lymphoid tissues and the genital tract and cross-clade protection against IVAG challenge. Mucosal immunization must be considered as a critical alternative vaccination strategy against HIV.
Acknowledgments
This work was supported by research grants from the Canadian Institutes for Health Research (CIHR) and the Canadian Network for Vaccines & Immunotherapeutics (CANVAC). J.Q.J. is supported by a Studentship Award from the Ontario HIV Treatment Network (OHTN), and K.L.R. is a Career Scientist of the OHTN.
REFERENCES
- 1.Amara, R. R., F. Villinger, J. D. Altman, S. L. Lydy, S. P. O'Neil, S. I. Staprans, D. C. Montefiori, Y. Xu, J. G. Herndon, L. S. Wyatt, M. A. Candido, N. L. Kozyr, P. L. Earl, J. M. Smith, H. L. Ma, B. D. Grimm, M. L. Hulsey, J. Miller, H. M. McClure, J. M. McNicholl, B. Moss, and H. L. Robinson. 2001. Control of a mucosal challenge and prevention of AIDS by a multiprotein DNA/MVA vaccine. Science 292:69-74. [DOI] [PubMed] [Google Scholar]
- 2.Ashkar, A. A., and K. L. Rosenthal. 2002. Toll-like receptor 9, CpG DNA and innate immunity. Curr. Mol. Med. 2:545-556. [DOI] [PubMed] [Google Scholar]
- 3.Baba, T. W., V. Liska, R. Hofmann-Lehmann, J. Vlasak, W. Xu, S. Ayehunie, L. A. Cavacini, M. R. Posner, H. Katinger, G. Stiegler, B. J. Bernacky, T. A. Rizvi, R. Schmidt, L. R. Hill, M. E. Keeling, Y. Lu, J. E. Wright, T. C. Chou, and R. M. Ruprecht. 2000. Human neutralizing monoclonal antibodies of the IgG1 subtype protect against mucosal simian-human immunodeficiency virus infection. Nat. Med. 6:200-206. [DOI] [PubMed] [Google Scholar]
- 4.Bernstein, D. I. 2000. Effect of route of vaccination with vaccinia virus expressing HSV-2 glycoprotein D on protection from genital HSV-2 infection. Vaccine 18:1351-1358. [DOI] [PubMed] [Google Scholar]
- 5.Choi, D. J., S. Dube, T. P. Spicer, H. B. Slade, F. C. Jensen, and B. J. Poiesz. 1997. HIV type 1 isolate Z321, the strain used to make a therapeutic HIV type 1 immunogen, is intersubtype recombinant. AIDS Res. Hum. Retrovir. 13:357-361. [DOI] [PubMed] [Google Scholar]
- 6.Cocchi, F., A. L. DeVico, A. Garzino-Demo, S. K. Arya, R. C. Gallo, and P. Lusso. 1995. Identification of RANTES, MIP-1 alpha, and MIP-1 beta as the major HIV-suppressive factors produced by CD8+ T cells. Science 270:1811-1815. [DOI] [PubMed] [Google Scholar]
- 7.Davila, E., M. G. Velez, C. J. Heppelmann, and E. Celis. 2002. Creating space: an antigen-independent, CpG-induced peripheral expansion of naive and memory T lymphocytes in a full T-cell compartment. Blood 100:2537-2545. [DOI] [PubMed] [Google Scholar]
- 8.Devito, C., J. Hinkula, R. Kaul, J. Kimani, P. Kiama, L. Lopalco, C. Barass, S. Piconi, D. Trabattoni, J. J. Bwayo, F. Plummer, M. Clerici, and K. Broliden. 2002. Cross-clade HIV-1-specific neutralizing IgA in mucosal and systemic compartments of HIV-1-exposed, persistently seronegative subjects. J. Acquir. Immune Defic. Syndr. 30:413-420. [DOI] [PubMed] [Google Scholar]
- 9.Douek, D. C., L. J. Picker, and R. A. Koup. 2003. T cell dynamics in HIV-1 infection. Annu. Rev. Immunol. 21:265-304. [DOI] [PubMed] [Google Scholar]
- 10.Dumais, N., A. Patrick, R. B. Moss, H. L. Davis, and K. L. Rosenthal. 2002. Mucosal immunization with inactivated human immunodeficiency virus plus CpG oligodeoxynucleotides induces genital immune responses and protection against intravaginal challenge. J. Infect. Dis. 186:1098-1105. [DOI] [PubMed] [Google Scholar]
- 11.Ferrantelli, F., M. Kitabwalla, R. A. Rasmussen, C. Cao, T. C. Chou, H. Katinger, G. Stiegler, L. A. Cavacini, Y. Bai, J. Cotropia, K. E. Ugen, and R. M. Ruprecht. 2004. Potent cross-group neutralization of primary human immunodeficiency virus isolates with monoclonal antibodies—implications for acquired immunodeficiency syndrome vaccine. J. Infect. Dis. 189:71-74. [DOI] [PubMed] [Google Scholar]
- 12.Ferrantelli, F., and R. M. Ruprecht. 2002. Neutralizing antibodies against HIV—back in the major leagues? Curr. Opin. Immunol. 14:495-502. [DOI] [PubMed] [Google Scholar]
- 13.Ferrari, G., W. Humphrey, M. J. McElrath, J. L. Excler, A. M. Duliege, M. L. Clements, L. C. Corey, D. P. Bolognesi, and K. J. Weinhold. 1997. Clade B-based HIV-1 vaccines elicit cross-clade cytotoxic T lymphocyte reactivities in uninfected volunteers. Proc. Natl. Acad. Sci. USA 94:1396-1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fukada, K., H. Tomiyama, C. Wasi, T. Matsuda, S. Kusagawa, H. Sato, S. Oka, Y. Takebe, and M. Takiguchi. 2002. Cytotoxic T-cell recognition of HIV-1 cross-clade and clade-specific epitopes in HIV-1-infected Thai and Japanese patients. AIDS 16:701-711. [DOI] [PubMed] [Google Scholar]
- 15.Gallichan, W. S., D. C. Johnson, F. L. Graham, and K. L. Rosenthal. 1993. Mucosal immunity and protection after intranasal immunization with recombinant adenovirus expressing herpes simplex virus glycoprotein B. J. Infect. Dis. 168:622-629. [DOI] [PubMed] [Google Scholar]
- 16.Gallichan, W. S., and K. L. Rosenthal. 1996. Long-lived cytotoxic T lymphocyte memory in mucosal tissues after mucosal but not systemic immunization. J. Exp. Med. 184:1879-1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gallichan, W. S., and K. L. Rosenthal. 1998. Long-term immunity and protection against herpes simplex virus type 2 in the murine female genital tract after mucosal but not systemic immunization. J. Infect. Dis. 177:1155-1161. [DOI] [PubMed] [Google Scholar]
- 18.Gallichan, W. S., R. N. Woolstencroft, T. Guarasci, M. J. McCluskie, H. L. Davis, and K. L. Rosenthal. 2001. Intranasal immunization with CpG oligodeoxynucleotides as an adjuvant dramatically increases IgA and protection against herpes simplex virus-2 in the genital tract. J. Immunol. 166:3451-3457. [DOI] [PubMed] [Google Scholar]
- 19.Hofmann-Lehmann, R., J. Vlasak, R. A. Rasmussen, B. A. Smith, T. W. Baba, V. Liska, F. Ferrantelli, D. C. Montefiori, H. M. McClure, D. C. Anderson, B. J. Bernacky, T. A. Rizvi, R. Schmidt, L. R. Hill, M. E. Keeling, H. Katinger, G. Stiegler, L. A. Cavacini, M. R. Posner, T.-C. Chou, J. Andersen, and R. M. Ruprecht. 2001. Postnatal passive immunization of neonatal macaques with a triple combination of human monoclonal antibodies against oral simian-human immunodeficiency virus challenge. J. Virol. 75:7470-7480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Horner, A. A., A. Ronaghy, P. M. Cheng, M. D. Nguyen, H. J. Cho, D. Broide, and E. Raz. 1998. Immunostimulatory DNA is a potent mucosal adjuvant. Cell. Immunol. 190:77-82. [DOI] [PubMed] [Google Scholar]
- 21.Jin, X., D. E. Bauer, S. E. Tuttleton, S. Lewin, A. Gettie, J. Blanchard, C. E. Irwin, J. T. Safrit, J. Mittler, L. Weinberger, L. G. Kostrikis, L. Zhang, A. S. Perelson, and D. D. Ho. 1999. Dramatic rise in plasma viremia after CD8(+) T cell depletion in simian immunodeficiency virus-infected macaques. J. Exp. Med. 189:991-998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kaul, R., F. A. Plummer, J. Kimani, T. Dong, P. Kiama, T. Rostron, E. Njagi, K. S. MacDonald, J. J. Bwayo, A. J. McMichael, and S. L. Rowland-Jones. 2000. HIV-1-specific mucosal CD8+ lymphocyte responses in the cervix of HIV-1-resistant prostitutes in Nairobi. J. Immunol. 164:1602-1611. [DOI] [PubMed] [Google Scholar]
- 23.Kaul, R., D. Trabattoni, J. J. Bwayo, D. Arienti, A. Zagliani, F. M. Mwangi, C. Kariuki, E. N. Ngugi, K. S. MacDonald, T. B. Ball, M. Clerici, and F. A. Plummer. 1999. HIV-1-specific mucosal IgA in a cohort of HIV-1-resistant Kenyan sex workers. AIDS 13:23-29. [DOI] [PubMed] [Google Scholar]
- 24.Klavinskis, L. S., C. Barnfield, L. Gao, and S. Parker. 1999. Intranasal immunization with plasmid DNA-lipid complexes elicits mucosal immunity in the female genital and rectal tracts. J. Immunol. 162:254-262. [PubMed] [Google Scholar]
- 25.Klenerman, P., Y. Wu, and R. Phillips. 2002. HIV: current opinion in escapology. Curr. Opin. Microbiol. 5:408-413. [DOI] [PubMed] [Google Scholar]
- 26.Kozlowski, P. A., and M. R. Neutra. 2003. The role of mucosal immunity in prevention of HIV transmission. Curr. Mol. Med. 3:217-228. [DOI] [PubMed] [Google Scholar]
- 27.Krieg, A. M. 2002. CpG motifs in bacterial DNA and their immune effects. Annu. Rev. Immunol. 20:709-760. [DOI] [PubMed] [Google Scholar]
- 28.Kwant, A., and K. L. Rosenthal. 2004. Intravaginal immunization with viral subunit protein plus CpG oligodeoxynucleotides induces protective immunity against HSV-2. Vaccine 22:3098-3104. [DOI] [PubMed] [Google Scholar]
- 29.Mascola, J. R., G. Stiegler, T. C. VanCott, H. Katinger, C. B. Carpenter, C. E. Hanson, H. Beary, D. Hayes, S. S. Frankel, D. L. Birx, and M. G. Lewis. 2000. Protection of macaques against vaginal transmission of a pathogenic HIV-1/SIV chimeric virus by passive infusion of neutralizing antibodies. Nat. Med. 6:207-210. [DOI] [PubMed] [Google Scholar]
- 30.Mazzoli, S., D. Trabattoni, S. Lo Caputo, S. Piconi, C. Ble, F. Meacci, S. Ruzzante, A. Salvi, F. Semplici, R. Longhi, M. L. Fusi, N. Tofani, M. Biasin, M. L. Villa, F. Mazzotta, and M. Clerici. 1997. HIV-specific mucosal and cellular immunity in HIV-seronegative partners of HIV-seropositive individuals. Nat. Med. 3:1250-1257. [DOI] [PubMed] [Google Scholar]
- 31.McAdam, S., P. Kaleebu, P. Krausa, P. Goulder, N. French, B. Collin, T. Blanchard, J. Whitworth, A. McMichael, and F. Gotch. 1998. Cross-clade recognition of p55 by cytotoxic T lymphocytes in HIV-1 infection. AIDS 12:571-579. [DOI] [PubMed] [Google Scholar]
- 32.McCluskie, M. J., and H. L. Davis. 1998. CpG DNA is a potent enhancer of systemic and mucosal immune responses against hepatitis B surface antigen with intranasal administration to mice. J. Immunol. 161:4463-4466. [PubMed] [Google Scholar]
- 33.Moldoveanu, Z., L. Love-Homan, W. Q. Huang, and A. M. Krieg. 1998. CpG DNA, a novel immune enhancer for systemic and mucosal immunization with influenza virus. Vaccine 16:1216-1224. [DOI] [PubMed] [Google Scholar]
- 34.Moss, R. B., W. K. Giermakowska, J. R. Savary, G. Theofan, A. E. Daigle, S. P. Richieri, F. C. Jensen, and D. J. Carlo. 1998. A primer on HIV type 1-specific immune function and REMUNE. AIDS Res. Hum. Retrovir. 14(Suppl. 2):S167-S175. [PubMed] [Google Scholar]
- 35.O'Hagan, D., C. Goldbeck, M. Ugozzoli, G. Ott, and R. L. Burke. 1999. Intranasal immunization with recombinant gD2 reduces disease severity and mortality following genital challenge with herpes simplex virus type 2 in guinea pigs. Vaccine 17:2229-2236. [DOI] [PubMed] [Google Scholar]
- 36.Parren, P. W. H. I., P. A. Marx, A. J. Hessell, A. Luckay, J. Harouse, C. Cheng-Mayer, J. P. Moore, and D. R. Burton. 2001. Antibody protects macaques against vaginal challenge with a pathogenic R5 simian/human immunodeficiency virus at serum levels giving complete neutralization in vitro. J. Virol. 75:8340-8347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Richieri, S. P., R. Bartholomew, R. C. Aloia, J. Savary, R. Gore, J. Holt, F. Ferre, R. Musil, H. R. Tian, R. Trauger, P. Lowry, F. Jensen, D. J. Carlo, R. Z. Maigetter, and C. P. Prior. 1998. Characterization of highly purified, inactivated HIV-1 particles isolated by anion exchange chromatography. Vaccine 16:119-129. [DOI] [PubMed] [Google Scholar]
- 38.Rowland-Jones, S. L., T. Dong, K. R. Fowke, J. Kimani, P. Krausa, H. Newell, T. Blanchard, K. Ariyoshi, J. Oyugi, E. Ngugi, J. Bwayo, K. S. MacDonald, A. J. McMichael, and F. A. Plummer. 1998. Cytotoxic T cell responses to multiple conserved HIV epitopes in HIV-resistant prostitutes in Nairobi. J. Clin. Investig. 102:1758-1765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schmitz, J. E., M. J. Kuroda, S. Santra, V. G. Sasseville, M. A. Simon, M. A. Lifton, P. Racz, K. Tenner-Racz, M. Dalesandro, B. J. Scallon, J. Ghrayeb, M. A. Forman, D. C. Montefiori, E. P. Rieber, N. L. Letvin, and K. A. Reimann. 1999. Control of viremia in simian immunodeficiency virus infection by CD8+ lymphocytes. Science 283:857-860. [DOI] [PubMed] [Google Scholar]
- 40.Shattock, R. J., and J. P. Moore. 2003. Inhibiting sexual transmission of HIV-1 infection. Nat. Rev. Microbiol. 1:25-34. [DOI] [PubMed] [Google Scholar]
- 41.Veazey, R. S., M. DeMaria, L. V. Chalifoux, D. E. Shvetz, D. R. Pauley, H. L. Knight, M. Rosenzweig, R. P. Johnson, R. C. Desrosiers, and A. A. Lackner. 1998. Gastrointestinal tract as a major site of CD4+ T cell depletion and viral replication in SIV infection. Science 280:427-431. [DOI] [PubMed] [Google Scholar]
- 42.Yi, A. K., P. Hornbeck, D. E. Lafrenz, and A. M. Krieg. 1996. CpG DNA rescue of murine B lymphoma cells from anti-IgM-induced growth arrest and programmed cell death is associated with increased expression of c-myc and bcl-xL. J. Immunol. 157:4918-4925. [PubMed] [Google Scholar]