Abstract
Ebola virus infection causes a highly lethal hemorrhagic fever syndrome associated with profound immunosuppression through its ability to induce widespread inflammation and cellular damage. Though GP, the viral envelope glycoprotein, mediates many of these effects, the molecular events that underlie Ebola virus cytopathicity are poorly understood. Here, we define a cellular mechanism responsible for Ebola virus GP cytotoxicity. GP selectively decreased the expression of cell surface molecules that are essential for cell adhesion and immune function. GP dramatically reduced levels of αVβ3 without affecting the levels of α2β1 or cadherin, leading to cell detachment and death. This effect was inhibited in vitro and in vivo by brefeldin A and was dependent on dynamin, the GTPase. GP also decreased cell surface expression of major histocompatibility complex class I molecules, which alters recognition by immune cells, and this effect was also dependent on the mucin domain previously implicated in GP cytotoxicity. By altering the trafficking of select cellular proteins, Ebola virus GP inflicts cell damage and may facilitate immune escape by the virus.
Ebola virus causes a highly pathogenic infection resulting in rapid failure of multiple organ systems and in death in many cases. The highest mortality rates are observed with the Zaire subtype, one of four types identified to date (7, 14). Though the pathogenesis of Ebola virus infection at the molecular level has not been fully explained, the envelope glycoproteins likely contribute to adverse events in the host (17, 18). The envelope gene of Ebola virus gives rise to two products from a single gene, a nonstructural secreted form of the glycoprotein, sGP, encoded by the predominant transcript (∼80%), and the virion envelope glycoprotein, GP, the result of RNA editing during formation of the message (∼20%). The tight control of virion GP synthesis may be necessary due to the ability of the full-length glycoprotein to elicit cytopathic effects in cells targeted by the virus (4, 17, 18). The mechanism by which GP causes cytopathicity in target cells remains to be elucidated, but its effects are evident from its ability to cause cell rounding and detachment (18). Here we report that Ebola virus GP-induced cytotoxicity involves a cellular trafficking pathway that is dependent upon dynamin, a GTPase that mediates transport vesicle formation.
MATERIALS AND METHODS
Expression vectors and cell lines.
ADV-GP(Z), the recombinant adenovirus expressing Zaire GP, was made according to the standard protocol for making first-generation recombinant adenovirus (1) and has been described previously. Expression vectors p1012, pGP(Z), and pGPΔMUC contain a cytomegalovirus enhancer promoter and have been described previously. The dynamin and K44E-dynamin expression vectors are pcDNA3.1 based and were a generous gift from P. Okamoto and R. Vallee and were received from D. Ganem (9). Human umbilical vein endothelial cells (HUVEC) and EGM-2 culture medium were obtained from BioWhittaker/Clonetics. 293 human embryonic kidney cells were cultured in Dulbecco’s modified Eagle’s medium supplemented with 10% fetal bovine serum (GIBCO). Transfections were performed using Fugene6 transfection reagent (Roche) according to the manufacturer's instructions.
Flow cytometry and antibodies.
HUVEC were infected with the indicated vectors at a multiplicity of infection (MOI) of 500, or 293 cells were transfected with plasmid vectors and analyzed by fluorescence-activated cell sorting for cell surface expression of the indicated glycoproteins after 12 to 24 h. Cells were collected after incubation with phosphate-buffered saline (PBS) (3 mM EDTA) and incubated with control immunoglobulin (Ig), rabbit anti-sGP/GP serum (generously provided by A. Sanchez), anti-integrin monoclonal antibodies (Chemicon International, Inc., Temecula, Calif.), or an HLA class I antibody (Biosource International) for 30 min on ice. The cells were washed twice with ice-cold PBS containing 2.5% fetal bovine serum, incubated with fluorescein isothiocyanate (FITC) (Jackson ImmunoResearch Laboratories, West Grove, Pa.)- or phycoerythrin (Sigma)-conjugated secondary antibodies for 30 min on ice, followed by washing. Analysis was conducted using a Becton Dickinson 4-color Calibur flow cytometer and FlowJo analysis software (Tree Star, Inc.).
Confocal microscopy.
HUVEC were infected with adenovirus vectors in chamber slides, fixed, and permeabilized 10 to 16 h postinfection. Cells were stained using the primary antibodies described above for 1 h at room temperature, washed, and incubated with Alexa 488-, Alexa 568-, or Alexa 594-conjugated secondary antibodies (Jackson ImmunoResearch Laboratories). Images were collected on a Leica TCS-NT/SP confocal microscope (Leica Microsystems, Exton, Pa.) using a 63× oil immersion objective (NA 1.32, zoom X). Fluorochromes were excited using an argon laser at 488 nm for FITC and a krypton laser at 568 nm for Alexa 568. Detector slits were configured to minimize any cross-talk between the channels. Differential interference contrast images were collected simultaneously with the fluorescence images by use of a transmitted light detector. Images were processed using Leica TCS-NT/SP (version 1.6.587), Imaris 3.1.1 (Bitplane AG, Zurich, Switzerland), and Adobe Photoshop 5.5 (Adobe Systems) software.
Metabolic labeling and immunoprecipitation.
Cells were metabolically labeled with 75 μCi each of [35S]cysteine and [35S]methionine per 10-cm-diameter tissue culture dish overnight. Radioactive medium was removed, and cells were washed, lysed with NP-40 buffer (1% NP-40, 0.15 M NaCl, 10 mM Tris, pH 7.5), and immunoprecipitated with antibodies against integrins and GP (described above) or dynamin (Transduction Laboratories, San Diego, Calif.) by use of immobilized protein G (Pierce, Rockford, Ill.). For some experiments metabolic labeling was omitted, and immunoprecipitated material was analyzed after sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) by Western blotting.
Expression of GP in vasculature and peroxidase perfusion experiments.
Porcine carotid arteries were infected with 2.5 × 108 PFU of ADV-ΔE1 or ADV-GP (Zaire)/ml in PBS for 1 h at room temperature. Vessels were then incubated with M199 medium containing 20% fetal calf serum for 48 h in a tissue culture incubator. Subsequently, the vessels were divided and processed for peroxidase perfusion experiments to determine permeability changes of the vessels or for scanning electronic microscopy as described previously (18). Briefly, for perfusion the vessels were perfused with 200 U of peroxidase (P8250; Sigma)/ml in PBS for 3 min at a constant Hg pressure (90 ± 2 [standard error of the mean] mm), fixed in 4% paraformaldehyde-10% glutaraldehyde for 2 h at 4°C, and incubated in diaminobenzidine solution (2 ng/ml) for 1 h.
RESULTS
Down-regulation of cell surface proteins by GP is selective.
Expression of Ebola virus GP disrupts cell adhesion in 293 embryonic kidney cells or HUVEC and is followed by cell death in 24 to 48 h (18). We therefore examined whether GP could alter cell surface expression of adhesion molecules. HUVEC were infected with replication-defective adenovirus vectors with no insert (ADV-ΔE1), the negative control, or with an insert encoding Ebola virus GP (ADV-GP). After 24 h, cells infected with ADV-GP showed rounding and detachment from the substratum whereas ADV-ΔE1 cells were unchanged, as reported previously (18). Because cadherin, α2β1, and αVβ3 are representative cell surface molecules known to mediate cell adhesion via their interaction with receptors on neighboring cells or the extracellular matrix, their expression levels were measured by flow cytometry (Fig. 1A). Cell surface αVβ3 levels were dramatically reduced in GP-expressing cells, in contrast to the results seen with cadherin, which was similarly expressed in endothelial cells infected with ADV-ΔE1 or ADV-GP. The α2β1 heterodimer expression was also unaffected by GP, whereas the β1 subunit was shown to be down-modulated in 293 cells (16), suggesting specificity of GP effects. Ebola virus GP contains a region approximately 200 amino acids in length that is rich in serines and threonines, in similarity to mucin proteins, and this mucin-like region is responsible for GP-mediated cell rounding and cytotoxicity (18). Expression of the GP lacking mucin (ADV-GPΔMUC), which is functionally active (18), abolished GP-mediated cell detachment and also completely reversed the decrease in αVβ3 levels (Fig. 1B). These data suggested that one effect of Ebola virus GP expression in endothelial target cells was disruption of cell adhesion through an effect on the αVβ3 integrin.
FIG. 1.
Effect of Ebola virus GP on cell surface adhesion molecules and down-regulation of αV integrins. (A) Selective down-regulation of cell surface adhesion molecules by Ebola virus GP. HUVEC were infected with the indicated vectors at an MOI of 500 and analyzed by fluorescence-activated cell sorting for cell surface expression of the indicated glycoproteins after 15 h. Cells were collected after incubation with PBS (3 mM EDTA) and incubated with anti-integrin anti-bodies (solid lines) as indicated for 30 min on ice. The cells were washed once with ice-cold PBS and incubated with FITC-conjugated sheep anti-mouse IgG for 30 min on ice, followed by washing. A matched isotype antibody was used as a negative control in each case (dashed line). Results are representative of three independent experiments. (B) Down-regulation of αV integrin in 293 cells. 293 cells were transfected with a plasmid encoding vector control, Ebola virus GP, or Ebola virus GP(ΔMUC). Both floating and adherent cells were collected 18 h after transfection, using 3 mM EDTA to remove adherent cells. Flow cytometry was performed on cells double stained for Ebola virus GP and αV integrin as described in Materials and Methods. Results are shown for events in the live cell gate. (C) Analysis of αV integrin degradation by metabolic labeling. HUVEC cells were infected with adenovirus vectors expressing the indicated inserts and metabolically labeled with [35S]cysteine and [35S]methionine. At 8 h postinfection, cells were washed and harvested either immediately (8 h) or after further incubation in unlabeled medium (11 and 19 h). After harvesting, cells were lysed in NP-40 buffer, immunoprecipitated with an αVβ3 antibody, clone LM609 (Chemicon International), and analyzed by SDS-PAGE on 4 to 15% gels.
GP alters recycling of αVβ3.
To define the mechanism by which GP reduced αVβ3 expression, we examined the effect of Ebola virus GP on αVβ3 turnover. Pulse-chase experiments ([35S]cysteine-methionine) were conducted using HUVEC infected with the ADV-ΔE1 or ADV-GP adenovirus vector to label the intracellular pool of αVβ3, and its degradation was monitored. Over a 19-h period, total cell-associated αVβ3 levels remained constant in cells infected with ADV-GP and were comparable to the levels in cells infected with empty vector (ADV-ΔE1) (Fig. 1C), despite the marked decrease in cell surface expression. The αVβ3 bands at 19 h in the control cells are somewhat lighter than those seen at the same time point in the GP-expressing cells. However, the difference in levels of band intensity (by scanning densitometry) is less than twofold. These data suggested that the reduction of cell surface αVβ3 levels was independent of its total turnover within the cell and that GP affected its transport or recycling.
GP effects on cell surface proteins are sensitive to brefeldin A.
Brefeldin A, a fungal metabolite, is known to block protein trafficking between the endoplasmic reticulum and Golgi complex, as well as recycling. To determine whether the effects of GP were mediated by a brefeldin-sensitive pathway, HUVEC were infected with ADV-ΔE1 or ADV-GP and treated with this drug for 10 h after infection. Treatment with brefeldin A completely blocked the ability of GP to induce cell rounding and detachment, and these cultures appeared to be similar to those of control cells infected with the empty vector (ADV-ΔE1). In untreated cells that express GP, rounding was observed in approximately 50% of the cells (Fig. 2A). Moreover, brefeldin A was able to block GP cytopathicity in intact blood vessels. Porcine carotid arteries were surgically removed and infused with an adenovirus vector expressing Ebola virus GP (Fig. 2B). In the absence of brefeldin A, GP destroyed the integrity of the endothelial cell layer of the vessels, exposing the basement membrane, as previously shown (18). Brefeldin A reversed the GP cytopathic effect completely. The results with brefeldin A suggested that the ability of GP to cause down-regulation of αVβ3 and disruption of blood vessel integrity was dependent upon the presence of a brefeldin-sensitive protein-trafficking pathway.
FIG. 2.
Brefeldin A inhibits the cytopathic effects of GP in cell culture and in porcine arteries. (A) Effect of brefeldin A on Ebola virus GP cytopathicity. HUVEC cells were infected by ADV-ΔE1 or ADV-GP at an MOI of 500. At 6 h after infection, brefeldin A was added (+) or the cells were left untreated (−). At 16 h after infection, pictures were taken. The percentage of rounding was calculated by counting the cells of three slides. (B) Cultured porcine arteries infected with ADV-GP. Peroxidase perfusion analysis to determine permeability changes was performed in the absence (None) (0 ng/ml) or presence (Brefeldin A) of brefeldin A treatment (100 ng/ml) as described in Materials and Methods. (C) Scanning electron micrograph of porcine arteries infected with ADV-GP and treated with brefeldin A (Brefeldin A) or left untreated (None). Vessels were infected with ADV-GP as described in Materials and Methods and processed for scanning electron microscopy (11).
αVβ3 and GP colocalize and interact biochemically.
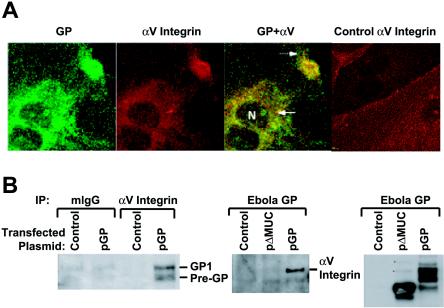
We next examined whether there was a relationship between the subcellular localizations of αVβ3 and GP by use of confocal microscopy. HUVEC were infected with ADV-GP and costained with a monoclonal antibody against αV and a polyclonal antiserum raised against GP. This analysis revealed that GP and αV colocalized in the perinuclear region and within a small area at the plasma membrane (Fig. 3A). The colocalization of GP and αV suggested that GP might directly cause the redistribution of αV within cells. Coimmunoprecipitation studies were therefore performed to determine whether the two molecules interact biochemically. 293 cells were transfected with an empty, control vector or with a GP expression vector plasmid, and cell lysates were immunoprecipitated with a monoclonal antibody against αV integrin or an isotype control. These samples were run on SDS-PAGE and immunoblotted with a polyclonal antiserum to Ebola virus GP. The monoclonal antibody specific for αV, in contrast to a control antibody, was able to coimmunprecipitate GP (Fig. 3B, left panel). We then performed the converse experiment in which an antibody to αV integrin was used to coprecipitate GP (Fig. 3B, middle panel). It is noteworthy that GPΔMUC, when expressed at levels similar to those seen with wild-type GP (Fig. 3B, right panel), which does not down-regulate αV, did not coprecipitate αV. These results demonstrated a protein association between Ebola virus GP and αV integrin that likely contributes to the intracellular redistribution of αV.
FIG. 3.
Subcellular distribution of Ebola virus GP and biochemical interaction with αV integrin. (A) Cellular distribution of Ebola virus GP and αV integrin by confocal microscopy. HUVEC were infected with ADV-ΔE1 or ADV-GP at an MOI of 300 for 1 h. At 10 h later, the cells were fixed, permeabilized, and stained for the indicated protein by use of specific monoclonal antibodies followed by secondary staining with Alexa 488 (green [GP])- or Alexa 568 (red [αV])- conjugated antibodies. The “GP+αV” panel shows an overlay of the red and green panels. GP (green) and αV (red) colocalized in the perinuclear region (solid arrow) and within a small area at the plasma membrane (dashed arrow). The far right panel shows the distribution of αV integrin in control cells not expressing GP. Slides were analyzed by confocal microscopy as described in Materials and Methods. (B) Coprecipitation of Ebola virus GP and αV integrin. 293 cells were transfected with Ebola virus GP (pGP), Ebola virus GP lacking the mucin domain (pΔMUC), or empty vector (Control) and incubated for 20 h. Supernatant cells were collected and pooled with adherent cells harvested by incubation with 3 mM EDTA. Lysis was performed with NP-40 buffer, and immunoprecipitation was performed using an isotype control mouse IgG (mIgG) or a monoclonal antibody against αV integrin. Immunoprecipitated proteins were separated by SDS-4 to 15% PAGE, and gels were immunoblotted using an antibody specific for Ebola virus GP. The left panel shows coprecipitation of GP by αV integrin antibody. The middle panel shows the inverse coprecipitation of αV integrin with GP antibody. The right panel shows the total amount of GP in the cell lysates.
GP-mediated down-regulation of αVβ3 requires functional dynamin.
The biosynthesis of mammalian αV integrins is slow; full maturation to the biologically active form requires 20 h. Once on the cell surface, integrins accumulate within microdomains called caveolae or lipid rafts (8). Recycling of molecules that reside in lipid rafts is influenced by the GTPase dynamin, which mediates pinching off of transport vesicles from the cytoplasmic face of rafts (12). K44E, a mutant form of dynamin, contains a mutation in the GTP binding domain that renders the molecule nonfunctional and causes it to interfere with the normal function of wild-type dynamin in a dominant-negative manner (9). We employed dynamin K44E to investigate the possibility that GP reduces cell surface αV integrin through a dynamin-dependent transport pathway. 293 cells were cotransfected with expression vectors encoding GP and dynamin K44E, and the amount of αV integrin on the cell surface was measured by flow cytometry. Dynamin K44E alone had no effect on basal αV cell surface expression in vector control-transfected cells (data not shown); however, dynamin K44E reversed approximately 40% of the GP-mediated decrease in surface αV integrin levels (Fig. 4A).
FIG. 4.
Interaction of Ebola virus GP with dynamin and inhibition of cell surface class I MHC expression. (A) Dominant-negative dynamin inhibition of αV integrin down-regulation. 293 cells were transfected with a vector encoding Ebola virus GP without (GP) or with (GPK+44E) dynamin K44E as indicated. Empty vector was transfected in the absence of dynamin K44E. Floating and adherent cells were harvested 18 h after transfection, pooled, and double stained with αV integrin and GP antibodies, followed by secondary staining as described in Materials and Methods. Results are shown for events in the live-cell gate. (B) Coprecipitation of GP with dynamin. 293 cells were transfected with Ebola virus GP (pGP) or empty vector (Control) and incubated for 20 h. Supernatant cells were collected and pooled with adherent cells harvested by incubation with 3 mM EDTA. Lysis was performed with NP-40 buffer, and immunoprecipitation was performed using a monoclonal antibody against dynamin or an unrelated control antibody (α1 integrin). Immunoprecipitated proteins were separated by SDS-4 to 15% PAGE, and gels were immunoblotted using an antibody specific for Ebola virus GP. (C) Down-regulation of MHC-I molecules and dynamin dependence. 293 cells were transfected with a vector control (Control), Ebola virus GP (GP), Ebola virus GP lacking the mucin domain (GPΔMUC), or Ebola virus GP plus dynamin K44E. Floating and adherent cells were harvested and double stained with antibodies against MHC-I and GP (left three panels) and against MHC-I alone, in GP-positive cells, in the absence (light line) or presence (bold line) of dynamin K44E (right panel). Analysis is shown for events in the live-cell gate.
Coimmunoprecipitation was performed to determine whether Ebola virus GP could also interact with dynamin. 293 cells were transfected with GP, and cell lysates were immunoprecipitated with a monoclonal antibody against dynamin, followed by Western blotting with a polyclonal antiserum to Ebola virus GP. GP coprecipitated with dynamin, indicating that there was also a colocalization of the two molecules (Fig. 4B). In contrast to the interaction of αV integrin with both mature GP and an intermediate form, dynamin coprecipitates with only the mature GP. These results suggested that Ebola virus GP down-regulated αV integrin through its ability to influence the dynamin protein-trafficking pathway. Through its interaction with dynamin and αV integrin, GP may redirect the normal cycling of one or both of these molecules in a way that alters the steady-state levels of αV integrin on the cell surface.
Down-regulation of MHC-I by Ebola virus GP.
Many viruses have evolved to circumvent immune responses by modulating proteins essential for immune signaling and function. Major histocompatibility complex class I (MHC-I) molecules are targeted by several pathogens, including cytomegalovirus, human immunodeficiency viruses, and herpesviruses (13). One of these viruses, Kaposi's sarcoma-associated herpesvirus (5), reduces cell surface expression of MHC-I molecules through a dynamin-dependent pathway (6). Since the down-regulation of αV integrin is dynamin dependent, we investigated whether Ebola virus GP also reduced MHC-I expression and thus altered protective immunity to Ebola virus. To test this possibility, cell surface expression of MHC-I was determined in 293 cells transfected with a control or GP expression vector by flow cytometry. At 24 h after transfection, cells expressing Ebola virus GP exhibited a dramatic reduction in cell surface MHC-I levels (Fig. 4C). In similarity to αV integrin, down-modulation was dependent on the mucin domain of GP and was partially reversed by the dominant-negative dynamin, K44E (Fig. 4C). As expected, the decrease in surface MHC-I levels caused by GP expression also reduced recognition of GP-expressing target cells by allogeneic effector T cells (data not shown).
DISCUSSION
Ebola virus typically avoids clearance by the host immune response in humans, resulting in uncontrolled replication, damage to host cells, and, ultimately, fatal organ destruction. Though some patients who mount a strong, early immune response can recover from infection (3), the fatality rates remain high. An understanding of Ebola virus pathogenic mechanisms facilitates the development of antiviral and immune therapies, which are presently unavailable. Though previous reports have suggested that Ebola virus GP alters expression of cell surface proteins (15, 16), the specificity and mechanisms of these effects have not been defined. The findings presented here demonstrate that Ebola virus GP exerts this effect through a specific mechanism involving a pathway that is dependent upon the GTPase dynamin. Through its interaction with dynamin, GP disrupts the normal intracellular trafficking of cell surface proteins that are essential for cell attachment, viability, and immune signaling. The functional interactions shown herein appear to indicate a direct binding of GP to dynamin and a specific subset of cell surface molecules. However, Ebola virus GP might also influence trafficking in particular regions of the cell surface membrane in which these specific sets of molecules reside.
We now have several lines of evidence to suggest that αV down-regulation is highly dependent on GP-trafficking within the cell. For example, both transmembrane deletion and alternate soluble forms of GP have no effect on αV levels, implying that residence of GP at the plasma membrane (or at least within membrane compartments that are in equilibrium with the plasma membrane) is necessary for the effect. The brefeldin A results reinforce this interpretation, because brefeldin A acts between the endoplasmic reticulum and Golgi complex to inhibit membrane traffic and therefore likely prevents GP from accessing specific membrane compartments or, perhaps, impedes posttranslational modifications of GP that occur late in trafficking and that are required for the effect. It is noteworthy that the GP-induced cytopathic effects require cell surface expression of Ebola virus GP (16). A previous report suggested that global down-regulation of cell surface molecules is exerted by GP (15), but our present experiments and those of others (15, 16) demonstrate that the expression of at least some cell surface molecules is unaffected. With human kidney 293T cells, minimal to nonexistent down-regulation was observed for several α-integrins (16), whereas β1 integrin is unaffected in HUVEC (15) but is down-regulated in 293T cells (15).
These disparate results suggest that Ebola virus GP does not cause global down-regulation but, rather, is linked to particular groups of molecules depending on cell type. Moreover, specific intracellular trafficking pathways that affect cell surface expression seem to be particularly relevant to down-regulation. Brefeldin A prevents Ebola virus GP from accessing cell surface microcompartments, and dynamin influences cycling to and from these same areas of the cell. We have observed that GP expression appears to be lost from the cell surface at time points when αV down-regulation is maximal. A likely explanation for this observation is that at late stages, down-regulation of GP is linked to removal of αV from the cell surface. Our biochemical and confocal results indicate that these two molecules reside in close proximity to one another. Therefore, a mechanism (e.g., increased endocytosis) that down-regulates αV may be expected to also influence cell surface levels of GP. The results presented herein provide a mechanism by which Ebola virus GP might influence groups of molecules, specified by particular cell trafficking pathways or by microlocalization at the cell membrane.
Unlike many enveloped viruses, the envelope glycoprotein gene of Ebola virus does not exhibit the high sequence variability that readily allows immune escape. These results suggest that Ebola virus has evolved in its natural host a mechanism that allows immune evasion in humans. Through its effects on specific cell surface molecules, Ebola virus disrupts several processes essential for immune activation and recognition, such as cell trafficking and antigen presentation. Adhesion and accessory proteins such as αV are involved in immune cell homing and signaling (2), and down-regulation by GP may further affect homing, acute inflammation, and costimulation that would reduce both innate and acquired immune responses. At the same time, the cytotoxic effects of GP on macrophage and endothelial cell function affect the function of inflammatory cells (10, 19) and disrupt the integrity of the vasculature (18). Together, these cytotoxic effects are likely responsible for the inflammatory dysregulation, immune suppression, and vascular dysfunction that are hallmarks of lethal Ebola virus infection.
Acknowledgments
We thank Ati Tislerics, Karen Stroud, Toni Garrison, Brenda Hartman, and Tina Suhana for manuscript preparation, Judy Stein for discussions and comments, and Owen Schwartz (National Institutes of Health-National Institute of Allergy and Infectious Diseases) for assistance with confocal microscopy. We are grateful to Patricia Okamoto, Richard Vallee, and Donald Ganem for kindly supplying the dominant-negative dynamin expression vector. We also thank members of the Roederer and Seder labs (National Institutes of Health, Vaccine Research Center, NIAID) for helpful discussions.
REFERENCES
- 1.Aoki, K., C. Barker, X. Danthinne, M. J. Imperiale, and G. J. Nabel. 1999. Efficient generation of recombinant adenoviral vectors by Cre-lox recombination in vitro. Mol. Med. 5:224-231. [PMC free article] [PubMed] [Google Scholar]
- 2.Arroyo, A. G., D. Taverna, C. A. Whittaker, U. G. Strauch, B. L. Bader, H. Rayburn, D. Crowley, C. M. Parker, and R. O. Hynes. 2000. In vivo roles of integrins during leukocyte development and traffic: insights from the analysis of mice chimeric for α5, αv, and α4 integrins. J. Immunol. 165:4667-4675. [DOI] [PubMed] [Google Scholar]
- 3.Baize, S., E. M. Leroy, M.-C. Georges-Courbot, M. Capron, J. Lansoud-Soukate, P. Debre, S. P. Fisher-Hoch, J. B. McCormick, and A. J. Georges. 1999. Defective humoral responses and extensive intravascular apoptosis are associated with fatal outcome in Ebola virus-infected patients. Nat. Med. 5:423-426. [DOI] [PubMed] [Google Scholar]
- 4.Chan, S. Y., M. C. Ma, and M. A. Goldsmith. 2000. Differential induction of cellular detachment by envelope glycoproteins of Marburg and Ebola (Zaire) viruses. J. Gen. Virol. 81:2155-2159. [DOI] [PubMed] [Google Scholar]
- 5.Chang, Y., E. Cesarman, M. S. Pessin, F. Lee, J. Culpepper, D. M. Knowles, and P. S. Moore. 1994. Identification of herpesvirus-like DNA sequences in AIDS-associated Kaposi's sarcoma. Science 266:1865-1869. [DOI] [PubMed] [Google Scholar]
- 6.Coscoy, L., and D. Ganem. 2000. Kaposi's sarcoma-associated herpesvirus encodes two proteins that block cell surface display of MHC class I chains by enhancing their endocytosis. Proc. Natl. Acad. Sci. USA 97:8051-8056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Feldmann, H., S. T. Nichol, H. D. Klenk, C. J. Peters, and A. Sanchez. 1994. Characterization of filoviruses based on differences in structure and antigenicity of the virion glycoprotein. Virology 199:469-473. [DOI] [PubMed] [Google Scholar]
- 8.Giancotti, F. G., and E. Ruoslahti. 1999. Integrin signaling. Science 285:1028-1032. [DOI] [PubMed] [Google Scholar]
- 9.Herskovits, J. S., C. C. Burgess, R. A. Obar, and R. B. Vallee. 1993. Effects of mutant rat dynamin on endocytosis. J. Cell Biol. 122:565-578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kindzelskii, A. L., Z. Yang, G. J. Nabel, R. F. Todd III, and H. R. Petty. 2000. Ebola virus secretory glycoprotein (sGP) diminishes FcγRIIIB-to-CR3 proximity on neutrophils. J. Immunol. 164:953-958. [DOI] [PubMed] [Google Scholar]
- 11.Mbiene, J. P., D. K. Maccallum, and C. M. Mistretta. 1997. Organ cultures of embryonic rat tongue support tongue and gustatory papilla morphogenesis in vitro without intact sensory ganglia. J. Comp. Neurol. 377:324-340. [DOI] [PubMed] [Google Scholar]
- 12.Oh, P., D. P. McIntosh, and J. E. Schnitzer. 1998. Dynamin at the neck of caveolae mediates their budding to form transport vesicles by GTP-driven fission from the plasma membrane of endothelium. J. Cell Biol. 141:101-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ploegh, H. L. 1998. Viral strategies of immune evasion. Science 280:248-253. [DOI] [PubMed] [Google Scholar]
- 14.Sanchez, A., S. G. Trappier, B. W. J. Mahy, C. J. Peters, and S. T. Nichol. 1996. The virion glycoproteins of Ebola viruses are encoded in two reading frames and are expressed through transcriptional editing. Proc. Natl. Acad. Sci. USA 93:3602-3607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Simmons, G., R. J. Wool-Lewis, F. Baribaud, R. C. Netter, and P. Bates. 2002. Ebola virus glycoproteins induce global surface protein down-modulation and loss of cell adherence. J. Virol. 76:2518-2528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Takada, A., S. Watanabe, H. Ito, K. Okazaki, H. Kida, and Y. Kawaoka. 2000. Downregulation of β1 integrins by Ebola virus glycoprotein: implication for virus entry. Virology 278:20-26. [DOI] [PubMed] [Google Scholar]
- 17.Volchkov, V. E., V. A. Volchkova, E. Muhlberger, L. V. Kolesnikova, M. Weik, O. Dolnik, and H.-D. Klenk. 2001. Recovery of infectious Ebola virus from complementary DNA: RNA editing of the GP gene and viral cytotoxicity. Science 291:1965-1969. [DOI] [PubMed] [Google Scholar]
- 18.Yang, Z.-Y., H. J. Duckers, N. J. Sullivan, A. Sanchez, E. G. Nabel, and G. J. Nabel. 2000. Identification of the Ebola virus glycoprotein as the main viral determinant of vascular cell cytotoxicity and injury. Nat. Med. 6:886-889. [DOI] [PubMed] [Google Scholar]
- 19.Yang, Z., R. Delgado, L. Xu, R. F. Todd, E. G. Nabel, A. Sanchez, and G. J. Nabel. 1998. Distinct cellular interactions of secreted and transmembrane Ebola virus glycoproteins. Science 279:1034-1037. [DOI] [PubMed] [Google Scholar]