Abstract
To determine whether identifying haemoglobin genotype, and providing education and counselling to senior school students will influence their choice of partner and reduce the frequency of births with sickle cell disease. The Manchester Project provided free voluntary blood tests to determine haemoglobin genotype to the fifth and sixth forms (grades 11–13), median age of 16.7 years, of all 15 secondary schools in the parish of Manchester in south central Jamaica. A total of 16,636 students complied, and counselling was offered to carriers of abnormal genes over 6 years (2008–2013). The genotypes of their offspring were determined by newborn screening of 66,892 deliveries in 12 regional hospitals over 8 years (2008–2015). The study focused on the genotypes of live deliveries to female students with the four most common haemoglobin genotypes: 7905 with an AA genotype, 898 with the sickle cell trait, 326 with the HbC trait and 78 with the beta thalassaemia trait. A total of 2442 live deliveries were identified by the end of 2015 in mothers screened at school. Eleven babies had clinically significant genotypes, and the prevalence of SS and SC disease did not differ from that predicted by random mating. First pregnancy was not delayed in AS or AC mothers. There was no evidence that knowledge of maternal haemoglobin genotype influenced choice of partner. On an interview, mothers of affected babies correctly recalled their genotype, but either did not discuss this with their partners or the latter refused to be tested. Subjects delaying child bearing for tertiary education would be largely excluded from the present study of first pregnancies and may make greater use of this information. Future options are a greater role for prenatal diagnosis.
Introduction
Homozygous sickle cell (SS) disease occurs in an estimated 312,000 births annually1, and all forms of sickle cell disease are likely to exceed 400,000 births annually. In sub-Saharan Africa, 240,000 babies with SS disease are estimated to be born each year (Piel et al. 2013), and while major management improvements have occurred in more developed societies, most sub-Saharan countries do not have the required resources or medical infrastructure. Whereas, the median survival of SS disease in the USA (Platt et al. 1994) and Jamaica (Wierenga et al. 2001) exceeds 40–50 years, it is likely, although not formally documented, that median survival of SS disease in many African countries is less than 5 years. Faced with a public health problem of this scale, disease prevention must be a priority if the limited resources are to provide better care for existing patients. Since the disease results from the inheritance of abnormal genes from both parents, it may be prevented if one parent has a normal haemoglobin (AA) genotype. In Bahrain, where 16% of the population carry the HbS gene, voluntary premarital screening for the sickle cell gene reduced the prevalence of SS births from 2.0 to 0.9% (Al Arrayed 2005) and became mandatory in June 2004. Similar legislation was passed in the same year for Saudi Arabia (Alswaidi et al. 2012; Memish and Saeed 2011), and the published data for 2009 show that out of nearly 300,000 marriage proposals, 1171 received ‘incompatible certificates’ of which, 608 (52%) marriages were cancelled. However, experience from Islamic societies with traditions of arranged marriages may not be applicable to other communities.
In the Orchomenos Program (Stammatoyannopoulos 1974), a farming community north of Athens, Greece, with a 23% prevalence of the sickle cell trait and characterised by arranged marriages in the mid 1960’s, screening for the sickle cell gene was performed in 2300 families between 1966 and 1970. Seven years later, review showed that although subjects correctly recalled their status and the genetics of sickle cell disease, four marriages had occurred between AS couples compared with 4.5 marriages predicted from random mating. Of these four, two got married while conscious of the risks and two concealed their carrier status with the conclusion that the program had failed to achieve its goals.
The current study addresses whether knowledge of haemoglobin genotype in Jamaicans of predominantly West African ancestry would influence choice of partner and reduce the births with sickle cell disease. Free haemoglobin genotype screening was performed in 16,636 high-school students over a 6-year period, and newborn screening was set up in the south and west of the island to determine the genotypes of their offspring.
Material and methods
Following discussions with the Ministry of Health, the project was based in the Parish of Manchester with an area of 830 km2 stretching from the coast in the south to an altitude of approximately 3000 ft in the north, a population of 192,000 in 2008 (Statistical Institute of Jamaica), and the parish capital Mandeville is 100-km west of the country capital Kingston. Screening was offered to 15 secondary schools, 13 in Manchester and two were close to the border in the neighbouring parish of Clarendon. The target population of fifth and sixth forms (grades 11–13) were offered free screening over six academic years (from 2007/8 to 2012/13) and grades 7–9 over three academic years (from 2010/11 to 2012/13) for a total of 16,636 students (9303 females). Because of the difficulty of confirming paternity, the current analysis was confined to 9207 females with the four most common genotypes: 7905 with the normal AA genotype, 898 with the sickle cell trait (AS), 326 with the HbC trait (AC) and 78 with the beta thalassaemia trait (Aβthal). Excluded were 96 students with other genotypes (30 with hereditary persistence of foetal haemoglobin (HPFH) trait, 21 with SS disease, 20 with SC disease, 11 with other variant traits, seven with Sbeta+ thalassaemia, four with CC disease, one each of Svariant, S-HPFH and an AS out of the age range).
Student screening
Sensitisation and awareness
The programme was approved by the Ministry of Health and the Ministry of Education and was preceded by discussions with the school staff, Parent-Teachers Associations and illustrated lectures on the disease and its genetics to the students. All students were given an information letter for their parents outlining the objectives of the study, the possible advantages and the option of declining for their child to be tested (the opt-out approach having been approved by the Ministry of Health).
Procedure
At pre-arranged times, a team of a physician, clerical assistants and 3–4 phlebotomists visited the school, and students were informed of the opportunity of testing by their form teachers. Each student completed a form providing demographic data (class, date of birth, address, family and contact details), and one 5-ml EDTA sample was taken by venepuncture. The average turn-round time for individuals was about 20 min, and depending on local factors, 150–200 students could be screened within a 2–2½-h period. Most schools required at least two visits, and larger schools received 4–5 visits.
Laboratory procedures
Haematological indices were measured in electronic cell counters (Abbott CellDyn 1700, Sysmex XS-1000i), and haemoglobin electrophoresis was performed in a TEB buffer at pH 8.4 on mylar-backed cellulose acetate (Helena Laboratories) and stained with benzidine. Samples with bands in the position of HbS were confirmed by the slide sickling test and bands in the position of HbC by agar gel at pH 6.2. Samples with MCH ≤26 pg and RDW(CV) <18.0 consistent with the beta thalassaemia trait, had estimations of HbA2 levels, which if ≥3.5%, were referred for DNA sequencing. The detailed methods and overall results are published elsewhere (Mason et al. 2016a).
Distribution of results and counselling
All tested students received laminated cards bearing personal details (name, school, date of birth), laboratory ID number and the haemoglobin genotype with interpretation. Normal subjects (AA genotype) received a card stating that they were not at risk of a baby with sickle cell disease. Carriers of abnormal genes received a card stating the genotype, for example AS with designation ‘Sickle Cell Trait’ and the notation that ‘This will not affect your health but you could pass it on to your children. If your partner is normal, you cannot have a child with sickle cell disease’. The reverse side of the card for those with abnormal genes had a simple pedigree as an example and contact information for the screening laboratory. The cards for normal (AA) genotypes were given in batches to the teacher or guidance counsellor for distribution. Students with abnormal genotypes were individually contacted by the screening team, were given the cards and were offered genetic counselling either individually or in small groups as desired and were given explanation sheets along with pedigrees for common potential partner genotypes and information on how to get their partner tested.
Student database
All student data were maintained in excel files listing basic demography, haematology and haemoglobin genotype. These files needed careful auditing because of the tendency of some students to be tested twice, especially those receiving abnormal results.
Newborn screening
Recruitment of hospitals throughout the south and west of Jamaica started with Mandeville Regional Hospital in August 2008 and covered 12 hospitals by July 2014 with total annual deliveries exceeding 15,000 babies. Samples were collected from the cut umbilical cord onto ‘Guthrie cards’ which were then dried, batched and delivered to the reference laboratory at the Southern Regional Health Authority in Mandeville. All deliveries were listed in excel files bearing mother’s name, date of birth and result of cord blood analysis and periodically reconciled by examination of the delivery books in each hospital.
Procedures
After eluting in water, haemolysates were analysed by high pressure liquid chromatography (Bio-Rad Variant or NBS, Hercules, California), and suspected clinically significant, abnormal genotypes were confirmed at age 2–4 weeks by heel prick with parental studies were possible. Confirmed patients were referred to local Sickle Cell Clinics for follow-up. The distribution of haemoglobin genotypes has been published (Mason et al. 2015).
Collating student and maternal data
Computerised matching of names and dates of birth was not feasible because of variations in spelling, and the process was achieved with databases bearing names and dates of birth of screened students alongside similar information for hospital deliveries. Two formats were used, ranked chronologically and alphabetically by date of birth, and the matching process was conducted by at least two observers, doubtful matches being resolved by phone contact.
Completeness of dataset
It was assumed that the databases derived from the 12 hospitals covered the great majority of deliveries in screened students, and this was supported when interviews with 200 AS mothers confirmed that over 95% deliveries occurred in these hospitals (Mason et al. 2016b).
Contact with mothers delivering clinically abnormal babies
All mothers were informed of significant results and requested to attend for confirmation and family studies. Mothers were interviewed by an individual intimately involved in screening and counselling.
Statistical analysis
Analysis of deliveries to the end of December 2015 excluded twins and pregnancies occurring prior to school screening and those delivering within 9 months of screening, since these may have been pregnant at the time of screening and therefore could not have factored genotype information into their reproductive decisions. Only first pregnancies were assessed since their outcome may have influenced partner choice for subsequent pregnancies. Based on the gene frequency observed among students in the Manchester Project (Mason et al. 2016a) and the assumption of random mating, the probability of an AS mother having an offspring with SS disease was estimated to be 0.02555. Since the primary question was whether the intervention had decreased this prevalence, a decrease was examined by a one-sided exact binomial test. Analysis of 216 offspring of AS mothers and 75 AC mothers indicated powers of 83 and 60%, respectively, to detect a decrease below an expected decrease of 75% in affected offspring at the statistical level of 5%. The possibility that genotype knowledge changed their reproductive behaviour was also considered by estimating the age at first delivery in the different maternal genotypes by the (inverse) Kaplan–Meier estimators, and differences between maternal genotype groups (AA, AS, and AC) were tested by the log rank test. The Bonferroni correction was applied for multiple testing. Calculations used the statistical package R, version 3.1.3, with power calculations using the command binom.power from the library binom.
Ethical issues
Haemoglobin genotypes were requested by senior school students following illustrated lectures to the islands in 160 secondary schools from 2000 to 2006, but the only option then available was referral to private laboratories where tests were expensive and often lacked the required accuracy. This need was met in the current study planned with the Ministry of Health. Details of the procedures prior to screening are detailed in the ‘Sensitisation and Awareness’ section of the methods. Blood tests were voluntary, except for some possible coercion from peers, and disruption of school schedules were minimised. For newborn screening, the Ministry of Health took the position that the benefits of screening so far outweighed any disadvantages that written consent would not be required. Only mothers of babies with clinically significant disease were contacted in order to confirm the diagnosis and recruit the child to local Sickle Cell Clinics.
Results
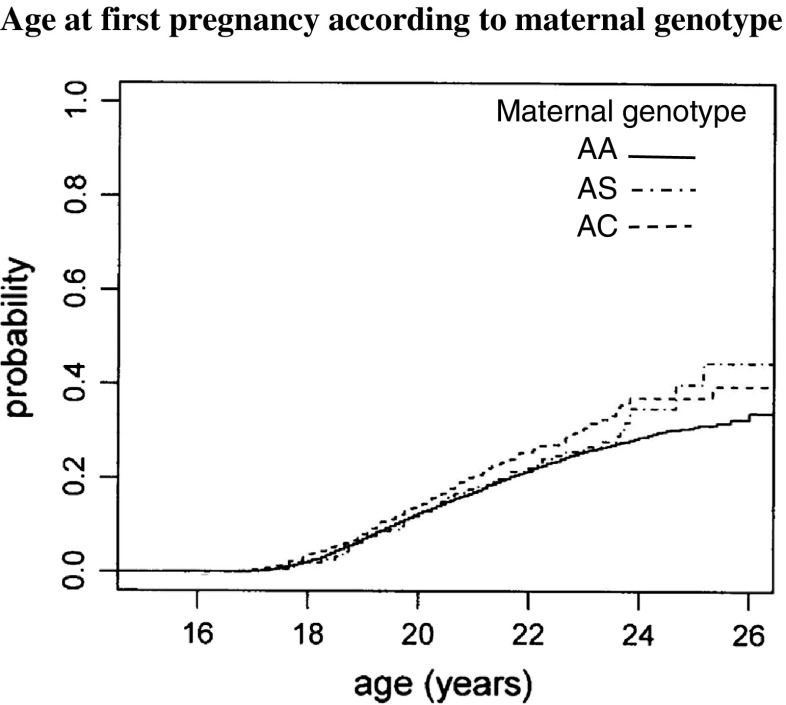
By the end of 2015, there were 2442 pregnancies among the four principal maternal haemoglobin genotypes, of which 2076 were first pregnancies 1742/7905 (22.0%) AA genotype, 231/898 (25.7%) with the sickle cell trait, 78/326 (23.9%) with the HbC trait, and in 25/78 (32.1%) with the beta thalassaemia trait (Table 1). Of these, 119 were excluded (106 delivered or may have been pregnant prior to screening, 13 twin-pairs) leaving 1957 eligible first-pregnancies. Of 216 eligible first-pregnancies among mothers with the sickle cell trait, there were five babies with SS disease compared with 5.5 babies predicted from random mating (p value 0.55), and of 75 eligible first-pregnancies among mothers with the AC trait, there were four babies with SC disease compared with 2.0 predicted from random mating (p value 0.96). Examining the age at first delivery (Figure 1), there was a significant difference between maternal genotypes (log rank test p = 0.0127). Comparing individual maternal genotypes, the differences between AA and AC mothers (log rank test p = 0.375), or between AS and AC mothers (log rank test p = 0.441) lacked statistical significance, but comparison of AA and AS mothers (log rank test p = 0.00388) suggested an earlier age at first pregnancy in AS mothers.
Table 1.
Pregnancies in subjects screened at school (up to Dec 2015)
| Maternal genotype | Total pregn. | Pregnant <screening | Twins (pairs) | Genotype of singleton births | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AA | AS | AC | SS | SC | Sβthal | Variants | Unknown | ‘Eligible’ pregnancies | ||||
| First pregnancy | ||||||||||||
| AA | 1742 | 91 | 10 | 1473 | 84 | 35 | 0 | 0 | 0 | 0 | 49 | 1641 |
| AS | 231 | 13 | 2 | 105 | 104 | 1 | 5 | 1 | 0 | 0 | 0 | 216 |
| AC | 78 | 2 | 1 | 30 | 3 | 37 | 0 | 4 | 0 | 0 | 1 | 75 |
| Aβthal | 25 | 0 | 0 | 21* | 2 | 0 | 0 | 0 | 1 | 0 | 1 | 25 |
| Total | 2076 | 106 | 13 | 1629 | 193 | 73 | 5 | 5 | 1 | 0 | 51 | 1957 |
| Second pregnancy | ||||||||||||
| AA | 286 | 1 | 3 | 257 | 11 | 8 | 0 | 0 | 0 | 2 | 4 | 282 |
| AS | 33 | 0 | 1 | 15 | 17 | 0 | 0 | 0 | 0 | 0 | 0 | 32 |
| AC | 11 | 0 | 0 | 6 | 0 | 5 | 0 | 0 | 0 | 0 | 0 | 11 |
| Aβthal | 4 | 0 | 0 | 4* | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 4 |
| Total | 334 | 1 | 4 | 282 | 28 | 13 | 0 | 0 | 0 | 2 | 4 | 329 |
| Third pregnancy | ||||||||||||
| AA | 26 | 0 | 1 | 23 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 25 |
| AS | 2 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 |
| AC | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Aβthal | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Total | 28 | 0 | 1 | 25 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 27 |
| Fourth pregnancy | ||||||||||||
| AA | 4 | 0 | 0 | 3 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 4 |
| Grand total | 2442 | 107 | 18 | 1939 | 222 | 86 | 5 | 5 | 1 | 2 | 57 | 2317 |
*For mothers with beta thalassaemia trait (Aβthal), these would be an AA phenotype, since approximately half would be Aβthal
Fig. 1.
Age at first pregnancy according to maternal genotype
Babies born to AS mothers
Case No.1 A mother was screened at age 17.9 years, and 3.3 years later, she delivered a baby with SS disease. She knew her AS status but ‘did not remember’ to enquire of her boyfriend who was a secondary school teacher and subsequently shown to be AS. They were later married with no further pregnancies in past 4 years.
Case No.2 A mother was screened at age 17.0 years, and 1.1 years later, she delivered a baby with SS disease. She knew her AS status and asked the baby-father, now a taxi-driver, early in the relationship about his genotype status; he replied that he was ‘normal’, and later tests were confirming an AS genotype. They have a continuing relationship; he is supporting, and there are no further pregnancies.
Case No.3 A mother was screened at age 17.7 years, and 6.2 years later, she delivered a baby with SS disease. She knew her AS status and asked the baby-father, now a mechanic, who declined to be tested during the school screening programme and refused to be tested after the birth of the child or to provide support. The mother is in denial; the baby is cared for primarily by the maternal grandmother, and there have been no further pregnancies.
Case No.4 A mother was screened at age 17.3 years, and 6.3 years later, she delivered a baby with SS disease. She knew her AS status but had a casual relationship with a gas station attendant, working at an adjacent site. There was no discussion of genotype beforehand, but the baby-father was subsequently confirmed as AS. The mother, who had a half-brother with SS disease noted that ‘this should never have happened’. There is no continuing relationship or paternal support and no further pregnancies.
Case No.5 A mother was screened at age 16.8 years, and 5.0 years later, she delivered a baby with SS disease. She knew her AS status, but although this was disclosed to the boyfriend, with whom she had a relationship for at least 5 years, he was not asked to attend for blood test. He attended a school outside the area where screening was offered and has repeatedly defaulted appointments for confirmatory blood tests.
Case No.6 A mother was screened at age 17.0 years, and 3.7 years later, she delivered a baby with SC disease. She knew her AS status but ‘forgot’ to disclose genotype before pregnancy, and since delivery, the father was found to be AC. They have a continuing relationship; the father supports, and there have been no further pregnancies.
Babies born to AC mothers
Case No.7 A mother was screened at age 16.9 years, and 6.3 years later, she delivered a baby with SC disease. She knew her AC status, and the baby-father knew that he was AS, so they went ahead realising that the offspring may have been SC. She currently continues relationship with the father who supports, but no further pregnancies.
Case No.8 A mother was screened at age 16.9 years, and 1.6 years later, she delivered a baby with SC disease. She knew her AC status but did not discuss this with the baby-father. He refused to be tested, has no continuing relationship and does not support.
Case No.9 A mother was screened at age 16.9 years, and 1.8 years later, she delivered a baby with SC disease. She knew that she was AC, the father was screened elsewhere and was told that test was ‘negative’, but after birth of the child, he was confirmed as AS genotype. They were married and have one further child with an AC genotype.
Case No.10 A mother was screened at age 17.4 years, and 1.8 years later, she delivered baby with SC disease. She knew her AC status, and the baby-father suspected that he was AS (a sister died at age 22 years from sickle cell disease), and this was subsequently confirmed. Convinced that she was not at risk of a baby with SS disease, they went ahead realising that the offspring may have been SC disease. She currently continues relationship with father who supports but no further pregnancies.
Baby born to beta thalassaemia trait mother
Case No.11 A mother was screened at age 16.9 years, and 1.4 years later, she delivered baby with Sbeta+ thalassaemia. She originally received a card as an HPFH trait on the basis of an HbF 23.1% and HbA2 4.0%, but analysis of the DNA 7 months later confirmed the beta thalassaemia trait with the −88 C > T mutation. By the time that she received the card with the revised diagnosis, she was already pregnant with a boyfriend who was known to be AS. They have a continuing good relationship but no further pregnancies.
Discussion
Mandatory premarital screening in Bahrain and Saudi Arabia has the advantage of removing any stigma since it applies to all. Even with ‘incompatible certificates’ implying that both prospective partners carry abnormal genes placing them at risk of a child with sickle cell disease, there need be no element of stigma as the families may choose a different partnership to identify a relationship that does not carry this risk. Voluntary premarital screening, as proposed in the Manchester Project relies upon a knowledge and significance of the abnormal genes in the population. Awareness of sickle cell conditions in Jamaica is relatively high. A survey of secondary school students nearly 40 years ago showing 45% had heard of sickle cell disease; the figure rising with educational level, socio-economic status and female gender (Desai and Serjeant 1976). Since then, increasing publicity and using sickle cell as the model for teaching genetics at school are likely to have increased this awareness. This knowledge would have been increased by illustrated lectures on sickle cell disease, genetics and clinical features to the fifth and sixth form students of almost all 160 secondary schools island-wide, sponsored by the Sickle Cell Trust (Jamaica) from 2000 to 2006. It was this lecture series that identified the need for genotype determination as a major request of the students. Specific interventions in the current project included illustrated lectures on the disease and its genetics offered at schools prior to screening, a letter providing further details to the parents, the distribution of results in laminated cards with further information and the offer of counselling and educational materials to carriers of abnormal genes. Voluntary compliance with school screening increased from 56 to 92% over the 6-year period11, and phone interviews of students with the sickle cell trait 1–7 years later showed correct recall of genotype in 95% and of the genetics in 91% (Mason et al. 2016b).
The present study fails to produce evidence that this knowledge has reduced the prevalence of affected births in first pregnancies in Jamaica. Of the 11 mothers delivering children with clinically significant disease, ten knew that their haemoglobin status placed them at risk of a child with sickle cell disease, but five of these ‘did not remember to discuss this’ with their partners; two partners refused to be tested believing themselves to be ‘normal’; two knew or suspected that they had an abnormal genotype, and one had been tested elsewhere and received an incorrect ‘negative’ test but was later shown to have an AS genotype. In the 11th mother, there was diagnostic confusion on her genotype, and believing she had the HPFH gene and was not at risk of a baby with sickle cell disease, she became pregnant with an AS partner, but she was subsequently shown to have the beta+ thalassaemia trait with an unusually high HbF level, and the baby had the mild sickle cell-beta+ thalassaemia. These 11 cases raise a series of issues but failure to discuss genetic information or refusal of the male partner to be tested accounted for seven cases and highlights the frailty of many Jamaican friendships where the girl does not wish to compromise a potential relationship by discussing a possibly contentious issue. The reason for Jamaican men declining to be tested may be fear of the result or the mild discomfort of a blood test, but only 14/315 (4%) male partners of students, known to carry abnormal genes, took advantage of a free blood test (Mason et al. 2016b).
Exploring the effect of genetic information on other indices of child bearing showed no difference between maternal genotypes in the proportion of becoming pregnant. The hypothesis that carriers of abnormal genes may delay pregnancy was not supported by the Kaplan–Meier estimates which showed a shorter interval in AS mothers compared with the AA mothers. The difference, though small, was significant and totally unexpected and seemingly illogical, but there was no known selection bias which could have created this difference; one possible explanation being that carriers of abnormal genes were concerned about fecundity and may have exposed themselves to pregnancy earlier.
Concepts of genetic counselling are closely linked to the educational, social and behavioural practices of a society, and a concern of the present study is the extent to which these observations are applicable elsewhere. Jamaica has a cultural and social history which has resulted in a different concept of marriage and has one of the highest mean ages for females at first marriage at 33.2 years (World Bank Cross Country Data 2016). The great majority of first pregnancies precede marriage and often precede the formation of stable relationships. Many first pregnancies result from short-term and even casual relationships, and concepts traditional in genetic practice such as counselling couples before planned pregnancies may not apply. Even the concept of a planned pregnancy may be difficult to interpret in Jamaica; some mothers stating that they just wanted to have a baby. Of the 11 mothers with offspring with sickle cell disease, two later married the father of their child, five had a continuing relationship and in four, the relationship had ceased without support for the child. The social complexity of these issues is beyond the scope of this paper, but the results must be considered within the context of this very different social framework. They also imply a heavier responsibility in decision making rests with the mother.
Much remains to be learnt on the attitude of the male students screened at school, but with impaternity rates exceeding 10% (GR Serjeant unpublished observations) and the limited ability for DNA confirmation, it was decided to focus on the reproductive outcome of the female students. However, the male attitudes are a vital component of prevention and need to be addressed in the future. Extending screening and education to male partners do not seem realistic since if carriers were identified, the only options for female carriers would to abandon the relationship or prenatal diagnosis with termination of an affected pregnancy, neither of which is entirely satisfactory (Jones et al. 1988). Screening and education at an earlier age might allow children to assimilate this genetic information, but prenatal diagnosis may play an important role. Furthermore, although there is no evidence that knowledge of haemoglobin genotype reduced the frequency of affected births in first pregnancies, it may assume greater importance among those who have delayed child-bearing in pursuit of tertiary education, and this hypothesis will be tested by continued observations of reproductive performance over the next few years.
Acknowledgements
This work was supported by grants from the National Health Fund in Jamaica, the Alcoa Foundation and the Chase Fund of Jamaica. These funders played no role in the study other than providing financial support to a grant proposal. The Southern Regional Health Authority of the Ministry of Health, Jamaica provided valuable infrastructural support to the Mandeville laboratory.
Statement by corresponding author
The corresponding author states that he has had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Contributions of Authors
Graham Serjeant, Beryl Serjeant and Karlene Mason conceived and organised the study including presentations to the school staff, Parent-Teacher Associations and illustrated lecture to the students. They were also intimately involved with the school screening component and collation of the newborn screening data.
Graham Serjeant, Beryl Serjeant, Karlene Mason and Felicea Gibson planned and conducted the school screening along with teams of phlebotomists.
Karlene Mason conducted the follow-up, dissemination of results and counselling of carriers.
Felicea Gibson, Beryl Serjeant and Karlene Mason analysed the haematology and electrophoretic results of the school screening.
Ruth-Ann Gardner and Lansford Warren organised the collection of cord blood samples and analysis by HPLC.
Marianne Jonker advised on the data collection and performed the statistical analysis.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Funding
The National Health Fund of Jamaica, the Alcoa Foundation, the Chase Fund of Jamaica and organisations within the private sector of Jamaica supplied funding for the conduct of the study but had no role in the design, conduct or publication of the work.
References
- Al Arrayed S. Campaign to control genetic blood diseases in Bahrain. Community Genetics. 2005;8(1):52–55. doi: 10.1159/000083340. [DOI] [PubMed] [Google Scholar]
- Alswaidi FM, Memish ZA, O'Brien SJ, Al-Hamdan NA, Al-Enzy FM, Alhayani OA, Al-Wadey AM. At-risk marriages after compulsory premarital testing and counselling for β-thalassaemia and sickle cell disease in Saudi Arabia, 2005-2006. J Genet Counsel. 2012;21(2):243–255. doi: 10.1007/s10897-011-9395-4. [DOI] [PubMed] [Google Scholar]
- Desai P, Serjeant GR. Awareness of sickle cell disease among high school students in Kingston, Jamaica. Public Health Rep. 1976;91(3):265–267. [PMC free article] [PubMed] [Google Scholar]
- Jones S, Shickle DA, Goldstein AR, Serjeant GR. Acceptability of antenatal diagnosis for sickle cell disease among Jamaican mothers and female patients. West Indian Med J. 1988;37(1):12–15. [PubMed] [Google Scholar]
- Mason K, Gibson F, Gardner R, Warren L, Fisher C, Higgs D, Happich M, Kulozik A, Hambleton I, Serjeant BE, Serjeant GR (2015) Newborn screening for sickle cell disease; Jamaican experience. West Ind Med J 65(1). doi:10.7727/wimj.2015.492 [DOI] [PubMed]
- Mason K, Gibson F, Higgs D, Fisher C, Thein SL, Clark B, Kulozik A, Happich M, Serjeant B, Serjeant G. Haemoglobin variant screening in Jamaica: meeting student’s request. Br J Haematol. 2016;172(4):634–636. doi: 10.1111/bjh.13531. [DOI] [PubMed] [Google Scholar]
- Mason K, Gibson F, Gardner R, Serjeant B, Serjeant G. Prevention of sickle cell disease: observations on females with the sickle cell trait from the Manchester project, Jamaica. J Comm Genet. 2016;7(2):127–132. doi: 10.1007/s12687-015-0258-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Memish ZA, Saeed MY. Six-year outcome of the national premarital screening and genetic counseling program for sickle cell disease and β-thalassaemia in Saudi Arabia. Ann Saudi Med. 2011;31(3):229–235. doi: 10.4103/0256-4947.81527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piel FB, Patil AP, Howes RE, Nyangiri OA, Gething PW, Dewi M, et al. Global epidemiology of sickle haemoglobin in neonates: a contemporary geostatistical model-based map and population estimates. Lancet. 2013;381(9861):142–151. doi: 10.1016/S0140-6736(12)61229-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platt OS, Brambilla DJ, Rosse WF, et al. Mortality in sickle cell disease. Life expectancy and risk factors for early death. N Engl J Med. 1994;330(23):1639–1644. doi: 10.1056/NEJM199406093302303. [DOI] [PubMed] [Google Scholar]
- Stammatoyannopoulos G (1974) Problems of screening and counselling in the haemoglobinopathies in birth defects. In: Motulsky AG, Lenz W (eds) Excerpta Medica Int Congr Ser, pp 268–276
- Statistical Institute of Jamaica, http://statinja.gov.jm/
- Wierenga KJ, Hambleton IR, Lewis NA. Survival estimates for patients with homozygous sickle-cell disease in Jamaica: a clinic-based population study. Lancet. 2001;357(9257):680–683. doi: 10.1016/S0140-6736(00)04132-5. [DOI] [PubMed] [Google Scholar]
- World Bank Cross Country Data (2016) http://www.NationMaster.com