Abstract
The purpose of this study is to investigate the role of serum calprotectin (CP), lactoferrin (LF), and high-mobility group protein B1 (HMGB-1) levels and fecal CP and LF levels in differential diagnosis of acute uncomplicated appendicitis from other causes of abdominal pain and further from complicated appendicitis. Totally, 120 children were included grouped into 4 as: healthy controls, patients with right lower quadrant pain with other than surgical causes, patients with uncomplicated appendicitis, and patients with complicated appendicitis. Serum CP, LF, HMGB-1, C-reactive protein (CRP) levels, and white blood cell (WBC) count were studied as well as the fecal CP and LF levels. There was a statistically significant difference between control group and both uncomplicated and complicated acute appendicitis groups, regarding all parameters. In diagnosis of complicated acute appendicitis, area under curve (AUC) for fecal LF, serum CP, and serum HMGB-1 were determined as 1.00 and the cutoff level was determined as 25 μg/g feces, 670 ng/mL, and 30 ng/mL, respectively. In differential diagnosis of uncomplicated and complicated AA, the most accurate parameter was fecal LF with an AUC of 0.977. At a 60 μg/g cutoff value for this variable, sensitivity, specificity, and accuracy were 96.7, 93.3, and 95.0 %, respectively. In conclusion, HMGB-1, calprotectin, and lactoferrin constitute novel markers in diagnosis of AA. Moreover, their levels may be helpful for the clinicians to judge about the severity of the condition. Larger studies are warranted to determine the diagnostic potential of HMGB-1, LF, and CP in AA diagnosis.
Keywords: Calprotectin, Lactoferrin, High-mobility group box 1 protein, Diagnosis, Acute appendicitis, Children
Introduction
Acute appendicitis (AA) is the most common disease requiring abdominal surgery through whole life with an approximate prevalence of 7 % [1]. However, since it overlaps with some other diseases commonly causing diagnostic difficulties, it may result in significant mortality and morbidities [2, 3]. Though noteworthy improvements are present in diagnosis and treatment of AA, the rate of negative appendectomy is still as high as 5–40 %, while the complicated appendicitis rates reach 5–30 % [2]. Unfortunately, C-reactive protein (CRP) and white blood cell (WBC) count are not very useful in correct diagnosis [4]. It remains a challenge to make the correct diagnosis, mainly because the clinical presentation may be nonspecific, and there is a lack of adequate specific biomarkers for AA [2]. Negative appendectomy was strongly associated with 9-fold increase in mortality [5]. Therefore, the need for a novel, non-invasive, and inexpensive laboratory marker is obvious to decrease the mortality and morbidity in such a common disease.
Calprotectin (CP) and lactoferrin (LF) are granular proteins located in neutrophil cytoplasm [6]. CP, also known as S100A8/A9, is a member of calgranulin family with a calcium-zinc binding structure. Mainly, CP is secreted by neutrophils and forms about 30–60 % of total cytoplasmic proteins [7]. The CP gene is located in 1q21 locus, and it has antibacterial, apoptotic, and chemotactic features. During inflammation, neutrophil transformation and exocytosis of granules result in CP and LF secretion [8]. These proteins are also present in small intestine epithelial cells, and they are secreted to the intestinal lumen during bacterial infections of intestine [9]. Since their plasma levels increase in 8 h, they can be regarded as acute phase reactants [7]. In inflammatory bowel diseases such as Crohn’s disease and ulcerative colitis, increased levels of fecal CP are determined [10]. The levels of fecal CP and LF are directly proportional with the efflux of neutrophils to the intestinal lumen and histological inflammation of the intestine [11]. Their secretion properties suggest that, before the increase of other systemic reactants and before endoscopic and macroscopic changes, these parameters can be determined in increased levels in early periods of the disease [12]. Thus, there are some studies reporting that fecal CP is more valuable in diagnosis of inflammatory bowel diseases than serum CRP levels [13, 14]. Fecal CP is an objective and non-invasive test reflecting various pathological processes occurring in the mucosa of pediatric patients.
During inflammation, bacterial endotoxins and pro-inflammatory cytokines may activate monocytes resulting in high-mobility group box 1 protein (HMGB-1) secretion and distal organ damage. HMGB-1 is a recently identified inflammatory factor which is highly expressed in severe sepsis, acute pancreatitis, hemorrhagic shock, severe trauma, and acute lung injury. Moreover, its expression is reported to be closely correlated with the severity of these symptoms [15, 16]. Recently, serum HMGB-1 levels have been reported to increase in patients with AA [17].
In this study, we aimed to investigate the role of serum CP, LF, and HMGB-1 levels and fecal CP and LF levels in differential diagnosis of acute uncomplicated appendicitis from other causes of abdominal pain and further from complicated appendicitis.
Methods
Patients
Totally, 120 children admitted to the Pediatric Surgery Department of Gaziantep Children Hospital from January 2013 to January 2014 were included in the study. Informed consent was obtained from the parents of all participants. The study was approved by the local ethics committee (Conclusion no: 13.03.2012/81). This work was supported by a Scientific Research Projects Unit of the Gaziantep University, project no: TF.13.17.
Children with a previous history of any abdominal surgery or chronic diseases were not included in the study. Participants were grouped into 4 as: healthy controls, patients with right lower quadrant pain other than surgical causes, patients with uncomplicated appendicitis, and patients with complicated appendicitis. Differential diagnosis of complicated and uncomplicated appendicitis was based on the pathological findings. Serum samples at EDTA containing tubes and fecal specimens of all participants are obtained and stored at −20 °C until analyses.
Methods
The human LF, CP, and HMGB-1 Elisa kits HyCult Biotechnology (Hbt, Uden, the Netherlands) are studied in Gaziantep University, Biochemistry Department, on Elisa instrument (Biotek Instruments, USA) with the solid-phase ELISAs sandwich principle. Blood samples of children were collected during admission using the standard venipuncture technique to analyze CP, LF, HMGB-1, WBC, and CRP levels. The WBC levels were measured with an automatic blood count device (Beckman Coulter Inc. Brea, CA, USA), and CRP concentrations were quantified with a particle enhanced immunonephelometry (Beckman Coulter Inc. Brea, CA, USA). Normal WBC and CRP levels were accepted as 4.5–11 × 103/mm3 and <0.8 mg/dl, respectively.
Statistical Analysis
One-way ANOVA was used in comparison of numerical variables between groups. According to the homogeneities of variables, or multiple comparisons, Tukey-HSD and Tamhane tests were performed. In comparison of categorical variables, Pearson chi-square test was used. In determining diagnostic accuracy of those biochemical tests, CP, LF, and HMGB-1, ROC analysis was performed. For the parameters with a high area under curve (AUC) value, cutoff levels were determined. Specificity, sensitivity, and accuracy of these biochemical parameters are calculated. The statistical analyses of this study were performed with SPSS 19.0 software program. The significance level was regarded as p < 0.05.
Results
There was not a statistically significant difference between groups in regards to the gender (p = 0.991) or mean age (p = 0.073). In comparison of groups regarding the biochemical parameters, statistically significant differences were present. The p value of comparison of groups for those biochemical parameters are summarized in Table 1. There was a statistically significant difference between healthy controls and both uncomplicated and complicated AA groups, regarding all parameters of this study. Moreover, there was a statistically significant difference between uncomplicated and complicated AA groups, regarding all parameters except WBC count. In patients with right lower quadrant pain other than surgical causes, serum WBC count, serum HMGB-1 levels, serum CP, and fecal LF levels were statistically significantly lower than those of uncomplicated AA group. There was a statistically significant difference between patients with right lower quadrant pain with other than surgical causes and complicated AA group, regarding all parameters.
Table 1.
Comparison of groups regarding the biochemical parameters
| p1 | p2 | p3 | p4 | p5 | p6 | |
|---|---|---|---|---|---|---|
| Serum WBC | 0.71 | <0.001 | <0.001 | <0.001 | <0.001 | 0.817 |
| Serum CRP | 0.18 | 0.004 | <0.001 | 0.4 | <0.001 | <0.001 |
| Serum HMGB-1 | 0.01 | 0.004 | <0.001 | 0.043 | <0.001 | <0.001 |
| Serum calprotectin | 0.98 | <0.001 | <0.001 | <0.001 | <0.001 | 0.003 |
| Fecal calprotectin | <0.001 | <0.001 | <0.001 | 0.94 | <0.001 | <0.001 |
| Serum Lactoferrin | <0.001 | <0.001 | <0.001 | 0.21 | <0.001 | 0.049 |
| Fecal Lactoferrin | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
p1: p value of comparison of groups 1 and 2; p2: p value of comparison of groups 1 and 3; p3: p value of comparison of group 1 and 4; p4: p value of comparison of groups 2 and 3; p5: p value of comparison of groups 2 and 4; p6: p value of comparison of groups 3 and 4
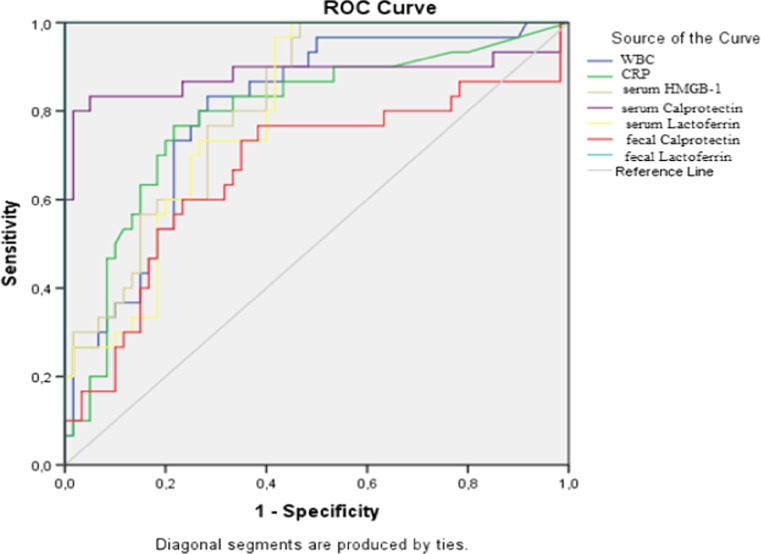
Areas under the curve are summarized in Table 2 regarding the ROC curve analysis in determining the diagnostic accuracy of biochemical parameters in identifying cases with uncomplicated appendicitis (group 3; n = 30) from patients without AA (groups 1 and 2; n = 60) (Fig. 1). In diagnosis of uncomplicated AA, AUC for fecal LF was determined as 1.00 and the cutoff level was determined as 25 μg/g feces. For this level, its sensitivity, specificity, and accuracy were 100 %. The sensitivity and specificity of serum CP in the diagnosis of AA were 73.3 and 100 %, respectively, for the 670 ng/ml cutoff value. For the 30 ng/ml cutoff value of serum HMGB-1 levels, the sensitivity was 76.7 % and the specificity was 100 %. Though serum LF levels were also different between groups, with an AUC ratio of 0.797, it was not determined to be appropriate as a diagnostic tool. Similarly, AUC ratio was 0.669 for fecal CP.
Table 2.
Area under the curve regarding the ROC curve analysis between cases with uncomplicated appendicitis (group 3; n = 30) and patients without acute appendicitis (groups 1 and 2; n = 60)
| AUC | 95 % Confidence interval | p | |
|---|---|---|---|
| WBC | 0.801 | 0.707–0.895 | <0.001 |
| CRP | 0.789 | 0.68–0.894 | <0.001 |
| Serum HMGB-1 | 0.814 | 0.729–0.900 | <0.001 |
| Serum calprotectin | 0.882 | 0.780–0.985 | <0.001 |
| Serum lactoferrin | 0.797 | 0.707–0.886 | <0.001 |
| Fecal calprotectin | 0.669 | 0.539–0.799 | 0.009 |
| Fecal lactoferrin | 1.000 | 1.000–1.000 | <0.001 |
Fig. 1.
ROC curve analysis in determining the diagnostic accuracy of biochemical parameters in identifying cases with uncomplicated appendicitis (group 3; n = 30) from patients without acute appendicitis (groups 1 and 2; n = 60)
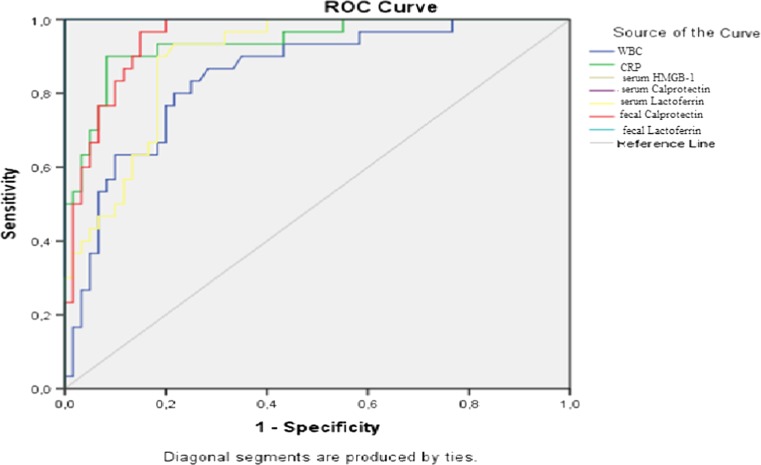
Areas under the curve are summarized in Table 3 regarding the ROC curve analysis in determining the diagnostic accuracy of biochemical parameters in identifying cases with complicated appendicitis (group 4; n = 30) from patients without AA (groups 1 and 2; n = 60) (Fig. 2). In diagnosis of complicated AA, AUC for fecal LF, serum CP, and serum HMGB-1 were determined as 1.00 and the cutoff level was determined as 25 μg/g feces, 670 ng/mL, and 30 ng/mL respectively. For these values, sensitivity, specificity, and accuracy were 100 %.
Table 3.
Area under the curve regarding the ROC curve analysis between cases with complicated appendicitis (group 4; n = 30) and patients without acute appendicitis (groups 1 and 2; n = 60)
| AUC | 95 % Confidence interval | p | |
|---|---|---|---|
| WBC | 0.847 | 0.764–0.931 | <0.001 |
| CRP | 0.938 | 0.886–0.990 | <0.001 |
| Serum HMGB-1 | 1.000 | 1.000–1.000 | <0.001 |
| Serum calprotectin | 1.000 | 1.000–1.000 | <0.001 |
| Serum lactoferrin | 0.895 | 0.832–0.958 | <0.001 |
| Fecal calprotectin | 0.951 | 0.910–0.991 | <0.001 |
| Fecal lactoferrin | 1.000 | 1.000–1.000 | <0.001 |
Fig. 2.
ROC curve analysis in determining the diagnostic accuracy of biochemical parameters in identifying cases with complicated appendicitis (group 4; n = 30) from patients without acute appendicitis (groups 1 and 2; n = 60)
In differential diagnosis of uncomplicated and complicated AA, the most accurate parameter was fecal lactoferrin with an AUC of 0.977. At a 60 μg/g cutoff value for this variable, sensitivity, specificity, and accuracy were 96.7, 93.3, and 95.0 %, respectively. In 1 of the 30 complicated appendicitis cases, false positivity was reported, and in 2 of the 30 uncomplicated appendicitis cases, false negativity was reported.
Discussion
With the aim of defining role of serum and fecal CP and LF, and serum HMGB-1 levels in differential diagnosis of acute uncomplicated appendicitis from other causes of abdominal pain and further from complicated appendicitis, we have determined that intracellular molecules of CP, LF, and HMGB-1 functioning in natural defense are valuable in diagnosis of both complicated and uncomplicated AA, even if in differentiation of complicated and uncomplicated AA. In this study, at the 25 μg/g fecal LF cutoff value, sensitivity, specificity, and accuracy were 100 % in diagnosis of complicated and uncomplicated AA cases. At the cutoff levels of 670 and 30 ng/mL, respectively, serum CP and serum HMGB-1 were also 100 % sensitive and specific in diagnosis of complicated AA.
Acute appendicitis is an acute suppurative infection which can cause systemic inflammatory symptoms when left untreated. Unnecessary appendectomy brings surgical risks for patients and waste of the money. Identification of non-invasive, inexpensive, easily available, and accurate diagnostic markers of AA is clinically highly desired.
Serum HMGB-1 levels have been studied in diagnosis of AA in a few studies. Wu et al. studied the diagnostic role of HMGB-1 in AA and determined the serum mean HMGB-1 levels in acute simple, suppurative, and gangrenous appendicitis cases as 10.97, 14.42, and 18.08 ng/ml, respectively [18]. For this finding, they suggested that with the expansion of bacteria to the appendix wall, bacterial endotoxins and proinflammatory cytokines activate monocytes to secrete HMGB-1 which worsens the local inflammation and causes the distal organ damage. They reported that HMGB1 might play an important role in the pathological progress of AA and can be clinically treated as a therapeutic target. In a study of Albayrak et al., the mean HMGB-1 level was 36.92 ng/ml in adult cases with AA, while it was 21.71 ng/ml in healthy control cases [17]. Though the HMGB-1 levels were similar with the AA cases in our study, they have determined higher serum HMGB-1 levels in healthy controls which may be due to the different age distribution of two studies. Recently, Hu et al. studied on the children with acute abdomen who had a diagnosis of suspected AA and healthy children [19]. They determined that serum HMGB-1 levels in the appendicitis and non-appendicitis groups were significantly higher than that of healthy children. Moreover, the appendicitis group showed higher serum HMGB-1 levels compared with the non-appendicitis group. They reported the sensitivity and specificity of serum HMGB-1 for the diagnosis of AA was 71.4 and 82.9 % respectively at the best cutoff of 28.0 ng/mL [18]. In our study, we have determined that the sensitivity was 76.7 % and the specificity was 100 % for the 30 ng/ml cutoff value of serum HMGB-1 levels. These findings are consistent with the data reported by Hu et al.
Serum CP levels have been studied previously in diagnosis of AA. Bealer et al. tested plasma samples from emergency department patients with acute abdominal pain (n = 181) for CP and reported that the sensitivity and specificity for CP in diagnosing AA were as 93 and 54 %, respectively. In the same study, performance characteristics of elevated WBC count were as sensitivity of 63 % and specificity of 67 % [20]. Schellekens et al. studied on 233 patients suspected of having AA and 52 healthy individuals to evaluate the diagnostic accuracy of CP. Among those 233 cases, 77 (33 %) had proven AA and median plasma levels for CP were significantly higher in patients with AA than in those with another final diagnosis. However, the area under the ROC curve was 0.67 for CP [21]. Mills et al. performed a prospective, double-blinded, single-arm, multicenter investigation in 13 emergency departments of 848 patients presenting with acute right lower quadrant abdominal pain and reported the sensitivity and specificity for the investigational plasma CP assay in diagnosing AA as 96 and 16 %, respectively [22]. Kharbanda et al. reported that median plasma CP levels were higher in appendicitis versus nonappendicitis, and it was also higher in perforated appendicitis compared to nonperforated appendicitis. In that study, at a cutoff value of 159 ng/mL, plasma CP provided a sensitivity of 100 % and a specificity of 27 % to identify children at risk for AA [23]. In our study, we have determined the serum CP levels at the cutoff value of 670 ng/ml as 73.3 % sensitive and 100 % specific.
Voganatsi et al. reported that LF is secreted from cells while CP is presented only after cell disruption or death [24]. This phenomenon suggests that in order to increase CP, leucocyte count should also be increased. On the other hand, for the increases in fecal CP, mucosal damage should be present and many polymorphonuclear leucocytes should be present on intestinal lumen. This explains the higher serum and fecal CP levels in complicated AA cases. Thuijls reported that median plasma concentrations for LF and CP were significantly higher in 51 patients with proven AA than in 27 healthy volunteers. They reported the median plasma concentrations for LF and CP as 665 and 766 ng/mL, respectively [25]. In our study, we have determined similar serum LF but higher CP levels in appendicitis groups.
To the best of our knowledge, fecal CP or LF has not been studied much in patients with AA before. We have determined an AUC of 0.951 in complicated AA but an AUC of 0.669 in uncomplicated AA for fecal CP. In this study, in diagnosis of complicated AA, serum CP at a cutoff value of 670 ng/ml, fecal LF at a cutoff value of 25 μg/g feces and serum HGMB-1 at a cutoff value of 30 ng/ml were determined to be 100 % sensitive and specific.
We have determined higher values of serum HMGB-1, serum CP, fecal CP, serum LF, and fecal LF levels in patients who were admitted with abdominal pain with other than surgical causes compared with the healthy controls. We believe that in all inflammatory conditions such as diarrhea, these increases may be seen. However, they were not as high as AA cases.
There are some limitations that should be mentioned. First, small sample size does not let us make any generalization. Second, we did not ask or record the time passed from the beginning of the pain so we could not assess the effect of time on these parameters. Moreover, these parameters are not inexpensive and/or easily available yet and studying those tests take a few hours which may be an important time period in emergency department.
In conclusion, HMGB-1, CP, and LF constitute novel markers in diagnosis of AA. Moreover, their levels may be helpful for the clinicians to judge about the severity of the condition. Since misdiagnosis results in unnecessary surgeries, new diagnostic tools that can support a diagnosis of AA are still required. Further, larger studies are warranted to determine the diagnostic potential of HMGB-1, LF, and CP in AA.
Acknowledgments
This study is supported by the University of Gaziantep, Faculty of Medicine, Scientific Investigations Project Department (BAP Project no: T.F.13.17).
Abbreviations
- CP
Calprotectin
- LF
Lactoferrin
- HMGB 1
High-mobility group box 1 protein
- CRP
C-reactive protein
- WBC
White blood cell
- AUC
Area under the curve
- AA
Acute appendicitis
- IBD
Inflammatory bowel diseases
Compliance with Ethical Standards
Conflict of Interest
There are non-financial competing interests (political, personal, religious, ideological, academic, intellectual, commercial, or any other) to declare in relation to this manuscript.
Authorship
Sevgi Buyukbese Sarsu designed the study, acquired the data, and drafted the article. Ayse Binnur Erbagci and Hasan Ulusal revised it critically for important intellectual content. Suleyman Cuneyt Karakus and Ozlem Gümüstekin Bülbül prepared the final approval of the version.
Contributor Information
Sevgi Buyukbese Sarsu, Phone: 00 90 5335172849, Email: sarsusevgi@yahoo.com.tr.
Ayse Binnur Erbagci, Email: aerbegci@gantep.edu.tr.
Hasan Ulusal, Email: hasan_ulusal@mynet.com.
Suleyman Cuneyt Karakus, Email: sckarakus@yahoo.com.
Özlem Gümüstekin Bulbul, Email: ozlem_45@yahoo.com.
References
- 1.Gwynn LK. The diagnosis of acute appendicitis: clinical assessment versus computed tomography evaluation. J Emerg Med. 2001;21(2):119–123. doi: 10.1016/S0736-4679(01)00353-5. [DOI] [PubMed] [Google Scholar]
- 2.Anderson RE. Meta-analysis of clinical and laboratory diagnosis of appendicitis. Br J Surg. 2004;91(1):28–37. doi: 10.1002/bjs.4464. [DOI] [PubMed] [Google Scholar]
- 3.Bundy DG, Byerley JS, Liles EA, Perrin EM, Katznelson J, Rice HE. Does this child have appendicitis? JAMA. 2007;298:438–451. doi: 10.1001/jama.298.4.438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beltrán MA, Almonacid J, Vicencio A, Gutiérrez J, Cruces KS, Cumsille MA. Predictive value of white blood cell count and C-reactive protein in children with appendicitis. J Pediatr Surg. 2007;42:1208–1214. doi: 10.1016/j.jpedsurg.2007.02.010. [DOI] [PubMed] [Google Scholar]
- 5.Andersson MN, Andersson RE. Causes of short-term mortality after appendectomy: a population-based case-controlled study. Ann Surg. 2011;254(1):103–107. doi: 10.1097/SLA.0b013e31821ad9c4. [DOI] [PubMed] [Google Scholar]
- 6.Angriman I, Scarpa M, D’Inca R, Basso D, Ruffalo C, Polese L, et al. Enzymes in feces: useful markers of chronic inflammatory bowel disease. Clin Chim Acta. 2007;381:63–68. doi: 10.1016/j.cca.2007.02.025. [DOI] [PubMed] [Google Scholar]
- 7.Vaos G, Kostakis ID, Zavras N, Chatzemichael A. The role of calprotectin in pediatric disease. Biomed Res Int. 2013;2013:542363. doi: 10.1155/2013/542363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lundberg JO, Hellstrom PM, Fagerhol MK, Weitzberg E, Roseth AG. Technology insight: calprotectin, lactoferrin and nitric oxide as novel markers of inflammatory bowel disease. Nat Clin Pract Gastroenterol Hepatol. 2005;2:96–102. doi: 10.1038/ncpgasthep0094. [DOI] [PubMed] [Google Scholar]
- 9.Sudan D, Vargas L, Sun Y, Bok L, Dijkstra G, Langnas A. Calprotectin: a novel noninvasive marker for intestinal allograft monitoring. Ann Surg. 2007;246:311–315. doi: 10.1097/SLA.0b013e3180f61af4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kolho KL, Turner D, Veereman-Wauters G, Sladek M, de Ridder L, Shaoul R, et al. Rapid test for fecal calprotectin levels in children with Crohn’s disease. J Pediatr Gastroenterol Nutr. 2012;55(4):436–439. doi: 10.1097/MPG.0b013e318253cff1. [DOI] [PubMed] [Google Scholar]
- 11.Vermeire S, Van Assche G, Rutgeerts P. Laboratory markers in IBD: useful, magic, or unnecessary toys? Gut. 2006;55:426–431. doi: 10.1136/gut.2005.069476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tibble JA, Sigthosson G, Foster R, Forgacs I, Bjarnason I. Use of surrogate markers of inflammation and Rome criteria to distinguish organic from nonorganic intestinal disease. Gastroenterology. 2002;123:450–460. doi: 10.1053/gast.2002.34755. [DOI] [PubMed] [Google Scholar]
- 13.Schoepfer AM, Trummler M, Seeholzer P, Criblez DH, Seibold F. Accuracy of four fecal assays in the diagnosis of colitis. Dis Colon Rectum. 2007;50:1697–1706. doi: 10.1007/s10350-007-0303-9. [DOI] [PubMed] [Google Scholar]
- 14.Langhorst J, Elsenbruch S, Koelzer J, Rueffer A, Michalsen A, Dobos GJ. Noninvasive markers in the assessment of intestinal inflammation in inflammatory bowel disease: performance of fecal lactoferrin, calprotectin, and PMN-elastase, CRP, and clinical indices. Am J Gastroenterol. 2008;103:162–169. doi: 10.1111/j.1572-0241.2007.01556.x. [DOI] [PubMed] [Google Scholar]
- 15.Lu GZ, Wu CX, Liu Q, Gong JP, Sun H. Increased expression of high-mobility group box chromosomal protein 1 in patients with acute pancreatitis. Basic Clin Med. 2010;30:1202–1205. [Google Scholar]
- 16.Abraham E. Unraveling the role of high mobility group box protein 1 in severe trauma. Crit Care. 2009;13:1004–1005. doi: 10.1186/cc8141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Albayrak Y, Albayrak A, Celik M, Gelincik I, Demiryılmaz I, Yildirim R, et al. High mobility group box protein-1 (HMGB-1) as a new diagnostic marker in patients with acute appendicitis. Scand J Trauma Resusc Emerg Med. 2011;19:27. doi: 10.1186/1757-7241-19-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wu C, Sun H, Wang H, Chi J, Liu Q, Guo H, et al. Evaluation of high mobility group box 1 protein as a presurgical diagnostic marker reflecting the severity of acute appendicitis. Scand J Trauma Resusc Emerg Med. 2012;20:61. doi: 10.1186/1757-7241-20-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hu JF, Wu JY, Zhang L, Yang LG, Long CX. Diagnostic value of high mobility group box 1 for acute appendicitis in children. Zhongguo Dang Dai Er Ke Za Zhi. 2014;16(9):919–921. [PubMed] [Google Scholar]
- 20.Bealer JF, Colgin M. S100A8/A9: a potential new diagnostic aid for acute appendicitis. Acad Emerg Med. 2010;17(3):333–336. doi: 10.1111/j.1553-2712.2010.00663.x. [DOI] [PubMed] [Google Scholar]
- 21.Schellekens DH, Hulsewé KW, van Acker BA, van Bijnen AA, de Jaegere TM, Sastrowijoto SH, et al. Evaluation of the diagnostic accuracy of plasma markers for early diagnosis in patients suspected for acute appendicitis. Acad Emerg Med. 2013;20(7):703–710. doi: 10.1111/acem.12160. [DOI] [PubMed] [Google Scholar]
- 22.Mills AM, Huckins DS, Kwok H, Baumann BM, Ruddy RM, Rothman RE, et al. Diagnostic characteristics of S100A8/A9 in a multicenter study of patients with acute right lower quadrant abdominal pain. Acad Emerg Med. 2012;19(1):48–55. doi: 10.1111/j.1553-2712.2011.01259.x. [DOI] [PubMed] [Google Scholar]
- 23.Kharbanda AB, Rai AJ, Cosme Y, Liu K, Dayan PS. Novel serum and urine markers for pediatric appendicitis. Acad Emerg Med. 2012;19(1):56–62. doi: 10.1111/j.1553-2712.2011.01251.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Voganatsi A, Panyutich A, Miyasaki KT, Murthy RK. Mechanism of extracellular release of human neutrophil calprotectin complex. J Leukoc Biol. 2001;70(1):130–134. [PubMed] [Google Scholar]
- 25.Thuijls G, Derikx JP, Prakken FJ, Huisman B, van BijnenIng AA, van Heurn EL, et al. A pilot study on potential new plasma markers for diagnosis of acute appendicitis. Am J Emerg Med. 2011;29(3):256–260. doi: 10.1016/j.ajem.2009.09.029. [DOI] [PubMed] [Google Scholar]