Abstract
A brain-enriched secreting signal peptide, NELL2, has been suggested to play multiple roles in the development, survival, and activity of neurons in mammal. We investigated here a possible involvement of central NELL2 in regulating feeding behavior and metabolism. In situ hybridization and an im-munohistochemical approach were used to determine expression of NELL2 as well as its colocalization with proopiomelanocortin (POMC) and neuropeptide Y (NPY) in the rat hypothalamus. To investigate the effect of NELL2 on feeding behavior, 2 nmole of antisense NELL2 oligodeoxynucleotide was administered into the lateral ventricle of adult male rat brains for 6 consecutive days, and changes in daily body weight, food, and water intake were monitored. Metabolic state-dependent NELL2 expression in the hypothalamus was tested in vivo using a fasting model. NELL2 was noticeably expressed in the hypothalamic nuclei controlling feeding behavior. Furthermore, all arcuatic POMC and NPY positive neurons produced NELL2. The NELL2 gene expression in the hypothalamus was up-regulated by fasting. However, NELL2 did not affect POMC and NPY gene expression in the hypothalamus. A blockade of NELL2 production in the hypothalamus led to a reduction in daily food intake, followed by a loss in body weight without a change in daily water intake in normal diet condition. NELL2 did not affect short-term hunger dependent appetite behavior. Our data suggests that hypothalamic NELL2 is associated with appetite behavior, and thus central NELL2 could be a new therapeutic target for obesity.
Keywords: feeding behavior, hypothalamus, metabolic state, NELL2, neural plasticity
INTRODUCTION
It has been proposed that the central nervous system plays a major function in appetite behavior, as well as whole body energy homeostasis. Within the central nervous system, a great focus has been made in and around the circumventricular organs of the hypothalamus, including the arcuate nucleus (ARC) and ventromedial hypothalamus (VMH) which are a first responder of circulating metabolic signal molecules as well as the paraventricular nucleus (PVN) as a deeper brain area integrating both peripheral and central metabolic signals, in energy metabolism strong underlining with an anatomical circuitry as well as molecular cascades among these hypothalamic areas (Baskin et al., 1999; Elias et al., 1999; King, 2006; Schwartz et al., 1997). In addition, several recent reports have also implied a critical function of synaptic plasticity in the hypothalamus in maintaining energy homeostasis (Horvath, 2005; Horvath and Diano, 2004; Horvath and Gao, 2005; Pinto et al., 2004).
Originally found as a NEL in chicken as a brain specific epidermal growth factor family protein, a novel molecule NELL2, a mammalian homologue of chicken NEL is also expressed dominantly in rat brain as a glycosylated secreting protein (Jeong et al., 2008a; Matsuhashi et al., 1995; Watanabe et al., 1996). Up to date, initial studies have proposed NELL2 functions in neuronal proliferation, differentiation, synaptic formation and plasticity during development and postnatal life (Choi et al., 2014; Jeong et al., 2008b; Kim et al., 2002; Matsuyama et al., 2004; Nelson et al., 2002; Oyasu et al., 2000). Our group has established the antisense oligodeoxynucleotide (AS ODN)-dependent gene modification system and applied NELL2 AS ODN into the rat brain hypothalamus for a cessation of NELL2 production in this region (Jeong et al., 2008b; Kim et al., 2002). Using this approach, our group has demonstrated that NELL2 is an active downstream signaling molecule in estrogen pathway during neuroprotection in the rat brain (Jeong et al., 2008b). We also reported a crucial function of central NELL2 in certain behavior in rats, such as sexual behavior as well as in regulating female estrous cycle (Jeong et al., 2008b; Ryu et al., 2011).
Although NELL2 is broadly distributed in the brain, considerably high expression of NELL2 is observed within certain hypothalamic areas, including the PVN, VMH and the ARC, all of which are known to be important in regulating metabolism (Jeong et al., 2008a). Therefore, we sought to investigate here a possible role of central NELL2 in the regulation of metabolism in rats. We first determined the expression of NELL2 in the hypothalamic areas that are important in feeding behavior and metabolism regulation using histological approaches. Then, we tested a potential role of central NELL2 in feeding behavior by administration of NELL2 AS ODN in the hypothalamus. Lastly, we determined whether metabolic state could also affect NELL2 expression in the hypothalamus using a fasting model. NELL2 is expressed in several hypothalamic regions that regulate metabolism, and rats lacking NELL2 production in the hypothalamus showed an attenuation of daily food intake on regular chow diet without a change in water intake, and therefore developed a lean phenotype compared to the control group. The NELL2 expression in the hypothalamus was elevated in fasting conditions compared to the fed state. However, NELL2 did not affect gene expression of either proopiomelanocortin (POMC) or neuropeptide Y (NPY) although NELL2 is expressed in these neurons. Our data suggests that a novel secreting protein NELL2 is one of the hypothalamic regulatory molecules in controlling appetite behavior in rats, and central NELL2 signaling may be a potential therapeutic target for obesity and type 2 diabetes.
MATERIALS AND METHODS
Animals
We purchased adult male Sprague-Dawley rats (8 weeks old, about 230–250 g) from a local breeder (Daehan Animal Breeding Company, Korea) and maintained them in our animal facility at the University of Ulsan. The rats had ad libitum access to a standard diet and tap water under 12 h dark/12 h light (from 6:00 a.m. to 6:00 p.m.) conditions. Temperature was kept at 19°C during the experiment. All procedures involving rats were approved by the Regulation of Animal Care at the University of Ulsan and were in accordance with the NIH Guide for the Care and Use of Laboratory Animals.
Radioactive in situ hybridization (ISH)
We designed a PCR primer set (forward: 5′-agtctcatcagatcgccttg-3′; backward: 5′-acagagcacctgtcgttctc-3′) based on the rat NELL2 mRNA sequence (NM 031070 from NCBI GenBank) and cloned an 899 bp PCR product in a pEZ-T vector (RNA Incorp., South Korea). We generated 33P-labeled sense and antisense riboprobes for NELL2 and purified them using G-50 columns as described previously (Mello et al., 1997). These riboprobes were then used for radioactive ISH. Adult male rats (n = 4) were decapitated and their brains were dissected, placed in an embedding mold with a Tissue-tek (Sakura, USA), and frozen in dry ice. Brains were then cut at 10 μm thickness coronally on a cryostat (Leica’s CM 1850, Wetzlar, Germany) and stored at −80°C until use. After post-fixation for 5 min at room temperature (RT) in 4% paraformaldehyde in 0.1 M phosphate buffered saline (PBS) followed by air dehydration, the slides were acetylated for 10 min at RT in 0.0135% triethanola-mine and 0.0025% acetic anhydride in RNase free water. After the air dehydration, the sections were hybridized overnight at 65°C in a hybridization buffer [50% formamide, 2× SSPE (17.53 g of sodium chloride, 2.76 g of sodium phosphate monobasic hydrous and 0.74 g of EDTA), 32 μg of tRNA, 16 μg of BSA and 16 μg of poly A per section], containing sense or antisense riboprobes (500,000 cpm per section), in an oil bath. The sections were washed sequentially for 1 h at RT in 2× SSPE and for 1 h at 65°C in 2× SSPE/50% formamide, followed by two high stringency washes for 30 min each at 65°C in 0.1× SSPE. The sections were then dehydrated and subjected to a phosphorimager autoradiography (Optic Quant Phosphorimage Analysis System, Packard Bioscience, USA).
Immunohistochemistry (IHC)
Animals were anesthetized with tribromoethanol (250 mg/kg B.W., Sigma), then perfused transcardially with 100 mL of 0.1 M phosphate buffer (PB, pH 7.4), followed by 100 mL of 4% paraformaldehyde in PB. Brains were then decapitated and post-fixed with 20% sucrose overnight at 4°C. Brains were coronally sectioned using a cryostat with a 20μm thickness after Tissue-tek embedding and stored at −80°C until use. The sections were air-dried overnight at RT before IHC and then incubated sequentially for 30 min each in PB, 0.3 M H2O2, and blocking buffer (4% skim milk, 0.3% Triton X-100 in PB) at RT. After 3 washes in PB for 5 min each at RT, the sections were incubated in an Avidin/Biotin blocking system following the manufacturer’s protocol (Vector, USA). The sections were then incubated for 2 h at RT with primary antibodies in a humidified chamber. Antibodies used in this study were anti-NELL2 antiserum (1:1,000, Jeong et al., 2008a; 2008b), anti-POMC (1:500, Santa Cruz Biotechnology, USA) and anti-NPY (1:50, Santa Cruz Bio-technology). The sections were then incubated for 2 h with biotinylated secondary antibodies (anti-rabbit IgG, 1:500 for NELL2; anti-goat IgG, 1:500 for POMC and NPY; Vector, USA). This incubation was followed by 3 washes with a slow agitation in PB for 10 min each. The sections underwent another 2 h incubation with an ABC kit (Vector) to amplify signals at RT, and finally, signals were visualized using a Tyramide Signal Amplification (TSA) system (TSA-plus fluorescein system; Cat. No. NEL741 for green and NEL744 for red, PerkinElmer, USA). Three washes in PB for 10 min each with a slow agitation at RT were done between each step above. The sections were coverslipped with a GVA mounting solution (Zymed Lab., USA) and subjected to a microscopy. For double IHC, we used two primary antibodies together (NELL2 and other) and then developed one signal first. Slides were incubated in 0.3% H2O2 for 30 min at RT and were followed by 3 washes for 10 min each with a slow agitation in PB. The second signal was then developed and the slides were imaged using a confocal microscope (Fluoview FV500, Olympus, Japan).
Animal behavioral study after a cessation of NELL2 production in the hypothalamus
We have previously used the AS ODN to block de novo synthesis of NELL2 in the adult rat hypothalamus (Kim et al., 2002). In this study, rats were divided into 3 groups; 1) 2 nmole of NELL2-AS in ACSF (n = 9), 2) 2 nmole of NELL2-SCR in ACSF (n = 6), and 3) ACSF (n = 5). Each treatment was injected into the lateral ventricle daily at 12:00 p.m. for 6 days without anesthesia via a stereotaxically implanted cannula (Kim et al., 2002). Range of body weight at the first day of injection was 280–300g. Food and water consumption and body weight were measured daily at 12:30 p.m. during the experimental period. For the fastedrefed study, NELL2-AS (n = 3) or NELL2-SCR (n = 4) was administered into the brain as above at 12:00 p.m. for 3 days. On second day of the experimental period, food was removed for overnight. Food was provided again at 12:00 p.m. following day, and animal’s food intake was measured for 1 h, 4 h, 8 h and 24 h period.
Determination of NELL2 transcript after food deprivation
Food was removed for 48 h starting at 10:00 a.m. with water available ad libitum to create a fasting condition for the animals (n = 4), while control animals (n = 4) had a free access to food and water during the experiment. The animals were later sacrificed, and the hypothalamus was obtained and used for RT-PCR to detect NELL2 transcript.
NELL2 on POMC and NPY gene expression
To test whether NELL2 affects POMC or NPY transcription, NELL2-AS (n = 5) or NELL2-SCR (n = 4) was administrated for 3 consecutive days at 12:00 p.m. everyday. Animals from both groups were sacrificed at 7:00 p.m., and the MBH was carefully removed and used for RT-PCR to detect POMC and NPY transcripts (Kim et al., 2006).
Relative reverse transcription (RT)-PCR
For the study of metabolic state-dependent NELL2 gene expression, total RNA from the rat hypothalamus of the control (n = 4) and the 48-h fasted animals (n = 4) was isolated by a TRIzol reagent (Sigma) and reverse transcribed using 2 μg of the total RNA. PCR was performed for 35 cycles at 94°C for 30 s, 57°C for 30 s and 72°C for 45 s, using a primer set (forward: 5′-ATG GAA TCC CGG GTA TTA CTG-3′, backward: 5′-GGC TAA GGA GAG CTT GTG CC-3′) to obtain a 500 bp NELL2 fragment. For the study of NELL2 effects on POMC and NPY synthesis, the MBH from the NELL2 AS or NELL2 SCR subjects was isolated, reverse transcribed, and amplified by PCR for 25 cycles at 94°C for 30 s, 56°C for 30 s, and 72°C for 35 s, using two sets of primers: POMC forward, 5′-AGT ACG TCA TGG GTC ACT TC-3′; backward, 5′-CAG CTC CCT CTT GAA CTC TA-3′; NPY forward, 5′-GCT AGG TAA CAA ACG AAT GG-3′; backward, 5′-GCA TTT TCT GTG CTT TCT CT-3′. For a quantity control of all PCR experiments, GAPDH was amplified using a primer set (forward, 5′-TGT GAA CGG ATT TGG CCG TA-3′; backward, 5′-ACT TGC CGT GGG TAG AGT CA-3′) for 25 cycles under the same conditions. All PCR products were analyzed with the NIH image software and normalized to the GAPDH.
Statistic analysis
Changes in food, water intake, and body weight were analyzed by two-way ANOVA using a Turkey’s multiple comparison test with Prism 7 software (GraphPad, USA). Data in the figures and text are presented as means. Unpaired student’s t test was also used to compare between values of NELL2 AS ODN and NELL2 SCR ODN. In all cases, a difference at P < 0.05 was considered statistically significant and the range of significance was indicated as *P < 0.05, **P < 0.01 and *** P < 0.001.
RESULTS
NELL2 expression in the hypothalamic areas important for metabolism regulation
In the previous study, we have shown that NELL2 gene was profoundly expressed in diverse mammalian brain areas including several hypothalamic nuclei, such as the VMH, PVN and the ARC (Jeong et al., 2008a), all of which known to play a key role in energy homeostasis. In addition, AS ODN-mediated cessation of NELL2 production in the hypothalamus of rats induced a lean phenotype under regular diet condition compared to the control group (our unpublished data). Therefore, we studied here a potential role of central NELL2 in energy homeostasis in rats with a primary focus on NELL2-dependent animal feeding behavior. To this end, we first performed radioactive in situ hybridization as well as immunohistochemistry to clearly define NELL2 expression in the hypothalamus. As expected, both mRNA and protein level of NELL2 are strongly expressed in the hypothalamic regions as mentioned above (Fig. 1).
Fig. 1. NELL2 expression in the adult male rat hypothalamus.
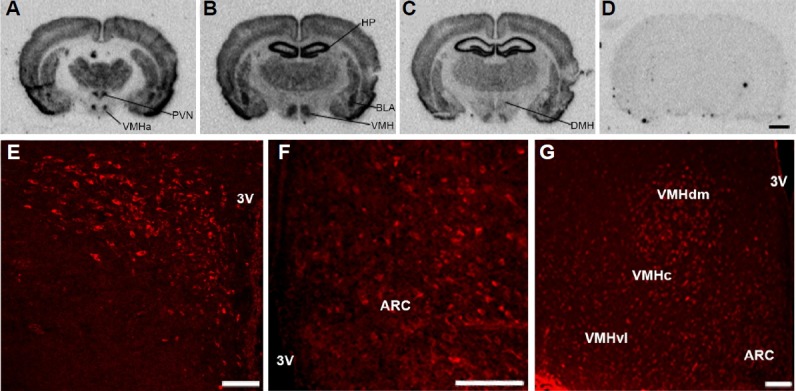
(A–C). NELL2 transcripts in coronally sectioned brain tissues from the rostral-to-caudal axis by radioactive ISH. (D) No signal was detected by a sense probe. (E) A relatively high expression of NELL2 protein was found within the PVN. (F) NELL2-ir was also detected within the ARC. (G) Many cells within the dorsomedial (VMHdm) and ventrolateral (VMHvl) parts of the VMH showed a relatively higher expression of NELL2-ir than those in the central (VMHc) part. Abbreviations: BLA, basolateral amygdala; DMH, dorsomedial hypothalamus; HP, hippocampus; VMHa, anterior part of VMH. Scale bar = 2 mm for (A–D), 100 μm for (E–G).
NELL2 affects feeding behavior
To determine the importance of central NELL2 signaling in regulating appetite behavior and energy balance, either the NELL2 AS ODN, its scrambled (NELL2 SCR ODN) sequence, or an ACSF was injected ICV daily into the adult male rat brain as reported previously (Kim et al., 2002) for six consecutive days (from day 0 to day 5, marked in Fig. 2). Daily food intake, water intake and body weight change were monitored during the experimental period (Fig. 2). During the 6 days of injection, rats with the ACSF and the NELL2 SCR ODN showed a similar pattern of daily body weight gain (average body weight gain for ACSF: 5.3 ± 0.7 g/day, and for NELL2 SCR ODN: 3.2 ± 0.8 g/day; Figs. 2A and 2D), while rats with the NELL2 AS ODN showed a significant reduction of daily body weight gain (−1.8 ± 1.3 g/day, p<0.001 compared to ACSF or SCR; Figs. 2A and 2D). This attenuation of body weight gain appeared in NELL2 AS ODN group is due to an attenuation of food consumption, as rats in this group ate significantly less than that of the control groups (average of daily food intake for NELL2 AS ODN: 19.9 ± 1.2 g/day, p<0.001 compared to ACSF or SCR, for ACSF: 28.1 ± 1.0 g/day, and for NELL2 SCR ODN: 25.1 ± 0.9 g/day; Figs. 2B and 2E). After the experimental period, daily food intake and body weight gain of the NELL2 AS ODN animals gradually returned to normal range (data not shown). Amount of daily water consumption was not differ between groups (43.3 ± 0.3 ml/day in ACSF, 35.5 ± 3.8 ml/day in NELL2 SCR ODN, and 35.7 ± 3.3 ml/day in NELL2 AS ODN; Figs. 2C and 2F).
Fig. 2. A cessation of NELL2 production in the hypothalamus affects feeding behavior in rats.
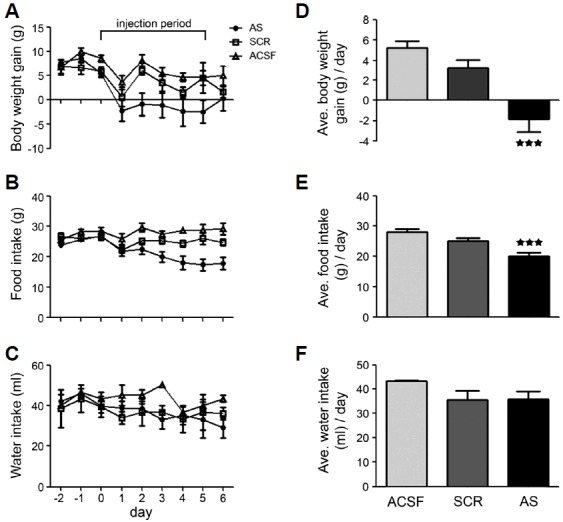
NELL2 AS ODN (AS; n = 9), NELL2 SCR ODN (SCR; n = 6) or ACSF (n = 5) were injected ICV daily into the lateral ventricle using a stereotaxically implanted cannula for six consecutive days from day 0 to day 5 (marked by line) as previously reported (Kim et al., 2002), and daily change in body weight (A, D), food intake (B, E), and water intake (C, F) were measured during the experimental period. The NELL2 AS ODN group showed a significant reduction in daily food intake (P < 0.001) followed by a significant loss in daily body weight gain (P < 0.001) without a change in water drinking behavior compared with NELL2 SCR ODN or ACSF animals.
As rats with NELL2 AS ODN consumed less food in freely food access condition, we next determined whether blockade of NELL2 production in the hypothalamus also affects hunger-dependent appetite behavior. We used NELL2 SCR ODN only as a control group as rats with the NELL2 SCR ODN and ACSF showed a similar appetite behavior. Using the same experimental set up as above, food from both groups was removed on the second day, and then rats were fasted overnight. Then, food was re-provided at following day and hunger-dependent eating behavior from each group was monitored. Interestingly, rats from both group consumed similar amount of food at early time period after overnight fasting (NELL2 AS ODN vs NELL2 SCR ODN in gram, in 1 h: 4.0 ± 1.0 vs 5.8 ± 0.7, p = 0.19; in 4 h: 6.5 ± 1.5 vs 9.5 ± 1.3, p = 0.19; in 8 h: 13.6 ± 1.8 vs 19.7 ± 2.9, p = 0.16), while rats with NELL2 AS ODN consumed significant less amount of food in 24 h period (17.8 ± 1.1 vs 30.9 ± 3.9 g, p = 0.04), indicating that central NELL2 signaling is not associated with a short-term hunger-dependent eating behavior (Fig. 3).
Fig. 3. A comparison of cumulative food intake between the NELL2 AS ODN and NELL2 SCR ODN after 24 h fasting.
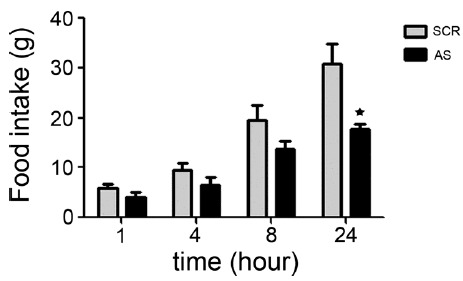
Food was removed for 24 h for both groups, and cumulative food intake at 1 h, 4 h, 8 h and overnight was measured and compared between the groups. Rats with NELL2 AS ODN (AS, n = 3) showed a similar food intake behavior during early time period at refed compared to the control (SCR, n = 4; p = 0.19 for 1 h, p = 0.19 for 4 h, p = 0.16 for 8 h). However, 24 h food intake in NELL2 AS ODN was significantly reduced compared to the control (p = 0.04).
Another set of animals was used to verify the knock down of NELL2 elicited by NELL2 AS ODN using the same experimental setup described above. Protein was extracted from the mediobasal hypothalamus of both group and western blotting was performed as previously reported (Jeong et al., 2008b; Kim et al., 2002). NELL2 AS ODN efficiently blocks NELL2 expression in the mediobasal hypothalamus by ~65% when compared to NELL2 SCR ODN animals (data not shown).
These data clearly suggest that central NELL2 signaling is, at least in some degree, associated with feeding behavior in rats. If so, we hypothesized that metabolic state might also affect NELL2 expression in the hypothalamus. Therefore, we determined an effect of fasting on the NELL2 gene transcription in the hypothalamus using a RT-PCR analysis of RNA samples from the control and the 48-h fasted animals (Fig. 4). Band densities of the PCR products were normalized by the GAPDH and compared between groups, and found that the expression level of NELL2 mRNA was significantly increased by fasting (P < 0.001 compared to control). Our data all together indicate that NELL2 plays an orexigenic role in rat brain.
Fig. 4. Fasting promoted NELL2 synthesis in the hypothalamus.
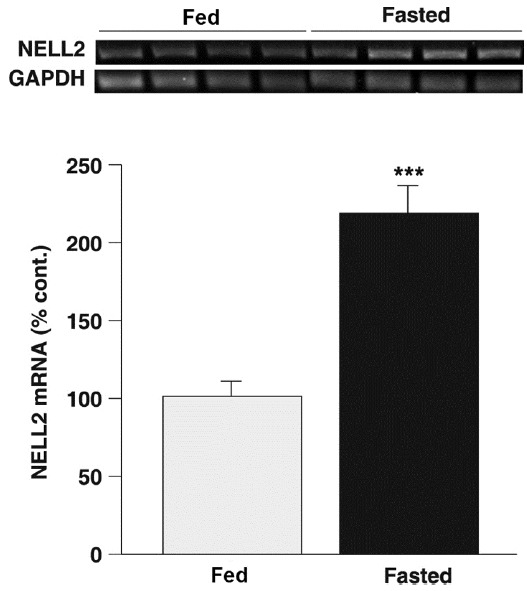
RT-PCR was performed using the hypothalamic total RNA to detect a transcriptional level of NELL2 from control fed (n = 4) and 48-h fasted animals (n = 4). The PCR products for NELL2 were normalized by GAPDH. Hypothalamic NELL2 synthesis was significantly increased in the fasted animals compared with that in control (P < 0.001).
Effect of NELL2 on POMC and NPY synthesis
Given that hypothalamic POMC and NPY neurons play a critical role in whole body energy metabolism as well as appetite behavior, and NELL2 is strongly expressed in the ARC, we tested whether NELL2 is associated with POMC and/or NPY expression in the rat ARC. NELL2 is indeed expressed in both POMC (Figs. 5A–5C) and NPY cells (Figs. 5D–5F) in the ARC. Using the similar experimental set, the gene expression of POMC and NPY in NELL2 AS ODN and NELL2 SCR ODN was measured. RT-PCR products of POMC and NPY from the medial basal hypothalamus (MBH) were compared between the groups, and we could not find any transcriptional difference of NPY (Fig. 5G) or POMC (Fig. 5H).
Fig. 5. NELL2 is expressed in both POMC and NPY neuronal populations in the ARC without affecting their gene expression.
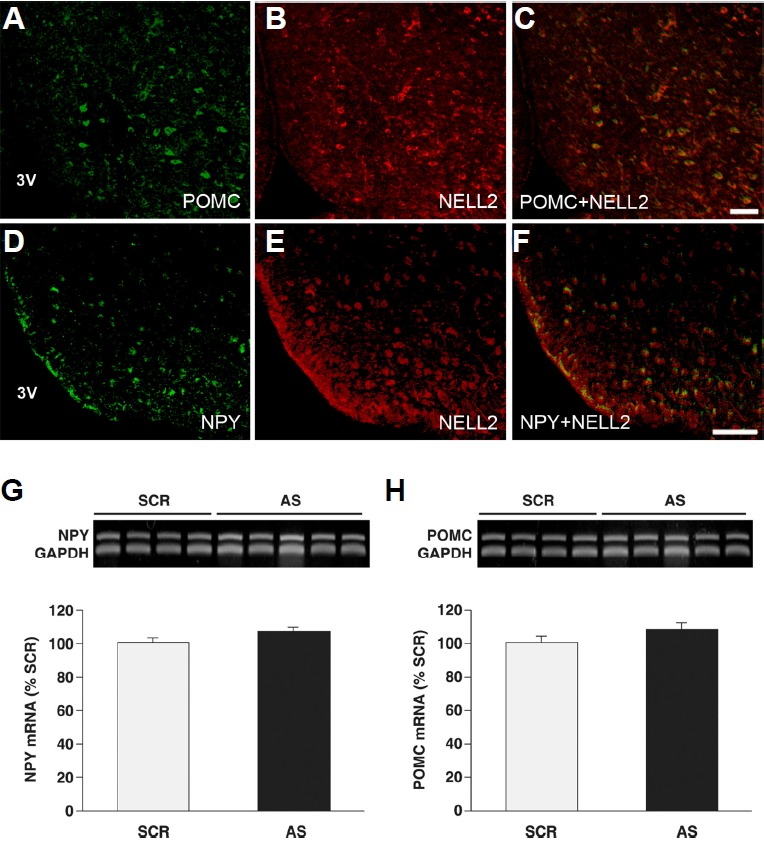
(A) A cytoplasmic distribution of POMC-ir developed by a green fluorescence was found mostly in the ventromedial part of the ARC through the rostral-to-caudal axis. (B, E) NELL2-ir was developed by a red fluorescence. (C) All POMC immuno-positive cells were colocalized with the NELL2-ir. (D) NPY-ir developed by a green fluorescence showed a clear cytoplasmic expression along the rostral-to-caudal axis. (F) All NPY-ir was found within the NELL2 immuno-positive cells in this area. Single NELL2-ir was also found in the experiment. Scale bar = 50 μm. (G, H) NPY and POMC transcripts in the MBH were measured by a relative RT-PCR in the NELL2 AS ODN (AS, n = 4) and NELL2 SCR ODN groups (SCR, n = 5), and then compared between the groups. The transcriptional level of NPY (G) and POMC (H) was not different between groups.
DISCUSSION
In our previous studies we developed and successfully applied AS ODN-mediated gene blocking system for the cessation of central NELL2 production in studying NELL2-dependent signal cascades in the hypothalamus using a cDNA expression array (Kim et al., 2002). In that study, we observed that several proteins involving the transport and release of vesicles in neurons, such as synaptophysin and rab7, as well as Ca2+-dependent intracellular signaling were significantly down-regulated by the NELL2 AS ODN, suggested that NELL2 is participating, at least in some part, in synaptic plasticity in rat brain. We also observed by accident that rats with NELL2 AS ODN showed an attenuated daily body weight gain during the experimental period under normal diet condition (unpublished data). Therefore, we hypothesized and tested here a potential role of central NELL2 in metabolism regulation. Our data clearly shows that hypothalamic NELL2 is associated with appetite behavior with its orexigenic tone.
In general, brain manipulation by drug or virus administration to study central metabolism regulation can be achieved by: 1) cannulation of the lateral (LV), or third ventricle, and by 2) stereological injection directly into the target brain region. As CSF is mainly produced around the LV region and circulates throughout the brain ventricular system, administration into the LV is an efficient way to study the chronic effect of a drug on the brain with minimal surgical damage. Compared to the LV system, drug administration into the third ventricle can also be useful when studying an acute effect of a drug in metabolism regulation because many brain regions that are important for energy metabolism and behavior are located around the third ventricle. However, drugs can freely travel within the brain via CSF in both cases and thus could affect multiple brain regions. To overcome this issue, a drug can be directly administered into a target region and requires minimal dosage. Nevertheless, a pipette injector must travel the brain tissue and may damage various brain regions during surgery. This may generate unknown surgical effects. For this study of NELL2 AS ODN in central metabolism regulation, we used the LV system for oligonucleotide delivery and report minimal brain damage.
Unnecessary or excessive food intake results in obesity and causes a variety of metabolic and cardiovascular disorders, and the hypothalamus has long been highlighted for its participation in feeding behavior and whole body metabolism regulation (Frago and Chowen, 2015; Hassouna et al., 2016). Our data show that the expression of NELL2 in the hypothalamus is directly associated with the metabolic state, as fasting enhanced NELL2 synthesis in this brain region. Furthermore, rats lacking NELL2 in the hypothalamus developed a lean phenotype and reduced food consumption, which clearly demonstrates a role for NELL2 in appetite behavior as an orexigen in the brain. Rats with NELL2 AS ODN possess an integrate system for water drinking behavior as well as a short-term hunger-dependent feeding behavior. However, NELL2 AS ODN induced a significant decrease in 24 h food intake after fasting. This is in line with our behavior data from the ad libitum condition, and provides clear evidence that central NELL2 may play a critical role in controlling unnecessary food intake. Further studies testing NELL2 in diverse eating behaviors, including food preference, addiction, compulsive eating, as well as high fat diet-induced appetite behavior are necessary to clearly determine central NELL2 function in metabolic behavior.
NELL2 is a glycoprotein containing multifunctional domains such as a protein kinase C-interacting domain, von Willebrand factor C motifs, a thrombospondin (TSP)-1-like structure and six EGF-like repeats (Kuroda et al., 1999), and has been suggested to play multiple roles in the development and function of neurons during embryonic and post-natal life (Aihara et al., 2003; Matsuyama et al., 2005; Nelson et al., 2002; 2004). The astrocyte-derived TSP-1 is an important glycoprotein for synaptic formation and remodeling during development (Christopherson et al., 2005) or after brain injury (Lin et al., 2003), through cell-extracellular matrix interactions. The EGF-like domains have been suggested to have diverse functions for cellular events by Ca2+-dependent protein-protein interactions (Kuroda et al., 1999). Considering our previous report (Hwang et al., 2007), the major isoform of NELL2 is secreted in the rat brain and thus, may act as an extracellular signaling molecule for the communication between cells through TSP-1-like and/or EGF-like structures. However, mice with a universal NELL2 knock out (KO) showed a normal phenotype without pathophysiologi-cal conditions compared with control animals (Matsuyama et al., 2005). This is probably due to unknown compensatory pathways. To challenge this issue, a group of scientists recently developed a mice model for conditional NELL2 KO using the NELL2loxP system, and demonstrated that NELL2 through binding and activating to its receptor Robo3 is indeed playing an important function in guiding axonal projections in spinal cord (Jaworski et al., 2015). This is in line with our previous report suggested a potential NELL2 role in neuronal plasticity in the hypothalamus (Kim et al., 2002). However, the cellular mechanism by which NELL2 controls neuronal activity remains unclear. Our unpublished in vitro study shows that NELL2 overexpression causes elongation of the axons in hippocampal neurons, while knock down of NELL2 results in shortened axonal development. This appears to contradict findings by the Jaworski et al. (2015) who report that NELL2 blocked axonal projections of other neurons. However, it could be explained that neurons producing NELL2 develops axonal projection, while neurons receiving NELL2 signaling from NELL2-producing neurons withhold their axons. If this is a true, NPY neurons may produce more of NELL2 in a fasted state and possess dominant axonal projections into the target area against POMC neurons to promote food intake. In this case, further studies are required to demonstrate cell-type specific, metabolic state-dependent NELL2 expression.
In this study, NELL2 did not affect either POMC or NPY gene expression in the hypothalamus. It is possible that NELL2 may affect downstream target signaling of both POMC and NPY, not by their gene expression, but via their axonal development as mentioned above. It is also possible that NELL2 may affect vesicle transport and/or release of POMC/NPY neurons as it is previously reported in the hypo-thalamic GnRH neurons (Ha et al., 2008). However, we compared POMC and NPY gene expression at single metabolic condition. In the ARC, POMC gene expression is high in fed condition with a low NPY level. In contrast, in the fasted state, POMC levels were low and NPY expression was high. Therefore, another study is necessary to clearly confirm the effect of NELL2 on POMC and NPY gene expression by comparing the effect of NELL2 (NELL2 AS ODN or NELL2 SCR ODN) on both genes between fed and fasting conditions. Alternately, it is also reasonable to assum that NELL2 may affect animal’s appetite behavior, not by POMC/NPY system in the ARC, but through other hypothalamic regions such as the PVN and VMH. This is in line with our histological data showing higher NELL2 expression in these hypothalamic nuclei when compared with the ARC (Fig. 1). Finally, further study is necessary to demonstrate a direct target of NELL2 action in the brain on metabolism regulation.
Our group first found NELL2 as a positively regulated downstream target molecule of estrogen in the rat hypothalamus (Choi et al., 2001; 2010), and NELL2 is expressed in neurons producing estrogen receptors in the adult rat brain (Choi et al., 2010; Jeong et al., 2008a). Interestingly, sex steroids are strongly associated with energy homeostasis; ovariectomy or estrogen receptor alpha KO mice showed similar features associated with obesity in female rats and estrogen treatment in this group reduced food intake and body weight (Butera and Beikirch, 1989; Czaja et al., 1983; Musatov et al., 2007; Roesch, 2006). In the adult male guinea pigs, on the other hand, testosterone administration after gonadectomy resulted in an up-regulation of food intake and body weight (Czaja, 1984), suggesting some degree of discrepancy exist for sex steroid dependent metabolism regulation. Anyhow, central NELL2 may play a role to mediate sex steroid-dependent feeding behavior and metabolism regulation.
In summary, we report here that a neural tissue-enriched multifunctional protein, NELL2 is a regulatory component of feeding behavior, possibly through a modulation of neuronal activity in the hypothalamus. Further investigation into the effect of NELL2 on synaptic plasticity of the hypothalamic neurons in metabolism regulation is necessary.
ACKNOWLEDGMENTS
This work was supported by Mid-career Researcher Program through NRF grant (NRF-2010-0009998). THL was supported by Priority Research Centers Program through NRF (2014R1A6A1030318).
REFERENCES
- Aihara K., Kuroda S., Kanayama N., Matsuyama S., Tanizawa K., Horie M. A neuron-specific EGF family protein, NELL2, promotes survival of neurons through mitogen-activated protein kinases. Brain Res Mol Brain Res. 2003;116:86–93. doi: 10.1016/s0169-328x(03)00256-0. [DOI] [PubMed] [Google Scholar]
- Baskin D.G., Breininger J.F., Bonigut S., Miller M.A. Leptin binding in the arcuate nucleus is increased during fasting. Brain Res. 1999;828:154–158. doi: 10.1016/s0006-8993(99)01252-4. [DOI] [PubMed] [Google Scholar]
- Butera P.C., Beikirch R.J. Central implants of diluted estradiol: independent effects on ingestive and reproductive behaviors of ovariectomized rats. Brain Res. 1989;491:266–273. doi: 10.1016/0006-8993(89)90062-0. [DOI] [PubMed] [Google Scholar]
- Choi E.J., Kim D.H., Kim J.G., Kim D.Y., Kim J.D., Seol O.J., Jeong C.S., Park J.W., Choi M.Y., Kang S.G., et al. Estrogen-dependent transcription of the NEL-like 2 (NELL2) gene and its role in protection from cell death. J Biol Chem. 2010;285:25074–25084. doi: 10.1074/jbc.M110.100545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi E.J., Ha C.M., Choi J., Kang S.S., Choi W.S., Park S.K., Kim K., Lee B.J. Low-density cDNA array-coupled to PCR differential display identifies new estrogen-responsive genes during the postnatal differentiation of the rat hypothalamus. Brain Res Mol Brain Res. 2001;97:115–128. [Google Scholar]
- Choi E.J., Kim D.H., Kim H.R., Yun C.H., Kim D.Y., Park J.W., Lee B.J. Neural epidermal growth factor-like like protein 2 (NELL2) promotes aggregation of embryonic carcinoma P19 Cells by inducing N-cadherin expression. PLos One. 2014;9:e85898. doi: 10.1371/journal.pone.0085898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christopherson K.S., Ullian E.M., Stokes C.C., Mullowney C.E., Hell J.W., Agah A., Lawler J., Mosher D.F., Bornstein P., Barres B.A. Thrombospondins are astrocyte-secreted proteins that promote CNS synaptogenesis. Cell. 2005;120:421–433. doi: 10.1016/j.cell.2004.12.020. [DOI] [PubMed] [Google Scholar]
- Czaja J.A. Sex differences in the activational effects of gonadal hormones on food intake and body weight. Physiol Behav. 1984;33:553–558. doi: 10.1016/0031-9384(84)90370-6. [DOI] [PubMed] [Google Scholar]
- Czaja J.A., Butera P.C., McCaffrey T.A. Independent effects of estradiol on water and food intake. Behav Neurosci. 1983;97:210–220. doi: 10.1037//0735-7044.97.2.210. [DOI] [PubMed] [Google Scholar]
- Elias C.F., Aschkenasi C., Lee C., Kelly J., Ahima R.S., Bjorbaek C., Flier J.S., Saper C.B., Elmquist J.K. Leptin differentially regulates NPY and POMC neurons projecting to the lateral hypothalamic area. Neuron. 1999;23:775–786. doi: 10.1016/s0896-6273(01)80035-0. [DOI] [PubMed] [Google Scholar]
- Frago L.M., Chowen J.A. Hypothalamic leptin and ghrelin signaling as targets for improvement in metabolic control. Curr Phar Des. 2015;21:3596–3605. doi: 10.2174/1381612821666150710145428. [DOI] [PubMed] [Google Scholar]
- Ha C.H., Choi J., Choi E.J., Costa M.E., Lee B.J., Ojeda S.R. Nell2, a neuron-specific EGF-like protein, is selectively expressed in glutamatergic neurons and contributes to the glutamatergic control of GnRH neurons at puberty. Neuroendocrinology. 2008;88:199–211. doi: 10.1159/000139579. [DOI] [PubMed] [Google Scholar]
- Hassouna R., Labarthe A., Tolle V. Hypothalamic regulation of body growth and appetite by ghrelin-derived peptides during balanced nutrition or undernutrition. Mol Cell Endocrinol. 2016;438:42–51. doi: 10.1016/j.mce.2016.09.027. [DOI] [PubMed] [Google Scholar]
- Horvath T.L. The hardship of obesity: a soft-wired hypothalamus. Nat Neurosci. 2005;8:561–565. doi: 10.1038/nn1453. [DOI] [PubMed] [Google Scholar]
- Horvath T.L., Diano S. The floating blueprint of hypothalamic feeding circuits. Nat Rev Neurosci. 2004;5:662–667. doi: 10.1038/nrn1479. [DOI] [PubMed] [Google Scholar]
- Horvath T.L., Gao X.B. Input organization and plasticity of hypocretin neurons: possible clues to obesity’s association with insomnia. Cell Metab. 2005;1:279–286. doi: 10.1016/j.cmet.2005.03.003. [DOI] [PubMed] [Google Scholar]
- Hwang E.M., Kim D.G., Lee B.J., Choi J., Kim E., Park N., Kang D., Han J., Choi W.S., Hong S.G., et al. Alternative splicing generates a novel non-secretable cytosolic isoform of NELL2. Biochem Biophys Res Commun. 2007;353:805–811. doi: 10.1016/j.bbrc.2006.12.115. [DOI] [PubMed] [Google Scholar]
- Jaworski A., Tom I., Tong R.K., Gildea H.K., Koch A.W., Gonzalez L.C., Tessier-Lavigne M. Operational reducdancy in axon guidance through the multifunctional receptor Robo3 and its ligand NELL2. Science. 2015;350:961–965. doi: 10.1126/science.aad2615. [DOI] [PubMed] [Google Scholar]
- Jeong J.K., Kim H.R., Hwang S.M., Park J.W., Lee B.J. Region- and neuronal phenotype-specific expression of NELL2 in the adult rat brain. Mol Cells. 2008a;26:186–192. [PubMed] [Google Scholar]
- Jeong J.K., Ryu B.J., Choi J., Kim D.H., Choi E.J., Park J.W., Park J.J., Lee B.J. NELL2 participates in formation of the sexually dimorphic nucleus of the preoptic area in rats. J Neurochem. 2008b;106:1604–1613. doi: 10.1111/j.1471-4159.2008.05505.x. [DOI] [PubMed] [Google Scholar]
- Kim H., Ha C.M., Choi J., Choi E.J., Jeon J., Kim C., Park S.K., Kang S.S., Kim K., Lee B.J. Ontogeny and the possible function of a novel epidermal growth factor-like repeat domain-containing protein, NELL2, in the rat brain. J Neurochem. 2002;83:1389–1400. doi: 10.1046/j.1471-4159.2002.01245.x. [DOI] [PubMed] [Google Scholar]
- Kim J.G., Nam-Goong I.S., Yun C.H., Jeong J.K., Kim E.S., Park J.J., Lee Y.C., Kim Y.I., Lee B.J. TTF-1, a homeodomain-containing transcription factor, regulates feeding behavior in the rat hypothalamus. Biochem Biophys Res Commun. 2006;349:969–975. doi: 10.1016/j.bbrc.2006.08.147. [DOI] [PubMed] [Google Scholar]
- King B.M. The rise, fall, and resurrection of the ventromedial hypothalamus in the regulation of feeding behavior and body weight. Physiol Behav. 2006;87:221–244. doi: 10.1016/j.physbeh.2005.10.007. [DOI] [PubMed] [Google Scholar]
- Kuroda S., Tanizawa K. Involvement of epidermal growth factor-like domain of NELL proteins in the novel protein-protein interaction with protein kinase C. Biochem Biophys Res Commun. 1999;265:752–757. doi: 10.1006/bbrc.1999.1753. [DOI] [PubMed] [Google Scholar]
- Kuroda S., Oyasu M., Kawakami M., Kanayama N., Tanizawa K., Saito N., Abe T., Matsuhashi S., Ting K. Biochemical characterization and expression analysis of neural thrombospondin-1-like proteins NELL1 and NELL2. Biochem Biophys Res Commun. 1999;265:79–86. doi: 10.1006/bbrc.1999.1638. [DOI] [PubMed] [Google Scholar]
- Lin T.N., Kim G.M., Chen J.J., Cheung W.M., He Y.Y., Hsu C.Y. Differential regulation of thrombospondin-1 and thrombospondin-2 after focal cerebral ischemia/reperfusion. Stroke. 2003;34:177–186. doi: 10.1161/01.str.0000047100.84604.ba. [DOI] [PubMed] [Google Scholar]
- Matsuhashi S., Noji S., Koyama E., Myokai F., Ohuchi H., Taniguchi S., Hori K. New gene, nel, encoding a M(r) 93 K protein with EGF-like repeats is strongly expressed in neural tissues of early stage chick embryos. Dev Dyn. 1995;203:212–222. doi: 10.1002/aja.1002030209. [DOI] [PubMed] [Google Scholar]
- Matsuyama S., Aihara K., Nishino N., Takeda S., Tanizawa K., Kuroda S., Horie M. Enhanced long-term potentiation in vivo in dentate gyrus of NELL2-deficient mice. Neuroreport. 2004;15:417–420. doi: 10.1097/00001756-200403010-00007. [DOI] [PubMed] [Google Scholar]
- Matsuyama S., Doe N., Kurihara N., Tanizawa K., Kuroda S., Iso H., Horie M. Spatial learning of mice lacking a neuron-specific epidermal growth factor family protein, NELL2. J Pharmacol Sci. 2005;98:239–243. doi: 10.1254/jphs.fp0050211. [DOI] [PubMed] [Google Scholar]
- Mello C.V., Jarvis E.D., Denisenko N., Rivas M. Isolation of song-regulated genes in the brain of songbirds. Methods Mol Biol. 1997;85:205–217. doi: 10.1385/0-89603-489-5:205. [DOI] [PubMed] [Google Scholar]
- Musatov S., Chen W., Pfaff D.W., Mobbs C.V., Yang X.J., Clegg D.J., Kaplitt M.G., Ogawa S. Silencing of estrogen receptor alpha in the ventromedial nucleus of hypothalamus leads to metabolic syndrome. Proc Natl Acad Sci USA. 2007;104:2501–2506. doi: 10.1073/pnas.0610787104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson B.R., Matsuhashi S., Lefcort F. Restricted neural epidermal growth factor-like like 2 (NELL2) expression during muscle and neuronal differentiation. Mech Dev. 2002;119(Suppl 1):S11–19. doi: 10.1016/s0925-4773(03)00084-4. [DOI] [PubMed] [Google Scholar]
- Nelson B.R., Claes K., Todd V., Chaverra M., Lefcort F. NELL2 promotes motor and sensory neuron differentiation and stimulates mitogenesis in DRG in vivo. Dev Biol. 2004;270:322–335. doi: 10.1016/j.ydbio.2004.03.004. [DOI] [PubMed] [Google Scholar]
- Oyasu M., Kuroda S., Nakashita M., Fujimiya M., Kikkawa U., Saito N. Immunocytochemical localization of a neuron-specific thrombospondin-1-like protein, NELL2: light and electron microscopic studies in the rat brain. Brain Res Mol Brain Res. 2000;76:151–160. doi: 10.1016/s0169-328x(99)00342-3. [DOI] [PubMed] [Google Scholar]
- Pinto S., Roseberry A.G., Liu H., Diano S., Shanabrough M., Cai X., Friedman J.M., Horvath T.L. Rapid rewiring of arcuate nucleus feeding circuits by leptin. Science. 2004;304:110–115. doi: 10.1126/science.1089459. [DOI] [PubMed] [Google Scholar]
- Roesch D.M. Effects of selective estrogen receptor agonists on food intake and body weight gain in rats. Physiol Behav. 2006;87:39–44. doi: 10.1016/j.physbeh.2005.08.035. [DOI] [PubMed] [Google Scholar]
- Ruy B.J., Kim H.R., Jeong J.K., Lee B.J. Regulation of the female rat estrous cycle by a neural cell-specific epidermal growth factor-like repeat domain containing protein, NELL2. Mol Cells. 2011;32:203–207. doi: 10.1007/s10059-011-0086-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz M.W., Seeley R.J., Woods S.C., Weigle D.S., Campfield L.A., Burn P., Baskin D.G. Leptin increases hypothalamic pro-opiomelanocortin mRNA expression in the rostral arcuate nucleus. Diabetes. 1997;46:2119–2123. doi: 10.2337/diab.46.12.2119. [DOI] [PubMed] [Google Scholar]
- Watanabe T.K., Katagiri T., Suzuki M., Shimizu F., Fujiwara T., Kanemoto N., Nakamura Y., Hirai Y., Maekawa H., Takahashi E. Cloning and characterization of two novel human cDNAs (NELL1 and NELL2) encoding proteins with six EGF-like repeats. Genomics. 1996;38:273–276. doi: 10.1006/geno.1996.0628. [DOI] [PubMed] [Google Scholar]


