Abstract
Background
Balance problems contribute to reduced quality of life in Parkinson’s disease (PD) and available treatments are often insufficient for treating axial and postural motor symptoms.
Objective
To investigate the safety of use and possible effects of stochastic vestibular stimulation (SVS) alone and combined with LDOPA in patients with PD.
Methods
SVS or sham stimulation was administered to 10 PD patients in a double-blind placebo controlled cross-over pilot study. Motor symptoms and balance were evaluated in a defined off-medication state and after a 200 mg test dose of LDOPA, using UPDRS-III, Posturo- Locomotor-Manual (PLM) movement times (MT), static posturography and force plate measurements of the correcting response to a balance perturbation.
Results
Patients did not detect when SVS was active, but SVS increased nausea after LDOPA in two patients. Mixed model analysis demonstrated that SVS improved balance corrections after a backward perturbation and shortened the postural response time. In static posturography there was significant interaction between effects of SVS, medication and proprioceptive input (standing on foam vs. on hard support) and SVS decreased the total sway-path with eyes closed and off medication.
As expected, LDOPA improved the UPDRS-III scores and MT. There was an interaction between the effect of SVS and LDOPA on UPDRS-III partly because of reduced UPDRS-III scores with SVS in the off-medication state.
Conclusions
Short term use of SVS is safe, improves corrective postural responses and may have a small positive effect on motor symptoms in PD patients off treatment.
Keywords: Parkinson’s disease, vestibular stimulation, levodopa, posture1
Introduction
Treatments for Parkinson’s disease (PD) motor symptoms are usually more effective for appendicular extremity symptoms than axial symptoms. Postural instability in particular, is often partly treatment resistant to both L-DOPA and deep brain stimulation (DBS) [1]. Dopaminergic treatments can sometimes increase balance problems, especially when they elicit dyskinesia [2] or cognitive impairments [3]. Non-dopaminergic treatments such as deep brain stimulation of the subthalamic nucleus (STN-DBS) is not suitable in patients biologically aged over 70, with cognitive decline or psychiatric comorbidity [3, 4]. As balance problems have a strong negative impact on quality of life, there is need for other treatments that either improve balance or improve Parkinson symptoms without adverse effects on balance.
Stochastic galvanic vestibular stimulation (SVS) is a non-invasive method which activates the vestibular system in a random fashion, so that the stimulation is not perceived as a perturbation of balance. Balance can be improved by SVS in healthy controls and in patients with Parkinson’s disease [5, 6]. There is also some evidence that SVS improves other symptoms in other neurodegenerative diseases, including autonomic responses, motor function and frontal executive function when currents higher than detection threshold are used [7, 8]. A proposed explanation for both sub- and supra-threshold effects is that SVS induces a phenomenon of stochastic resonance (SR), something that can be observed in threshold activated systems with suboptimal function and can result in improved signal detection as well as improved network function on higher levels. For detailed discussions on SR see recent reviews [9–11]. The exact mechanism of how SVS may influence the activity of the brain is not known, but may theoretically involve SR effects on thresholds in the activation pattern of basal ganglia output. SVS has been demonstrated to influence resting state electroencephalography activity indicating that cortical-subcortical activity is also modulated [12]. A recent rodent study demonstrated improved balance and locomotion in the rotarod test in 6-OHDA hemilesioned rats during near detection threshold SVS [13]. SVS was additionally found to increase GABA-levels in the substantia nigra pars reticulata (SNr) in a fashion similar to that seen after LDOPA treatment or subthalamic stimulation [13, 14], suggesting a possible neurochemical mechanism for improved motor function in PD during SVS, involving inhibition of the SNr, a nucleus which is overactive in PD [15, 16].
In previous studies of SVS in neurodegenerative diseases, motor improvement effects have not been evaluated with standard clinical scales, thus it is not clear if the improvement is large enough to be of clinical significance [5, 7, 8]. Importantly, despite the encouraging findings mentioned, the clinical efficacy of SVS in PD has not been determined and it is not known whether SVS effects interact at all with the effect of LDOPA. The current investigation is a randomized cross-over placebo controlled pilot study of the safety and feasibility of SVS, with efficacy on balance and motor symptoms as secondary objectives. Although it was not powered to detect minor improvements it can indicate what kind of effects can be expected using the widely accepted UPDRS-III motor symptom rating scale. Unlike previous studies, the effects of SVS were also compared with the effect of LDOPA.
Material and methods
The study was approved by the regional Ethical Review Board in Gothenburg, Sweden (permission no 754-11) and informed written consent was obtained prior to inclusion.
Subjects
Ten patients with Parkinson’s disease fulfilling UKPDS brain bank criteria [17] (6 males and 4 females, 61±8 years of age, Table 1) were recruited from the Neurology Clinic at Sahlgrenska University Hospital in Gothenburg, Sweden. Inclusion criteria were a clinical effect of LDOPA medication and Hoehn and Yahr stage ≤3. Exclusion criteria were implanted electronic devices as well as ongoing or previously diagnosed vestibular diseases.
Table 1. Study subject characteristics.
Half of the subjects received active stimulation and half received sham stimulation (0 mA) on test day 1 (T1). The reversed stimulation condition was applied on day 2 (T2). AE, adverse effects reported by the subjects.
| Subj. | Sex | Age of onset | Age at testing | PD dominant side | Daily LDE1 (mg) | Hoehn & Yahr scale | T1 mA | T2 mA | AE |
|---|---|---|---|---|---|---|---|---|---|
| 1 | M | 45 | 52 | Right | 845 | 2.5 | 0.9 | 0 | N/A |
| 2 | F | 53 | 62 | Right | 825 | 3 | 0 | 0.6 | N/A |
| 3 | M | 64 | 70 | Left | 1000 | 3 | 0 | 0.45 | N/A |
| 4 | M | 50 | 62 | Right | 154 | 2 | 0 | 0.6 | N/A |
| 5 | F | 38 | 44 | Right | 300 | 2 | 0 | 0.4 | 2) |
| 6 | M | 62 | 66 | Left | 1670 | 2.5 | 0 | 0.9 | N/A |
| 7 | F | 59 | 64 | Right | 650 | 2.5 | 0.35 | 0 | 3) |
| 8 | M | 60 | 62 | Right | 400 | 2 | 0.1 | 0 | N/A |
| 9 | F | 52 | 60 | Right | 710 | 2 | 0.3 | 0 | 4) |
| 10 | M | 52 | 70 | Left | 820 | 2.5 | 0.4 | 0 | 5) |
Calculated as recommended in Tomlinson CL et al. Systematic review of levodopa dose equivalency reporting in Parkinson’s disease. Mov Disord, 2010. 25(15): p. 2649–53.
T1=slight nausea + stomach discomfort 1h post LDOPA, T2=slight nausea 30min post LDOPA + vomit 45min and 90min post LDOPA
T2=slight vertigo during movement
T1=mild headache + mild dizziness, T2=dry mouth 1h post LDOPA
T1=slight nausea 45min post LDOPA
Procedure
The study was carried out on 2 different days in a randomized cross-over design. Subjects were block-randomized to treatment arm A or B, which differed in whether active stimulation or sham stimulation of 0mA was present on the first or the second test day (Suppl. Fig. S1). The effects of SVS or sham SVS were evaluated after 12h of medication abstinence as well as after a single open label dose of dispersed LDOPA, Madopar Quick, 200 mg.
Stochastic electric currents were applied to the vestibular system through oval 4×6cm electrodes (Axelgaard Manufacturing, CA, USA) placed over the mastoid process behind both ears, in a bipolar binaural configuration. For best possible electrode contact and to avoid skin sensations during the stimulation, the skin behind the ears was cleaned thoroughly with Nuprep® skin prep gel (Weaver and Company, USA). A generous amount of Skintact® ECG electrode gel (Leonhard Lang, Austria) was applied on the electrodes which were placed firmly on the cleaned area. The electrodes were held in place by a soft pad and secured by an elastic head band. The electrode impedance was measured repeatedly and was confirmed to be no more than 1 kΩ across evaluations. A portable and programmable constant current stimulator [6] was used to deliver the stimulus. Before evaluations commenced, the individual threshold for stimulation induced perceptible sway was determined. Whilst blindfolded, subjects were seated on a stool placed on a Kistler force plate (model 9260AA, Kistler Nordic AB, Sweden). A sinusoid-shaped bipolar signal with a frequency of 1Hz was applied at eleven amplitude levels ranging between ±0.1mA – ±0.7mA (peak to peak), in two subsequent trials. Each amplitude level was presented for a period of 10s followed by 5s of 0mA in a fixed pseudorandomized order. The lowest amplitude level where rhythmic sway was recorded by the force plate software (BioWare software version 5.0.3.0, Kistler Nordic AB, Sweden) or consistently reported by the subject was recorded as the individual sinusoidal threshold amplitude. Two subjects did not respond to ±0.7mA and a similar protocol with eight amplitude levels between ±0.5mA and ±1.2mA was used. The individual stimulation threshold was used as the maximum allowed amplitude of the SVS protocol. A white noise stimulation pattern (0–30Hz) was generated using a white noise generator and then filtered using a 10th order low-pass Butterworth filter with the cutoff frequency set at 30 Hz. The generated signal was confirmed to have a zero mean (± 1%) and root mean square (RMS) [(30 μA RMS/100 μA) ±5%]. Like in previous animal study [13], the SVS current was never higher than the threshold amplitude and for 90% of the time it was less than 45% of the threshold and was within the top 10% of current values for less than 5% of the time. This can be compared with the sinusoidal test current where the current level distribution is skewed toward the maximum and minimum values so that it is within the top 10% of current levels for 30% of the time. The used SVS current waveform allows for blinded procedures and has been demonstrated to alter basal ganglia GABA release and motor performance in 6-OHDA hemilesioned rats [13].
SVS was either on or off during the entire evaluation period (≤3h). When SVS was started, the stimulator ramped up the amplitude over the first 3 s to avoid sudden stimulation sensations.
Neither participants nor examiners were blinded to LDOPA medication. The study was carried out according to the intended double-blind design for SVS by using pre-programmed stimulation protocols and masking all current indications on the device with black tape.
Evaluations
Patients were encouraged to report any discomfort or adverse reaction during the test day. At the end of the day subjects were debriefed using a structured interview protocol (Suppl. Appendix A).
A dynamic perturbed balance response test was carried out on a force plate (Kistler Nordic AB, Sweden, Fig. 1). A thin rope was connected to a figure-of-8 harness worn by the subject. The rope was loaded with 3% of the subject’s body weight, which pulled at the height of the manubrium sterni. The subject stood on the force plate while counterbalancing the pull and looking straight ahead at a marker at eye level. Without prior notice the examiner released the pull force by disengaging an electromagnetic holding magnet. This created a spontaneous backward sway which the subject reflectively corrected. The perturbation achieved with this setup is analogous to the clinical push/release test [18], but much less pronounced so will not elicit a stepping response, only a COP sway. The COP sway movements in anterioposterior (Y) and mediolateral (X) directions, as well as the perturbation correction time (s) were recorded with BioWare acquisition software (Kistler Nordic AB, Sweden) and analyzed. The mean COP position during 1.1 seconds immediately before the pull-release was used as the starting point for assessing sway-response to the perturbation. The perturbed balance test was repeated 3–4 times for each treatment condition and mean responses were used for statistical calculations.
Figure 1. Dynamic “pull-release” posturography.
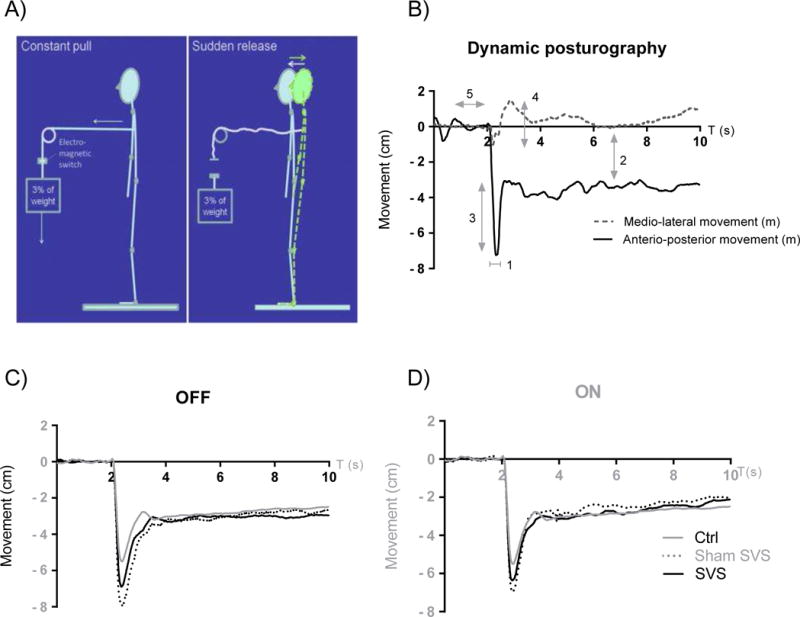
A) Schematic explanation of the procedure where the subject counterbalances 3% of his/her weight and the weight is suddenly released without prior notice.
B) Typical dynamic posturography centre of pressure (COP) registration: 1 the corrective response time was the time (s) between T=2.0 s and the change of movement direction; 2 the difference between stable anterior-posterior (AP) position before and after release was calculated from a stable 2 s period after the corrective movement (usually at t=6–8s); 3 the release AP excursion (cm) was calculated as the maximum deviation in relation to the new stable AP CO) position; 4 medio-lateral movement (m) was calculated as the maximum deviation to either side of the medial axis during the corrective response, resulting in two values per trial; 5 the baseline average over T=[0.9 s – 2.0 s] was used as start reference point. C) Mean AP COP of registrations from all subjects during OFF medication state. Data from 9 healthy controls (age 22–68) are also included for comparison. D) Mean AP COP registrations after L-DOPA. Data from healthy controls is included as in C.
Two static balance tests were also executed. During the first, subjects stood barefoot directly on the force plate with feet together, arms folded over the chest and eyes closed. Four consecutive 10s trials were recorded. The procedure was repeated with the subject standing on a 10×50×50 cm pad of medium density foam to decrease proprioceptive input. The overall center of pressure (COP) sway-path and maximum sway (absolute distance from the mean COP position derived from the COP postural measurements) were analyzed.
A trained examiner performed UPDRS section III scoring while blinded to stimulation, but not to medication status. The examination was recorded using a full HD camcorder and was scored immediately and on a later occasion off line, with maintained blinding for stimulation status. For rigidity items the first assessment was used, for the other measurements the off-line scoring was used. When total scores (excluding rigidity items) differed by more than 5% between first and second assessment, the recording was re-assessed by a second trained UPDRS examiner and a consensus score was agreed.
The Posturo-Locomotor-Manual test (PLM) is a repeated movement where the subject picks up an object and transfers it to a platform at chin height and two meters ahead. The total movement time MT(s) is composed of three partly overlapping movement phases: Postural, where the test person stands up while lifting the object, Locomotive, when the person walks and Manual, where the person transfers the object from a holding position to the top of the platform. The movement is recorded using infrared motion capture technique as previously described [19]. The optoelectronic measuring system consisted of an infrared camera, reflective markers, and an automated tracking software system (Qbtech/PDMonitor, Qbtech AB, Sweden). The test movement was carried out in 10 triplets at each evaluation point and the mean movement time of the three fastest consecutive movements was used as MT(s).
Statistical analysis
Statistical analysis was performed after unblinding. Logarithmic transformations were made to normalize data distributions. All data except maximum sway passed normal distribution tests. Non parametric Friedman test, with Wilcoxon’s paired test as a post-hoc measure was use to analyses maximum sway. Other variables were analyzed with linear mixed model analyses (fixed-effect, repeated measures) to assess the main effects of SVS and LDOPA treatment as well as interaction between the two. In the static posturography, reduced proprioceptive input was used as a third main factor. All statistical analysis was performed using SPSS (PASW Statistics 18). All data are reported as mean±SD, unless otherwise indicated.
Results
The sinusoidal current thresholds determined prior to 0 mV sham stimulation were 0.500±0.245 mA and the SVS maximum currents (based on another sinusoidal threshold determination) of the active stimulation sessions were 0.500±0.255 mA (mean±SD) (Table 1). None of the subjects could determine in which session SVS was active. The examiners were also successfully blinded throughout the course of the data collection. Four patients reported six adverse effects during the evaluation. There were four reports of adverse effects during active stimulation and two in the sham condition. The adverse effects during active SVS consisted of slight or more pronounced nausea with vomiting in response to the LDOPA administration and one patient that reported mild headache and dizziness. One patient reported slight vertigo during movement in the sham condition, but not during active stimulation. The patient that vomited in response to LDOPA during SVS only reported slight discomfort in the sham condition (Table 1).
In the dynamic balance test, SVS shortened the mean response time (s) of the perturbation correction (FSVS(1,8.9)=16.3, p≤0.01, Table 2, Fig. 2A). Post-hoc analysis was not significant but the largest difference between SVS and sham SVS was observed in the off medication state where the correction time was 0.42±0.14s during SVS and 0.46±0.10s during sham SVS. The new stable AP position after the balance correction during SVS was slightly posterior to that during sham SVS (FSVS(1,7.54)=5.48, p≤0.05, Table 2). Furthermore, the maximum backward COP excursion in relation to the new stable position was decreased by SVS (FSVS(1,8)=12.94, p≤0.01, Table 2, Fig 2B), in particular in the off medication state where the excursion was reduced from −0.057±0.025 to −0.041±0.019 m, with a mean reduction of 0.017 m (p<0.01). There was also a main effect of SVS reducing the mediolateral deviation during the correcting response (FSVS(1,8.3)=14.4, p≤0.01, Table 2, Fig 2C). Although maximum excursion can be expected to depend on the response time, there was no correlation between the two variables (Spearman r= − 0.1811, p=0.30) No significant main effect of LDOPA was observed for perturbed postural responses.
Table 2. Summary of effects and collapsed estimates.
Mean difference ± SEM of the collapsed estimates for the main effects of the mixed model ANOVA are given with p-values in brackets. Significant interactions indicated with p-values. The different measurements 1–4 are explained in Fig 1B.
| Mean ± SD difference of collapsed estimates (p- value for main effect) | p-values for interactions | ||||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| SVS vs shamSVS | LDOPA ON vs OFF | No Foam vs Foam | SVS × LDOPA | SVS × Foam | LDOPA × Foam | SVS × LDOPA × Foam | |
| Correction time1 | −0.05 ± 0.03 s (≤ 0.001) |
−0.04 ± 0.09 s (0.21) |
0.054 | ||||
| Stable pre-post release position2, Δy | −0.5 ± 0.6 cm (≤ 0.05) |
0.1 ± 1.3 cm (0.72) |
0.12 | ||||
| Release AP deviation3, Δy | 0.9 ± 1.0 cm (≤ 0.01) |
0.1 ± 1.3 cm (0.77) |
0.02 | ||||
| Release ML deviation4, Δx | −0.2 ± 0.3 cm (≤ 0.01) |
−0.2 ± 0.6 cm (0.28) |
0.051 | ||||
| Sway-path | −9.2 ± 9.5 cm (≤ 0.01) |
−2.8 ± 2 cm (0.16) |
9.7 ± 9.5 cm (0.02) |
0.13 | 0.14 | 0.84 | ≤0.01 |
| Max sway | −0.3 ± 0.8 cm (>0.05) |
−0.19 ± 1.1 cm (>0.05) |
−1.7 ± 2.1 cm (≤0.01) |
N/A | |||
| UPDRS | −0.3 ± 3.4 (0.82) |
−10.6 ± 5.9 (≤ .001) |
0.02 | ||||
| PLM.MT | 0.02 ± 0.06 s (0.30) |
−0.11 ± 0.06 s (≤ 0.001) |
0.34 | ||||
The different measurements are explained in Fig 1B.
Figure 2. Posturography and UPDRS-III results.
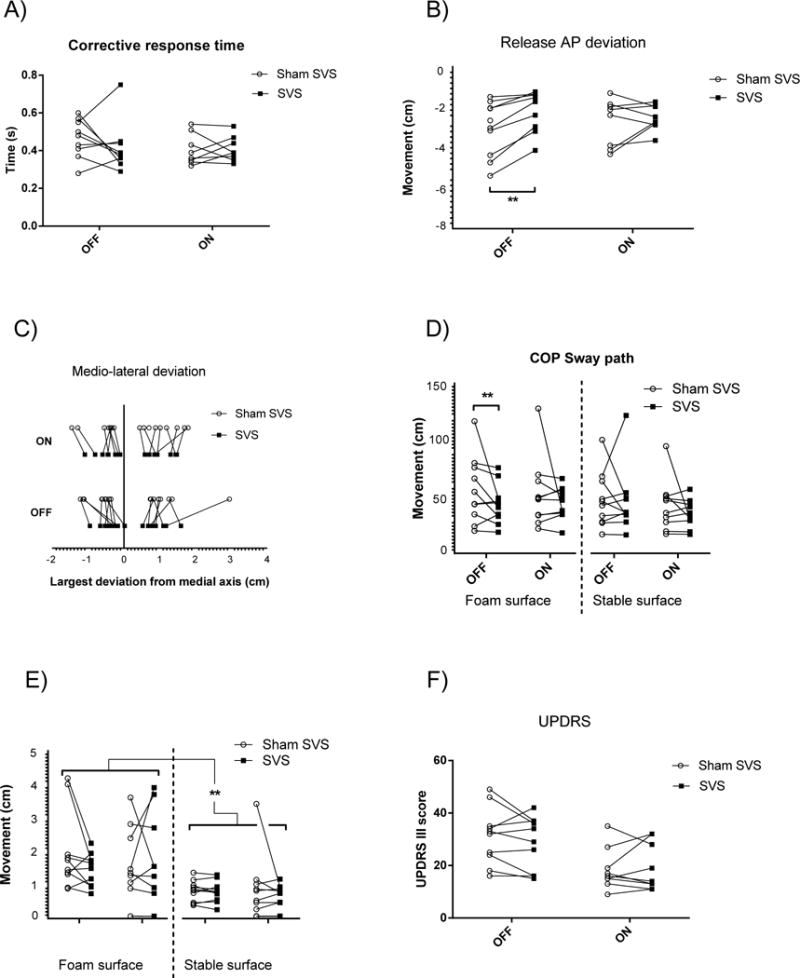
Statistics given in Table 2; A) SVS shortened the response time (measure 1 in Fig. 1) from force release until the reactive centre of pressure (COP) movement had changed direction; B) Maximum anterioposterior (AP) centre of pressure (COP) deviation (cm) from the post release stable position (measure 3 in Fig. 1); C) COP deviations in the medio-lateral direction during the corrective response after force release were reduced during SVS (measure 4 in Fig. 1); D) total COP sway-path distance while standing with eyes closed during 10s of standing still on narrow support; E) the maximum sway deviation during 10s of standing still with eyes closed; F) UPDRS scores before and after LDOPA in the SVS and sham stimulation conditions. SVS=stochastic vestibular stimulation, OFF=no medication, ON=after 200 mg LDOPA. Post hoc test statistics: ** p ≤ 0.01.
In the static balance test there was a main effect of SVS with shorter COP sway-path compared to sham (FSVS(1,9.9)=11.02, p≤0.01, Fig. 2D). As expected, sway-path was significantly increased during reduced proprioceptive input (FFoam(1, 10.0)=9.0, p=0.02, Table 2, Fig. 2D) and there was also a significant three-way interaction (FSVS*LDOPA*Foam(1,10)=5.2, p≤0.05). Post-hoc analysis demonstrated a significantly reduced sway-path with SVS (0.73±0.3 m) compared to sham SVS (0.93±0.5 m, p≤0.01) off medication. The Friedman non-parametric test showed that the max sway deviation was different between the eight different conditions (χ2(7)=40.13, p<0.001, Table 2, Fig. 2E). A paired post-hoc (Wilcoxon’s signed rank) confirmed that maximum sway was always smaller on a stable surface than on a foam surface (all post hoc comparisons foam vs. no foam, Z≥−2.6, p<0.01), but significant effects of LDOPA or SVS were not found.
There was a significant main effect of LDOPA with decreased UPDRS-III motor scores (FLDOPA(1,9.5)=76.15, p≤0.001, Table 2, Fig 2F) and a significant interaction effect between SVS and LDOPA (FSVS*LDOPA (1, 9.9)=7.05, p=0.02). The interaction effect is mainly explained by reduced UPDRS-III scores during active SVS (28.8±10.1) compared to sham SVS (31.2±10.8) off medication (mean change −2.4±2.0), but the post hoc test was not significant.
There was a main effect of LDOPA treatment on PLM movement times (FLDOPA(1,9.5)=27.08, p≤0.001, Table 2), but no main effect of SVS. The PLM test is clinically categorized as positive if there is a significant improvement in MT after LDOPA. With SVS 7 of the patients were positive in the PLM LDOPA test versus 5 of the patients during sham, a difference that was not significant (Wilcoxon matched pair, p=0.25).
Discussion
The primary aim of this pilot study was to evaluate the safety and feasibility of SVS in PD patients. A secondary aim was to provide an indication of possible efficacy regarding balance and motor symptoms in PD. In this small sample, SVS was overall safe to use in PD patients, but exaggerated nausea after LDOPA challenge was observed. The LDOPA challenge with 200 mg of LDOPA will in our experience rarely lead to vomiting, although nausea is not uncommon. If SVS is used outside the experimental setting the potential interaction with LDOPA leading to increased side effects of the dopaminergic drugs should be considered. SVS improved both static and dynamic balance indices whereas improvements of overall motor symptoms were not clearly demonstrated.
To maintain the blinding of the study, great care was taken to minimize any skin sensation of the SVS. Those elaborate procedures would make SVS unpractical for domestic use, but are not necessary when slight skin sensations are acceptable. As more pronounced sensations are norm with transcutaneous electrical nerve stimulation (TENS), we think home use of SVS with regular adhesive electrodes is feasible.
Previous studies have evaluated the effect of SVS on motor symptoms using waist and wrist-born accelerometers [7, 8] that provide measures that are difficult to translate to a clinically meaningful effect. We therefore used the better validated and widely accepted UPDRS motor rating section. This is the first study evaluating the effect of SVS with UPDRS. Although the effect of SVS on UPDRS-III was small and not in the range of what is usually considered as clinically significant in individual cases [20] it is interesting that all improvements (also balance responses) were found in the off-medication state, as it suggests that SVS may reduce motor off fluctuations. The effect size of the improved dynamic balance is similar to what we have previously observed with DBS in PD patients [21], which is promising. Ultimately, novel treatments for balance problems need to demonstrate reduced frequency of falls and that would be an important objective of future studies of SVS in PD. It should be noted that previous studies have used a somewhat different stimulation protocol. Instead of white noise, a “pink” 1/f noise profile was used and a nociceptive threshold was used to decide the maximum amplitude [7,8]. The stimulation used here is a near-threshold paradigm, where the threshold for subjective or objective detection of rhythmic synchronized stimulation was used as the maximum allowed current. This does not mean that the vestibular system is not activated, only that the activation is not perceived. The SVS amplitudes were similar to those reported in previous work [7, 8] where stimulation currents where slightly lower (around 0.3 mA) compared to this study (0.5 mA). In a study of SVS effects on balance function in healthy individuals [6], maximum improvements occurred with stimulation amplitudes in a range predominantly between 0.1 and 0.4 mA (mean 0.26, one subject with the optimal stimulation level 0.7 mA) using the same 0–30Hz filtered stochastic protocol that was used here. Often SR is described in the context of signal detection. There is some controversy in regard to the linearity of the afferent side of the vestibular system [22], but regardless of whether there can be SR at the level of the vestibular system the corrective postural motor responses are gated by basal ganglia activity which is a non-linear selective function where SR can take place. If SR is involved in the observed effects we find it more likely that it occurs in the selection and activation of motor programs. There are theoretical models that predict that low dopamine levels are associated with less neuronal noise and that increasing the noise in a low dopamine system will be associated with larger noise benefit [23, 24], and also that more noise is needed to reach optimal benefit [24]. Those predictions are in agreement with the observation that SVS improved functions more before than after LDOPA administration. A ceiling effect of LDOPA in the current study is also possible.
It was previously shown that patients with Parkinson’s disease have a decreased vestibulocollic reflex as measured with vestibular-evoked myogenic potentials, and that LDOPA restores this deficit [25]. An expected effect of subthreshold SVS is to increase the responsivity of a suppressed vestibular system. It is therefore possible that SVS, similar to LDOPA, ameliorates the reduced vestibulospinal responses in PD. In agreement with that, we found that SVS reduced the overall COP sway-path, particularly in the off medication state when standing on foam. Standing on foam makes the proprioceptive inputs less reliable, so with eyes closed the subject has to rely more on the vestibular system to maintain balance. SVS also had a positive effect on perturbed dynamic balance with reduced excursions in the anterioposterior and mediolateral plane as well as improvement of the balance response time. We expected the anterioposterior COP sway distance to be strongly correlated to the response time, but this was not the case (data not shown). Independent improvements of response time and COP excursion suggest that SVS has a positive effect on the reactivity of the balance system, as well as on the correcting motor response.
The finding that LDOPA medication had little positive effect on the static and perturbed balance conditions is consistent with previous observations [26]. One reason for this could be that dopaminergic drugs decrease stiffness and can lead to dyskinesia, which, even when subtle, could affect postural balance negatively [2]. Overall dopaminergic treatment ameliorates appendicular symptoms more than axial symptoms. Automatic postural control may be more improved by facilitating the vestibulospinal control system or other non-dopaminergic pathways that are activated by SVS. One of those pathways may involve altered activity in the SNr, as increases in GABA release in the SNr can result from both SVS and from LDOPA treatment [13]. Interestingly, it was recently reported that high-frequency DBS of the SNr improves axial symptoms and gait, when combined with DBS in the STN [27]. We suggest that SVS and DBS-SNr may in part act through a common mechanism by inhibiting the overactive SNr [28]. There was a significant interaction between SVS and LDOPA in the overall motor score suggestive of reduced symptoms in OFF. The same pattern was however not observed in the PLM test, which assesses mainly speed of movement. Although it is interesting that more patients improved significantly in MT after LDOPA during active SVS, this may be a random result because the study is underpowered for categorical data. Effect sizes of SVS on overall motor symptoms appear to be much smaller than those of LDOPA, so to confirm or dismiss a general motor improvement of SVS in PD, large studies would be required.
One of the most devastating complications to Parkinson’s disease is falls [29]. This problem increases and becomes less treatment responsive as the disease progresses. Although the dynamic balance test used here is a surrogate marker, and responses were not completely normalized by SVS, PD patients who report falls could potentially benefit from long term use of SVS. We conclude that it is feasible to perform blinded or open studies of SVS in PD and that the effects observed in this pilot study indicate that such studies should focus on patients with balance and gait problems in particular.
Supplementary Material
Highlights.
Short term use of stochastic vestibular stimulation (SVS) is safe.
SVS improves postural reflexes in Parkinson’s disease.
Positive effects of SVS are mainly found off pharmacological treatment.
SVS can potentially improve balance during off-fluctuations.
Acknowledgments
The study was supported by the Swedish Research Council, grant no 2009-2618 and in part through the NASA Cooperative agreement NCC9-58 with NSBRI (SA2001) and NIH grant RO1-DC009031.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
This study was in part presented as an abstract at the 18th International Congress of Parkinson’s Disease and Movement Disorders in Stockholm 2014
Abbreviations: 6-OHDA 6-hydroxydopamine, COP center of pressure, PLM posturo- locomotor- manual test, MT movement time, SR stochastic resonance, STN-DBS subthalamic nucleus deep brain stimulation, SVS stochastic vestibular stimulation
References
- 1.Maurer C, et al. Effect of chronic bilateral subthalamic nucleus (STN) stimulation on postural control in Parkinson’s disease. Brain. 2003;126(Pt 5):1146–63. doi: 10.1093/brain/awg100. [DOI] [PubMed] [Google Scholar]
- 2.Hely MA, Morris JGL, Reid WGJ, Trafficante R. Sydney multicenter study of Parkinson’s disease: Non-L-dopa–responsive problems dominate at 15 years. Movement Disorders. 2004;20(2):190–199. doi: 10.1002/mds.20324. [DOI] [PubMed] [Google Scholar]
- 3.Russmann H, Ghika J, Villemure JG, Robert B, Bogousslavsky J, Burkhard PR, Vingerhoets FJG. Subthalamic nucleus deep brain stimulation in Parkinson disease patients over age 70 years. Neurology. 2004;63(10):1952–1954. doi: 10.1212/01.wnl.0000144198.26309.d8. [DOI] [PubMed] [Google Scholar]
- 4.Derost PP, Ouchchane L, Morand D, Ulla M, Llorca PM, Barget M, Debilly B, Lemaire JJ, Durif F. Is DBS-STN appropriate to treat severe Parkinson disease in an elderly population? Neurology. 2007;68(17):1345–55. doi: 10.1212/01.wnl.0000260059.77107.c2. [DOI] [PubMed] [Google Scholar]
- 5.Pal S, Rosengren SM, Colebatch JG. Stochastic galvanic vestibular stimulation produces a small reduction in sway in Parkinson’s disease. J Vestib Res. 2009;19(3–4):137–42. doi: 10.3233/VES-2009-0360. [DOI] [PubMed] [Google Scholar]
- 6.Mulavara AP, et al. Improving balance function using vestibular stochastic resonance: optimizing stimulus characteristics. Experimental Brain Research. 2011;210(2):303–12. doi: 10.1007/s00221-011-2633-z. [DOI] [PubMed] [Google Scholar]
- 7.Pan W, et al. Improvement of motor functions by noisy vestibular stimulation in central neurodegenerative disorders. J Neurol. 2008;255(11):1657–61. doi: 10.1007/s00415-008-0950-3. [DOI] [PubMed] [Google Scholar]
- 8.Yamamoto Y, et al. Noisy vestibular stimulation improves autonomic and motor responsiveness in central neurodegenerative disorders. Ann Neurol. 2005;58(2):175–81. doi: 10.1002/ana.20574. [DOI] [PubMed] [Google Scholar]
- 9.Collins JJ, Imhoff TT, Grigg P. Noise-enhanced Information Transmission in Rat SA1 Cutaneous Mechanoreceptors via Aperiodic Stochastic Resonance. Journal of Neurophysiology. 1996;76(1) doi: 10.1152/jn.1996.76.1.642. [DOI] [PubMed] [Google Scholar]
- 10.Funke K, Kerscher NJ, Wörgötter F. Noise-improved signal detection in cat primary visual cortex via a well-balanced stochastic resonance-like procedure. European Journal of Neuroscience. 2007;26(5):1322–32. doi: 10.1111/j.1460-9568.2007.05735.x. [DOI] [PubMed] [Google Scholar]
- 11.Moss F, Ward LM, Sannita WG. Stochastic resonance and sensory information processing: a tutorial and review of application. Clinical Neurophysiology. 2004;115:267–281. doi: 10.1016/j.clinph.2003.09.014. [DOI] [PubMed] [Google Scholar]
- 12.Kim DJ, et al. Noisy galvanic vestibular stimulation modulates the amplitude of EEG synchrony patterns. PLoS One. 2013;8(7):e69055. doi: 10.1371/journal.pone.0069055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Samoudi G, et al. Noisy galvanic vestibular stimulation promotes GABA release in the substantia nigra and improves locomotion in hemiparkinsonian rats. PloS One. 2012;7(1):e29308. doi: 10.1371/journal.pone.0029308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Windels F, et al. Effects of high frequency stimulation of subthalamic nucleus on extracellular glutamate and GABA in substantia nigra and globus pallidus in the normal rat. European Journal of Neuroscience. 2000;12:4141–4146. doi: 10.1046/j.1460-9568.2000.00296.x. [DOI] [PubMed] [Google Scholar]
- 15.Kravitz AV, et al. Regulation of parkinsonian motor behaviours by optogenetic control of basal ganglia circuitry. Nature. 2010;466(7306):622–U7. doi: 10.1038/nature09159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.DeLong MR, Wichmann T. Circuits and circuit disorders of the basal ganglia. Archives of Neurology. 2007;64(1):20–24. doi: 10.1001/archneur.64.1.20. [DOI] [PubMed] [Google Scholar]
- 17.Hughes AJ, et al. Accuracy of clinical diagnosis of idiopathic Parkinson’s disease: a clinicopathological study of 100 cases. J Neurol Neurosurg Psychiatry. 1992;55:181–184. doi: 10.1136/jnnp.55.3.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jacobs J, et al. An alternative clinical postural stability test for patients with Parkinson’s disease. J Neurol. 2006;253(11):1404–1013. doi: 10.1007/s00415-006-0224-x. [DOI] [PubMed] [Google Scholar]
- 19.Zackrisson T, et al. Evaluation of the objective posturo-locomotor-manual method in patients with parkinsonian syndromes. Front Neurol. 2013;4:95. doi: 10.3389/fneur.2013.00095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schrag A, et al. Minimal clinically important change on the unified Parkinson’s disease rating scale. Movement Disorders. 2006;21(8):1200–7. doi: 10.1002/mds.20914. [DOI] [PubMed] [Google Scholar]
- 21.Bergquist F. 15th Congress of the EFNS. Budapest: Eur J Neurol; 2011. Standardized force plate pull-release test in Parkinson’s disease, P2450. [Google Scholar]
- 22.Bagnall MW, et al. Frequency-independent synaptic transmission supports a linear vestibular behavior. Neuron. 2008;60(2):343–52. doi: 10.1016/j.neuron.2008.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li SC, von Oertzen T, Lindenberger U. A neurocomputational model of stochastic resonance and aging. Neurocomputing. 2006;69:1553–1560. [Google Scholar]
- 24.Sikström S, Söderlund G. Stimulus-dependent dopamine release in attention-deficit/hyperactivity disorder. Psychol Rev. 2007;114(4):1047–75. doi: 10.1037/0033-295X.114.4.1047. [DOI] [PubMed] [Google Scholar]
- 25.Poetter-Nerger M, et al. Differential effect of dopa and subthalamic stimulation on vestibular activity in Parkinson’s disease. Movement Disorders. 2012;27(10):1268–1275. doi: 10.1002/mds.25061. [DOI] [PubMed] [Google Scholar]
- 26.Nardone A, Schieppati M. Balance in Parkinson’s disease under static and dynamic conditions. Movement Disorders. 2006;21(9):1515–1520. doi: 10.1002/mds.21015. [DOI] [PubMed] [Google Scholar]
- 27.Weiss D, et al. Nigral stimulation for resistant axial motor impairment in Parkinson’s disease? A randomized controlled trial. Brain. 2013;136:2098–2108. doi: 10.1093/brain/awt122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Avila I, et al. Beta frequency synchronization in basal ganglia output during rest and walk in a hemiparkinsonian rat. Experimental Neurology. 2010;221(2):307–319. doi: 10.1016/j.expneurol.2009.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schrag A, et al. Caregiver-burden in parkinson’s disease is closely associated with psychiatric symptoms, falls, and disability. Parkinsonism Relat Disord. 2006;12(1):35–41. doi: 10.1016/j.parkreldis.2005.06.011. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


