Abstract
Expression of the poliovirus receptor (PVR) on cells is a major host determinant of infection by poliovirus. Previously, the only immune cell type known to express PVR was the blood-derived monocyte, which is susceptible to infection at very low frequency. We demonstrate that professional antigen-presenting cells—macrophages and dendritic cells, generated upon differentiation of monocytes—retain expression of PVR and are highly susceptible to infection by type 1 Mahoney strain of poliovirus. Maximal cell-associated titers of virus are obtained within 6 to 8 h postinfection, and cell death and lysis occurs within 24 h postinfection. Similar kinetics are observed in cells infected with the Sabin 1 vaccine strain. Although protein synthesis and receptor-mediated endocytosis are inhibited upon poliovirus infection of these critical antigen-presenting cells, we demonstrate for the first time that functional presentation of antigen occurs in these infected cells via the HLA class II pathway.
Poliovirus (PV), a prototypical member of the Picornaviridae family, is a small, nonenveloped, positive-stranded RNA virus whose replication is limited to specific cells and tissues that express the PV receptor (PVR; CD155) (30, 40) on the cell surface. Most cells of the immune system, such as T and B lymphocytes, are not susceptible to PV infection (35, 41), as they do not express PVR. In contrast, Freistadt et al. demonstrated that human peripheral blood monocytes do express PVR (22). However, monocytes are not very permissive to PV infection; viral protein production could only be demonstrated at very low frequency in a subpopulation of monocytes (18, 21).
Monocytes are precursor cells that are able to differentiate into macrophages or dendritic cells (DCs) (10-12, 36, 44). In response to pathogens, inflammatory cytokines, or necrotic cells, DCs and macrophages play central roles in the induction of immune responses. These antigen-presenting cells (APCs) acquire and process antigens, displaying them in the context of HLA class I and II molecules at the cell surface (9, 31, 43, 45). Subsequent interaction of the HLA-antigen complexes and costimulatory molecules on APCs with T cells in the presence of relevant secreted cytokines induces immune responses (26). DCs are critical APCs that, in contrast to macrophages, are unique in being able to induce primary immune responses from naive T cells to novel antigens in humans (6).
To better understand the interaction of PV with the immune system, we characterized the susceptibility of monocyte-derived macrophages and DCs to PV infection. We show here that these in vitro-differentiated macrophages and DCs retain PVR expression and can be productively infected with PV. The kinetics of viral infection in these APCs follows that seen for PV infection with well-characterized laboratory cell lines (7, 38) and results in cell death. Furthermore, PV-induced cytopathic effects typically observed during viral infection of standard cell lines (1, 3, 37, 41, 48) are also seen here upon infection with either the Mahoney or Sabin vaccine strain. Viral infection of DCs also alters production of inflammatory cytokines. However, antigen-specific T cells lyse PV-infected DCs presenting HLA class II-restricted peptides. Thus, PV-infected DCs are able to functionally present antigen via the HLA class II pathway.
MATERIALS AND METHODS
Donors.
This study was approved by the Human Research Advisory Committee and the Institutional Review Board, with a minimal-risk-for-adults rating. Adult volunteers (between the ages of 25 and 65 years) donated blood for this study.
Cell cultures. (i) Monocyte isolation and DC culture.
Peripheral blood mononuclear cells (PBMCs) were isolated from heparinized blood by centrifugation on Ficoll-Hypaque (Bio-One Inc., Longwood, Fla.). The buffy coats were washed twice with phosphate-buffered saline (PBS) and resuspended in RPMI 1640 medium (Invitrogen Life Technologies, Carlsbad, Calif.) supplemented with 10% fetal calf serum (FCS) (Invitrogen) and 2 mM glutamine (RPMI-10). Monocytes from the PBMCs were allowed to adhere to plastic six-well dishes for 2 h at 37°C, 7% CO2, and nonadherent cells were subsequently removed by aspiration. These cells were cultured for 6 days according to established protocols (44, 46). Briefly, monocytes were cultivated in RPMI-10 containing granulocyte-macrophage colony-stimulating factor at 800 U/ml and interleukin-4 (R&D Systems, Minneapolis, Minn.) at 500 U/ml for 6 days to obtain immature DCs. Mature DCs were generated from immature DCs by the addition of tumor necrosis factor alpha (R&D Systems) at 1,000 U/ml and 1 mM prostaglandin E2 (Sigma Chemicals, St. Louis, Mo.) for 48 h. Monocytes were maintained in RPMI-10 containing macrophage colony-stimulating factor (R&D Systems) at 100 U/ml for 6 days to generate macrophages. All cultures were supplemented with fresh medium and cytokines every 3 days. Monocyte-derived cells were always maintained at 37°C, 7% CO2.
(ii) Other cells.
HeLa-S3 cells were maintained in suspension (3 × 105 to 4 × 105 cells/ml) in Joklik's modified minimal essential medium (S-MEM; Sigma) supplemented with 7.5% horse serum (Invitrogen Life Technologies) and 1 mM MEM sodium pyruvate (S-MEM-7.5). For monolayer growth, cells were transferred into Dulbecco's modified Eagle's medium (DMEM; Invitrogen Life Technologies) supplemented with 5% FCS, penicillin at 50 U/ml, streptomycin at 50 μg/ml, and 1 mM sodium pyruvate (DMEM-5) in a 5% CO2 incubator at 37°C.
Virus stocks.
PV serotype 1 strains were used for all studies. Mahoney (PVM) and Sabin1 (PVS) viral stocks were propagated as previously described from a plaque obtained by transfection of the infectious cDNAs into HeLa cells (47).
UV inactivation of poliovirus (uvPV).
The Mahoney strain in serum-free RPMI (109 PFU/ml; 35-mm dish) was irradiated on ice by using the UV cross-linker FB-UVXL-1000 (Fisher Biotech, Pittsburg, Pa.) at a distance of 10 cm for 10 min at optimal cross-linking values (1,200 U, 100 μJ/cm2). These conditions are sufficient to reduce the viral infectivity to below that detectable by plaque assays on confluent HeLa cell monolayers (data not shown). Binding assays showed that the UV inactivation did not alter the ability of radiolabeled virus to bind the PVR on HeLa cells (data not shown).
MAbs, flow cytometry, and fluorescence microscopy.
Monoclonal antibodies (MAbs) directed against human surface markers CD14, CD83, HLA-DR, HLA class I, CD86, CD1a, CD68, CD54, and CD58 (Caltag Laboratories, Burlingame, Calif.) were used at the supplier's recommended concentrations. All were direct phycoerythrin (PE)- or fluorescein isothiocyanate (FITC)-conjugated antibodies. PVR antibodies (D-171) were obtained from Neomarkers (Lab Vision Corporation, Fremont, Calif.) and used with a secondary goat anti-mouse antibody conjugated to FITC (Caltag Laboratories).
Suspensions of monocyte-derived cells (5 × 105/200 μl) or adherent cells on chamber slides (5 × 104/chamber) in serum-free C-RPMI (RPMI 1640 supplemented with 2 mM glutamine) were incubated at 4°C for 30 min with the appropriate antibodies. Where the PVR antibody was used, cells were washed and incubated for a further 30 min at 4°C with the secondary antibody following primary antibody incubation. The stained cells were washed twice with cold PBS, fixed in 2% paraformaldehyde, and analyzed by flow cytometry on a FACSCalibur apparatus (BD Biosciences, San Diego, Calif.) or visualized with a fluorescence microscope.
PV infections.
Virus (PVM or PVS) was bound to monocyte-derived cells at a high multiplicity of infection (MOI = 10) for 30 min at room temperature (RT) in C-RPMI. Where noted, uvPV was also bound to DCs at RT for 30 min in C-RPMI medium. The inoculum was removed by aspiration. Infection (or internalization of uvPV) was initiated with the addition of medium prewarmed to 37°C and incubating the cells at 37°C, 7% CO2. At various times postinfection (p.i.), cells (105) were collected by scraping adherent macrophages and immature DCs and by collecting the growth medium for nonadherent mature DCs. Cell pellets were obtained by centrifugation. Media supernatants were stored separately at −20°C. Cell pellets were lysed in PBS (100 μl) by three freeze-thaw cycles. Titers of the cell pellets and supernatants were subsequently determined by plaque assays on confluent HeLa cell monolayers.
Protein synthesis.
Mock- or virus-infected DCs (approximately 5 × 105 mature DCs in suspension or 1 × 105 adherent immature DCs/well) were incubated in methionine-free S-MEM (Sigma) supplemented with 10% FCS. At 3 h p.i., [35S]methionine (50 μCi/ml; 1,000 Ci/mmol; Amersham Biosciences Corporation, Piscataway, N.J.) was added to the mock- and virus-infected cultures. At various times p.i., 105 cells were harvested. The cells were spun down, washed twice with PBS, and resuspended in 50 μl of PBS. The cell pellets were lysed by three freeze-thaw cycles, and the labeled proteins were analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE).
Endocytosis. (i) Bulk-phase endocytosis.
Immature DCs were cultured on poly-l-lysine (Sigma)-coated glass coverslips. At various times p.i., Alexa Fluor 568-conjugated 10,000-MW dextran (250 μg/ml; Molecular Probes Inc., Eugene, Oreg.) was added to either mock- or virus-infected cells and incubated for 1 h at 37°C. The cells were washed extensively with PBS and fixed with 2% paraformaldehyde. To identify virally infected cells, the fixed cells were permeabilized with a solution of 0.5% saponin plus 1% bovine serum albumin (BSA) in PBS and stained for viral capsid proteins using polyclonal rabbit anti-PV antibody at a 1:200 dilution for 20 min at RT. The cells were washed with 0.5% saponin-1% BSA, and the PE-conjugated goat anti-rabbit secondary antibody (Caltag) was added for 20 min at the recommended concentration. The cells were washed and fixed again with 2% paraformaldehyde solution, and the coverslips were mounted on glass slides using the Prolong antifade kit (Molecular Probes). The cells were examined by confocal microscopy.
(ii) Receptor-mediated endocytosis.
Immature DCs (105 adherent cells in 35-mm plates) were mock infected or infected with PV (MOI = 10). In addition, separate cultures of immature DCs were treated continuously with 20 mM ammonium chloride. BODIPY-conjugated DQ-ovalbumin (DQ-OVA; Molecular Probes), a self-quenched conjugate of ovalbumin that exhibits bright green fluorescence upon proteolytic processing due to the released dye molecules, was added (200 μg/ml in C-RPMI) at 6 h p.i. to all DCs for 20 min at 4°C. The cells were washed three times with ice-cold PBS containing 2% serum to remove unbound or nonspecifically associated DQ-OVA, and then fresh RPMI-10 was added. The cells were incubated at 4°C for 10 min or for various intervals at 37°C. The cells were then removed from the plates by trypsinization, washed with ice-cold PBS, fixed with 2% paraformaldehyde, and analyzed by flow cytometry.
Apoptosis.
A CaspaTag kit (Intergen Company, Purchase, N.Y.) was used according to the manufacturer's protocol to measure the levels of activated caspases. Briefly, mature DCs (3 × 105) were either mock infected, infected with PV (MOI = 10), or treated with 1 μM staurosporine (STS) for 10 h. At 0 and 6 h p.i., the cells were incubated with CaspaTag solution for 1 h at 37°C in a 300-μl volume/sample. The cells were washed, stained externally for CD83, washed with PBS, and then fixed in 2% paraformaldehyde. Samples were analyzed by flow cytometry.
In separate experiments, the levels of sub-G1 DNA in infected DCs were determined. Cells were harvested at various times p.i., washed with PBS, and fixed in cold 70% ethanol overnight. Following fixation, cells were pelleted by centrifugation, washed twice with PBS, and treated with RNase (5 μg). Samples were then stained in 200 μl of propidium iodide solution (50 μg/ml) and analyzed by flow cytometry.
Cytotoxicity assays. (i) CD8 T cells.
A CD8+ cytotoxic T-lymphocyte line specific for the HLA A2-restricted hepsin 42-51 peptide (VLLRSDQEPL; peptide 19) was generated by stimulation of T cells from an HLA A2-positive healthy adult volunteer. Mature DCs were pulsed for 1 to 2 h at 37°C with 50 μg of peptide/ml and washed with PBS twice before culture with peripheral blood leukocytes at a responder/stimulator ratio of 30:1. After 7 days, T cells were collected and restimulated with peptide-pulsed DCs. For the second and third DC stimulations, the medium was supplemented with interleukin-Z at 50 to 100 U/ml, and the culture period was extended to 14 days. After the third cycle, CD8+ T cells were recovered by positive selection with anti-CD8 magnetic beads (Dynal Biotech Inc., Lake Success, N.Y.). Subsequent passages of CD8+ T-cell lines used peptide-loaded autologous peripheral blood leukocytes as antigen-presenting cells.
Targets were DCs or macrophages which were mock infected or infected with PVM. In addition, the mock- or virus-infected APCs were pulsed with peptide 19. The DCs and macrophages were pulsed with peptide (50 μg/ml) for 1 h at 37°C, 7% CO2 in 200 μl of C-RPMI. The target cells were then labeled with chromium-51 (50 μCi/106 cells in 200 μl of PBS; 417.1 μCi/mg; Perkin-Elmer, Boston, Mass.) for 1 h at 37°C, 7% CO2, washed, resuspended in RPMI-10, and incubated in triplicate (104 target cells/well) with various ratios of autologous CD8 T cells specific for peptide 19. The assay mixtures were harvested after 4 h at 37°C, 7% CO2, and the supernatant activities were determined.
(ii) CD4 T cells.
SCCE 110 is a 30-amino-acid peptide with the following sequence: VNDLMLVKLNSQARLSSMVKKVRLPSRCEP. This peptide after processing is presented by HLA class II molecules and is recognized by CD4 cytotoxic T cells. Autologous DCs loaded with SCCE 110 were used to generate a class II-restricted, peptide-specific human CD4+ T-cell line. Prior to use in the cytotoxicity assays described below, the specificity of these CD4 T cells was tested. The cells proliferated in response to peptide-loaded DCs. In addition, these T cells were also strongly cytotoxic against autologous HLA class II-positive peptide-loaded targets but did not lyse peptide-free targets. Addition of an anti-HLA class II MAb inhibited lysis of targets, indicating that the T cells were HLA class II restricted (data not shown)
DCs were matured for 24 h and then either mock infected or infected with PVM and incubated with SCCE 110 (100 μg/ml) at the start of infection. Alternatively, immature DCs were incubated with SCCE 110 (50 μg/ml) and the maturation cytokine cocktail for 24 h and then mock infected or infected with PVM. Following 2 h of infection, cells were labeled with chromium-51 (100 μCi/106 cells) for 1 h at 37°C, 7% CO2, washed, resuspended in RPMI-10, and incubated in triplicate (104 target cells/well) with various ratios of autologous CD4 T cells specific for SCCE 110. The assay mixtures were harvested after 3 h at 37°C, 7% CO2, and the supernatant activities were determined.
RESULTS
PVR expression on cells during culture and differentiation of monocytes.
PBMCs are precursors to DCs in vivo and can be differentiated in vitro to DCs (10, 36, 44). To determine whether the monocyte-derived cells retained expression of PVR, monocytes were cultured under standard conditions to induce differentiation first to immature DCs and then, subsequently, to mature DCs. The resultant cells were analyzed by flow cytometry. Mature DCs were CD14− CD83+ CD86+ CD1a+ CD54+ CD58+ and expressed high levels of HLA-DR and HLA class I molecules (data not shown). Coexpression of PVR with various cell surface markers was followed by flow cytometry over the several days of culture as monocytes (CD14+high; day zero) differentiated into immature DCs (CD14− CD83−; day 6) (Table 1) and then mature DCs (CD14− CD83+) (Fig. 1A). To confirm that mature DCs indeed expressed PVR, CD83+ cells were sorted (with the FACSCalibur) and then examined for the expression of PVR. All CD83+ cells were also positive for PVR expression (data not shown). PVR expression was maintained throughout differentiation of monocytes to mature DCs.
TABLE 1.
PVR expression on cells during culture of monocyte-derived DCs
| Day of culture | Surface moleculea | MFIb |
|---|---|---|
| 0 | PVR | 20 |
| CD14 | 1,608 | |
| PVR/CD14 | 27/1,612 | |
| 2 | PVR | 16 |
| CD14 | 561 | |
| PVR/CD14 | 15/454 | |
| 4 | PVR | 17 |
| CD14 | 158 | |
| PVR/CD14 | 18/39 | |
| 6 | PVR | 19 |
| CD14 | 31 | |
| PVR/CD14 | 20/34 | |
| CD83 | — |
Cells were either singly or doubly stained with the indicated antibody.
MFI, Mean fluorescence intensity. —, negative.
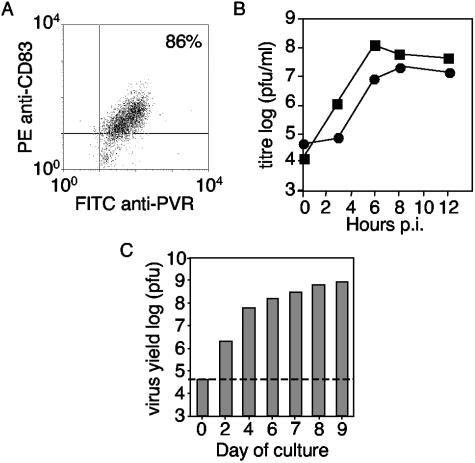
FIG. 1.
Monocyte-derived DCs express PVR and are permissive for PV infection. (A) Mature DCs were double stained for the expression of CD83 and PVR and analyzed by flow cytometry. (B) Single-cycle growth curves of PV infection in immature and mature DCs. Immature (•) and mature (▪) DCs were infected with PVM, and the titers of cell-associated virus were determined at various times p.i. (C) Permissivity of cells to PV infection over days 0 to 9 of DC culture. On each day, monocyte-derived cells (106) were infected with PVM. At 6 h p.i., cells were harvested and the titers of cell-associated virus were determined. The dashed line indicates input titer.
Productive infection and apoptosis of monocyte-derived cells.
To determine whether DCs were permissive for PV infection, cells were infected with PVM. In contrast to monocytes (18, 21), both immature and mature DCs were highly permissive to viral infection (Fig. 1B). Virus titers increased rapidly during the period of infection, with maximal cell-associated titers at 6 to 8 h p.i. Maximal viral release into the supernatant was also between 6 and 8 h p.i., although virus titers continued to increase slightly at even later times p.i. (data not shown). Mature DCs appeared to be more susceptible to the infection than immature DCs. In repeated experiments, PVM grew consistently to higher titers in cultures of fully differentiated mature DCs than in the monocyte or immature DC cultures (Fig. 1B and C). DCs showed a similar pattern of susceptibility to PVS infection. PVS grew to high titers within immature DCs and to higher titers in mature DCs, but there was no detectable increase of viral titers in infected monocytes (data not shown).
To determine whether productive infection by poliovirus was associated with a specific stage during the differentiation process, cells were infected at different days of culture. Total viral yields from 106 cells were determined (Fig. 1C). Consistent with previous studies (18, 21), infection of monocytes (on day zero of culture) with PVM showed no detectable increase in viral titers from that of the original input titer over a 6-h infection period. In contrast, virus yields continuously increased over the subsequent days of culture as the cells differentiated from monocytes to DCs. Thus, cells became increasingly susceptible to PVM during the culture period, with mature DCs being the most permissive to PV infection.
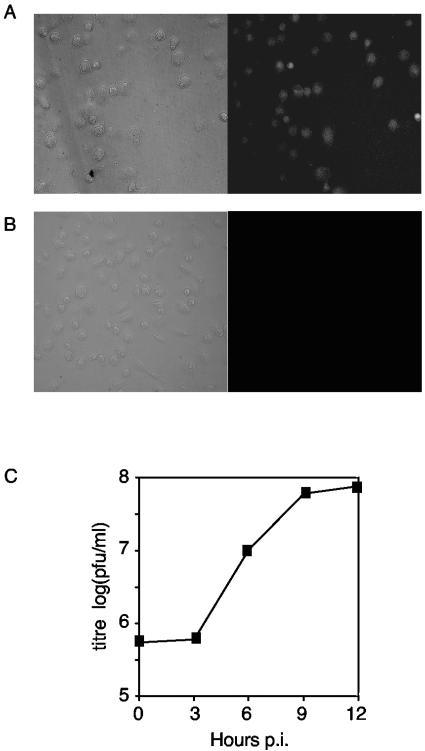
Monocytes are also precursors of macrophages (11, 12). Following in vitro differentiation, the macrophages were CD14high CD1a+ CD68+ (data not shown) and retained expression of PVR (Fig. 2A). Macrophages were also productively infected by PVM, with infected macrophages yielding a rapid increase in viral titer over a 12-h period of infection (Fig. 2C).
FIG. 2.
PV infects monocyte-derived macrophages. (A and B) Macrophages stained with PVR antibody (A) or with only secondary antibody as a control (B) and visualized with a fluorescence microscope (right panel). Magnification, ×20. (C) Single-cycle growth curve of PVM infection in macrophages. Macrophages were infected with PVM, and the titers of the cell-associated virus were determined at various times p.i.
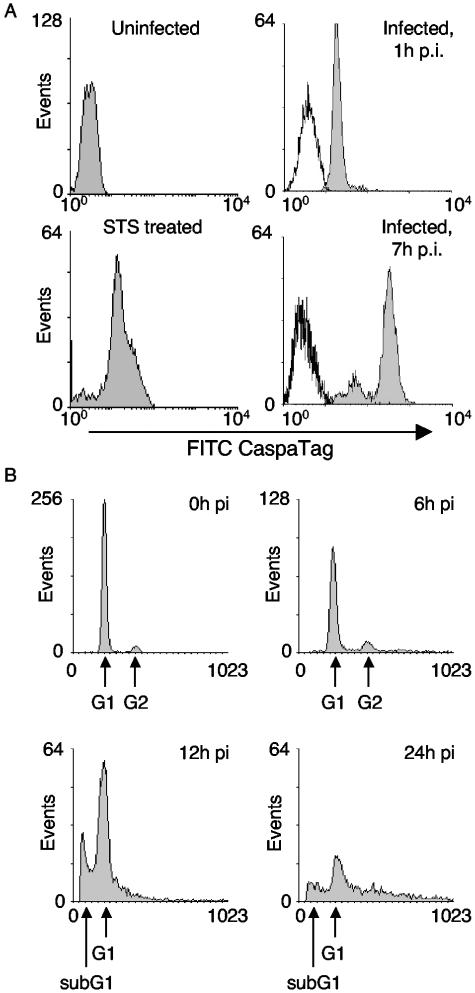
Viral infection of DCs and macrophages results in cell death and lysis (data not shown) between 12 and 24 h p.i., similar to that observed with established PV-sensitive cell lines. Of note is that cell death occurred irrespective of the PV strain (PVM or PVS) used to infect DCs and macrophages. Cell death by apoptosis is known to allow antigen presentation on HLA class I (4, 20) and class II (19) molecules following uptake by unaffected cells and to induce stimulation of antigen-specific CD8 and CD4 T cells. Thus, the potential hallmarks of apoptosis were analyzed in infected DCs (Fig. 3). CaspaTag (FAM-VAD-FMK) is a carboxyfluorescein (FAM) derivative of zVAD-FMK and a potent inhibitor of caspase activity that binds irreversibly to the active site of activated caspases 1 to 10 and 12. Consistent with the ability of STS to induce apoptosis (33), flow cytometric analyses of CaspaTag-stained, STS-treated, uninfected DCs showed increased staining above the background levels of control untreated cells, indicating caspase activation. CaspaTag staining of PVM-infected DCs showed that, at even early times of infection, caspase activation was comparable to levels seen in STS-treated cells and that the levels of activated caspases continued to increase as infection progressed (Fig. 3A). In addition, at late stages of infection there was an associated increase in the number of terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end labeling-positive cells (data not shown) and a shift in DNA content to sub-G1 levels (Fig. 3B). Together these data suggest that DCs undergo apoptosis as a result of PV infection.
FIG. 3.
PV infection induces apoptosis in DCs. (A) Caspases are activated in infected DCs. Mock-infected, STS-treated uninfected mature DCs or virus-infected DCs were harvested at 0 and 6 h p.i. and stained with CaspaTag for 1 h at 37°C. Cells at 1 and 7 h p.i. were analyzed by flow cytometry. Black line, unstained cells; grey fill, FITC CaspaTag. (B) Cellular DNA is fragmented in infected DCs. Infected DCs were stained with propidium iodide at various times p.i. Arrows indicate cellular DNA in the G1, G2, and sub-G1 phases.
Effects of viral infection on DC cellular processes. (i) Protein translation.
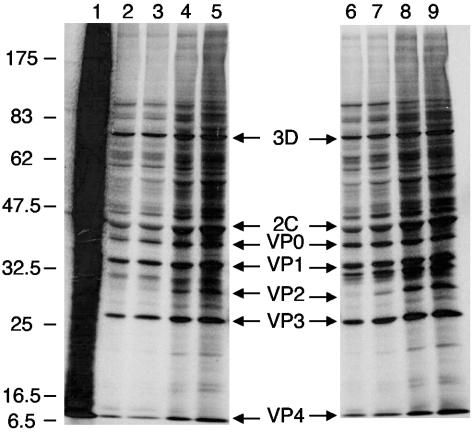
Protein synthesis in DCs is important for aspects of antigen acquisition and processing and the production of immune cytokines necessary for induction of immune responses. PV infection of laboratory cell lines causes shut-off of host transcription, translation (32), and the secretory pathway (16, 17) within the first few hours of infection. As inhibition of host translation and secretion may potentially inhibit the ability of these cells to induce immune responses, the effects of virus infection on DC biology were studied. Protein synthesis was examined at different times p.i. in mature DCs infected with PVM or PVS (Fig. 4). The pattern of radiolabeled proteins was that expected for viral infection in other standard cell lines, with visible synthesis of both nonstructural and capsid proteins. Host protein synthesis was inhibited by approximately 3 h p.i. in infected DCs. Analysis of PVS-infected mature DC lysates showed similar patterns of viral protein synthesis and inhibition of host translation. In addition, host translation is similarly inhibited in infected immature DCs and macrophages (data not shown). Interestingly, although the virus yields from infected DCs and HeLa cells are similar, the amount of viral proteins produced in DCs is much less than that seen with equivalent numbers of infected HeLa cells (data not shown).
FIG. 4.
Host translation is inhibited in PVM- and PVS-infected DCs. Mature DCs were mock infected (lane 1) or infected with PVS (lanes 2 to 5) or with PVM (lanes 6 to 9) and continuously labeled with [35S]methionine. Cell lysates were prepared at 3.5 h p.i. (lanes 2 and 6), 4 h p.i. (lanes 3 and 7), 5 h p.i. (lanes 4 and 8), and 6 h p.i. (lanes 5 and 9). Samples normalized for cell equivalence were resolved by SDS-PAGE on a 12% acrylamide gel and exposed to film. The positions of the molecular weight markers and labeled viral proteins are indicated.
(ii) Endocytosis.
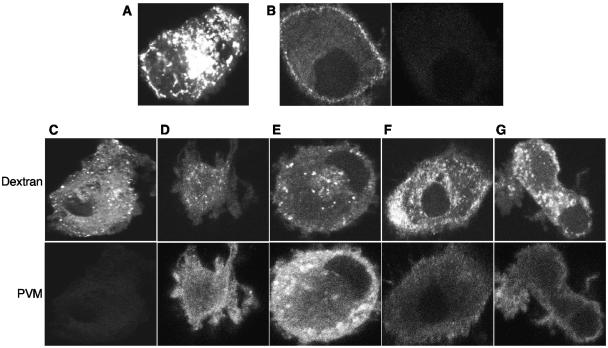
Antigen acquisition in immature DCs occurs primarily through endocytosis and is critical to the immunological function of these cells. To determine whether bulk-phase endocytosis is affected, uninfected and infected immature DCs were incubated with fluorescent dextran beads and examined by confocal microscopy (Fig. 5). Immature DCs were able to endocytose high levels of dextran beads, as can be seen by the large number of fluorescent vesicles within each cell (Fig. 5A and C). Although the level of fluorescence varied from cell to cell, there was punctate staining at even late stages of infection, indicating continued uptake of dextran beads into endocytic vesicles (Fig. 5D to G, upper panels). Staining for viral capsid proteins increased over time (Fig. 5C to E, lower panels), confirming that these cells were indeed infected. Staining for viral proteins decreased at 5 to 6 h p.i. (Fig. 5F and G, lower panels) due to release of viral particles from the infected DCs. These data suggest that bulk-phase endocytosis remains relatively unaffected by PV infection.
FIG. 5.
Bulk-phase endocytosis is unaffected in PVM-infected immature DCs. Alexa Fluor 568-conjugated dextran was incubated with either mock-infected (A) or infected (B to G) cells at 4 or 37°C for 1 h at various times p.i. Cells were also stained with rabbit anti-PV capsid antibodies and examined with a confocal microscope. (A) Mock-infected DCs and dextran at 37°C. (B) Dextran uptake (left panel) and PVM staining (right panel) of infected DCs at 4°C at 0 h p.i. (C to G) Dextran uptake (upper panels) and PVM staining (lower panels) of infected DCs at 2 (C), 3 (D), 4 (E), 5 (F), or 6 (G) h p.i. Magnification, ×100.
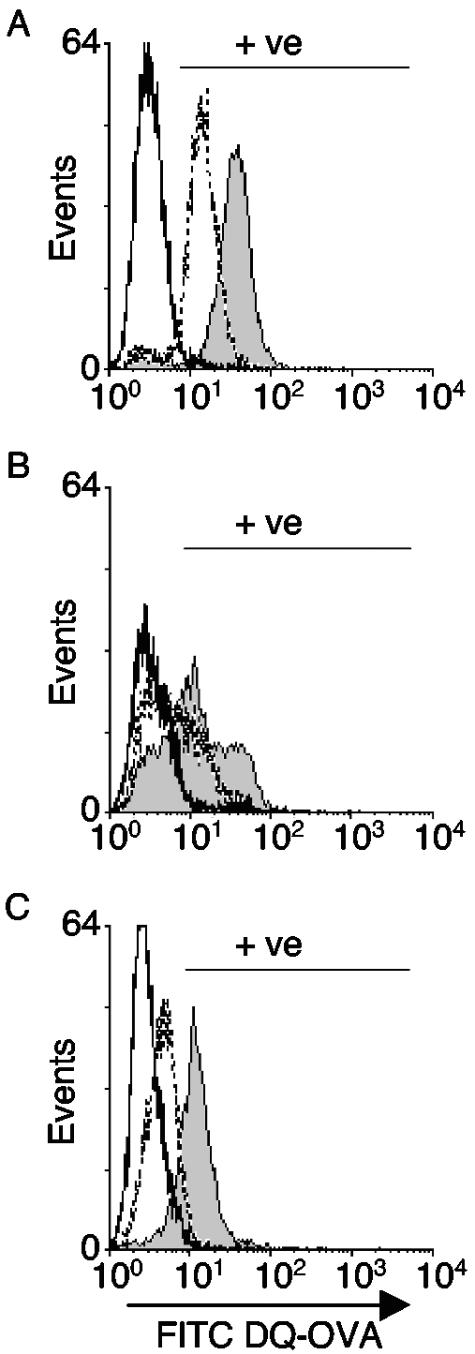
Although bulk-phase endocytosis appears unaffected in infected cells, previous studies had suggested that receptor-mediated endocytosis was inhibited in PV-infected cells (15). To determine whether this pathway was similarly inhibited in infected immature DCs, receptor-mediated uptake was analyzed at late stages of infection by using ovalbumin labeled with the pH-insensitive fluorescent dye BODIPY (DQ-OVA). The DQ-OVA conjugate is endocytosed via the mannose receptor, after which the levels of fluorescence detected are proportional to the extent of DQ-OVA processing (13, 45), and this is a measure of the extent of receptor-mediated internalization within the cell (42). Uninfected (Fig. 6A) and infected (Fig. 6B) cells were incubated with DQ-OVA, and the extent of ovalbumin processing was followed by flow cytometry. Uninfected DCs were able to endocytose and process DQ-OVA at high levels. Although the levels of DQ-OVA processing varied in infected cells, the percentage of positive cells in infected cultures was significantly decreased. Thus, PV-infected DCs are able to process DQ-OVA but at reduced levels in comparison to the uninfected DCs. Confocal imaging also showed decreased uptake of transferrin into infected DCs (data not shown). These data indicate that receptor-mediated uptake is inhibited in infected DCs.
FIG. 6.
Receptor-mediated endocytosis is inhibited in PVM-infected immature DCs. BODIPY-conjugated DQ-OVA was bound to uninfected, infected, or NH4Cl-treated DCs at 4°C and subsequently incubated at 37°C for 10 or 25 min. The fluorescence intensities were measured by flow cytometry. Grey fill, DQ-OVA at 37°C for 25 min; dashed line, DQ-OVA at 37°C for 10 min; black line, DQ-OVA at 4°C for 10 min. (A) Uninfected immature DCs; (B) infected DCs at 6 h p.i.; (C) NH4Cl-treated uninfected cells.
Infection does not affect cell surface levels of HLA molecules or T-cell recognition.
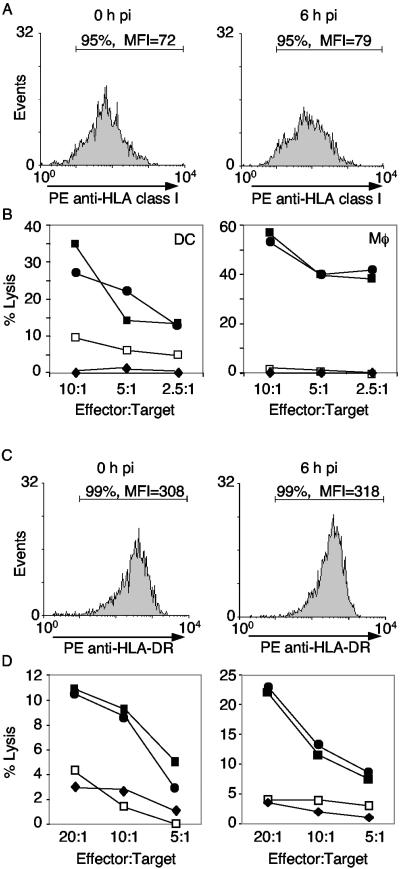
The inhibitory effects of the virus on cellular processes of the DCs suggest that the ability of the DCs to load and present antigen at the cell surface may be compromised. It is known that inhibition of secretion by the nonstructural protein 3A also inhibits transport of new HLA class I molecules from the endoplasmic reticulum (ER) to the Golgi and, thus, to the cell surface. These molecules are synthesized and loaded with antigen within the ER, and the absence of antigen-loaded HLA molecules on the cell surface inhibits lysis of infected target cells by cytotoxic T lymphocytes specific to the new antigen (14). Similar inhibition in APCs would inhibit cell surface expression of new HLA molecules and potentially alter the ability of DCs to induce T cells. The cell surface levels of both HLA class I and class II molecules were examined, as these are the molecules that present antigen to CD8 and CD4 T cells, respectively. At different times p.i., DCs and macrophages were examined by flow cytometry for the surface levels of HLA class I and class II molecules. The levels remained unchanged over a 6-h period of infection (Fig. 7A and C). Thus, the HLA molecules already present on the surface of the DCs or macrophages remained unaffected by PV infection, indicating that PV does not actively downregulate cell surface HLA molecules as a mechanism of immune evasion.
FIG. 7.
PVM infection does not affect cell surface levels of HLA molecules or T-cell recognition. Levels of HLA class I (A) and class II (C) molecules on the surface of virus-infected mature DCs were measured by flow cytometry at 0 and 6 h p.i. The percentages of positive cells and mean fluorescence intensities (MFI) are indicated. (B) Mature DCs and macrophages were exogenously loaded with peptide 19 and then mock infected or infected with PVM. The cells were then incubated with peptide 19-specific CD8 T cells in a standard chromium release assay. ⧫, DCs or macrophages alone; □, infected DCs or macrophages; •, DCs or macrophages plus peptide 19; ▪, infected DCs or macrophages plus peptide 19. (D) Cytotoxicity assays with SCCE 110-loaded DCs. (Left) DCs were simultaneously incubated with SCCE 110 and either mock infected or infected with PVM for 3 h prior to addition of peptide-specific CD4+ T cells in a standard chro mium release assay. (Right) DCs were loaded with SCCE 110 for 24 h and then mock infected or infected with PVM. Peptide-specific CD4+ T cells were added at 3 h p.i. in a standard chromium release assay. ⧫, DCs alone; □, infected DCs alone; •, DCs plus SCCE 110; ▪, infected DCs plus SCCE 110.
To determine whether antigen presented by infected DCs or macrophages could be recognized by CD8+ cytotoxic T cells, infected DCs or macrophages were loaded with an HLA class I peptide (peptide 19) and incubated with cytotoxic T cells (Fig. 7B). Peptide-specific CD8+ T cells were able to kill peptide-loaded, infected targets, indicating that exogenously loaded peptides could be functionally presented by cell surface HLA class I molecules on infected APCs.
Antigen presentation via the HLA class II molecules is dependent upon cellular uptake of exogenous protein by phagocytosis or endocytosis. The protein is then processed into smaller peptides and loaded onto HLA class II molecules stored within the CIIV compartment of APCs. Immature DCs are known to express low levels of surface HLA class II molecules but have high levels of intracellular HLA class II molecules. Conversely, mature DCs express high levels of HLA class II molecules on the cell surface (5, 26). To examine whether the class II pathway was intact in infected DCs, immature DCs were pulsed with the large SCCE 110 peptide and matured overnight prior to the start of infection and incubation with SCCE-specific CD4 T cells (Fig. 7D, right panel). Under these conditions, sufficient time is provided for peptide processing and presentation on HLA II molecules prior to the start of infection. The CD4 T cells lysed uninfected and infected peptide-pulsed DCs at similar levels, demonstrating that viral infection did not interfere with the overall levels or turnover of peptide-loaded HLA class II complexes at the surface of target cells. To further examine whether PV infection affected the ability of DCs to process and present antigens in the class II pathway, mature DCs were infected and pulsed with the SCCE 110 peptide at the same time (Fig. 7D, left panel). The SCCE 110-specific CD4 T cells recognized and lysed infected, peptide-pulsed DCs at levels similar to that of uninfected, peptide-pulsed DCs. The low levels of lysis observed in Fig. 7D (left panel) are consistent with the short time available to the mature DCs to process and present antigen at the cell surface and with the fact that mature DCs are less efficient at uptake and processing of antigen. Nevertheless, PV infection does not appear to affect the HLA class II presentation pathway.
DISCUSSION
We found that DCs and macrophages express PVR but, unlike monocytes, they are highly permissive for PVM and PVS infection. Susceptibility to PV increases as the cells differentiate from monocytes to DCs. Virus yields are greatest from mature DCs compared to cells at earlier stages of differentiation. The low susceptibility of monocytes to PV, demonstrated in this paper, is consistent with previous work (18, 22, 39) and the view that the PVR is not the sole determinant of cell susceptibility to infection by PV. Several trans-acting cellular proteins have been identified that bind to specific stem-loop structures within the internal ribosome entry site of PV RNAs and are required for efficient translation (8, 23, 25, 51). Expression of these factors or others may account for the increased permissivity to PV infection as monocytes differentiate into DCs or macrophages.
Infection in DCs follows a typical course, with rapid growth of the virus eventually causing cell death and lysis. The observed cytopathic effects are similar to those previously documented in other susceptible cell types (1, 37, 48). Cell death as well as the inhibition of intracellular protein trafficking would be predicted to perturb the immunological functions of the APCs and to affect the ability of these cells to initiate and stimulate immune responses to PV in vivo. Although the Sabin strains are growth attenuated in certain cell lines (27, 28, 34), the kinetics of virus production and induction of cytopathic effects in PVS-infected DCs are similar to those in PVM-infected cells. Therefore, the immune function of PVS-infected DCs may be predicted to be comparably perturbed in vivo. In addition, the production of inflammatory cytokines is altered during both PMV and PVS infection (data not shown). Therefore the question arises, how do individuals develop the strong and lasting immunity (29, 50, 52) associated with the Sabin vaccine? Several models can be hypothesized.
Antigen acquisition in immature DCs occurs primarily through bulk-phase and receptor-mediated endocytosis. Although receptor-mediated endocytosis is inhibited in infected cells, bulk-phase endocytosis appears to be unaffected. If the bulk-phase endocytic pathway is unaffected, then processing of such acquired antigens and presentation of viral antigens on HLA class II molecules may occur. The facts that presentation of antigen via the class II pathway appears to be unaffected in DCs, as demonstrated in Fig. 7D, and that PV-specific helper T cells are observed in vaccinated individuals (24, 49) are consistent with the possibility that the class II antigen presentation pathway remains relatively intact in infected APCs.
Another potential model is that immune stimulation of T cells occurs due to presentation of PV antigens by uninfected APCs. The data suggest that PV-infected DCs undergo apoptosis, which may allow viral proteins to be taken up along with the apoptotic bodies by uninfected APCs. The immune response would be induced by uninfected APCs that have acquired viral antigens and can, therefore, present antigens in the context of HLA class II (19) and HLA class I (2, 2, 4, 20) molecules at the cell surface.
Finally, the data in this paper as well as previous studies indicate that PV does not appear to actively downregulate HLA molecules from the surface of cells (Fig. 7). Although processes required to present antigen via the class I pathway (protein synthesis and transport from the ER to the plasma membrane) are inhibited at late stages of infection, during the early stages of viral infection significant amounts of viral antigens may be processed, bound to HLA molecules, and transported to the cell surface. As the half-life of HLA molecules on mature DCs is relatively long, viral antigens that are presented at the cell surface can be displayed over the 12 to 24 h that the cells are intact. This may be sufficient for specific T cells to interact with the viral antigens in the context of the appropriate HLA molecules and become activated by the infected cells. Furthermore, it must be noted that our results were obtained following synchronized infection of all cells in culture at a high MOI. In vivo, PV is unlikely to infect all APCs at the same time, and certainly the MOI is likely to be very low, allowing processing and presentation of viral peptides and interaction of specific T cells. Therefore, the efficiency of induction of immune responses to PV by professional APCs in vivo may not be compromised.
Acknowledgments
This work was supported by Public Health Service grants AI22627 and AI42390 from the National Institute of Allergy and Infectious Diseases.
We thank Usha Ponnappan and Mark Crew for helpful suggestions. We also thank Richard Kurten and the DCM Laboratory for assistance with confocal microscopy and Ashley Whitlow and Conrad Browning for excellent technical assistance.
REFERENCES
- 1.Agol, V. I., G. A. Belov, K. Bienz, D. Egger, M. S. Kolesnikova, L. I. Romanova, L. V. Sladkova, and E. A. Tolskaya. 2000. Competing death programs in poliovirus-infected cells: commitment switch in the middle of the infectious cycle. J. Virol. 74:5534-5541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Albert, M. L., B. Sauter, and N. Bhardwaj. 1998. Dendritic cells acquire antigen from apoptotic cells and induce class I-restricted CTLs. Nature 392:86-89. [DOI] [PubMed] [Google Scholar]
- 3.Ammendolia, M. G., A. Tinari, A. Calcabrini, and F. Superti. 1999. Poliovirus infection induces apoptosis in CaCo-2 cells. J. Med. Virol. 59:122-129. [PubMed] [Google Scholar]
- 4.Bachmann, M. F., M. B. Lutz, G. T. Layton, S. J. Harris, T. Fehr, M. Rescigno, and P. Ricciardi-Castagnoli. 1996. Dendritic cells process exogenous viral proteins and virus-like particles for class I presentation to CD8+ cytotoxic T lymphocytes. Eur. J. Immunol. 26:2595-2600. [DOI] [PubMed] [Google Scholar]
- 5.Banchereau, J., F. Briere, C. Caux, J. Davoust, S. Lebecque, Y. J. Liu, B. Pulendran, and K. Palucka. 2000. Immunobiology of dendritic cells. Annu. Rev. Immunol. 18:767-811. [DOI] [PubMed] [Google Scholar]
- 6.Banchereau, J., and R. M. Steinman. 1998. Dendritic cells and the control of immunity. Nature 392:245-252. [DOI] [PubMed] [Google Scholar]
- 7.Bouchard, M. J., and V. R. Racaniello. 1997. CD44 is not required for poliovirus replication. J. Virol. 71:2793-2798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boussadia, O., M. Niepmann, L. Creancier, A. C. Prats, F. Dautry, and H. Jacquemin-Sablon. 2003. Unr is required in vivo for efficient initiation of translation from the internal ribosome entry sites of both rhinovirus and poliovirus. J. Virol. 77:3353-3359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brossart, P., and M. J. Bevan. 1997. Presentation of exogenous protein antigens on major histocompatability complex class I molecules by dendritic cells: pathway of presentation and regulation by cytokines. Blood 90:1594-1599. [PMC free article] [PubMed] [Google Scholar]
- 10.Chapuis, F., M. Rosenzwajg, M. Yagello, M. Ekman, P. Biberfeld, and J. C. Gluckman. 1997. Differentiation of human dendritic cells from monocytes in vitro. Eur. J. Immunol. 27:431-441. [DOI] [PubMed] [Google Scholar]
- 11.Chomarat, P., J. Banchereau, J. Davoust, and A. K. Palucka. 2000. IL-6 switches the differentiation of monocytes from dendritic cells to macrophages. Nat. Immunol. 1:510-514. [DOI] [PubMed] [Google Scholar]
- 12.Chomarat, P., C. Dantin, L. Bennett, J. Banchereau, and A. K. Palucka. 2003. TNF skews monocyte differentiation from macrophages to dendritic cells. J. Immunol. 171:2262-2269. [DOI] [PubMed] [Google Scholar]
- 13.Daro, E., B. Pulendran, K. Brasel, M. Teepe, D. Pettit, D. H. Lynch, D. Vremec, L. Robb, K. Shortman, H. J. McKenna, C. R. Maliszewski, and E. Maraskovsky. 2000. Polyethylene glycol-modified GM-CSF expands CD11bhigh CD11chigh but notCD11blow CD11chigh murine dendritic cells in vivo: a comparative analysis with Flt3 ligand. J. Immunol. 165:49-58. [DOI] [PubMed] [Google Scholar]
- 14.Deitz, S. B., D. A. Dodd, S. Cooper, P. Parham, and K. Kirkegaard. 2000. MHC I-dependent antigen presentation is inhibited by poliovirus protein 3A. Proc. Natl. Acad. Sci. USA 97:13790-13795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.DeTulleo, L., and T. Kirchhausen. 1998. The clathrin endocytic pathway in viral infection. EMBO J. 17:4585-4593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dodd, D. A., T. H. Giddings, Jr., and K. Kirkegaard. 2001. Poliovirus 3A protein limits interleukin-6 (IL-6), IL-8, and beta interferon secretion during viral infection. J. Virol. 75:8158-8165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Doedens, J. R., and K. Kirkegaard. 1995. Inhibition of cellular protein secretion by poliovirus proteins 2B and 3A. EMBO J. 14:894-907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Eberle, K. E., V. T. Nguyen, and M. S. Freistadt. 1995. Low levels of poliovirus replication in primary human monocytes: possible interactions with lymphocytes. Arch. Virol. 140:2135-2150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fonteneau, J. F., M. Larsson, and N. Bhardwaj. 2002. Interactions between dead cells and dendritic cells in the induction of antiviral CTL responses. Curr. Opin. Immunol. 14:471-477. [DOI] [PubMed] [Google Scholar]
- 20.Fonteneau, J. F., D. G. Kavanagh, M. Lirvall, C. Sanders, T. L. Cover, N. Bhardwaj, and M. Larsson. 2003. Characterization of the MHC class I cross-presentation pathway for cell-associated antigens by human dendritic cells. Blood 102:4448-4455. [DOI] [PubMed] [Google Scholar]
- 21.Freistadt, M. S., and K. E. Eberle. 1996. Correlation between poliovirus type 1 Mahoney replication in blood cells and neurovirulence. J. Virol. 70:6486-6492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Freistadt, M. S., H. B. Fleit, and E. Wimmer. 1993. Poliovirus receptor on human blood cells: a possible extraneural site of poliovirus replication. Virology 195:798-803. [DOI] [PubMed] [Google Scholar]
- 23.Gamarnik, A. V., and R. Andino. 1996. Replication of poliovirus in Xenopus oocytes requires two human factors. EMBO J. 15:5988-5998. [PMC free article] [PubMed] [Google Scholar]
- 24.Graham, S., E. C. Wang, O. Jenkins, and L. K. Borysiewicz. 1993. Analysis of the human T-cell response to picornaviruses: identification of T-cell epitopes close to B-cell epitopes in poliovirus. J. Virol. 67:1627-1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gromeier, M., B. Bossert, M. Arita, A. Nomoto, and E. Wimmer. 1999. Dual stem loops within the poliovirus internal ribosomal entry site control neurovirulence. J. Virol. 73:958-964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guermonprez, P., J. Valladeau, L. Zitvogel, C. Thery, and S. Amigorena. 2002. Antigen presentation and T cell stimulation by dendritic cells. Annu. Rev. Immunol. 20:621-667. [DOI] [PubMed] [Google Scholar]
- 27.Gutierrez, A. L., M. Denova-Ocampo, V. R. Racaniello, and R. M. del Angel. 1997. Attenuating mutations in the poliovirus 5′ untranslated region alter its interaction with polypyrimidine tract-binding protein. J. Virol. 71:3826-3833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Haller, A. A., S. R. Stewart, and B. L. Semler. 1996. Attenuation stem-loop lesions in the 5′ noncoding region of poliovirus RNA: neuronal cell-specific translation defects. J. Virol. 70:1467-1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hogg, K., G. Hogg, R. Lester, and E. Uren. 2002. Immunity to poliomyelitis in Victorians. Aust. N. Z. J. Public Health 26:432-436. [DOI] [PubMed] [Google Scholar]
- 30.Holland, J. J., and B. H. Hoyer. 1962. Early stages of enterovirus infection. Cold Spring Harbor Symp. Quant. Biol. 27:101-112. [DOI] [PubMed] [Google Scholar]
- 31.Inaba, K., S. Turley, T. Iyoda, F. Yamaide, S. Shimoyama, C. R. Sousa, R. N. Germain, I. Mellman, and R. M. Steinman. 2000. The formation of immunogenic major histocompatibility complex class II-peptide ligands in lysosomal compartments of dendritic cells is regulated by inflammatory stimuli. J. Exp. Med. 191:927-936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kaariainen, L., and M. Ranki. 1984. Inhibition of cell functions by RNA-virus infections. Annu. Rev. Microbiol. 38:91-109. [DOI] [PubMed] [Google Scholar]
- 33.Kabir, J., M. Lobo, and I. Zachary. 2002. Staurosporine induces endothelial cell apoptosis via focal adhesion kinase dephosphorylation and focal adhesion disassembly independent of focal adhesion kinase proteolysis. Biochem. J. 367:145-155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.La Monica, N., and V. R. Racaniello. 1989. Differences in replication of attenuated and neurovirulent polioviruses in human neuroblastoma cell line SH-SY5Y. J. Virol. 63:2357-2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lange, R., X. Peng, E. Wimmer, M. Lipp, and G. Bernhardt. 2001. The poliovirus receptor CD155 mediates cell-to-matrix contacts by specifically binding to vitronectin. Virology 285:218-227. [DOI] [PubMed] [Google Scholar]
- 36.Leon, B., G. Martinez del Hoyo, V. Parrillas, H. Hernandez Vargas, P. Sanchez-Mateos, N. Longo, M. Lopez-Bravo, and C. Ardavin. 2004. Dendritic cell differentiation potential of mouse monocytes: monocytes represent immediate precursors of CD8− and CD8+ splenic dendritic cells. Blood 103:2668-2676. [DOI] [PubMed] [Google Scholar]
- 37.Lopez-Guerrero, J. A., M. Alonso, F. Martin-Belmonte, and L. Carrasco. 2000. Poliovirus induces apoptosis in the human U937 promonocytic cell line. Virology 272:250-256. [DOI] [PubMed] [Google Scholar]
- 38.Lopez-Guerrero, J. A., C. Cabanas, C. Bernabeu, M. Fresno, and M. A. Alonso. 1991. Effects of poliovirus replication on undifferentiated and differentiated monocytic U937 cells: comparative studies with human macrophages. Intervirology 32:137-148. [DOI] [PubMed] [Google Scholar]
- 39.Lopez-Guerrero, J. A., L. Carrasco, F. Martinez-Abarca, M. Fresno, and M. A. Alonso. 1989. Restriction of poliovirus RNA translation in a human monocytic cell line. Eur. J. Biochem. 186:577-582. [DOI] [PubMed] [Google Scholar]
- 40.Mendelsohn, C., B. Johnson, K. A. Lionetti, P. Nobis, E. Wimmer, and V. R. Racaniello. 1986. Transformation of a human poliovirus receptor gene into mouse cells. Proc. Natl. Acad. Sci. USA 83:7845-7849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Okada, Y., G. Toda, H. Oka, A. Nomoto, and H. Yoshikura. 1987. Poliovirus infection of established human blood cell lines: relationship between the differentiation stage and susceptibility of cell killing. Virology 156:238-245. [DOI] [PubMed] [Google Scholar]
- 42.Polyak, S., H. Chen, D. Hirsch, I. George, R. Hershberg, and K. Sperber. 1997. Impaired class II expression and antigen uptake in monocytic cells after HIV-1 infection. J. Immunol. 159:2177-2188. [PubMed] [Google Scholar]
- 43.Rodriguez, A., A. Regnault, M. Kleijmeer, P. Ricciardi-Castagnoli, and S. Amigorena. 1999. Selective transport of internalized antigens to the cytosol for MHC class I presentation in dendritic cells. Nat. Cell Biol. 1:362-368. [DOI] [PubMed] [Google Scholar]
- 44.Romani, N., S. Gruner, D. Brang, E. Kampgen, A. Lenz, B. Trockenbacher, G. Konwalinka, P. O. Fritsch, R. M. Steinman, and G. Schuler. 1994. Proliferating dendritic cell progenitors in human blood. J. Exp. Med. 180:83-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Santambrogio, L., A. K. Sato, G. J. Carven, S. L. Belyanskaya, J. L. Strominger, and L. J. Stern. 1999. Extracellular antigen processing and presentation by immature dendritic cells. Proc. Natl. Acad. Sci. USA 96:15056-15061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Santin, A. D., P. L. Hermonat, A. Ravaggi, M. Chiriva-Internati, M. J. Cannon, J. C. Hiserodt, S. Pecorelli, and G. P. Parham. 1999. Expression of surface antigens during the differentiation of human dendritic cells vs macrophages from blood monocytes in vitro. Immunobiology 200:187-204. [DOI] [PubMed] [Google Scholar]
- 47.Sarnow, P. 1989. Role of 3′-end sequences in infectivity of poliovirus transcripts made in vitro. J. Virol. 63:467-470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schaefer, A., R. Zibirre, P. Kabus, J. Kuhne, and G. Koch. 1982. Alterations in plasma-membrane functions after poliovirus infection. Biosci. Rep. 2:613-615. [DOI] [PubMed] [Google Scholar]
- 49.Simons, J., M. Kutubuddin, and M. Chow. 1993. Characterization of poliovirus-specific T lymphocytes in the peripheral blood of Sabin-vaccinated humans. J. Virol. 67:1262-1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Taffs, R. E., Y. V. Chernokhvostova, E. M. Dragunsky, T. Nomura, K. Hioki, E. C. Beuvery, E. A. Fitzgerald, I. S. Levenbook, and D. M. Asher. 1997. Inactivated poliovirus vaccine protects transgenic poliovirus receptor mice against type 3 poliovirus challenge. J. Infect. Dis. 175:441-444. [DOI] [PubMed] [Google Scholar]
- 51.Toyoda, H., N. Koide, M. Kamiyama, K. Tobita, K. Mizumoto, and N. Imura. 1994. Host factors required for internal initiation of translation on poliovirus RNA. Arch. Virol. 138:1-15. [DOI] [PubMed] [Google Scholar]
- 52.Winter, P. A., J. D. Krynauw, and G. A. Marais. 1981. Artificial herd immunity to poliomyelitis in a semirural community in South Africa. S. Afr. Med. J. 60:889-890. [PubMed] [Google Scholar]