Abstract
Background and Purpose
Dipeptidyl‐peptidase 4 (DPP4) is expressed by resident renal cells, including glomerular cells. DPP4 inhibitors (gliptins) exert albuminuria lowering effects, but the role of renal DPP4 as a pharmacological target has not been elucidated. To better understand the actions of gliptins, the effects of linagliptin on the behaviour of immortalized human podocytes and mesangial cells were evaluated.
Experimental Approach
The expression of DPP4 was measured at both the mRNA and protein levels. The effects of linagliptin on DPP4 activity, cell growth and cell cycle progression were determined. The contribution of the stromal cell‐derived factor‐1‐ CXCR4/CXCR7 signalling pathways was evaluated by studying the effects of AMD3100 (a CXCR4 antagonist and CXCR7 agonist) alone and in combination with linagliptin. The contribution of ERK1/2 activation was analysed by studying the effects of the MAPK kinase 1/2 inhibitor AZD6244.
Key Results
DPP4 was highly expressed in podocytes. The activity of DPP4 and podocyte growth were reduced by linagliptin. The effects of sitagliptin on podocyte growth were similar to those of linagliptin, were associated with inhibition of cell proliferation and mimicked by AMD3100. Moreover, linagliptin and AMD3100 were found to have a synergistic interaction, whereas no interaction was seen between linagliptin and AZD6244.
Conclusions and Implications
Our cultures of human glomerular cells represent a reliable system for investigating the actions of gliptins. Moreover, DPP4 contributes to the regulation of podocyte behaviour. Inhibition of DPP4 in podocytes could underlie the effects of linagliptin on glomerular cells.
Abbreviations
- DPP4
dipeptidyl‐peptidase 4
- GLP‐1
glucagon‐like peptide‐1
- MEK
MAPK kinase
- MTT
3‐(4,5‐dimethylthiazol‐2‐yl)‐2,5‐diphenyltetrazolium bromide
- PI
propidium iodide
- SDF
stromal cell‐derived factor
Tables of Links
| TARGETS | |
|---|---|
| GPCRs a | Enzymes b |
| CXCR4 | DPP4 |
| CXCR7 (ACKR3) | MEK1 |
| MEK2 |
These Tables list key protein targets and ligands in this article which are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Southan et al., 2016), and are permanently archived in the Concise Guide to PHARMACOLOGY 2015/16 (a,bAlexander et al., 2015a,b).
Introduction
Dipeptidyl‐peptidase 4 (DPP4)/CD26 inhibitors (gliptins) exert beneficial effects in diabetic patients and currently are registered in many countries as drugs for the treatment of type 2 diabetes mellitus (Baetta and Corsini, 2011; Davidson, 2013). Inhibition of the DPP4‐mediated incretin [e.g. glucagon‐like peptide‐1 (GLP‐1)] inactivation is thought to mediate the therapeutic effects of these agents. Nevertheless, given the recognized involvement of DPP4 in many intra‐ and inter‐cellular signalling pathways (Gorrell et al., 2001; Lambeir et al., 2003; Mulvihill and Drucker, 2014), a more complex and pleiotropic mechanism of action, beyond the sole inhibition of incretin inactivation, may explain some of the therapeutic effects of gliptins.
Dipeptidyl‐peptidase 4 is a glycoprotein endowed with both enzymatic and non‐enzymatic functions. It is a member of the serine peptidase/prolyl oligopeptidase, which preferentially cleaves X‐proline dipeptides from the N‐terminus of polypeptides. Many substrates have been identified, including chemokines, growth factors, neuropeptides and hormones (Lambeir et al., 2003; Mulvihill and Drucker, 2014). The non‐enzymatic functions can result from the interaction between DPP4 and cell‐surface macromolecules, including adenosine deaminase (Kameoka et al., 1993), chemokine C‐X‐C motif receptor (CXCR)4 (Herrera et al., 2001) and integrin β1 (Ghersi et al., 2006) among others.
Dipeptidyl‐peptidase 4 is widely distributed throughout the body, and high levels have been found in many tissues (Gorrell et al., 2001; Lambeir et al., 2003). The expression/activity of DPP4 by glomerular cells was originally reported by Fukasawa et al. (1981) and subsequently confirmed by other groups (Hartel et al., 1988; Kettmann et al., 1992; Stefanovic et al., 1993; Mentzel et al., 1997). Nevertheless, the pathophysiological and pharmacological roles of the glomerular DPP4 remain elusive, although convergent lines of evidence suggest its potential importance. Indeed, increased DPP4 expression has been measured in human glomerular endothelial cells exposed to high glucose concentrations (Pala et al., 2003), whole kidneys (Kirino et al., 2009) and glomeruli of rats receiving a high‐fat diet and streptozotocin (Yang et al., 2007), db/db mice (Sharkovska et al., 2014) and insulin‐dependent diabetic patients (Sharkovska et al., 2014; Maeda et al., 2015). Moreover, albuminuria‐lowering effects resulting from DPP4 inhibition have been described in both preclinical and clinical studies using different gliptins, including sitagliptin, vildagliptin and linagliptin (Hattori, 2011; Mega et al., 2011; Liu et al., 2012; Groop et al., 2013; Nistala et al., 2014; Eun Lee et al., 2016). Whether these effects result from an action on the enzyme expressed by resident renal cells is unknown. However, a contribution of the glomerular DPP4 as a therapeutic target of these drugs cannot be excluded.
Members of the stromal cell‐derived factor (SDF)‐1 family are plausible mediators of the effects of gliptins on glomerular cells. Indeed, SDF‐1 peptides are released by resident renal cells (e.g. podocytes) and play essential roles during glomerular development, in the maintenance of glomerular integrity and may sustain regenerative processes (Mazzinghi et al., 2008; Stokman et al., 2010; Chen et al., 2014). In addition, they are processed rapidly by DPP4 (Lambeir et al., 2003; De La Luz Sierra et al., 2004). To date, however, the role of SDF‐1 peptides in mediating the renal effects of gliptins has not been clearly established. Interestingly, Takashima et al. (2016) showed that SDF‐1 can mediate the renal effects of DPP4 inhibition in animal models of diabetes mellitus. Clearly, elucidation of the mechanism underlying the renal effects of gliptins, as well as the role of the SDF‐1 signalling pathway needs appropriate analytical studies.
In general, the findings of analytical investigations, especially those on subtle pharmacological actions, are more informative when you can limit the number of variables that might confound interpretation of the measured responses. Compared with more complex experimental systems – such as in vivo models – cellular systems meet this premise and could be helpful by providing data to (dis)prove and generate novel hypotheses. Therefore, in the present series of investigations, the effects of linagliptin on immortalized human podocytes and mesangial cells were evaluated.
Methods
Cell cultures
In this study, we used lines of immortalized human podocytes and mesangial cells. Immortalized cells were obtained from primary podocytes and mesangial cells by infection with a hybrid Adeno5/SV40 virus. Cells were characterized as described previously (Conaldi et al., 1998; Doublier et al., 2001; Miglio et al., 2011, 2012). Under standard conditions, cells were cultured in DMEM supplemented with FBS (10%), penicillin G (100 U·mL−1), streptomycin (100 μg·mL−1) and L‐glutamine (2.0 mM). Cell culture medium was replaced every 2 days, and cultures were maintained at 37°C, 95% air‐5% CO2 in a humidified incubator.
RNA isolation and RT‐PCR analyses
Total RNA was extracted from cell cultures using the EuroGold Trifast kit, according to the manufacturer's instructions. First‐strand cDNA was synthesized from 10 ng of total RNA using the RevertAid First Strand cDNA Synthesis kit. Reactions were performed in 25 μL reaction mixtures containing 2 μL of cDNA, 2.5 μL of 10× reaction buffer, dNTPs (0.2 mM), MgCl2 (2.5 mM), EuroTaq Thermostable DNA polymerase (2.5 U) and a specific primer pair (0.5 μM; Supporting Information Table S1). ACTIN was adopted as an internal standard to control for unwanted sources of variation. Amplicons were resolved in agarose gels by electrophoresis and visualized with ethidium bromide.
Enzymatic assays
Dipeptidyl‐peptidase 4 activity was measured in extracts prepared from confluent cell cultures and in fresh/conditioned cell culture media. Cell extracts were prepared as described by Thomas et al. (2008) with minor modifications. In brief, cells were washed twice with Mg2+‐free PBS and lysed at 4°C in a buffered solution (10 mM Tris‐HCl, 150 mM NaCl, 0.04 U·mL−1 aprotinin, 0.5% Nonidet P40, pH 8.0). The resulting samples were centrifuged at 16 000 g for 30 min. Supernatants were stored at −80°C. Assays were performed by mixing 20 μL of either vehicle alone or linagliptin with 50 μL of the DPP4 substrate, H‐Ala‐Pro‐7‐amido‐4‐trifluoromethylcoumarin (final concentration in the assay buffer 100 μM), and 30 μL of cell extract/culture media (100‐fold diluted in the assay buffer: 100 mM Tris‐HCl, 100 mM NaCl, pH 7.8). Plates were maintained at room temperature for 1 h, and fluorescence was measured at 5 min intervals at excitation/emission wavelengths of 405/535 nm by using a VICTOR X4 plate reader (PerkinElmer, Waltham, MA, USA). Enzymatic activity measured in different samples was normalized to protein content of the samples.
Western blot analyses
Western blot analyses were performed as previously described (Miglio et al., 2011; 2012). Dipeptidyl‐peptidase 4 was detected following incubation with a goat anti‐DPP4 polyclonal antibody (0.2 μg·mL−1). Expression of p21 and phosphorylated ERK1/2 was detected with mouse anti‐p21 or anti‐pERK1/2 monoclonal antibodies (at dilution factors of 1:200 and 1:2000 respectively). Cyclin D1 and p27 expression was detected with rabbit anti‐cyclin D1 and anti‐p27 polyclonal antibodies (at dilution factors of 1:200 and 1:100 respectively). To confirm equal protein loading, membranes were stripped and incubated with an anti‐β‐actin monoclonal antibody. Finally, membranes were overlaid with Western Lightning Chemiluminescence Reagent Plus and luminescence detected using Hyperfilm ECL film.
Measurement of cell growth and cell cycle analyses
Cells were plated (2 × 103 cells per well) in 24‐well culture plates and exposed to vehicle alone (control), linagliptin, sitagliptin, AZD6244 and/or AMD3100. Cell growth was evaluated in sub‐confluent cultures using the 3‐(4,5‐dimethylthiazol‐2‐yl)‐2,5‐diphenyltetrazolium bromide (MTT) colorimetric assay; results were confirmed by determining cell density, as previously described (Miglio et al., 2011, 2012).
The percentage of cells in different phases of the cell cycle was determined as described previously (Miglio et al., 2005). In brief, at the end of each treatment, cells were washed with PBS, harvested then centrifuged. Pellets were resuspended in ice‐cold ethanol (70%) and maintained at −20°C for at least 24 h. Afterwards, cells were washed twice with PBS and treated (1 h, 37°C) with RNase (0.5 mg·mL−1, final concentration). Finally, propidium iodide (PI; 50 μg·mL−1) was added. Fluorescence from individual nuclei was measured on a FACSCalibur flow cytometer (BD Bioscience, San Jose, CA, USA). Cell cycle analysis was performed using the Flowing Software version 2.5 (Centre for Biotechnology, Turku, Finland).
Measurement of SDF‐1α concentration
Cell culture supernatants were collected, and the level of SDF‐1α was quantified with an ELISA kit according to the manufacturer's instructions.
Data and analysis
The data and statistical analysis in this study comply with the recommendations on experimental design and analysis in pharmacology (Curtis et al., 2015). Experimental groups (treatments) were labelled with anonymous codes (random numbers) by a Principal Investigator (G.M. or E.B.) and preparations (e.g. wells, plates or cell extracts) were randomly assigned (completely randomized design) to the experimental groups. To decrease bias, assignment of preparations to groups, data recording and data analysis were blinded to the operator and analyst (a second different person, G.M., G.V. or E.B.). To control for unwanted sources of variation, data were normalized to an internal standard (see Results for more details). The −Log value of the molar concentration of an agent that decreases the baseline response measured in control samples by 50% (pIC50) was calculated with the Origin 6.0 software (Microcal Software, Northampton, MA, USA). Concentration–response data were analysed by adopting the Hill regression model and those on the effects exerted by mixtures of two agents by the Bliss model (Bliss, 1939). In particular, given two agents, A and B, both exerting overtly similar effects: Agent A at concentration a exerts the effect Y a, and agent B at concentration b exerts the effect Y b. If A and B act independently (no interaction), the combined effect, Y ab,P, can be predicted using the additivity of probability theory as
An alternative scenario (interaction) can be established by comparing the observed combined effect, Y ab,O, with Y ab,P and considering the following criteria: Y ab,O > Y ab,P, synergy; Y ab,O < Y ab,P, antagonism.
Differences between data sets were evaluated by either Student's t‐test or ANOVA in conjunction with a Bonferroni post hoc test (Prism 5, GraphPad Software, La Jolla, CA, USA). Differences were judged to be statistically significant when P < 0.05 and the post hoc test was run only if F achieved P < 0.05, and there was no significant variance inhomogeneity.
Materials
Linagliptin and sitagliptin were kindly provided by Boehringer Ingelheim GmbH (Biberach, Germany). AZD6244 was generously supplied by Dr M. Gallicchio (Dipartimento di Scienza e Tecnologia del Farmaco, Università degli Studi di Torino, Turin, Italy). DMEM, FBS, penicillin G, streptomycin and L‐glutamine were obtained from Lonza (Basel, Switzerland). EuroGold Trifast kit and EuroTaq Thermostable DNA polymerase were from EuroClone (Milan, Italy). RevertAid First Strand cDNA Synthesis kit was from Thermo Scientific (Waltham, MA, USA). PCR primers were from Sigma Life Science (Milan, Italy). H‐Ala‐Pro‐7‐amido‐4‐trifluoromethylcoumarin was obtained from Bachem (Bubendorf, Switzerland). The ELISA kit to quantify SDF‐1α was from Peprotech House (London, UK). Anti‐DPP4 antibody was obtained from RD System (Minneapolis, MN, USA). Antibodies against phosphorylated ERK1/2, anti‐mouse and anti‐rabbit horseradish peroxidase‐linked‐antibodies were obtained from Cell Signaling Technology (Danvers, MA, USA). Anti‐β‐actin antibody was obtained from Sigma‐Aldrich (Milan, Italy). Antibodies against cyclin D1, p21, p27 and goat horseradish peroxidase‐linked antibodies were obtained from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Western Lightning Chemiluminescence Reagent Plus was obtained from PerkinElmer Life Science (Norwalk, CT, USA). Hyperfilm ECL film was obtained from Amersham Biosciences (Piscataway, NJ, USA). All other reagents and chemicals were obtained from Sigma‐Aldrich (Milan, Italy).
Results
Expression of DPP4 by immortalized human glomerular cells
The expression of DPP4 was studied in immortalized human podocytes and mesangial cells by RT‐PCR. A single product of the predicted size was detected in all reactions. Moreover, as shown in Figure 1A, the gene encoding for DPP4 was expressed by podocytes and at a markedly lower level by mesangial cells. To extend these findings, DPP4 enzymatic activity was measured in both cell extracts and cell culture media. As shown in Figure 1B, compared with podocytes, a significantly (P < 0.05) lower activity was observed in extracts from mesangial cells, thus confirming the PCR findings. Enzymatic activity in podocyte extracts was even higher than those measured in culture media, indicating that DPP4 is mainly retained by cells. To confirm these findings, DPP4 expression was assessed at protein level by western blot analyses. A single band at the expected molecular weight was found in podocyte extracts (Figure 1C), although no band corresponding to DPP4 was detected consistently in mesangial cell extracts (data not shown). Finally, to further confirm the identity of the enzymatic activity, the effects of increasing linagliptin concentrations (0.01–100 nM) on the enzymatic activity were evaluated. Enzymatic activity measured in podocyte extracts was inhibited by linagliptin in a concentration‐dependent manner and abolished at 30–100 nM (Figure 1D). Notably, the pIC50 value (8.9) was consistent with the expected value (Thomas et al., 2008). Enzymatic activity measured in mesangial cell extracts and culture media was also abolished by 100 nM linagliptin (data not shown).
Figure 1.
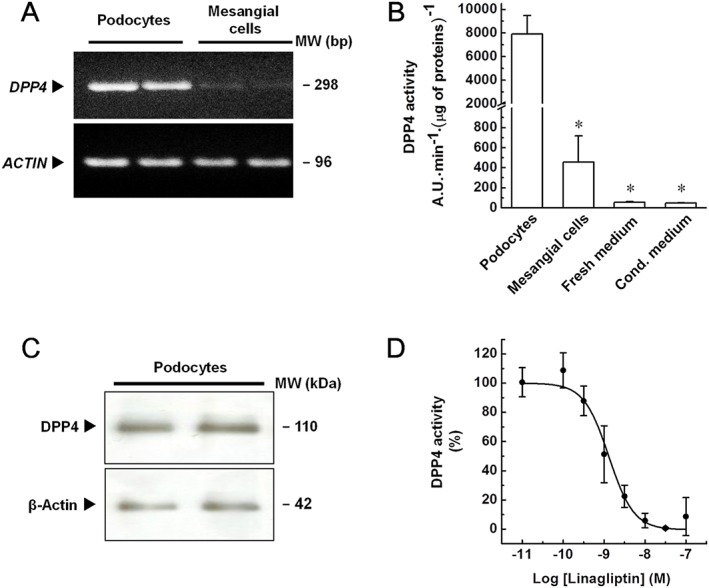
Expression and activity of DPP4 in immortalized human podocytes and mesangial cells. (A) Expression of the gene encoding for DPP4 was evaluated in immortalized human podocytes and mesangial cells by RT‐PCR analyses. Actin (ACTIN) was adopted as an internal standard to control for unwanted sources of variation. Image is representative of five experiments run in duplicate for each experimental group. (B) Activity of DPP4 in cell extracts, fresh and podocyte‐conditioned (5 days) media was evaluated by measuring the rate of increase in fluorescence intensity, expressed as arbitrary units (A.U.)·min−1 and normalized to protein content. Data are expressed as mean ± SEM of five experiments run in duplicate for each experimental group. (C) Expression of DPP4 in immortalized human podocytes was evaluated by western blot analyses. β‐Actin was adopted as an internal standard to control for unwanted sources of variation. Image is representative of five experiments run in duplicate for each experimental group. (D) Effects of increasing linagliptin concentrations on the enzymatic activity measured in immortalized human podocytes. Activity of DPP4 was measured in cell extracts treated with either vehicle alone (control) or increasing linagliptin concentrations (0.01–100 nM), as above described. To set the Y axis, all data were normalized to the mean value of the control group (100%). Data are expressed as mean ± SEM of five experiments run in duplicate for each experimental group. * P < 0.05 versus podocytes.
Therefore, by expressing high DPP4 levels, our cultures of immortalized human podocytes have an essential property for them to be evaluated as a system to study the effects of DPP4 inhibitors on this cell type.
Effects of glucose on DPP4 activity in immortalized mesangial cells
Contrary to podocytes, DPP4 is expressed at very low levels by our immortalized human mesangial cells. These cells were cultured under the same conditions as podocytes, except for glucose concentrations in the culture media that were 1.0 g·L−1 and 4.5 g·L−1 respectively. Given that glucose concentration has been reported to influence DPP4 expression/activity (Pala et al., 2003), its effect on the enzymatic activity in mesangial cells was studied. Mesangial cells were exposed to higher glucose concentration (4.5 g·L−1) for up to 48 h, and enzymatic activity was assessed in cell extracts. As shown in Figure 2, compared with the basal level (0 h), no significant change in the enzymatic activity was measured in cells exposed to the higher glucose concentration for 24 or 48 h (longer exposure times significantly affected cell viability; data not shown).
Figure 2.
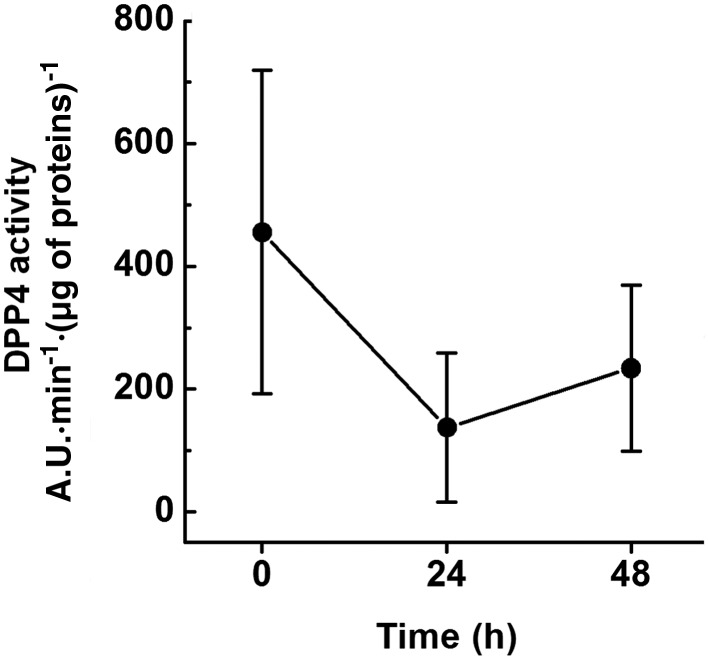
Effects of glucose on DPP4 activity in immortalized human mesangial cells. Immortalized human mesangial cells were exposed to high glucose concentration (4.5 g·L−1) for up to 48 h. Enzymatic activity was evaluated in cell extracts by measuring the rate of increase in fluorescence intensity, expressed as arbitrary units (A.U.)·min−1, normalized to protein content. Data are expressed as mean ± SEM of five experiments run in duplicate for each experimental group.
Thus, low DPP4 levels are expressed by our immortalized human mesangial cells, irrespective of the glucose concentration in the culture media. However, these cells could be utilized to evaluate any DPP4‐independent effects of gliptins.
Effects of DPP4 inhibitors on the cell growth of immortalized human glomerular cells
Inhibitors of DPP4 have been reported to decrease proliferation of different cell types, including T cells (Schön et al., 1985; Reinhold et al., 1997) and smooth muscle cells (Ervinna et al., 2013; Wronkowitz et al., 2014). To investigate whether linagliptin modulates glomerular cell behaviour, its effect on cell growth in culture was studied. Podocytes and mesangial cells were exposed to linagliptin (30 nM; 1–5 days), and cell growth was measured using the MTT assay. As shown in Figure 3A, podocyte growth was decreased by linagliptin in a time‐dependent manner. Significant effects (P < 0.05) were observed at 3–5 days (differences between control and linagliptin‐treated cells decreased at longer treatment times due to cell confluency; data not shown). By contrast, mesangial cell growth was unaffected by linagliptin. These data were confirmed by determining cell number in each well (Supporting Information Figure S1A, B), thus indicating that the observed changes in the rate of cell growth actually reflect differences in terms of cell density.
Figure 3.
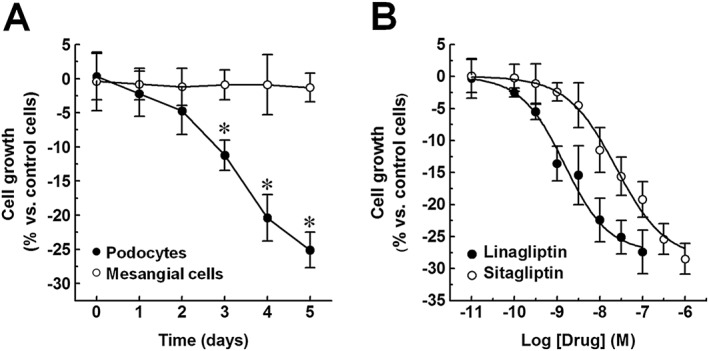
Effects of DPP4 inhibitors on cell growth of immortalized human podocytes and mesangial cells. (A) Immortalized human podocytes and mesangial cells were exposed to either vehicle alone (control) or linagliptin (30 nM; 1–5 days), and cell growth was measured by a colorimetric assay. (B) Immortalized human podocytes were exposed to vehicle alone (control) or increasing concentrations of either linagliptin (0.01–100 nM; 5 days) or sitagliptin (0.01–1000 nM; 5 days), and cell growth was measured as described above. To set the Y axis, all data were normalized to the mean value of the control group (0%). Data are expressed as mean ± SEM of six experiments run in triplicate for each experimental group. *P < 0.05 versus control group.
As the effects of linagliptin were observed on podocytes, but not on mesangial cells, they may depend on DPP4 inhibition. To confirm this hypothesis, podocytes were exposed to increasing concentrations of either linagliptin (0.01–100 nM) or sitagliptin (0.01–1000 nM), and cell growth was measured at 5 days. Cell growth was decreased by both drugs in a concentration‐dependent manner (Figure 3B). The pIC50 values (8.8 and 7.6, calculated with respect to their maximal effects: −27.4 ± 3.4% and −28.5 ± 2.4% vs. vehicle alone respectively) were comparable with the expected values for the inhibition of DPP4 activity (Kim et al., 2005; Thomas et al., 2008), thus indicating a probable link between the two effects.
Therefore, as already observed in other cell types, significant changes in the rate of podocyte growth results from DPP4 inhibition.
Effects of linagliptin on cell cycle progression and apoptosis
A decreased rate of cell growth can result from inhibition of cell proliferation and/or toxicity. To better understand the effects of linagliptin on podocyte growth, cells were exposed to linagliptin (1 or 100 nM) for 5 days. After the exposure, nuclei were stained with PI to determine the percentage of cells in different phases of the cell cycle. As shown in Table 1, cell cycle progression was altered by linagliptin. Significant differences (P < 0.05), with respect to the control cells, were determined for linagliptin 100 nM. In particular, changes in the percentage of cells in the G0/G1 (increase) and S (decrease) phases were observed. No significant difference was measured in the percentage of cells in the sub‐G1 (apoptotic) phase.
Table 1.
Effects of linagliptin on cell cycle progression
| [Linagliptin] (nM) | |||
|---|---|---|---|
| Cell cycle phase | Vehicle | 1 | 100 |
| Sub‐G1 | 4.8 ± 0.3 | 5.0 ± 0.3 | 4.8 ± 0.3 |
| G0/G1 | 56.7 ± 3.0 | 63.2 ± 1.4 | 66.7 ± 1.7a |
| S | 16.0 ± 0.9 | 13.4 ± 0.4 | 10.9 ± 0.5a |
| G2/M | 22.5 ± 2.1 | 18.4 ± 0.9 | 17.7 ± 1.4 |
Immortalized human podocytes were exposed to either vehicle alone (control) or linagliptin for 5 days, then they were harvested, stained with propidium iodide and examined by flow cytometry to determine the percentage of cells in the different phases of the cell cycle. Data are the mean ± SEM of five experiments run in duplicate for each experimental group.
P < 0.05 versus control group.
To further investigate these findings, the expression of cyclin D1, p27 and p21 was tested by western blot analyses. Compared with control cells, a lower level of cyclin D1 was observed in linagliptin‐treated (100 nM, 5 days) cells (Figure 4). The constitutive low expression of p27 was unchanged by linagliptin (Figure 4); p21 was not detected (data not shown).
Figure 4.
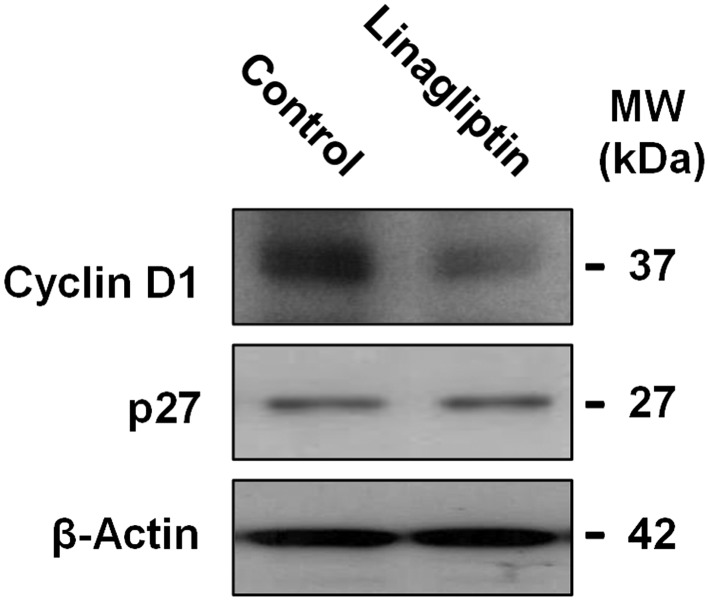
Effects of linagliptin on the expression of cyclin D1 and p27 in immortalized human podocytes. Immortalized human podocytes were exposed to either vehicle alone (control) or linagliptin (100 nM) for 5 days, then the expression of cyclin D1 and p27 was evaluated by western blot analyses. β‐Actin was adopted as an internal standard to control for unwanted sources of variation. Each image is representative of five experiments run in duplicate for each experimental group.
Therefore, in our immortalized human podocytes, a slowed‐down cell cycle progression results from DPP4 inhibition.
Expression of SDF‐1, CXCR4 and CXCR7 by immortalized human podocytes
The effects of gliptins on cell growth could result from an interference in signalling pathways involving molecules that are both DPP4 substrates and play a role in the control of cell proliferation. By fulfilling these prerequisites, the SDF‐1 signalling pathway could be suggested to mediate the effects of linagliptin on podocyte growth. To assess this hypothesis, the expression of the genes encoding for SDF‐1 and its cognate receptors (CXCR4 and CXCR7) by our immortalized podocytes was studied by RT‐PCR. As shown in Figure 5A, SDF‐1, CXCR4 and CXCR7 were constitutively expressed by our cells. In order to strengthen these findings, the local production of SDF‐1α (as a representative member of the SDF‐1 chemokine family) was evaluated by measuring the peptide levels in the extracellular milieu by ELISA. Compared with the basal value (2.72 ± 0.18 ng·mL−1), SDF‐1α concentration significantly increased (P < 0.05) throughout the culture growth and was 7.76 ± 0.17 ng·mL−1 after 5 days of culture (Figure 5B).
Figure 5.
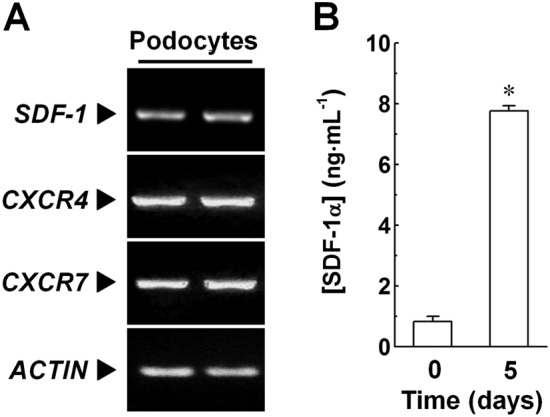
Expression of components of the SDF‐1‐CXCR4/CXCR7 axes in immortalized human podocytes. (A) Expression of the gene encoding for SDF‐1, CXCR4 and CXCR7 was evaluated in immortalized human podocytes by RT‐PCR analyses. Actin (ACTIN) was adopted as an internal standard to control for unwanted sources of variation. Each image is representative of five experiments run in duplicate for each experimental group. (B) Release of SDF‐1α by immortalized human podocytes was evaluated by measuring concentrations of this chemokine in fresh (0 days; baseline) and podocyte‐conditioned (5 days) media by ELISA. Data are expressed as mean ± SEM of five experiments run in triplicate for each experimental group. *P < 0.05 versus baseline.
Therefore, by expressing all components of the SDF‐1‐CXCR4/CXCR7 pathways, our cultures of human glomerular cells could be helpful to study the role of these pathways in mediating the effects resulting from DPP4 inhibition.
Effects of AMD3100 on the growth of immortalized human podocytes
To assess whether pharmacological modulation of the SDF‐1‐CXCR4/CXCR7 pathways mediates the effects of linagliptin in our system, a pharmacological analysis was performed. In particular, the effects of AMD3100/plerixafor – a CXCR4 competitive antagonist (Zhang et al., 2002) and CXCR7 agonist (Kalatskaya et al., 2009; Gravel et al., 2010) – were studied. Moreover, to evaluate whether an interaction between AMD3100 and linagliptin could be established, the effects exerted by mixtures of these agents were measured. Podocytes were exposed to increasing concentrations of AMD3100 (0.01–1 μM), either in the absence or presence of linagliptin (1 nM), and cell growth was measured at 5 days by MTT assay. Podocyte growth was decreased by AMD3100 in a concentration‐dependent manner (pIC50 = 6.4; Figure 6). The value of the slope parameter (3.1 ± 1.6) was consistent with a heterogeneous effector function. Of note, when the effects of mixtures of AMD3100 + linagliptin were compared with the hypothetical independence (no interaction), potentiation was observed (the pIC50 was 7.1 ± 0.1 and the slope parameter 2.8 ± 1.2). Therefore, a synergistic interaction between the two agents was established. These data were confirmed by determining cell number in each well (Supporting Information Figure S2).
Figure 6.
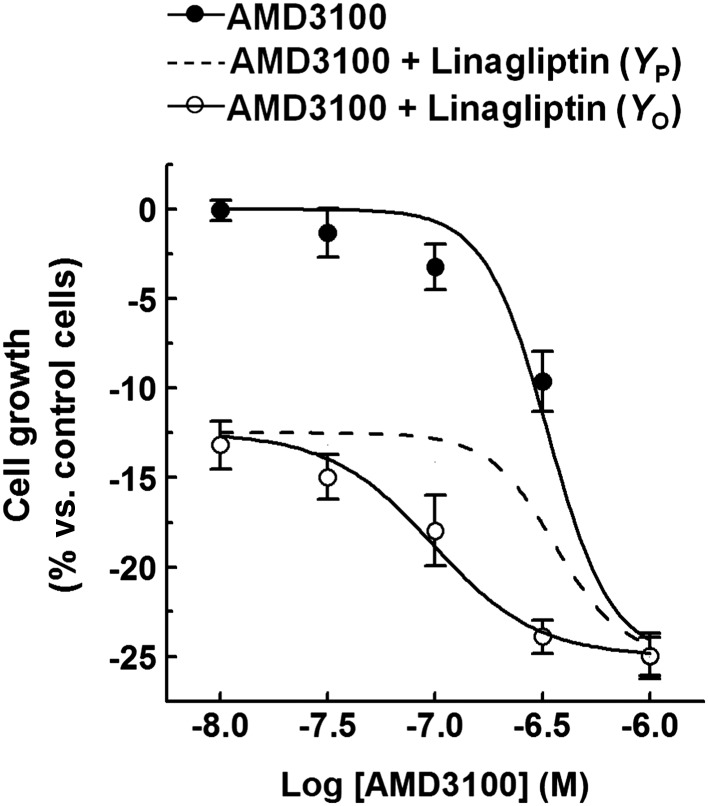
Effects of AMD3100 on the growth of immortalized human podocytes. (A) Immortalized human podocytes were exposed to either vehicle alone (control) or increasing AMD3100 concentrations (0.01–1 μM; 5 days), in the absence or presence of linagliptin (1 nM), and cell growth was measured by a colorimetric assay. To set the Y axis, all data were normalized to the mean value of the control group (0%). Data are the mean ± SEM of five experiments run in triplicate for each experimental group. Combined effects were predicted by assuming Bliss independence.
Thereby, a pharmacological modulation of the SDF‐1‐CXCR4/CXCR7 signalling pathways contributes to the regulation of podocyte growth in our experiments.
Effects of linagliptin and AMD3100 on ERK1/2 activation in immortalized human podocytes
To further evaluate the contribution of the SDF‐1‐CXCR4/CXCR7 pathways in mediating the effects of linagliptin on podocytes, activation of ERK1/2 – intracellular signalling molecules activated in response to CXCR4 and CXCR7 stimulation (Wang et al., 2008; Gravel et al., 2010) – was investigated. Podocytes were exposed to linagliptin (1 nM), AMD3100 (0.3 μM), or linagliptin + AMD3100, and activation of ERK1/2 (pERK1/2) at 18 h was assessed by Western blot analyses. Compared with control cells, a marked increase in pERK1/2 level was measured in linagliptin and/or AMD3100‐treated cells (Figure 7), thus supporting the hypothesis that linagliptin interferes with the SDF‐1‐CXCR4/CXCR7 pathways.
Figure 7.
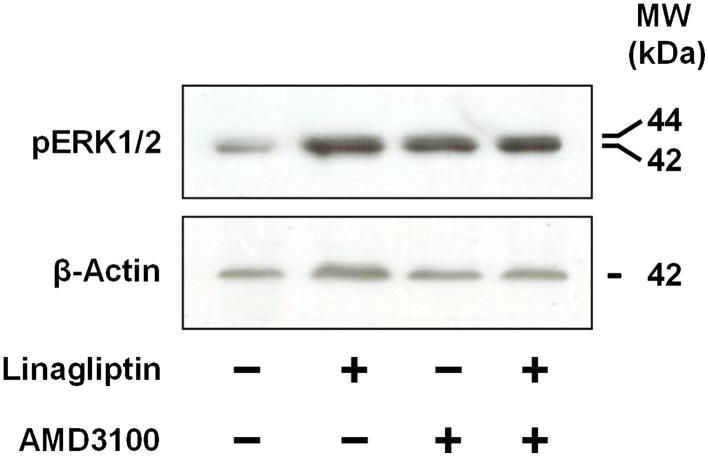
Effects of linagliptin and AMD3100 on ERK1/2 activation in immortalized human podocytes. Immortalized human podocytes were exposed to vehicle alone (control), linagliptin (1 nM), AMD3100 (0.3 μM) or both for 18 h. ERK1/2 activation was evaluated by western blot analyses. β‐Actin was adopted as an internal standard to control for unwanted sources of variation. Each image is representative of five experiments run in duplicate for each experimental group.
Effects of AZD6244 on the growth of immortalized human podocytes
If the effects of linagliptin on cell growth depend on ERK1/2 activation, they should be sensitive to mitogen‐activated protein kinase kinase (MEK)1/2 inhibition. To assess this hypothesis, the effects of AZD6244/selumetinib – a MEK1/2 inhibitor (Huynh et al., 2007) –were studied. In particular, podocytes were exposed to increasing concentrations of this drug (0.1–10 μM) either alone or in combination with linagliptin (1–100 nM), and cell growth was measured after 5 days by MTT assay. Podocyte growth was decreased by both AZD6244 alone and AZD6244 + linagliptin (Figure 8 and Table 2). In addition, when the observed and predicted effects were compared, no interaction was observed. These results were confirmed by assessing cell density in each well (Supporting Information Figure S3).
Figure 8.
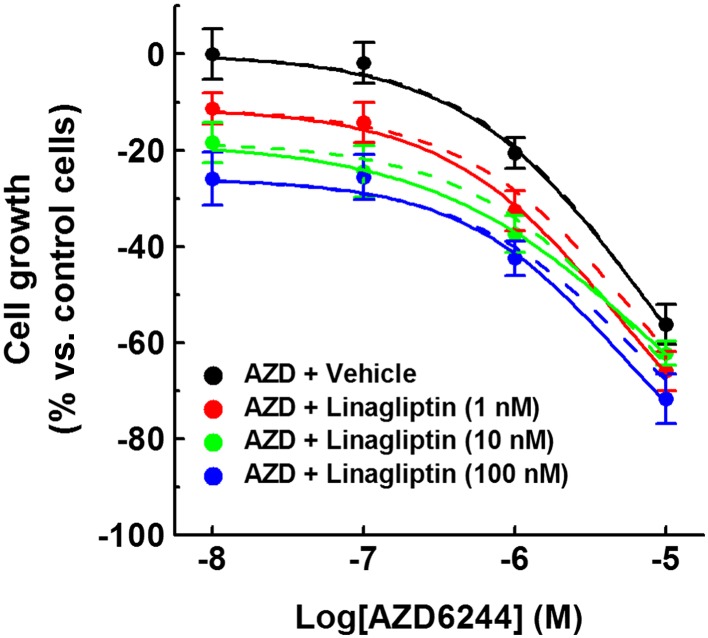
Effects of AZD6244 on the growth of immortalized human podocytes. Immortalized human podocytes were exposed to vehicle alone (control), increasing AZD6244 concentrations (0.1–10 μM; 5 days), in the absence or presence of linagliptin (1–100 nM), and cell growth was measured by a colorimetric assay. To set the Y axis, all values were normalized to the mean value of the control group (0%). Data are the mean ± SEM of five experiments run in triplicate for each experimental group. Solid lines represent the combined observed effects. Dashed lines represent the combined effects predicted by assuming Bliss independence.
Table 2.
Combined effects of AZD6244 and linagliptin on podocyte growth
| pIC50 | ||
|---|---|---|
| [Linagliptin] (nM) | Y P | Y O |
| – | 5.2 | 5.2 |
| 1 | 5.2 | 5.3 |
| 10 | 5.2 | 5.1 |
| 100 | 5.2 | 5.3 |
Immortalized human podocytes were exposed to vehicle alone, AZD6244 or AZD6244 + linagliptin for 5 days, then cell growth was evaluated. Data are the mean ± SEM of five experiments run in duplicate for each experimental group.
Therefore, ERK1/2 activation does not mediate the effects of linagliptin on podocyte growth.
Discussion and conclusion
Dipeptidyl‐peptidase 4 inhibition underlies the beneficial effects of gliptins, especially on blood glucose levels (Baetta and Corsini, 2011; Davidson, 2013). The wide distribution of the glycoprotein, together with the demonstrated broad spectra of its biological roles (Gorrell et al., 2001; Lambeir et al., 2003; Mulvihill and Drucker, 2014), suggests multiple additional consequences of DPP4 inhibition, some of which may go some way to explain the clinical data. Nevertheless, the actions of DPP4 inhibitors are difficult to appreciate in some cases. A notable example is the renal actions of gliptins. Beneficial, partially glycaemia‐independent renal effects of these drugs have been reported (Hattori, 2011; Mega et al., 2011; Liu et al., 2012; Groop et al., 2013; Nistala et al., 2014; Eun Lee et al., 2016). However, their intrarenal actions remain poorly understood. Whether inhibition of the renal DPP4 contributes to the beneficial effects of gliptins is an intriguing hypothesis which merits further investigation.
Here, the effects of linagliptin on cultures of immortalized human podocytes and mesangial cells have been studied. Our cellular systems have been characterized previously. For example, our lines of human podocytes phenotypically reassemble primary normal cells; they appear as arborized epithelial cells with a large cytoplasmic‐to‐nuclear area ratio, express podocyte‐specific markers (e.g. synaptopodin and nephrin; Conaldi et al., 1998; Doublier et al., 2001; Miceli et al., 2010; Miglio et al., 2011; 2012) and grow in vitro under typical culture conditions. Moreover, they have been employed to study the effects of agents acting on angiotensin II receptors (Miceli et al., 2010; Rosa et al., 2012), PPARs (Miceli et al., 2010; Miglio et al., 2011; 2012) among others. In these circumstances, they have proved to be reliable systems for analytical pharmacology studies.
Robust expression of active DPP4 has been observed in our cultures of human podocytes. In addition, a decrease in the rate of podocyte growth has been observed when the effects of either linagliptin or sitagliptin were evaluated. These effects were concentration‐dependent, observed at drug concentrations that inhibit DPP4 activity both in vitro and in vivo and are achieved after oral administration of therapeutic doses in healthy individuals and diabetic patients (Kim et al., 2005; Thomas et al., 2008; Baetta and Corsini, 2011). Moreover, they have been associated with a decreased cell proliferation without toxicity. The significance of these results should however be treated with caution. In mature glomeruli, podocytes behave as post‐mitotic cells, while immortalized podocytes proliferate. Hence, the behaviour of normal cells in mature glomeruli is not identical to that in our model. Nevertheless, the ability of our cells to proliferate offers a convenient parameter (measurement of cell growth) to study the actions of gliptins. Notably, DPP4 inhibitors have been reported to decrease the rate of growth of different cell types, including mitogen‐treated T cells (Schön et al., 1985; Reinhold et al., 1997) and smooth muscle cells (Ervinna et al., 2013; Wronkowitz et al., 2014). The mechanism underlying these effects remains uncertain in most cases. However, the disruption of intra/intercellular signalling following DPP4 inhibition is one possibility. Consistent with this argument, the effects exerted by two structurally different gliptins on the rate of growth of our cells suggest the involvement of DPP4 inhibition. Acquisition of a ‘quiescent phenotype’ could be considered a potentially favourable response, with regard to the maintenance of the glomerular integrity, but this speculation deserves further evaluation.
Since observing the relationship between DPP4 activity and podocyte proliferation, we investigated the role of plausible signalling pathways. The GLP‐1‐GLP1 receptor axis was excluded. GLP‐1 is not produced by renal cells, and the GLP‐1 receptor was not found in our glomerular cell cultures (data not shown). Notably, this finding confirms previous data which exclude a prominent action of incretin hormones on podocytes in mature glomeruli (Fujita et al., 2014; Pyke et al., 2014). In contrast, a role for the SDF‐1‐CXCR4/CXCR7 signalling pathways is supported by converging lines of evidence: (i) a strong expression of CXCR4 and CXCR7 by our podocytes; (ii) the constitutive production of SDF‐1α; (iii) activation of the cognate intracellular signalling molecules (ERK1/2) after a relatively short‐term exposure to linagliptin; (iv) the effects exerted by AMD3100 alone; and (v) the synergistic interaction between AMD3100 and linagliptin. Thus, our data corroborate previous findings on the expression of DPP4 by podocytes, the role played by DPP4 in SDF‐1 proteolytic processing (SDF‐1 being one of the best DPP4 substrates) and the expression of SDF‐1 peptides and their receptors by podocytes (Lambeir et al., 2003; De La Luz Sierra et al., 2004; Mazzinghi et al., 2008; Stokman et al., 2010; Chen et al., 2014). More importantly, these observations allow us to propose a linkage between renal effects of gliptins resulting from the inhibition of the glomerular DPP4 and interference with the SDF‐1‐CXCR4/CXCR7 pathways.
The complexity of the SDF‐1 signalling pathways makes study of this linkage difficult. Indeed, for example SDF‐1α (1–68) is known to act as a full CXCR4 and CXCR7 agonist (Crump et al., 1997; Gravel et al., 2010), whereas SDF‐1α (3–68) is a CXCR4 antagonist (Crump et al., 1997) and has been proposed to act as a CXCR7 agonist (Gravel et al., 2010). In addition, CXCR7 activation interferes with the CXCR4‐mediated responses in some cellular systems (Levoye et al., 2009; Uto‐Konomi et al., 2013). Nevertheless, data resulting from our pharmacological analysis allows us to propose the following mechanistic model: (i) mixtures of CXCR4‐ and CXCR7‐ligands (SDF‐1 peptides) are produced by podocytes; (ii) some of the SDF‐1 peptides act at their cognate receptors, thus establishing autocrine/paracrine circuits which govern cell behaviour (Figure 9A); and (iii) changes in the peptide mixture composition and/or in the protein–protein interaction (e.g. DPP4‐CXCR4 complexes; Herrera et al., 2001) result from DPP4 inhibition, and these could be directly upstream of the effects of gliptins on podocyte behaviour (Figure 9B). These changes on the composition of the SDF‐1 peptide mixture and the function of DPP4‐CXCR4 complexes cannot be easily detected by conventional molecular assays. However, they most likely take place in our systems. The effects of linagliptin and sitagliptin on cell growth were mimicked by AMD3100, which alters CXCR4 and CXCR7 signalling in a DPP4‐independent manner. Therefore, under normal conditions, proliferation of our podocytes is sustained by a stimulus mediated by CXCR4 activation. In the presence of DPP4 inhibitors and/or AMD3100, CXCR7 seems, however, to play an opposing role. Although further studies are needed to confirm these findings, a novel mechanism of action of gliptins can be inferred: namely, the modulation of intrarenal autocrine/paracrine signals resulting from DPP4 inhibition through a mechanism that involves SDF‐1‐CXCR4/CXCR7 signalling.
Figure 9.
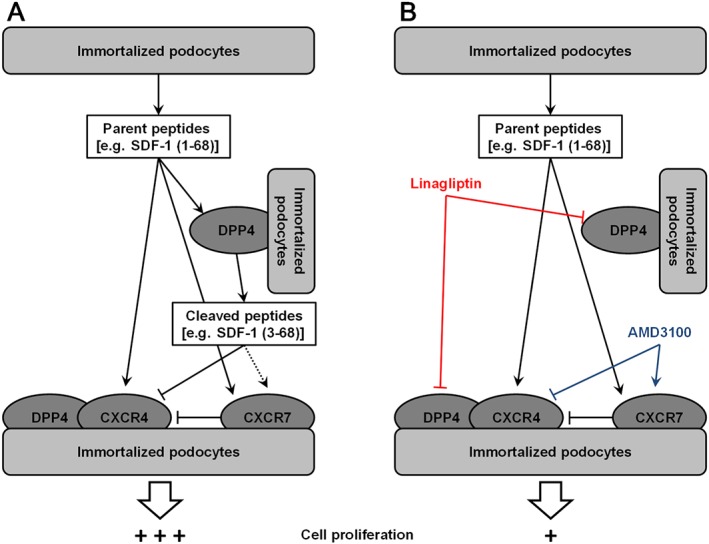
A proposed mechanistic model to interpret the effects of linagliptin and AMD3100 on the growth of immortalized podocytes. (A) DPP4 is highly expressed by podocytes and is likely to participate in the SDF‐1‐CXCR4/CXCR7 signalling pathway both by mediating the local processing of SDF‐1 and interacting with CXCR4. (B) A decrease in podocyte proliferation could result from the interruption of SDF‐1 signalling, which is altered by both linagliptin and AMD3100, in a DPP4‐dependent and DPP4‐independent manner respectively.
Several intra‐ and extra‐renal cell types are targeted by SDF‐1, and it acts together with other signalling molecules (including DPP4 substrates). For example, the expression at the mRNA level of SDF‐1, CXCR4 and CXCR7 was observed not only in podocytes but also in mesangial cells (see Supporting Information Figure S4). Therefore, a cascade of autocrine and paracrine actions could be triggered by inhibiting intra‐glomerular DPP4‐mediated SDF‐1 processing. Until now, conflicting results have been reported on the role of the SDF‐1‐CXCR4/CXCR7 pathways in the kidney. Indeed, some studies have suggested a beneficial function for SDF‐1 (Mazzinghi et al., 2008; Stokman et al., 2010; Chen et al., 2014; Nistala et al., 2014). In contrast, it has also been shown that a CXCR4‐mediated podocyte proliferation could contribute to the development of certain glomerular diseases (Ding et al., 2006; Rizzo et al., 2013). As discussed above, disruption of the SDF‐1 signalling is proposed to mediate the potentially favourable effects of gliptins on podocyte behaviour observed in our experiments. These findings contribute to the compelling evidence on the role of the pharmacological modulation of the intrarenal SDF‐1 signalling pathways. Recently, an in vivo study on the renal effects of linagliptin has been published (Takashima et al., 2016). By using different animal models of diabetes, the beneficial effects resulting from DPP4 inhibition were shown to be mediated by the SDF‐1 signalling pathway, although the exact mechanism remains unclear. Therefore, consistent with our conclusion, pharmacological modulation of the intrarenal SDF‐1 signalling pathways may be one mechanism through which gliptins exert their therapeutic effects.
In conclusion, DPP4 expressed by glomerular cells could be a clinically relevant target for gliptins. In particular, by acting on DPP4 expressed by podocytes, these drugs could promote potentially beneficial changes with respect to the maintenance of the glomerular integrity. These effects could be exerted at therapeutic concentrations. Moreover, they are incretin‐independent effects, mediated by disruption of the SDF‐1‐CXCR4/CXCR7 pathways. Thus, collectively, our findings give rise to a novel hypothesis and could contribute to a better understanding of the renal actions of gliptins.
Author contributions
G.M. devised the experiments; G.M, G.V. and E.B. performed the experiments; G.M. and E.B. analysed and interpreted the data and wrote the manuscript; and T.K. contributed to the discussion.
Conflict of interest
T. K. is a research employee of Boehringer Ingelheim Pharma.
Declaration of transparency and scientific rigour
This Declaration acknowledges that this paper adheres to the principles for transparent reporting and scientific rigour of preclinical research recommended by funding agencies, publishers and other organisations engaged with supporting research.
Supporting information
Table S1 PCR primers used in this study.
Figure S1 Effects of linagliptin on cell growth of immortalized mesangial cells and podocytes. Immortalized human mesangial cells (A) or podocytes (B) were exposed to either vehicle alone or linagliptin (1 or 100 nM; 1–5 days), and cell growth was evaluated by determining cell number in each well. Data are expressed as mean ± SEM of five experiments run in triplicate for each experimental group.
Figure S2 Effects of AMD3100 on growth of immortalized human podocytes. Immortalized human podocytes were exposed to vehicle alone (control, white bar), linagliptin, AMD3100 or linagliptin + AMD3100 for 5 days and cell growth was evaluated by determining cell number in each well. Data are expressed as mean ± SEM of five experiments run in triplicate for each experimental group. Combined effects (dashed line) were predicted by assuming Bliss independence. * P < 0.05 versus control group.
Figure S3 Effects of AZD6244 on growth of immortalized human podocytes. Immortalized human podocytes were exposed to vehicle alone (control, white bar), linagliptin, AZD6244 or linagliptin + AZD6244 for 5 days and cell growth was evaluated by determining cell number in each well. Data are expressed as mean ± SEM of five experiments run in triplicate for each experimental group. Combined effects (dashed line) were predicted by assuming Bliss independence. * P < 0.05 versus control group.
Figure S4 Expression of the gene encoding for SDF‐1, CXCR4 and CXCR7 in immortalized mesangial cells. Expression was evaluated in immortalized mesangial cells by RT‐PCR analyses. Image is representative of five experiments run in duplicate for each experimental group.
Acknowledgements
We thank Prof Giovanni Camussi (Dipartimento di Scienze Mediche, University of Turin) for providing immortalized human glomerular cells. This work was supported by a grant from Boehringer Ingelheim Pharma GmbH & Co. KG.
Miglio, G. , Vitarelli, G. , Klein, T. , and Benetti, E. (2017) Effects of linagliptin on human immortalized podocytes: a cellular system to study dipeptidyl‐peptidase 4 inhibition. British Journal of Pharmacology, 174: 809–821. doi: 10.1111/bph.13739.
References
- Alexander SPH, Davenport AP, Kelly E, Marrion N, Peters JA, Benson HE et al. (2015a). The concise guide to PHARMACOLOGY 2015/16: G protein‐coupled receptors. Br J Pharmacol 172: 5744–5869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Fabbro D, Kelly E, Marrion N, Peters JA, Benson HE et al. (2015b). The concise guide to PHARMACOLOGY 2015/16: enzymes. Br J Pharmacol 172: 6024–6109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baetta R, Corsini A (2011). Pharmacology of dipeptidyl peptidase‐4 inhibitors: similarities and differences. Drugs 71: 1441–1467. [DOI] [PubMed] [Google Scholar]
- Bliss CI (1939). The toxicity of poisons applied jointly. Ann Appl Biol 26: 585–615. [Google Scholar]
- Chen LH, Advani SL, Thai K, Kabir MG, Sood MM, Gibson IW et al. (2014). SDF‐1/CXCR4 signaling preserves microvascular integrity and renal function in chronic kidney disease. PLoS One 9: e92227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conaldi PG, Biancone L, Bottelli A, Wade‐Evans A, Racusen LC, Boccellino M et al. (1998). HIV‐1 kills renal tubular epithelial cells in vitro by triggering an apoptotic pathway involving caspase activation and Fas upregulation. J Clin Invest 102: 2041–2049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crump MP, Gong JH, Loetscher P, Rajarathnam K, Amara A, Arenzana‐Seisdedos F et al. (1997). Solution structure and basis for functional activity of stromal cell‐derived factor‐1; dissociation of CXCR4 activation from binding and inhibition of HIV‐1. EMBO J 16: 6996–7007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis MJ, Bond RA, Spina D, Ahluwalia A, Alexander SP, Giembycz MA et al. (2015). Experimental design and analysis and their reporting: new guidance for publication in BJP. Br J Pharmacol 172: 3461–3471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson JA (2013). The placement of DPP4 inhibitors in clinical practice recommendations for the treatment of type 2 diabetes. Endocr Pract 19: 1050–1061. [DOI] [PubMed] [Google Scholar]
- De La Luz Sierra M, Yang F, Narazaki M, Salvucci O, Davis D, Yarchoan R et al. (2004). Differential processing of stromal‐derived factor‐1alpha and stromal‐derived factor‐1beta explains functional diversity. Blood 103: 2452–2459. [DOI] [PubMed] [Google Scholar]
- Ding M, Cui S, Li C, Jothy S, Haase V, Steer BM et al. (2006). Loss of the tumor suppressor Vhlh leads to upregulation of Cxcr4 and rapidly progressive glomerulonephritis in mice. Nat Med 12: 1081–1087. [DOI] [PubMed] [Google Scholar]
- Doublier S, Ruotsalainen V, Salvidio G, Lupia E, Biancone L, Conaldi PG et al. (2001). Nephrin redistribution on podocytes is a potential mechanism for proteinuria in patients with primary acquired nephritic syndrome. Am J Pathol 158: 1723–1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ervinna N, Mita T, Yasunari E, Azuma K, Tanaka R, Fujimura S et al. (2013). Anagliptin, a DPP4 inhibitor, suppresses proliferation of vascular smooth muscles and monocyte inflammatory reaction and attenuates atherosclerosis in male apo E‐deficient mice. Endocrinology 154: 1260–1270. [DOI] [PubMed] [Google Scholar]
- Eun Lee J, Kim JE, Lee MH, Song HK, Ghee JY, Kang YS et al. (2016). DA‐1229, a dipeptidyl peptidase IV inhibitor, protects against renal injury by preventing podocyte damage in an animal model of progressive renal injury. Lab Invest 96: 547–560. [DOI] [PubMed] [Google Scholar]
- Fujita H, Morii T, Fujishima H, Sato T, Shimizu T, Hosoba M et al. (2014). The protective roles of GLP‐1R signaling in diabetic nephropathy: possible mechanism and therapeutic potential. Kidney Int 85: 579–589. [DOI] [PubMed] [Google Scholar]
- Fukasawa KM, Fukasawa K, Sahara N, Harada M, Kondo Y, Nagatsu I (1981). Immunohistochemical localization of dipeptidyl aminopeptidase IV in rat kidney, liver, and salivary glands. J Histochem Cytochem 29: 337–343. [DOI] [PubMed] [Google Scholar]
- Ghersi G, Zhao Q, Salamone M, Yeh Y, Zucker S, Chen WT (2006). The protease complex consisting of dipeptidyl peptidase IV and seprase plays a role in the migration and invasion of human endothelial cells in collagenous matrices. Cancer Res 66: 4652–4661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorrell MD, Gysbers V, McCaughan GW (2001). CD26: a multifunctional integral membrane and secreted protein of activated lymphocytes. Scand J Immunol 54: 249–264. [DOI] [PubMed] [Google Scholar]
- Gravel S, Malouf C, Boulais PE, Berchiche YA, Oishi S, Fujii N et al. (2010). The peptidomimetic CXCR4 antagonist TC14012 recruits beta‐arrestin to CXCR7: roles of receptor domains. J Biol Chem 285: 37939–37943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groop PH, Cooper ME, Perkovic V, Emser A, Woerle HJ, von Eynatten M (2013). Linagliptin lowers albuminuria on top of recommended standard treatment in patients with type 2 diabetes and renal dysfunction. Diabetes Care 36: 3460–3468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartel S, Gossrau R, Hanski C, Reutter W (1988). Dipeptidyl peptidase (DPP) IV in rat organs. Comparison of immunohistochemistry and activity histochemistry. Histochemistry 89: 151–161. [DOI] [PubMed] [Google Scholar]
- Hattori S (2011). Sitagliptin reduces albuminuria in patients with type 2 diabetes. Endocr J 58: 69–73. [DOI] [PubMed] [Google Scholar]
- Herrera C, Morimoto C, Blanco J, Mallol J, Arenzana F, Lluis C et al. (2001). Comodulation of CXCR4 and CD26 in human lymphocytes. J Biol Chem 276: 19532–11959. [DOI] [PubMed] [Google Scholar]
- Huynh H, Soo KC, Chow PK, Tran E (2007). Targeted inhibition of the extracellular signal‐regulated kinase kinase pathway with AZD6244 (ARRY‐142886) in the treatment of hepatocellular carcinoma. Mol Cancer Ther 6: 138–146. [DOI] [PubMed] [Google Scholar]
- Kalatskaya I, Berchiche YA, Gravel S, Limberg BJ, Rosenbaum JS, Heveker N (2009). AMD3100 is a CXCR7 ligand with allosteric agonist properties. Mol Pharmacol 75: 1240–1247. [DOI] [PubMed] [Google Scholar]
- Kameoka J, Tanaka T, Nojima Y, Schlossman SF, Morimoto C (1993). Direct association of adenosine deaminase with a T cell activation antigen, CD26. Science 261: 466–469. [DOI] [PubMed] [Google Scholar]
- Kettmann U, Humbel B, Holzhausen HJ (1992). Ultrastructural localization of dipeptidylpeptidase IV in the glomerulum of the rat kidney. Acta Histochem 92: 225–227. [DOI] [PubMed] [Google Scholar]
- Kim D, Wang L, Beconi M, Eiermann GJ, Fisher MH, He H et al. (2005). (2R)‐4‐oxo‐4‐[3‐(trifluoromethyl)‐5,6‐dihydro[1,2,4]triazolo[4,3‐a]pyrazin‐7(8H)‐yl]‐1‐(2,4,5‐trifluorophenyl)butan‐2‐amine: a potent, orally active dipeptidyl peptidase IV inhibitor for the treatment of type 2 diabetes. J Med Chem 48: 141–151. [DOI] [PubMed] [Google Scholar]
- Kirino Y, Sato Y, Kamimoto T, Kawazoe K, Minakuchi K, Nakahori Y (2009). Interrelationship of dipeptidyl peptidase IV (DPP4) with the development of diabetes, dyslipidaemia and nephropathy: a streptozotocin‐induced model using wild‐type and DPP4‐deficient rats. J Endocrinol 200: 53–61. [DOI] [PubMed] [Google Scholar]
- Lambeir AM, Durinx C, Scharpé S, De Meester I (2003). Dipeptidyl‐peptidase IV from bench to bedside: an update on structural properties, functions, and clinical aspects of the enzyme DPP IV. Crit Rev Clin Lab Sci 40: 209–294. [DOI] [PubMed] [Google Scholar]
- Levoye A, Balabanian K, Baleux F, Bachelerie F, Lagane B (2009). CXCR7 heterodimerizes with CXCR4 and regulates CXCL12‐mediated G protein signaling. Blood 113: 6085–6093. [DOI] [PubMed] [Google Scholar]
- Liu WJ, Xie SH, Liu YN, Kim W, Jin HY, Park SK et al. (2012). Dipeptidyl peptidase IV inhibitor attenuates kidney injury in streptozotocin‐induced diabetic rats. J Pharmacol Exp Ther 340: 248–255. [DOI] [PubMed] [Google Scholar]
- Maeda Y, Ishikado A, Park K, Khamaisi M, Qi W, Hastings SM et al. (2015). Characterization of podocyte dipeptidyl peptidase‐4 expression in glomeruli after 50 years of diabetes. Diabetes 64: A147–A148. [Google Scholar]
- Mazzinghi B, Ronconi E, Lazzeri E, Sagrinati C, Ballerini L, Angelotti ML et al. (2008). Essential but differential role for CXCR4 and CXCR7 in the therapeutic homing of human renal progenitor cells. J Exp Med 205: 479–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mega C, de Lemos ET, Vala H, Fernandes R, Oliveira J, Mascarenhas‐Melo F et al. (2011). Diabetic nephropathy amelioration by a low‐dose sitagliptin in an animal model of type 2 diabetes (Zucker diabetic fatty rat). Exp Diabetes Res . doi:10.1155/2011/162092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mentzel S, Van Son JP, De Jong AS, Dijkman HB, Koene RA, Wetzels JF et al. (1997). Mouse glomerular epithelial cells in culture with features of podocytes in vivo express aminopeptidase A and angiotensinogen but not other components of the renin‐angiotensin system. J Am Soc Nephrol 8: 706–719. [DOI] [PubMed] [Google Scholar]
- Miceli I, Burt D, Tarabra E, Camussi G, Perin PC, Gruden G (2010). Stretch reduces nephrin expression via an angiotensin II‐AT(1)‐dependent mechanism in human podocytes: effect of rosiglitazone. Am J Physiol Renal Physiol 298: F381–F390. [DOI] [PubMed] [Google Scholar]
- Miglio G, Rosa AC, Rattazzi L, Grange C, Camussi G, Fantozzi R (2012). Protective effects of peroxisome proliferator‐activated receptor agonists on human podocytes: proposed mechanisms of action. Br J Pharmacol 167: 641–653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miglio G, Rosa AC, Rattazzi L, Grange C, Collino M, Camussi G et al. (2011). The subtypes of peroxisome proliferator‐activated receptors expressed by human podocytes and their role in decreasing podocyte injury. Br J Pharmacol 162: 111–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miglio G, Varsaldi F, Lombardi G (2005). Human T lymphocytes express N‐methyl‐D‐aspartate receptors functionally active in controlling T cell activation. Biochem Biophys Res Commun 338: 1875–1883. [DOI] [PubMed] [Google Scholar]
- Mulvihill EE, Drucker DJ (2014). Pharmacology, physiology, and mechanisms of action of dipeptidyl peptidase‐4 inhibitors. Endocr Rev 35: 992–1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nistala R, Habibi J, Aroor A, Sowers JR, Hayden MR, Meuth A et al. (2014). DPP4 inhibition attenuates filtration barrier injury and oxidant stress in the zucker obese rat. Obesity 22: 2172–2179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pala L, Mannucci E, Pezzatini A, Ciani S, Sardi J, Raimondi L et al. (2003). Dipeptidyl peptidase‐IV expression and activity in human glomerular endothelial cells. Biochem Biophys Res Commun 310: 28–31. [DOI] [PubMed] [Google Scholar]
- Pyke C, Heller RS, Kirk RK, Orskov C, Reedtz‐Runge S, Kaastrup P et al. (2014). GLP‐1 receptor localization in monkey and human tissue: novel distribution revealed with extensively validated monoclonal antibody. Endocrinology 155: 1280–1290. [DOI] [PubMed] [Google Scholar]
- Reinhold D, Bank U , Bühling F, Lendeckel U, Faust J, Neubert K et al. (1997). Inhibitors of dipeptidyl peptidase IV induce secretion of transforming growth factor‐beta 1 in PWM‐stimulated PBMC and T cells. Immunology 91: 354–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzo P, Perico N, Gagliardini E, Novelli R, Alison MR, Remuzzi G et al. (2013). Nature and mediators of parietal epithelial cell activation in glomerulonephritides of human and rat. Am J Pathol 183: 1769–1778. [DOI] [PubMed] [Google Scholar]
- Rosa AC, Rattazzi L, Miglio G, Collino M, Fantozzi R (2012). Angiotensin II induces tumor necrosis factor‐α expression and release from cultured human podocytes. Inflamm Res 61: 311–317. [DOI] [PubMed] [Google Scholar]
- Schön E, Mansfeld HW, Demuth HU, Barth A, Ansorge S (1985). The dipeptidyl peptidase IV, a membrane enzyme involved in the proliferation of T lymphocytes. Biomed Biochim Acta 44: K9–K15. [PubMed] [Google Scholar]
- Sharkovska Y, Reichetzeder C, Alter M, Tsuprykov O, Bachmann S, Secher T et al. (2014). Blood pressure and glucose independent renoprotective effects of dipeptidyl peptidase‐4 inhibition in a mouse model of type‐2 diabetic nephropathy. J Hypertens 32: 2211–2223. [DOI] [PubMed] [Google Scholar]
- Stefanovic V, Ardaillou N, Vlahovic P, Placier S, Ronco P, Ardaillou R (1993). Interferon‐gamma induces dipeptidylpeptidase IV expression in human glomerular epithelial cells. Immunology 80: 465–470. [PMC free article] [PubMed] [Google Scholar]
- Stokman G, Stroo I, Claessen N, Teske GJ, Florquin S, Leemans JC (2010). SDF‐1 provides morphological and functional protection against renal ischaemia/reperfusion injury. Nephrol Dial Transplant 25: 3852–3859. [DOI] [PubMed] [Google Scholar]
- Southan C, Sharman JL, Benson HE, Faccenda E, Pawson AJ, Alexander SPH et al. (2016). The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. Nucleic Acids Res 44 (Database Issue): D1054–D1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takashima S, Fujita H, Fujishima H, Shimizu T, Sato T, Morii T et al. (2016). Stromal cell–derived factor‐1 is upregulated by dipeptidyl peptidase‐4 inhibition and has protective roles in progressive diabetic nephropathy. Kidney Int 90: 783–796. [DOI] [PubMed] [Google Scholar]
- Thomas L, Eckhardt M, Langkopf E, Tadayyon M, Himmelsbach F, Mark M (2008). (R)‐8‐(3‐Amino‐piperidin‐1‐yl)‐7‐but‐2‐ynyl‐3‐methyl‐1‐(4‐methyl‐quinazolin‐2‐ylmethyl)‐3,7‐dihydro‐purine‐2,6‐dione (BI 1356), a novel xanthine‐based dipeptidyl peptidase 4 inhibitor, has a superior potency and longer duration of action compared with other dipeptidyl peptidase‐4 inhibitors. J Pharmacol Exp Ther 325: 175–182. [DOI] [PubMed] [Google Scholar]
- Uto‐Konomi A, McKibben B, Wirtz J, Sato Y, Takano A, Nanki T et al. (2013). CXCR7 agonists inhibit the function of CXCL12 by down‐regulation of CXCR4. Biochem Biophys Res Commun 431: 772–776. [DOI] [PubMed] [Google Scholar]
- Wang J, Shiozawa Y, Wang J, Wang Y, Jung Y, Pienta KJ et al. (2008). The role of CXCR7/RDC1 as a chemokine receptor for CXCL12/SDF‐1 in prostate cancer. J Biol Chem 283: 4283–4294. [DOI] [PubMed] [Google Scholar]
- Wronkowitz N, Görgens SW, Romacho T, Villalobos LA, Sánchez‐Ferrer CF, Peiró C et al. (2014). Soluble DPP4 induces inflammation and proliferation of human smooth muscle cells via protease‐activated receptor 2. Biochim Biophys Acta 1842: 1613–1621. [DOI] [PubMed] [Google Scholar]
- Yang J, Campitelli J, Hu G, Lin Y, Luo J, Xue C (2007). Increase in DPP‐IV in the intestine, liver and kidney of the rat treated with high fat diet and streptozotocin. Life Sci 81: 272–279. [DOI] [PubMed] [Google Scholar]
- Zhang WB, Navenot JM, Haribabu B, Tamamura H, Hiramatu K, Omagari A et al. (2002). A point mutation that confers constitutive activity to CXCR4 reveals that T140 is an inverse agonist and that AMD3100 and ALX40‐4C are weak partial agonists. J Biol Chem 277: 24515–24521. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 PCR primers used in this study.
Figure S1 Effects of linagliptin on cell growth of immortalized mesangial cells and podocytes. Immortalized human mesangial cells (A) or podocytes (B) were exposed to either vehicle alone or linagliptin (1 or 100 nM; 1–5 days), and cell growth was evaluated by determining cell number in each well. Data are expressed as mean ± SEM of five experiments run in triplicate for each experimental group.
Figure S2 Effects of AMD3100 on growth of immortalized human podocytes. Immortalized human podocytes were exposed to vehicle alone (control, white bar), linagliptin, AMD3100 or linagliptin + AMD3100 for 5 days and cell growth was evaluated by determining cell number in each well. Data are expressed as mean ± SEM of five experiments run in triplicate for each experimental group. Combined effects (dashed line) were predicted by assuming Bliss independence. * P < 0.05 versus control group.
Figure S3 Effects of AZD6244 on growth of immortalized human podocytes. Immortalized human podocytes were exposed to vehicle alone (control, white bar), linagliptin, AZD6244 or linagliptin + AZD6244 for 5 days and cell growth was evaluated by determining cell number in each well. Data are expressed as mean ± SEM of five experiments run in triplicate for each experimental group. Combined effects (dashed line) were predicted by assuming Bliss independence. * P < 0.05 versus control group.
Figure S4 Expression of the gene encoding for SDF‐1, CXCR4 and CXCR7 in immortalized mesangial cells. Expression was evaluated in immortalized mesangial cells by RT‐PCR analyses. Image is representative of five experiments run in duplicate for each experimental group.


