Abstract
Background and purpose
Our previous studies revealed that hypidone hydrochloride (YL‐0919), which acts as a selective 5‐HT (serotonin) reuptake inhibitor (SSRI) and displays partial 5‐HT1A receptor agonist properties, exerts a significant antidepressant effect in various animal models. The aim of present research was to further investigate the pharmacology of YL‐0919.
Experimental approach
We first investigated the target profile of YL‐0919 using [35S]‐GTPγS binding and microdialysis. To determine whether the 5‐HT or noradrenergic systems are involved in the antidepressant‐like effect of YL‐0919, the 5‐hydroxytryptophan (5‐HTP)‐induced head‐twitch test and antagonism with a high dose of apomorphine were performed. Using the learned helplessness paradigm, the novelty suppressed feeding test, the Vogel‐type conflict and elevated plus‐maze test, we further verified the antidepressant‐like and anxiolytic‐like effects of YL‐0919. The effects of YL‐0919 on hippocampal long‐term potentiation (LTP) and sexual behaviour were also evaluated.
Key results
Data from the present study demonstrated that YL‐0919 displays partial 5‐HT1A receptor agonist properties, producing a greater impact on extracellular 5‐HT levels than a conventional SSRI (fluoxetine), as well as significant antidepressant and anxiolytic effects. Furthermore, YL‐0919 treatment rapidly influenced the synaptic plasticity (enhancing LTP) of rats. Finally, at doses close to those producing antidepressant‐like effects, YL‐0919 did not result in a marked inhibition of sexual function.
Conclusions and implications
These data suggest that YL‐0919 is probably a fast‐onset potent antidepressant with few side effects.
Abbreviations
- fEPSP
field EPSP
- LPP
lateral perforant path
- NA
noradrenaline
- NSF
novelty suppressed feeding
- PS
population spike
- SSRIs
selective 5‐HT reuptake inhibitors
Tables of Links
| TARGETS |
|---|
| GPCRs a |
| 5‐HT1A receptor |
| Transporters b |
| SERT |
| LIGANDS | |
|---|---|
| 5‐HT | Dopamine |
| 5‐hydroxytryptophan | Fluoxetine (FLX) |
| 5‐OH‐DPAT | GDP |
| Apomorphine | Noradrenaline |
| Diazepam (DZP) | Vilazodone |
| Desipramine | WAY‐100635 |
These Tables list key protein targets and ligands in this article which are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Southan et al., 2016), and are permanently archived in the Concise Guide to PHARMACOLOGY 2015/16 (a,bAlexander et al., 2015a,b).
Introduction
Major depressive disorder (MDD), a serious and debilitating mental illness, is a major contributor to personal and societal burden worldwide. Selective 5‐HT (serotonin) reuptake inhibitors (SSRIs) are widely used antidepressants; however, they have several major side effects, including sexual dysfunction, cognitive dysfunction and weight gain. Most notably, SSRIs require 4–6 weeks to achieve a clinically therapeutic effect, and approximately, only two‐thirds of patients respond to them, leaving patients with severe MDD at risk of suicide (Tollefson, 1991; Stassen et al., 1996; Culpepper et al., 2004). These findings indicate a clear unmet medical need for stronger and faster‐acting antidepressant drugs with fewer side effects.
The delayed onset of SSRIs is thought to be mainly attributable to the desensitization of the 5‐HT1A autoreceptor. Growing evidence suggests that augmenting serotonergic transmission, either via the activation of 5‐HT1A receptors and/or by the inhibition of 5‐HT reuptake, can be an effective treatment for MDD (Hjorth and Auerbach, 1996; Stahl, 2014). Several compounds that target 5‐HT1A receptors as agonists or with partial agonistic activity, including pindolol and buspirone, can enhance or hasten the antidepressant effects of SSRIs (Artigas et al., 2001; Plenge and Mellerup, 2003).
Therefore, an alternative approach is to develop drugs that combine two mechanisms in a single drug, namely SSRIs with partial 5‐HT1A receptor agonists; these are termed 5‐HT partial agonist‐reuptake inhibitors (SPARIs). SPARIs could be a novel strategy for obtaining antidepressants with higher therapeutic efficacies. Indeed, the Food and Drug Administration has approved the first such drug, vilazodone (Viibryd®), for the treatment of MDD (Owen, 2011; Choi et al., 2012). Evidence has suggested that the partial agonism of vilazodone at the 5‐HT1A receptor may increase the 5‐HT level to a greater extent than conventional SSRIs, thereby enhancing and broadening the antidepressant efficacy (Laughren et al., 2011; Lindsey, 2011; Schwartz et al., 2011).
Our previous studies revealed that hypidone hydrochloride (YL‐0919), a novel small‐molecule compound that showed high affinity and selectivity for both the 5‐HT transporter and 5‐HT1A receptor, exerted a significant antidepressant effect in various animal models (Chen et al., 2013; Qin et al., 2014). Moreover, YL‐0919 was identified as a potential 5‐HT1A agonist and SSRI. Compared with vilazodone, which has a complicated structure and is difficult to dissolve in water, YL‐0919 can be easily synthesized and dissolved in water. Metabolic studies showed that after treatment with a YL‐0919 prototype, the concentration of YL‐0919 reached micromolar levels in rat and mouse brain tissues. All of these results warrant further pharmacological characterization of this compound.
In the present study, we first investigated the target profile of YL‐0919 using [35S]‐GTPγS binding and microdialysis. To determine whether the 5‐HT or noradrenaline (NA) systems were involved in the antidepressant‐like effect of YL‐0919, the 5‐hydroxytryptophan (5‐HTP)‐induced head‐twitch test and antagonism with a high dose of apomorphine were performed. Using the learned helplessness paradigm, we further verified the antidepressant‐like activity of subchronic treatment with YL‐0919. To identify the onset time of YL‐0919 efficacy, the novelty‐suppressed feeding (NSF) test was conducted. The Vogel‐type conflict and elevated plus‐maze test were also used to measure the anxiolytic‐like effect of YL‐0919. The effect of YL‐0919 on LTP in the lateral perforant path (LPP)‐granule cell synapses of the rat dentate gyrus was evaluated after YL‐0919 treatment. Finally, the effects of YL‐0919 on sexual behaviour were assessed by examining copulatory behaviour and noncontact penile erections in sexually experienced male rats.
Methods
Animals and housing
Male Sprague Dawley (SD) rats (240 ± 20 g), male and female Wistar rats (240 ± 20 g) and male imprinting control region (ICR) mice (20 ± 2 g) (Vital River, Beijing, China) were housed at constant room temperature (23 ± 1°C), a relative humidity of 50–60% and a 12 h light/dark cycle, with water and food freely available. All procedures followed the National Institutes of Health's Guide for the Care and Use of Laboratory Animals (NIH Publications No. 80–23) and were approved by the Institutional Animal Care and Use Committee. Efforts were made to reduce the number of animals used for the experiments and to minimize animal suffering. Animal studies are reported in compliance with the ARRIVE guidelines (Kilkenny et al., 2010; McGrath & Lilley, 2015).
Target profile
[35S]‐GTPγS binding studies in rat hippocampal membranes
This assay was performed mainly as described by Odagaki and Toyoshima (2005). Male SD rats were killed by decapitation, and the hippocampus was rapidly removed and placed on dry ice. The tissues were weighed and sonicated in Tris buffer (50 mM Tris, pH 7.4) on ice. The homogenate was centrifuged for 10 min at 4°C and 39 800 g. The pellet was resuspended in Tris buffer and incubated for 10 min at 37°C. Then, the suspension was centrifuged again, and the pellet was resuspended in Tris buffer. After the final centrifugation, the pellet was resuspended in Tris buffer to yield a concentration of approximately 100 mg protein mL−1. The suspension was aliquoted and stored at −70°C until needed. For the [35S]‐GTPγS assay, membranes (50 μg of protein per well) were resuspended in assay buffer (50 mM Tris; 100 mM NaCl; 5 mM MgCl2·6H2O; 0.1 mM EDTA‐Na2·6H2O; 0.2 mM EGTA, pH 7.4) and incubated with the test compound, 100 μM GDP (Sigma) and 0.2 nM [35S]‐GTPγS (NEN™ Lifetime, Inc, USA) at 28°C for 60 min. The reaction was terminated by filtration through Whatman GF/C filters, which were presoaked with distilled water and washed with 4 mL of ice‐cold Tris buffer. Non‐specific binding was defined by GTPγS (BioLog, Germany, 40 μM), and radioactivity was detected by liquid scintillation counting (LS6500, Beckman, USA). All data were analysed using a non‐linear regression method with GraphPad Prism software (GraphPad, San Diego, CA, USA), and the concentration eliciting the half‐maximal effect (EC50) and the % maximal increase above the basal value (% Emax) were calculated.
Microdialysis experiment in freely moving rats
After an acclimatization period of approximately 1 week, male SD rats (6–9 rats per group) were anaesthetized with 4% chloral hydrate (400 mg·kg−1, i.p.) and placed into a stereotaxic apparatus. Stainless steel guide cannulae were stereotaxically implanted into the ventral hippocampus at the following coordinates using a stereotaxic instrument: AP −5.2, ML ±5.4 and DV 7.4 mm from bregma (Paxinos et al., 1980). Following surgery, the animals were individually housed for at least 7 days and handled approximately 4 days before the microdialysis experiments.
On the day before the experiments, the rats were placed in microdialysis cages for overnight habituation. The following morning, the pins were removed from the guide cannulae, and microdialysis probes were lowered into the left ventral hippocampus. The microdialysis probes were continuously perfused with artificial CSF at a flow rate of 1.0 μL·min−1. Baseline samples were collected every 30 min for 90 min of washing to obtain a stable extracellular neurotransmitter level. Then the animals were treated with vehicle, FLX [10 mg·kg−1, intragastric gavage (i.g.)] or YL‐0919 (1.25, 2.5 mg·kg−1, i.g.), and microdialysis samples were collected every 30 min for another 240 min. The extracellular levels of 5‐HT, dopamine and NA were determined using a Waters e2695 HPLC system with electrochemical detection. The perfusate levels of the neurotransmitter were expressed as percentages of the mean value of the absolute quantity of the transmitter collected in the four pre‐injection control samples (basal level).
Experimental design and behavioural tests
As illustrated in Table 1, behavioural tests (8–10 animals per group) including the 5‐HTP‐induced head‐twitch test, antagonism of a high dose of apomorphine, Vogel‐type conflict test in rats and elevated plus‐maze test were performed after acute drug treatments. Other experiments, such as the NSF test, learned‐helplessness test and sexual behaviour observations, were conducted after subchronic or chronic drug treatment. The dose of YL‐0919 was chosen based on our previous studies, and the vehicle or drugs were administered by i.g.
Table 1.
Experimental design
| Experiment | Dose of YL‐0919 (mg·kg−1) | Time span |
|---|---|---|
| Microdialysis experiment | 1.25, 2.5 | single |
| Hippocampal LTP recordings | 0.625, 1.25, 2.5 | 7 or 21 days |
| Antidepressant effect | ||
| 5‐HTP‐induced head‐twitch test | 0.625, 1.25, 2.5 | single |
| Antagonism of a high dose of apomorphine | 0.625, 1.25, 2.5 | single |
| Novelty suppressed feeding test | 0.625, 1.25, 2.5 | 7 or 14 days |
| Learned‐helplessness Paradigm | 0.625, 1.25, 2.5 | 4 days |
| Anxiolytic effect | ||
| Vogel‐type conflict test in rats | 1.25, 2.5 | single |
| Elevated plus‐maze test | 1.25, 2.5 | single |
| Sexual behaviour | ||
| Noncontact penile erections | 1.25, 2.5, 5 | 9 days |
| Copulatory test | 1.25, 2.5, 5 | 13 days |
5‐HTP‐induced mouse head‐twitch test
This test was performed following the protocol of Fischer (Fischer and Muller, 1984). ICR mice were administered YL‐0919 (0.625–2.5 mg·kg−1, i.g.), FLX (30 mg·kg−1, i.g.) or saline 60 min before the injection of 5‐HTP (120 mg·kg−1, i.p.). After the injection of 5‐HTP, the mice were immediately placed in the plastic cages, and the total number of head twitches (rapid movements of the head with little or no involvement of the trunk) was recorded over a 20 min period.
Antagonism of a high dose of apomorphine
The high‐dose apomorphine‐induced hypothermia test was adopted to assess the possible effect of YL‐0919 on the NA system (Puech et al., 1981). Thirty minutes after the administration of YL‐0919 (0.625–2.5 mg·kg−1, i.g.) or vehicle, a high dose of apomorphine (16 mg·kg−1) was s.c. injected into ICR mice. To evaluate the effect of the drugs on the basal body temperature and apomorphine‐induced hypothermia, the rectal temperature was measured immediately before apomorphine injection and 30 min later. Throughout the experiment, the room temperature was strictly maintained at 20 ± 1°C.
NSF test
NSF was measured after subchronic (7 days) or chronic (14 days) drug treatments (once daily, YL‐0919 was administered i.g. at 0.625–2.5 mg·kg−1, and FLX was administered i.g. at 10 mg·kg−1). Briefly, all ICR mice were deprived of food but not water for 24 h before the test. On the day of the test, each mouse was placed singly in the test chamber (60 × 60 cm) with food pellets in the centre. The latency to feeding was recorded for 5 min; non‐feeding behaviours (e.g. smelling and touching) were ignored. If food was not taken within 5 min, the feeding latency was recorded as 5 min.
Learned‐helplessness paradigm
To further verify the antidepressant‐like activity of subchronic treatment with the drug, the ICR mice were subjected to the learned helplessness paradigm (Anisman and Merali, 2001; Chourbaji et al., 2005). In this study, mice were placed in a shuttle‐box apparatus (40 × 10 × 13 cm) divided into two compartments to which they had free access. To induce helplessness, each mouse received 120 inescapable shocks (18–44 s, average 30 s; 0.45 mA for 15 s) over two consecutive training days. To screen helpless mice, the mice were subjected to 30 avoidance trials (18–44 s, average 30 s; 0.45 mA for 3 s). Animals that developed helplessness (>10 escape failures) were then treated with vehicle, FLX (10 mg·kg−1, i.g.) or YL‐0919 (0.625–2.5 mg·kg−1, i.g.) once daily for 4 days. The number of escape failures and the latency to escape was automatically recorded using Graphic State software (Coulbourn Instruments Inc., Allentown, PA, USA).
Vogel‐type conflict test in rats
In this test, behaviour boxes (50 × 30 × 20 cm) with a stainless steel grid floor (Columbus Instruments, Ohio, USA) were used. A water bottle with a metal drinking tube was fitted outside the box to ensure that only the drinking tube extended into the box. Electric shocks (0.3 mA, 2 s) were delivered to each rat by automatically switching the connections to the drinking tube and the grid floor from the drinkometer to an electric stimulator. The rats were individually placed in the test chamber after 48 h of water deprivation. After every 20 licks, the number of shocks that each animal received was recorded for 3 min. Diazepam (DZP, 1.5 mg·kg−1) was administered i.p. 30 min before the test, and YL‐0919 (1.25 and 2.5 mg·kg−1) was administered p.o. 1 h before the test session.
Elevated plus‐maze test
In this test, each SD rat was placed in the centre of the maze (70 cm above the floor), facing an enclosed arm. The time spent in and the number of entries into (with all four paws) both the open and enclosed arms (50 × 10 cm and 50 × 10 × 25 cm high respectively) were recorded for 5 min. The percentages of time spent and entries into the open arms were calculated. Diazepam (1.5 mg·kg−1) was administered i.p. 30 min before the test, and YL‐0919 (1.25 and 2.5 mg·kg−1) was administered p.o. 1 h before the test.
Electrode implantation and hippocampal LTP recordings
Male SD rats were treated with YL‐0919 (0.625–2.5 mg·kg−1, i.g.) or saline for 7 or 21 days for LTP recordings (5–6 rats per group). Twelve hours after the final drug treatment, the animals were anaesthetized (urethane, 1.2 g·kg−1, i.p.), and then LTP recordings were obtained. A bipolar enamel‐coated stainless steel electrode was used as the stimulating or recording electrode. The stimulating electrode was inserted 4.0 mm right of the midline and 8.0 mm posterior to the bregma, and the recording electrode was positioned 2.0 mm right of the midline and 4.0 mm posterior to the bregma (Lu et al., 2010; Yong et al., 2013). Responses were evoked by stimulation at a low frequency (0.03 Hz, 0.1 ms stimulus duration). The field EPSP (fEPSP) slope and the population spike (PS) amplitude in the dentate gyrus were monitored. Alterations in the fEPSP reflect dendritic changes, and the PS amplitude indicates the summated action potentials from the somatic layer of granule cells in the dentate gyrus. LTP was induced by high‐frequency stimulation using 20 pulses at 200 Hz; this procedure was repeated three times at 30 s intervals. To assess the basal synaptic transmission and ensure the stability of the recordings, the fEPSP slope and baseline PS amplitude of all of the rats were obtained by averaging the response to stimulation every 5 min over a period of 30 min. Then the PS amplitude and alterations in the fEPSP were measured and averaged every 5 min over a 60 min period. All stimulations and recordings were performed using an online computerized oscilloscope‐stimulator and data analysis interface system.
Effects of YL‐0919 on sexual behaviour
Preparation of sexually receptive females
This test was performed in Wistar rats as previously described (Agmo, 1997). Ovariectomized, adult female rats were individually housed, brought into behavioural oestrus weekly and injected with 30 μg of 17‐β oestradiol benzoate (in 0.1 mL of corn oil) 52 h before testing, followed by the s.c. administration of 500 μg of progesterone ( in 0.1 mL of corn oil) 4–6 h before testing.
Noncontact penile erections
After the daily administration of drugs for 9 days, sexually receptive female Wistar rats were brought into the test room at least 30 min before the behavioural test. Male rats (8–10 per group) were observed for noncontact penile erections in individual test cages (40 × 25 × 18 cm). A penile erection was noted when the male rat was observed in a hunched position grasping the penis with the forepaws, followed by a series of pelvic thrusts. The number of penile erections was quantified over a 30 min observation session.
Copulatory test
All animals underwent a copulatory test after chronic administration of the drugs for 13 days (8–10 rats per group). The test sessions were conducted under dim red light illumination during the dark phase (18–24 h). Each male rat was placed in a test box (40 × 60 × 40 cm), and a sexually receptive female was introduced into the chamber 5 min later. Then the following behavioural parameters were recorded within a 30 min interval after the introduction of the female: (1) mount latency (ML), the time from the introduction of the female until the first mount with incomplete mounts (without pelvic thrusting) and badly orientated mounts disregarded; (2) intromission latency (IL), the time from the introduction of the female until the first intromission (vaginal penetration); (3) intromission frequency (IF), the number of intromissions preceding ejaculation; (4) mount frequency (MF), the number of mounts preceding ejaculation; and (5) ejaculation latency (EL), the time from the first intromission until ejaculation. In cases of failure to ejaculate, the test ended 30 min after the introduction of the female rat.
Statistical analysis
All data are expressed as the means ± SEM and were analysed with GraphPad Prism 5.0 (GraphPad Software Inc., San Diego, CA, USA). Differences between two groups were analysed with Student's t‐test, and differences between the vehicle‐treated group and the YL‐0919‐treated group were accessed with a one‐way ANOVA followed by Dunnett's test. For all of the tests, differences with P < 0.05 were considered significant. The data and statistical analysis comply with the recommendations on experimental design and analysis in pharmacology (Curtis et al., 2015).
Drugs and reagents
YL‐0919 was synthesized by the Department of Medicinal Chemistry at our institute (white powder with purity >99.8%). Fluoxetine hydrochloride (FLX), desipramine (DMI), 5‐HTP, apomorphine, 8‐hydroxy‐2‐(di‐n‐propylamino) tetralin (8‐OH‐DPAT, 5‐HT1A receptor agonist), N‐[2‐[4‐(2‐methoxyphenyl)‐1‐piperazinyl]ethyl]‐N‐(2‐pyridinyl) cyclohexanecarboxamide (WAY‐100635, 5‐HT1A receptor antagonist), mianserin hydrochloride, tropisetron hydrochloride, GDP and chemical standards including 5‐HT, NA and dopamine were purchased from Sigma‐Aldrich (St. Louis, MO, USA). Vilazodone (dissolved in 2% DMSO) was purchased from Toronto Research Chemicals (North York, Canada). Unless otherwise specified, all compounds were dissolved in saline and administered at a dose of 2 mL·kg−1 in rats or 10 mL·kg−1 in mice.
Results
Target profile
[35S]‐GTPγS binding studies in rat hippocampal membranes
The EC50 values of YL‐0919, 8‐OH‐DPAT and vilazodone were 1.20 nM, 0.035 nM and 2.69 nM respectively. The intrinsic activity of YL‐0919 was higher than that of vilazodone and lower than that of 8‐OH‐DPAT. The maximal efficacy of YL‐0919 was 85% of that induced by 8‐OH‐DPAT, which was slightly greater than that of vilazodone (76%) (Table 2, Figure 1A). In the antagonist studies, WAY‐100635 (1–10 μM) markedly and completely prevented the maximal efficacy of 8‐OH‐DPAT (Figure 1B) and YL‐0919, but mianserin (5‐HT2 receptor antagonist) and tropisetron (5‐HT3 receptor antagonist) did not affect the maximal efficacy of YL‐0919 (Figure 1C).
Table 2.
Potency (EC50) and efficacy (Emax) of YL‐0919 in the rat hippocampus ([35S]‐GTPγS binding assay)
| Drugs | EC50 (nM) | Emax (%) |
|---|---|---|
| YL‐0919 | 1.20 ± 0.21* | 85.11 ± 9.70 |
| Vilazodone | 2.69 ± 0.42* | 76.01 ± 8.18 |
| 8‐OH‐DPAT | 0.035 ± 0.003 | 100 |
P < 0.05 versus Vehicle.
Figure 1.

(A) Effect of YL‐0919, vilazodone and 8‐OH‐DPAT on [35S]‐GTPγS binding to rat hippocampal membranes. (B) Effect of WAY‐100635 on 8‐OH‐DPAT‐induced stimulation of [35S]‐GTPγS binding to rat hippocampal membranes. (C) Effect of WAY‐100635, mianserin or tropisetron on YL‐0919‐induced stimulation of [35S]‐GTPγS binding to rat hippocampal membranes. The results are expressed as the mean ± SEM. Values for the % of the respective basal binding were obtained from four experiments performed in duplicate. * P < 0.05 versus Vehicle, # P < 0.05 versus 8‐OH‐DPAT or YL‐0919 (Two‐way ANOVA followed by Dunnett's test).
Microdialysis experiment in freely moving rats
As shown in Figure 2, YL‐0919 in a dosage range of 1.25–2.5 mg·kg−1 i.g. significantly increased the extracellular 5‐HT levels to approximately 407.7% of the basal level 60 min after drug treatment (two‐way ANOVA). In addition, the levels of extracellular NA and dopamine after the injection of YL‐0919 (2.5 mg·kg−1) into the rat hippocampus were also measured, but no significant alterations were observed among the groups. Significant increases in 5‐HT levels were observed 60 min after single FLX (10 mg·kg−1) treatment, but these increases were significantly lower than those caused by YL‐0919 (P < 0.05 vs. control).
Figure 2.

Effect of YL‐0919 on extracellular 5‐HT, NA or dopamine (DA) levels in rat hippocampus. Rats were treated with YL‐0919 (1.25 or 2.5 mg·kg−1, i.g.), FLX (10 mg·kg−1, i.g.) or vehicle at t = 0. And then a rapid and validated HPLC‐MS method coupled with microdialysis was used to monitor the levels of monoamine levels after YL‐0919 treatment. Data are expressed as a percentage of basal 5‐HT (NA, DA) (n = 6–9 per group). * P < 0.05 compared with the Vehicle group.
Effects of YL‐0919 on behavioural models of antidepressants
Figure 3A illustrates the acute effects of YL‐0919 on 5‐HTP‐induced head twitch in mice. At a dose of 120 mg·kg−1, 5‐HTP produced robust frequencies of the head‐twitch response in mice. YL‐0919 increased head twitches in mice pretreated with 5‐HTP in a dose‐dependent manner, with significant effects observed at 1.25 mg·kg−1 and 2.5 mg·kg−1 (one‐way ANOVA, F[3,36] = 7.315, P < 0.05), similar to the positive control drug FLX (Student's t‐test, P < 0.05), which also significantly increased head twitches.
Figure 3.
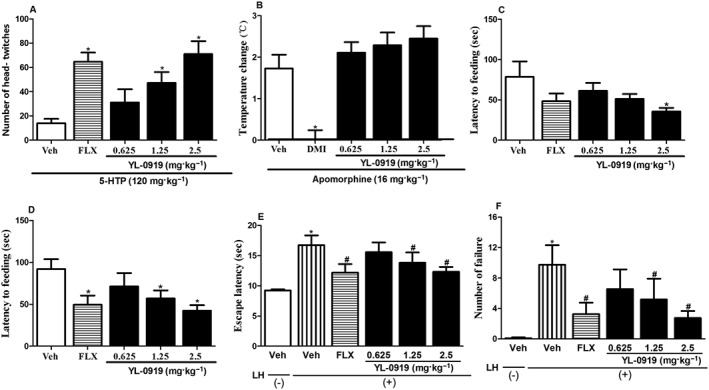
Effect of YL‐0919 in several behavioural and pharmacological models. Pretreatment with YL‐0919 (1.25 and 2.5 mg·kg−1, i.g.) potentiated the ability of 5‐HTP‐induced head‐twitch response in 5‐HTP‐induced head‐twitch test (A); single YL‐0919 administration had no effect on high dose of apomorphine (16 mg·kg−1) induced hypothermia (B); subchronic (7 days) treatment with YL‐0919 decreased the latency to feed (C). A similar result was observed after chronic (14 days) treatment with YL‐0919 treatment (D); sub‐chronic treatment with YL‐0919 significantly decreased escape latencies (E) and number of failures to escape (F) in comparison with control mice. Data are presented as the means ± SEM (n = 8–10). * P < 0.05, compared with the Vehicle (Veh) control group (ANOVA followed by Dunnett's test); # P < 0.05, compared with LH(+)‐Veh group.
As shown in Figure 3B, hypothermia developed as a result of the injection of a high dose of apomorphine (16 mg·kg−1), and the maximum response occurred at the 30 min point. Pretreatment with DMI significantly attenuated the hypothermia response in mice (Student's t‐test, P < 0.05 vs. vehicle), whereas no such effect was observed when YL‐0919 (0.625–2.5 mg·kg−1) was given as a pretreatment.
In the NSF test, subchronic (7 days) treatment with YL‐0919 decreased the latency to feed (one‐way ANOVA, F[3,36] = 2.574, P < 0.05). A similar result was observed after chronic (14 days) treatment with YL‐0919 (one‐way ANOVA, F[3,36] = 2.977, P < 0.05). The latency to feed remained significantly decreased only after chronic (14 days) treatment with FLX (Student's t‐test, P < 0.05 vs. vehicle) but not after subchronic (7 days) treatment (Figure 3C and D). The amount of food consumed in the home cage was also measured as a control for the feeding behaviour, and no differences were detected among the groups (data not shown).
The antidepressant‐like effects of YL‐0919 were further evaluated using the learned helplessness paradigm, which is a well‐known and widely used model of antidepressant efficacy that resembles the passive and withdrawn behaviour associated with human depression. As shown in Figure 3E and F, Student's t‐test revealed that the learned helplessness (LH)‐vehicle mice had significant increases in the number of failures to escape and the escape latency compared with the control (P < 0.05). One‐way ANOVA demonstrated that the subchronic administration of YL‐0919 (1.25 and 2.5 mg·kg−1) significantly decreased the escape latency (F[3,36] = 1.809, P < 0.05) and the number of failures to escape (F[3,36] = 1.691, P < 0.05), as did repeated administration of the positive control drug FLX at 10 mg·kg−1 (Student's t‐test, vs. LH‐vehicle mice).
Effects of YL‐0919 on behavioural models of anxiety
The Vogel‐type conflict test in rats is a paradigm that is widely used for anxiolytic testing. In this test, the anxiolytic effect is indicated by an increase in the number of shocks that the rats receive as a result of the inhibition of the conflict between the desire to drink water and the aversion to electric shocks. When given p.o. at doses of 1.25–2.5 mg·kg−1, YL‐0919 significantly increased the number of shocks that the rats received (one‐way ANOVA, F[2,27] = 1.624, P < 0.05). Similarly, diazepam at a dose of 1.5 mg·kg−1 also produced a significant increase in the number of shocks (P < 0.05 vs. vehicle) (Figure 4A).
Figure 4.
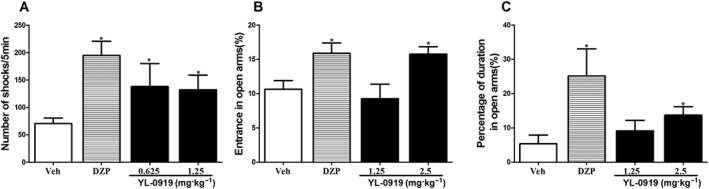
Effects of YL‐0919 on behavioural models of anxiety. YL‐0919 significantly increased the number of shocks in Vogel‐type conflict test in rats (A) and the percentage of both the time spent and the entries into the open arms in the elevated plus‐maze test (B and C). Data are presented as the means ± SEM (n = 10). * P < 0.05, compared with the vehicle (Veh) group.
The elevated plus‐maze test is another well‐established model for detecting both anxiolytic‐ and anxiogenic‐like behaviours. In this test, the proven anxiolytic diazepam at 1.5 mg·kg−1 increased the percentages of both the time spent and the entries into the open arms (Student's t‐test, P < 0.05 for the percentages of entries and time). A similar effect was produced by YL‐0919 (2.5 mg·kg−1), which increased the percentages of both the time spent (one‐way ANOVA, F[2,27] = 4.845, P < 0.05) and entries into the open arms (one‐way ANOVA, F[2,27] = 2.306, P < 0.05) (Figure 4B and C). At the doses used, neither YL‐0919 nor diazepam altered the total arm entries or the total time spent in arm exploration (data not shown).
Effect of YL‐0919 on hippocampal LTP
A baseline experiment revealed no significant differences between the vehicle and drug‐treated groups. Chronic (21 days) but not subchronic (7 days) treatment with FLX significantly increased the fEPSP and PS amplitude (Student's t‐test, P < 0.05). After subchronic YL‐0919 (0.625–2.5 mg·kg−1, i.g.) treatment for 7 days, significant increases in the fEPSP (F[3,36] = 1.742, P < 0.05) and PS amplitude (F[3,36] = 2.072, P < 0.05) were observed at the higher dose (2.5 mg·kg−1) of YL‐0919, whereas neither fEPSP nor the PS amplitude was different from the vehicle group after treatment with the lower dose of YL‐0919. Chronic treatment with YL‐0919 for 21 days significantly facilitated LTP, and further, post hoc analysis revealed that the PS amplitude was significantly increased after YL‐0919 treatment (F[3,36] = 7.098, P < 0.05). A similar result was observed for the fEPSP, which also exhibited significantly enhanced potentiation after YL‐0919 treatment (F[3,36] = 3.391, P < 0.05). These results indicated that YL‐0919 treatment produced a faster and greater LTP of the LPP‐dentate granule cell synapses than FLX treatment (Figure 5).
Figure 5.

Hippocampal LTP (PS amplitude or fEPSP slope) in rats was greatly enhanced by YL‐0919 treatment in adult freely behaving rats. LTP was induced 12 h after the termination of sub‐chronic (7 days) or chronic (21 days) treatment with saline, FLX (10 mg·kg−1, i.g.) or YL‐0919 (0.625–2.5 mg·kg−1, i.g.) respectively. Results were summarized from all animals (n = 5–6). Each value represents the mean ± SEM. * P < 0.05, compared with the Vehicle group.
Effects of YL‐0919 on sexual behaviour
As shown in Figure 6, unlike FLX, neither YL‐0919 (1.25–5 mg·kg−1) nor vilazodone (2 mg·kg−1) treatment significantly affected the number of non‐contact penile erections (9 days). In addition, the daily administration of YL‐0919 or vilazodone for 13 days had no effect on the copulatory behaviour of adult male rats compared with the vehicle control. Conversely, FLX increased the ML, IL and EL but significantly reduced the MF and IF relative to those of the vehicle‐treated rats (Student's t‐test, P < 0.05).
Figure 6.
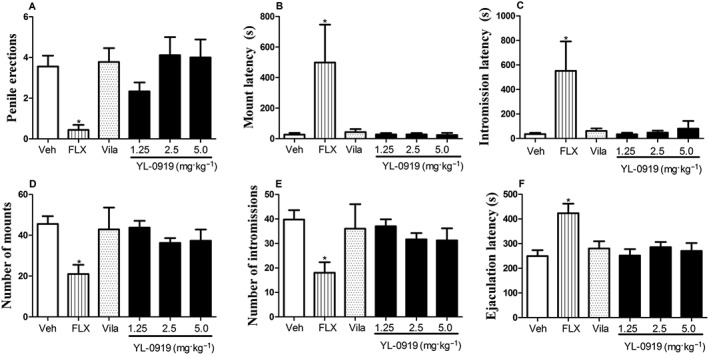
Effects of repeated YL‐0919 treatments on the sexual behaviours. Data are expressed as means ± SEM. * P < 0.05, compared with vehicle (Veh).
Discussion
Data from the present study demonstrate that YL‐0919 displayed partial 5‐HT1A receptor agonist properties, producing a greater impact on extracellular 5‐HT levels than conventional SSRIs (FLX), as well as significant antidepressant and anxiolytic effects. Furthermore, YL‐0919 treatment rapidly influenced the synaptic plasticity (enhancing LTP) of rats. Finally, at doses close to those producing antidepressant‐like effects, YL‐0919 did not result in a marked inhibition of sexual function.
In the present study, we first examined the ability of YL‐0919 to promote [35S]‐GTPγS binding in the rat hippocampus and found that the maximal efficacy of YL‐0919 was 85% of that induced by 8‐OH‐DPAT (a full 5‐HT1A agonist), which was slightly greater than that exhibited by vilazodone (76%), These values suggest that YL‐0919 is a high‐efficacy partial agonist of 5‐HT1A receptors.
The results obtained using the rapid and validated HPLC‐MS method coupled with microdialysis showed that the acute administration of YL‐0919 dose‐dependently increased the extracellular levels of 5‐HT in the rat ventral hippocampus to a greater extent than FLX. The augmentation of extracellular 5‐HT achieved by the acute administration of YL‐0919 demonstrated that vilazodone has 5‐HT neurotransmission‐enhancing potential. The combined inhibition of the 5‐HT transporter with partial agonist effects at the 5‐HT1A autoreceptor of YL‐0919 may account, at least in part, for the augmentation of extracellular 5‐HT. A possible mechanism by which the acute administration of YL‐0919 enhanced 5‐HT neurotransmission is that YL‐0919 rapidly desensitized 5‐HT1A autoreceptors (partial agonists of YL‐0919) and enhanced serotonergic activity, in part due to the robust blockade of the 5‐HT transporter (SERT). However, further work is needed to investigate this hypothesis. In addition, no significant changes in the extracellular levels of dopamine or NA were found after acute YL‐0919 treatment, which is consistent with the results, suggesting that YL‐0919 displayed low affinities for dopamine transporter (DAT) and noradrenaline transporter (NET).
In the present study, we found that pretreatment with YL‐0919 could potentiate the 5‐HTP‐induced head‐twitch response. Although 5‐HTP‐induced head twitches are known to be mainly mediated by the 5‐HT2A receptor, the elevated 5‐HT levels in the brains of mice induced by several types of antidepressants (e.g. fluoxetine and moclobemide) have been reported to increase the number of head twitches (Nakamura et al., 1976). Furthermore, our previous study showed that except for the 5‐HT1A receptor and SERT, YL‐0919 showed no affinity for the 5‐HT2A receptor (Chen et al., 2013). However, because several 5‐HT receptor subtypes exist that show different sensitivities to 5‐HT, increasing the endogenous levels of 5‐HT by YL‐0919 should indirectly stimulate 5‐HT2A activity. Thus, the behavioural effects of YL‐0919 may occur via an indirect mechanism. Antagonism against high‐dose apomorphine‐induced hypothermia is considered an indicator of marked noradrenergic antidepressant activity (Puech et al., 1981). The data presented here demonstrated that a single YL‐0919 administration had no effect on high‐dose apomorphine (16 mg·kg−1)‐induced hypothermia, indicating that the NA system was not related to the antidepressant effect of YL‐0919. This finding is consistent with the results obtained in the microdialysis experiment reported above.
To date, the learned helplessness model has been demonstrated by different laboratories to resemble the withdrawn and passive behaviour exhibited by patients with depression. Animals were exposed to inescapable foot‐shock and then tested for shuttle‐escape performance in a two‐way shuttle box (Anisman and Merali, 2001; Chourbaji et al., 2005). Using this model, we investigated the antidepressant‐like effect of YL‐0919. This study demonstrated that subchronic treatment with YL‐0919 caused an antidepressant effect in this animal model, as indicated by significant decreases in the number of failures and escape latency relative to those exhibited by control mice. This finding is similar to that obtained with a first‐line SSRI antidepressant (FLX). In addition, the dosage range of YL‐0919 in this animal model was similar to that in a rat model of chronic unpredictable stress (Chen et al., 2013). Most SSRIs have also been reported to exert anxiolytic effects in several animal models of anxiety (Handley, 1995). Evidence suggests that the anxiolytic effects of 5‐HT receptor agonists are mediated by activating the 5‐HT1A autoreceptors directly in the raphe nucleus (Jolas et al., 1995). Based on these reports, YL‐0919 may reduce anxiety in animal models. Our results from behavioural studies demonstrated that YL‐0919 exerted anxiolytic effects in the Vogel drinking and elevated plus‐maze tests; these results were consistent with those obtained with vilazodone, which displayed anxiolytic effects in the shock‐probe test over a specific dosage range (Treit et al., 2001).
To rule out possible false‐positive activity attributable to YL‐0919, locomotor activity was assessed in our previous study (Chen et al., 2013). YL‐0919 was not found to influence locomotor activity at the effective doses, suggesting that the antidepressant‐like effect of YL‐0919 was unlikely to be attributable to a change in basal locomotor activity.
Activation of the somatodendritic 5‐HT1A receptors has been widely suggested to indirectly reduce serotonergic transmission in the raphe and consequently reduce 5‐HT release. This decrease is thought to be responsible for the delay in the onset of the therapeutic action of SSRIs (Stassen et al., 1996; Stuart et al., 2015).
SSRIs take several weeks to become clinically effective, and this delayed onset may be partly due to the activation of the somatodendritic 5‐HT1A autoreceptors, which indirectly reduce serotonergic transmission in the dorsal raphe. In support of this, SPARIs may theoretically shorten the onset of antidepressant‐like efficacy (Hogg and Dalvi, 2004). Chronic, but not acute, treatment with antidepressants reduced hyponeophagia (Sartori et al., 2012; Duman and Newton, 2013); thus, the NSF test is a sensitive and reliable method to detect the time course of antidepressant efficacy. In the present study, subchronic (7 days) and chronic (21 days) treatment with YL‐0919 contributed to reduced feeding latency, while subchronic administration of FLX (7 days) did not affect the feeding latency. All of these results indicated that YL‐0919 had a faster onset than conventional SSRIs (FLX).
More recent studies have focused on long‐term effects on hippocampal neuroplasticity and neurogenesis as common mechanisms for the delayed action of antidepressants (Racagni and Popoli, 2008). In addition, hippocampal LTP is thought to be one of the primary methods for investigating the cellular mechanisms underlying synaptic plasticity. The depletion of 5‐HT or the 5‐HT1A antagonist WAY‐100635 has been reported to attenuate perforant path‐dentate gyrus LTP (Wheal et al., 1983), whereas 8‐OH‐DPAT (5‐HT1A agonist) increased LTP (Hjorth et al., 1997). Furthermore, recent studies have demonstrated that activating the 5‐HT1A receptors or 5‐HT release facilitated LTP via a phasic attenuation of GABAergic inhibition.
Based on these factors, we examined the effects of YL‐0919 on LTP in the LPP‐granule cell synapses of the rat dentate gyrus. We found that treatment with FLX for 21 days facilitated LTP, whereas treatment for 7 days did not affect hippocampal LTP. In contrast, rats treated with YL‐0919 for 7 and 21 days showed enhanced LTP, consistent with the onset latency of behavioural effects (NSF test). All of these results suggested that YL‐0919 rapidly influenced synaptic plasticity, although further work is needed to clarify the exact molecular mechanisms.
Sexual dysfunction is the most common side effect of repeated treatment with SSRIs and is one of the leading causes of nonadherence to treatment (Baldwin and Foong, 2013; Graf et al., 2014). In studies in both humans and animals, increased 5‐HT levels (e.g. after SSRI treatment) have been demonstrated to inhibit sexual function (Snoeren et al., 2014a,b). In addition, preclinical studies have indicated that 5‐HT1A receptor agonists facilitate sexual performance in male rats (Giuliano and Clement, 2006; Waldinger, 2006). Different behavioural methods, including paired mating studies, intracavernous pressure assessments and noncontact penile erection measurements in sexually experienced male rats, have been employed to model SSRI‐induced sexual dysfunction (Sukoff Rizzo et al., 2008).
Based on these findings, the current study was designed to assess the possible side effects of YL‐0919 on sexual function in sexually experienced male rats. Unlike FLX, YL‐0919 and vilazodone, at doses close to those producing antidepressant‐like effects preclinically, did not result in a marked inhibition of sexual function sensitization. This result suggests that YL‐0919 may be a promising antidepressant that does not cause the sexual impairment that is typically associated with conventional SSRIs.
In conclusion, our report presents a novel small‐molecule compound, YL‐0919, that acts as both an SSRI and a partial 5‐HT1A receptor agonist and is likely a fast‐onset, potent antidepressant with reduced sexual dysfunction side effects compared with SSRIs alone. Our findings provide useful insights into the development of antidepressants with good efficacy profiles and without the side effect of sexual dysfunction.
Author contributions
L.‐M.Z. designed the research, analysed the data and wrote the manuscript. N.Z., Y.‐Q.L., Y.‐H.R. and Y.‐L.W. performed the behavioural tests. X.‐Y.W. and X.‐X.H. participated in investigating the molecular and cellular mechanisms of YL‐0919. R.‐F.Y. performed the chemical synthesis of YL‐0919. Y.‐Z.Z. and Y.‐F.L. contributed to the research design, data analysis and manuscript revision.
Conflict of interest
The authors declare no conflicts of interest.
Declaration of transparency and scientific rigour
This Declaration acknowledges that this paper adheres to the principles for transparent reporting and scientific rigour of preclinical research recommended by funding agencies, publishers and other organisations engaged with supporting research.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (nos 81102423, 81302761, 81001653 and 81173036) and the Beijing Natural Science Foundation (no. 7164290). The content is solely the responsibility of the authors and may not represent the official views of the National Natural Science Foundation of China. The funders had no role in the study design, data collection or analysis; the decision to publish; or the preparation of the manuscript.
Zhang, L.‐M. , Wang, X.‐Y. , Zhao, N. , Wang, Y.‐L. , Hu, X.‐X. , Ran, Y.‐H. , Liu, Y.‐Q. , Zhang, Y.‐Z. , Yang, R.‐F. , and Li, Y.‐F. (2017) Neurochemical and behavioural effects of hypidone hydrochloride (YL‐0919): a novel combined selective 5‐HT reuptake inhibitor and partial 5‐HT1A agonist. British Journal of Pharmacology, 174: 769–780. doi: 10.1111/bph.13675.
References
- Agmo A (1997). Male rat sexual behavior. Brain Res Brain Res Protoc 1: 203–209. [DOI] [PubMed] [Google Scholar]
- Alexander SPH, Davenport AP, Kelly E, Marrion N, Peters JA, Benson HE et al. (2015a). The Concise Guide to PHARMACOLOGY 2015/16: G protein‐coupled receptors. Br J Pharmacol 172: 5744–5869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Kelly E, Marrion N, Peters JA, Benson HE, Faccenda E et al. (2015b). The Concise Guide to PHARMACOLOGY 2015/16: Transporters. Br J Pharmacol 172: 6110–6202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anisman H, Merali Z (2001). Rodent models of depression: learned helplessness induced in mice. Curr Protoc Neurosci Chapter 8: Unit 8 10C. [DOI] [PubMed] [Google Scholar]
- Artigas F, Celada P, Laruelle M, Adell A (2001). How does pindolol improve antidepressant action? Trends Pharmacol Sci 22: 224–228. [DOI] [PubMed] [Google Scholar]
- Baldwin DS, Foong T (2013). Antidepressant drugs and sexual dysfunction. Br J Psychiatry 202: 396–397. [DOI] [PubMed] [Google Scholar]
- Chen HX, Jin ZL, Zhang LM, Xue R, Xu XD, Zhao N et al. (2013). Antidepressant‐like activity of YL‐0919: a novel combined selective serotonin reuptake inhibitor and 5‐HT1A receptor agonist. PLoS One 8: e83271. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Choi E, Zmarlicka M, Ehret MJ (2012). Vilazodone: a novel antidepressant. Am J Health Syst Pharm 69: 1551–1557. [DOI] [PubMed] [Google Scholar]
- Chourbaji S, Zacher C, Sanchis‐Segura C, Dormann C, Vollmayr B, Gass P (2005). Learned helplessness: validity and reliability of depressive‐like states in mice. Brain Res Brain Res Protoc 16: 70–78. [DOI] [PubMed] [Google Scholar]
- Culpepper L, Davidson JR, Dietrich AJ, Goodman WK, Kroenke K, Schwenk TL (2004). Suicidality as a possible side effect of antidepressant treatment. Prim Care Companion J Clin Psychiatry 6: 79–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis MJ, Bond RA, Spina D, Ahluwalia A, Alexander SP, Giembycz MA et al. (2015). Experimental design and analysis and their reporting: new guidance for publication in BJP. Br J Pharmacol 172: 3461–3471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duman CH, Newton SS (2013). Evaluating effects of EPO in rodent behavioral assays related to depression. Methods Mol Biol 982: 127–140. [DOI] [PubMed] [Google Scholar]
- Fischer W, Muller M (1984). Antiserotonergic effect of antidepressives in L‐5‐HTP‐induced head shaking behavior in the rat. Pharmazie 39: 277–278. [PubMed] [Google Scholar]
- Giuliano F, Clement P (2006). Serotonin and premature ejaculation: from physiology to patient management. Eur Urol 50: 454–466. [DOI] [PubMed] [Google Scholar]
- Graf H, Walter M, Metzger CD, Abler B (2014). Antidepressant‐related sexual dysfunction – perspectives from neuroimaging. Pharmacol Biochem Behav 121: 138–145. [DOI] [PubMed] [Google Scholar]
- Handley SL (1995). 5‐Hydroxytryptamine pathways in anxiety and its treatment. Pharmacol Ther 66: 103–148. [DOI] [PubMed] [Google Scholar]
- Hjorth S, Auerbach SB (1996). 5‐HT1A autoreceptors and the mode of action of selective serotonin reuptake inhibitors (SSRI). Behav Brain Res 73: 281–283. [DOI] [PubMed] [Google Scholar]
- Hjorth S, Westlin D, Bengtsson HJ (1997). WAY100635‐induced augmentation of the 5‐HT‐elevating action of citalopram: relative importance of the dose of the 5‐HT1A (auto)receptor blocker versus that of the 5‐HT reuptake inhibitor. Neuropharmacology 36: 461–465. [DOI] [PubMed] [Google Scholar]
- Hogg S, Dalvi A (2004). Acceleration of onset of action in schedule‐induced polydipsia: combinations of SSRI and 5‐HT1A and 5‐HT1B receptor antagonists. Pharmacol Biochem Behav 77: 69–75. [DOI] [PubMed] [Google Scholar]
- Jolas T, Schreiber R, Laporte AM, Chastanet M, De Vry J, Glaser T et al. (1995). Are postsynaptic 5‐HT1A receptors involved in the anxiolytic effects of 5‐HT1A receptor agonists and in their inhibitory effects on the firing of serotonergic neurons in the rat? J Pharmacol Exp Ther 272: 920–929. [PubMed] [Google Scholar]
- Kilkenny C, Browne W, Cuthill IC, Emerson M, Altman DG (2010). Animal research: reporting in vivo experiments: the ARRIVE guidelines. Br J Pharmacol 160: 1577–1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laughren TP, Gobburu J, Temple RJ, Unger EF, Bhattaram A, Dinh PV et al. (2011). Vilazodone: clinical basis for the US Food and Drug Administration's approval of a new antidepressant. J Clin Psychiatry 72: 1166–1173. [DOI] [PubMed] [Google Scholar]
- Lindsey WT (2011). Vilazodone for the treatment of depression. Ann Pharmacother 45: 946–953. [DOI] [PubMed] [Google Scholar]
- Lu W, Dong HJ, Bi GH, Zhao YQ, Yang Z, Su RB et al. (2010). Effect of agmatine on long‐term potentiation in morphine‐treated rats. Pharmacol Biochem Behav 96: 125–129. [DOI] [PubMed] [Google Scholar]
- McGrath JC, Lilley E (2015). Implementing guidelines on reporting research using animals (ARRIVE etc.): new requirements for publication in BJP. Br J Pharmacol 172: 3189–3193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura M, Fukushima H, Kitagawa S (1976). Effects of amitriptyline and isocarboxazid on 5‐hydroxytryptophan induced head twitches in mice. Psychopharmacology (Berl) 48: 101–104. [DOI] [PubMed] [Google Scholar]
- Odagaki Y, Toyoshima R (2005). Detailed pharmacological characterization of 5‐HT1A‐receptor‐mediated [35S]GTP gamma S binding in rat hippocampal membranes. J Pharmacol Sci 98: 66–76. [DOI] [PubMed] [Google Scholar]
- Owen RT (2011). Vilazodone: a new treatment option for major depressive disorder. Drugs Today (Barc) 47: 531–537. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson CR, Emson PC (1980). AChE‐stained horizontal sections of the rat brain in stereotaxic coordinates. J Neurosci Methods 3: 129–149. [DOI] [PubMed] [Google Scholar]
- Plenge P, Mellerup ET (2003). Pindolol and the acceleration of the antidepressant response. J Affect Disord 75: 285–289. [DOI] [PubMed] [Google Scholar]
- Puech AJ, Chermat R, Poncelet M, Doare L, Simon P (1981). Antagonism of hypothermia and behavioral response to apomorphine: a simple, rapid and discriminating test for screening antidepressants and neuroleptics. Psychopharmacology (Berl) 75: 84–91. [DOI] [PubMed] [Google Scholar]
- Qin JJ, Chen HX, Zhao N, Yuan L, Zhang YZ, Yang RF et al. (2014). The role of activation of the 5‐HT1A receptor and adenylate cyclase in the antidepressant‐like effect of YL‐0919, a dual 5‐HT1A agonist and selective serotonin reuptake inhibitor. Neurosci Lett 582: 104–108. [DOI] [PubMed] [Google Scholar]
- Racagni G, Popoli M (2008). Cellular and molecular mechanisms in the long‐term action of antidepressants. Dialogues Clin Neurosci 10: 385–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sartori SB, Whittle N, Hetzenauer A, Singewald N (2012). Magnesium deficiency induces anxiety and HPA axis dysregulation: modulation by therapeutic drug treatment. Neuropharmacology 62: 304–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz TL, Siddiqui UA, Stahl SM (2011). Vilazodone: a brief pharmacological and clinical review of the novel serotonin partial agonist and reuptake inhibitor. Ther Adv Psychopharmacol 1: 81–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snoeren EM, Veening JG, Olivier B, Oosting RS (2014a). Serotonin 1A receptors and sexual behavior in female rats: a review. Pharmacol Biochem Behav 121: 43–52. [DOI] [PubMed] [Google Scholar]
- Snoeren EM, Veening JG, Olivier B, Oosting RS (2014b). Serotonin 1A receptors and sexual behavior in male rats: a review. Pharmacol Biochem Behav 121: 102–114. [DOI] [PubMed] [Google Scholar]
- Southan C, Sharman JL, Benson HE, Faccenda E, Pawson AJ, Alexander SP et al. (2016). The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. Nucl Acids Res 44: D1054–D1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahl SM (2014). Mechanism of action of the SPARI vilazodone: serotonin 1A partial agonist and reuptake inhibitor. CNS Spectr 19: 105–109. [DOI] [PubMed] [Google Scholar]
- Stassen HH, Angst J, Delini‐Stula A (1996). Delayed onset of action of antidepressant drugs? Survey of results of Zurich meta‐analyses. Pharmacopsychiatry 29: 87–96. [DOI] [PubMed] [Google Scholar]
- Stuart SA, Butler P, Munafo MR, Nutt DJ, Robinson ES (2015). distinct neuropsychological mechanisms may explain delayed‐ versus rapid‐onset antidepressant efficacy. Neuropsychopharmacology 40: 2165–2174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sukoff Rizzo SJ, Schechter LE, Rosenzweig‐Lipson S (2008). A novel approach for predicting antidepressant‐induced sexual dysfunction in rats. Psychopharmacology (Berl) 195: 459–467. [DOI] [PubMed] [Google Scholar]
- Tollefson GD (1991). Antidepressant treatment and side effect considerations. J Clin Psychiatry 52 (Suppl): 4–13. [PubMed] [Google Scholar]
- Treit D, Degroot A, Kashluba S, Bartoszyk GD (2001). Systemic EMD 68843 injections reduce anxiety in the shock‐probe, but not the plus‐maze test. Eur J Pharmacol 414: 245–248. [DOI] [PubMed] [Google Scholar]
- Waldinger MD (2006). Emerging drugs for premature ejaculation. Expert Opin Emerg Drugs 11: 99–109. [DOI] [PubMed] [Google Scholar]
- Wheal HV, Lancaster B, Bliss TV (1983). Long‐term potentiation in Schaffer collateral and commissural systems of the hippocampus: in vitro study in rats pretreated with kainic acid. Brain Res 272: 247–253. [DOI] [PubMed] [Google Scholar]
- Yong Z, Yan L, Dong Z, Wang X, Su R, Gong Z (2013). The effect of chronic thienorphine administration on long‐term potentiation and synaptic structure in rat hippocampus. Synapse 67: 779–785. [DOI] [PubMed] [Google Scholar]


