Plasmacytoid dendritic cells (pDCs) are one of two principal subsets of human dendritic cells (DCs). pDCs were initially identified in pathological specimens of reactive or neoplastic lymph nodes (LNs), in close association with high endothelial venules (HEVs) (44). Their plasma cell-like morphology and localization led to their designation as “plasmacytoid T cells,” or “plasmacytoid monocytes” (reviewed in reference 31). Subsequently, these cells were found to correspond to a subset of circulating blood DCs, an immature CD11c− population, now referred to as pre-pDCs (an immediate precursor of pDCs), and to be distinct from “conventional” myeloid CD11c+ DCs (mDCs) (60). Pre-pDCs express CD4 but lack T-cell receptor alpha (TCRα), TCRβ, TCRγ, TCRδ, or CD3 chains. Furthermore, they do not express B-cell lineage (CD19, CD21) or myeloid (CD13, CD14, CD33) markers. Pre-pDCs typically mature and produce large amounts of alpha/beta interferons (IFN-α/β) in response to viral and bacterial stimuli (76). This property further identified them as the enigmatic “natural type I IFN-producing cell” in blood (25, 51, 131), a rare CD4+, MHC class II+ cell with potential to secrete significant amounts of IFN-α (1, 29, 48). pDCs display an antigen-presenting function, thereby firmly establishing them as members of the DC family. While mDCs comprise several subsets of DCs, including Langerhans cells (LCs), dermal dendritic cells, and interstitial DCs, pDCs have not yet been segregated into subpopulations.
Recently, several groups simultaneously identified the murine pDC equivalent as a CD11c lo-int, B220+, IFN-α/β-producing cell in spleen, LNs, thymus (7, 17, 105, 109), and blood (110). Murine pDCs differ from their human counterparts in expressing low to intermediate levels of CD11c, with lymphoid tissue pDCs also expressing CD4 and/or CD8α. Furthermore, murine pDCs from the spleen, but not blood, secrete interleukin-12 (IL-12) (p70) after stimulation with Staphylococcus aureus Cowan (SAC), in contrast to human pDCs (17).
ORIGIN AND PRECURSORS
For many reasons, pDCs were considered to derive from a lineage (a common lymphoid progenitor) distinct from that of mDCs. Human blood pre-pDCs lack most myeloid markers and have growth requirements distinct from those of mDCs. They express CD123 (IL-3R) and depend upon IL-3 but not granulocyte-macrophage colony-stimulating factor (GM-CSF) to differentiate into immature pDCs (60). In the thymus, pre-pDCs express pre-TCRα, which after assembly with the TCRβ chain forms a pre-TCR. Transfection of fetal liver CD34+, CD38− precursors with Id2 and Id3 blocks their differentiation into T cells, B cells, and pDCs but not NK cells or myeloid cells (136). Furthermore, in the absence of IFN consensus sequence binding protein ICSBP/IRF-8 there is a failure in the development of pDCs and but not certain myeloid DC subsets (143, 144). However, the concept that pDCs arise from a specific lymphoid lineage has been challenged by studies with mice showing that a common CD11c+ DC precursor in blood can develop into both pDCs and mDCs (CD8α+ and CD8α− subsets) (142). In addition, murine pDCs and mDCs can be generated from both common lymphoid and myeloid progenitors following their transfer into irradiated recipients (152). Further studies are required for confirmation, and it remains to be seen whether there is a dual contribution of myeloid and lymphoid differentiation pathways in the generation of pDCs that reside in different organs (32, 108). Moreover, we do not know whether pre-pDCs derive from progenitors in the bone marrow, from resident precursors in peripheral organs, as recently demonstrated for Langerhans cells in the skin (101), from precursors recruited in response to infection or inflammation, or all three.
DIFFERENTIATION FROM PRECURSORS AND ISOLATION
FLT-3 ligand (FLT-3L) is a key differentiation factor for pDCs from hematopoietic progenitor cells (HPCs). Its injection in vivo dramatically increases the numbers of both mDCs and pDCs in human blood (98, 116) and in the blood, lymphoid tissues, liver, and lungs of mice (97, 117, 118, 129). In vitro, FLT-3L and thrombopoietin synergistically induce the generation of large numbers of pDCs, in addition to CD11c+ immature DCs and CD14+ monocytes from human fetal liver-derived or blood HPCs (57). pDCs, which comprise <1% of total peripheral blood mononuclear cells (PBMCs), can also be directly isolated from blood through removal of lineage-positive cells and selection of CD123+ or CD11c−, CD4+, DR+ cells (60). The identification of two markers on pDCs (BDCA-2 and BDCA-4) has facilitated their isolation from PBMCs by positive selection with magnetic-bead-coupled monoclonal antibodies to BDCA-4 (Miltenyi Biotec, Bergisch Gladbach, Germany). In contrast to pDCs, human mDCs can be generated from either CD34+ HPCs with GM-CSF, c-kit ligand and tumor necrosis factor alpha (TNF-α) (23), or monocytes with GM-CSF and IL-4/IL-13 (12, 21, 22, 57, 124).
LOCALIZATION AND TRAFFICKING PATTERNS
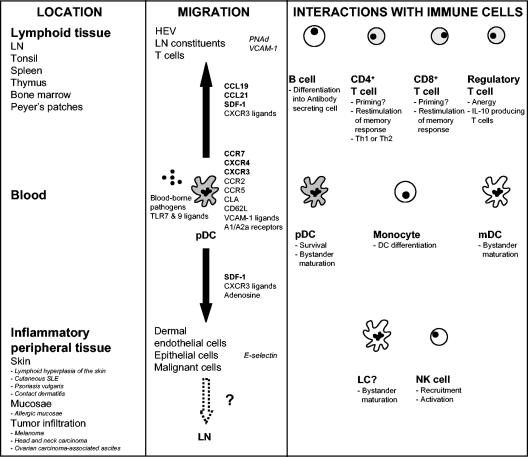
In humans, pDCs circulate in the blood of adults and neonates (107, 134) and can be located in lymphoid tissues (LNs, tonsils, spleen, thymus, bone marrow, and Peyer's patches) and certain peripheral tissues (fetal liver). pDCs accumulate in inflammatory sites, e.g., lymphoid hyperplasia of the skin (42), cutaneous systemic lupus erythematosus (SLE), psoriasis vulgaris (basal epidermis and papillary dermis, but not normal skin), contact dermatitis, and allergic mucosa (151). pDCs (identified either as CD123+ or BDCA-2+ cells) also infiltrate primary and malignant melanoma-, head and neck carcinoma-, and ovarian carcinoma-associated ascites (61, 122, 148, 157). Recruitment into these sites suggests that pDCs may contribute to the ongoing inflammatory response through release of cytokines and chemokines and activation of lymphocytes (153) or, alternatively, to the induction of tolerogenic responses (157).
How do pDCs enter LNs and inflammatory sites? Chemokines are important regulators of DC trafficking in vivo. Similar to mDCs, blood pre-pDCs after activation with microbial stimuli or CD40 ligation undergo maturation and upregulate functional CCR7, thereby acquiring responsiveness towards SLC (CCL19) and ELC (CCL21) expressed by HEVs and LN constituents (25, 72). Furthermore, pDCs express L-selectin (CD62L), which recognizes corresponding ligands (peripheral lymph node addressin [PNAd]) on HEVs. These observations may account for the localization of pDCs around HEVs and in T-cell-rich areas of LNs. pDCs also express ligands for VCAM-1, an inducible molecule on endothelial cells which may enhance migration to draining nodes (157).
Pre-pDCs express several additional chemokine receptors, e.g., CCR2, CCR5, and CXCR3 (112, 146). Unlike mDCs, however, they marginally respond to the corresponding ligands (MCP-1; RANTES, MIP-1α, and MIP-1β; and Mig [CXCL9], IP-10 [CXCL10], and I-TAC [CXCL11], respectively). Instead, they migrate efficiently to the CXCR4 ligand SDF-1/CXCL12, which is expressed on dermal endothelial cells, in LN HEVs, and in malignant cells (157). However, CXCR3 ligands produced by Th1 cells, while relatively inactive on their own, can enhance the responsiveness of pre-pDCs to SDF-1 20- to 50-fold (25, 146). During microbial infection or inflammation, the induction of CXCR3 ligands might drive the recruitment of immature pDCs to sites of SDF-1 production. In tonsils and in psoriatic skin, epithelial cells expressing SDF-1 have been associated with the expression of CXCR3 ligands (146). Interestingly, pDCs express CLA, which binds to E-selectin on dermal endothelial cells and which may enhance their recruitment to cutaneous inflammatory lesions (8). Once differentiated, however, pDCs lose their responsiveness to SDF-1 (112).
Adenosine has recently been identified as a potent chemotactic factor for immature pDCs via an A1 receptor-mediated mechanism. Upon maturation, the receptor is downregulated, resulting in a loss of migratory function. In turn, the A2a receptor is upregulated, through which adenosine reduces the production of proinflammatory cytokines. Thus, adenosine, in addition to SDF-1 and CXCR3 ligands, may recruit immature pDCs from blood to inflammatory sites but subsequently limit their contribution to an inflammatory response upon maturation after an encounter with virus, bacteria, or activated T cells (127).
Maturation “locally” would upregulate CCR7, allowing pDCs to migrate to LNs in response to CCL19 and CCL21 and resist apoptosis (125). At this site, pDCs could potentially present antigens they have acquired peripherally to T cells. Recently, IL-18 produced by mDCs in inflamed sites was shown to attract pre-pDCs and modulate their function to skew Th cells towards Th1 cells (78).
ACTIVATION OF pDCs BY PATHOGENS
Pathogens which activate human pDCs.
Virtually all cell types are able to produce IFN-α/β in response to viral exposure. The amounts, kinetics, and types depend to a large extent on the cell type, with pDCs being the “professional IFN-α/β-producing cells” (30, 74). IFN-α/βs are produced by pDCs in response to a wide range of enveloped viruses, including herpes simplex virus (HSV), Sendai virus, human immunodeficiency virus type 1 (HIV-1), influenza virus, Newcastle disease virus, and vesicular stomatitis virus, as well as parasites (Plasmodium falciparum) (114), bacteria (e.g., SAC) (137), and DNA containing unmethylated CpG sequences, typical of microbial DNA (10, 25, 76, 131). More than 1 × 10e5 to 2 × 10e5 IU/10e6 (0.1 to 0.2 million IU/million cells), or the equivalent of 10 pg/cell, can be produced, making the pDC the most efficient producer of IFN-α (monocytes are at least 10-fold less efficient) (47). It is important to note that pDCs are not activated to produce IFN-α by all viruses, do not need to be infected, and may even respond differently depending on the virus. For example, in mice, pDCs are the main IFN-α/β, IL-12, and TNF-α producers in response to murine cytomegalovirus (MCMV) but not lymphocytic choriomeningitis virus (33, 34). To activate pDCs, viruses need not to be replication competent (UV irradiated or chemically inactivated virus is sufficient), but envelope structure should remain intact. IFN-α/βs induce MxA, an IFN-α-inducible intracellular protein well established as a surrogate marker for local IFN-α production (148), oligoadenylate synthetase, and double-stranded RNA (dsRNA)-dependent protein kinase (PKR), which impart cellular resistance, inhibit viral replication, and block viral spread. IFN-α/βs also modulate several aspects of the immune response, including pDC survival, mDC differentiation (104), DC-mediated Th1 and CD8+ T-cell responses, cross presentation and cross priming (87), upregulation of costimulatory and MHC molecules, activation of NK cells, and induction of primary antibody responses (88).
pDCs produce a diverse array of cytokines and chemokines following activation with pathogens or CpG oligodeoxynucleotides (ODNs) e.g., TNF-α, IL-1, and/or IL-6. Murine, but not human, pDCs have the capacity to synthesize bioactive IL-12, although this capacity remains controversial (39, 82, 127). Depending upon the activation stimulus, pDCs produce CCL3 (MIP-1α), CCL4 (MIP-1β), CCL5 (RANTES), CXCL8 (IL-8), and CXCL10 (IP-10). The latter attracts CXCR3 bearing Th1 polarized cells, while CCL4 recruits NK cells (100). Therefore, pDCs can facilitate leukocyte homing into their local environment.
Regulation of IFN synthesis.
pDCs express a broader profile of IFNA genes than other antigen-presenting cells (APCs) and respond to a wider range of viral and nonviral stimuli in synthesizing IFN-α, and more rapidly. In humans, the IFN family consists of 13 IFN-α subtypes, IFN-β, and IFN-ω. IFN-α1 is the major subtype expressed by pDCs, but other forms, including IFN-α2, -α5, -α8, -α10, and -α14 and a newly described family of IFN-λ1-3 (30, 71), are also elicited. Interferon regulatory factors (IRFs) play an important role in the regulation of IFN-α/β gene transcription. Nine cellular IRFs have been identified, and three of these IRFs are direct transducers of virus-mediated signaling. Expression of IRF-3 is sufficient to support induction of IFN-β, while IRF-5 or IRF-7 is required for stimulation of IFN-α gene expression. In general, pDCs constitutively express most of the IRF genes and higher levels of IRF-5 and IRF-7 mRNA and IRF-7 protein than monocytes (71). The high constitutive levels of IRF-7 in pDCs that are available for activation by virus is thought to contribute to rapid IFN-α synthesis (79, 139). While the precise pathways of IRF-7 activation are unknown, the nuclear translocation of IRF-7 by CpG DNA is preceded by p38 mitogen-activated protein-kinase-dependent phosphorylation of STAT-1 (79, 139).
In mDCs, the transcriptional induction for genes IFNA and IFNB is also dependent on various mechanisms, including tropism of virus (35). Activation of TLR3 with poly(I:C), dsRNA, or RNA viruses, like influenza virus, induces mDC maturation (upregulation of costimulatory molecules and CD83), induction of IFNA and IFNB genes, and IL-12 (35, 68, 93). mDCs can also be activated to produce IFN-α/βs through a TLR3-, MyD88-independent pathway which requires the introduction of dsRNA into the cytosol (mimicking viral infection), PKR activation, and phosphorylation of IRF-3 and IRF-7 (36). Finally, there is evidence for a PKR-independent pathway that results in the induction of IFN-α/β (36, 68, 133).
TLRs.
How are pDCs activated by viruses or bacteria? One pathway involves Toll-like receptors (TLRs), a family of 11 pattern recognition receptors which mediate the recognition of many pathogens through the detection of distinct pathogen-associated molecular patterns (PAMPs) (73, 140). pDCs and mDCs each have a different TLR expression profile (Fig. 1). In humans, mDCs can express TLR1, -2, -3, -4, -5, -7, and -8, while pDCs express TLR7 and -9 (67, 82). TLR7, -8, and -9 belong to a functional subfamily and detect PAMPs in endosomal/lysosomal compartments following acidification (64, 86). Following exposure to synthetic TLR7 or TLR9 agonists (e.g., imidazoquinoline compounds or guanosine analogs for TLR7/8, CpG ODN for TLR9), pDCs secrete IFN-α and proinflammatory cytokines (IL-8 and TNF-α) and undergo maturation, a differentiation program characterized by upregulation of the costimulatory molecules CD80, CD86, and CD40, expression of functional CCR7 and the maturation marker CD83, and heightened T-cell stimulatory capacity (2, 67, 70, 94). TLR expression may itself be modulated in response to IFN-α/β, TNF-α, and virus exposure (3, 102).
FIG. 1.
Human blood mDCs and pDCs differ in their expression of TLRs and CLRs. The repertoire of CLRs expressed by pDCs includes BDCA-2, dectin-1, and possibly DEC-205. The functions of these CLRs, especially BDCA-2, are largely unknown but may include antigen uptake and presentation. mDCs express DC-SIGN, DC-LAMP, mannose receptor, and DEC-205. It is well known that mDCs can rely on their CLRs for antigen uptake and viral transmission. pDCs and mDCs express different and complementary sets of TLRs. Recently, a few reports have demonstrated cross talks between CLRs and TLRs in mDCs. It has yet to be shown whether CLRs and TLRs interact either synergistically or competitively in pDCs.
Natural agonists for murine TLR7 have recently been identified and include the single-stranded RNA (ssRNA) viruses vesicular stomatitis virus and influenza virus (35, 96). Guanosine- and uridine-rich ssRNA, including RNA derived from the HIV-1 U5 region, are also recognized by murine TLR7 and human TLR8 (65). In response to short ssRNA segments of 20 nucleotides, human CD123+ DCs secrete IFN-α, while CD11c+ cells produce IL-12p40, IL-6, and TNF-α (65). TLR7 recognition of ssRNA viruses appears to require an intact endocytic pathway, induces MyD88 adaptor protein-dependent signaling via the Toll-IL-1 receptor pathway, and is essential for optimal production of IFN-α in vivo (96). These results are consistent with observations that influenza virus and HIV-1 mature human pDCs (upregulation of costimulatory molecules, CD83, and CCR7), and induce production of IFN-α (49, 50, 154).
TLR9 agonists include synthetic CpG ODNs (10, 63, 77, 81, 82). Interestingly, the response of human pDCs is dependent upon the class of CpG ODN used to stimulate them. Stimulation with CpGA (D)/2216 ODN induces sustained high IFN-α production by pDCs but minimal upregulation of cell surface maturation markers CD80, CD86, and major histocompatibility complex class II (MHC-II) (66, 79, 81, 149) and has no effect on B cells (which also express TLR9). On the other hand, stimulation with CpGB (K)/2006, a strong B-cell activator, results in increased expression of costimulatory and antigen-presenting molecules and heightened IL-8 and TNF-α secretion but low levels of IFN-α production by pDCs. Two distinct pathways of IFN-α/β production have been identified regarding stimulation with CpGA versus CpGB (79). pDCs constitutively express IRF-7 and synthesize high levels of IFN-α in response to CpGA, which also triggers an autocrine feedback loop involving the IFN receptor-dependent pathway. In contrast, IFN-α/β induction by CpGB is independent of the IFN-α/β receptor loop (79, 139). Recently, a new class of CpG ODN, CpGC, in which structural elements of CpGA and CpGB have been combined, has emerged, and this sequence activates B cells and induces IFN-α production by pDCs (62). Furthermore, non-CpG-containing ODNs have been shown to bind human TLR9 (86) and to stimulate pDCs (43; our unpublished data).
The concept that microbial DNA could be a physiological activator of pDCs gained credence with the discovery that the genome of HSV-1 and -2 stimulated murine pDCs to produce IFN-α through a viral-replication-independent, TLR9/MyD88-dependent pathway (80, 95). Interestingly, mice lacking TLR9 or MyD88 were still able to control HSV-1 replication after local infection, suggesting that the immune system can compensate for the impaired function of the pDCs (80). Indeed, MyD88-independent and PKR-dependent or -independent pathways leading to IFN-α production by mDCs or pDCs have been described (35, 133), indicating that while TLR ligation is critical for inducing IFN-α pDC function, it is not essential. On the other hand, mice lacking TLR9 are impaired in their response to systemic MCMV infection (138).
A role for envelope-receptor interactions has been proposed as an additional mechanism by which IFN-α production is induced in pDCs. The cell binding component of the hemagglutinin-neuraminidase glycoprotein of paramyxovirus members must interact with sialic acid-expressing receptors on human natural IFN-producing cells to elicit IFN-α production (155). Similarly, envelope-deficient HIV-1 fails to elicit IFN-α production from pDCs (50). It remains to be clarified whether envelope-receptor interactions trigger signaling by themselves or whether they are needed to route and/or concentrate PAMPs to the appropriate compartment expressing TLRs. Further studies will be required to identify the various mechanisms and pathways utilized by pDC activators to elicit IFN-α production.
CLRs.
A diversity of C-type lectin receptors (CLRs) have been identified on DC subsets, including DC-SIGN (CD209), DEC-205 (CD205), langerin (CD207), mannose receptor (CD206), BDCA-2, and dectin-1 (Fig. 1). CLRs typically recognize carbohydrate-rich structures on microbes and self-antigens (55). They have been implicated in cell adhesion and regulation of signaling events (e.g., BDCA-2), migration and homing (e.g., DC-SIGN), antigen uptake and processing for MHC-II presentation to T cells (e.g., DC-SIGN, BDCA-2, langerin, and mannose receptor), cell-cell transmission of pathogens (e.g., DC-SIGN), and tolerance to self-antigens (e.g., DEC-205). pDCs express BDCA-2 and BDCA-4, dectin-1, and possibly DEC-205 but lack DC-SIGN and langerin, found on CD34+ and monocyte-derived DCs and LCs, respectively (147). The physiologic function of CLRs on pDCs remains unknown. Anti-BDCA-2 antibodies are rapidly internalized and efficiently presented to T cells, suggesting a role in antigen capture and presentation (41).
Interesting relationships between CLRs and TLRs have been documented. In mDCs, interaction of DC-SIGN with lipoarabinomannan secreted by mycobacteria inhibits lipopolysaccharide (LPS)-induced DC activation through TLR4 (56). This mechanism may permit pathogens to evade immune responses and perpetuate tolerance to self-antigens in the face of TLR activation by microbes. On the other hand, it has been shown that dectin-1 collaborates with TLR2 in inducing proinflammatory cytokine secretion in murine macrophages and DCs (53). Whether BDCA-2 has any connection to TLRs in pDCs remains to be seen. However, early reports have shown that induction of IFN secretion by pDCs in response to influenza virus (most likely triggering TLR7/8) or to complexes of plasmid DNA and anti-DNA antibodies (possibly stimulating both FcR and TLR9) is significantly inhibited by ligation of BDCA-2 with anti-BDCA-2 antibody (41). It is worth noting that BDCA-2 is downregulated after pDCs mature and that mature pDCs secrete less IFN-α/β in response to viral stimuli than immature pDCs do (16, 40, 131). BDCA-2 has an intracellular domain of 21 amino acids without known motifs implicated in signal transduction; however, ligation induces Src-family protein-tyrosine kinase-dependent intracellular calcium mobilization and protein-tyrosine phosphorylation of intracellular proteins (41). BDCA-4 (neuropilin-1) is also upregulated on blood mDCs after overnight culture and may participate in DC-lymphocyte interactions (141).
pDCs PROVIDE A LINK BETWEEN THE INNATE AND ADAPTIVE IMMUNE SYSTEMS
An emerging paradigm is a central role for pDCs in activating host innate and adaptive immune responses. Activation of pDCs results in the indirect and/or direct activation of many other cell types, e.g., monocytes, mDCs, B cells, NK cells, and T cells (Fig. 2).
FIG. 2.
The many fates of pDCs. pDCs play a central role in immunity, not restricted to their capacity to secrete IFN-α/β in response to viruses and induce an antiviral innate immune response. Upon encountering blood-borne pathogens, they can undergo maturation which can lead to the bystander maturation of surrounding pDCs and mDCs. Mature pDCs can migrate to LNs, where they activate antigen-specific B cells and CD4+ and CD8+ T cells. In response to inflamed tissue, pDCs can also traffic to the periphery. It is not clear whether pDCs reach the site of inflammation with an immature or mature phenotype. It has yet to be demonstrated whether interactions of immature pDCs with non-blood-borne pathogens in inflammatory tissue induce IFN-α production as well as maturation and migration to the draining LN to stimulate adaptive immune response.
pDC-APC interactions.
Interactions of pDCs with viruses have profound impacts on surrounding APCs and leukocytes, both positive and negative. For example, HIV-1 enhances pDC viability and induces the phenotypic maturation of pDC, upregulates CCR7, and leads to the production of IFN-α/β, TNF-α, and proinflammatory chemokines (50, 154). Virus-induced IFNs act as an autocrine survival factor, while TNF-α has a role in the maturation and differentiation of immature pDCs (76). Supernatants derived from HIV-1-stimulated pDCs mature bystander immature blood or monocyte-derived mDCs, which are not activated by themselves following HIV-1 exposure (50). The maturation is inhibited by antibodies to TNF-α, IFN-α/β, and IFN-α/β receptor. In response to these cytokines, blood mDCs also upregulate CCR7 and migrate towards CCL19. These findings provide one explanation for the depressed levels of DC subsets in the blood of untreated HIV-infected individuals, namely, enhanced migration to LNs. The exposure of pDCs to CpG sequences similarly leads to the bystander activation of human mDCs (61). IFN-α/β produced by pDCs in response to MCMV infection is also required for CD8α+ DC maturation in vivo (33) and for promoting CD8+ T-cell IFN-γ production (87, 106). The released IFNs control the accumulation, maturation, and cytokine production of DC subsets in lymphoid organs (33, 34).
pDCs amplify antimicrobial responses through IFN-α-mediated effects on monocytes. When monocytes are cultured with GM-CSF and IFN-α in place of IL-4, they differentiate into DCs with the capacity to drive a Th1 response that is independent of IL-12 and partially dependent on IFN-α (103). They also acquire TLR7 mRNA, CD123 and BDCA-4 expression, and responsiveness to TLR7 agonists and secrete IFN-α in response to HSV, although they do not express TLR9. CpG ODN induces pDC secretion of IFN-α, and other soluble factors secondarily induce purified monocytes to secrete high levels of the Th1 promoting chemokine IFN-γ-inducible protein 10 (IP-10) (18). Therefore, IFN-α, by influencing DC precursor differentiation, can affect the development of immune responses. In a reciprocal manner of regulation, IL-10 produced by monocytes stimulated with LPS can inhibit IFN-α production by activated pDCs (156). IL-10 can inhibit production of both IFN-α and IL-12 by pDCs stimulated with CD40L together with CpG (39). These data confirm a regulatory role for IL-10 in the control of virus- or bacterium-induced cytokine production by pDCs as previously suggested (54).
pDCs can also inhibit certain mDC functions, at least in mice. In MCMV-infected mice, pDCs are the primary source of IFN-α and IL-12, and the IFN-α/βs they produce inhibit the synthesis of IL-12 from mDCs (34).
B cells.
Following activation with viral antigens or CpG ODNs, pDCs secrete IFN-α/β, which, together with LPS and CD40 ligation, induces the expression of BAFF (a B-cell activating and survival factor) by mDCs in vitro (90). IFN-α and IL-6 secreted by influenza virus-activated pDCs induce CD40-activated B cells to develop into plasmablasts and differentiate into antibody-secreting plasma cells (75). CD40 ligation (i.e., a T-cell contribution) may not be essential for pDC-induced B-cell activation, as B cells can differentiate into plasmablasts after coculture with CpG and B-cell receptor ligation (115).
These data suggest a critical role for pDCs during generation of plasma cells and the antibody response and are consistent with observations for animal models showing that IFN-α is involved in the development of humoral immunity, including class switching (88).
T-cell-activating function.
Pre-pDCs have poor T-cell-stimulating capacity, and maturation is necessary to acquire this function. Early experiments reported that CD40L- and IL-3-stimulated pDCs developed into a functionally distinct DC type that promoted the development of IL-4 secreting Th2 cells (119). However, pDCs also prime Th1 or Th0 allogeneic responses (24, 50, 69, 76). For example, virus-stimulated pDCs activate T cells which make IL-10 and IFN-γ (24, 76), while CpG stimulation enhances CXCR3 and CCR5 expression on activated T cells, concomitant with a potent Th1 polarization (85). Differences in IFN-α-inducing capacity as well as in OX40L expression may partly underlie the Th1/Th2 polarizing capacity of pDCs, in addition to the stimulant, antigen dose, stage of differentiation, and environment (69, 91). pDCs stimulated with TLR agonists and pulsed with peptide antigens or infected with viruses (influenza virus, cytomegalovirus) also expand antigen-specific memory CD4+ and CD8+ T cells (49, 94).
While pDCs approach or are equivalent to mDCs in memory T-cell activation, pDCs seem less efficient in priming antigen-specific naïve T cells, even after maturation with a potent stimulus such as CpG or virus (17, 50, 108, 110). This finding raises the issue of whether pDCs are involved in the initiation of T-cell responses or simply amplify responses first induced by mDCs. However, male FLT-3L-mobilized pDCs or pDCs matured in vivo with CpG induce male-specific transplantation antigen H-Y-specific cytotoxic T lymphocytes (CTLs) when adoptively transferred to female murine recipients (123). Peptide-pulsed murine pDCs can also induce antigen-specific CTLs which are protective for melanoma (123), and vaccinia virus Ankara (a replication-deficient poxvirus)-infected or peptide-pulsed and CD40L-activated human pDCs have the ability to prime antigen-specific naïve CD8+ T cells (122), firmly placing them in the category of professional APCs.
Unlike mDCs, pDCs cannot cross present soluble antigens to prime CTLs (123), probably because of their lesser ability to phagocytose soluble or particulate antigens (49, 60). Studies examining priming of CD4+ T cells indicate that pDCs expressing endogenous antigens (hen egg lysosome expressed on the cell membrane) are less efficient than mDCs in their ability to assemble peptide-MHC-II complexes and stimulate naïve T cells (83). However, once primed by mDCs, T cells can be skewed towards Th1 cells by pDCs, supporting an amplifying role for these APCs.
In summary, depending upon the circumstances, pDCs may play both initiating and amplifying roles in T-cell activation.
NK cells.
Along with pDCs, NK cells are on the front line of defense against viruses. The activity of NK cells is predominantly regulated by IFN-α/β and IL-12. IFN-α/βs increase cytotoxicity, while IL-12 elicits IFN-γ production (15). In mice, following infection with MCMV, pDCs are the main DC subset to rapidly activate NK cells (33). Similarly, matured human pDCs recruit and activate NK cells in vitro (5, 100). pDCs therefore amplify the immune response through activation of additional members of the innate arm of immunity.
Regulatory T cells and tolerance.
pDCs are predominant in the thymic medulla (13) and are speculated to play a role in shaping the T-cell repertoire (128). A number of studies have implicated pDCs in the development of tolerance. In mice, pDCs induce the development of T regulatory 1 cells (Tr1) which block naïve T cell activation through an IL-10-dependent mechanism (14). Furthermore, a CD8α+, CD11c+, B220+, CD19− cell population in bone marrow-derived populations resembling pDCs facilitates the engraftment of allogeneic stem cells (52). Interestingly, transplantation of GM-CSF-mobilized human blood cells (which have increased numbers of pDCs) induces less severe graft-versus-host disease (6, 121, 150).
In human cell cultures, immature pDCs can induce the anergy of CD4+ tetanus toxoid-specific T-cell clones (84). Presentation of alloantigen by IL-3- and CD40L-matured pDCs induces IL-10-producing CD8+ T cells which exhibit poor cytolytic activity and are unresponsive to secondary stimulation. These cells share many properties with CD4+ Tr1 cells: anergic profile, IL-10 dependence for their production, and suppression of primary T cell responses with IL-10 (58).
Several questions remain regarding a role for pDCs in the induction of tolerance. What distinguishes a tolerance-inducing pDC from a stimulatory pDC? It is not as simple as a maturation difference, since CD40L- and IL-3-matured pDCs induce Th2 responses in addition to CD8+ T regulatory cells. Furthermore, the injection of immature pDCs in vivo does not induce antigen-specific tolerance (123). Clearly, further studies will be necessary to identify the factors directing pDCs to become tolerogenic.
CLINICAL SIGNIFICANCE OF pDCs
Several studies have correlated pDC frequency and function with disease progression. Selected conditions are discussed below.
HIV-1 infection.
In both acute and chronic infection, there is a progressive decline in pDC and mDC numbers, only partially reversible with antiretroviral treatment (28, 111, 130, 132). The decline of pDCs is inversely correlated with viral load and associated with a functional drop in the amount of IFN-α production per cell and a fall in CD4+ T-cell counts (9, 28, 37, 38, 46, 131, 135). An increased susceptibility to opportunistic infection has been documented with a reduction in IFN-α/β-producing cells (130). Furthermore, blood pDCs and mDCs may become infected with HIV-1 and display impaired T-cell stimulatory function (37). Chronic HIV-1 infection therefore may lead to depletion, infection, and impairment of both cytokine-secreting and T-cell-activating properties of pDCs. Similar observations have been made for patients with HCV infection and HCV-HIV coinfection (4, 59). On the other hand, in HIV infection, the frequency of pDCs is higher in long-term survivors than in healthy donors (89). Altogether, these data suggest that preserving pDC function and number is critical to maintaining antiviral immune responses.
Dengue virus infection.
An increase in the frequency of circulating pDCs soon after dengue virus infection was observed in nonhuman primates who developed very mild or asymptomatic disease and in children with mild nonasymptomatic acute infection (113), whereas an early rise was absent in children who subsequently developed dengue hemorrhagic fever. Live but not UV- or heat-inactivated forms of flavivirus induce pDCs to make IFN-α. Whether IFN-α/βs are essential for protection against disease progression remains to be determined.
SLE and allergy.
Lupus sera contain elevated levels of IFN-α (45, 120) and an inducer of IFN-α. The inducer can be mimicked by stimulation of pDCs with a combination of anti-dsDNA antibodies and immunostimulatory plasmid DNA, implicating SLE-associated autoantibodies in IFN-α production (145). Activation may depend upon internalization of immune complexes after binding to CD32, the Fc gamma RIIa (11), and/or ligation of TLR9 by autologous DNA. Furthermore, lupus sera can promote the differentiation of monocytes into APCs in an IFN-α-dependent manner (19). These findings suggest that SLE might be triggered following an infection in genetically susceptible persons, in which release of DNA from infected apoptotic cells triggers the production of autoantibodies, including anti-DNA antibodies. Antibody-DNA complexes activate pDCs, and the resulting production of IFN-α/β induces and amplifies innate and adaptive immune responses. Indeed, subjects undergoing IFN-α therapy for cancer can develop anti-DNA antibodies and clinical symptoms resembling SLE, and IFN-α induces a lupus-like syndrome in mice. Anti-BDCA-2 antibody blocks IFN-α production by pDCs in response to immune complexes (20), suggesting the possibility that the targeting of this molecule may be beneficial in treating SLE.
pDCs also accumulate in the epidermis and papillary dermis of the skin of patients with cutaneous SLE, psoriasis vulgaris, and allergic contact dermatitis but not in patients with atopic dermatitis (45, 151). pDCs may enter the dermis through CLA/E-selectin and CD62L-PNAd interactions (151). It remains to be determined whether pDCs contribute to disease onset at these sites or are recruited by inflammatory chemokines and cytokines. pDCs also accumulate in the nasal mucosa in experimentally induced nasal allergy (72). Recent studies show that histamine, via the H2 receptor, blocks the CpG ODN or influenza virus stimulation of IFN-α and TNF-α and shifts T-cell-stimulating capacity from Th1 to Th2 (99). By impacting the balance of Th1/Th2 immunity, histamine would enhance the severity of allergic diseases. The potent Th1-priming abilities of CpG DNA, presumably mediated through pDCs, are the basis for current clinical trials for asthma and allergic rhinitis.
Tumors.
pDCs have been located in head and neck cancer, ovarian cancer, and primary melanoma lesions (61, 122, 148, 157). Tumor-infiltrating pDCs have a diminished capacity to produce IFN-α in response to stimuli like CpG ODN, suggesting either that they are matured or that their functions are impaired. Furthermore, supernatants from tumor cell cultures inhibit pDC function, possibly through transforming growth factor β, vascular endothelial growth factor β, IL-10, downregulation of specific TLRs (TLR9), or other means. Tumor- or tumor supernatant-exposed pDCs induce IL-10-producing regulatory T cells (61, 157), which in turn suppress the ability of tumor-infiltrating T cells to recognize autologous tumor antigens cross presented by mDCs (157). Therefore, tumors may dysregulate immunity by attracting and manipulating pDC activation potential and by blocking accumulation of mDCs. How do pDCs localize in tumors? Stromal cell-derived factor 1 (SDF-1) (or SDF-1 together with CXCR3 ligands) is involved in the recruitment of pDCs into ovarian cancer (157). It has been detected in peritumoral stromal cells and endothelium and upregulates pDC VLA-5 expression, which mediates adhesion to V-CAM-1 on endothelial cells.
Direct injection of CpG ODN into tumors reduces tumor size and, when coadministered with mDCs, cures chemotherapy-resistant tumors (92). Aldara (imiquimod, a TLR7 agonist) has been successfully used in the clinic to treat basal cell carcinoma, human papillomavirus-infected warts, and condylomata accuminata (126). TLR7 and -9 agonists, therefore, by stimulating pDC activation may overturn tumor-induced immunosuppressive effects directed at pDCs and thereby potentiate antitumor immunity.
The existence of rare hematological neoplasms, consisting of malignant counterparts of pDCs presenting with cutaneous nodules, lymphadenopthy, and bone marrow infiltration, has recently been described (26). Malignant cells resemble pDCs and express CD4, CD45RA, BDCA-2, BDCA-4, CD123, and CD56 but lack other lineage-specific markers. These cells survive in the presence of IL-3 and polarize T cells towards a Th2 phenotype after CD40L-mediated differentiation or towards a Th1 phenotype after influenza virus infection. Furthermore, they secrete significant amounts of IFN-α, TNF-α, IL-6, and IL-8 in response to influenza virus exposure (26). Leukemic pDCs process and present tetanus toxoid (although less efficiently than mDCs) and influenza virus antigens to CD4+ and CD8+ T-cell clones, respectively (27). These cells, because of their near identity to primary pDCs, will be a useful tool for investigators who study pDC function.
SUMMARY
DC subsets are coordinately specialized to activate innate and adaptive immune responses in vivo. pDCs can orchestrate events during the course of viral infections, atopy, autoimmune disease, and metastatic cancer. Subsequent studies will need to precisely identify the functional role of pDCs in these various conditions in order to determine whether they are targets for therapeutic interventions.
ADDENDUM IN PROOF
Some important studies which have more precisely confirmed and characterized the role of pDCs in linking innate and adaptive immunity have been published recently. First, pDCs, together with conventional DCs, were shown to activate NK cells through a coordinated cytokine- and TLR9-dependent response (A. Krug, A. R. French, W. Barchet, J. A. Fischer, A. Dzionek, J. T. Pingel, M. M. Orihuela, S. Akira, W. M. Yokoyama, and M. Colonna, Immunity 21:107-119, 2004). Furthermore, it was demonstrated that murine influenza virus-activated pDCs can prime antigen-specific CD8+ cells in vivo, and interestingly, CpG-activated pDCs were able to recall only antigen-specific responses (G. Schlecht, S. Garcia, N. Escriou, A. A. Freitas, C. Leclerc, and G. Dadaglio, Blood 104:1808-1815, 2004). Finally, the induction of IFN-α/β in response to TLR9 stimulation and likely to TLR7 triggering was shown to involve direct interactions between MyD88 and IRF-7 in a complex also including IRAK-4 and TRAF-6 (T. Kawai, S. Sato, K. J. Ishii, C. Coban, H. Hemmi, M. Yamamoto, K. Terai, M. Matsuda, J. Inoue, S. Uematsu, O. Takeuchi, and S. Akira, Nat. Immunol. 5:1061-1068, 2004; K. Honda, H. Yanai, T. Mizutani, H. Negishi, N. Shimada, N. Suzuki, Y. Ohba, A. Takaoka, W. C. Yeh, and T. Taniguchi, Proc. Natl. Acad. Sci. USA 101:15416-15421, 2004).
Acknowledgments
These studies were supported by grants from the NIH (AI44628), the NCI (CA84512), the Mary Kirkland Foundation, the Cancer Research Institute, and the Burroughs Wellcome Foundation (to N.B.). N.B. is a Doris Duke Distinguished Scientist and an Elizabeth Glaser Scientist. A.-S.B. was supported by La Fondation pour la Recherche Médicale and a grant from the CFAR at the NYU School of Medicine. The NYU Sackler Institute provided support for K.M.
We thank Marie Larsson and Mojca Skoberne (NYU School of Medicine) and Jean-François Fonteneau (INSERM U463, Nantes, France) for helpful discussions.
REFERENCES
- 1.Abb, J., H. Abb, and F. Deinhardt. 1983. Phenotype of human alpha-interferon producing leucocytes identified by monoclonal antibodies. Clin. Exp. Immunol. 52:179-184. [PMC free article] [PubMed] [Google Scholar]
- 2.Ahonen, C. L., S. J. Gibson, R. M. Smith, L. K. Pederson, J. M. Lindh, M. A. Tomai, and J. P. Vasilakos. 1999. Dendritic cell maturation and subsequent enhanced T-cell stimulation induced with the novel synthetic immune response modifier R-848. Cell. Immunol. 197:62-72. [DOI] [PubMed] [Google Scholar]
- 3.Akira, S. 2001. Toll-like receptors and innate immunity. Adv. Immunol. 78:1-56. [DOI] [PubMed] [Google Scholar]
- 4.Anthony, D. D., N. L. Yonkers, A. B. Post, R. Asaad, F. P. Heinzel, M. M. Lederman, P. V. Lehmann, and H. Valdez. 2004. Selective impairments in dendritic cell-associated function distinguish hepatitis C virus and HIV infection. J. Immunol. 172:4907-4916. [DOI] [PubMed] [Google Scholar]
- 5.Ardavin, C., S. Amigorena, and C. Reis e Sousa. 2004. Dendritic cells: immunobiology and cancer immunotherapy. Immunity 20:17-23. [DOI] [PubMed] [Google Scholar]
- 6.Arpinati, M., C. L. Green, S. Heimfeld, J. E. Heuser, and C. Anasetti. 2000. Granulocyte-colony stimulating factor mobilizes T helper 2-inducing dendritic cells. Blood 95:2484-2490. [PubMed] [Google Scholar]
- 7.Asselin-Paturel, C., A. Boonstra, M. Dalod, I. Durand, N. Yessaad, C. Dezutter-Dambuyant, A. Vicari, A. O'Garra, C. Biron, F. Briere, and G. Trinchieri. 2001. Mouse type I IFN-producing cells are immature APCs with plasmacytoid morphology. Nat. Immunol. 2:1144-1150. [DOI] [PubMed] [Google Scholar]
- 8.Bangert, C., J. Friedl, G. Stary, G. Stingl, and T. Kopp. 2003. Immunopathologic features of allergic contact dermatitis in humans: participation of plasmacytoid dendritic cells in the pathogenesis of the disease? J. Investig. Dermatol. 121:1409-1418. [DOI] [PubMed] [Google Scholar]
- 9.Barron, M. A., N. Blyveis, B. E. Palmer, S. MaWhinney, and C. C. Wilson. 2003. Influence of plasma viremia on defects in number and immunophenotype of blood dendritic cell subsets in human immunodeficiency virus 1-infected individuals. J. Infect. Dis. 187:26-37. [DOI] [PubMed] [Google Scholar]
- 10.Bauer, M., V. Redecke, J. W. Ellwart, B. Scherer, J. P. Kremer, H. Wagner, and G. B. Lipford. 2001. Bacterial CpG-DNA triggers activation and maturation of human CD11c−, CD123+ dendritic cells. J. Immunol. 166:5000-5007. [DOI] [PubMed] [Google Scholar]
- 11.Bave, U., M. Magnusson, M. L. Eloranta, A. Perers, G. V. Alm, and L. Ronnblom. 2003. Fc gamma RIIa is expressed on natural IFN-alpha-producing cells (plasmacytoid dendritic cells) and is required for the IFN-alpha production induced by apoptotic cells combined with lupus IgG. J. Immunol. 171:3296-3302. [DOI] [PubMed] [Google Scholar]
- 12.Bender, A., M. Sapp, G. Schuler, R. M. Steinman, and N. Bhardwaj. 1996. Improved methods for the generation of dendritic cells from nonproliferating progenitors in human blood. J. Immunol. Methods 196:121-135. [DOI] [PubMed] [Google Scholar]
- 13.Bendriss-Vermare, N., C. Barthelemy, I. Durand, C. Bruand, C. Dezutter-Dambuyant, N. Moulian, S. Berrih-Aknin, C. Caux, G. Trinchieri, and F. Briere. 2001. Human thymus contains IFN-alpha-producing CD11c−, myeloid CD11c+, and mature interdigitating dendritic cells. J. Clin. Investig. 107:835-844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bilsborough, J., and J. L. Viney. 2002. Getting to the guts of immune regulation. Immunology 106:139-143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Biron, C. A., K. B. Nguyen, G. C. Pien, L. P. Cousens, and T. P. Salazar-Mather. 1999. Natural killer cells in antiviral defense: function and regulation by innate cytokines. Annu. Rev. Immunol. 17:189-220. [DOI] [PubMed] [Google Scholar]
- 16.Bjorck, P. 2004. Dendritic cells exposed to herpes simplex virus in vivo do not produce IFN-alpha after rechallenge with virus in vitro and exhibit decreased T cell alloreactivity. J. Immunol. 172:5396-5404. [DOI] [PubMed] [Google Scholar]
- 17.Bjorck, P. 2001. Isolation and characterization of plasmacytoid dendritic cells from Flt3 ligand and granulocyte-macrophage colony-stimulating factor-treated mice. Blood 98:3520-3526. [DOI] [PubMed] [Google Scholar]
- 18.Blackwell, S. E., and A. M. Krieg. 2003. CpG-A-induced monocyte IFN-gamma-inducible protein-10 production is regulated by plasmacytoid dendritic cell-derived IFN-alpha. J. Immunol. 170:4061-4068. [DOI] [PubMed] [Google Scholar]
- 19.Blanco, P., A. K. Palucka, M. Gill, V. Pascual, and J. Banchereau. 2001. Induction of dendritic cell differentiation by IFN-α in systemic lupus erythematosus. Science 294:1540-1543. [DOI] [PubMed] [Google Scholar]
- 20.Blomberg, S., M. L. Eloranta, M. Magnusson, G. V. Alm, and L. Ronnblom. 2003. Expression of the markers BDCA-2 and BDCA-4 and production of interferon-alpha by plasmacytoid dendritic cells in systemic lupus erythematosus. Arthritis Rheum. 48:2524-2532. [DOI] [PubMed] [Google Scholar]
- 21.Brasel, K., T. De Smedt, J. L. Smith, and C. R. Maliszewski. 2000. Generation of murine dendritic cells from flt3-ligand-supplemented bone marrow cultures. Blood 96:3029-3039. [PubMed] [Google Scholar]
- 22.Brawand, P., D. R. Fitzpatrick, B. W. Greenfield, K. Brasel, C. R. Maliszewski, and T. De Smedt. 2002. Murine plasmacytoid pre-dendritic cells generated from flt3 ligand-supplemented bone marrow cultures are immature APCs. J. Immunol. 169:6711-6719. [DOI] [PubMed] [Google Scholar]
- 23.Caux, C., C. Massacrier, B. Vanbervliet, B. Dubois, I. Durand, M. Cella, A. Lanzavecchia, and J. Banchereau. 1997. CD34+ hematopoietic progenitors from human cord blood differentiate along two independent dendritic cell pathways in response to granulocyte-macrophage colony-stimulating factor plus tumor necrosis factor α. Blood 90:1458-1470. [PubMed] [Google Scholar]
- 24.Cella, M., F. Facchetti, A. Lanzavecchia, and M. Colonna. 2000. Plasmacytoid dendritic cells activated by influenza virus and CD40L drive a potent Th1 polarization. Nat. Immunol. 1:305-310. [DOI] [PubMed] [Google Scholar]
- 25.Cella, M., D. Jarrossay, F. Facchetti, O. Alebardi, H. Nakajima, A. Lanzavecchia, and M. Colonna. 1999. Plasmacytoid monocytes migrate to inflamed lymph nodes and produce large amounts of type I interferon. Nat. Med. 5:919-923. [DOI] [PubMed] [Google Scholar]
- 26.Chaperot, L., N. Bendriss, O. Manches, R. Gressin, M. Maynadie, F. Trimoreau, H. Orfeuvre, B. Corront, J. Feuillard, J. J. Sotto, J. C. Bensa, F. Briere, J. Plumas, and M. C. Jacob. 2001. Identification of a leukemic counterpart of the plasmacytoid dendritic cells. Blood 97:3210-3217. [DOI] [PubMed] [Google Scholar]
- 27.Chaperot, L., I. Perrot, M. C. Jacob, D. Blanchard, V. Salaun, V. Deneys, S. Lebecque, F. Briere, J. C. Bensa, and J. Plumas. 2004. Leukemic plasmacytoid dendritic cells share phenotypic and functional features with their normal counterparts. Eur. J. Immunol. 34:418-426. [DOI] [PubMed] [Google Scholar]
- 28.Chehimi, J., D. E. Campbell, L. Azzoni, D. Bacheller, E. Papasavvas, G. Jerandi, K. Mounzer, J. Kostman, G. Trinchieri, and L. J. Montaner. 2002. Persistent decreases in blood plasmacytoid dendritic cell number and function despite effective highly active antiretroviral therapy and increased blood myeloid dendritic cells in HIV-infected individuals. J. Immunol. 168:4796-4801. [DOI] [PubMed] [Google Scholar]
- 29.Chehimi, J., S. E. Starr, H. Kawashima, D. S. Miller, G. Trinchieri, B. Perussia, and S. Bandyopadhyay. 1989. Dendritic cells and IFN-alpha-producing cells are two functionally distinct non-B, non-monocytic HLA-DR+ cell subsets in human peripheral blood. Immunology 68:488-490. [PMC free article] [PubMed] [Google Scholar]
- 30.Coccia, E. M., M. Severa, E. Giacomini, D. Monneron, M. E. Remoli, I. Julkunen, M. Cella, R. Lande, and G. Uze. 2004. Viral infection and Toll-like receptor agonists induce a differential expression of type I and lambda interferons in human plasmacytoid and monocyte-derived dendritic cells. Eur. J. Immunol. 34:796-805. [DOI] [PubMed] [Google Scholar]
- 31.Colonna, M., A. Krug, and M. Cella. 2002. Interferon-producing cells: on the front line in immune responses against pathogens. Curr. Opin. Immunol. 14:373-379. [DOI] [PubMed] [Google Scholar]
- 32.Corcoran, L., I. Ferrero, D. Vremec, K. Lucas, J. Waithman, M. O'Keeffe, L. Wu, A. Wilson, and K. Shortman. 2003. The lymphoid past of mouse plasmacytoid cells and thymic dendritic cells. J. Immunol. 170:4926-4932. [DOI] [PubMed] [Google Scholar]
- 33.Dalod, M., T. Hamilton, R. Salomon, T. P. Salazar-Mather, S. C. Henry, J. D. Hamilton, and C. A. Biron. 2003. Dendritic cell responses to early murine cytomegalovirus infection: subset functional specialization and differential regulation by interferon alpha/beta. J. Exp. Med. 197:885-898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dalod, M., T. P. Salazar-Mather, L. Malmgaard, C. Lewis, C. Asselin-Paturel, F. Briere, G. Trinchieri, and C. A. Biron. 2002. Interferon α/β and interleukin 12 responses to viral infections: pathways regulating dendritic cell cytokine expression in vivo. J. Exp. Med. 195:517-528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Diebold, S. S., T. Kaisho, H. Hemmi, S. Akira, and E. S. C. Reis. 2004. Innate antiviral responses by means of TLR7-mediated recognition of single-stranded RNA. Science 303:1529-1531. [DOI] [PubMed] [Google Scholar]
- 36.Diebold, S. S., M. Montoya, H. Unger, L. Alexopoulou, P. Roy, L. E. Haswell, A. Al-Shamkhani, R. Flavell, P. Borrow, and C. R. Sousa. 2003. Viral infection switches non-plasmacytoid dendritic cells into high interferon producers. Nature 424:324-328. [DOI] [PubMed] [Google Scholar]
- 37.Donaghy, H., B. Gazzard, F. Gotch, and S. Patterson. 2003. Dysfunction and infection of freshly isolated blood myeloid and plasmacytoid dendritic cells in patients infected with HIV-1. Blood 101:4505-4511. [DOI] [PubMed] [Google Scholar]
- 38.Donaghy, H., A. Pozniak, B. Gazzard, N. Qazi, J. Gilmour, F. Gotch, and S. Patterson. 2001. Loss of blood CD11c+ myeloid and CD11c− plasmacytoid dendritic cells in patients with HIV-1 infection correlates with HIV-1 RNA virus load. Blood 98:2574-2576. [DOI] [PubMed] [Google Scholar]
- 39.Duramad, O., K. L. Fearon, J. H. Chan, H. Kanzler, J. D. Marshall, R. L. Coffman, and F. J. Barrat. 2003. IL-10 regulates plasmacytoid dendritic cell response to CpG-containing immunostimulatory sequences. Blood 102:4487-4492. [DOI] [PubMed] [Google Scholar]
- 40.Dzionek, A., Y. Inagaki, K. Okawa, J. Nagafune, J. Rock, Y. Sohma, G. Winkels, M. Zysk, Y. Yamaguchi, and J. Schmitz. 2002. Plasmacytoid dendritic cells: from specific surface markers to specific cellular functions. Hum. Immunol. 63:1133-1148. [DOI] [PubMed] [Google Scholar]
- 41.Dzionek, A., Y. Sohma, J. Nagafune, M. Cella, M. Colonna, F. Facchetti, G. Günther, I. Johnston, A. Lanzavecchia, T. Nagasaka, T. Okada, W. Vermi, G. Winkels, T. Yamamoto, M. Zysk, Y. Yamaguchi, and J. Schmitz. 2001. BDCA-2, a novel plasmacytoid dendritic cell-specific type II C-type lectin, mediates antigen-capture and is a potent inhibitor of interferon-α/β induction. J. Exp. Med. 194:1823-1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Eckert, F., and U. Schmid. 1989. Identification of plasmacytoid T cells in lymphoid hyperplasia of the skin. Arch. Dermatol. 125:1518-1524. [PubMed] [Google Scholar]
- 43.Elias, F., J. Flo, R. A. Lopez, J. Zorzopulos, A. Montaner, and J. M. Rodriguez. 2003. Strong cytosine-guanosine-independent immunostimulation in humans and other primates by synthetic oligodeoxynucleotides with PyNTTTTGT motifs. J. Immunol. 171:3697-3704. [DOI] [PubMed] [Google Scholar]
- 44.Facchetti, F., C. de Wolf-Peeters, J. J. van den Oord, R. de Vos, and V. J. Desmet. 1989. Plasmacytoid monocytes (so-called plasmacytoid T-cells) in Kikuchi's lymphadenitis. An immunohistologic study. Am. J. Clin. Pathol. 92:42-50. [DOI] [PubMed] [Google Scholar]
- 45.Farkas, L., K. Beiske, F. Lund-Johansen, P. Brandtzaeg, and F. L. Jahnsen. 2001. Plasmacytoid dendritic cells (natural interferon-α/β-producing cells) accumulate in cutaneous lupus erythematosus lesions. Am. J. Pathol. 159:237-243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Feldman, S., D. Stein, S. Amrute, T. Denny, Z. Garcia, P. Kloser, Y. Sun, N. Megjugorac, and P. Fitzgerald-Bocarsly. 2001. Decreased interferon-alpha production in HIV-infected patients correlates with numerical and functional deficiencies in circulating type 2 dendritic cell precursors. Clin. Immunol. 101:201-210. [DOI] [PubMed] [Google Scholar]
- 47.Fitzergerald-Bocarsly, P., M. Feldman, M. Mendelsohn, S. Curl, and C. Lopez. 1988. Human mononuclear cells which produce interferon-alpha during NK[HSV-FS] assays are HLA-DR positive cells distinct from cytolyltic natural killer effectors. J. Leukoc. Biol. 43:323-334. [DOI] [PubMed] [Google Scholar]
- 48.Fitzgerald-Bocarsly, P. 1993. Human natural interferon-alpha producing cells. Pharmacol. Ther. 60:39-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fonteneau, J. F., M. Gilliet, M. Larsson, I. Dasilva, C. Munz, Y. J. Liu, and N. Bhardwaj. 2003. Activation of influenza virus-specific CD4+ and CD8+ T cells: a new role for plasmacytoid dendritic cells in adaptive immunity. Blood 101:3520-3526. [DOI] [PubMed] [Google Scholar]
- 50.Fonteneau, J. F., M. Larsson, A. S. Beignon, K. McKenna, I. Dasilva, A. Amara, Y. J. Liu, J. D. Lifson, D. R. Littman, and N. Bhardwaj. 2004. Human immunodeficiency virus type 1 activates plasmacytoid dendritic cells and concomitantly induces the bystander maturation of myeloid dendritic cells. J. Virol. 78:5223-5232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Galy, A., I. Christopherson, G. Ferlazzo, G. Liu, H. Spits, and K. Georgopoulos. 2000. Distinct signals control the hematopoiesis of lymphoid-related dendritic cells. Blood 95:128-137. [PubMed] [Google Scholar]
- 52.Gandy, K. L., J. Domen, H. Aguila, and I. L. Weissman. 1999. CD8+TCR+ and CD8+TCR− cells in whole bone marrow facilitate the engraftment of hematopoietic stem cells across allogeneic barriers. Immunity 11:579-590. [DOI] [PubMed] [Google Scholar]
- 53.Gantner, B., R. Simmons, S. Canavera, and D. Underhill. 2003. Collaborative induction of inflammatory responses by dectin-1 and Toll-like receptor 2. J. Exp. Med. 197:1107-1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gary-Gouy, H., P. Lebon, and A. H. Dalloul. 2002. Type I interferon production by plasmacytoid dendritic cells and monocytes is triggered by viruses, but the level of production is controlled by distinct cytokines. J. Interferon Cytokine Res. 22:653-659. [DOI] [PubMed] [Google Scholar]
- 55.Geijtenbeek, T. B. H., S. J. van Vliet, A. Engering, B. A. 't Hart, and Y. van Kooyk. 2004. Self- and nonself-recognition by C-type lectins on dendritic cells. Annu. Rev. Immunol. 22:33-54. [DOI] [PubMed] [Google Scholar]
- 56.Geijtenbeek, T. B. H., S. J. van Vliet, E. A. Koppel, M. Sanchez-Hernandez, C. M. J. E. Vandenbroucke-Grauls, B. Appelmelk, and Y. van Kooyk. 2003. Mycobacteria target DC-SIGN to suppress dendritic cell function. J. Exp. Med. 197:7-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gilliet, M., A. Boonstra, C. Paturel, S. Antonenko, X. L. Xu, G. Trinchieri, A. O'Garra, and Y. J. Liu. 2002. The development of murine plasmacytoid dendritic cell precursors is differentially regulated by FLT3-ligand and granulocyte/macrophage colony-stimulating factor. J. Exp. Med. 195:953-958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gilliet, M., and Y.-J. Liu. 2002. Generation of human CD8 T regulatory cells by CD40 ligand-activated plasmacytoid dendritic cells. J. Exp. Med. 195:695-704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Goutagny, N., C. Vieux, E. Decullier, B. Ligeoix, A. Epstein, C. Trepo, P. Couzigou, G. Inchauspe, and C. Bain. 2004. Quantification and functional analysis of plasmacytoid dendritic cells in patients with chronic hepatitis C virus infection. J. Infect. Dis. 189:1646-1655. [DOI] [PubMed] [Google Scholar]
- 60.Grouard, G., M.-C. Rissoan, L. Filgueira, I. Durand, J. Banchereau, and Y.-J. Liu. 1997. The enigmatic plasmacytoid T cells develop into dendritic cells with IL-3 and CD40-ligand. J. Exp. Med. 185:1101-1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hartmann, E., B. Wollenberg, S. Rothenfusser, M. Wagner, D. Wellisch, B. Mack, T. Giese, O. Gires, S. Endres, and G. Hartmann. 2003. Identification and functional analysis of tumor-infiltrating plasmacytoid dendritic cells in head and neck cancer. Cancer Res. 63:6478-6487. [PubMed] [Google Scholar]
- 62.Hartmann, G., J. Battiany, H. Poeck, M. Wagner, M. Kerkmann, N. Lubenow, S. Rothenfusser, and S. Endres. 2003. Rational design of new CpG oligonucleotides that combine B cell activation with high IFN-alpha induction in plasmacytoid dendritic cells. Eur. J. Immunol. 33:1633-1641. [DOI] [PubMed] [Google Scholar]
- 63.Hartmann, G., G. J. Weiner, and A. M. Krieg. 1999. CpG DNA: a potent signal for growth, activation, and maturation of human dendritic cells. Proc. Natl. Acad. Sci. USA 96:9305-9310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Heil, F., P. Ahmad-Nejad, H. Hemmi, H. Hochrein, F. Ampenberger, T. Gellert, H. Dietrich, G. Lipford, K. Takeda, S. Akira, H. Wagner, and S. Bauer. 2003. The Toll-like receptor 7 (TLR7)-specific stimulus loxoribine uncovers a strong relationship within the TLR7, 8 and 9 subfamily. Eur. J. Immunol. 33:2987-2997. [DOI] [PubMed] [Google Scholar]
- 65.Heil, F., H. Hemmi, H. Hochrein, F. Ampenberger, C. Kirschning, S. Akira, G. Lipford, H. Wagner, and S. Bauer. 2004. Species-specific recognition of single-stranded RNA via Toll-like receptor 7 and 8. Science 303:1526-1529. [DOI] [PubMed] [Google Scholar]
- 66.Hemmi, H., T. Kaisho, K. Takeda, and S. Akira. 2003. The roles of Toll-like receptor 9, MyD88, and DNA-dependent protein kinase catalytic subunit in the effects of two distinct CpG DNAs on dendritic cell subsets. J. Immunol. 170:3059-3064. [DOI] [PubMed] [Google Scholar]
- 67.Hemmi, H., T. Kaisho, O. Takeuchi, S. Sato, H. Sanjo, K. Hoshino, T. Horiuchi, H. Tomizawa, K. Takeda, and S. Akira. 2002. Small anti-viral compounds activate immune cells via the TLR7 MyD88-dependent signaling pathway. Nat. Immunol. 3:196-200. [DOI] [PubMed] [Google Scholar]
- 68.Honda, K., S. Sakaguchi, C. Nakajima, A. Watanabe, H. Yanai, M. Matsumoto, T. Ohteki, T. Kaisho, A. Takaoka, S. Akira, T. Seya, and T. Taniguchi. 2003. Selective contribution of IFN-alpha/beta signaling to the maturation of dendritic cells induced by double-stranded RNA or viral infection. Proc. Natl. Acad. Sci. USA 100:10872-10877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ito, T., R. Amakawa, M. Inaba, T. Hori, M. Ota, K. Nakamura, M. Takebayashi, M. Miyaji, T. Yoshimura, K. Inaba, and S. Fukuhara. 2004. Plasmacytoid dendritic cells regulate Th cell responses through OX40 ligand and type I IFNs. J. Immunol. 172:4253-4259. [DOI] [PubMed] [Google Scholar]
- 70.Ito, T., R. Amakawa, T. Kaisho, H. Hemmi, K. Tajima, K. Uehira, Y. Ozaki, H. Tomizawa, S. Akira, and S. Fukuhara. 2002. Interferon-α and interleukin-12 are induced differentially by Toll-like receptor 7 ligands in human blood dendritic cell subsets. J. Exp. Med. 195:1507-1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Izaguirre, A., B. J. Barnes, S. Amrute, W. S. Yeow, N. Megjugorac, J. Dai, D. Feng, E. Chung, P. M. Pitha, and P. Fitzgerald-Bocarsly. 2003. Comparative analysis of IRF and IFN-alpha expression in human plasmacytoid and monocyte-derived dendritic cells. J. Leukoc. Biol. 74:1125-1138. [DOI] [PubMed] [Google Scholar]
- 72.Jahnsen, F. L., F. Lund-Johansen, J. F. Dunne, L. Farkas, R. Haye, and P. Brandtzaeg. 2000. Experimentally induced recruitment of plasmacytoid (CD123high) dendritic cells in human nasal allergy. J. Immunol. 165:4062-4068. [DOI] [PubMed] [Google Scholar]
- 73.Janeway, C. A., Jr., and R. Medzhitov. 2002. Innate immune recognition. Annu. Rev. Immunol. 20:197-216. [DOI] [PubMed] [Google Scholar]
- 74.Jarrossay, D., G. Napolitani, M. Colonna, F. Sallusto, and A. Lanzavecchia. 2001. Specialization and complementarity in microbial molecule recognition by human myeloid and plasmacytoid dendritic cells. Eur. J. Immunol. 31:3388-3393. [DOI] [PubMed] [Google Scholar]
- 75.Jego, G., A. K. Palucka, J. P. Blanck, C. Chalouni, V. Pascual, and J. Banchereau. 2003. Plasmacytoid dendritic cells induce plasma cell differentiation through type I interferon and interleukin 6. Immunity 19:225-234. [DOI] [PubMed] [Google Scholar]
- 76.Kadowaki, N., S. Antoneko, J. Y.-N. Lau, and Y.-J. Liu. 2000. Natural interferon-α/β-producing cells link innate and adaptive immunity. J. Exp. Med. 192:219-226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kadowaki, N., S. Antonenko, and Y. J. Liu. 2001. Distinct CpG DNA and polyinosinic-polycytidylic acid double-stranded RNA, respectively, stimulate CD11c− type 2 dendritic cell precursors and CD11c+ dendritic cells to produce type I IFN. J. Immunol. 166:2291-2295. [DOI] [PubMed] [Google Scholar]
- 78.Kaser, A., S. Kaser, N. C. Kaneider, B. Enrich, C. J. Wiedermann, and H. Tilg. 2004. Interleukin-18 attracts plasmacytoid dendritic cells (DC2s) and promotes Th1 induction by DC2s through IL-18 receptor expression. Blood 103:648-655. [DOI] [PubMed] [Google Scholar]
- 79.Kerkmann, M., S. Rothenfusser, V. Hornung, A. Towarowski, M. Wagner, A. Sarris, T. Giese, S. Endres, and G. Hartmann. 2003. Activation with CpG-A and CpG-B oligonucleotides reveals two distinct regulatory pathways of type I IFN synthesis in human plasmacytoid dendritic cells. J. Immunol. 170:4465-4474. [DOI] [PubMed] [Google Scholar]
- 80.Krug, A., G. D. Luker, W. Barchet, D. A. Leib, S. Akira, and M. Colonna. 2004. Herpes simplex virus type 1 activates murine natural interferon-producing cells through toll-like receptor 9. Blood 103:1433-1437. [DOI] [PubMed] [Google Scholar]
- 81.Krug, A., S. Rothenfusser, V. Hornung, B. Jahrsdorfer, S. Blackwell, Z. K. Ballas, S. Endres, A. M. Krieg, and G. Hartmann. 2001. Identification of CpG oligonucleotide sequences with high induction of IFN-alpha/beta in plasmacytoid dendritic cells. Eur. J. Immunol. 31:2154-2163. [DOI] [PubMed] [Google Scholar]
- 82.Krug, A., A. Towarowski, S. Britsch, S. Rothenfusser, V. Hornung, R. Bals, T. Giese, H. Engelmann, S. Endres, A. M. Krieg, and G. Hartmann. 2001. Toll-like receptor expression reveals CpG DNA as a unique microbial stimulus for plasmacytoid dendritic cells which synergizes with CD40 ligand to induce high amounts of IL-12. Eur. J. Immunol. 31:3026-3037. [DOI] [PubMed] [Google Scholar]
- 83.Krug, A., R. Veeraswamy, A. Pekosz, O. Kanagawa, E. R. Unanue, M. Colonna, and M. Cella. 2003. Interferon-producing cells fail to induce proliferation of naive T cells but can promote expansion and T helper 1 differentiation of antigen-experienced unpolarized T cells. J. Exp. Med. 197:899-906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kuwana, M., J. Kaburaki, T. M. Wright, Y. Kawakami, and Y. Ikeda. 2001. Induction of antigen-specific human CD4+ T cell anergy by peripheral blood DC2 precursors. Eur. J. Immunol. 31:2547-2557. [DOI] [PubMed] [Google Scholar]
- 85.Langenkamp, A., K. Nagata, K. Murphy, L. Wu, A. Lanzavecchia, and F. Sallusto. 2003. Kinetics and expression patterns of chemokine receptors in human CD4+ T lymphocytes primed by myeloid or plasmacytoid dendritic cells. Eur. J. Immunol. 33:474-482. [DOI] [PubMed] [Google Scholar]
- 86.Latz, E., A. Schoenemeyer, A. Visintin, K. A. Fitzgerald, B. G. Monks, C. F. Knetter, E. Lien, N. J. Nilsen, T. Espevik, and D. T. Golenbock. 2004. TLR9 signals after translocating from the ER to CpG DNA in the lysosome. Nat. Immunol. 5:190-198. [DOI] [PubMed] [Google Scholar]
- 87.Le Bon, A., N. Etchart, C. Rossmann, M. Ashton, S. Hou, D. Gewert, P. Borrow, and D. F. Tough. 2003. Cross-priming of CD8+ T cells stimulated by virus-induced type I interferon. Nat. Immunol. 4:1009-1015. [DOI] [PubMed] [Google Scholar]
- 88.Le Bon, A., G. Schiavoni, G. D'Agostinio, I. Gresser, F. Belardelli, and D. F. Tough. 2001. Type I interferons potently enhance humoral immunity and can promote isotype switching by stimulating dendritic cells in vivo. Immunity 14:461-470. [DOI] [PubMed] [Google Scholar]
- 89.Levy, J. A., I. Scott, and C. Mackewicz. 2003. Protection from HIV/AIDS: the importance of innate immunity. Clin. Immunol. 108:167-174. [DOI] [PubMed] [Google Scholar]
- 90.Litinskiy, M. B., B. Nardelli, D. M. Hilbert, B. He, A. Schaffer, P. Casali, and A. Cerutti. 2002. DCs induce CD40-independent immunoglobulin class switching through BLyS and APRIL. Nat. Immunol. 3:822-829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Liu, Y. J. 2001. Dendritic cell subsets and lineages, and their functions in innate and adaptive immunity. Cell 106:259-262. [DOI] [PubMed] [Google Scholar]
- 92.Lonsdorf, A. S., H. Kuekrek, B. V. Stern, B. O. Boehm, P. V. Lehmann, and M. Tary-Lehmann. 2003. Intratumor CpG-oligodeoxynucleotide injection induces protective antitumor T cell immunity. J. Immunol. 171:3941-3946. [DOI] [PubMed] [Google Scholar]
- 93.Lopez, C. B., A. Garcia-Sastre, B. R. G. Williams, and T. M. Moran. 2003. Type 1 interferon induction pathway, but not released interferon, participates in the maturation of dendritic cells induced by negative strand RNA viruses. J. Infect. Dis. 187:1126-1136. [DOI] [PubMed] [Google Scholar]
- 94.Lore, K., M. R. Betts, J. M. Brenchley, J. Kuruppu, S. Khojasteh, S. Perfetto, M. Roederer, R. A. Seder, and R. A. Koup. 2003. Toll-like receptor ligands modulate dendritic cells to augment cytomegalovirus- and HIV-1-specific T cell responses. J. Immunol. 171:4320-4328. [DOI] [PubMed] [Google Scholar]
- 95.Lund, J., A. Sato, S. Akira, R. Medzhitov, and A. Iwasaki. 2003. Toll-like receptor 9-mediated recognition of herpes simplex virus-2 by plasmacytoid dendritic cells. J. Exp. Med. 198:513-520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lund, J. M., L. Alexopoulou, A. Sato, M. Karow, N. C. Adams, N. W. Gale, A. Iwasaki, and R. A. Flavell. 2004. Recognition of single-stranded RNA viruses by Toll-like receptor 7. Proc. Natl. Acad. Sci. USA 101:5598-5603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Maraskovsky, E., K. Brasel, M. Teepe, E. R. Roux, S. D. Lyman, K. Shortman, and H. J. McKenna. 1996. Dramatic increase in the numbers of functionally mature dendritic cells in Flt3 ligand-treated mice: multiple dendritic cell subpopulations identified. J. Exp. Med. 184:1953-1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Maraskovsky, E., E. Daro, E. Roux, M. Teepe, C. R. Maliszewski, J. Hoek, D. Caron, M. E. Lebsack, and H. J. McKenna. 2000. In vivo generation of human dendritic cell subsets by Flt3 ligand. Blood 96:878-884. [PubMed] [Google Scholar]
- 99.Mazzoni, A., C. A. Leifer, G. E. Mullen, M. N. Kennedy, D. M. Klinman, and D. M. Segal. 2003. Cutting edge: histamine inhibits IFN-alpha release from plasmacytoid dendritic cells. J. Immunol. 170:2269-2273. [DOI] [PubMed] [Google Scholar]
- 100.Megjugorac, N. J., H. A. Young, S. B. Amrute, S. L. Olshalsky, and P. Fitzgerald-Bocarsly. 2004. Virally stimulated plasmacytoid dendritic cells produce chemokines and induce migration of T and NK cells. J. Leukoc. Biol. 75:504-514. [DOI] [PubMed] [Google Scholar]
- 101.Merad, M., M. G. Manz, H. Karsunky, A. Wagers, W. Peters, I. Charo, I. L. Weissman, J. G. Cyster, and E. G. Engleman. 2002. Langerhans cells renew in the skin throughout life under steady-state conditions. Nat. Immunol. 3:1135-1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Miettinen, M., T. Sareneva, I. Julkunen, and S. Matikainen. 2001. IFNs activate toll-like receptor gene expression in viral infections. Genes Immun. 2:349-355. [DOI] [PubMed] [Google Scholar]
- 103.Mohty, M., A. Vialle-Castellano, J. A. Nunes, D. Isnardon, D. Olive, and B. Gaugler. 2003. IFN-alpha skews monocyte differentiation into toll-like receptor 7-expressing dendritic cells with potent functional activities. J. Immunol. 171:3385-3393. [DOI] [PubMed] [Google Scholar]
- 104.Montoya, M., G. Schiavoni, F. Mattei, I. Gresser, F. Belardelli, P. Borrow, and D. F. Tough. 2002. Type I interferons produced by dendritic cells promote their phenotypic and functional activation. Blood 99:3263-3271. [DOI] [PubMed] [Google Scholar]
- 105.Nakano, H., M. Yanagita, and M. D. Gunn. 2001. Cd11c+B220+Gr-1+ cells in mouse lymph nodes and spleen display characteristics of plasmacytoid dendritic cells. J. Exp. Med. 194:1171-1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Nguyen, K. B., W. T. Watford, R. Salomon, S. R. Hofmann, G. C. Pien, A. Morinobu, M. Gadina, J. J. O'Shea, and C. A. Biron. 2002. Critical role for STAT4 activation by type 1 interferons in the interferon-gamma response to viral infection. Science 297:2063-2066. [DOI] [PubMed] [Google Scholar]
- 107.O'Doherty, U., M. Peng, S. Gezelter, W. J. Swiggard, M. Betjes, N. Bhardwaj, and R. M. Steinman. 1994. Human blood contains two subsets of dendritic cells, one immunologically mature, and the other immature. Immunology 82:487-493. [PMC free article] [PubMed] [Google Scholar]
- 108.O'Keeffe, M., H. Hochrein, D. Vremec, I. Caminschi, J. L. Miller, E. M. Anders, L. Wu, M. H. Lahoud, S. Henri, B. Scott, P. Hertzog, L. Tatarczuch, and K. Shortman. 2002. Mouse plasmacytoid cells: long-lived cells, heterogeneous in surface phenotype and function, that differentiate into CD8+ dendritic cells only after microbial stimulus. J. Exp. Med. 196:1307-1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.O'Keeffe, M., H. Hochrein, D. Vremec, J. Pooley, R. Evans, S. Woulfe, and K. Shortman. 2002. Effects of administration of progenipoietin 1, Flt-3 ligand, granulocyte colony-stimulating factor, and pegylated granulocyte-macrophage colony-stimulating factor on dendritic cell subsets in mice. Blood 99:2122-2130. [DOI] [PubMed] [Google Scholar]
- 110.O'Keeffe, M., H. Hochrein, D. Vremec, B. Scott, P. Hertzog, L. Tatarczuch, and K. Shortman. 2003. Dendritic cell precursor populations of mouse blood: identification of the murine homologues of human blood plasmacytoid pre-DC2 and CD11c+ DC1 precursors. Blood 101:1453-1459. [DOI] [PubMed] [Google Scholar]
- 111.Pacanowski, J., S. Kahi, M. Baillet, P. Lebon, C. Deveau, C. Goujard, L. Meyer, E. Oksenhendler, M. Sinet, and A. Hosmalin. 2001. Reduced blood CD123+ (lymphoid) and CD11c+ (myeloid) dendritic cell numbers in primary HIV-1 infection. Blood 98:3016-3021. [DOI] [PubMed] [Google Scholar]
- 112.Penna, G., S. Sozzani, and L. Adorini. 2001. Cutting edge: selective usage of chemokine receptors by plasmacytoid dendritic cells. J. Immunol. 167:1862-1866. [DOI] [PubMed] [Google Scholar]
- 113.Pichyangkul, S., T. P. Endy, S. Kalayanarooj, A. Nisalak, K. Yongvanitchit, S. Green, A. L. Rothman, F. A. Ennis, and D. H. Libraty. 2003. A blunted blood plasmacytoid dendritic cell response to an acute systemic viral infection is associated with increased disease severity. J. Immunol. 171:5571-5578. [DOI] [PubMed] [Google Scholar]
- 114.Pichyangkul, S., K. Yongvanitchit, U. Kum-arb, H. Hemmi, S. Akira, A. M. Krieg, D. G. Heppner, V. A. Stewart, H. Hasegawa, S. Looareesuwan, G. D. Shanks, and R. S. Miller. 2004. Malaria blood stage parasites activate human plasmacytoid dendritic cells and murine dendritic cells through a Toll-like receptor 9-dependent pathway. J. Immunol. 172:4926-4933. [DOI] [PubMed] [Google Scholar]
- 115.Poeck, H., M. Wagner, J. Battiany, S. Rothenfusser, D. Wellisch, V. Hornung, B. Jahrsdorfer, T. Giese, S. Endres, and G. Hartmann. 2003. Plasmacytoid dendritic cells, antigen and CpG-C license human B cells for plasma cell differentiation and immunoglobulin production in the absence of T-cell help. Blood 103:3058-3064. [DOI] [PubMed] [Google Scholar]
- 116.Pulendran, B., J. Banchereau, S. Burkeholder, E. Kraus, E. Guinet, C. Chalouni, D. Caron, C. Maliszewski, J. Davoust, J. Fay, and K. Palucka. 2000. Flt3-ligand and granulocyte colony-stimulating factor mobilize distinct human dendritic cell subsets in vivo. J. Immunol. 165:566-572. [DOI] [PubMed] [Google Scholar]
- 117.Pulendran, B., J. Lingappa, M. K. Kennedy, J. Smith, M. Teepe, A. Rudensky, C. R. Maliszewski, and E. Maraskovsky. 1997. Developmental pathways of dendritic cells in vivo: distinct function, phenotype, and localization of dendritic cell subsets in FLT3 ligand-treated mice. J. Immunol. 159:2222-2231. [PubMed] [Google Scholar]
- 118.Pulendran, B., J. L. Smith, G. Caspary, K. Brasel, D. Pettit, E. Maraskovsky, and C. E. Maliszweski. 1999. Distinct dendritic cell subsets differentially regulate the class of immune responses in vivo. Proc. Natl. Acad. Sci. USA 96:1036-1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Rissoan, M. C., V. Soumelis, N. Kadowaki, G. Grouard, F. Briere, R. de Waal Malefyt, and Y. J. Liu. 1999. Reciprocal control of T helper cell and dendritic cell differentiation. Science 283:1183-1186. [DOI] [PubMed] [Google Scholar]
- 120.Ronnblom, L., and G. V. Alm. 2001. A pivotal role for the natural interferon alpha-producing cells (plasmacytoid dendritic cells) in the pathogenesis of lupus. J. Exp. Med. 194:F59-F63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Rossi, M., M. Arpinati, D. Rondelli, and C. Anasetti. 2002. Plasmacytoid dendritic cells: do they have a role in immune responses after hematopoietic cell transplantation? Hum. Immunol. 63:1194-1200. [DOI] [PubMed] [Google Scholar]
- 122.Salio, M., M. Cella, W. Vermi, F. Facchetti, M. J. Palmowski, C. L. Smith, D. Shepherd, M. Colonna, and V. Cerundolo. 2003. Plasmacytoid dendritic cells prime IFN-gamma-secreting melanoma-specific CD8 lymphocytes and are found in primary melanoma lesions. Eur. J. Immunol. 33:1052-1062. [DOI] [PubMed] [Google Scholar]
- 123.Salio, M., M. J. Palmowski, A. Atzberger, I. F. Hermans, and V. Cerundolo. 2004. CpG-matured murine plasmacytoid dendritic cells are capable of in vivo priming of functional CD8 T cell responses to endogenous but not exogenous antigens. J. Exp. Med. 199:567-579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Sallusto, F., and A. Lanzavecchia. 1994. Efficient presentation of soluble antigen by cultured human dendritic cells is maintained by granulocyte/macrophage colony-stimulating factor plus interleukin 4 and downregulated by tumor necrosis factor α. J. Exp. Med. 179:1109-1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Sanchez-Sanchez, N., L. Riol-Blanco, G. de la Rosa, A. Puig-Kroger, J. Garcia-Bordas, D. Martin, N. Longo, A. Cuadrado, C. Cabanas, A. L. Corbi, P. Sanchez-Mateos, and J. L. Rodriguez-Fernandez. 2004. Chemokine receptor CCR7 induces intracellular signaling that inhibits apoptosis of mature dendritic cells. Blood 104:619-625. [DOI] [PubMed] [Google Scholar]
- 126.Sauder, D. N. 2003. Imiquimod: modes of action. Br. J. Dermatol. 149(Suppl. 66):5-8. [DOI] [PubMed] [Google Scholar]
- 127.Schnurr, M., T. Toy, A. Shin, G. Hartmann, S. Rothenfusser, J. Soellner, I. D. Davis, J. Cebon, and E. Maraskovsky. 2004. Role of adenosine receptors in regulating chemotaxis and cytokine production of plasmacytoid dendritic cells. Blood 103:1391-1397. [DOI] [PubMed] [Google Scholar]
- 128.Schotte, R., M. C. Rissoan, N. Bendriss-Vermare, J. M. Bridon, T. Duhen, K. Weijer, F. Briere, and H. Spits. 2003. The transcription factor Spi-B is expressed in plasmacytoid DC precursors and inhibits T-, B-, and NK-cell development. Blood 101:1015-1023. [DOI] [PubMed] [Google Scholar]
- 129.Shurin, M. R., P. P. Plandaripande, T. D. Zorina, C. Haluszazck, V. M. Suubotin, O. Hunter, A. Brumfiels, W. J. Storkus, E. Maraskovsky, and M. Lotze. 1997. Flt3-L induces the generation of functionally active dendritic cells in mice. Cell. Immunol. 179:174-184. [DOI] [PubMed] [Google Scholar]
- 130.Siegal, F. P., P. Fitzgerald-Bocarsly, B. K. Holland, and M. Shodell. 2001. Interferon-alpha generation and immune reconstitution during antiretroviral therapy for human immunodeficiency virus infection. AIDS 15:1603-1612. [DOI] [PubMed] [Google Scholar]
- 131.Siegal, F. P., N. Kadowaki, M. Shodell, P. A. Fitzgerald-Bocarsly, K. Shah, S. Ho, S. Antonenko, and Y. J. Liu. 1999. The nature of the principal type 1 interferon-producing cells in human blood. Science 284:1835-1837. [DOI] [PubMed] [Google Scholar]
- 132.Siegal, F. P., C. Lopez, P. A. Fitzgerald, K. Shah, P. Baron, I. Z. Leiderman, D. Imperato, and S. Landesman. 1986. Opportunistic infections in acquired immune deficiency syndrome result from synergistic defects of both the natural and adaptive components of cellular immunity. J. Clin. Investig. 78:115-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Smith, E. J., I. Marie, A. Prakash, A. Garcia-Sastre, and D. E. Levy. 2001. IRF3 and IRF7 phosphorylation in virus-infected cells does not require double-stranded RNA-dependent protein kinase R or IκB kinase but is blocked by vaccinia virus E3L protein. J. Biol. Chem. 276:8951-8957. [DOI] [PubMed] [Google Scholar]
- 134.Sorg, R. V., G. Kögler, and P. Wernet. 1999. Identification of cord blood dendritic cells as an immature CD11c− population. Blood 93:2302-2307. [PubMed] [Google Scholar]
- 135.Soumelis, V., I. Scott, F. Gheyas, D. Bouhour, G. Cozon, L. Cotte, L. Huang, J. A. Levy, and Y. J. Liu. 2001. Depletion of circulating natural type 1 interferon-producing cells in HIV-infected AIDS patients. Blood 98:906-912. [DOI] [PubMed] [Google Scholar]
- 136.Spits, H., F. Couwenberg, A. Q. Bakker, K. Weijer, and C. H. Uittenbogaart. 2000. Id2 and Id3 inhibit development of CD34+ stem cells into predendritic cell (Pre-DC)2 but not into pre-DC1: evidence for a lymphoid origin of pre-DC2. J. Exp. Med. 192:1775-1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Svensson, H., B. Cederblad, M. Lindahl, and G. Alm. 1996. Stimulation of natural interferon-alpha/beta-producing cells by Staphylococcus aureus. J. Interferon Cytokine Res. 16:7-16. [DOI] [PubMed] [Google Scholar]
- 138.Tabeta, K., P. Georgel, E. Janssen, X. Du, K. Hoebe, K. Crozat, S. Mudd, L. Shamel, S. Sovath, J. Goode, L. Alexopoulou, R. A. Flavell, and B. Beutler. 2004. Toll-like receptors 9 and 3 as essential components of innate immune defense against mouse cytomegalovirus infection. Proc. Natl. Acad. Sci. USA 101:3516-3521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Takauji, R., S. Iho, H. Takatsuka, S. Yamamoto, T. Takahashi, H. Kitagawa, H. Iwasaki, R. Iida, T. Yokochi, and T. Matsuki. 2002. CpG-DNA-induced IFN-alpha production involves p38 MAPK-dependent STAT1 phosphorylation in human plasmacytoid dendritic cell precursors. J. Leukoc. Biol. 72:1011-1019. [PubMed] [Google Scholar]
- 140.Takeda, K., T. Kaisho, and S. Akira. 2003. Toll-like receptors. Annu. Rev. Immunol. 21:335-376. [DOI] [PubMed] [Google Scholar]
- 141.Tordjman, R., Y. Lepelletier, V. Lemarchandel, M. Cambot, P. Gaulard, O. Hermine, and P. H. Romeo. 2002. A neuronal receptor, neuropilin-1, is essential for the initiation of the primary immune response. Nat. Immunol. 3:477-482. [DOI] [PubMed] [Google Scholar]
- 142.Traver, D., K. Akashi, M. Manz, M. Merad, T. Miyamoto, E. G. Engleman, and I. L. Weissman. 2000. Development of CD8α-positive dendritic cells from a common myeloid progenitor. Science 290:2152-2154. [DOI] [PubMed] [Google Scholar]
- 143.Tsujimura, H., T. Tamura, C. Gongora, J. Aliberti, E. S. C. Reis, A. Sher, and K. Ozato. 2003. ICSBP/IRF-8 retrovirus transduction rescues dendritic cell development in vitro. Blood 101:961-969. [DOI] [PubMed] [Google Scholar]
- 144.Tsujimura, H., T. Tamura, and K. Ozato. 2003. Cutting edge: IFN consensus sequence binding protein/IFN regulatory factor 8 drives the development of type I IFN-producing plasmacytoid dendritic cells. J. Immunol. 170:1131-1135. [DOI] [PubMed] [Google Scholar]
- 145.Vallin, H., A. Perers, G. V. Alm, and L. Ronnblom. 1999. Anti-double-stranded DNA antibodies and immunostimulatory plasmid DNA in combination mimic the endogenous IFN-alpha inducer in systemic lupus erythematosus. J. Immunol. 163:6306-6313. [PubMed] [Google Scholar]
- 146. Vanbervliet, B., N. Bendriss-Vermare, C. Massacrier, B. Homey, O. de Bouteiller, F. Briere, G. Trinchieri, and C. Caux. 2003. The inducible CXCR3 ligands control plasmacytoid dendritic cell responsiveness to the constitutive chemokine stromal cell-derived factor 1 (SDF-1)/CXCL12. J. Exp. Med. 198:823-830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. van Kooyk, Y., and T. B. Geijtenbeek. 2003. DC-SIGN: escape mechanism for pathogens. Nat. Rev. Immunol. 3:697-709. [DOI] [PubMed] [Google Scholar]
- 148.Vermi, W., R. Bonecchi, F. Facchetti, D. Bianchi, S. Sozzani, S. Festa, A. Berenzi, M. Cella, and M. Colonna. 2003. Recruitment of immature plasmacytoid dendritic cells (plasmacytoid monocytes) and myeloid dendritic cells in primary cutaneous melanomas. J. Pathol. 200:255-268. [DOI] [PubMed] [Google Scholar]
- 149.Verthelyi, D., R. T. Kenney, R. A. Seder, A. A. Gam, B. Friedag, and D. M. Klinman. 2002. CpG oligodeoxynucleotides as vaccine adjuvants in primates. J. Immunol. 168:1659-1663. [DOI] [PubMed] [Google Scholar]
- 150.Waller, E. K., H. Rosenthal, T. W. Jones, J. Peel, S. Lonial, A. Langston, I. Redei, I. Jurickova, and M. W. Boyer. 2001. Larger numbers of CD4(bright) dendritic cells in donor bone marrow are associated with increased relapse after allogeneic bone marrow transplantation. Blood 97:2948-2956. [DOI] [PubMed] [Google Scholar]
- 151.Wollenberg, A., M. Wagner, S. Gunther, A. Towarowski, E. Tuma, M. Moderer, S. Rothenfusser, S. Wetzel, S. Endres, and G. Hartmann. 2002. Plasmacytoid dendritic cells: a new cutaneous dendritic cell subset with distinct role in inflammatory skin diseases. J. Investig. Dermatol. 119:1096-1102. [DOI] [PubMed] [Google Scholar]
- 152.Wu, L., A. D'Amico, H. Hochrein, M. O'Keeffe, K. Shortman, and K. Lucas. 2001. Development of thymic and splenic dendritic cell populations from different hemopoietic precursors. Blood 98:3376-3382. [DOI] [PubMed] [Google Scholar]
- 153.Yoneyama, H., S. Narumi, Y. Zhang, M. Murai, M. Baggiolini, A. Lanzavecchia, T. Ichida, H. Asakura, and K. Matsushima. 2002. Pivotal role of dendritic cell-derived CXCL10 in the retention of T helper cell 1 lymphocytes in secondary lymph nodes. J. Exp. Med. 195:1257-1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Yonezawa, A., R. Morita, A. Takaori-Kondo, N. Kadowaki, T. Kitawaki, T. Hori, and T. Uchiyama. 2003. Natural alpha interferon-producing cells respond to human immunodeficiency virus type 1 with alpha interferon production and maturation into dendritic cells. J. Virol. 77:3777-3784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Zeng, J., P. Fournier, and V. Schirrmacher. 2002. Stimulation of human natural interferon-alpha response via paramyxovirus hemagglutinin lectin-cell interaction. J. Mol. Med. 80:443-451. [DOI] [PubMed] [Google Scholar]
- 156.Zou, W., J. Borvak, S. Wei, T. Isaeva, D. T. Curiel, and T. J. Curiel. 2001. Reciprocal regulation of plasmacytoid dendritic cells and monocytes during viral infection. Eur. J. Immunol. 31:3833-3839. [DOI] [PubMed] [Google Scholar]
- 157.Zou, W., V. Machelon, A. Coulomb-L'Hermin, J. Borvak, F. Nome, T. Isaeva, S. Wei, R. Krzysiek, I. Durand-Gasselin, A. Gordon, T. Pustilnik, D. T. Curiel, P. Galanaud, F. Capron, D. Emilie, and T. J. Curiel. 2001. Stromal-derived factor-1 in human tumors recruits and alters the function of plasmacytoid precursor dendritic cells. Nat. Med. 7:1339-1346. [DOI] [PubMed] [Google Scholar]