Abstract
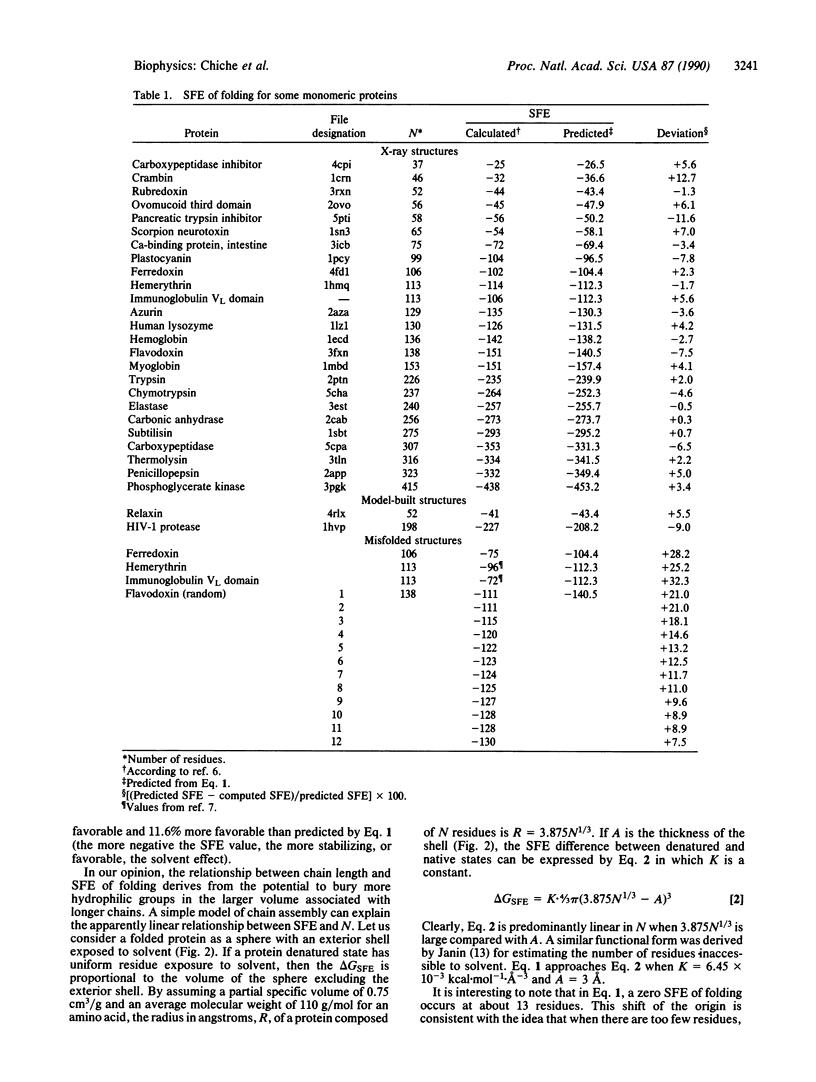
A systematic study of solvation free energy of folding for proteins with known crystallographic structures is presented. There is a linear relationship between the solvation free energy of folding and the protein size. This relationship, which can be rationalized by a simple model of chain folding, allows prediction of the solvation free energy of folding for proteins for which no high resolution structures are available. All misfolded structures analyzed show solvation free energies higher than predicted; however, some of the misfolded structures have values close enough to the predicted values so that one must be very careful when using such a criterion to check the correctness of a protein model.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bernstein F. C., Koetzle T. F., Williams G. J., Meyer E. F., Jr, Brice M. D., Rodgers J. R., Kennard O., Shimanouchi T., Tasumi M. The Protein Data Bank: a computer-based archival file for macromolecular structures. J Mol Biol. 1977 May 25;112(3):535–542. doi: 10.1016/s0022-2836(77)80200-3. [DOI] [PubMed] [Google Scholar]
- Blundell T., Sibanda B. L., Pearl L. Three-dimensional structure, specificity and catalytic mechanism of renin. Nature. 1983 Jul 21;304(5923):273–275. doi: 10.1038/304273a0. [DOI] [PubMed] [Google Scholar]
- Carlson W., Karplus M., Haber E. Construction of a model for the three-dimensional structure of human renal renin. Hypertension. 1985 Jan-Feb;7(1):13–26. doi: 10.1161/01.hyp.7.1.13. [DOI] [PubMed] [Google Scholar]
- Chiche L., Gaboriaud C., Heitz A., Mornon J. P., Castro B., Kollman P. A. Use of restrained molecular dynamics in water to determine three-dimensional protein structure: prediction of the three-dimensional structure of Ecballium elaterium trypsin inhibitor II. Proteins. 1989;6(4):405–417. doi: 10.1002/prot.340060407. [DOI] [PubMed] [Google Scholar]
- Cohen F. E., Sternberg M. J., Taylor W. R. Analysis and prediction of the packing of alpha-helices against a beta-sheet in the tertiary structure of globular proteins. J Mol Biol. 1982 Apr 25;156(4):821–862. doi: 10.1016/0022-2836(82)90144-9. [DOI] [PubMed] [Google Scholar]
- Eisenberg D., McLachlan A. D. Solvation energy in protein folding and binding. Nature. 1986 Jan 16;319(6050):199–203. doi: 10.1038/319199a0. [DOI] [PubMed] [Google Scholar]
- Ghosh D., Furey W., Jr, O'Donnell S., Stout C. D. Structure of a 7Fe ferredoxin from Azotobacter vinelandii. J Biol Chem. 1981 May 10;256(9):4185–4192. [PubMed] [Google Scholar]
- Isaacs N., James R., Niall H., Bryant-Greenwood G., Dodson G., Evans A., North A. C. Relaxin and its structural relationship to insulin. Nature. 1978 Jan 19;271(5642):278–281. doi: 10.1038/271278a0. [DOI] [PubMed] [Google Scholar]
- Janin J. Surface and inside volumes in globular proteins. Nature. 1979 Feb 8;277(5696):491–492. doi: 10.1038/277491a0. [DOI] [PubMed] [Google Scholar]
- Novotný J., Bruccoleri R., Karplus M. An analysis of incorrectly folded protein models. Implications for structure predictions. J Mol Biol. 1984 Aug 25;177(4):787–818. doi: 10.1016/0022-2836(84)90049-4. [DOI] [PubMed] [Google Scholar]
- Novotný J., Rashin A. A., Bruccoleri R. E. Criteria that discriminate between native proteins and incorrectly folded models. Proteins. 1988;4(1):19–30. doi: 10.1002/prot.340040105. [DOI] [PubMed] [Google Scholar]
- Reid L. S., Thornton J. M. Rebuilding flavodoxin from C alpha coordinates: a test study. Proteins. 1989;5(2):170–182. doi: 10.1002/prot.340050212. [DOI] [PubMed] [Google Scholar]
- Stout C. D. 7-Iron ferredoxin revisited. J Biol Chem. 1988 Jul 5;263(19):9256–9260. doi: 10.2210/pdb3fd1/pdb. [DOI] [PubMed] [Google Scholar]
- Weber I. T., Miller M., Jaskólski M., Leis J., Skalka A. M., Wlodawer A. Molecular modeling of the HIV-1 protease and its substrate binding site. Science. 1989 Feb 17;243(4893):928–931. doi: 10.1126/science.2537531. [DOI] [PubMed] [Google Scholar]


