Abstract
Intracellular pathogens are known to manipulate host cell regulatory pathways to establish an optimal environment for their growth and survival. Pathogens employ active mechanisms to hijack host cell metabolism and acquire existing nutrient and energy store. The role of the cellular energy sensor AMP-activated protein kinase (AMPK) in the regulation of cellular energy homeostasis is well documented. Here, we highlight recent advances showing the importance of AMPK signaling in pathogen-host interactions. Pathogens interact with AMPK by a variety of mechanisms aimed at reprogramming host cell metabolism to their own benefit. Stimulation of AMPK activity provides an efficient process to rapidly adapt pathogen metabolism to the major nutritional changes often encountered during the different phases of infection. However, inhibition of AMPK is also used by pathogens to manipulate innate host response, indicating that AMPK appears relevant to restriction of pathogen infection. We also document the effects of pharmacological AMPK modulators on pathogen proliferation and survival. This review illustrates intricate pathogen-AMPK interactions that maybe exploited to the development of novel anti-pathogen therapies.
Introduction
Essential requirement for survival, multicellular organisms have developed a variety of mechanisms to recognize and eliminate invading bacterial, parasite and viral pathogens. Infection triggers powerful cellular signaling events, which result in a wide range of possible immune responses. Innate and adaptive host immunity is essential for inducing and maintaining an optimal immune response and protection against infection. However, in return, pathogens have evolved specific mechanisms to circumvent the immune response to survive in infected hosts. In addition, successful pathogens remodel the host cell to establish an optimal environment for their persistence and to reallocate resources for their replication. To acquire essential nutrient and energy for their own growth and proliferation, intracellular pathogens exploit the existing host nutrient stores and energy producing sources[1]. The metabolic manipulation of host cells resources is currently recognized to play an important role in the pathology of infection and there is growing interest in identifying the underlying mechanisms. Here, we detail how intracellular pathogens hijack cellular metabolism by suppressing or increasing the activity of the energy sensor AMP-activated protein kinase (AMPK).
AMPK regulates cellular energy homeostasis
AMPK, a cellular fuel gauge
A critical requirement for cell survival and growth is the maintenance of energy balance. This coordination is achieved through the function of AMPK, a cellular “fuel gauge” that directs metabolic adaptation to support the growth demands[2]. At a critical level of signals related to impaired cellular energy status (high AMP/ATP and ADP/ATP ratios), occurring when cells are exposed to metabolic stress (e.g., nutrient deprivation, hypoxia and viral infection), AMPK functions to restore energy homeostasis by switching off biosynthetic pathways consuming ATP while switching on catabolic pathways that produce ATP. AMPK has been conserved throughout eukaryote evolution as a central sensor and regulator of energy homeostasis.
AMPK structure and regulation
Mammalian AMPK is a heterotrimeric complex consisting of a catalytic (α) and two regulatory (β and γ) subunits, encoded by different genes (α1, α2, β1, β2, γ1, γ2, and γ3), enabling the formation of a diverse collection of αβγ heterotrimer combinations. AMPK is activated by binding of AMP and/or ADP to the γ-subunit, causing structural changes and subsequent phosphorylation of a conserved residue within the activation loop (Thr172) of the catalytic α subunit, which is required for AMPK activity[2]. In addition, another effcet of AMP and ADP binding is to prevent dephosphorylation of Thr172 and subsequent inactivation of the AMPK complex by cellular phosphatases. Furthermore, the binding of AMP (but not ADP) enhances AMPK activity by allosteric activation. Of note, all the effects of AMP and ADP are antagonized by binding of ATP, indicating that cellular AMP/ATP and ADP/ATP ratios primarily define the levels of AMPK activation. The major upstream kinase is liver kinase B1 (LKB1), a tumour suppressor mutated in Peutz Jeghers syndrome. Interestingly, LKB1 appears to be constitutively active, reinforcing the importance of AMP/ ADP binding in the resistance of AMPK to dephosphorylation in the mechanism of AMPK activation. Anon-canonical activation mechanism involves the phosphorylation of Thr172 by calcium/calmodulin-dependent protein kinase kinase β (CaMKKβ) in response to a rise in intracellular Ca2+[2].
Downstream effects of AMPK activation
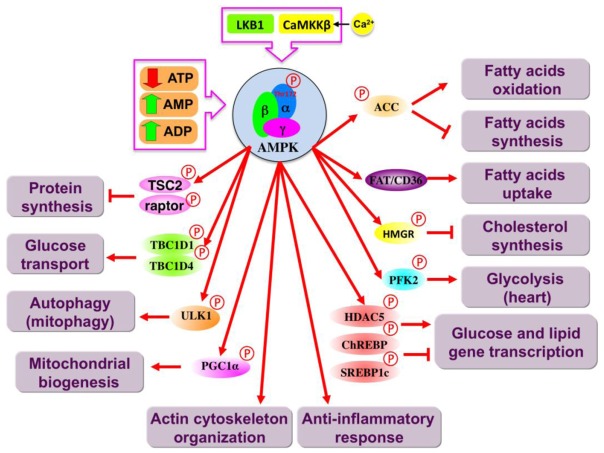
It is well established that AMPK represents a point of conversion of regulatory signals monitoring cellular energy status. In addition, recent evidence indicates that AMPK may also maintain cell survival through the regulation of processes other than metabolic pathways, such as autophagy, cytoskeletal organization, cell cycle and inflammation. The many proposed downstream responses to AMPK activation are summarized in Figure 1. There have been many excellent reviews recently published which cover the downstream effects of AMPK activation (e.g., [2, 3]). Briefly, in response to energy stress, activation of AMPK maintains cellular energy homeostasis by changing the balance between anabolism and catabolism. The overall result is the inhibition of energy-consuming biosynthetic pathways, such as lipid and protein synthesis, and activation of ATP-producing catabolic pathways, such as fatty acid oxidation, glucose uptake, and glycolysis (at least in the heart). AMPK regulates these pathways via acute and long term mechanisms, involving the phosphorylation of key enzymes and regulation of gene expression. It is important to note that AMPK phosphorylates acetyl CoA carboxylase (ACC), a key enzyme for fatty acid synthesis and oxidation. AMPK-dependent phosphorylation of ACC suppresses malonyl-CoA synthesis. As malonyl-CoA is both a critical precursor of biosynthesis of fatty acids and an inhibitor of fatty acid uptake into mitochondria via the transport system involving carnitine palmitoyltransferase-1, this has the dual effect of inhibiting fatty acid synthesis and enhancing fatty acid oxidation[4]. In complement to this metabolic switch, AMPK stimulates expression and translocation to the plasma membrane of glucose transporter 4 (GLUT4) through inhibition of HDAC5 activity and TBC1D1 and AS160, two Rab-GAP (GTPase-activating protein) proteins[5]. AMPK positively regulates glycolysis by phosphorylating 6-phosphofructo-2-kinase (PFK-2)[6]. AMPK decreases protein synthesis by phosphorylating the tuberous sclerosis complex 1/2 (TSC1/2), an inhibitor of the mammalian target of rapamycin (mTOR) signaling pathway, and the regulatory associated protein of mTOR (raptor), and targeting the eEF2K/ eEF2 signaling pathway[7–10]. Furthermore, it has been postulated that AMPK-dependent phosphorylation of PGC1α stimulates mitochondrial biogenesis[11]. In addition to a role in regulating metabolism, AMPK is a critical component of autophagy induction by phosphorylating and activating ULK1, the protein kinase that initiates the process[12, 13] and also through the inhibition of mTOR signaling pathway. Recent studies have also implicated AMPK in actin polymerization and phosphorylation of cytoskeletal targets[14].
Figure 1. Target proteins and pathways regulated by AMPK.
Main catabolic and anabolic pathways activated and inhibited, respectively, by AMPK activation are depicted. The proteins that are likely to mediate the effects of AMPK are shown (Direct AMPK targets are labeled with a phosphorylation mark). ACC, acetyl CoA carboxylase; AMPK, AMP-activated protein kinase; CaMKKβ, calcium/calmodulin-dependent protein kinase kinase β; ChREBP, carbohydrate response element-binding protein; FAT/CD36, fatty acid translocase/cluster of differentiation; HDAC5, histone deacetylase 5; HMGR, 3-hydroxy-3-methylglutaryl-CoA reductase; LKB1, liver kinase B1; PFK2, 6-phosphofructokinase 2; PGC1α, peroxisome proliferator-activated receptor γ co-activator-1α; SREBP1c, sterol regulatory element binding protein TCB1D1/4, Tre-2/BUB2/cdc 1 domain 1/4; TSC2, Tuberous sclerosis 2; ULK1, Unc-51-like kinase 1.
A wide variety of AMPK agonists (i.e., AICAR and metformin) have been commonly used to define the downstream actions of AMPK activation (Table 1). However, several recent studies have reported that many of the effects of these pharmacological compounds are AMPK-independent [15, 16] and activates AMPK indirectly through the modulation of cellular AMP:ATP ratio [17]. In the recent years, the use of more specific AMPK agonists that directly bind to the AMPK heterotrimer complex has emerged as a valuable pharmacological alternative to assess the physiological role of AMPK activation [18–20] (Table 1). However, it is highly recommended to confirm the results obtained with pharmacological AMPK activators with genetic knockdown/ inactivation of AMPK subunits as specific controls for activity rather than using the only available AMPK inhibitor, compound C, originally described as a specific inhibitor[21]. This compound, also known as dorsomorphin, has many AMPK-independent effects due to the inhibition of many other kinases with greater potency[22].
Table 1.
Drugs acting through AMPK signaling to restrict intracellular pathogens survival and proliferation.
| Drug | Description | Pathogen | Survival | ref |
|---|---|---|---|---|
|
| ||||
| AICAR | AMPK activator | HCMV | − | [43] |
| HCV | − | [51] | ||
| HIV-1 | − | [55] | ||
| Influenza | − | [60] | ||
| Mtb | − | [68, 69] | ||
| L. infantum | + | [73] | ||
| Pl. falciparum | − | [89] | ||
|
| ||||
| Metformin | AMPK activator | HCV | − | [90] |
| Mtb | − | [51, 69] | ||
|
| ||||
| A-769662 | AMPK activator | RVFV | − | [50] |
| KUNV | − | [50] | ||
| HCV | − | [51] | ||
|
| ||||
| Salicylate | AMPK activator | PRRSV | − | [91] |
|
| ||||
| EGCG | AMPK activator | T. brucei | (−) | [92, 93] |
|
| ||||
| Resveratrol | SIRT1 activator | HIV-1 | − | [55] |
|
| ||||
| AM251 | CB1 antagonist | HCV | − | [59] |
|
| ||||
| Tanshinone II A | Redox balance | HIV-1 | − | [61] |
|
| ||||
| Bryostatin | PKC inhibitor | HIV-1 | − | [58] |
|
| ||||
| Compound C | AMPK inhibitor | Ebolavirus | − | [27] |
| ARV | − | [34] | ||
|
| ||||
| STO-609 | CaMKK inhibitor | HCMV | − | [32] |
Abbreviations: AICAR, 5-aminoimidazole-4-carboxamide-1-β-D-ribofuranoside; AMPK, AMP-activated protein kinase; ARV, avian reovirus; CB1, cannabinoid 1; CaMKK, calcium/calmodulin-dependent protein kinase kinase; EGCG, (−)-epigallocatechin-3-gallate (EGCG) HCMV, human cytomegalovirus ; HCV, hepatitis C virus; HIV-1, human immunodeficiency virus-1; KUNV, kunjin virus; L. infantum, Leishmania infantum; Mtb, Mycobacterium tuberculosis; Pl. falciparum, Plasmodium falciparum; PKC, protein kinase C; PRRSV, porcine reproductive and respiratory syndrome virus; RVFV, Rift Valley fever virus; SIRT1, Sirtuin 1, T. brucei, Trypanosomabrucei.
AMPK in the control of viral infection
To achieve optimal levels of proliferation and spread, viruses are dependent on a balanced interaction between viral and cellular proteins. Especially protein kinases are important regulators of virus-host interaction and many different host cell signaling pathways are subverted to enable the generation of the next round of infectious virions. In one hand, viruses turn off anti-viral and pro-apoptotic pathways, and in the other hand activate anti-apoptotic and pro-survival pathways. To identify host factors influencing the replication and spread of viruses, functional high throughput small interfering RNA (siRNA) and bioinformatics-based screens as well as kinome profiling approaches have been carried out and have highlighted the important role of the cellular protein kinase AMPK during viral infection [23–27]. Given the recognized role of AMPK in the control of cell metabolism, it is not surprising that this energy sensor appears as a specific target for virus-mediated reprogrammation of host cells. All over the sophisticated lifecycle of viruses, a number of temporally regulated steps have been linked with the suppression or increase in AMPK activation, including virus entry, expression of early gene products, replication, and assembly (Figures 2A and B).
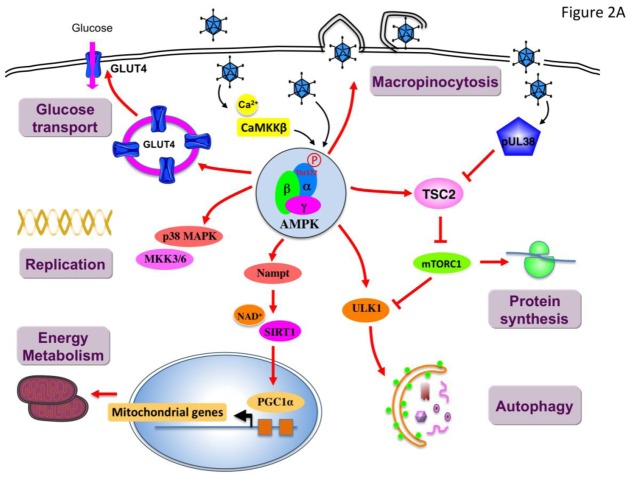
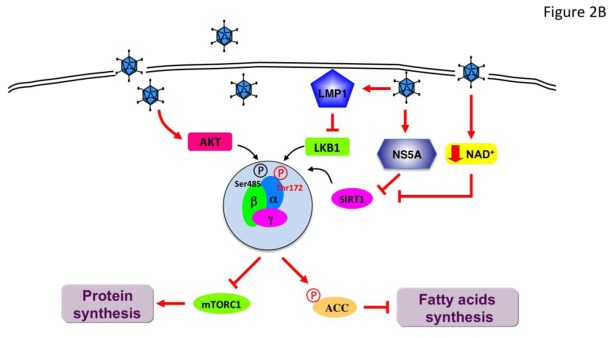
Figure 2. Viral infection mediates a differential regulation of AMPK signaling pathway.
During infection, manipulation of host AMPK activity is essential in the establishment and/or maintenance of infection. Virus infection may result in the stimulation (A) or inhibition (B) of AMPK to support virus growth and replication. AMPK, AMP-activated protein kinase; CaMKKβ, calcium/calmodulin-dependent protein kinase kinase β; GLUT4, glucose transporter 4; Nampt, nicotinamidephosphoribosyltransferase; MKK3/6, MAPK kinase 3/6; mTORC1, mechanistic target of rapamycin complex 1; LKB1, liver kinase B1; LMP1, latent membrane protein 1; NS5A, viral nonstructural protein 5A; p38 MAPK, p38 mitogen-activated protein kinase; SIRT1, Sirtuin 1; TSC2, Tuberous sclerosis 2; ULK1, Unc-51-like kinase 1.
AMPK contribution to virus entry
Entry is a critical step for the initial establishment of infection. Internalization by macropinocytosis is the primary entry mechanism used by a number of viruses to enter host cells. Macropinocytosis is a well-established endocytic process that involves extensive actin-cytoskeletal rearrangement, leading to membrane ruffling and internalization of large cargo. By performing a kinome RNAi screen, in Drosophila cells Moser et al. found that AMPK promotes early steps of the vaccinia virus lifecycle and is specifically required for vaccinia-induced macropinocytic entry (Figure 2A) [25]. The regulation of macropinocytosis by AMPK is achieved through metabolic-independent functions on actin cytoskeletal remodeling[25] and phosphorylation of key cytoskeletal targets [28–30]. Interestingly, the requirement of AMPK in macropinocytic events has been also reported for the entry of the Zaire Ebola virus in Vero (African green monkey kidney) cells [27]. These results raise the possibility that AMPK may act as an important component for other viruses, such as influenza A and African swine fever viruses, also depending on this endocytic pathway to enter host cells. However, future studies investigating the contribution of AMPK in the entry of these viruses are warranted.
AMPK contribution to virus replication
AMPK has been identified in different high-throughput screens as a pro-viral host factor that influence the replication of human cytomegalovirus (HCMV) [24, 26, 31] and vaccinia virus in a panel of human tumor cell lines [23]. The importance of AMPK in HCMV replication is supported by the increase of AMPK phosphorylation at residue Thr172 in HCMV-infected cells [24, 31]. This has been further confirmed by the use of AMPK inhibitor Compound C showing an antiviral action [24, 31]. Inhibition of AMPK attenuates the expression of early gene expression as well as viral DNA replication efficiency. Interestingly, the expression of the AMPK upstream kinase CaMKK is induced by viral infection and appears also to be an important host factor for HCMV replication [32]. The inhibition of CaMKK by the specific inhibitor STO-609 or the expression of a CaMKK kinase-dead allele inhibits the production of HCMV viral progeny. Furthermore, inhibition of CaMKK reduced the phosphorylation of AMPK and downstream targets during HCMV infection, indicating that HCMV infection requires CaMKK activity to activate AMPK [31]. However, the exact mechanism responsible for CaMKK activation remains unclear, but may potentially involve the release of endoplasmicreticulumCa2+stores during HCMV infection [33]. Of note, LKB1 is probably not involved in the activation of AMPK in response to viral infection as this protein kinase has never been identified as hit in the siRNA kinome screens performed to date [24–26].
It has been shown that AMPK plays a major role in the interaction between avian reovirus (ARV) and host cells. AMPK is activated during ARV infection and contributes to maintain the efficiency of replication in Vero cells and transformed chicken embryo fibroblasts[34]. AMPK facilitates mitogen-activated protein kinase (MAPK) kinase (MKK) 3/6 - MAPK p38 signaling that is critical for ARV replication. The action of AMPK is independent of the inhibition of phosphatidylinositol 3-kinase (PI3K) or mechanistic target of rapamycin complex 1 (mTORC1) signaling, as wortmannin and rapamycin have no negative effects on ARV replication. Inhibition of AMPK by compound C causes down-regulation of MKK3/6 and MAPK p38 phosphorylation and ARV replication, indicating that MAPK p38 is a critical downstream factor involved in the regulation of ARV replication. Interestingly, activation of MAPK p38 signaling appears to be specific to avian viruses because MAPK p38phosphorylation is not observed after infection with the mammalian counterpart viruses [34].
As an energy sensor, not surprisingly, AMPK is involved in the regulation of autophagy and may serve as a logical target for manipulation to enhance virus replication (Figure 2A). Indeed, it is now recognized that many viruses exploit the autophagy machinery to facilitate their replication and survival in host cells. Autophagy is a catabolic cellular process that involves the degradation and turnover of damaged organelles and long-lived proteins after forming a cytoplasmic double-membrane vesicle or autophagosome. Activation of autophagy during nutrient starvation is a way to provide a limited amount of nutrients for the survival of cells. Thus, many viruses have evolved mechanisms to promote autophagy to support the high energy cost of replication. Some viruses (mainly positive stranded RNA viruses) use the autophagy machinery to hide their RNA replication intermediate into the cytoplasmic vesicles. However, autophagy can also destroy cytosolic pathogens by targeting viral components to degradation through lysosomes, in a process called xenophagy. While the evasion of autophagy by pathogens has been demonstrated, recent work suggests that some viruses require components of the autophagic machinery to benefit their replication and is therefore a critical process in viral pathogenesis [35]. Recent evidence have showed that dengue virus (DENV) induces autophagy to degrade lipid droplets (lipophagy) and release free fatty acids required for efficient virus replication in human hepatoma cell lines [36]. This concept is further supported by the rescue of DENV infection by addition of exogenous fatty acid when autophagy is inhibited[36]. Interestingly, some viruses encode autophagy-promoting proteins to induce autophagy. Simian virus 40 (SV40) small T antigen activates AMPK through the inactivation of protein phosphatase 2A (PP2A) which results in the inactivation of mTOR and induction of autophagy for survival during glucose deprivation of cancer cells[37]. Similarly, the nonstructural protein p17 of ARV functions as a positive regulator of autophagy by activating AMPK Vero cells and transformed chicken embryo fibroblasts[38]. Another example is the capsid protein from porcine circovirus type 2 (PCV2) that induces autophagosome formation and enhances autophagic flux by increasing AMPK phosphorylation with a concomitant inhibition of mTOR signaling in porcine kidney cells[39]. In the context of the human immunodeficiency virus (HIV-1) infection, inhibition of autophagy reduces viral replication. Although in this model the role of AMPK has not yet explored, it is interesting to note that infection of CD4+ T cells is associated with a mitochondrial depolarization and ATP loss, concomitantly with the activation of the autophagy pathway [40]. Thus AMPK could be involved in the regulation of HIV replication.
AMPK-mediated coordination of cellular metabolism during viral infection
A growing body of evidence implicates a key role for metabolic function during viral infection and recent studies have reported that viruses target the host cell metabolic machinery to favor their propagation. For instance, upon infection, HCMV takes control of numerous cellular processes and reprograms the metabolic activity of the host cell by increasing glucose uptake through the increase of glucose transporter GLUT4 levels at the plasma membrane [32, 41]. Consistent with the role of AMPK in the modulation of key glycolytic enzymes, including glucose transporters (Glut1 and Glut4), hexokinase, and phospho-fructokinase-2 (PFK-2), to increase glycolytic flux[2], AMPK has been linked to a substantial subset of metabolic changes induced during HCMV infection in human fibroblasts [26, 31]. By stimulating glucose uptake, AMPK provides fuel for glycolysis and the TCA cycle. These results demonstrate that AMPK is required to create a favorable metabolic environment for HCMV replication by phosphorylating multiple substrates that switch on catabolic pathways producing ATP. However, AMPK activation also induces the phosphorylation of substrates that block ATP-consuming anabolic pathways, such as fatty acid and protein synthesis, disfavoring viral replication[42]. The substrates of AMPK are ACC, playing a key role in the synthesis and metabolism of fatty acids, and the tuberous sclerosis protein complex TSC1/2, which negatively regulates translation through inhibition of mTOR[2]. Thus, HCMV-induced activation of AMPK would be detrimental to infection by inhibition of both fatty acid synthesis and protein translation. To resolve this paradigm, HCMV uncouples the catabolic and anabolic regulation by AMPK. An HCMV-encoded protein (pUL38) binds to the TSC1/2 complex and prevents the inactivation of mTOR signaling by activated AMPK in human foreskin fibroblasts[43, 44]. Although mTOR is an essential component of protein translation in the productive viral growth cycle, it is also required for HCMV-mediated induction of ACC expression and activation of fatty acid synthesis through the proteolytic processing of sterol regulatory element-binding protein 1c (SREBP1c)[42]. HCMV also exploits host defense mechanisms to increase lipogenesis in infected cells. Upon infection, HCMV induces the expression of interferon-inducible host protein, viperin[45]. Interaction with the viral mitochondrial inhibitor of apoptosis (vMIA) relocalizes viperin to the mitochondria where it inhibits β-oxidation as shown in human fibroblasts [45]. This results in reduced cellular ATP levels and activation of AMPK. In turn, AMPK activation induces GLUT4 expression, stimulates glucose uptake and increases glycolytic flux in infected cells. Increase in intracellular glucose, in addition to the induction of glycolysis, leads to the enhanced transcription of genes encoding lipogenic enzymes through the translocation to the nucleus of the glucose-regulated transcription factor carbohydrate response element-binding protein (ChREBP)[46]. These data indicates that viperin is a major effector in the remodeling of host cell lipid metabolism during infection, facilitating the formation of HCMV membrane envelope.
During the course of infection, herpes simplex virus type 1 (HSV-1) differentially regulates the activity of AMPK for its propagation and survival in primary cortical neurons[47]. At early times post infection, HSV-1 downregulates AMPK phosphorylation but this inhibition is gradually reversed during the course of infection. Initial inhibition of AMPK would be beneficial for the synthesis of viral proteins and lipids, as AMPK activation inhibits protein translation and lipid synthesis [2]. At later times point, AMPK activation represents a strategy to counteract antiviral host mechanism (e.g., induction of apoptosis) and to hijack the cellular metabolic pathways to maintain cellular energy status and establish latency in infected cells [47]. The effects of AMPK are carried out through the modulation of cellular NAD+ levels and activation of sirtuin 1 (SIRT1), a NAD+-dependent histone deacetylase, interfering with the p53-dependent pro-apoptotic response and metabolic pathways. SIRT1 and AMPK are known to both regulate each other and share similar effects on diverse processes such as cellular fuel metabolism and mitochondrial function [48]. On one hand, AMPK can function as a SIRT1 activator by increasing the cellular NAD+/NADH ratio via transcription of the NAD+ biosynthetic enzyme nicotinamidephosphoribosyltransferase (Nampt), converting nicotinamide into NAD+, leading to SIRT1 activation. On other hand, SIRT1 modulation of the acetylation status of LKB1 is involved in the activation of AMPK, consistent with the concept that AMPK and SIRT1 are components of a cycle. During HSV-1 infection, activation of the AMPK/SIRT1 pathway contributes to the metabolic homeostasis of cell host by activation of peroxisome proliferator-activated receptor γ co-activator-1α (PGC1α), a master regulator of mitochondrial biogenesis [47].
AMPK in innate antiviral response
Recent discoveries have revealed a critical relationship between innate immune response to pathogens and cellular metabolic sensing pathways to confer an efficient defense against infection [49]. Emerging evidence indicate that AMPK have intrinsic innate immune functions and restricts the replication of viruses (Figure 2B). Moser et al. showed evidence that AMPK activation with Rift Valley fever virus (RVFV) infection restricts its replication by blocking fatty acid synthesis through ACC phosphorylation in mouse embryonic fibroblasts[50]. This was further supported by the rescue of AMPK-mediated RVFV restriction by pre-treatment with palmitate, the first product of fatty acid synthesis. Inhibition of AMPK by compound C significantly increased RVFV infection but treatment with the mTORC1 inhibitor rapamycin showed no significant difference in RVFV infection, indicating that mTORC1 signaling is not required for the antiviral activity of AMPK. Activation of AMPK is dependent on the upstream AMPK kinase LKB1, and is triggered by incoming particles but not viral replication. Inhibition of the alternative upstream kinase CaMKK by STO-609 had no effect on infection level. Additionally, AMPK and LKB1 also appears to act as intrinsic immune components to inhibit viral replication of multiple arboviruses, including flavivirus kunjin virus (KUNV), togavirus sindbis virus (SINV), and rhabdovirus vesicular stomatitis virus (VSV) [50]. These results indicate that LKB1/AMPK signaling represents a potential pharmacological target to limit viral replication, at least for viruses known to have important lipid dependencies. Interestingly, RFVF and KUNV infection was significantly decreased in the presence of the direct AMPK activator A-769662 in a dose-dependent manner (Table 1).
Since AMPK acts as a restriction factor for virus replication, mainly by inhibiting host catabolic and biosynthetic activities, viruses has evolved mechanisms of evasion to limit AMPK activity during the course of infection (Figure 2B). Different mechanisms for repression of AMPK signaling have been reported. Hepatitis C virus (HCV)-encoded NS5A protein has been shown to down-regulate AMPK activation by inhibiting AMPK Thr172 phosphorylation in human hepatoma cell lines[51]. HCV infection activates the protein kinase B (PKB)/(AKT) pathway to phosphorylate AMPK at Ser485, resulting in the inhibition of AMPK activation. The importance of Ser485 phosphorylation for HCV genome replication of Ser485was confirmed by over-expression of a nonphosphorylable mutant (S485A)[51]. This mechanism of AMPK inhibition may be shared with HMCV during the early time points post-infection where expression of immediate early proteins is associated with increased PKB/AKT activation [43, 52]. Similarly, it has been shown that PKB/AKT antagonizes AMPK activation by increasing AMPK Ser485 phosphorylation in ischemic heart in response to insulin[53]. Reduced AMPK activity during HCV infection permits the synthesis of lipids at high levels, which are required for virus replication. Conversely, activation of AMPK with pharmacological agonists (Table 1) abrogates lipid accumulation and reduced HCV replication [51]. To promote proliferation and transformation, the Epstein-Barr virus (EBV)-encoded latent membrane protein 1 (LMP1) oncogenic protein inhibits the activity of AMPK through the phosphorylation of LKB1 at Ser325 and Ser428 mediated by the ERK-MAPK signaling pathway in human nasopharyngeal epithelial cells[54]. By altering the cellular redox state (decreased NAD+ levels), the HIV-1-encoded Tat protein inhibits the activity of host NAD+-dependent histone deacetylase SIRT1 leading to decreased AMPK activity in HeLa cell line that expresses high levels of CD4 [55]. In this context, the inhibition of the SIRT1/AMPK cellular checkpoint is associated with increased Tat-induced HIV-1 LTR transcription and enhanced HIV-1 replication [55]. Inhibition of HIV-1 transcription transactivation may be related to the SIRT1-mediated deacetylation of Tat[56] because Tat transcriptional activity is dependent on cellular acetylation by targeting cellular histone acetyltransferases [57]. However, the role of AMPK appears more complex. Indeed, AMPK activation was found to coordinate the reactivation of latent HIV-1 proviruses process via protein kinase C (PKC) signaling[58]. Therefore these results suggest - that AMPK has differential roles during HIV-1 lifecycle.
Antiviral therapeutics opportunities
Given the importance of virus-host interaction in controlling the different steps of viral infection, drugs targeting host proteins are of attractive therapeutic potential. Manipulation of the energy sensor AMPK has emerged as a promising antiviral strategy. In Table 1are listed pharmacological compounds with significant antiviral activity acting either through the activation or inhibition of AMPK signaling. Notably, cannabinoid 1 (CB1) antagonist AM251 shows effective antiviral effects against HCV in human hepatoma cells[59]. These effects are, at least in part, due to the inhibition of lipogenesis mediated by activation of AMPK. This is consistent with previous results obtained with the AMPK agonists5-aminoimidazole-4-carboxamide-1-β-D-ribofuranoside (AICAR), metformin and A-769662 on the inhibition of HCV replication by decreasing lipid accumulation [51] (Table 1). In addition, the use of AMPK agonist AICAR has been reported to protect mice from the deleterious effect of influenza infection [60].
AMPK activation was shown to be a crucial component in the inhibitory effect of Tanshinone II A (a lipid-soluble monomer extracted from the root of Salvia miltiorrhiza) on Tat-induced HIV-1 LTR transactivation in a HeLa-derived cell line expressing surface CD4, CXCR4, CCR5, and containing a chromatin-integrated HIV-1 LTR[61]. Tanshinone II A plays an important role in the regulation of cellular redox balance during HIV-1 infection and can revert Tat-induced inhibition of SIRT1 activity. These effects are mediated by redox-regulated Nampt activity and modulation of Nampt through activation of AMPK [61]. Thus, targeting redox signaling emerges as a novel therapeutic strategy to fight against HIV-1 infection and may lead to the development of new class of drug with potential antiviral activity.
Bacterial infection and AMPK
Control of bacterial infection by xenophagy
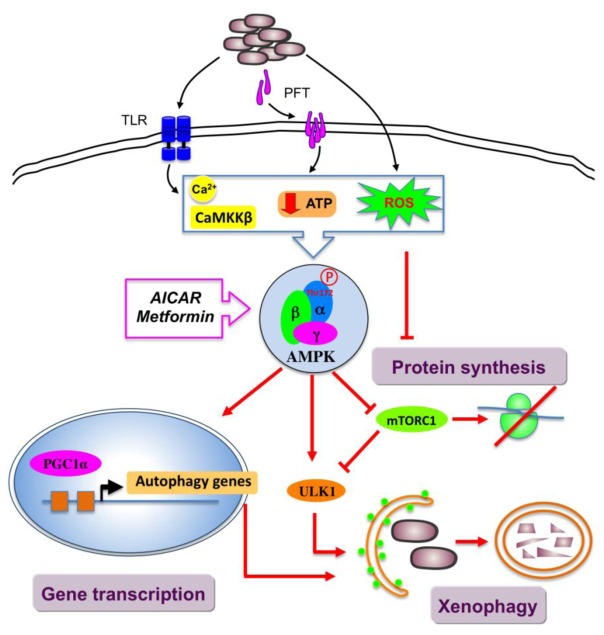
Recent evidence demonstrates that autophagy, best known as a mechanism for surviving starvation, is now considered as a critical arm of the host defense against intracellular bacterial pathogens. This selective autophagic destruction of intracellular pathogens, also known as xenophagy, plays a critical role in innate immune response to bacterial invasion[62]. Several pathways critical for the specific control of xenophagy have been identified. Recently, AMPK has emerged as a potential key player in the signaling pathways that contribute to the induction of autophagy during infection (Figure 3). It has been reported that infection of intestinal epithelial cells by enterotoxigenic Escherichia coli (ETEC) induces autophagy through activation of AMPK [63]. Induction of autophagy may serve as a host defense against infection because inhibition of autophagy resulted in decreased survival of infected cells. In addition, Toll-like receptor (TLR) stimulation by the lipoprotein LpqH from Mycobacterium tuberculosis (Mtb) was found to activate antibacterial autophagy through increases in intracellular calcium following AMPK activation in human primary monocytes[64]. Consistently, siRNA-mediated knockdown of CaMKK-β and AMPK significantly abrogated LpqH-induced autophagy. Another important upstream signal triggering xenophagy defense pathway is host membrane damage (e.g., by bacterial toxins) [65, 66]. Indeed, damages of the plasma membrane are known to cause cellular amino acids starvation and energy shortage, the classic inducers of autophagy. Various bacterial pore-forming toxins (PFT), including α-hemolysin from Staphilococcus aureus and cytolysin from Vibrio cholerae, have been demonstrated to activate AMPK by causing a massive, but transient drop of intracellular ATP in a non-virally transformed human keratinocyte cell line[65]. In turn, AMPK activation leads to the deactivation of mTORC1 signaling. HeLa cells infected with Shigella flexneri triggers a rapid induction of an intracellular amino acid starvation, which leads to the deactivation of mTOR signaling and induction of autophagy [66].
Figure 3. AMPK in innate host response to bacterial infection.
AMPK acts as a restriction factor targeting bacteria to xenophagy. AMPK activation by the drugs AICAR and metformin induces antibacterial autophagy. AICAR, 5-aminoimidazole-4-carboxamide-1-β-D-ribofuranoside, AMPK, AMP-activated protein kinase; CaMKKβ, calcium/calmodulin-dependent protein kinase kinase β; mTORC1, mechanistic target of rapamycin complex 1; PGC1α, peroxisome proliferator-activated receptor γ co-activator-1α; ULK1, Unc-51-like kinase 1.
The importance of this innate immune defense mechanism has been highlighted by the identification of bacterial strategies that were evolved to escape from degradation mediated by autophagy. Salmonella Typhimurium escapes this defense pathway by the recruitment and reactivation of mTOR at the surface of the Salmonella-containing vacuole in infected HeLa cells[66]. Pseudomonas entomophila paralyzes the global host response to infection in gut Drosophila by causing a severe reduction in protein synthesis, thereby inhibiting immune and repair gene programs [67]. After ingestion of bacteria, the severity of cellular damages induced by monalysin, a P. entomophila PFT, and microbicidal reactive oxygen species (ROS) production in the Drosophila gut induces an excessive and detrimental stress response that ultimately disrupts gut integrity. Interestingly, reduction in AMPK activity partially restored host translation in P. entomophila infected flies and makes flies more resistant to P. entomophila infection, indicating that activation of AMPK by monalysin and ROS production plays a detrimental role in the pathogenesis [67].
Antibacterial therapeutics opportunities
The increasing prevalence of drug-resistant bacteria has stimulated the development of host-directed therapies to amplify endogenous effector mechanisms. It is now clear that autophagy is associated with innate immune response and contributes to antibacterial defense during infection. Thus, autophagy is an attractive candidate. Interestingly, AMPK-activating drugs have already demonstrated beneficial outcomes on antimicrobial responses (Figure 3). It has been established that AICAR promotes antibacterial autophagy against Mtb infection in murine bone marrow-derived macrophages (Table 1), in part through the induction of PGC1α and subsequent transcriptional upregulation of mitochondrial biogenesis and autophagy-associated genes [68]. AICAR-induced autophagy activation is mediated through the down-regulation of mTOR signaling and the phosphorylation of ULK1, the upstream kinase of autophagy machinery. In addition, another AMPK activator, the oral anti-diabetic biguanide metformin has been recently shown to control the growth of Mtb in infected mice by improving the immune response [69]. The effects of metformin were abolished in human monocyte-derived macrophages where AMPK is knocked-down or inhibited chemically. The protective effect of metformin is mediated by its cellular innate antimicrobicidal functions and increased acidification of mycobacterial phagosome. Metformin-mediated inhibition of mitochondrial complex I (NADH dehydrogenase) activity is known to induce the production of mitochondrial ROS [70]. Importantly, metformin therapy has been associated with beneficial clinical outcomes in patients infected with Mtb[69]. Furthermore, metformin enhances the efficacy of conventional anti-Mtb drugs, indicating that this drug can be use as adjuvant therapy to improve the effective treatment of Mtb infection.
Interaction between AMPK and parasites
Like bacteria or viruses, parasites manipulate host cells to establishment a successful infection after parasite entrance. Once inside the host, intracellular or extracellular parasites must negotiate their surrounding nutritional and signaling environment to obtain energy and biosynthetic precursors that support their survival and high growth rate. At this level a nutrient competition is established between the manipulative parasite trying to obtain usable energy and metabolites and the host attempting to sequester the same precursors from the pathogen. Moreover, some parasites are auxotrophic for several metabolites being the host the only viable source for acquisition. The balance between these mechanisms becomes crucial for the parasite survival. In the core of this metabolic network, AMPK has emerged as a potential candidate modulated by parasites due to its crucial role on key metabolic pathways.
AMPK on the core of host-parasite metabolic coupling
Metabolic manipulation of host metabolism is regularly identified as required for intracellular pathogen growth. Recent studies with Toxoplasma gondii [71], Trypanosomabrucei[72], Leishmania spp[73, 74], Schistosomamansoni [72] and Plasmodium berghei [75] have paved the way to our understanding on the molecular mechanisms used by parasites to take advantage of the nutritive host resources. Nevertheless, the role of AMPK during the metabolic host-parasite crosstalk remains largely unexplored.
Caradonna et al. by employing a genome-wide RNA interference screen, targeting host genes in HeLa cells infected with Trypanosomacruzi for the identification of cellular processes that fuel parasite growth showed that sustained AKT-mTORC1 pathway regulate intracellular T. cruzi growth [76]. The maintenance of high cellular ATP/ADP ratios at higher levels provided a distinct advantage for the parasite, which therefore kept AMPK activity in check. Thus, acute silencing of AMPK catalytic (PRKAA1) or the regulatory subunit (PRKAB1) provides a more favorable growth environment for intracellular T. cruzi. Although so far it has not been confirmed in vivo, AMPK inhibition is suggested to contribute for T. cruzi survival. Recently we demonstrated that AMPK is in contrary crucial for the establishment of a microenvironment more prone to L. infantum survival in macrophages [73]. Previous analysis on the transcriptomic signature of L. major infected macrophages has revealed that carbohydrate and lipid metabolism were among the most altered pathways during infection. Increased mRNA levels of glucose transporters as well as key glycolytic enzymes encoding genes, such as hexokinases (Hk), pyruvate kinase M2 (Pkm2) and lactate dehydrogenase a (Ldha) were induced in the presence of live but not heat-killed L. major promastigotes. L. major also induced a down-regulation of a number of genes implicated in the tricarboxylic acid (TCA) cycle and oxidative phosphorylation suggesting that infected macrophages mainly rely on increased glycolytic flow for energy production. On the other hand, L. major led to cholesterol and triglycerides accumulation on infected macrophages by enhancing the expression of scavenger receptors involved in the uptake of Low-Density Lipoprotein (LDL), inhibiting cholesterol efflux and increasing the synthesis of triacylglycerides[74]. The accumulation of lipid droplets in close proximity to parasitophorous vacuoles advocates the former structure as a potential high-energy substrate source for the intracellular parasite. We observed that following L. infantum infection, macrophages switch from an early glycolytic to an oxidative metabolism, in a process requiring SIRT1 and LKB1/AMPK. In the absence of SIRT1 or LKB1, infected macrophages are not able to induce AMPK activation leading to an impairment of the metabolic switch. In that sense, the AICAR-induced AMPK activation contributes to parasite survival while inhibition of AMPK using compound C resulted in lower parasite numbers in vitro. Interestingly we demonstrated that AMPK inactivation specifically in the myeloid population led to a reduce L. infantum burden in vivo[73]. Gastrointestinal nematodes also affects profoundly host metabolism. The infection of CD11c-specific AMPK α−/− (DC-AMPK−/−) mice with the wookworm Nippostrongylus brasiliensis led to a dysregulation of Th2 immune response concomitantly with a failure to regenerate tissue damage mediated by pathogen. Deregulated responses generated in DC-AMPK−/− mice were associated with increased Type-1 responses, greater numbers of Th17 cells, and defects in the generation of alternatively activated macrophages. Therefore, AMPK activity in myeloid cells was shown to regulate host protection against GI parasites [77]. Although still insufficient, these examples highlight the strategies used by the parasites to explore host resources shedding some light on cellular metabolic subversion mechanisms induced by microbe infections within the host. In this context, the modulation of AMPK activity has been put forward as a possible therapeutic target against parasitic diseases.
Conservation of the AMPK machinery in parasite
With the exception of the Encephalitozoon cuniculi obligate intracellular parasite, which lack of an identifiable AMPK and presumably relies on host cell AMPK to regulate energy balance[78], several members of this highly conserved kinase family were already described in yeasts and plants, named sucrose non-fermenting 1 (SNF1) and SNF1-related protein kinase 1 (SnRK1), respectively [79, 80]. SNF1 and SnRK1 homologs develop similar functions in what concerns the surveillance of the metabolic status in response to nutrient and environmental stress through the induction of catabolic processes and a general repression of anabolism. Similarly to these organisms, some reports have defined the presence of AMPK related proteins in eukaryote parasites. A systematic functional analysis of protein kinases of Plasmodium berghei identified a homolog of SNF1 described as SNF1/KIN. This protein was shown to be important for sporozoite development, particularly in the egression to the salivary gland of the mosquito Anopheles stephensi by acting as a regulator of energy metabolism [81]. Similarly, a PfKIN gene was identified in Pl. falciparum where it is predominantly expressed in the gametocyte stage being involved in the transmission and adaptation of the malaria parasite from the human bloodstream to the mosquito midgut [82]. A comparative analysis of the kinomes of representative members of pathogenic trypanosomatids, namely Trypanosomabrucei, Trypanosomacruzi and Leishmania major have highlighted that AMPK homologues are relatively poorly represented within trypanosomatid genomes as compared to humans, although are predicted to be active [83]. The procyclic forms of T. brucei monitor changes in glucose levels to regulate surface molecule expression, which is important for survival in the tsetse fly vector. While the α AMPK subunit remains elusive, the β and γ subunits (TbAMPKβ and TbAMPKγ, respectively) play a role in surface molecule expression, as silencing of the genes leads to upregulation of procyclin expression. Moreover, the localization of the scaffold (β) subunit suggests positioning in the cell consistent with a role as an intermediary between surface molecule expression and glycolysis providing thus a molecular connection between these two mechanisms [84]. Finally, a SNF1 type protein kinase gene from Toxoplasma gondii (TOXPK1) with 58% identity to human AMPKα was shown to be transiently expressed to up-regulate glycogen biosynthesis during the development of tachyzoites into bradyzoites [85, 86]. Although efforts were made to characterize AMPK homologs in parasite organisms, their role during parasite adaptation to host remains elusive.
Conclusion and perspectives
It is now clear that pathogens have evolved multiple mechanisms to manipulate host regulatory pathways and hijack host cell metabolism to their own benefit. Modulation of AMPK activity appears to be a fundamental process involved in pathogen-host interaction. Although activation of AMPK helps pathogens to obtain sufficient amounts of energy and nutrient for their replication, inhibition of AMPK is also a strategy used by pathogens to evade innate host defense. This intricate pathogen-AMPK interaction is not limited to mammalian systems and seems to be conserved through evolution. Recent data suggest the existence of plant-specific SnRK1 (SNF1-related protein kinase) trimeric complexes involved in plant-pathogen interactions [87]. The SnRK1 heterotrimeric complex containing AKINβγ could be implicated in plant pathogen resistance through the interaction in the cytoplasm with AtHSPRO1/2 proteins, two defense-related genes, putatively involved in plant defense response [87]. In further support of this role, the expression of an antisense SNF1 transgene in Nicotiana benthamiana plants causes enhanced susceptibility similar to that conditioned by the geminivirus AL2 and L2 transgenes, whereas SNF1 overexpression leads to enhanced resistance. These observations suggest that the metabolic alterations mediated by SNF1 are a component of innate antiviral defenses and that SNF1 inactivation by AL2 and L2 is a counter-defensive measure[88].
Determining how intracellular pathogens manipulate host metabolic functions to support their own growth and survival is essential to identify mechanisms of pathogenicity and host adaptation. Blocking or activating specific metabolic pathways may improve host cells response to intracellular pathogens and could be used in the treatment of infectious diseases. Exciting translational opportunities are arising from the use of pharmacological AMPK agonists/ antagonists (Table 1) and could pave the way towards the design of novel host-directed therapies. Thus, a major future challenge will be to dissect the precise role that AMPK plays in the life cycle of viruses, bacteria and parasites, allowing a better rationale to identify new targets and design novel anti-pathogen clinical interventions.
Acknowledgments
This work was supported by grants from INSERM, CNRS and Université Paris Descartes. BV and MF are working in the Département Hospitalo-Universitaire (DHU) AUToimmune and HORmonal diseaseS. JE acknowledges the support of the Canada Research Chair program. JE and ABC received funding from the European Community’s Seventh Framework Programme under grant agreement No. 602773 (Project KINDRED). DM is supported by SFRH/BD/91543/2012.
Footnotes
Conflict of interest
The authors have no conflict of interest related to this work.
References
- 1.Brunton J, Steele S, Ziehr B, Moorman N, Kawula T. Feeding uninvited guests: mTOR and AMPK set the table for intracellular pathogens. PLoS Pathog. 2013;9:e1003552. doi: 10.1371/journal.ppat.1003552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hardie DG, Ross FA, Hawley SA. AMPK: a nutrient and energy sensor that maintains energy homeostasis. Nat Rev Mol Cell Biol. 2012;13:251–62. doi: 10.1038/nrm3311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hardie DG. AMPK-Sensing Energy while Talking to Other Signaling Pathways. Cell Metab. 2014;20:939–52. doi: 10.1016/j.cmet.2014.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Foretz M, Viollet B. Regulation of hepatic metabolism by AMPK. J Hepatol. 2011;54:827–9. doi: 10.1016/j.jhep.2010.09.014. [DOI] [PubMed] [Google Scholar]
- 5.Richter EA, Hargreaves M. Exercise, GLUT4, and skeletal muscle glucose uptake. Physiol Rev. 2013;93:993–1017. doi: 10.1152/physrev.00038.2012. [DOI] [PubMed] [Google Scholar]
- 6.Marsin AS, Bertrand L, Rider MH, et al. Phosphorylation and activation of heart PFK-2 by AMPK has a role in the stimulation of glycolysis during ischaemia. Curr Biol. 2000;10:1247–55. doi: 10.1016/s0960-9822(00)00742-9. [DOI] [PubMed] [Google Scholar]
- 7.Demeulder B, Zarrinpashneh E, Ginion A, et al. Differential regulation of eEF2 and p70S6K by AMPKalpha2 in heart. Biochim Biophys Acta. 2013;1832:780–90. doi: 10.1016/j.bbadis.2013.02.015. [DOI] [PubMed] [Google Scholar]
- 8.Horman S, Browne G, Krause U, et al. Activation of AMP-activated protein kinase leads to the phosphorylation of elongation factor 2 and an inhibition of protein synthesis. Current Biology. 2002;12:1419–23. doi: 10.1016/s0960-9822(02)01077-1. [DOI] [PubMed] [Google Scholar]
- 9.Inoki K, Ouyang H, Zhu T, et al. TSC2 integrates Wnt and energy signals via a coordinated phosphorylation by AMPK and GSK3 to regulate cell growth. Cell. 2006;126:955–68. doi: 10.1016/j.cell.2006.06.055. [DOI] [PubMed] [Google Scholar]
- 10.Gwinn DM, Shackelford DB, Egan DF, et al. AMPK phosphorylation of raptor mediates a metabolic checkpoint. Mol Cell. 2008;30:214–26. doi: 10.1016/j.molcel.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jager S, Handschin C, St-Pierre J, Spiegelman BM. AMP-activated protein kinase (AMPK) action in skeletal muscle via direct phosphorylation of PGC-1alpha. Proc Natl Acad Sci U S A. 2007;104:12017–22. doi: 10.1073/pnas.0705070104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Egan DF, Shackelford DB, Mihaylova MM, et al. Phosphorylation of ULK1 (hATG1) by AMP-activated protein kinase connects energy sensing to mitophagy. Science. 2011;331:456–61. doi: 10.1126/science.1196371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim J, Kundu M, Viollet B, Guan KL. AMPK and mTOR regulate autophagy through direct phosphorylation of Ulk1. Nat Cell Biol. 2011;13:132–41. doi: 10.1038/ncb2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Onselaer MB, Oury C, Hunter RW, et al. The Ca(2+)/calmodulin-dependent kinase kinase beta-AMP-activated protein kinase-alpha1 pathway regulates phosphorylation of cytoskeletal targets in thrombin-stimulated human platelets. J Thromb Haemost. 2014;12:973–86. doi: 10.1111/jth.12568. [DOI] [PubMed] [Google Scholar]
- 15.Foretz M, Hebrard S, Leclerc J, et al. Metformin inhibits hepatic gluconeogenesis in mice independently of the LKB1/AMPK pathway via a decrease in hepatic energy state. J Clin Invest. 2010;120:2355–69. doi: 10.1172/JCI40671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vincent EE, Coelho PP, Blagih J, et al. Differential effects of AMPK agonists on cell growth and metabolism. Oncogene. 2014 doi: 10.1038/onc.2014.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hawley SA, Ross FA, Chevtzoff C, et al. Use of cells expressing gamma subunit variants to identify diverse mechanisms of AMPK activation. Cell Metab. 2010;11:554–65. doi: 10.1016/j.cmet.2010.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goransson O, McBride A, Hawley SA, et al. Mechanism of action of A-769662, a valuable tool for activation of AMP-activated protein kinase. J Biol Chem. 2007;282:32549–60. doi: 10.1074/jbc.M706536200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hunter RW, Foretz M, Bultot L, et al. Mechanism of action of compound-13: an alpha1-selective small molecule activator of AMPK. Chem Biol. 2014;21:866–79. doi: 10.1016/j.chembiol.2014.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lai YC, Kviklyte S, Vertommen D, et al. A small-molecule benzimidazole derivative that potently activates AMPK to increase glucose transport in skeletal muscle: comparison with effects of contraction and other AMPK activators. Biochem J. 2014;460:363–75. doi: 10.1042/BJ20131673. [DOI] [PubMed] [Google Scholar]
- 21.Zhou G, Myers R, Li Y, et al. Role of AMP-activated protein kinase in mechanism of metformin action. J Clin Invest. 2001;108:1167–74. doi: 10.1172/JCI13505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bain J, Plater L, Elliott M, et al. The selectivity of protein kinase inhibitors: a further update. Biochem J. 2007;408:297–315. doi: 10.1042/BJ20070797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Beard PM, Griffiths SJ, Gonzalez O, et al. A loss of function analysis of host factors influencing Vaccinia virus replication by RNA interference. PLoS One. 2014;9:e98431. doi: 10.1371/journal.pone.0098431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hutterer C, Wandinger SK, Wagner S, et al. Profiling of the kinome of cytomegalovirus-infected cells reveals the functional importance of host kinases Aurora A, ABL and AMPK. Antiviral Res. 2013;99:139–48. doi: 10.1016/j.antiviral.2013.04.017. [DOI] [PubMed] [Google Scholar]
- 25.Moser TS, Jones RG, Thompson CB, Coyne CB, Cherry S. A kinome RNAi screen identified AMPK as promoting poxvirus entry through the control of actin dynamics. PLoS Pathog. 2010;6:e1000954. doi: 10.1371/journal.ppat.1000954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Terry LJ, Vastag L, Rabinowitz JD, Shenk T. Human kinome profiling identifies a requirement for AMP-activated protein kinase during human cytomegalovirus infection. Proc Natl Acad Sci U S A. 2012;109:3071–6. doi: 10.1073/pnas.1200494109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kondratowicz AS, Hunt CL, Davey RA, Cherry S, Maury WJ. AMP-activated protein kinase is required for the macropinocytic internalization of ebolavirus. J Virol. 2013;87:746–55. doi: 10.1128/JVI.01634-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bae HB, Zmijewski JW, Deshane JS, et al. AMP-activated protein kinase enhances the phagocytic ability of macrophages and neutrophils. Faseb J. 2011;25:4358–68. doi: 10.1096/fj.11-190587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Miranda L, Carpentier S, Platek A, et al. AMP-activated protein kinase induces actin cytoskeleton reorganization in epithelial cells. Biochem Biophys Res Commun. 2010;396:656–61. doi: 10.1016/j.bbrc.2010.04.151. [DOI] [PubMed] [Google Scholar]
- 30.Blume C, Benz PM, Walter U, et al. AMP-activated protein kinase impairs endothelial actin cytoskeleton assembly by phosphorylating vasodilator-stimulated phosphoprotein. J Biol Chem. 2007;282:4601–12. doi: 10.1074/jbc.M608866200. [DOI] [PubMed] [Google Scholar]
- 31.McArdle J, Moorman NJ, Munger J. HCMV targets the metabolic stress response through activation of AMPK whose activity is important for viral replication. PLoS Pathog. 2012;8:e1002502. doi: 10.1371/journal.ppat.1002502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McArdle J, Schafer XL, Munger J. Inhibition of calmodulin-dependent kinase kinase blocks human cytomegalovirus-induced glycolytic activation and severely attenuates production of viral progeny. J Virol. 2011;85:705–14. doi: 10.1128/JVI.01557-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sharon-Friling R, Goodhouse J, Colberg-Poley AM, Shenk T. Human cytomegalovirus pUL37x1 induces the release of endoplasmic reticulum calcium stores. Proc Natl Acad Sci U S A. 2006;103:19117–22. doi: 10.1073/pnas.0609353103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ji WT, Lee LH, Lin FL, Wang L, Liu HJ. AMP-activated protein kinase facilitates avian reovirus to induce mitogen-activated protein kinase (MAPK) p38 and MAPK kinase 3/6 signalling that is beneficial for virus replication. J Gen Virol. 2009;90:3002–9. doi: 10.1099/vir.0.013953-0. [DOI] [PubMed] [Google Scholar]
- 35.Sir D, Ou JH. Autophagy in viral replication and pathogenesis. Mol Cells. 2010;29:1–7. doi: 10.1007/s10059-010-0014-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Heaton NS, Randall G. Dengue virus-induced autophagy regulates lipid metabolism. Cell Host Microbe. 2010;8:422–32. doi: 10.1016/j.chom.2010.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kumar SH, Rangarajan A. Simian virus 40 small T antigen activates AMPK and triggers autophagy to protect cancer cells from nutrient deprivation. J Virol. 2009;83:8565–74. doi: 10.1128/JVI.00603-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chi PI, Huang WR, Lai IH, Cheng CY, Liu HJ. The p17 nonstructural protein of avian reovirus triggers autophagy enhancing virus replication via activation of phosphatase and tensin deleted on chromosome 10 (PTEN) and AMP-activated protein kinase (AMPK), as well as dsRNA-dependent protein kinase (PKR)/eIF2alpha signaling pathways. J Biol Chem. 2013;288:3571–84. doi: 10.1074/jbc.M112.390245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhu B, Zhou Y, Xu F, et al. Porcine circovirus type 2 induces autophagy via the AMPK/ERK/TSC2/mTOR signaling pathway in PK-15 cells. J Virol. 2012;86:12003–12. doi: 10.1128/JVI.01434-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Laforge M, Limou S, Harper F, et al. DRAM triggers lysosomal membrane permeabilization and cell death in CD4(+) T cells infected with HIV. PLoS Pathog. 2013;9:e1003328. doi: 10.1371/journal.ppat.1003328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yu Y, Maguire TG, Alwine JC. Human cytomegalovirus activates glucose transporter 4 expression to increase glucose uptake during infection. J Virol. 2011;85:1573–80. doi: 10.1128/JVI.01967-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Spencer CM, Schafer XL, Moorman NJ, Munger J. Human cytomegalovirus induces the activity and expression of acetyl-coenzyme A carboxylase, a fatty acid biosynthetic enzyme whose inhibition attenuates viral replication. J Virol. 2011;85:5814–24. doi: 10.1128/JVI.02630-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kudchodkar SB, Del Prete GQ, Maguire TG, Alwine JC. AMPK-mediated inhibition of mTOR kinase is circumvented during immediate-early times of human cytomegalovirus infection. J Virol. 2007;81:3649–51. doi: 10.1128/JVI.02079-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Moorman NJ, Cristea IM, Terhune SS, et al. Human cytomegalovirus protein UL38 inhibits host cell stress responses by antagonizing the tuberous sclerosis protein complex. Cell Host Microbe. 2008;3:253–62. doi: 10.1016/j.chom.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Seo JY, Yaneva R, Hinson ER, Cresswell P. Human cytomegalovirus directly induces the antiviral protein viperin to enhance infectivity. Science. 2011;332:1093–7. doi: 10.1126/science.1202007. [DOI] [PubMed] [Google Scholar]
- 46.Seo JY, Cresswell P. Viperin regulates cellular lipid metabolism during human cytomegalovirus infection. PLoS Pathog. 2013;9:e1003497. doi: 10.1371/journal.ppat.1003497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Martin C, Leyton L, Arancibia Y, et al. Modulation of the AMPK/Sirt1 axis during neuronal infection by herpes simplex virus type 1. J Alzheimers Dis. 2014;42:301–12. doi: 10.3233/JAD-140237. [DOI] [PubMed] [Google Scholar]
- 48.Ruderman NB, Xu XJ, Nelson L, et al. AMPK and SIRT1: a long-standing partnership? Am J Physiol Endocrinol Metab. 2010;298:E751–60. doi: 10.1152/ajpendo.00745.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tsalikis J, Croitoru DO, Philpott DJ, Girardin SE. Nutrient sensing and metabolic stress pathways in innate immunity. Cell Microbiol. 2013;15:1632–41. doi: 10.1111/cmi.12165. [DOI] [PubMed] [Google Scholar]
- 50.Moser TS, Schieffer D, Cherry S. AMP-activated kinase restricts Rift Valley fever virus infection by inhibiting fatty acid synthesis. PLoS Pathog. 2012;8:e1002661. doi: 10.1371/journal.ppat.1002661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mankouri J, Tedbury PR, Gretton S, et al. Enhanced hepatitis C virus genome replication and lipid accumulation mediated by inhibition of AMP-activated protein kinase. Proc Natl Acad Sci U S A. 2010;107:11549–54. doi: 10.1073/pnas.0912426107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Johnson RA, Wang X, Ma XL, Huong SM, Huang ES. Human cytomegalovirus up-regulates the phosphatidylinositol 3-kinase (PI3-K) pathway: inhibition of PI3-K activity inhibits viral replication and virus-induced signaling. J Virol. 2001;75:6022–32. doi: 10.1128/JVI.75.13.6022-6032.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Horman S, Vertommen D, Heath R, et al. Insulin antagonizes ischemia-induced Thr172 phosphorylation of AMP-activated protein kinase alpha-subunits in heart via hierarchical phosphorylation of Ser485/491. J Biol Chem. 2006;281:5335–40. doi: 10.1074/jbc.M506850200. [DOI] [PubMed] [Google Scholar]
- 54.Lo AK, Lo KW, Ko CW, Young LS, Dawson CW. Inhibition of the LKB1-AMPK pathway by the Epstein-Barr virus-encoded LMP1 promotes proliferation and transformation of human nasopharyngeal epithelial cells. J Pathol. 2013;230:336–46. doi: 10.1002/path.4201. [DOI] [PubMed] [Google Scholar]
- 55.Zhang HS, Wu MR. SIRT1 regulates Tat-induced HIV-1 transactivation through activating AMP-activated protein kinase. Virus Res. 2009;146:51–7. doi: 10.1016/j.virusres.2009.08.005. [DOI] [PubMed] [Google Scholar]
- 56.Pagans S, Pedal A, North BJ, et al. SIRT1 regulates HIV transcription via Tat deacetylation. PLoS Biol. 2005;3:e41. doi: 10.1371/journal.pbio.0030041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kiernan RE, Vanhulle C, Schiltz L, et al. HIV-1 tat transcriptional activity is regulated by acetylation. Embo J. 1999;18:6106–18. doi: 10.1093/emboj/18.21.6106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mehla R, Bivalkar-Mehla S, Zhang R, et al. Bryostatin modulates latent HIV-1 infection via PKC and AMPK signaling but inhibits acute infection in a receptor independent manner. PLoS One. 2010;5:e11160. doi: 10.1371/journal.pone.0011160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shahidi M, Tay ES, Read SA, et al. Endocannabinoid CB1 antagonists inhibit hepatitis C virus production, providing a novel class of antiviral host-targeting agents. J Gen Virol. 2014;95:2468–79. doi: 10.1099/vir.0.067231-0. [DOI] [PubMed] [Google Scholar]
- 60.Moseley CE, Webster RG, Aldridge JR. Peroxisome proliferator-activated receptor and AMP-activated protein kinase agonists protect against lethal influenza virus challenge in mice. Influenza Other Respir Viruses. 2010;4:307–11. doi: 10.1111/j.1750-2659.2010.00155.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhang HS, Chen XY, Wu TC, Zhang FJ. Tanshinone II A inhibits tat-induced HIV-1 transactivation through redox-regulated AMPK/Nampt pathway. J Cell Physiol. 2014;229:1193–201. doi: 10.1002/jcp.24552. [DOI] [PubMed] [Google Scholar]
- 62.Sorbara MT, Girardin SE. Emerging themes in bacterial autophagy. Curr Opin Microbiol. 2014;23C:163–70. doi: 10.1016/j.mib.2014.11.020. [DOI] [PubMed] [Google Scholar]
- 63.Tang Y, Li F, Tan B, et al. Enterotoxigenic Escherichia coli infection induces intestinal epithelial cell autophagy. Vet Microbiol. 2014;171:160–4. doi: 10.1016/j.vetmic.2014.03.025. [DOI] [PubMed] [Google Scholar]
- 64.Shin DM, Yuk JM, Lee HM, et al. Mycobacterial lipoprotein activates autophagy via TLR2/1/CD14 and a functional vitamin D receptor signalling. Cell Microbiol. 2010;12:1648–65. doi: 10.1111/j.1462-5822.2010.01497.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kloft N, Neukirch C, Bobkiewicz W, et al. Pro-autophagic signal induction by bacterial pore-forming toxins. Med Microbiol Immunol. 2010;199:299–309. doi: 10.1007/s00430-010-0163-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tattoli I, Sorbara MT, Vuckovic D, et al. Amino acid starvation induced by invasive bacterial pathogens triggers an innate host defense program. Cell Host Microbe. 2012;11:563–75. doi: 10.1016/j.chom.2012.04.012. [DOI] [PubMed] [Google Scholar]
- 67.Chakrabarti S, Liehl P, Buchon N, Lemaitre B. Infection-induced host translational blockage inhibits immune responses and epithelial renewal in the Drosophila gut. Cell Host Microbe. 2012;12:60–70. doi: 10.1016/j.chom.2012.06.001. [DOI] [PubMed] [Google Scholar]
- 68.Yang CS, Kim JJ, Lee HM, et al. The AMPK-PPARGC1A pathway is required for antimicrobial host defense through activation of autophagy. Autophagy. 2014;10:785–802. doi: 10.4161/auto.28072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Singhal A, Jie L, Kumar P, et al. Metformin as adjunct antituberculosis therapy. Sci Transl Med. 2014;6:263ra159. doi: 10.1126/scitranslmed.3009885. [DOI] [PubMed] [Google Scholar]
- 70.Foretz M, Guigas B, Bertrand L, Pollak M, Viollet B. Metformin: from mechanisms of action to therapies. Cell Metab. 2014;20:953–66. doi: 10.1016/j.cmet.2014.09.018. [DOI] [PubMed] [Google Scholar]
- 71.MacRae JI, Sheiner L, Nahid A, et al. Mitochondrial metabolism of glucose and glutamine is required for intracellular growth of Toxoplasma gondii. Cell Host Microbe. 2012;12:682–92. doi: 10.1016/j.chom.2012.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wang Y, Utzinger J, Saric J, et al. Global metabolic responses of mice to Trypanosoma brucei brucei infection. Proc Natl Acad Sci U S A. 2008;105:6127–32. doi: 10.1073/pnas.0801777105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Moreira D, Rodrigues V, Abengozar M, et al. Leishmania infantum Modulates Host Macrophage Mitochondrial Metabolism by Hijacking the SIRT1-AMPK Axis. PLoS Pathog. 2015 doi: 10.1371/journal.ppat.1004684. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Rabhi I, Rabhi S, Ben-Othman R, et al. Transcriptomic signature of Leishmania infected mice macrophages: a metabolic point of view. PLoS Negl Trop Dis. 2012;6:e1763. doi: 10.1371/journal.pntd.0001763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Li JV, Wang Y, Saric J, et al. Global metabolic responses of NMRI mice to an experimental Plasmodium berghei infection. J Proteome Res. 2008;7:3948–56. doi: 10.1021/pr800209d. [DOI] [PubMed] [Google Scholar]
- 76.Caradonna KL, Engel JC, Jacobi D, Lee CH, Burleigh BA. Host metabolism regulates intracellular growth of Trypanosoma cruzi. Cell Host Microbe. 2013;13:108–17. doi: 10.1016/j.chom.2012.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Nieves W, Oniskey T, Hung L, Herbert D. Metabolic regulation of Type 2 immunity controls tissue repair. 63rd annual ASTMH meeting; New Orleans, USA. November 2–6, 2014. [Google Scholar]
- 78.Miranda-Saavedra D, Stark MJ, Packer JC, et al. The complement of protein kinases of the microsporidium Encephalitozoon cuniculi in relation to those of Saccharomyces cerevisiae and Schizosaccharomyces pombe. BMC Genomics. 2007;8:309. doi: 10.1186/1471-2164-8-309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hardie DG. AMP-activated/SNF1 protein kinases: conserved guardians of cellular energy. Nat Rev Mol Cell Biol. 2007;8:774–85. doi: 10.1038/nrm2249. [DOI] [PubMed] [Google Scholar]
- 80.Polge C, Thomas M. SNF1/AMPK/SnRK1 kinases, global regulators at the heart of energy control? Trends Plant Sci. 2007;12:20–8. doi: 10.1016/j.tplants.2006.11.005. [DOI] [PubMed] [Google Scholar]
- 81.Tewari R, Straschil U, Bateman A, et al. The systematic functional analysis of Plasmodium protein kinases identifies essential regulators of mosquito transmission. Cell Host Microbe. 2010;8:377–87. doi: 10.1016/j.chom.2010.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bracchi V, Langsley G, Thelu J, Eling W, Ambroise-Thomas P. PfKIN, an SNF1 type protein kinase of Plasmodium falciparum predominantly expressed in gametocytes. Mol Biochem Parasitol. 1996;76:299–303. doi: 10.1016/0166-6851(96)02564-9. [DOI] [PubMed] [Google Scholar]
- 83.Parsons M, Worthey EA, Ward PN, Mottram JC. Comparative analysis of the kinomes of three pathogenic trypanosomatids: Leishmania major, Trypanosoma brucei and Trypanosoma cruzi. BMC Genomics. 2005;6:127. doi: 10.1186/1471-2164-6-127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Clemmens CS, Morris MT, Lyda TA, Acosta-Serrano A, Morris JC. Trypanosoma brucei AMP-activated kinase subunit homologs influence surface molecule expression. Exp Parasitol. 2009;123:250–7. doi: 10.1016/j.exppara.2009.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ng HC, Singh M, Jeyaseelan K. Identification of two protein serine/threonine kinase genes and molecular cloning of a SNF1 type protein kinase gene from Toxoplasma gondii. Biochem Mol Biol Int. 1995;35:155–65. [PubMed] [Google Scholar]
- 86.Ng HC, Singh M, Jeyaseelan K. Nucleotide sequence of ToxPK1 gene from Toxoplasma gondii. DNA Seq. 1997;7:179–91. doi: 10.3109/10425179709034033. [DOI] [PubMed] [Google Scholar]
- 87.Gissot L, Polge C, Jossier M, et al. AKIN beta gamma contributes to SnRK1 heterotrimeric complexes and interacts with two proteins implicated in plant pathogen resistance through its KIS/GBD sequence. Plant Physiol. 2006;142:931–44. doi: 10.1104/pp.106.087718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hao L, Wang H, Sunter G, Bisaro DM. Geminivirus AL2 and L2 proteins interact with and inactivate SNF1 kinase. Plant Cell. 2003;15:1034–48. doi: 10.1105/tpc.009530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bulusu V, Thakur SS, Venkatachala R, Balaram H. Mechanism of growth inhibition of intraerythrocytic stages of Plasmodium falciparum by 5-aminoimidazole-4-carboxamide ribonucleoside (AICAR) Mol Biochem Parasitol. 2011;177:1–11. doi: 10.1016/j.molbiopara.2011.01.001. [DOI] [PubMed] [Google Scholar]
- 90.Huang H, Kang R, Wang J, et al. Hepatitis C virus inhibits AKT-tuberous sclerosis complex (TSC), the mechanistic target of rapamycin (MTOR) pathway, through endoplasmic reticulum stress to induce autophagy. Autophagy. 2013;9:175–95. doi: 10.4161/auto.22791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Sang Y, Brichalli W, Rowland RR, Blecha F. Genome-wide analysis of antiviral signature genes in porcine macrophages at different activation statuses. PLoS One. 2014;9:e87613. doi: 10.1371/journal.pone.0087613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Guida MC, Esteva MI, Camino A, et al. Trypanosoma cruzi: in vitro and in vivo antiproliferative effects of epigallocatechin gallate (EGCg) Exp Parasitol. 2007;117:188–94. doi: 10.1016/j.exppara.2007.04.015. [DOI] [PubMed] [Google Scholar]
- 93.Vigueira PA, Ray SS, Martin BA, Ligon MM, Paul KS. Effects of the green tea catechin (−)-epigallocatechin gallate on Trypanosoma brucei. Int J Parasitol Drugs Drug Resist. 2012;2:225–9. doi: 10.1016/j.ijpddr.2012.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]