Abstract
The role of tumor necrosis factor (TNF) in regulating various phases of the antiviral T-cell response is incompletely understood. Additionally, despite strong evidence ascribing a role for TNF in protecting against T-cell-dependent autoimmunity, the underlying mechanisms are still obscure. To address these issues, we have investigated the role of tumor necrosis factor receptors (TNFRs) I (p55R) and II (p75R) in regulating CD8 T-cell responses to lymphocytic choriomeningitis virus (LCMV) with wild-type, p55R-deficient (p55−/−), p75R-deficient (p75−/−), and p55R- and p75R-deficient (DKO) mice. Loss of p55R increased the number of memory CD8 T cells to only one of the two immunodominant epitopes, and p75R deficiency had a minimal impact on the T-cell response to LCMV. Strikingly, deficiency of both p55R and p75R had a more dramatic effect on the LCMV-specific CD8 T-cell response; in the DKO mice, as a sequel to enhanced expansion and a reduction in contraction of CD8 T cells, there was a substantial increase in the number of memory CD8 T cells (specific to the two immunodominant epitopes). While the majority of LCMV-specific memory CD8 T cells in wild-type mice were CD62Lhi CCR7hi (central memory), a major proportion of memory CD8 T cells in DKO mice were CD62Llo CCR7hi. TNFR deficiency did not affect the proliferative renewal of memory CD8 T cells. Taken together, these data suggested that TNFRs p55R and p75R have overlapping roles in downregulating CD8 T-cell responses and establishment of immune homeostasis during an acute viral infection.
It is well established that CD8 T cells play a critical role in defense against viral, intracellular bacterial, and protozoan pathogens. Typically, acute systemic viral infections in humans and mice elicit massive expansion of antigen-specific CD8 T cells (7-9, 36-38, 59). This phase of intense antigen-driven proliferation is followed by the contraction phase, when 90 to 95% of the expanded virus-specific CD8 T cells are lost, presumably by apoptosis (2, 38, 52-53). The remaining ≈5% of the CD8 T cells survive to become memory cells, which are responsible for accelerated T-cell responses upon reinfection (2, 52). The number of memory CD8 T cells generated depends upon the extent of expansion (clonal burst size) and ensuing apoptosis (clonal downsizing) of activated T cells during the primary T-cell response (2, 18, 38). A thorough understanding of the mechanisms that regulate the expansion and apoptosis of antigen-specific CD8 T cells during an immune response should be helpful towards engineering effective vaccines that induce potent T-cell memory and development of immunotherapeutic strategies to curb T-cell-dependent autoimmune diseases.
Tumor necrosis factor alpha (TNF-α) is a proinflammatory cytokine produced primarily by activated T cells and macrophages. TNF exerts its effect on cells primarily via two receptors: TNF receptor (TFNR) I (p55R) and TNFR II (p75R) (30, 51). Apart from its well-established role as a proinflammatory cytokine, TNF has also been shown to play an important role in maintaining immune homeostasis. Germline mutations in p55R have been shown to be the underlying cause of dominantly inherited autoinflammatory syndromes in humans (35). p55R deficiency accelerates lymphadenopathy, autoimmunity, and early mortality seen in Fas mutant (lpr) mice (71). Further, there is substantial evidence that TNF has protective effects against T-cell-mediated autoimmunity (14, 15, 25). It was recently reported that TNF-deficient mice exhibit severe tissue pathology and mortality during a mycobacterial infection as a result of uncontrolled T-cell activation and cytokine production (68). However, the mechanisms underlying the regulation of antigen-specific T-cell responses by TNF-TNFR interactions are incompletely understood.
Lymphocytic choriomeningitis virus (LCMV) infection of mice is one of the best-characterized systems to study CD8 T-cell responses. Infection of adult immunocompetent mice with the Armstrong strain of LCMV induces a potent CD8 T-cell response that results in viral clearance within 8 to 10 days postinfection (28). Following LCMV infection, at the peak of CD8 T-cell response (day 8 postinfection), 50 to 70% of the CD8 T cells in the spleen are virus specific (17, 38). A majority of these LCMV-specific effector CD8 T cells undergo apoptosis after day 8 postinfection, leaving behind a finite number of memory T cells (38). In immune mice, LCMV-specific memory CD8 T cells (all epitopes combined) exist at a remarkably high frequency, constituting up to 10% of the total CD8 T cells in the spleen (17, 38). The primary CD8 T-cell response to LCMV-Armstrong is not dependent upon the presence of CD4 T cells or B cells or CD28- and CD40L-mediated costimulatory interactions (3, 6, 13, 27, 34, 54, 62, 66). Although the expansion phase (days 0 to 8 postinfection) has been well studied, the mechanisms that regulate the contraction (days 8 to 30 postinfection) phase of LCMV-specific CD8 T-cell response are not well understood. Previous studies have indicated that apoptosis of LCMV-specific CD8 T cells occurs in a Fas-independent fashion (44, 72) and gamma interferon (IFN-γ)-deficient mice exhibit a delay in contraction of LCMV-specific CD8 T-cell responses (5, 31). The role of TNFR p55R in regulating LCMV-specific CD8 T-cell responses has been investigated with T-cell receptor transgenic mice (39, 45). These studies with monoclonal T cells bearing the transgenic T-cell receptor showed that p55R deficiency did not affect the expansion or contraction of activated CD8 T cells during acute LCMV infection (39, 45). However, the role of the other TNFR, p75R, which has been directly implicated in activated T-cell apoptosis (70), is not known. Additionally, the existence of functional redundancies between p55R and p75R (48) in regulating CD8 T-cell responses has not been examined. Therefore, in this study, we have performed a detailed and systematic analysis of the role of TNFRs p55R and p75R in regulating the expansion, contraction, and memory phases of the polyclonal CD8 T-cell response to acute LCMV infection in mice.
MATERIALS AND METHODS
Mice.
The generation and characterization of the p55R-deficient (p55−/−), p75R-deficient (p75−/−), and p55R- and p75R-deficient (DKO) mice have been reported previously (41, 42). The p55−/− mice (42) on the C57BL/6 background and the wild-type C57BL/6 mice were purchased from Jackson Laboratories (Bar Harbor, Maine). The p75−/− and DKO mice on the C57BL/6 background (41) were kindly provided by Jacques J. Peschon (Immunex Corp., Seattle, Wash.). The mice used in these studies were between 6 and 8 weeks of age at the time of infection. All mice were provided sterilized food and drinking water and were maintained under specific-pathogen-free conditions. Animal experiments were conducted in accordance with approved institutional animal welfare guidelines.
Virus.
Mice were infected with 2 × 105 PFU of the Armstrong CA 1371 strain of LCMV (LCMV-Armstrong) by intraperitoneal injection (1). All LCMV stocks used in this study were triple plaque purified on Vero cells, and stocks were grown in BHK-21 cells. Infectious LCMV in the tissues and serum of infected mice was quantitated by a plaque assay on Vero cells as described previously (1).
Cytotoxicity assay.
The major histocompatibility complex (MHC) class I-restricted CD8 T-cell-mediated cytotoxic activity in the spleens of LCMV-infected mice was measured directly ex vivo with MC57 cells (H-2b) as targets in a standard 51Cr release assay as described elsewhere (1).
Quantitation of LCMV-specific CD8 T cells by MHC class I tetramers.
In C57BL/6 mice (H-2b), the two dominant Db-restricted LCMV cytotoxic T-lymphocyte epitopes are the amino acid residues 396 to 404 and 33 to 41 in the viral nucleoprotein (NP) and glycoprotein (GP), respectively. The preparation of MHC class I tetramers (Db) loaded with LCMV cytotoxic T-lymphocyte epitope peptides NP396-404 (NP396) and GP33-41 (GP33) has been described previously (38). Erythrocyte-depleted single-cell suspensions of the spleen were prepared by standard procedures. Mononuclear cells were isolated from phosphate-buffered saline-perfused livers as described by Masopust et al. (33). Freshly explanted splenocytes or mononuclear cells from the liver were stained with allophycocyanin-labeled MHC class I tetramers, anti-CD8, anti-CD44, and anti-CD62L antibodies in fluorescence-activated cell sorting buffer (phosphate-buffered saline containing 2% bovine serum albumin and 0.1% sodium azide) for 1 h at 4 C.
CCR7 expression on LCMV-specific memory CD8 T cells was assessed by staining with CCL19-Fc fusion proteins as described previously (56). CCL19-Fc fusion proteins were provided by Jason Cyster (University of California, San Francisco). In some experiments, splenocytes were stained with anti-CD122 and anti-Ly-6C in conjunction with anti-CD8 antibodies to enumerate activated and memory CD8 T cells. All antibodies were purchased from BD-Pharmingen (San Diego, Calif.). Following staining, cells were fixed in phosphate-buffered saline containing 2% paraformaldehyde and acquired on a FACScalibur flow cytometer (Becton Dickinson, San Jose, Calif.), and the data were analyzed with CellQuest software (Becton Dickinson).
Quantitation of LCMV-specific CD8 T cells by intracellular staining for IFN-γ.
Intracellular staining for IFN-γ was performed as described previously (38). Briefly, freshly explanted splenocytes (106 cells/well) were cultured with or without the LCMV cytotoxic T-lymphocyte epitope peptides (0.1 μg/ml) in the presence of brefeldin A (Golgistop; Pharmingen) and human recombinant interleukin-2 (10 U/well; Pharmingen) in 96-well flat-bottomed plates. After 6 h of culture, cells were stained for surface CD8 and intracellular IFN-γ with the Cytofix/Cytoperm kit (Pharmingen) according to the manufacturer's instructions. The anti-CD8 and anti-IFN-γ antibodies used in this procedure were purchased from BD-Pharmingen.
Assessment of in vivo proliferation of LCMV-specific CD8 T cells with BrdU.
The proliferation of LCMV-specific CD8 T cells in LCMV-infected mice was assessed by administering 5-bromo-2′-deoxyuridine (BrdU) (0.8 mg/ml) in drinking water for 8 days. After 8 days of BrdU treatment, splenocytes were stained with allophycocyanin-labeled MHC class I tetramers and phycoerythrin-labeled anti-CD8 antibodies. Following surface staining, cells were permeabilized and stained for BrdU with fluorescein isothiocyanate-conjugated anti-BrdU antibodies (B44; Becton Dickinson) as described previously (54). Flow cytometry and data analysis were performed as described earlier. BrdU-free LCMV-infected mice served as controls for BrdU staining.
Statistical analysis.
Experimental data were analyzed by using commercially available software (SYSTAT [Chicago, Ill.], version 10.2).
RESULTS
Primary CD8 T-cell responses in TNFR-deficient mice.
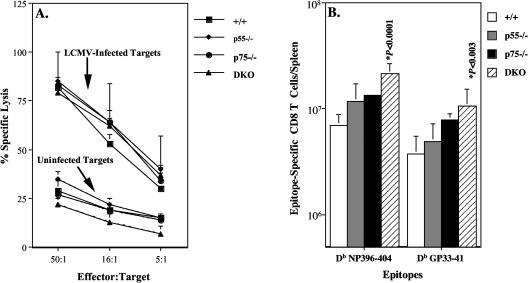
To examine the role of TNFRs in the induction of the primary CD8 T-cell response, groups of wild-type, p55−/−, p75−/−, and DKO mice were infected with LCMV-Armstrong. On day 8 postinfection, we quantitated MHC class I-restricted cytotoxic T-lymphocyte activity in the spleens of LCMV-infected wild-type, p55−/−, p75−/−, and DKO mice directly ex vivo. As shown in Fig. 1A, splenocytes from wild-type mice exhibited potent LCMV-specific cytotoxic T-lymphocyte activity. The data in Fig. 1A also show that cytotoxic T-lymphocyte activity in the spleens of p55−/−, p75−/−, and DKO mice was vigorous and comparable to that of wild-type mice. These data suggested that development of MHC class I-restricted cytotoxicity, an effector function of CD8 T cells, was not dependent upon TNFR signaling.
FIG. 1.
TNFR-deficient mice generate a normal primary LCMV-specific cytotoxic T-lymphocyte effector response. We infected 6- to 8-week-old C57BL/6 wild-type (+/+), p55R-deficient (p55−/−), p75R-deficient (p75−/−), and p55R- and p75R-deficient (DKO) mice with LCMV-Armstrong. (A) Eight days after infection, the MHC class I-restricted cytotoxic activity in the spleens was measured directly ex vivo in a 6-h 51Cr release assay. Uninfected and LCMV-infected MC57 cells (H-2b) were used as target cells. Data are the means for three mice per group. (B) On the eighth day after infection, CD8 T cells specific to the cytotoxic T-lymphocyte epitopes NP396-404 and GP33-41 were quantitated with MHC I tetramers. Data are the means for three to seven mice per group ± standard deviation and representative of three independent experiments.
In mice on the H-2b background, the immunodominant LCMV cytotoxic T-lymphocyte epitopes (Db restricted) are NP396 and GP33 (38). The primary CD8 T-cell response to LCMV in TNFR-deficient mice was further characterized by determining the expansion of virus-specific CD8 T cells with MHC class I tetramers specific to the LCMV cytotoxic T-lymphocyte epitopes NP396 (Db NP396-404) and GP33 (Db GP33-41). On day 8 postinfection, splenocytes from wild-type, p55−/−, p75−/−, and DKO mice were stained with anti-CD8, anti-CD44, and MHC class I tetramers, and analyzed by three-color flow cytometry. The data in Fig. 1B show the absolute numbers of LCMV-specific CD8 T cells in the spleens of wild-type, p55−/−, p75−/−, and DKO mice. Compared to that of wild-type mice, the absolute numbers of LCMV-specific CD8 T cells in the spleens of p75−/− and DKO mice were enhanced but not significantly different from each other. However, the total numbers of LCMV-specific CD8 T cells in the spleens of DKO mice and not p75−/− mice were significantly (P ≤ 0.05) higher than in wild-type mice. Quantitation of LCMV-specific CD8 T cells by intracellular cytokine staining also showed that the spleens of p75−/− and DKO mice contained two to threefold more LCMV-specific CD8 T cells than wild-type mice (data not shown). These data show that TNFR signaling is not required for optimal activation and expansion of CD8 T cells specific to the dominant cytotoxic T-lymphocyte epitopes of LCMV. Indeed, these findings suggested that TNFRs might suppress primary CD8 T-cell responses during an acute viral infection.
To examine the effect of TNFR deficiency on viral clearance, we quantitated infectious LCMV in the spleen and lung on days 3, 5, and 8 after infection with LCMV-Armstrong. As shown in Table 1, in all groups of mice, peak levels of LCMV were attained on day 3 postinfection, and viral titers dropped rapidly thereafter. No infectious LCMV was detected in the brain, kidney, and liver at all times examined. As illustrated in Table 1, the magnitude of viral replication and the kinetics of LCMV clearance in TNFR-deficient mice were similar to that of wild-type mice. These data show that loss of TNFR signaling did not affect the resolution of an acute LCMV infection. Furthermore, these findings are consistent with the generation of normal CD8 cytotoxic T-lymphocyte responses in TNFR-deficient mice (Fig. 1).
TABLE 1.
Effect of TNFR deficiency on LCMV clearancea
| Mice | Viral titer (log10 PFU/spleen or PFU/g)
|
|||||
|---|---|---|---|---|---|---|
| Spleen
|
Lung
|
|||||
| Day 3 | Day 5 | Day 8 | Day 3 | Day 5 | Day 8 | |
| Wild type | 6.1 | 5.4 | 3.3 | 4.4 | 3.3 | <2.6 |
| 6.1 | 5.0 | 2.6 | 3.4 | 3.8 | 2.9 | |
| 6.1 | 5.2 | 2.8 | 4.1 | 4.5 | <2.6 | |
| p55−/− | 5.9 | 4.4 | 1.6 | 3.5 | 3.0 | <2.6 |
| 5.8 | 4.0 | 2.3 | 3.4 | 2.5 | <2.6 | |
| 6.0 | 4.6 | 2.0 | 3.3 | 4.5 | <2.6 | |
| DKO | 5.7 | 5.0 | <1.6 | 3.1 | 3.1 | <2.6 |
| 5.8 | 5.9 | 2.1 | 3.7 | 4.1 | <2.6 | |
| 5.5 | 5.0 | 2.8 | 3.3 | 4.6 | 3.3 | |
Mice were infected with LCMV, and viral titers in the spleen and lung were determined by a plaque assay on the indicated day after infection. The data are the viral titers of individual mice.
Contraction of LCMV-specific CD8 T cells in TNFR-deficient mice.
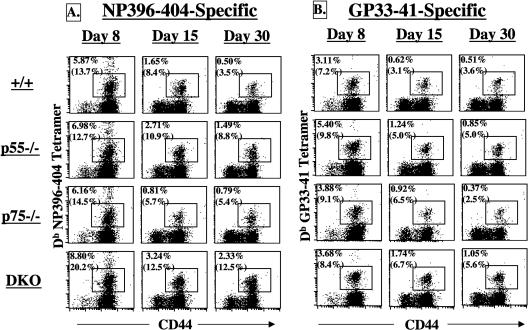
Previous studies have shown that ≈90% of the expanded LCMV-specific CD8 T cells are eliminated between days 8 and 30 postinfection (38). Here, we investigated the effect of TNFR deficiency on the contraction phase of CD8 T-cell response to LCMV (Fig. 2 and Table 2). The frequencies of NP396- and GP33-specific CD8 T cells in the spleens of wild-type mice exhibited a sharp decline between days 8 and 30 postinfection. The drop in the frequencies of LCMV-specific CD8 T cells in p75−/− mice was comparable to that in wild-type mice. As shown in Table 2, the contraction of both NP396- and GP33-specific CD8 T cells was substantially attenuated in DKO mice; while the drop in the frequencies of NP396- and GP33-specific CD8 T cells was ≈10- and 9-fold, respectively, in wild-type mice, and there was only a ≈5-fold decline in the percentages of LCMV-specific CD8 T cells in DKO mice. Interestingly, the contraction of only NP396-specific CD8 T cells but not GPP33-specific CD8 T cells was significantly altered in p55−/− mice compared to wild-type mice.
FIG. 2.
Effect of TNFR deficiency on the contraction of LCMV-specific CD8 T cells. Groups of wild-type, p55−/−, p75−/−, and DKO mice were infected with LCMV-Armstrong. On the indicated days after infection, splenocytes were stained with anti-CD8, anti-CD44, and MHC class I tetramers. The dot plots are gated on total CD8 T cells, and the numbers are the percentages of epitope-specific CD8 T cells among total splenocytes. The numbers in parentheses are the percentages of LCMV-specific CD8 T cells among total CD8 T cells. Data are representative of three independent experiments.
TABLE 2.
Contraction of LCMV-specific CD8 T cells in TNFR-deficient micea
| Mice (no. in group) | % CD8 T cells ± SD
|
|||||
|---|---|---|---|---|---|---|
| Day 8 p.i.
|
Day 15 p.i.
|
Day 30 p.i.
|
||||
| NP396-404 | GP33-41 | NP396-404 | GP33-41 | NP396-404 | GP33-41 | |
| Wild type (6) | 7.08 ± 2.50 | 3.82 ± 0.72 | 1.73 ± 0.43 | 1.00 ± 0.32 | 0.68 ± 0.30 | 0.41 ± 0.08 |
| p55−/− (6) | 8.39 ± 3.45 | 3.16 ± 1.50 | 2.84 ± 0.67 | 1.08 ± 0.20 | 1.25 ± 0.36 | 0.78 ± 0.10 |
| p75−/− (3) | 6.11 ± 0.47 | 3.57 ± 0.78 | 0.86 ± 0.13 | 0.90 ± 0.02 | 0.73 ± 0.30 | 0.45 ± 0.07 |
| DKO (6) | 9.46 ± 2.80 | 4.96 ± 1.40 | 2.83 ± 0.31 | 1.97 ± 0.27 | 1.84 ± 0.31 | 1.05 ± 0.26 |
Mice were infected with LCMV, and on the indicated days postinfection (p.i.), the percentage of CD8 T cells in the spleen that are specific to the epitopes NP396-404 and GP33-41 was determined by flow cytometry. The numbers are the percentages of epitope-specific CD8 T cells ± standard deviation.
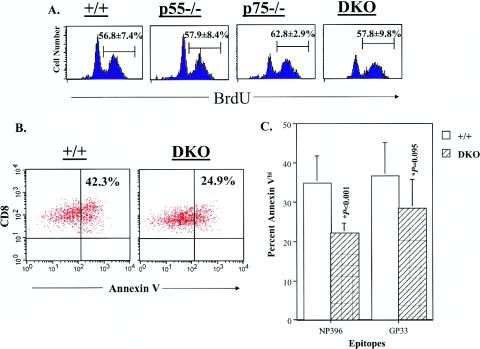
Alterations in cellular proliferation and/or apoptosis can affect the contraction phase of the CD8 T-cell response. To address this issue, first we quantitated the proliferation rates of LCMV-specific CD8 T cells in vivo in wild-type and TNFR-deficient mice between days 8 and 15 postinfection. The data in Fig. 3A show that similar proportions of LCMV-specific CD8 T cells incorporated BrdU between days 8 and 15 postinfection in all groups of mice. These data suggested that attenuated contraction of LCMV-specific CD8 T cells in DKO mice might not be due to increased proliferation. Previous work has shown that on day 8 postinfection, a significant proportion of LCMV-specific CD8 T cells in the spleen exhibit a proapoptotic phenotype, as determined by annexin V binding (64). We examined the effect of TNFR deficiency on the apoptosis of LCMV-specific CD8 T cells in the spleen directly ex vivo on day 8 postinfection. As shown in Fig. 3B and 3C, the relative proportions of annexin Vhi NP396-specific CD8 T cells in the spleens of DKO mice were significantly lower than in wild-type mice. The percentages of annexin Vhi GP33-specific CD8 T cells were lower in DKO mice compared to wild-type mice, albeit not highly significant at P < 0.01. Taken together, these data indicated that TNFR might modulate the contraction phase of the CD8 T-cell response by regulating cellular apoptosis.
FIG. 3.
Effect of TNFR deficiency on the proliferation and apoptosis of LCMV-specific CD8 T cells. (A) Groups of wild-type, p55−/−, p75−/−, and DKO mice were infected with LCMV-Armstrong and administered BrdU between days 8 and 15 postinfection. On day 15 postinfection, splenocytes were stained with anti-CD8, MHC class I tetramer (NP396 specific), and anti-BrdU antibodies. The number of BrdU-positive LCMV-specific CD8 T cells was determined by flow cytometry. The histograms are gated on tetramer-binding CD8 T cells and the numbers are the percentages of BrdU-positive cells of tetramer-binding CD8 T cells ± standard deviation (data are the means for three to four mice per group). (B and C) Eight days after infection, the number of apoptotic LCMV-specific CD8 T cells in the spleen was quantitated directly ex vivo by staining with anti-CD8, MHC class I tetramers, and annexin V. The percentage of annexin V-binding tetramer-positive CD8 T cells was determined by flow cytometry. The dot plots in panel B are gated on Db NP396 tetramer-binding CD8 T cells, and the numbers shown are the percentages of apoptotic cells of NP396-specific CD8 T cells. The data in panel C are the means for five to six mice per group ± standard deviation from three independent experiments.
CD8 T-cell memory in TNFR-deficient mice.
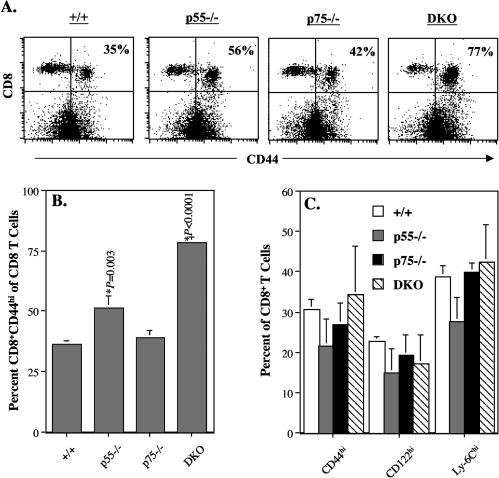
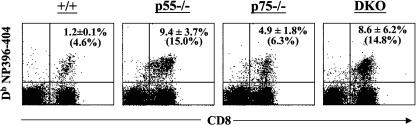
Recovery from an acute LCMV infection results in potent CD8 T-cell memory that provides life-long immunity to reinfection (28, 38). To determine the role of TNFRs in the regulation of long-term CD8 T-cell memory, groups of wild-type, p55−/−, p75−/−, and DKO mice were infected with LCMV-Armstrong and CD8 T-cell responses were analyzed at multiple time points between days 120 and 480 postinfection. It is well recognized that in C57BL/6 mice, naïve CD8 T cells express low levels of CD44 (CD44lo) and activated/memory CD8 T cells are CD44hi. To examine the effect of TNFR deficiency on the homeostasis of naïve and memory CD8 T cells in LCMV-immune mice, we stained splenocytes with anti-CD8 and anti-CD44 antibodies and analyzed them by flow cytometry (Fig. 4A and 4B). At all times examined, ≈35% of the CD8 T cells were of the CD44hi phenotype in wild-type and p75−/− mice. The proportion of CD44hi CD8 T cells in p55−/− mice was ≈50%, which was slightly higher than in wild-type mice. Strikingly, in the DKO mice, 70 to 80% of the CD8 T cells in the spleen were CD44hi (Fig. 4A and 4B). Interestingly, the relative proportions of activated/memory phenotype (CD44hi, CD122hi, and Ly-6Chi) CD8 T cells in the spleens of age-matched uninfected wild-type and TNFR-deficient mice were comparable (Fig. 4C). Taken together, these data provided the first indication that TNFRs might regulate memory CD8 T-cell homeostasis in LCMV-infected mice but not in uninfected mice.
FIG. 4.
Memory phenotype CD8 T cells in TNFR-deficient mice. (A and B) Groups of wild-type, p55−/−, p75−/−, and DKO mice were infected with LCMV-Armstrong. Two hundred and ten days after infection, splenocytes were stained with anti-CD8 and anti-CD44 antibodies. The dot plots in panel A are gated on total splenocytes, and the numbers are the percentages of CD44hi CD8 T cells among total CD8 T cells. Data in panel B are the means for three mice per group ± standard deviation; data shown is representative of six independent experiments. (C) Groups of uninfected wild-type, p55−/−, p75−/−, and DKO mice (age matched with mice used in experiments in panels A and B) were sacrificed, and splenocytes were stained with anti-CD8, anti-CD44, anti-CD122, and anti-Ly-6C antibodies. The percentages of CD44hi, CD122hi, and Ly-6Chi cells among CD8 T cells were determined by flow cytometry. The data are the means for two to three mice per group ± standard deviation.
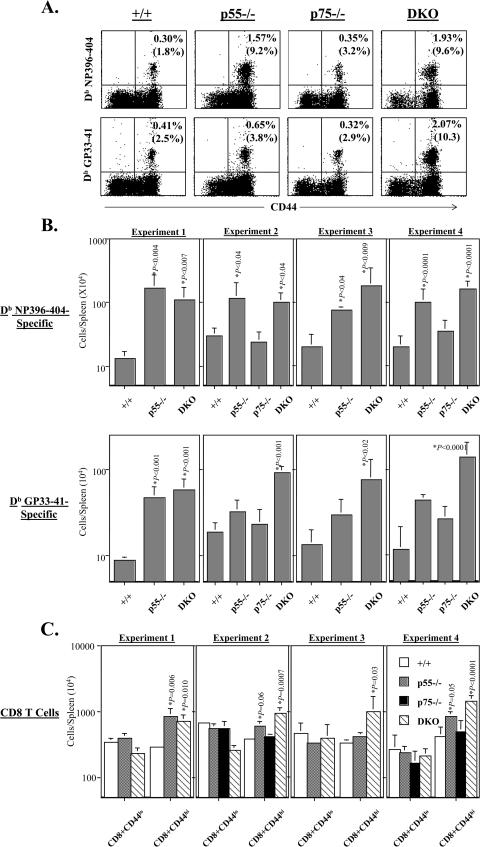
Next, we quantitated LCMV-specific memory CD8 T cells in the spleens with fluorochrome-labeled MHC class I tetramers. Representative flow cytometry profiles of staining for LCMV-specific memory CD8 T cells on day 480 postinfection are shown in Fig. 5A. The data in Fig. 5A shows that LCMV-specific memory CD8 T cells were readily detected in the spleens of all groups of LCMV-immune mice. It is worth noting that all the LCMV-specific memory CD8 T cells are CD44hi, which is typical of memory T cells. The data presented in Fig. 5A illustrate the striking enhancement in the frequencies (≈6-fold higher) of NP396-specific memory CD8 T cells in the spleens of p55−/− and DKO mice compared to wild-type mice. The frequencies of NP396-404-specific memory CD8 T cells in the spleens of p75−/− mice were comparable to that of wild-type mice. These data suggested that signaling via p55R might play an important role in determining the number of NP396-specific memory CD8 T cells.
FIG.5.
CD8 T-cell memory in TNFR-deficient mice. Between days 120 and 480 after infection with LCMV-Armstrong, the number of LCMV-specific memory CD8 T cells in the spleens of wild-type, p55−/−, p75−/−, and DKO mice was determined by staining with anti-CD8 antibodies, MHC class I tetramers (loaded with NP396 or GP33 peptides), and anti-CD44 antibodies. The data in panel A were obtained on day 480 after infection. Dot plots are gated on total CD8 T cells and show staining for CD44 and the indicated MHC class I tetramers. The numbers are the percentages of tetramer-binding CD8 T cells among total splenocytes. Note the enhanced frequencies of NP396-404-specific memory CD8 T cells in p55−/− and DKO mice compared to wild-type mice. A notable enhancement in the frequencies of GP33-41-specific memory CD8 T cells is seen in DKO mice. Panel B shows the total number of LCMV-specific memory CD8 T cells in the spleens of wild-type, p55−/−, p75−/−, and DKO mice in several independent experiments (experiment 1, day 120 postinfection; experiment 2, day 210 postinfection; experiment 3, day 300 postinfection; experiment 4, day 480 postinfection). Panel C shows the total number of naïve (CD44lo) and activated/memory (CD44hi) CD8 T cells in the spleens of wild-type, p55−/−, p75−/−, and DKO mice in various experiments. Data in panels B and C are the means for 3 to 10 mice per group for each experiment ± standard deviation. Please note the log scale in the graph.
The effect of TNFR deficiency on CD8 T-cell memory seems to be epitope dependent; while loss of p55R alone led to a notable increase in the number of NP396-specific memory CD8 T cells, loss of p55R or p75R alone had a minimal impact on the frequencies of GP33-specific memory CD8 T cells. However, the frequency of GP33-specific memory CD8 T cells in DKO mice was considerably higher compared to wild-type, p55−/−, and p75−/− mice. These findings indicate that the function of TNFRs p55R and p75R in regulating the number of GP33-specific memory CD8 T cells might be cooperative and/or redundant.
The absolute numbers of LCMV-specific memory CD8 T cells in the spleens of wild-type, p55−/−, p75−/−, and DKO mice is shown in Fig. 5B (please note the log scale in the figure). In all experiments, irrespective of epitope specificity, the spleens of DKO mice contained substantially greater numbers of LCMV-specific memory CD8 T cells (8- to 12-fold) compared to wild-type mice. Loss of p75R did not have a significant impact on the number of LCMV-specific memory CD8 T cells in the spleen. While the spleens of LCMV-immune p55−/− mice contained significantly more (≈5-fold) NP396-specific memory CD8 T cells than those of wild-type mice, loss of p55R did not affect the number of GP33-specific memory CD8 T cells on a consistent basis. Consistent with the LCMV-specific memory CD8 T-cell data, the total number of activated/memory CD8 T cells (CD44hi) in the spleens of DKO mice was significantly higher than in wild-type mice (Fig. 5C). Taken together, these data provide strong evidence supporting a negative regulatory role for TNFRs in determining the magnitude of CD8 T-cell memory.
Previous studies have shown that a substantial number of memory T cells reside in the nonlymphoid organs in addition to the secondary lymphoid organs (33, 46). Therefore, it was important to examine the role of TNFRs in regulating the number of memory CD8 T cells in the nonlymphoid organs. Furthermore, it is well established that TNF-TNFR interactions play critical roles in leukocyte trafficking (50), and it could be argued that enhanced numbers of LCMV-specific memory CD8 T cells in the secondary lymphoid organs of p55−/− or DKO mice may be due to anatomic redistribution. To address this issue, we quantitated the number of antigen-specific memory CD8 T cells in the livers of wild-type, p55−/−, p75−/−, and DKO mice about 15 months after an acute LCMV infection. As shown in Fig. 6, NP396-specific memory CD8 T cells were readily detected at high frequencies in the livers of wild-type mice. In comparison to wild-type mice, the frequencies of NP396-specific memory CD8 T cells in the livers of p75−/− mice were two- to threefold higher. Remarkably, the livers of p55−/− and DKO mice contained substantially more NP396-specific memory CD8 T cells than those of wild-type mice (Fig. 6). Comparable numbers of mononuclear cells were isolated from the livers in all groups of mice (data not shown). Therefore, increased frequencies of LCMV-specific memory CD8 T cells in p55−/− and DKO mice reflect a true enhancement in the absolute numbers of memory T cells in the liver. Further studies revealed that ≈90% of LCMV-specific memory CD8 T cells isolated from the liver from all groups of mice were CD44hi and CD62Llo (data not shown), which is the phenotype of the effector memory CD8 T cells. In summary, the data presented in Fig. 5 and 6 provide strong evidence that TNFRs might play a critical role in regulating the number of LCMV-specific memory CD8 T cells in both lymphoid and nonlymphoid organs.
FIG. 6.
LCMV-specific memory CD8 T cells in the nonlymphoid organs of TNFR-deficient mice. Fifteen months after LCMV infection, mononuclear cells were isolated from the livers of wild-type, p55−/−, p75−/−, and DKO mice. Mononuclear cells isolated from the livers were stained with anti-CD8 antibodies and MHC class I tetramers. The dot plots are gated on viable mononuclear cells based on forward and side scatter and show staining for CD8 and MHC class I tetramers (loaded with NP396-404 peptide). The numbers are mean percentages of tetramer-binding CD8 T cells among mononuclear cells ± standard deviation; numbers in parentheses are percentages of tetramer-binding CD8 T cells among total CD8 T cells in a representative mouse. The data are the means for three to four mice per group and representative of two independent experiments.
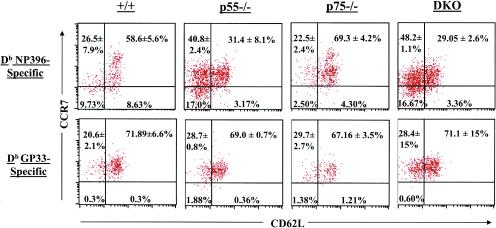
Next we investigated whether TNFR deficiency affected the expression of cell surface molecules on LCMV-specific memory CD8 T cells in the spleen. Loss of p55R and/or p75R did not affect the expression of CD44 on LCMV-specific memory CD8 T cells (Fig. 5A); LCMV-specific memory CD8 T cells from all groups of mice were uniformly CD44hi. However, notable differences were evident when expression of CD62L and CCR7 was compared between NP396-specific memory CD8 T cells from wild-type and TNFR-deficient mice (Fig. 7). Consistent with published data, ≈60% of NP396-specific memory CD8 T cells in wild-type mice exhibited the CD62Lhi CCR7hi phenotype (65). In contrast, only ≈30% of NP396-specific CD8 T cells were of the CD62Lhi CCR7hi phenotype in LCMV-immune p55−/− and DKO mice. Strikingly, 40 to 50% of NP396-specific CD8 T cells were CD62Llo CCR7hi in p55−/− and DKO mice compared to ≈17% in wild-type mice. Compared to NP396-specific CD8 T cells, the effect of TNFR deficiency on the expression of CD62L on GP33-specific CD8 T cells was variable and less remarkable; while 20 to 30% of the GP33-specific memory CD8 T cells in wild-type mice were CD62Llo, the relative proportions of CD62Llo cells varied between 30 and 50% in DKO mice. Taken together, these data show that TNFR deficiency affected the cell surface expression of CD62L and to a lesser extent CCR7 on antigen-specific memory CD8 T cells in an epitope-dependent fashion.
FIG. 7.
Expression of CD62L and CCR7 on LCMV-specific memory CD8 T cells in TNFR-deficient mice. Three hundred and eighty days after infection with LCMV, splenocytes were stained with anti-CD8, anti-CD62L, MHC class I tetramers (specific to NP396 and GP33 epitopes), and CCL19-Fc (ligand for CCR7). The dot plots are gated on tetramer-binding CD8 T cells. The numbers in each quadrant represent percentages of cells (± standard deviation) of a particular phenotype among NP396-specific CD8 T cells (upper panel) or GP33-specific CD8 T cells (lower panel). Data are the means for two to three mice per group and representative of two independent experiments.
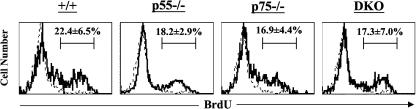
One of the hallmark features of memory T cells is their ability to undergo proliferative renewal under the influence of cytokines (25, 32, 57, 69). Proliferative renewal is essential to prevent cellular attrition and promote survival of memory CD8 T cells (51). During proliferative renewal, cellular proliferation is matched by cell death, as a result of which the numbers of memory CD8 T cells remain stable. Since there is evidence that TNFR signaling can regulate apoptosis of activated T cells (70), it was of importance to examine the role of TNFRs in regulating the proliferative renewal of LCMV-specific memory CD8 T cells. To this end, groups of wild-type, p55−/−, p75−/−, and DKO mice were infected with LCMV-Armstrong, and 120 days later, proliferation of LCMV-specific memory CD8 T cells was determined by measuring BrdU incorporation in vivo. Figure 8 shows the incorporation of BrdU by LCMV-specific CD8 T cells over a period of 8 days. Consistent with published findings (54), 20 to 30% of NP396-specific CD8 T cells in the wild-type mice incorporated BrdU during a span of 8 days (Fig. 8). Figure 8 also shows that the relative proportions of LCMV-specific CD8 T cells that incorporated BrdU in p55−/−, p75−/−, and DKO mice were comparable to those of wild-type mice. The turnover of GP33-specific memory CD8 T cells in p55−/−, p75−/−, and DKO mice was comparable to that of wild-type mice (data not shown). These data suggested that proliferative renewal of LCMV-specific memory CD8 T cells is not dependent upon signaling via TNFRs.
FIG. 8.
Proliferative renewal of LCMV-specific memory CD8 T cells in TNFR-deficient mice. Groups of wild-type, p55−/−, p75−/−, and DKO mice were infected with LCMV-Armstrong. One hundred and twenty days after infection, LCMV-immune mice were administered BrdU in drinking water for 8 days. After 8 days of BrdU treatment, mice were sacrificed and splenocytes were stained with anti-CD8 antibodies, MHC class I tetramers (specific to NP396-404), and anti-BrdU antibodies. The histograms showing BrdU staining are gated on tetramer-binding CD8 T cells. The bold and broken lines represent staining of tetramer-binding CD8 T cells for BrdU in BrdU-treated and untreated LCMV-immune mice, respectively. The numbers are the percentages of NP396-404-specific memory CD8 T cells that incorporated BrdU in the 8-day period, and the data are the means for four mice per group± standard deviation from two independent experiments.
DISCUSSION
In this report we have documented the role of TNFRs p55R and p75R in regulating various phases of the CD8 T-cell response to an acute viral infection. We show that TNF-TNFR interactions have a negative regulatory role during the expansion and contraction phases of the CD8 T-cell response to LCMV. Deficient TNF-TNFR interactions increased the expansion of CD8 T cells and attenuated the ensuing contraction phase of the T-cell response. As a sequel, there was a significant enhancement in the number of LCMV-specific memory CD8 T cells in TNFR-deficient mice compared to wild-type mice. These findings have implications for the development of vaccines and treatment of autoimmune diseases.
In vitro experiments have suggested a role for TNF-TNFR interactions as costimulators during T-cell activation (51). A recent report has indicated that TNFR p75R provides a CD28-independent costimulatory signal for optimal T-cell proliferation in vitro (23). Although p55R−/− mice mount normal CD8 cytotoxic T-lymphocyte responses to LCMV (47), the effect of p75R deficiency or double deficiency in p55R and p75R on the induction of LCMV-specific CD8 T-cell responses was not known. Our studies revealed that p55R and p75R are not required for optimal activation and expansion of CD8 T cells during an acute LCMV infection. Following LCMV infection, CD8 T cells from p55−/−, p75−/−, and DKO mice underwent normal activation, proliferation, and differentiation into potent cytotoxic effector cells. Interestingly, our studies showed that loss of both p55R and p75R resulted in significant enhancement of the expansion of LCMV-specific CD8 T cells, which suggests that TNFRs may inhibit primary CD8 T-cell responses in vivo. This inference is consistent with a recent report which shows that TNF suppresses T-cell responses during a mycobacterial infection in mice (68). Lack of TNF during a mycobacterial infection results in enhanced activation of T cells and fatal immunopathology (68).
The antiviral effect of TNF is well established (60). TNF activity is important in control of ectromelia virus, cytomegalovirus, and adenovirus infections in mice (48). However, treatment with exogenous TNF or blocking TNF activity with antibodies or p55R deficiency had no detectable effects on the replication of LCMV in mice (24, 29). Our present study confirms and extends these findings. Mice deficient in p55R and/or p75R successfully controlled an acute infection with LCMV-Armstrong with kinetics comparable to those in wild-type mice. Resolution of an acute LCMV infection is dependent upon IFN-γ and perforin (20, 31, 63). Compared to an acute infection with LCMV, the resolution of a chronic LCMV infection is dependent upon TNFRs (55).
During an acute LCMV infection, the peak of the CD8 T-cell response is attained on day 8 postinfection, which coincides with viral clearance. Between days 8 and 30 postinfection, 90 to 95% of the expanded CD8 T cells are eliminated by programmed contraction (19). The mechanisms that underlie this contraction are a subject of intense investigation because of the implications for memory T-cell development and therapy of T-cell-dependent immunopathologies. It has been reported that the contraction phases of the CD8 T-cell response to Listeria monocytogenes and LCMV are impaired in IFN-γ-deficient mice (5). Here we show that TNF-TNFR interactions might play a role in regulating the contraction phase of the CD8 T-cell response to LCMV. Deficiency of both p55R and p75R consistently reduced the magnitude of contraction of CD8 T cells specific to the two immunodominant cytotoxic T-lymphocyte epitopes NP396 and GP33. However, the antigenic specificity of CD8 T cells might determine whether a deficiency of p55R alone or a double deficiency of p55R and p75R is necessary to affect the contraction phase of the anti-LCMV CD8 T-cell response. Deficiency of p55R alone attenuated the contraction of NP396-specific CD8 T cells but not GP33-specific CD8 T cells. Deficiency of both p55R and p75R was required to reduce the contraction of GP33-specific CD8 T cells. Epitope-specific effects of TNFR deficiency on the CD8 T-cell response have also been reported in an influenza virus infection model in mice (58). Nevertheless, this finding might explain why the contraction of GP33-specific transgenic CD8 T cells was unaffected by p55R deficiency alone in previous studies (39, 45). Notably, our results emphasize the importance of studying polyclonal multiepitope-specific CD8 T-cell responses in lieu of using monoclonal CD8 T cells carrying a transgenic T-cell receptor (39, 45).
It is worth noting that p75R deficiency did not affect the clonal downsizing of LCMV-specific CD8 T cells in vivo, despite strong evidence that had ascribed a role for p75R in activated T-cell apoptosis in vitro (70). Similar to our results, the downregulation of primary CD8 T-cell responses following influenza virus infection was independent of p75R (58). However, as indicated above, our studies show that p75R does play an overlapping role with p55R in mediating the contraction of GP33-specific CD8 T cells, which concurs with a report that p75R augments p55R-induced apoptosis in T lymphocytes (10).
How did TNFR deficiency affect the contraction phase of the anti-LCMV CD8 T-cell response? The number of CD8 T cells at a given time is dependent upon the proliferation and/or apoptosis rates. Therefore, a delay in clonal downsizing of LCMV-specific CD8 T cells in DKO mice could be a sequel to increased proliferation and/or reduced apoptosis. It is less likely that continued cellular proliferation resulted in an increased number of LCMV-specific CD8 T cells in DKO mice because the proliferation rates of LCMV-specific CD8 T cells in wild-type and TNFR-deficient mice were similar during the contraction phase of the T-cell response. Consistent with the recognized ability of TNF to induce apoptosis of activated CD8 T cells (70), at the onset of the contraction phase, the relative proportions of “proapoptotic” LCMV-specific CD8 T cells were significantly lower in the spleens of DKO mice than in wild-type mice. Previous work has shown that the “proapoptotic” status of LCMV-specific CD8 T cells on day 8 postinfection was associated with downregulation of BcL-2 expression (16). However, we did not detect differences in BcL-2 expression between NP396-specific CD8 T cells from wild-type and DKO mice (data not shown). It was recently shown that the proapoptotic molecule Bim might be important in clonal downsizing of activated CD8 T cells following infection of mice with herpes simplex virus (40). It would be important to examine whether TNFR-mediated apoptosis is dependent upon Bim. Nonetheless, the data presented in this paper strongly suggest that TNFRs might play a role in regulating the clonal downsizing of activated CD8 T cells in vivo.
The generation of T-cell memory is of fundamental importance to the development of effective vaccines. The development of T-cell memory is associated with both quantitative and qualitative alterations in antigen-specific T cells (2, 52, 53). Quantitatively, there are more antigen-specific CD8 T cells in immune mice compared to naive mice. The number of memory CD8 T cells generated is a function of the extent of clonal expansion and the magnitude of clonal downsizing that occurs during the primary T-cell response (2, 19). Our studies indicated that a combined deficiency of TNFRs p55R and p75R resulted in a significant increase (≈10-fold) in the number of antigen-specific memory CD8 T cells following acute LCMV infection. What is the mechanism(s) of enhancement in the memory CD8 T-cell number in DKO mice? The increase in the number of LCMV-specific memory CD8 T cells in the DKO mice is likely a result of increased expansion coupled with reduced contraction during the primary T-cell response. Following the phase of antigen-driven expansion, between days 8 and 480 postinfection, there was an approximately 35-fold drop in the number of NP396-specific CD8 T cells in wild-type and p75−/− mice. In striking contrast, in the DKO mice, there was only an 18-fold contraction in the number of NP396-specific CD8 T cells after day 8 postinfection. After day 8 postinfection, the magnitude of contraction of GP33-specific CD8 T cells in the wild-type and DKO mice was 34-fold and 8-fold, respectively.
Two lines of evidence argue against the possibility that prolonged antigenic stimulation in DKO mice resulted in enhancing the number of memory CD8 T cells. First, clearance of infectious LCMV in all tissues of the mice was more expedient in DKO mice compared to wild-type mice. Second, the proliferation rate of LCMV-specific CD8 T cells between days 8 and 15 postinfection was comparable between wild-type and TNFR-deficient mice. Additionally, viral persistence during an LCMV infection results in loss (exhaustion) and not inflation of NP396-specific CD8 T cells (65, 67). The enhancement in the number of memory CD8 T cells in DKO mice was not restricted to the spleen and lymph nodes. Substantially greater numbers of LCMV-specific memory CD8 T cells were recovered from the nonlymphoid organs of DKO mice compared to wild-type mice. This finding decreases the likelihood of anatomic redistribution of memory CD8 T cells as the underlying cause of the observed increase in the memory CD8 T cells in the spleen.
Qualitative differences between naïve and memory T cells have been well recognized. Compared to naïve T cells, memory T cells express higher levels of adhesion molecules and exhibit hyperreactivity to antigenic stimulation (2, 43, 61). Cell surface expression of CD62L and CCR7 has been used to identify subpopulations of human memory CD8 T cells, namely, central memory and effector memory (49). It was of interest to investigate whether TNFR deficiency affected the relative proportions of LCMV-specific central and effector memory CD8 T cells in the memory CD8 T-cell pool. Approximately 60 and 10% of NP396-specific memory CD8 T cells in the spleens of wild-type mice exhibited the “classic” central memory (CD62Lhi CCR7hi) and effector memory (CD62Llo CCR7lo) phenotypes, respectively. In contrast, in the DKO mice, there was a significant reduction in the percentage of central memory NP396-specific CD8 T cells. Strikingly, ≈50% of the NP396-specific memory CD8 T cells in DKO mice were CD62Llo CCR7hi, a phenotype intermediate between those of central and effector memory.
GP33-specific memory CD8 T cells in all groups of mice were uniformly CCR7hi. However, the relative proportions of CD62Llo cells among GP33-specific memory CD8 T cells were marginally increased in the DKO mice compared to wild-type mice. We can only speculate about the cause of the observed increase in the relative proportions of CD62Llo memory CD8 T cells in DKO mice. One possibility is that TNFR deficiency leads to increased survival of memory T-cell precursors that give rise to CD62Llo memory CD8 T cells. Since it has been shown previously that CD62Llo memory CD8 T cells convert to the CD62Lhi phenotype over time (65), it is possible that the transition of memory CD8 T cells from the CD62Llo to CD62Lhi phenotype is delayed in the DKO mice. Indeed, 250 days later, a great majority of LCMV-specific memory CD8 T cells in DKO mice exhibited the CD62Lhi CCR7hi central memory phenotype (data not shown). However, the observed phenotypic differences between wild-type and DKO mice did not appear to affect the ability of memory CD8 T cells to produce cytokines such as IFN-γ upon antigenic stimulation. Preliminary studies have indicated that the activation threshold of LCMV-specific memory CD8 T cells (measuring the ability of a CD8 T-cell to produce IFN-γ as a function of peptide concentration) in wild-type and DKO mice is comparable (data not shown). Furthermore, the unique ability of memory CD8 T cells to undergo proliferative renewal was unaffected by TNFR deficiency, which is consistent with long-term maintenance of a relatively stable number of LCMV-specific memory CD8 T cells in TNFR-deficient mice. In striking contrast to the spleen, TNFR deficiency did not affect the expression of CD62L on LCMV-specific memory CD8 T cells in the liver; ≈90% of NP396-specific memory CD8 T cells were CD62Llo in both wild-type and TNFR-deficient mice. Our observation is in complete agreement with published work by Wherry and Ahmed (65). However, in contrast to our observation, it has been reported that, following intranasal LCMV infection, a significant proportion of memory CD8 T cells in the liver expressed the CD62Lhi phenotype (22). Although the exact cause of this discrepancy is not known, it is possible that differences in the routes of infection (intraperitoneal versus intranasal) might alter the magnitude and the kinetics of viral replication in different tissues, which in turn can affect the phenotype and tissue localization of virus-specific CD8 T cells.
In conclusion, we have documented for the first time that TNFRs might play a critical role in regulating all phases of the CD8 T-cell response during an acute viral infection. What are the implications of this finding? The induction of protective immunity depends on the generation of a threshold number of memory T cells (2). Understanding the mechanisms that regulate the magnitude of T-cell memory should aid in the development of immune modalities to enhance the generation of memory T cells. Therefore, modulation of TNFR-mediated effects may be a fruitful strategy to increase the number of vaccine-induced memory CD8 T cells. Second, the findings presented in this article have clear implications for the treatment of T-cell-dependent immunopathologies. Although several studies have shown a protective role for TNF-TNFR interactions in autoimmune diseases (4, 14, 15), the underlying mechanisms are not well understood. It has recently been shown that exacerbation of experimental autoimmune encephalomyelitis (a T-cell-dependent disease) in TNF-deficient mice is associated with prolonged myelin basic protein-specific T-cell reactivity, as measured by proliferation assays (21). Studies with a transgenic mouse model of LCMV-induced diabetes have shown that induction of TNF-α late in the infection reduced the incidence of autoimmune disease by diminishing the number of autoreactive NP396-specific CD8 T cells in the islets (11). In another study, viral infection-induced cure of prediabetic mice was shown to be dependent upon TNF-α-dependent effects, possibly by causing the apoptosis of NP396-specific CD8 T cells (12).
Our studies suggested that TNFRs might exert their immunoregulatory effects by inhibiting the expansion of CD8 T cells during the primary response and limiting the number of memory CD8 T cells that survive the contraction phase. Taken together, these findings suggest that caution needs to be exercised in administering anti-TNF therapies to treat T-cell-dependent inflammatory diseases because TNF deficiency might lead to augmentation of the autoaggressive T-cell response.
Acknowledgments
We thank John Altman (Emory University) for providing the MHC class I tetramers. Technical assistance by Shuning Zhan, Nicole Miller, and Katie Skell is greatly appreciated.
This work was supported in part by grants from National Institutes of Health (AI48785) and the National Multiple Sclerosis Society (RG3092A1/T) to M. Suresh.
REFERENCES
- 1.Ahmed, R., A. Salmi, L. D. Butler, J. M. Chiller, and M. B. Oldstone. 1984. Selection of genetic variants of lymphocytic choriomeningitis virus in spleens of persistently infected mice. Role in suppression of cytotoxic T lymphocyte response and viral persistence. J. Exp. Med. 160:521-540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ahmed, R., and D. Gray. 1996. Immunological memory and protective immunity: understanding their relation. Science 272:54-60. [DOI] [PubMed] [Google Scholar]
- 3.Asano, M. S., and R. Ahmed. 1996. CD8 T-cell memory in B cell-deficient mice. J. Exp. Med. 183:2165-2174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bachmann, R., H. P. Eugster, K. Frei, A. Fontana, and H. Lassmann. 1999. Impairment of TNF-receptor-1 signaling but not fas signaling diminishes T-cell apoptosis in myelin oligodendrocyte glycoprotein peptide-induced chronic demyelinating autoimmune encephalomyelitis in mice. Am. J. Pathol. 154:1417-1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Badovinac, V. P., A. R. Tvinnereim, and J. T. Harty. 2000. Regulation of antigen-specific CD8+ T-cell homeostasis by perforin and interferon-gamma. Science 290:1354-1358. [DOI] [PubMed] [Google Scholar]
- 6.Borrow, P., A Tishon, S. Lee, J. Xu, I. S. Grewal, M. B. Oldstone, and R. A. Flavell. 1996. CD40L-deficient mice show deficits in antiviral immunity and have an impaired memory CD8+ CTL response. J. Exp. Med. 183:2129-2142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Butz, E. A., and M. J. Bevan. 1998. Massive expansion of antigen-specific CD8+ T cells during an acute virus infection. Immunity 8:167-175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Callan, M. F., C. Fazou, H. Yang, T. Rostron, K. Poon, C. Hatton, and A. J. McMichael. 2000. CD8+ T-cell selection, function, and death in the primary immune response in vivo. J. Clin. Investig. 106:1251-1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Callan, M. F., L. Tan, N. Annels, G. S. Ogg, J. D. K. Wilson, C. A. O'Callaghan, N. Steven, A. J. McMichael, and A. B. Rickinson. 1998. Direct visualization of antigen-specific CD8+ T cells during the primary immune response to Epstein-Barr virus in vivo. J. Exp. Med. 187:1395-1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chan, F. K., and M. J. Lenardo. 2000. A crucial role for p80 TNF-R2 in amplifying p60 TNF-R1 apoptosis signals in T lymphocytes. Eur. J. Immunol. 30:652-660. [DOI] [PubMed] [Google Scholar]
- 11.Christen, U., T. Wolfe, U. Mohrle, A. C. Hughes, E. Rodrigo, E. A. Green, R. A. Flavell, and M. G. von Herrath. 2001. A dual role for TNF-alpha in type 1 diabetes: islet-specific expression abrogates the ongoing autoimmune process when induced late but not early during pathogenesis. J. Immunol. 166:7023-7032. [DOI] [PubMed] [Google Scholar]
- 12.Christen, U., D. Benke, T. Wolfe, E. Rodrigo, A. Rhode, A. C. Hughes, M. B. Oldstone, and M. G. von Herrath. 2004. Cure of prediabetic mice by viral infections involves lymphocyte recruitment along an IP-10 gradient. J. Clin. Investig. 113:74-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Christensen, J. P., O. Marker, and A. R. Thomsen. 1994. The role of CD4+ T cells in cell-mediated immunity to LCMV: studies in MHC class I and class II deficient mice. Scand. J. Immunol. 40:373-382. [DOI] [PubMed] [Google Scholar]
- 14.Cope, A. P. 1998. Regulation of autoimmunity by proinflammatory cytokines. Curr. Opin. Immunol. 10:669-676. [DOI] [PubMed] [Google Scholar]
- 15.Falcone, M., and N. Sarvetnick. 1999. Cytokines that regulate autoimmune responses. Curr. Opin. Immunol. 11:670-676. [DOI] [PubMed] [Google Scholar]
- 16.Grayson, J. M., A. J. Zajac, J. D. Altman, and R. Ahmed. 2000. Cutting edge: increased expression of Bcl-2 in antigen-specific memory CD8+ T cells. J. Immunol. 164:3950-3954. [DOI] [PubMed] [Google Scholar]
- 17.Homann, D., L. Teyton, and M. B. Oldstone. 2001. Differential regulation of antiviral T-cell immunity results in stable CD8+ but declining CD4+ T-cell memory. Nat. Med. 7:913-919. [DOI] [PubMed] [Google Scholar]
- 18.Hou, S., L. Hyland, K. W. Ryan, A. Portner, and P. C. Doherty. 1994. Virus-specific CD8+ T-cell memory determined by clonal burst size. Nature 369:652-654. [DOI] [PubMed] [Google Scholar]
- 19.Kaech, S. M., E. J. Wherry, and R. Ahmed. 2002. Effector and memory T-cell differentiation: implications for vaccine development. Nat. Rev. Immunol. 2:251-262. [DOI] [PubMed] [Google Scholar]
- 20.Kagi, D., B. Ledermann, K. Burki, P. Seiler, B. Odermatt, K. J. Olsen, E. R. Podack, R. M. Zingernagel, and H. Hengartner. 1994. Cytotoxicity mediated by T cells and natural killer cells is greatly impaired in perforin-deficient mice. Nature 369:31-37. [DOI] [PubMed] [Google Scholar]
- 21.Kassiotis, G., and G. Kollias. 2001. Uncoupling the proinflammatory from the immunosuppressive properties of tumor necrosis factor (TNF) at the p55 TNF receptor level: implications for pathogenesis and therapy of autoimmune demyelination. J. Exp. Med. 193:427-434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Khanolkar, A., M. J. Fuller, and A. J. Zajac. 2004. CD4 T-cell-dependent CD8 T-cell maturation. J. Immunol. 172:2834-2844. [DOI] [PubMed] [Google Scholar]
- 23.Kim, E. Y., and H. S. Teh. 2001. TNF type 2 receptor (p75) lowers the threshold of T-cell activation. J. Immunol. 167:6812-6820. [DOI] [PubMed] [Google Scholar]
- 24.Klavinskis, L. S., R. Geckeler, and M. B. Oldstone. 1989. Cytotoxic T lymphocyte control of acute lymphocytic choriomeningitis virus infection: interferon gamma, but not tumor necrosis factor alpha, displays antiviral activity in vivo. J. Gen. Virol. 70:3317-3325. [DOI] [PubMed] [Google Scholar]
- 25.Kollias, G., D. Kontoyiannis, E. Douni, and G. Kassiotis. 2002. The role of TNF/TNFR in organ-specific and systemic autoimmunity: implications for the design of optimized ‘anti-TNF’ therapies. Curr. Dir. Autoimmun. 5:30-50. [DOI] [PubMed] [Google Scholar]
- 26.Ku, C. C., M. Murakami, A. Sakamoto, J. Kappler, and P. Marrack. 2000. Control of homeostasis of CD8+ memory T cells by opposing cytokines. Science 288:675-678. [DOI] [PubMed] [Google Scholar]
- 27.Kundig, T. M., A. Shahinian, K. Kawai, H. W. Mittrucker, E. Sebzda, M. F. Bachmann, T. W. Mak, and P. S. Ohashi. 1996. Duration of TCR stimulation determines costimulatory requirement of T cells. Immunity 5:41-52. [DOI] [PubMed] [Google Scholar]
- 28.Lau, L. L., B. D. Jamieson, T. Somasundaram, and R. Ahmed. 1994. Cytotoxic T-cell memory without antigen. Nature 369:648-652. [DOI] [PubMed] [Google Scholar]
- 29.Leist, T. P., and R. M. Zinkernagel. 1990. Treatment with anti-tumor necrosis factor alpha does not influence the immune pathological response against lymphocytic choriomeningitis virus. Cytokine 2:29-34. [DOI] [PubMed] [Google Scholar]
- 30.Locksley, R. M., N. Killeen, and M. J. Lenardo. 2001. The TNF and TNF receptor superfamilies: integrating mammalian biology. Cell 104:487-501. [DOI] [PubMed] [Google Scholar]
- 31.Lohman, B. L., and R. M. Welsh. 1998. Apoptotic regulation of T cells and absence of immune deficiency in virus-infected gamma interferon receptor knockout mice. J. Virol. 72:7815-7821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Marrack, P., J. Bender, D. Hildeman, M. Jordan, T. Mitchell, M. Murakami, A. Sakamoto, B. C. Schaefer, B. Swanson, and J. Kappler. 2001. Homeostasis of alpha beta TCR+ T cells. Nat. Immunol. 1:107-111. [DOI] [PubMed] [Google Scholar]
- 33.Masopust, D., V. Vezys, A. L. Marzo, and L. Lefrancois. 2001. Preferential localization of effector memory cells in nonlymphoid tissue. Science 291:2413-2417. [DOI] [PubMed] [Google Scholar]
- 34.Matloubian, M., R. J. Concepcion, and R. Ahmed. 1994. CD4+ T cells are required to sustain CD8+ cytotoxic T-cell responses during chronic viral infection. J. Virol. 68:8056-8063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McDermott, M. F., I. Aksentijevich, J. Galon, E. M. McDermott, B. W. Ogunkolade, M. Centola, E. Mansfield, M. Gadina, L. Karenko, T. Pettersson, J. McCarthy, D. M. Frucht, M. Aringer, Y. Torosyan, A. M. Teppo, M. Wilson, H. M. Karaarslan, Y. Wan, I. Todd, G. Wood, R. Schlimgen, T. R. Kumarajeewa, S. M. Cooper, J. P. Vella, C. I. Amos, J. Mulley, K. A. Quane, M. G. Molloy, A. Ranki, R. J. Powell, G. A. Hitman, J. J. O'Shea, and D. L. Kastner. 1999. Germline mutations in the extracellular domains of the 55 kDa TNF receptor, TNFR1, define a family of dominantly inherited autoinflammatory syndromes. Cell 97:133-144. [DOI] [PubMed] [Google Scholar]
- 36.McMichael, A. J., and C. A. O'Callaghan. 1998. A new look at T cells. J. Exp. Med. 187:1367-1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mongkolsapaya, J., A. Jaye, M. F. C. Callan, A. F. Magnusen, A. J. McMichael, and H. C. Whittle. 1999. Antigen-specific expansion of cytotoxic T lymphocytes in acute measles virus infection. J. Virol. 73:67-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Murali-Krishna, K., J. D. Altman, M. Suresh, D. J. D. Sourdive, A. J. Zajac, J. Miller, and R. Ahmed. 1998. Counting antigen-specific CD8 T cells: a reevaluation of bystander activation during viral infection. Immunity 8:177-187. [DOI] [PubMed] [Google Scholar]
- 39.Nguyen, L. T., K. McKall-Faienza, A. Zakarian, D. E. Speiser, T. W. Mak, and P. S. Ohashi. 2000. TNF receptor 1 (TNFR1) and CD95 are not required for T-cell deletion after virus infection but contribute to peptide-induced deletion under limited conditions. Eur. J. Immunol. 30:683-688. [DOI] [PubMed] [Google Scholar]
- 40.Pellegrini, M., G. Belz, P. Bouillet, and A. Strasser. 2003. Shutdown of an acute T-cell immune response to viral infection is mediated by the proapoptotic Bcl-2 homology 3-only protein Bim. Proc. Natl. Acad. Sci. USA 100:14175-14180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Peschon, J. J., D. S. Torrance, K. L. Stocking, M. B. Glaccum, C. Otten, C. R. Willis K. Charrier, P. J. Morrissey, C. B. Ware, and K. M. Mohler. 1998. TNF receptor-deficient mice reveal divergent roles for p55 and p75 in several models of inflammation. J. Immunol. 160:943-952. [PubMed] [Google Scholar]
- 42.Pfeffer, K., T. Matsuyama, T. M. Kundig, A. Wakeham, K. Kishihara, A. Shahinian, K. Wiegmann, P. S. Ohashi, M. Kronke, and T. W. Mak. 1993. Mice deficient for the 55 kd tumor necrosis factor receptor are resistant to endotoxic shock, yet succumb to L. monocytogenes infection. Cell 73:457-467. [DOI] [PubMed] [Google Scholar]
- 43.Pihlgren, M., P. M. Dubois, M. Tomkowiak, T. Sjögren, and J. Marvel. 1996. Resting memory CD8+ T cells are hyperreactive to antigenic challenge in vitro. J. Exp. Med. 184:2141-2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Razvi, E. S., Z. Jiang, B. A. Woda, and R. M. Welsh. 1995. Lymphocyte apoptosis during the silencing of the immune response to acute viral infections in normal, lpr, and Bcl-2-transgenic mice. Am. J. Pathol. 147:79-91. [PMC free article] [PubMed] [Google Scholar]
- 45.Reich, A., H. Korner, J. D. Sedgwick, and H. Pircher. 2000. Immune down-regulation and peripheral deletion of CD8 T cells does not require TNF receptor-ligand interactions nor CD95 (Fas, APO-1). Eur. J. Immunol. 30:678-682. [DOI] [PubMed] [Google Scholar]
- 46.Reinhardt, R. L., A. Khoruts, R. Merica, T. Zell, and M. K. Jenkins. 2001. Visualizing the generation of memory CD4 T cells in the whole body. Nature 410:101-105. [DOI] [PubMed] [Google Scholar]
- 47.Rothe, J., W. Lesslauer, H. Lotscher, Y. Lang, P. Koebel, F. Kontgen, A. Althage, R. Zinkernagel, M. Steinmetz, and H. Bluethmann. 1993. Mice lacking the tumor necrosis factor receptor 1 are resistant to TNF-mediated toxicity but highly susceptible to infection by Listeria monocytogenes. Nature 364:798-802. [DOI] [PubMed] [Google Scholar]
- 48.Ruby, J., H. E. Bluethmann, and J. J. Peschon. 1997. Antiviral activity of tumor necrosis factor (TNF) is mediated via p55 and p75 TNF receptors. J. Exp. Med. 9:1591-1596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sallusto, F., J. Geginat, and A. Lanzavecchia. 2004. Central memory and effector memory T-cell subsets: function, generation, and maintenance. Annu. Rev. Immunol. 22:745-763. [DOI] [PubMed] [Google Scholar]
- 50.Sedgwick, J. D., D. S. Riminton, J. G. Cyster, and H. Korner. 2000. Tumor necrosis factor: a master-regulator of leukocyte movement. Immunol. Today 21:110-113. [DOI] [PubMed] [Google Scholar]
- 51.Smith, C. A., T. Farrah, and R. G. Goodwin. 1994. The TNF receptor superfamily of cellular and viral proteins: activation, costimulation, and death. Cell 76:959-962. [DOI] [PubMed] [Google Scholar]
- 52.Sprent, J., and C. D. Surh. 2002. T-cell memory. Annu. Rev. Immunol. 20:551-579. [DOI] [PubMed] [Google Scholar]
- 53.Sprent, J., and D. F. Tough. 2001. T-cell death and memory. Science 293:245-248. [DOI] [PubMed] [Google Scholar]
- 54.Suresh, M., J. K. Whitmire, L. E. Harrington, C. P. Larsen, T. C. Pearson, J. D. Altman, and R. Ahmed. 2001. Role of CD28-B7 interactions in generation and maintenance of CD8 T-cell memory. J. Immunol. 167:5565-5573. [DOI] [PubMed] [Google Scholar]
- 55.Suresh, M., X. Gao, C. Fischer, N. E. Miller, and K. Tewari. 2004. Dissection of antiviral and immune regulatory functions of tumor necrosis factor receptors in a chronic lymphocytic choriomeningitis virus infection. J. Virol. 78:3906-3918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tewari, K., J. Sacha, X. Gao, and M. Suresh. 2004. Effect of chronic viral infection on epitope selection, cytokine production, and surface phenotype of CD8 T cells and the role of IFN-gamma receptor in immune regulation. J. Immunol. 172:1491-1500. [DOI] [PubMed] [Google Scholar]
- 57.Tough, D. F., X. Zhang, J. Sprent. 2001. An IFN-gamma-dependent pathway controls stimulation of memory phenotype CD8+ T-cell turnover in vivo by IL-12, IL-18, and IFN-gamma. J. Immunol. 166:6007-6011. [DOI] [PubMed] [Google Scholar]
- 58.Turner, S. J., N. L. La Gruta, J. Stambas, G. Diaz, and P. C. Doherty. 2004. Differential tumor necrosis factor receptor 2-mediated editing of virus-specific CD8+ effector T cells. Proc. Natl. Acad. Sci. USA 101:3545-3550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Usherwood, E. J., R. J. Hogan, G. Crowther, S. L. Surman, T. L. Hogg, J. D. Altman, and D. L. Woodland. 1999. Functionally heterogeneous CD8(+) T-cell memory is induced by Sendai virus infection of mice. J. Virol. 9:7278-7286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Vassalli, P. 1992. The pathophysiology of tumor necrosis factors. Annu. Rev. Immunol. 10:411-452. [DOI] [PubMed] [Google Scholar]
- 61.Veiga-Fernandes, H., U. Walter, C. Bourgeois, A. McLean, and B. Rocha. 2000. Response of naive and memory CD8+ T cells to antigen stimulation in vivo. Nat. Immunol. 1:47-53. [DOI] [PubMed] [Google Scholar]
- 62.von Herrath, M. G., M. Yokoyama, J. Dockter, M. B. Oldstone, and J. L. Whitton. 1996. CD4-deficient mice have reduced levels of memory cytotoxic T lymphocytes after immunization and show diminished resistance to subsequent virus challenge. J. Virol. 70:1072-1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Walsh, C. M., M. Matloubian, C-C. Liu, R. Ueda, G. G. Kurahara, and W. R. Clark. 1994. Immune function in mice lacking the perforin gene. Proc. Natl Acad. Sci. USA 91:10854-10858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wang, X. Z., S. E. Stepp, M. A. Brehm, H. D. Chen, L. K. Selin, and R. M. Welsh. 2003. Virus-specific CD8 T cells in peripheral tissues are more resistant to apoptosis than those in lymphoid organs. Immunity 18:631-642. [DOI] [PubMed] [Google Scholar]
- 65.Wherry, E. J., V. Teichgraber, T. C. Becker, D. Masopust, S. M. Kaech, R. Antia, U. H. von Andrian, and R. Ahmed. 2003. Lineage relationship and protective immunity of memory CD8 T-cell subsets. Nat. Immunol. 4(3):225-234. [DOI] [PubMed] [Google Scholar]
- 66.Whitmire, J. K., M. K. Slifka, I. S. Grewal, R. A. Flavell, and R. Ahmed. 1996. CD40 ligand-deficient mice generate a normal primary cytotoxic T-lymphocyte response but a defective humoral response to a viral infection. J. Virol. 12:8375-8381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zajac, A. J., J. N. Blattman, K. Murali-Krishna, D. J. Sourdive, M. Suresh, J. D. Altman, and R. Ahmed. 1998. Viral immune evasion due to persistence of activated T cells without effector function. J. Exp. Med. 188:2205-2213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zganiacz, A., M. Santosuosso, J. Wang, T. Yang, L. Chen, M. Anzulovic, S. Alexander, B. Gicquel, Y. Wan, J. Bramson, M. Inman, and Z. Xing. 2004. TNF-alpha is a critical negative regulator of type 1 immune activation during intracellular bacterial infection. J. Clin. Investig. 113:401-413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhang, X., S. Sun, I. Hwang, D. F. Tough, and J. Sprent. 1998. Potent and selective stimulation of memory-phenotype CD8+ T cells in vivo by IL-15. Immunity 8:591-599. [DOI] [PubMed] [Google Scholar]
- 70.Zheng, L., G. Fisher, R. E. Miller, J. Peschon, D. H. Lynch, and M. J. Lenardo. 1995. Induction of apoptosis in mature T cells by tumour necrosis factor. Nature 377:348-351. [DOI] [PubMed] [Google Scholar]
- 71.Zhou, T., C. K3rd. Edwards, P. Yang, Z. Wang, H. Bluethmann, and J. D. Mountz. 1996. Greatly accelerated lymphadenopathy and autoimmune disease in lpr mice lacking tumor necrosis factor receptor I. J. Immunol. 156:2661-2665. [PubMed] [Google Scholar]
- 72.Zimmermann, C., M. Rawiel, M., C. Blaser, M. Kaufmann, and H. Pircher. 1996. Homeostatic regulation of CD8+ T cells after antigen challenge in the absence of Fas (CD95). Eur. J. Immunol. 12:2903-2910. [DOI] [PubMed] [Google Scholar]