Abstract
Heme oxygenase 1 (HMOX1) plays an important role in the development of chronic obstructive pulmonary disease (COPD). However, the association of HMOX1 length polymorphism in promoter region to the risk and severity of COPD has not been well studied. In this study, we searched the databases including PubMed, EMBASE, Cochrane Library and China National Knowledge Infrastructure (CNKI) and extracted the information from related articles. Pooled odds ratios (ORs) and 95% confidence intervals (CIs) were calculated to study the effect of HMOX1 polymorphism on the risk and severity of COPD. As a result, nine studies were included for this meta‐analysis. Higher frequencies of L allele and type I genotype (containing at least one L allele) were found in patients with COPD (for L allele, OR 2.02, 95% CI: 1.32–3.11, P = 0.001; for type I genotype, OR 1.82, 95% CI: 1.28–2.61, P = 0.001), especially in Asian population (for L allele, OR 2.23, 95% CI: 1.68–2.95, P < 0.001; for type I genotype, OR 2.02, 95% CI: 1.51–2.70, P < 0.001). Genotyping method, source of control subjects, literature quality and language also affected the results to some extent. However, there was little difference in HMOX1 genotypes distribution in patients with COPD with different severity. Our study indicated L allele and type I genotype were related to the susceptibility but not the severity of COPD.
Keywords: HMOX1, COPD, length polymorphism, susceptibility, severity
Introduction
COPD is a common airway disease, leading to an increasing mortality and morbidity in the world 1, 2. Despite its high incidence in recent years, the detail mechanism of this disease has not been fully elucidated so far 3. It is widely accepted that the imbalance of oxidation and reduction reaction plays an important role in the development of COPD 4, 5. The overproduction of oxidative substance is attributed to the pathogenesis of COPD.
Heme oxygenase has the ability to resist damage caused by oxidative stress. There are three types of heme oxygenase isozymes 6, 7. Among these, HMOX1 or HO‐1, the inducible isoform responding to various stimuli in the environment, was confirmed to play a pivotal role in protecting against mucus hypersecretion 8, emphysema 9, 10, 11, airway inflammation 12, which were the main characteristics of COPD, in a series of studies. The activity of this gene is dependent on its promoter, and length polymorphism caused by various kinds of GT repeat numbers ((GT)n) exists in promoter sequence. In view of the great variety of GT repeat numbers in different populations, these polymorphisms are generally divided into three types of alleles, named S, M and L alleles, representing short, medium and long GT repeat sequence, respectively 13. Different genotypes lead to different activity levels of HMOX1, affect the degree of oxidative stress in the organism and finally influence the susceptibility to COPD. Thus, many researchers focused their attention on the relationship between (GT)n polymorphism and COPD in the past decades. However, they obtained inconsistent results, which might be due to ethnicity, sample size and selection bias 14, 15, 16, 17, 18, 19, 20, 21, 22. Based on these, we conducted this meta‐analysis to determine the association between genetic polymorphism of heme oxygenase 1 promoter and COPD occurrence and severity.
Materials and methods
Search strategy
We performed a comprehensive search strategy in several databases including PubMed, EMBASE, Cochrane Library and CNKI to find out the articles about the association between HMOX1 polymorphisms and COPD. The terms we used as follows: ‘chronic obstructive pulmonary disease’, ‘COPD’, ‘emphysema’, ‘chronic bronchitis’; ‘heme oxygenase1’, ‘hmox1’, ‘ho1’; and ‘genetic polymorphism’, ‘variant’, ‘variation’, ‘association’. Additional studies were identified by a manual search from references of original studies or review articles on this topic. Only studies with full‐text articles published until October 2015 were included.
Study selection
The criteria for the papers selection were as follows: (i) studies with case–control or prospective longitudinal cohort design; (ii) studies with at least two comparison groups (COPD versus control or less severe COPD patients versus more severe COPD patients (measured by lung function)); (iii) the study including HMOX1 length polymorphisms (GT repeat number) in COPD cases and controls; and (iv) provide the available allele and/or genotype frequency in each group.
Quality assessment
All the included studies were assessed in three aspects consisting of selection, comparability and exposure, with the use of Newcastle–Ottawa Scale (NOS) 23, which has been widely applied in observational studies. Each study was assigned a score from 0 to 9 points, and higher points meant higher quality.
Data extraction
The data were carefully extracted from all eligible publications independently by two authors according to the inclusion criteria listed above. Once encountering disagreements, we resolved them by discussions with the third person. The information we extracted from papers contains basic information of study (author, publication year), population (sample size, ethnicity, age, source of control subjects, lung function and smoking status), COPD definition, genotype distribution in cases and controls, genotype identification method, etc.
Data synthesis
OR and 95% CIs were used to assess the strength of association between HMOX1 polymorphisms and COPD risk and severity.
Heterogeneity assumption was checked by the Cochrane Q test. If P value for the Q test is over 0.10, we consider that there is lack of heterogeneity. We also used the statistic of I 2 to detect the degree of heterogeneity, with I 2 <25%, 25–75% and >75% to represent low, moderate and high degree of inconsistency, respectively 24, 25. In the analysis of pooled data, we used two different models according to the trait of the included studies: If no heterogeneity was found, a fixed effect model was adopted to determine the gene effect or the random effect model was used. Moreover, if heterogeneity across studies existed, subgroup analysis was performed to seek out the source of heterogeneity. Studies were subdivided by ethnicity (Asian versus Caucasian), genotyping methods (automated sequencing versus PCR‐PAGE), source of control subjects (general population‐based versus hospital‐based), study quality [higher quality (NOS ≥7) versus lower quality (NOS <7)] and language (Chinese versus English) to find the source of any heterogeneity.
Hardy–Weinberg equilibrium (HWE) was tested in control subjects in each study. Deviation from HWE was tested using the chi‐square test. Studies with controls that depart from HWE (P < 0.05) were subjected to a sensitivity analysis in order to check the consistency of the overall effect.
We made use of Begg's funnel plot to examine the underlying publication bias, and also used Egger's weighted regression method to calculate P for bias 26, 27. If no publication bias existed, the funnel plot looked symmetrical.
All analyses were conducted with the use of REVIEW MANAGER, V.5.2 (Revman, The Cochrane Collaboration) or STATA software, V.12.0 (STATA Corp, Lakeway Drive College Station, Texas, USA).
Results
Characteristics of included studies
We identified 79 related articles, of which 17 studies were potentially suitable. Four studies were given up because objective population were not patients with COPD (one for lung cancer and others for general population). One study did not examine any HMOX1 length polymorphism mentioned above. One study was excluded because of lack of proper control. Furthermore, two repeated studies were also ruled out. Thus, nine studies including 1447 cases and 891 controls met the including criteria (Fig. 1). HOMX1 polymorphism was mentioned in seven studies, and four studies provided the association of HMOX1 polymorphism with COPD severity. The study characteristics are listed in Tables 1 & 2. Patients with COPD were diagnosed through lung function index in all studies as well as radiography manifestation in some studies. General population and hospital‐based controls were involved in different studies. In addition, frequency‐matched controls to the cases by ethnicity, sex, age and smoking status were applied in some studies. Automated sequencer was applied to detect HMOX1 genotypes in five of the nine studies. The scores of included studies ranged from 5 to 7 by NOS.
Figure 1.
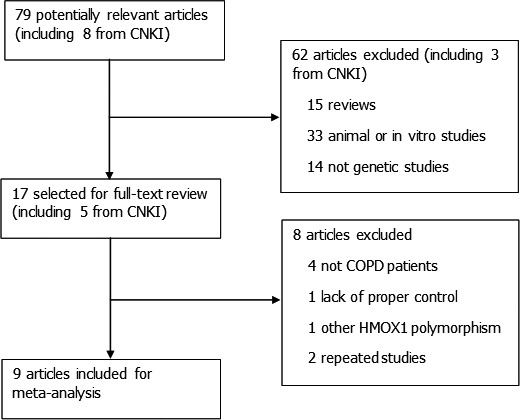
Study identification, inclusion and exclusion for meta‐analysis.
Table 1.
Summary of studies about genetic association of HMOX1 to COPD risk
| Author | Year | Ethnicity | Sample Size | Source of control | Age matched | Smoking index matched | Lung function of COPD | COPD diagnosis | Genotype identification | NOS§ | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Case | Control | FEV1/FVC% | FEV1/Pre% | |||||||||
| Budhi | 2003 | Asian | 63 | 172 | General population | No | Yes | Not mentioned | Not mentioned | Radiology and lung function† | Automated sequencing | 5 |
| Du | 2006 | Asian | 64 | 56 | General population | Yes | Yes | 49.6 ± 7.0 | 53.6 ± 8.4 | CMA guideline‡ | PCR‐PAGE | 7 |
| Fu | 2007 | Asian | 256 | 266 | Hospital | Yes | Yes | 48.2 ± 6.3 | 58.5 ± 10.0 | GOLD## | Automated sequencing | 6 |
| Ma | 2005 | Asian | 50 | 30 | Hospital | Yes | Yes | Not mentioned | Not mentioned | CMA guideline | PCR‐PAGE | 5 |
| Matokanović | 2012 | Caucasian | 130 | 95 | General population | No | Yes | 62.9 (54.9–67.1)* | 41.5 ± 13.9 | GOLD | Automated sequencing | 7 |
| Putra | 2013 | Asian | 48 | 172 | General population | No | Yes | Not mentioned | 59 ± 15.6 | GOLD | Automated sequencing | 6 |
| Yamada | 2000 | Asian | 101 | 100 | Hospital | Yes | Yes | 47 ± 2 | 84 ± 7 | Comprehensive*** | Automated sequencing | 7 |
*The data were presented as median (interquartile range).
†The patients were in accordance with early‐stage disease demonstrated by lung function test (the concrete data were not available) and radiology criteria as follows: chest CT indicated one with emphysematous changes showing low attenuation areas and/or bulla.
‡The guideline was published by Chinese Medical Association (CMA) for diagnosis and treatment of COPD in 2002.
##Global Initiative for Chronic Obstructive Lung Disease, the guideline for COPD diagnosis, management and prevention.
§Newcastle–Ottawa Scale, a tool for assess the quality of case–control study, ranged from 0 to 9.
***The patient with COPD was defined as a physical examination that demonstrated hyperresonant chest and flattened hemidiaphragms; a chest roentgenogram that demonstrated hyperinflation, flattened diaphragms and marked loss of vascularity; a computed tomography scan that demonstrated areas of low attenuation; and pulmonary function testing that demonstrated decreased FEV1:FVC ratios and impaired diffusion capacity
Table 2.
Summary of studies about genetic association of HMOX1 to COPD severity
| Author | Year | Ethnicity | Less severe | More severe | COPD diagnosis | Genotype identification | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Sample Size | FEV1/FVC% | FEV1/Pre% | Sample Size | FEV1/FVC% | FEV1/Pre% | |||||
| Fu | 2007 | Asian | 215 | 59.6 ± 10.4 | 76.6 ± 14.0 | 237 | 39.8 ± 9.3 | 37.5 ± 8.2 | GOLD | Automated sequencing |
| Jiang | 2006 | Asian | 60 | 46.3 ± 8.4 | 50.2 ± 10.0 | 45 | 25.9 ± 8.2 | 27.2 ± 7.6 | CMA guideline | PCR‐PAGE |
| Matokanović | 2012 | Caucasian | 37 | Not mentioned | GOLD Stage II | 93 | Not mentioned | GOLD Stage III‐IV | GOLD | Automated sequencing |
| Putra | 2013 | Asian | 25 | Not mentioned | 71.0 ± 5.8 | 23 | Not mentioned | 46.0 ± 10.8 | GOLD | Automated sequencing |
HMOX1 allele distribution in COPD and control groups
First, the distribution of each allele of HMOX1 in both patients with COPD and control subjects was analysed with the use of random effect model. The frequencies of S and M allele were not different between these two groups. However, compared with control subjects, there was much higher frequency of L allele in patients with COPD (OR 2.02, 95% CI: 1.32–3.11, P = 0.001). Further analysis indicated that S but not M allele was a protective factor for COPD in Asian people (OR 0.62, 95% CI: 0.40–0.96, P = 0.03). Conversely, L allele suggested higher risk to COPD in this subpopulation (OR 2.23, 95% CI: 1.68–2.95, P < 0.001). Other than ethnicity, subgroup analysis also showed gene detection method, source of control subjects, the quality and language of included literature which could affect the results to some extent. Whereas less S alleles were observed in patients with COPD in PCR‐PAGE subgroup, more L carriers were found in patients with COPD in PCR‐PAGE, hospital‐based, lower quality and Chinese subgroups (Fig. 2).
Figure 2.

Correlation between L allele and COPD risk. The studies were divided into two groups according to ethnicity (Asian or Caucasian) (A), genotyping methods (automated sequencing or PCR‐PAGE) (B), source of control subjects (general population‐based or hospital‐based) (C), study quality (higher quality (NOS ≥7) or lower quality (NOS <7)) (D) and language (Chinese or English) (E) under the comparison of L versus S+M.
HMOX1 genotypes distribution in COPD and control groups
HMOX1 genotypes from seven studies are presented in Table 3. As a result, the proportion of type I genotype (L allele carrier) was higher than that of type II genotype (non‐L allele carrier) in patients with COPD (OR 1.82, 95% CI: 1.28–2.61, P = 0.001). Further analysis suggested that type I genotype was of dominant position in patients with COPD in several subgroups, including Asian (OR 2.02, 95% CI: 1.51–2.70, P < 0.001), hospital‐based (OR 2.33, 95% CI: 1.50–3.60, P < 0.001), lower quality (OR 1.86, 95% CI: 1.17–2.96, P = 0.009) and Chinese subgroup (OR 2.37, 95% CI: 1.46–3.87, P < 0.001). Moreover, in both subgroups divided by detection method, more L allele carriers were observed in patients with COPD than those in control subjects (for automated sequencing subgroup, OR 1.61, 95% CI: 1.13–2.30, P = 0.008; for PCR‐PAGE subgroup, OR 3.30, 95% CI: 1.49–7.29, P = 0.003; Fig. 3).
Table 3.
the distribution of HMOX1 alleles and genotypes in patients with COPD and control subjects
| Author | Year | Allele | Genotype | HWE(P) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Case | Control | Case | Control | |||||||||
| S | M | L | S | M | L | Type I* | Type II† | Type I | Type II | |||
| Budhi | 2003 | – | – | – | – | – | – | 55 | 8 | 140 | 32 | – |
| Du | 2006 | 42 | 56 | 30 | 54 | 46 | 12 | 26 | 38 | 12 | 44 | 0.208 |
| Fu | 2007 | 233 | 195 | 84 | 243 | 240 | 49 | 72 | 184 | 45 | 221 | <0.001 |
| Ma | 2005 | 29 | 49 | 22 | 31 | 26 | 3 | 20 | 30 | 3 | 27 | 0.172 |
| Matokanović | 2012 | 107 | 140 | 13 | 70 | 108 | 12 | 12 | 118 | 11 | 84 | 0.088 |
| Putra | 2013 | – | – | – | – | – | – | 40 | 8 | 140 | 32 | – |
| Yamada | 2000 | 67 | 93 | 42 | 92 | 88 | 20 | 38 | 63 | 20 | 80 | 0.103 |
*The subjects with at least one L allele.
†The subjects without L allele.
Figure 3.

Correlation between HMOX1 genotype and COPD risk. The studies were divided into two groups according to ethnicity (Asian or Caucasian) (A), genotyping methods (automated sequencing or PCR‐PAGE) (B), source of control subjects (general population‐based or hospital‐based) (C), study quality (higher quality (NOS ≥7) or lower quality (NOS <7)) (D) and language (Chinese or English) (E) under the comparison of type I versus type II.
HMOX1 genotypes distribution in patients with COPD with different severity
Then, we further observed whether HMOX1 genotypes were associated with COPD patients with different severity. Patients with COPD were divided into two groups according to their lung function. Unexpectedly, no evident difference of type I or type II genotype frequency was found in both groups (OR 0.97, 95% CI: 0.49–1.92). Due to the limited study numbers, subgroup analysis was not performed (Table S1).
Heterogeneity and sensitivity analysis
For allele study, I 2 showed a distinct variation degree in different comparisons, from very low (M versus S+L) to quite high (S versus M+L) heterogeneity. In addition, moderate variation was found in genotype comparisons (Table 4). Subgroup analysis showed reduced heterogeneity in some subgroups in majority comparisons (Tables S2 & S3).
Table 4.
Pooled odds ratio for COPD susceptibility and severity, heterogeneity and publication bias in meta‐analysis: comparison of alleles and genotypes
| Comparison | Study number | OR [95% CI] | P value | Heterogeneity | Publication bias | ||
|---|---|---|---|---|---|---|---|
| I 2 | P heterogeneity | Begg | Egger | ||||
| COPD susceptibility | |||||||
| S versus M+L | 5 | 0.72 [0.49, 1.04] | 0.08 | 76% | 0.002 | 0.142 | 0.126 |
| M versus S+L | 5 | 0.91 [0.76, 1.10] | 0.33 | 15% | 0.32 | 0.142 | 0.021 |
| L versus S+M | 5 | 2.02 [1.31, 3.11] | 0.001 | 52% | 0.08 | 0.327 | 0.79 |
| type I versus type II | 7 | 1.82 [1.28, 2.61] | 0.001 | 37% | 0.14 | 0.453 | 0.949 |
| COPD severity | |||||||
| type I versus type II | 4 | 0.97 [0.49, 1.92] | 0.94 | 59% | 0.06 | 0.497 | 0.335 |
Sensitivity analysis was conducted to assess the effect of each study on the overall results. Among these studies, Budhi et al. recruited patients with COPD with different inclusion criteria, whereas Ma et al. enrolled specific control subjects who were all lung cancer patients without airflow limitation. Moreover, the population in Fu's report was not in accordance with HWE. However, discarding these studies did not affect the pooled OR value in genotype comparisons, and neither did other studies (Fig. S1).
Publication bias
Publication bias was detected by Begg's and Egger's tests. These tests did not show significant results in almost all comparisons (Table 4). The funnel plots exhibited approximate symmetry shape (Fig. 4). These results indicated little publication bias.
Figure 4.
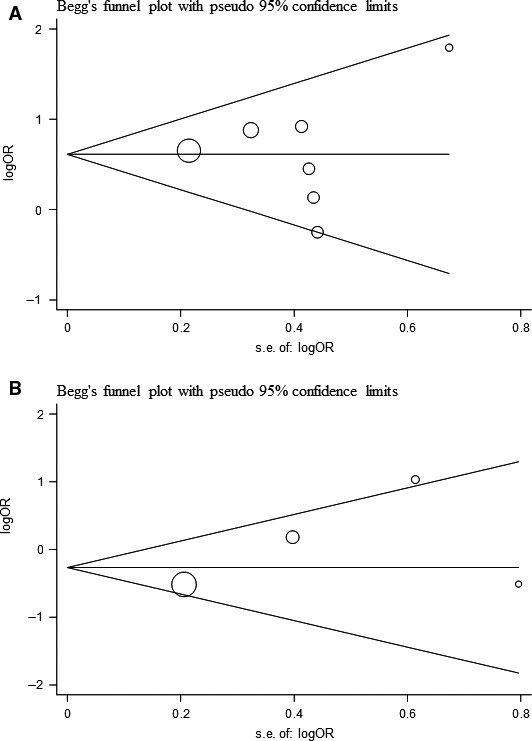
Publication bias on HMOX1 polymorphism. (A) Begg's funnel plot of the seven eligible studies of HMOX1 genotype distribution in COPD risk; (B) Begg's funnel plot of the four eligible studies of HMOX1 genotype distribution in COPD severity.
Discussion
As a complex disease, genetic background is considered to be another important factor related to COPD, other than environmental exposure 28. In the past years, genomewide association studies (GWAS) indicated several genes, including FAM13A (4q22), HHIP (4q31), CHRNA3/5 (15q25), IREB2 (15q25), MYO1D (17q11), VWA8 (13q14) and BICD1 (12p11), were related to COPD susceptibility 29, 30, 31, 32. However, heme oxygenase 1, a gene responsible for redox reactions in the microenvironment, has not been mentioned in these studies. There are several kinds of genetic polymorphisms, among which single nucleotide polymorphism (SNP) is the most common type. In our study, the genetic polymorphism we discussed is length polymorphism, which is the product of microsatellite DNA or short tandem repeat (STR) usually consisting of 2–6 repeated base pairs 33. GWAS mainly focused on SNP, but little attention has been paid to length polymorphism. Compared with SNP, the technique for accurate assessment of STR in whole genome is still under development 34. Moreover, the statistical models to analyse the association between STR phenotype and human disease in GWAS are considered to be defective 34. As a typical example of STR, previous researches suggested a long (GT)n sequence in HMOX1 promoter led to lower activity of antioxidation, which gave rise to the tendency to increased risk of COPD occurrence 19, 22, 35 and refractoriness to regular treatment 36, but there is still a lack of clear conclusion.
Our results indicated that L allele and type I genotype (L carriers) were the risk factors of COPD, especially for Asian population. These results were rather robust after sensitivity analysis. On the contrary, there was little association between HMOX1 and COPD risk in Caucasians. It was in line with several GWAS, which were carried out in Western countries, and the enrolled subjects were largely Caucasians. Despite the ethnic difference, several reasons might be worthy to point out. First, there were no unified standard for classification of three kinds of alleles. For example, L allele was defined when the GT repeat number was equal to or more than 30 in Yamada's study 22, but this allele was not recognized unless the GT repeat number was larger than 31 in the Caucasian population 20. The inconsistent definitions of allele and genotype might cause the different results. Second, the inclusion criteria of patients with COPD were different among studies. In the report by Budhi et al. 14 the researches selected the patients whose disease were at early stage, which was quite different from other studies. Third, the selection of control subjects also need to note. In three studies, hospital‐derived control subjects were recruited to compare with COPD. Coincidently, positive results were observed in all these studies, especially in the study by Ma et al. 19. So selection bias might influence the overall effects.
In contrast to the association between HMOX1 and COPD occurrence, we did not find that HMOX1 (GT)n polymorphism was related to COPD severity. Lung function is the most important index to assess the severity of COPD. So far, only a few studies have reported the relationship between HMOX1 polymorphism and lung function. Guenegou et al. found a long HMOX1 gene promoter was associated with accelerated decline in lung function in a general population, especially in heavy smokers 37, 38. In addition, the study by Nakayama et al. 39 revealed that the patients with COPD with L allele were at a much higher risk of rapid decrease in lung function than those without L allele. These results indicated that lung function was affected by HMOX1, which was observed in both Eastern and Western countries. However, our present study did not get positive result, and further research was needed to explain the discrepancy.
In our study, moderate to high heterogeneity existed in some comparisons, so subgroup analysis was introduced to seek out the source of heterogeneity. During the analysis, all the studies were divided into two subgroups according to ethnicity, source of control subjects, detection method, quality and language of literature. After stratification, one subgroup presented reduced or nearly disappeared heterogeneity in most comparisons, which demonstrated these factors were at least part source of heterogeneity. Among these factors, quality and language of literature were particularly noticeable because some papers were written in Chinese which might be considered as poor quality. However, there was little difference in quality between Chinese‐written papers and English‐written papers according to NOS score. As we did not set up language restriction of inclusion criteria, studies written in Chinese were also adopted into final analysis. In fact, we found in some subgroups, including Chinese subgroup, the role of L allele and genotype I as risk factor of COPD was reinforced. However, this phenomenon would rather be attributed to ethnicity rather than language. Compared with Chinese‐written studies, the subjects were mixed ethnicities in English‐written studies, including the one reported by Matokanovic et al. 20 which was the only study on Caucasian population. If this study was removed, positive results could be also observed in English subgroup. Nevertheless, it may remind us to distinguish confounder factors in future research.
There were some shortcomings in our work. First, the studies were limited in quantity and could not represent the whole population in the world. There were only about 1500 COPD subjects in these studies, which was a small part of patients with COPD worldwide. Moreover, the majority studies included in our present analysis were on Asian, and only one study mentioned Caucasian, to say nothing of Africans. Second, there were confounder factors in almost all the studies. As discussed above, the source of control subjects and method for genotype identification might amplify the main effect due to genetic factors. Last but not least, COPD is a chronic disease which is not fully reversible. So it is of significance to study the linkage between genotype and the degree of disease progression. However, we are lack of such data, which prevent us from further exploration.
In conclusion, we demonstrated L allele and genotype I were the risk factor of COPD, but not find HMOX1 length polymorphism in promoter region was associated with COPD severity. Due to the various deficiencies in present studies, future studies with larger sample size, covering different populations, selecting proper control subjects and detection methods should be carried out to further validate the relationship between HMOX1 and this disease.
Conflict of interest
The authors report no conflict of interests.
Author contribution
This study was designed by Zhou H and Li Y. The data were extracted by Ying X and Liu Y. Ye S and Yan J performed statistical analysis and graph drafting, respectively. This manuscript was originally written and finally approved by all the authors.
Supporting information
Figure S1 The influence of each study on overall result of COPD risk and severity
Table S1 The distribution of HMOX1 genotypes in COPD patients with different stages of severity
Table S2 Subgroup analysis of association between HMOX1 and COPD risk
Acknowledgements
This work was supported by grants from the National Natural Science Foundation of China (No. 81470241 and No. 81470109), The Foundation of Science and Technology Department of Zhejiang Province (No. 2014C37022) and Natural Science Foundation of Zhejiang Province (No. LQ17H010006).
References
- 1. Lopez‐Campos JL, Tan W, Soriano JB. Global burden of COPD. Respirology. 2016; 21: 14–23. [DOI] [PubMed] [Google Scholar]
- 2. Negewo NA, Gibson PG, McDonald VM. COPD and its comorbidities: impact, measurement and mechanisms. Respirology. 2015; 20: 1160–71. [DOI] [PubMed] [Google Scholar]
- 3. Decramer M, Janssens W, Miravitlles M. Chronic obstructive pulmonary disease. Lancet. 2012; 379: 1341–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Domej W, Oettl K, Renner W. Oxidative stress and free radicals in COPD–implications and relevance for treatment. Int J Chron Obstruct Pulmon Dis. 2014; 9: 1207–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mercado N, Ito K, Barnes PJ. Accelerated ageing of the lung in COPD: new concepts. Thorax. 2015; 70: 482–9. [DOI] [PubMed] [Google Scholar]
- 6. Ryter SW, Otterbein LE, Morse D, et al Heme oxygenase/carbon monoxide signaling pathways: regulation and functional significance. Mol Cell Biochem. 2002; 234–235: 249–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Haines DD, Lekli I, Teissier P, et al Role of haeme oxygenase‐1 in resolution of oxidative stress‐related pathologies: focus on cardiovascular, lung, neurological and kidney disorders. Acta Physiol. 2012; 204: 487–501. [DOI] [PubMed] [Google Scholar]
- 8. Almolki A, Guenegou A, Golda S, et al Heme oxygenase‐1 prevents airway mucus hypersecretion induced by cigarette smoke in rodents and humans. Am J Pathol. 2008; 173: 981–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Goven D, Boutten A, Lecon‐Malas V, et al Prolonged cigarette smoke exposure decreases heme oxygenase‐1 and alters Nrf2 and Bach1 expression in human macrophages: roles of the MAP kinases ERK(1/2) and JNK. FEBS Lett. 2009; 583: 3508–18. [DOI] [PubMed] [Google Scholar]
- 10. Goven D, Boutten A, Lecon‐Malas V, et al Altered Nrf2/Keap1‐Bach1 equilibrium in pulmonary emphysema. Thorax. 2008; 63: 916–24. [DOI] [PubMed] [Google Scholar]
- 11. Wu ML, Layne MD, Yet SF. Heme oxygenase‐1 in environmental toxin‐induced lung disease. Toxicol Mech Methods. 2012; 22: 323–9. [DOI] [PubMed] [Google Scholar]
- 12. Shinohara T, Kaneko T, Nagashima Y, et al Adenovirus‐mediated transfer and overexpression of heme oxygenase 1 cDNA in lungs attenuates elastase‐induced pulmonary emphysema in mice. Hum Gene Ther. 2005; 16: 318–27. [DOI] [PubMed] [Google Scholar]
- 13. Fredenburgh LE, Perrella MA, Mitsialis SA. The role of heme oxygenase‐1 in pulmonary disease. Am J Respir Cell Mol Biol. 2007; 36: 158–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Budhi A, Hiyama K, Isobe T, et al Genetic susceptibility for emphysematous changes of the lung in Japanese. Int J Mol Med. 2003; 11: 321–9. [PubMed] [Google Scholar]
- 15. Du Y, Li Y, Jiang P. Association between genetic polymorphisms of human Heme Oxygenase‐1 and chronic obstructive pulmonary disease. J Shandong Univ. 2006; 44: 519–23. [Google Scholar]
- 16. Fu WP, Sun C, Dai LM, et al Relationship between COPD and polymorphisms of HOX‐1 and mEPH in a Chinese population. Oncol Rep. 2007; 17: 483–8. [PubMed] [Google Scholar]
- 17. Fu WP, Zhao ZH, Fang LZ, et al Heme oxygenase‐1 polymorphism associated with severity of chronic obstructive pulmonary disease. Chin Med J. 2007; 120: 12–6. [PubMed] [Google Scholar]
- 18. Jiang P, Li Y. Association among genetic polymorphisms of HO‐1, TNF‐alpha and chronic obstructive pulmonary disease. J Shandong Univ. 2006; 44: 1253–7. [Google Scholar]
- 19. Ma ZM, Zhang ZX, Han ZM, et al A study on the relationship between the microsatellite polymorphism in the heme oxygenase‐1 gene promotor and cell apoptosis in the lung tissue from patients with COPD. Chin J Gerontol. 2005; 25: 1289–92. [Google Scholar]
- 20. Matokanovic M, Rumora L, Popovic‐Grle S, et al Association of hsp70‐2 (+1267A/G), hsp70‐hom (+2437T/C), HMOX‐1 (number of GT repeats) and TNF‐alpha (+489G/A) polymorphisms with COPD in Croatian population. Clin Biochem. 2012; 45: 770–4. [DOI] [PubMed] [Google Scholar]
- 21. Putra AC, Tanimoto K, Arifin M, et al Genetic variations in detoxification enzymes and HIF‐1alpha in Japanese patients with COPD. Clin Respir J. 2013; 7: 7–15. [DOI] [PubMed] [Google Scholar]
- 22. Yamada N, Yamaya M, Okinaga S, et al Microsatellite polymorphism in the heme oxygenase‐1 gene promoter is associated with susceptibility to emphysema. Am J Hum Genet. 2000; 66: 187–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wells GA, Shea B, O'Connell D, et al The Newcastle‐Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta‐analyses. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp.
- 24. Higgins JP, Thompson SG. Quantifying heterogeneity in a meta‐analysis. Stat Med. 2002; 21: 1539–58. [DOI] [PubMed] [Google Scholar]
- 25. Higgins JP, Thompson SG, Deeks JJ, et al Measuring inconsistency in meta‐analyses. BMJ. 2003; 327: 557–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994; 50: 1088–101. [PubMed] [Google Scholar]
- 27. Egger M, Davey Smith G, Schneider M, et al Bias in meta‐analysis detected by a simple, graphical test. BMJ. 1997; 315: 629–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Chen ZH, Kim HP, Ryter SW, et al Identifying targets for COPD treatment through gene expression analyses. Int J Chron Obstruct Pulmon Dis. 2008; 3: 359–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Cho MH, Castaldi PJ, Wan ES, et al A genome‐wide association study of COPD identifies a susceptibility locus on chromosome 19q13. Hum Mol Genet. 2012; 21: 947–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Castaldi PJ, Cho MH, San Jose Estepar R, et al Genome‐wide association identifies regulatory Loci associated with distinct local histogram emphysema patterns. Am J Respir Crit Care Med. 2014; 190: 399–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Cho MH, Boutaoui N, Klanderman BJ, et al Variants in FAM13A are associated with chronic obstructive pulmonary disease. Nat Genet. 2010; 42: 200–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Pillai SG, Ge D, Zhu G, et al A genome‐wide association study in chronic obstructive pulmonary disease (COPD): identification of two major susceptibility loci. PLoS Genet. 2009; 5: e1000421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Gymrek M, Willems T, Guilmatre A, et al Abundant contribution of short tandem repeats to gene expression variation in humans. Nat Genet. 2015; 48: 22–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Press MO, Carlson KD, Queitsch C. The overdue promise of short tandem repeat variation for heritability. Trends Genet. 2014; 30: 504–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kimpara T, Takeda A, Watanabe K, et al Microsatellite polymorphism in the human heme oxygenase‐1 gene promoter and its application in association studies with Alzheimer and Parkinson disease. Hum Genet. 1997; 100: 145–7. [DOI] [PubMed] [Google Scholar]
- 36. Zhang JQ, Zhang JQ, Fang LZ, et al Effect of oral N‐acetylcysteine on COPD patients with microsatellite polymorphism in the heme oxygenase‐1 gene promoter. Drug Des Devel Ther. 2015; 9: 6379–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Guenegou A, Boczkowski J, Aubier M, et al Interaction between a heme oxygenase‐1 gene promoter polymorphism and serum beta‐carotene levels on 8‐year lung function decline in a general population: the European Community Respiratory Health Survey (France). Am J Epidemiol. 2008; 167: 139–44. [DOI] [PubMed] [Google Scholar]
- 38. Guenegou A, Leynaert B, Benessiano J, et al Association of lung function decline with the heme oxygenase‐1 gene promoter microsatellite polymorphism in a general population sample. Results from the European Community Respiratory Health Survey (ECRHS), France. J Med Genet. 2006; 43: e43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Nakayama K, Kikuchi A, Yasuda H, et al Heme oxygenase‐1 gene promoter polymorphism and decline in lung function in Japanese men. Thorax. 2006; 61: 921. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 The influence of each study on overall result of COPD risk and severity
Table S1 The distribution of HMOX1 genotypes in COPD patients with different stages of severity
Table S2 Subgroup analysis of association between HMOX1 and COPD risk


