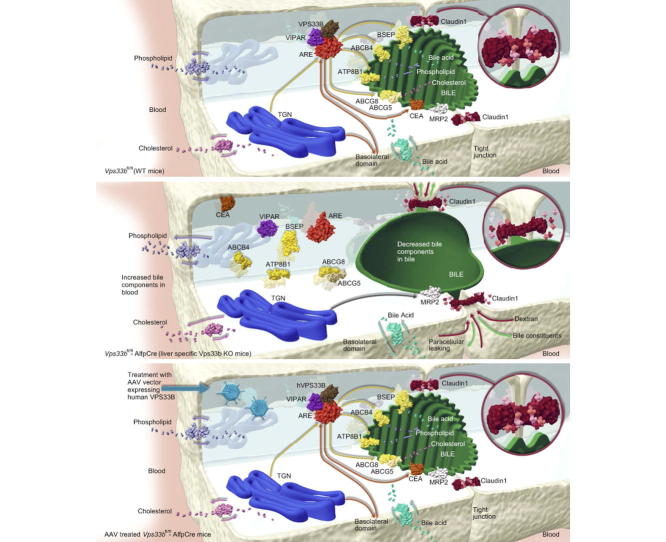
Graphical abstract
Keywords: Vps33b, Hepatocyte polarity, Canalicular membrane, Cholestasis, Protein trafficking, Rab11a, Gene transfer, Bile canaliculi, Bile, Liver diseases
Abstract
Background & Aims
In the normal liver, hepatocytes form a uniquely polarised cell layer that enables movement of solutes from sinusoidal blood to canalicular bile. Whilst several cholestatic liver diseases with defects of hepatocyte polarity have been identified, the molecular mechanisms of pathogenesis are not well defined. One example is arthrogryposis, renal dysfunction and cholestasis syndrome, which in most patients is caused by VPS33B mutations. VPS33B is a protein involved in membrane trafficking that interacts with RAB11A at recycling endosomes. To understand the pathways that regulate hepatocyte polarity better, we investigated VPS33B deficiency using a novel mouse model with a liver-specific Vps33b deletion.
Methods
To assess functional polarity, plasma and bile samples were collected from Vps33b liver knockout (Vps33bfl/fl-AlfpCre) and control (Vps33bfl/fl) mice; bile components or injected substrates were quantitated by mass spectrometry or fluorometry. For structural analysis, livers underwent light and transmission electron microscopy. Apical membrane and tight junction protein localisation was assessed by immunostaining. Adeno-associated virus vectors were used for in vivo gene rescue experiments.
Results
Like patients, Vps33bfl/fl-AlfpCre mice showed mislocalisation of ATP-binding cassette proteins that are specifically trafficked to the apical membrane via Rab11a-positive recycling endosomes. This was associated with retention of bile components in blood. Loss of functional tight junction integrity and depletion of apical microvilli were seen in knockout animals. Gene transfer partially rescued these defects.
Conclusions
Vps33b has a key role in establishing structural and functional aspects of hepatocyte polarity and may be a target for gene replacement therapy.
Lay summary
Hepatocytes are liver cells with tops and bottoms; that is, they are polarised. At their bottoms they absorb substances from blood. They then, at their tops, secrete these substances and their metabolites into bile. When polarity is lost, this directional flow of substances from blood to bile is disrupted and liver disease follows. In this study, using a new mouse model with a liver-specific mutation of Vps33b, the mouse version of a gene that is mutated in most patients with arthrogryposis, renal dysfunction and cholestasis (ARC) syndrome, we investigated how the Vps33b gene product contributes to establishing hepatocyte polarity. We identified in these mice abnormalities similar to those in children with ARC syndrome. Gene transfer could partly reverse the mouse abnormalities. Our work contributes to the understanding of VPS33B disease and hepatocyte polarity in general, and may point towards gene transfer mediated treatment of ARC liver disease.
Introduction
In metazoans, the development of three-dimensional body structures depends on the generation of polarised epithelial layers. Polarisation of epithelial cells is a complex process that requires the cooperation of multiple factors including cell junction formation, extracellular matrix interactions and intracellular protein trafficking [1]. Correct interaction of these factors enables the establishment of discrete apical and basolateral membrane domains. This then promotes normal epithelial cell function by allowing directional solute absorption and secretion [2].
In hepatocytes, the basolateral membrane faces sinusoidal blood and one or more apical domains contribute to the formation of bile canaliculi in conjunction with adjacent hepatocytes. This polarisation enables the directional flow of molecules from sinusoidal blood to canalicular bile and, as such, is crucial for normal biliary physiology [3]. Genetic disorders of hepatic tight junction formation and protein trafficking entail a loss of hepatocyte polarity. This impaired hepatocyte polarity causes pathophysiological features similar to those of cholestatic liver diseases caused by mutations in genes encoding apical ATP-binding cassette transporters [4], [5], [6], [7].
One such polarity-loss disorder is arthrogryposis renal dysfunction and cholestasis syndrome (ARC). ARC is an autosomal recessive multisystem disorder. It has a spectrum of severity and typically is characterised by congenital joint contractures, renal tubular acidosis and neonatal jaundice leading to death in infancy [8]. However, in milder cases patients survive into adulthood and have raised serum bile acid values but normal bilirubin levels [9]. Approximately 75% of ARC patients harbour germline mutations in VPS33B. VPS33B is orthologous to the yeast Sec-1-Munc18 family protein Vps33p: a class C vacuolar protein sorting (vps) protein that regulates SNARE-mediated vesicle fusion [10]. Class C vps proteins are core constituents of the homotypic fusion and vacuole protein sorting (HOPS) and class C core vacuole/endosome tethering (CORVET) multiprotein complexes, crucial in several stages of vesicular trafficking [11]. Whilst the Vps33p ortholog VPS33A is recognised as a member of the mammalian HOPS complex [12], the function of VPS33B is associated with the activity of Rab11 family members [13].
Rab11a is involved in protein trafficking via the apical recycling endosome (ARE) and, thereby, biogenesis of the apical membrane domain [14]. Canalicular proteins that are specifically trafficked via Rab11a-positive ARE are mislocalised in ARC patient liver [8], [11]. Whilst patients’ serum aminotransferase and gamma-glutamyl transpeptidase (γGT) activities are normal, serum alkaline phosphatase (ALP) activity is substantially elevated [8].
To treat the human liver diseases associated with failure to regulate apical-basolateral polarity, one must understand the pathways that govern hepatocyte polarisation. We therefore investigated the molecular pathology of ARC liver disease to elucidate the role of VPS33B in establishing hepatocyte polarity in mammals. We generated and characterised a novel murine model with a liver-specific Vps33b gene mutation (Vps33bfl/fl-AlfpCre) that recapitulates key aspects of ARC liver disease. In Vps33bfl/fl-AlfpCre animals we observed structural and functional defects of hepatocyte polarity associated with failure to generate the canalicular membrane correctly. We could, in part, reverse these defects by adeno-associated virus mediated gene transfer.
Materials and methods
Vps33bfl/fl-AlfpCre mouse generation
Mice with Vps33b exons 2–3 flanked by loxP sites were generated by Artemis Pharmaceuticals (Cologne, Germany) as described and crossed onto a C57BL/6 J background [15]. Mice were further crossed with AlfpCre-recombinase expressing animals to obtain Vps33bfl/fl-AlfpCre mice with hepatocyte and cholangiocyte specific Vps33b deletion [16]. Animals were sacrificed at 14–16 weeks of age and Cre-negative, age matched littermates were used as wild-type controls. For experiments on a cholic acid (CA) diet, 8 week old mice were fed 0.5% CA supplemented chow (TestDiet® Europe, London, UK) for 6 weeks and sacrificed immediately thereafter at age 14 weeks. For histologic assessment in older animals, mice were aged for 10 to 12 months and fed a normal chow diet. All experimental animal groups were of mixed gender. All procedures were undertaken with United Kingdom Home Office approval in accordance with the Animals (Scientific Procedures) Act of 1986.
Mass spectrometry
Bile acids were quantified by UPLC-MS/MS [17]. Bile acid concentrations in bile were corrected by flow rate. Details are provided in the Supplementary methods section.
Transmission electron microscopy (TEM)
Details of TEM procedures [13] are provided in the Supplementary methods section.
Biliary secretion analysis
For analysis of bile composition, bile was collected by cannulating the gall bladder of 14–16-week-old CA fed mice using polyethylene (PE-10) tubing following midline laparotomy and ligation of the common bile duct. Analysis of biliary cholyl-L-lysyl-fluorescein (CLF) secretion via MRP2 was carried out using in vivo methods [18]. Integrity of hepatic tight junction barrier function was measured using 40 kDa fluorescein isothiocyanate (FITC)-conjugated dextran (FD-40) [19]. Results obtained from CLF and FD-40 fluorometric analysis were corrected for bile flow rate.
Microarray analysis
Total RNA was extracted from liver samples using the RNAeasy Microkit per manufacturer’s instructions (Qiagen, Manchester, UK). RNA transcript levels were measured using Affymetrix Mouse Gene 2.0 microarrays. Details of in silico gene expression and pathway analysis are described in the Supplemental methods section.
The data discussed in this publication have been deposited in NCBI’s Gene Expression Omnibus [20] and are accessible through GEO series accession number GSE83192 (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE83192).
Adeno-associated virus (AAV) vector production
All ssAAV2/8 vectors were made by the adenovirus-free transient transfection method, using a chimeric AAV2 Rep-8Cap packaging plasmid (pAAV2-8) and an adenoviral helper plasmid [21]. Vectors were purified [22] and vector genome (vg) titres were determined by standard alkaline gel based methods [23]. Details of molecular cloning procedures are provided in the Supplemental methods section.
Serum and bile biochemical assays, immunoblotting and histological and immunostaining
All the above procedures are described in the Supplemental methods section.
Results
The ARC liver phenotype is recapitulated in Vps33bfl/fl-AlfpCre mice and exacerbated by CA feeding
Loss of Vps33b expression in hepatocytes of Vps33bfl/fl-AlfpCre animals was confirmed by fluorescence in situ hybridisation and Western blotting techniques (Supplementary Fig. 1A and B).
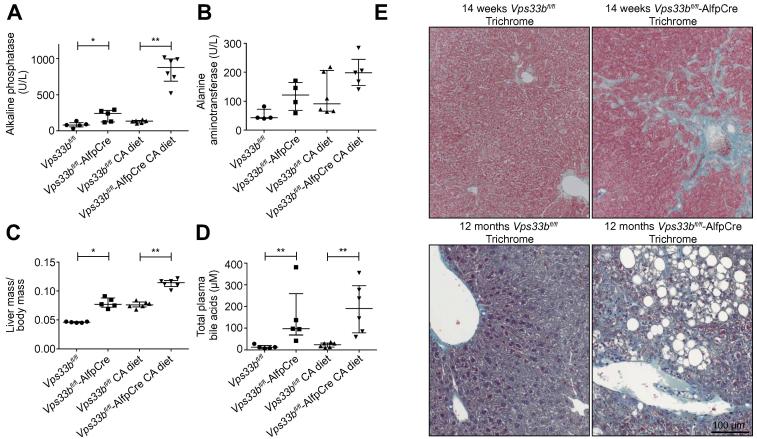
To assess hepatobiliary injury associated with Vps33b mutation, levels of plasma ALP activity; alanine aminotransferase (ALT) activity and bilirubin concentrations were measured in 14-week-old animals fed normal chow or a 0.5% CA diet (n = 4–6). As in ARC patients, a significant increase in plasma ALP activity was detected in normal chow fed Vps33bfl/fl-AlfpCre mice (3-fold, median: 241.0 U/L, IQR: 123.5–283.5 U/L) in comparison to Cre-negative Vps33bfl/fl littermates (median: 80.0 U/L, IQR: 54.0–113.50 U/L) (Fig.1A, p = 0.0317, Mann Whitney U test). In CA fed animals, a greater increase in ALP activity (6.5-fold) was observed in the plasma of Vps33bfl/fl-AlfpCre mice (median: 874.5 U/L, IQR: 688.0–994.5 U/L) than in that of Vps33bfl/fl mice (median: 134.0 U/L, IQR: 108–144.0 U/L) (Fig.1A, p = 0.0022, Mann Whitney U test). Whilst ALT activity levels appeared slightly raised in Vps33bfl/fl-AlfpCre animals, no significant difference in plasma ALT activity was found between Vps33bfl/fl and Vps33bfl/fl-AlfpCre mice under both diet conditions (Fig.1B, normal diet p = 0.0591, CA diet p = 0.1775, Mann Whitney U test). Although ALT activity levels increased in both Vps33bfl/fl and Vps33bfl/fl-AlfpCre animals following CA feeding, this increase was not statistically significant (Vps33bfl/fl p = 0.0691, Vps33bfl/fl-AlfpCre p = 0.1111). Addition of CA to the diet may therefore have a slight effect on liver injury in both wild-type and knockout animals. CA feeding consistently increased liver mass in Vps33bfl/fl and Vps33bfl/fl-AlfpCre mice (1.5-fold, Vps33bfl/fl p = 0.0078, Vps33bfl/fl-AlfpCre p = 0.0043) and liver mass was significantly increased between Vps33bfl/fl and Vps33bfl/fl-AlfpCre mice under both diet conditions (normal diet 1.7-fold; p = 0.0117, CA diet 1.5-fold; p = 0.0050) (Fig.1C). Plasma bilirubin concentrations were below detection level (<2 μmol/L) in all experimental groups.
Fig. 1.
ARC liver disease in Vps33bfl/fl-AlfpCre mice. (A) Plasma ALP activity, (B) plasma ALT activity (C) liver mass and (D) total plasma bile acid levels assessed in 14-week-old normal chow fed and cholic acid (CA) fed Vps33bfl/fl-AlfpCre and Vps33bfl/fl mice (n = 4–6). (E) Trichrome staining of liver sections from 14-week-old CA fed mice and 12-month-old mice fed with normal diet (n = 3). Graphs are presented with medians and IQR. p values (Mann Whitney U test): ALP activity, p = 0.0317 and p = 0.0022; ALT activity p = 0.0591 and p = 0.1775; plasma bile acids, p = 0.0043 and p = 0.0022; liver mass, p = 0.0117 and p = 0.0050 (normal chow fed and CA fed comparisons respectively).
As high plasma bile acid levels characterise ARC cholestasis [8], we measured bile acids in mouse plasma by mass spectrometry (n = 5–6). Concordantly, median total bile acid levels of 97.30 μM (IQR: 68.85–259.2 μM) and 12.65 μM (IQR: 7.65–20.90 μM) were observed in normal diet Vps33bfl/fl-AlfpCre and Vps33bfl/fl samples respectively (Fig.1D, 7.6-fold, p = 0.0043, Mann Whitney U test). In CA diet Vps33bfl/fl-AlfpCre and Vps33bfl/fl plasma samples, total bile acid levels were increased with respective concentrations of 190.4 μM (IQR: 78.2–295.9 μM) and 24.1 μM (IQR: 13.0–31.9 μM) detected (7.9-fold, p = 0.0022).
These results collectively show that key biomarkers of ARC cholestasis (raised plasma ALP and bile acid levels, with normal aminotransferase levels) are recapitulated in Vps33bfl/fl-AlfpCre mice fed either normal chow or a 0.5% CA diet. As CA feeding did not create artefactual phenotypes in Vps33bfl/fl-AlfpCre mice and only exacerbated disease phenotypes already observed at physiological level, CA feeding was not considered a confounding factor. The remaining experiments in this study were carried out in CA fed animals unless otherwise stated.
Histologic findings in livers of Vps33bfl/fl-AlfpCre mice
Light microscopy of 14-week-old, CA fed Vps33bfl/fl-AlfpCre mouse liver sections primarily revealed fibrotic changes that were not observed in Vps33bfl/fl sections. By trichrome staining (n = 3) we observed periportal and pericentral fibrous expansion with focal bridging fibrosis in Vps33bfl/fl-AlfpCre mice (Fig.1E). As leucocyte infiltration was observed in Vps33bfl/fl-AlfpCre liver (Supplementary Fig. 2), these fibrotic changes may indicate a response to inflammation caused by cholestasis in Vps33bfl/fl-AlfpCre animals.
Trichrome staining of liver sections from 12-month-old Vps33bfl/fl-AlfpCre mice fed normal chow revealed marked geographic fibrosis and focal steatosis (n = 3) (Fig.1E). This suggests a progression of fibrotic changes and possible defects of lipid metabolism and accumulation in aged animals.
To investigate the mechanism of liver damage in Vps33bfl/fl-AlfpCre mice, levels of apoptosis were assessed by immunostaining 14-week-old liver sections to detect cleaved caspase 3 (CC3) protein (n = 3). The percentage of CC3 positive cells per field of view was significantly increased in Vps33bfl/fl-AlfpCre sections in comparison to Vps33bfl/fl sections under both normal diet and CA diet conditions (p <0.0001 and p = 0.0058 respectively, Mann Whitney U test) (Supplementary Fig. 3). This suggests that apoptosis may contribute to the liver injury observed in Vps33bfl/fl-AlfpCre mice. However, in comparison to cholestatic mouse models of bile salt export pump (BSEP) deficiency, the percentage of apoptotic cells detected in Vps33bfl/fl-AlfpCre sections was relatively low [24].
Vps33bfl/fl-AlfpCre animals show mislocalisation of apical membrane proteins
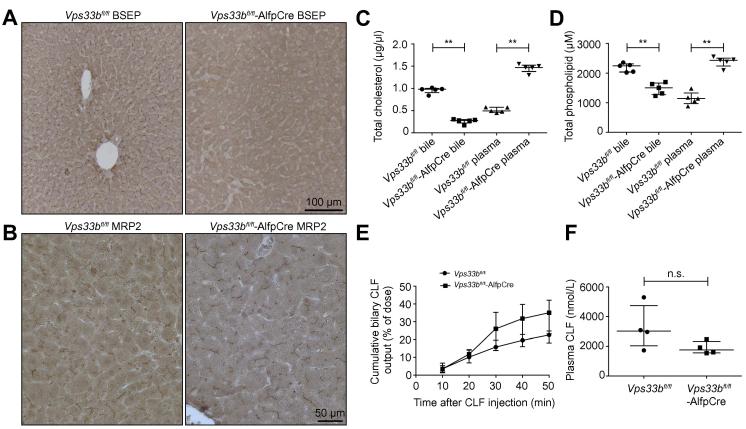
In vitro studies have suggested that VPS33B has a role in apical membrane protein trafficking in epithelial cells [11]. This is reported specifically to involve interaction with Rab11a-positive ARE. To investigate possible defects of protein trafficking to the canalicular membrane, we used immunostaining of Vps33bfl/fl and Vps33bfl/fl-AlfpCre mouse liver sections to assess the localisation of apical membrane proteins known to be trafficked via Rab11a-positive ARE (n = 3). In contrast to Vps33bfl/fl tissue, in Vps33bfl/fl-AlfpCre sections we observed absence of the ATP-binding cassette proteins BSEP and ABCG8 at the hepatocyte canalicular membrane (Fig.2A and Supplementary Fig. 4A). These proteins are responsible for secretion into bile of conjugated bile acids and cholesterol respectively [25], [26], [27]. We also observed mislocalisation of the GPI anchored glycoprotein carcinoembryonic antigen (CEA) in Vps33bfl/fl-AlfpCre liver with a diffuse stain appearing around the entire hepatocyte membrane as opposed to the clear and selective apical marking in Vps33bfl/fl liver (Supplementary Fig. 4B). Multidrug resistance-associated protein 2 (MRP2) reportedly bypasses the RE during apical trafficking [28], [29]. Interestingly, as in ARC patients, MRP2 did not appear mislocalised in Vps33bfl/fl-AlfpCre mouse liver (Fig.2B). Localisation of basolateral proteins appeared unaffected by loss of Vps33b (Supplementary Fig. 4C).
Fig. 2.
Functional polarity defects in Vps33bfl/fl-AlfpCre mice. (A) Immunostaining for detection of BSEP and (B) MRP2 localisation in Vps33bfl/fl-AlfpCre and Vps33bfl/fl liver sections. (C) Plasma and bile levels of cholesterol and (D) phospholipid in Vps33bfl/fl-AlfpCre and Vps33bfl/fl mice assessed by fluorometric analysis (n = 5, p = 0.0079 in all tests, Mann Whitney U test). (E) Cumulative biliary levels of CLF and (F) blood levels of CLF measured in Vps33bfl/fl-AlfpCre and Vps33bfl/fl (n = 4). All mice were fed a cholic acid supplemented diet and sacrificed at 14–15 weeks of age. Graphs are presented with medians and IQR.
Immunofluorescence detection of BSEP in normal chow fed mice established that protein mislocalisation was not an artefact of CA feeding and also showed intracellular punctate staining in Vps33bfl/fl-AlfpCre sections (Supplementary Fig. 5A). Immunoblotting analysis showed that BSEP and ABCG8 expression levels were not strongly affected in Vps33bfl/fl-AlfpCre liver (Supplementary Fig. 5B and C). These data collectively suggest that canalicular proteins were mislocalised to intracellular vesicles rather than downregulated in Vps33bfl/fl-AlfpCre liver.
Disruption of functional hepatocyte polarity in Vps33bfl/fl-AlfpCre mice
To investigate the physiological impact of apical protein mislocalisation, levels of bile components were assessed by mass spectrometry in plasma and bile samples of CA fed Vps33bfl/fl and Vps33bfl/fl-AlfpCre mice (n = 4–6). We observed a significant increase in plasma bile salt levels of Vps33bfl/fl-AlfpCre animals over those of Vps33bfl/fl mice (Table 1). The major accumulating bile acid in plasma was taurocholate, which in Vps33bfl/fl-AlfpCre mice was present at a median concentration of 97.9 μM: 49-fold higher than the median concentration in Vps33bfl/fl mice. The median concentrations of most other bile acid species were also increased: glycocholate 9.7-fold; cholate 7-fold; tauro-diOH-cholanoates 7.5-fold; glyco-diOH-cholanoates 9.3-fold; tauro-tetraOH-cholanoates 80-fold; glyco-tetraOH-cholanoates 26-fold.
Table 1.
Concentrations of bile acids in plasma from Vps33bfl/fl and Vps33bfl/fl-AlfpCre mice fed 0.5% cholic acid.
| Bile acid |
Vps33bfl/fl |
Vps33bfl/fl-AlfpCre |
Comparison | ||||
|---|---|---|---|---|---|---|---|
| Median, μM | 25th Percentile | 75th Percentile | Median, μM | 25th Percentile | 75th Percentile |
p value (Mann Whitney U) |
|
| TCA | 1.99 (9.69%) | 1.49 | 4.16 | 97.9 (54.55%) | 46.2 | 183 | 0.002 |
| GCA | 1.09 (5.31%) | 0.57 | 1.61 | 10.6 (5.91%) | 6.99 | 24.4 | 0.002 |
| CA | 3.44 (16.75%) | 2.19 | 5.84 | 24.2 (13.48%) | 5.50 | 33.0 | 0.02 |
| Tauro-diOH-C | 1.98 (9.64%) | 1.28 | 2.62 | 14.8 (8.25%) | 5.67 | 25.4 | 0.004 |
| Glyco-diOH-C | 0.08 (0.39%) | 0.038 | 0.15 | 0.74 (0.41%) | 0.20 | 2.30 | 0.002 |
| DiOH-C | 11.8 (57.45%) | 5.41 | 19.9 | 24.0 (13.37%) | 11.8 | 33.4 | 0.13 (ns) |
| Tauro-tetraOH-C | 0.08 (0.39%) | 0.068 | 0.16 | 6.40 (3.57%) | 1.77 | 10.8 | 0.0048 |
| Glyco-tetraOH-C | 0.03 (0.15%) | 0.018 | 0.057 | 0.78 (0.43%) | 0.64 | 2.68 | 0.0238 |
| TetraOH-C | 0.05 (0.23%) | 0.03 | 0.058 | 0.06 (0.03%) | 0.03 | 0.09 | 0.5097 (n.s.) |
Contributions of individual bile acids to the total bile acid pool are listed as percentages in parenthesis.
CA, cholic acid; C, cholanoate; T, tauro; G, glyco; DiOH, dihydroxy; tetra-OH, tetrahydroxy.
In comparison to Vps33bfl/fl mice, the main biliary bile acid, taurocholate, was present at 1.9-fold higher concentrations in Vps33bfl/fl-AlfpCre mouse bile (not statistically significant). Biliary levels of glyco-diOH-cholanoates were equivalent between Vps33bfl/fl-AlfpCre and Vps33bfl/fl animals (Table 2). However, median biliary concentrations of all other bile acids were lower in Vps33bfl/fl-AlfpCre mice than in Vps33bfl/fl mice (1.9-, 13.3-, 1.5- and 4.3-fold reduction in glycocholate, unconjugated cholate, tauro-diOH-cholanoates, and unconjugated diOH-cholanoates respectively). These reduced concentrations were not statistically significant excepting unconjugated cholate (p = 0.0190).
Table 2.
Concentrations of bile acids in bile from Vps33bfl/fl and Vps33bfl/fl-AlfpCre mice fed 0.5% cholic acid corrected by flow rate.
| Bile acid |
Vps33bfl/fl |
Vps33bfl/fl-AlfpCre |
Comparison | ||||
|---|---|---|---|---|---|---|---|
| Median (nmol/min.100 g) | 25th Percentile | 75th Percentile | Median (nmol/min.100 g) | 25th Percentile | 75th Percentile |
p value (Mann Whitney U) |
|
| TCA | 47.26 (68.48%) | 33.76 | 53.74 | 90.42 (92.26%) | 70.67 | 99.24 | 0.0667(n.s.) |
| GCA | 2.93 (4.25%) | 2.12 | 3.99 | 1.58 (1.61%) | 0.98 | 2.88 | 0.2571 (n.s.) |
| CA | 11.21 (16.24%) | 7.28 | 11.60 | 0.84 (0.86%) | 0.14 | 4.55 | 0.0190 |
| Tauro-diOH-C | 7.30 (10.58%) | 4.96 | 8.41 | 4.93 (5.03%) | 2.74 | 6.23 | 0.0667(n.s.) |
| Glyco-diOH-C | 0.18 (0.26%) | 0.15 | 0.21 | 0.21 (0.21%) | 0.19 | 0.27 | 0.1714 (n.s.) |
| DiOH-C | 0.13 (0.19%) | 0.08 | 0.31 | 0.03 (0.03%) | 0.00 | 0.08 | 0.1143(n.s.) |
Contributions of individual bile acids to the total bile acid pool are listed as percentages in parenthesis.
CA, cholic acid; C, cholanoate; T, tauro; G, glyco; DiOH, dihydroxy; tetra-OH, tetrahydroxy.
Total cholesterol and phospholipid levels were also significantly increased in plasma (2.8- and 2-fold respectively) and decreased in bile (3.6- and 1.5-fold respectively) of Vps33bfl/fl-AlfpCre animals (p = 0.0079 in all tests, Mann Whitney U test) (Fig.2C and D). Collectively these results suggest that the directional flow of bile components from blood to canalicular bile is disrupted in Vps33bfl/fl-AlfpCre mice. Increased levels of plasma total cholesterol were confirmed in normal chow fed Vps33bfl/fl-AlfpCre (Supplementary Fig. 6A).
In a further group of 14-week-old CA fed animals, we analysed the composition of plasma cholesterol as concentrations of total (Supplementary Fig. 6B), free and esterified cholesterol (n = 6–7). Levels of free cholesterol in plasma of Vps33bfl/fl-AlfpCre mice were significantly increased (3.6-fold; p = 0.0012, Mann Whitney U test) over those of Vps33bfl/fl animals (Supplementary Fig. 6C, median: 0.3678 μg/μl; IQR: 0.2996–0.5072 μg/μl and median: 0.1036 μg/μl; IQR: 0.0836–0.1615 μg/μl respectively) whilst levels of esterified cholesterol were equivalent between Vps33bfl/fl-AlfpCre and Vps33bfl/fl mice (Supplementary Fig. 6D, median: 0.5134 μg/μl; IQR: 0.3869–0.5459 μg/μl and median: 0.4371 μg/μl; IQR: 0.3998–0.5747 μg/μl respectively). This suggests that the increased levels of total cholesterol observed in plasma of Vps33bfl/fl-AlfpCre mice are largely due to the increase in concentration of free cholesterol as opposed to esterified cholesterol.
CLF is secreted into canalicular bile specifically via MRP2 [18]. To determine whether MRP2 protein was functional as well as correctly localised in Vps33bfl/fl-AlfpCre hepatocytes, anaesthetised animals were given an intravenous injection of CLF followed by collection of bile and plasma fractions for fluorometric quantification of CLF (n = 4). Plasma and bile levels of CLF did not significantly differ between Vps33bfl/fl-AlfpCre and Vps33bfl/fl mice, suggesting normal MRP2 function in Vps33b deficiency (Fig.2E and F). However, accumulation of CLF in bile appeared more rapid in Vps33bfl/fl-AlfpCre animals. This is consistent with reports that suggest an increase in mouse MRP2 expression levels in liver in response to bile acid retention [30].
Inflammatory and metabolic pathways are perturbed in Vps33bfl/fl-AlfpCre liver
To assess differential gene expression in liver following Vps33b deletion, we carried out microarray analysis of total RNA isolated from Vps33bfl/fl and Vps33bfl/fl-AlfpCre liver samples (n = 4–6) from mice fed a 0.5% CA diet and sacrificed at 10–14 weeks.
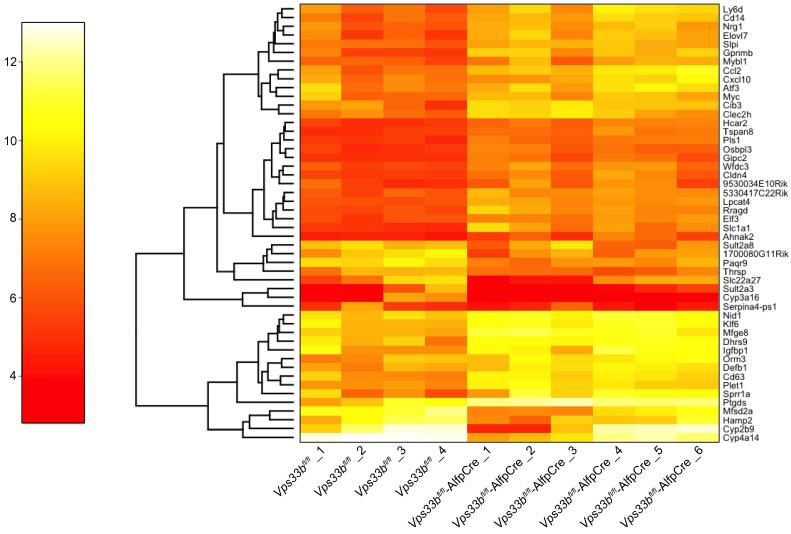
Using partial least squares (PLS) regression, we observed that the first PLS component clearly separated Vps33bfl/fl-AlfpCre from Vps33bfl/fl liver samples (Supplementary Fig. 7). Relevant genes were identified by selecting those with the largest contribution to the first component (Fig. 3).
Fig. 3.
Gene expression analysis. Heat map showing gene expression of the top 50 genes identified as differentially expressed by PLS loading in the first component. Analysis was performed on liver RNA samples from 10–14-week-old, cholic acid fed Vps33bfl/fl and Vps33bfl/fl-AlfpCre mice. Color Key: 4 (red) = lower expression level; 12 (yellow) = higher expression level. Left hand trees link genes with similar expression patterns but do not necessarily infer a functional relationship.
In comparison to samples from Cre-negative littermates, in Vps33bfl/fl-AlfpCre liver, we observed upregulation of several genes involved in the inflammatory response. This included markers of immune cells (Ly6d, Cd14) as well as chemokines that induce immune cell infiltration (Ccl2, Cxcl10). Using the Database for Annotation, Visualization and Integrated Discovery (DAVID), we identified many over-represented gene ontology annotations in the top 150 genes (Supplementary Fig. 8). In particular, we observed terms relating to ‘inflammatory response’ but annotations such as ‘membrane’ and ‘extracellular region’ were also elicited.
Additionally, numerous genes involved in metabolic pathways were identified as misregulated in Vps33bfl/fl-AlfpCre mouse liver. Gene set enrichment analysis consistently identified the Kegg pathway bile acid biosynthesis as the most perturbed pathway in Vps33bfl/fl-AlfpCre animals. Several other metabolic and inflammatory pathways also were perturbed (Supplementary Table 1).
Collectively, these data support our observations of inflammation and fibrotic changes in Vps33bfl/fl-AlfpCre liver and suggests that intrahepatic retention of bile components may directly result in dysregulation of bile acid metabolism in Vps33bfl/fl-AlfpCre mice.
The hepatocyte phenotype is rescued by AAV mediated gene transfer
To determine whether reintroduction of VPS33B to Vps33bfl/fl-AlfpCre hepatocytes could restore structural and functional polarity, we performed gene transfer experiments using ssAAV2/8 vectors containing either wild-type or codon optimised human VPS33B cDNA (ssAAV2/8-EFS-hVPS33B-WPRE and ssAAV2/8-EFS-hVPS33Bco-WPRE respectively) (Fig.4A). Vectors were administered to 5-week-old mice at a dose of 1 × 1012 vector genomes (vg) per animal via intraperitoneal injection. At 8 weeks of age, injected animals and control groups received CA supplemented feed for 6 weeks. At 14 weeks, without re-exposure to a normal diet, animals were sacrificed and transgene expression in hepatocytes was confirmed immunohistochemically (Supplementary Fig. 9).
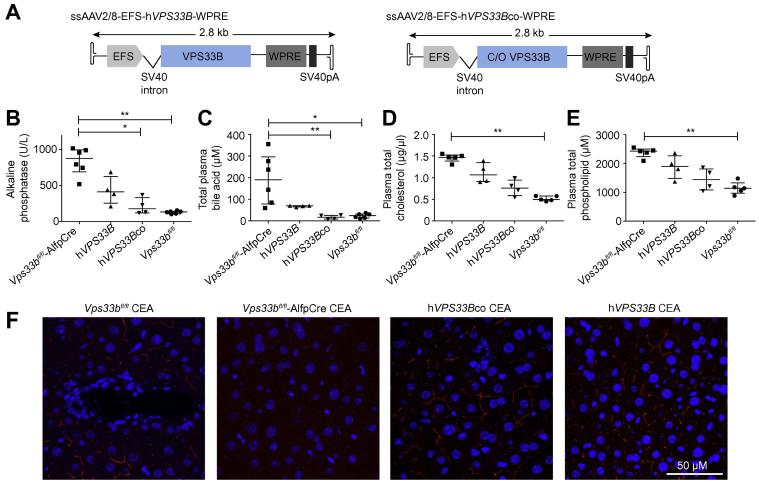
Fig. 4.
In vivo gene transfer of human VPS33B. (A) Schematic of ssAAV2/8 vectors containing wild-type (hVPS33B) and codon optimised (hVPS33Bco) human VPS33B cDNA. (B) Plasma alkaline phosphatase activity levels (p = 0.0016, Kruskal-Wallis test), (C) total plasma bile acid levels (p = 0.0015, Kruskal-Wallis test) (D) Plasma cholesterol levels (p = 0.0023, Kruskal-Wallis test) (E) plasma phospholipid levels (p = 0.0047, Kruskal-Wallis test) and (F) CEA protein localisation assessed 9 weeks after injection of ssAAV2/8-EFS-hVPS33B-WPRE and ssAAV2/8-EFS-hVPS33Bco-WPRE (1 × 1012 vg/mouse) in 5-week-old Vps33bfl/fl-AlfpCre mice (n = 4–6). All mice were fed cholic acid supplemented diet and sacrificed at 14 weeks of age. Graphs are presented with medians and IQR.
In animals treated with ssAAV2/8-EFS-hVPS33B-WPRE and ssAAV2/8-EFS-hVPS33Bco-WPRE vectors, we observed respective 2.1- and 4.9-fold decreases in median levels of plasma ALP vs. Vps33bfl/fl-AlfpCre mice (Fig.4B). This was accompanied by a 2.7-fold decrease of total plasma bile acid levels in ssAAV2/8-EFS-hVPS33B-WPRE treated mice and a significant 11-fold decrease in plasma bile acid levels of mice receiving ssAAV2/8-EFS-hVPS33Bco-WPRE (p = 0.0015, Kruskal-Wallis test) (Fig.4C). Plasma levels of cholesterol and phospholipid were also reduced in mice receiving ssAAV2/8-EFS-hVPS33B-WPRE and ssAAV2/8-EFS-hVPS33Bco-WPRE vectors (Fig.4D and E).
Immunostaining demonstrated partial restoration of canalicular membrane protein localisation following gene transfer with both vectors (Fig.4F; Supplementary Fig. 10). This suggests that, following gene transfer, functional polarity is rescued, as manifest by correction of protein localisation to the apical membrane.
Vps33bfl/fl-AlfpCre hepatocytes show tight junction defects
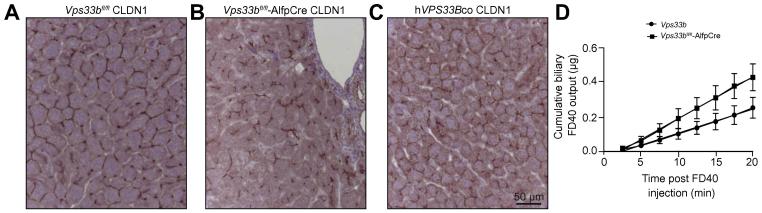
Hepatic tight junctions constitute the barrier that separates sinusoidal blood from canalicular bile. Following knockdown of Rab11a, WIF-B9 cells form tight junctions that appear morphologically abnormal [31]. To study the role of Vps33b in Rab11a dependent canalicular membrane biogenesis, we investigated tight junction abnormalities in Vps33bfl/fl-AlfpCre liver by immunostaining for Claudin1. In control Vps33bfl/fl liver, Claudin1 staining revealed normal belt-like tight junctions (Fig.5A). In Vps33bfl/fl-AlfpCre tissue, Claudin1 marking was irregular and tortuous, suggesting changes in tight junction organisation (Fig.5B). Following gene transfer of 1 x 1012 vg/animal ssAAV2/8-EFS-hVPS33Bco-WPRE vector, we observed partial correction of tight junction appearance (Fig.5C).
Fig. 5.
Tight junction defects in Vps33bfl/fl-AlfpCre mice. Appearance of hepatocellular tight junctions assessed by immunostaining for Claudin1 in (A) Vps33bfl/fl and (B) Vps33bfl/fl-AlfpCre mouse liver sections and in (C) sections of Vps33bfl/fl-AlfpCre mouse liver 9 weeks after injection of ssAAV2/8-EFS-hVPS33Bco-WPRE vector (1 × 1012 vg/mouse injected at 5 weeks of age) (n = 3). (D) Cumulative FD-40 levels measured in bile fractions from Vps33bfl/fl-AlfpCre and Vps33bfl/fl mice collected after intravenous FD-40 injection (n = 4–5, p = 0.0134). All mice were fed a cholic acid supplemented diet and sacrificed at 14–15 weeks of age.
To assess functional integrity of hepatic tight junctions in Vps33bfl/fl-AlfpCre and Vps33bfl/fl liver, we measured levels of FD-40 in bile following intravenous injection. Disruption of tight junction barrier function results in rapid biliary accumulation of FD-40. Fluorometry documented build-up of FD-40 in the bile of Vps33bfl/fl-AlfpCre mice earlier than in control Vps33bfl/fl littermates (p = 0.01342) (Fig.5D). This observation suggests that tight junctions in Vps33bfl/fl-AlfpCre mice, abnormal in Claudin1 distribution on immunostaining, were defective in function.
Structural abnormalities of Vps33bfl/fl-AlfpCre canaliculi
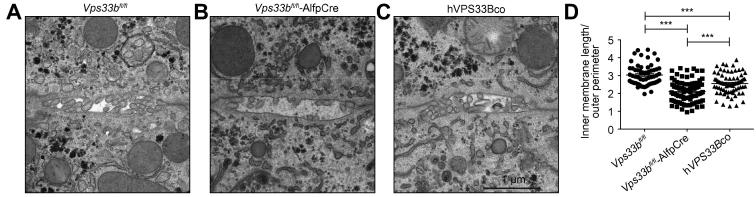
Ultrastructure of Vps33bfl/fl-AlfpCre and Vps33bfl/fl canaliculi was assessed by TEM. Relative to Cre-negative littermates, we observed a marked paucity of canalicular microvilli in Vps33bfl/fl-AlfpCre hepatocytes (Fig.6A and B). Ultrastructure of the bile canaliculi appeared restored in mice treated with ssAAV2/8-EFS-hVPS33Bco-WPRE vector (Fig.6C). To quantify these observations in terms of apical membrane length relative to canaliculus size, we measured the total inner membrane length of the bile canaliculi and normalised these values to the length of the outer canalicular perimeter (Fig.6D). In Vps33bfl/fl-AlfpCre cells we observed a significant decrease in inner membrane length in comparison to Vps33bfl/fl cells. This corresponds to an overall loss of canalicular membrane surface area in the absence of Vps33b. This observation was accompanied by a significant increase in canalicular membrane length in hepatocytes of AAV treated mice in comparison to untreated Vps33bfl/fl-AlfpCre animals (p <0.001, One way ANOVA, Bonferroni’s test). Measurements of tight junction length and breadth did not differ between Vps33bfl/fl and Vps33bfl/fl-AlfpCre micrographs (Supplementary Fig. 11).
Fig. 6.
Bile canaliculus ultrastructure. Transmission electron micrographs of bile canaliculi in (A) Vps33bfl/fl and (B) Vps33bfl/fl-AlfpCre mouse liver and in (C) liver of Vps33bfl/fl-AlfpCre mice injected with 1 × 1012 vg/mouse of ssAAV2/8-EFS-hVPS33Bco-WPRE vector. (D) Measurements of canalicular membrane length (± SEM) as a factor of canalicular perimeter assessed using ITEM software on mouse hepatocyte electron micrographs (n = 62–90, p <0.0001, One way ANOVA, Bonferroni’s test). All mice were fed a normal chow diet and sacrificed at 14–15 weeks of age.
Discussion
Loss of apical-basolateral hepatocyte polarity is observed in many types of inherited cholestatic liver disease and in liver cancer [4], [5], [6], [7], [32], [33]. However, the molecular mechanisms that cause this loss are not well determined. In this study, we aimed to improve understanding of the pathways that govern hepatocyte polarity. Specifically, we investigated the role of VPS33B in biogenesis of the bile canaliculus and subsequent maintenance of apical domain structure and function. To achieve this aim, we generated a novel murine model with a liver epithelial cell-specific deletion of Vps33b (Vps33bfl/fl-AlfpCre) that recapitulates hepatocyte polarity defects observed in patients with ARC syndrome.
ARC patients with VPS33B mutations show cholestasis with high plasma ALP activity and bile acid levels. Patients’ aminotransferase activity and γGT levels remain normal [8]. In agreement with these data, our Vps33bfl/fl-AlfpCre mice demonstrated significant increases in plasma ALP and total bile acid levels in comparison to Cre-negative littermates. As seen in other cholestasis models, these phenotypes were exacerbated by CA feeding [34]. No significant difference in plasma ALT activity was observed in Vps33bfl/fl-AlfpCre vs. Vps33bfl/fl mice and γGT levels were not assessed as mice do not express γGT in hepatocytes after birth [35]. Biomarkers in our Vps33bfl/fl-AlfpCre mouse thus accurately track those used to assess ARC liver disease. Like patients with clinically milder ARC, Vps33bfl/fl-AlfpCre animals did not manifest increased serum bilirubin levels.
As ALP is normally expressed at the apical hepatocyte membrane, the increase in plasma ALP levels in Vps33bfl/fl-AlfpCre animals may be due to defects in apical membrane biosynthesis. Further increases in plasma ALP caused by the CA diet may be related to canalicular damage, although increased ALP synthesis or shifts in localisation are also possible. Further evidence of canalicular injury is supplied by immunostaining of ARC patient liver biopsy specimens, which reveals mislocalisation of proteins typically located at the apical membrane. Among these is BSEP, an ATP-binding cassette transporter required for movement of conjugated bile acids from blood to bile [25]. The mislocalisation of BSEP was reproduced in our Vps33bfl/fl-AlfpCre animals under both normal diet and CA diet conditions. By immunofluorescence, we observed punctate intracellular BSEP staining, consistent with localisation of BSEP in intracellular vesicles.
In patients with progressive familial intrahepatic cholestasis type 2 (PFIC2), the loss of BSEP results in intrahepatic retention of bile salts [36]. In the Bsep knockout mouse, severe cholestatic liver disease is observed when mice are fed a diet containing 0.5% CA [24]. In agreement with these observations, by utilising a recently developed mass spectrometry technique, we detected significant increases (7 to 80-fold) in plasma concentrations of TCA, GCA, CA, tauro-diOH-cholanoates, glyco-diOH-cholanoates, tauro-tetra-OH-cholanoates and glyco-tetra-OH-cholanoates in Vps33bfl/fl-AlfpCre mice fed 0.5% CA. Concentrations of plasma bile acids in Vps33bfl/fl-AlfpCre animals were only slightly lower than values previously observed in the Bsep knockout mouse [24].
Our comparison of biliary bile acid concentrations in Vps33bfl/fl-AlfpCre and Vps33bfl/fl mice also yielded results similar to those obtained on comparison of Bsep knockout and wild-type mice [24]. Neither the concentration of the major biliary bile acid component, taurocholate, nor the total biliary bile acid concentration differed significantly between Vps33bfl/fl-AlfpCre and Vps33bfl/fl animals. This may indicate an alternative route of apical bile acid secretion following CA feeding such as via MRP2 [37]. For some of the lesser bile acids, biliary concentrations were lower in Vps33bfl/fl-AlfpCre mice than in Vps33bfl/fl mice. This reduction in biliary concentration was statistically significant only in the case of unconjugated cholate. As in the Bsep knockout mouse, this may indicate a higher rate of taurine conjugation and conversion to tetra-OH bile acid species in Vps33bfl/fl-AlfpCre animals as a mechanism of reducing bile acid toxicity [38]. Indeed, the proportion of unconjugated bile acids in plasma and bile of Vps33bfl/fl-AlfpCre mice was lower than that of Vps33bfl/fl littermates and a significant increase in conjugated tetra-OH species was observed in Vps33bfl/fl-AlfpCre plasma. Overall, these data suggest that mislocalisation of BSEP in Vps33bfl/fl-AlfpCre mice has a similar effect on transport of bile acids to the effect of Bsep knockout. However, even CA fed Vps33bfl/fl-AlfpCre animals had normal serum bilirubin concentrations, no obvious reduction in survival and only a small increase in apoptosis (as detected by CC3 staining), thus suggesting liver disease less severe than that in Bsep knockout mice [24].
In addition to BSEP mislocalisation, in Vps33bfl/fl-AlfpCre hepatocytes we observed mislocalisation of ABCG8, an apical ATP-binding cassette transporter involved in the directional movement of cholesterol [27]. Concordant with a loss of apical ABCG8, in comparison to Vps33bfl/fl mice, we detected reduced levels of total cholesterol in bile and accumulation of cholesterol in plasma of CA fed Vps33bfl/fl-AlfpCre animals. Collectively these results suggest that, in the absence of Vps33b, apical transporter proteins are mislocalised, causing an overall loss of functional polarity and accumulation of bile components in blood.
Interestingly, the increase in plasma total cholesterol observed in Vps33bfl/fl-AlfpCre animals was due to an increase in free cholesterol as opposed to esterified cholesterol. In combination with our observation of canalicular proteins mislocalised to possible intracellular vesicles, as in other cholestatic models, this increase in free cholesterol may imply that the majority of phospholipid and cholesterol in Vps33bfl/fl-AlfpCre plasma is present in the form of lipoprotein X, biliary lipid vesicles that contain mostly free cholesterol and phosphatidylcholine, ascribed in cholestasis to shedding of hepatocyte membranes into bile and thence, with bile, between hepatocytes into plasma [39], [40].
Proteins that appear mislocalised in Vps33bfl/fl-AlfpCre mouse hepatocytes are thought to be trafficked to the canalicular membrane specifically via Rab11a-positive ARE [41]. In our model we observed mislocalisation of apical proteins that are trafficked directly from the trans golgi network (TGN) to the ARE (BSEP and ABCG8) as well as proteins that are first delivered to the basolateral membrane from TGN and then transcytosed to the canalicular membrane via ARE (CEA) [41]. However, as in ARC patients, MRP2 was not mislocalised in Vps33bfl/fl-AlfpCre liver. MRP2 is reported to bypass Rab11a-positive recycling ARE en route to the hepatocyte apical domain [28], [29]. Functional integrity of MRP2 in Vps33bfl/fl-AlfpCre liver was confirmed by intravenous administration and subsequent quantification in bile of cholyl-lysyl-fluorescein (a compound specifically exported into bile via MRP2) [18]. As in zebrafish, these results suggest that mammalian VPS33B is specifically involved in trafficking of proteins to the apical domain via Rab11a-positive ARE [11]. Basolateral membrane proteins were not mislocalised in Vps33bfl/fl-AlfpCre hepatocytes, suggesting a specific role for VPS33B in biogenesis of the bile canaliculus.
As we observed in mutant mice that MRP2 is functional and normally sited, as is MRP2 in ARC patients [11], we infer that the collections of Dubin-Johnson-like pigment in some ARC patients’ hepatocytes [42] are unlikely to originate from MRP2 absence or dysfunction.
To assess defects in structural components of hepatocyte polarity, we analysed tight junction integrity in Vps33bfl/fl-AlfpCre and Vps33bfl/fl liver. As in cultured cells, immunohistochemical marking for Claudin1, a tight junction component, was irregular in Vps33bfl/fl-AlfpCre liver, with tortuous distribution [31]. Tight junctions between control Vps33bfl/fl hepatocytes appeared uniform and belt-like. This suggestion of a structural tight junction defect was supported by our functional analysis of tight junction integrity which showed rapid accumulation of fluorescence in the bile of Vps33bfl/fl-AlfpCre mice following intravenous injection of 40 kDa fluorescent dextran (FD-40). The observed leaking of FD-40 from blood into bile suggests a loss of tight junction barrier function in Vps33bfl/fl-AlfpCre hepatocytes [19]. Whether tight junction proteins are normally trafficked to the apical membrane by VPS33B or loss of tight junction integrity is a secondary effect of incorrect apical protein trafficking remains to be determined. In contrast to reports in Tjp2 knockout mice, our TEM analysis of tight junction ultrastructure revealed no overall increase in tight junction length or breadth following deletion of Vps33b [4], [7]. In future studies, freeze fracture electron microscopy may be used for a more detailed assessment of tight junction composition in Vps33bfl/fl-AlfpCre liver.
Analysis of ultrastructural images of Vps33bfl/fl-AlfpCre liver revealed a significant reduction in apical membrane area due to a partial loss of canalicular microvilli. This implies a loss of structural polarity at the apical membrane and an overall reduction of surface area for movement of solutes from the hepatocyte into bile. When Rab11a is mutated in mouse intestine, apical brush border microvilli are lost [43]. These results provide an interesting link between the observed interaction of VPS33B in Rab11a protein trafficking pathways and the maintenance of apical microvilli in hepatocytes.
To confirm the role of VPS33B in hepatocyte polarity, we performed in vivo gene rescue studies in Vps33bfl/fl-AlfpCre mice using ssAAV2/8 vectors containing either wild-type or codon optimised human VPS33B cDNA (ssAAV2/8-EFS-hVPS33B-WPRE and ssAAV2/8-EFS-hVPS33Bco-WPRE). At 9 weeks after gene transfer (1 × 1012 vg/mouse in 4–5 week old animals) we observed partial rescue of apical protein localisation, Claudin1 distribution at tight junctions, canalicular ultrastructure and plasma ALP activity, cholesterol, phospholipid and bile acid levels. This phenotype correction appeared more robust in animals treated with the codon optimised vector: all such animals had plasma total bile acid levels equivalent to those in control Vps33bfl/fl mice. As well as confirming that the observed loss of polarity in Vps33bfl/fl-AlfpCre hepatocytes is due to Vps33b mutation, these experiments establish an initial proof of principle for future therapeutic use of gene transfer in ARC patients. To meet a therapeutic aim, dose optimisation and safety of VPS33B gene transfer would need to be determined in future studies.
In conclusion, using our novel Vps33bfl/fl-AlfpCre mouse model, we have identified that VPS33B is vital for maintenance of mammalian structural and functional hepatocyte polarity. We have established that genetic hepatocyte polarity disorders can be rescued using gene transfer with AAV vectors and that ARC liver disease may be a potential target of therapeutic gene replacement.
Financial support
Children’s Liver Disease Foundation (CLDF), Great Ormond Street Hospital and UCL Institute of Child Health Biomedical Research Centre. European Research Council (grant ref. ERC-2013-StG-337057 CLOC), P.G. is a Welcome Trust senior clinical research fellow. J.J.B. is supported by core funding to MRC_UCL LMCB university unit (grant ref. MC_U122665002).
Conflict of interest
Dr. Clayton reports grants and personal fees from Actelion Pharmaceuticals UK, outside the submitted work. All the other authors who have taken part in this study declared that they do not have anything to disclose regarding funding or conflict of interest with respect to this manuscript.
Authors’ contributions
J.H. performed experiments, contributed to overall study design and wrote the manuscript which was edited by all co-authors. D.D., F.M., J.J.B., R.F., H.S., A.S-I., B.B., A.V. and S.W. performed experiments. A.M-L. performed in silico gene expression and pathway analysis. D.D., J.J.B., K.M., F.P.L., A.S.K., S.H., N.S., S.W., C.C.P., P.C. and P.G. contributed to overall study design. P.G. directed the study.
Acknowledgements
We would like to thank the Great Ormond Street Hospital, Camelia Botnar Laboratories, chemical pathology services for contribution towards assessment of liver function. Histologic samples were imaged in the UCL Institute of Child Health Imaging Facility.
Footnotes
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.jhep.2017.01.001.
Supplementary data
References
Author names in bold designate shared co-first authorship
- 1.Treyer A., Musch A. Hepatocyte polarity. Compr Physiol. 2013;3:243–287. doi: 10.1002/cphy.c120009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cereijido M., Contreras R.G., Shoshani L. Cell adhesion, polarity, and epithelia in the dawn of metazoans. Physiol Rev. 2004;84:1229–1262. doi: 10.1152/physrev.00001.2004. [DOI] [PubMed] [Google Scholar]
- 3.Slim C.L., Lazaro-Dieguez F., Bijlard M., Toussaint M.J., de Bruin A., Du Q. Par1b induces asymmetric inheritance of plasma membrane domains via LGN-dependent mitotic spindle orientation in proliferating hepatocytes. PLoS Biol. 2013;11 doi: 10.1371/journal.pbio.1001739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carlton V.E.H., Harris B.Z., Puffenberger E.G., Batta A.K., Knisely A.S., Robinson D.L. Complex inheritance of familial hypercholanemia with associated mutations in TJP2 and BAAT. Nat Genet. 2003;34:91–96. doi: 10.1038/ng1147. [DOI] [PubMed] [Google Scholar]
- 5.Girard M., Lacaille F., Verkarre V., Mategot R., Feldmann G., Grodet A. MYO5B and bile salt export pump contribute to cholestatic liver disorder in microvillous inclusion disease. Hepatology. 2014;60:301–310. doi: 10.1002/hep.26974. [DOI] [PubMed] [Google Scholar]
- 6.Hadj-Rabia S., Baala L., Vabres P., Hamel-Teillac D., Jacquemin E., Fabre M. Claudin-1 gene mutations in neonatal sclerosing cholangitis associated with ichthyosis: a tight junction disease. Gastroenterology. 2004;127:1386–1390. doi: 10.1053/j.gastro.2004.07.022. [DOI] [PubMed] [Google Scholar]
- 7.Sambrotta M., Strautnieks S., Papouli E., Rushton P., Clark B.E., Parry D.A. Mutations in TJP2 cause progressive cholestatic liver disease. Nat Genet. 2014;46:326. doi: 10.1038/ng.2918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gissen P., Tee L., Johnson C.A., Genin E., Caliebe A., Chitayat D. Clinical and molecular genetic features of ARC syndrome. Hum Genet. 2006;120:396–409. doi: 10.1007/s00439-006-0232-z. [DOI] [PubMed] [Google Scholar]
- 9.Smith H., Galmes R., Gogolina E., Straatman-Iwanowska A., Reay K., Banushi B. Associations among genotype, clinical phenotype, and intracellular localization of trafficking proteins in ARC syndrome. Hum Mutat. 2012;33:1656–1664. doi: 10.1002/humu.22155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Baker R.W., Jeffrey P.D., Zick M., Phillips B.P., Wickner W.T., Hughson F.M. A direct role for the Sec1/Munc18-family protein Vps33 as a template for SNARE assembly. Science. 2015;349:1111–1114. doi: 10.1126/science.aac7906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cullinane A.R., Straatman-Iwanowska A., Zaucker A., Wakabayashi Y., Bruce C.K., Luo G. Mutations in VIPAR cause an arthrogryposis, renal dysfunction and cholestasis syndrome phenotype with defects in epithelial polarization. Nat Genet. 2010;42:303–312. doi: 10.1038/ng.538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sriram V., Krishnan K.S., Mayor S. Deep-orange and carnation define distinct stages in late endosomal biogenesis in Drosophila melanogaster. J Cell Biol. 2003;161:593–607. doi: 10.1083/jcb.200210166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Banushi B., Forneris F., Straatman-Iwanowska A., Strange A., Lyne A.M., Rogerson C. Regulation of post-Golgi LH3 trafficking is essential for collagen homeostasis. Nat Commun. 2016;7:12111. doi: 10.1038/ncomms12111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Roland J.T., Bryant D.M., Datta A., Itzen A., Mostov K.E., Goldenring J.R. Rab GTPase-Myo5B complexes control membrane recycling and epithelial polarization. Proc Natl Acad Sci U S A. 2011;108:2789–2794. doi: 10.1073/pnas.1010754108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bem D., Smith H., Banushi B., Burden J.J., White I.J., Hanley J. VPS33B regulates protein sorting into and maturation of alpha-granule progenitor organelles in mouse megakaryocytes. Blood. 2015;126:133–143. doi: 10.1182/blood-2014-12-614677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kellendonk C., Opherk C., Anlag K., Schutz G., Tronche F. Hepatocyte-specific expression of Cre recombinase. Genesis. 2000;26:151–153. doi: 10.1002/(sici)1526-968x(200002)26:2<151::aid-gene17>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 17.Mazzacuva F., Mills P., Mills K., Camuzeaux S., Gissen P., Nicoli E.R. Identification of novel bile acids as biomarkers for the early diagnosis of Niemann-Pick C disease. FEBS Lett. 2016;11:1651–1662. doi: 10.1002/1873-3468.12196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.de Waart D.R., Hausler S., Vlaming M.L., Kunne C., Hanggi E., Gruss H.J. Hepatic transport mechanisms of cholyl-L-lysyl-fluorescein. J Pharmacol Exp Ther. 2010;334:78–86. doi: 10.1124/jpet.110.166991. [DOI] [PubMed] [Google Scholar]
- 19.Han X., Fink M.P., Uchiyama T., Yang R., Delude R.L. Increased iNOS activity is essential for hepatic epithelial tight junction dysfunction in endotoxemic mice. Am J Physiol Gastrointest Liver Physiol. 2004;286:G126–G136. doi: 10.1152/ajpgi.00231.2003. [DOI] [PubMed] [Google Scholar]
- 20.Edgar R., Domrachev M., Lash A.E. Gene Expression Omnibus: NCBI gene expression and hybridization array data repository. Nucleic Acids Res. 2002;30:207–210. doi: 10.1093/nar/30.1.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nathwani A.C., Davidoff A., Hanawa H., Zhou J.F., Vanin E.F., Nienhuis A.W. Factors influencing in vivo transduction by recombinant adeno-associated viral vectors expressing the human factor IX cDNA. Blood. 2001;97:1258–1265. doi: 10.1182/blood.v97.5.1258. [DOI] [PubMed] [Google Scholar]
- 22.Davidoff A.M., Ng C.Y., Sleep S., Gray J., Azam S., Zhao Y. Purification of recombinant adeno-associated virus type 8 vectors by ion exchange chromatography generates clinical grade vector stock. J Virol Methods. 2004;121:209–215. doi: 10.1016/j.jviromet.2004.07.001. [DOI] [PubMed] [Google Scholar]
- 23.Fagone P., Wright J.F., Nathwani A.C., Nienhuis A.W., Davidoff A.M., Gray J.T. Systemic errors in quantitative polymerase chain reaction titration of self-complementary adeno-associated viral vectors and improved alternative methods. Hum Gene Ther Methods. 2012;23:1–7. doi: 10.1089/hgtb.2011.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang R., Lam P., Liu L., Forrest D., Yousef I.M., Mignault D. Severe cholestasis induced by cholic acid feeding in knockout mice of sister of P-glycoprotein. Hepatology. 2003;38:1489–1499. doi: 10.1016/j.hep.2003.09.037. [DOI] [PubMed] [Google Scholar]
- 25.Gerloff T., Stieger B., Hagenbuch B., Madon J., Landmann L., Roth J. The sister of P-glycoprotein represents the canalicular bile salt export pump of mammalian liver. J Biol Chem. 1998;273:10046–10050. doi: 10.1074/jbc.273.16.10046. [DOI] [PubMed] [Google Scholar]
- 26.Stieger B., Fattinger K., Madon J., Kullak-Ublick G.A., Meier P.J. Drug- and estrogen-induced cholestasis through inhibition of the hepatocellular bile salt export pump (Bsep) of rat liver. Gastroenterology. 2000;118:422–430. doi: 10.1016/s0016-5085(00)70224-1. [DOI] [PubMed] [Google Scholar]
- 27.Yu L., Li-Hawkins J., Hammer R.E., Berge K.E., Horton J.D., Cohen J.C. Overexpression of ABCG5 and ABCG8 promotes biliary cholesterol secretion and reduces fractional absorption of dietary cholesterol. J Clin Invest. 2002;110:671–680. doi: 10.1172/JCI16001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bandler P.E., Westlake C.J., Grant C.E., Cole S.P., Deeley R.G. Identification of regions required for apical membrane localization of human multidrug resistance protein 2. Mol Pharmacol. 2008;74:9–19. doi: 10.1124/mol.108.045674. [DOI] [PubMed] [Google Scholar]
- 29.Nies A.T., Keppler D. The apical conjugate efflux pump ABCC2 (MRP2) Pflugers Arch. 2007;453:643–659. doi: 10.1007/s00424-006-0109-y. [DOI] [PubMed] [Google Scholar]
- 30.Slitt A.L., Allen K., Morrone J., Aleksunes L.M., Chen C., Maher J.M. Regulation of transporter expression in mouse liver, kidney, and intestine during extrahepatic cholestasis. Biochim Biophys Acta. 2007;1768:637–647. doi: 10.1016/j.bbamem.2006.10.008. [DOI] [PubMed] [Google Scholar]
- 31.Wakabayashi Y., Dutt P., Lippincott-Schwartz J., Arias I.M. Rab11a and myosin Vb are required for bile canalicular formation in WIF-B9 cells. Proc Natl Acad Sci U S A. 2005;102:15087–15092. doi: 10.1073/pnas.0503702102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Taniguchi K., Roberts L.R., Aderca I.N., Dong X., Qian C., Murphy L.M. Mutational spectrum of beta-catenin, AXIN1, and AXIN2 in hepatocellular carcinomas and hepatoblastomas. Oncogene. 2002;21:4863–4871. doi: 10.1038/sj.onc.1205591. [DOI] [PubMed] [Google Scholar]
- 33.Vilarinho S., Erson-Omay E.Z., Harmanci A.S., Morotti R., Carrion-Grant G., Baranoski J. Paediatric hepatocellular carcinoma due to somatic CTNNB1 and NFE2L2 mutations in the setting of inherited bi-allelic ABCB11 mutations. J Hepatol. 2014;61:1178–1183. doi: 10.1016/j.jhep.2014.07.003. [DOI] [PubMed] [Google Scholar]
- 34.Yeh T.H., Krauland L., Singh V., Zou B., Devaraj P., Stolz D.B. Liver-specific beta-catenin knockout mice have bile canalicular abnormalities, bile secretory defect, and intrahepatic cholestasis. Hepatology. 2010;52:1410–1419. doi: 10.1002/hep.23801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gallagher B.C., Rudolph D.B., Hinton B.T., Hanigan M.H. Differential induction of gamma-glutamyl transpeptidase in primary cultures of rat and mouse hepatocytes parallels induction during hepatocarcinogenesis. Carcinogenesis. 1998;19:1251–1255. doi: 10.1093/carcin/19.7.1251. [DOI] [PubMed] [Google Scholar]
- 36.Evason K., Bove K.E., Finegold M.J., Knisely A.S., Rhee S., Rosenthal P. Morphologic findings in progressive familial intrahepatic cholestasis 2 (PFIC2): correlation with genetic and immunohistochemical studies. Am J Surg Pathol. 2011;35:687–696. doi: 10.1097/PAS.0b013e318212ec87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Megaraj V., Iida T., Jungsuwadee P., Hofmann A.F., Vore M. Hepatobiliary disposition of 3alpha,6alpha,7alpha,12alpha-tetrahydroxy-cholanoyl taurine: a substrate for multiple canalicular transporters. Drug Metab Dispos. 2010;38:1723–1730. doi: 10.1124/dmd.110.033480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang Y., Li F., Patterson A.D., Wang Y., Krausz K.W., Neale G. Abcb11 deficiency induces cholestasis coupled to impaired beta-fatty acid oxidation in mice. J Biol Chem. 2012;287:24784–24794. doi: 10.1074/jbc.M111.329318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Elferink R.P., Ottenhoff R., van Marle J., Frijters C.M., Smith A.J., Groen A.K. Class III P-glycoproteins mediate the formation of lipoprotein X in the mouse. J Clin Invest. 1998;102:1749–1757. doi: 10.1172/JCI3597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Manzato E., Fellin R., Baggio G., Walch S., Neubeck W., Seidel D. Formation of lipoprotein-X. Its relationship to bile compounds. J Clin Invest. 1976;57:1248–1260. doi: 10.1172/JCI108393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gissen P., Arias I.M. Structural and functional hepatocyte polarity and liver disease. J Hepatol. 2015;63:1023–1037. doi: 10.1016/j.jhep.2015.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nezelof C., Dupart M.C., Jaubert F., Eliachar E. A lethal familial syndrome associating arthrogryposis multiplex congenita, renal dysfunction, and a cholestatic and pigmentary liver disease. J Pediatr. 1979;94:258–260. doi: 10.1016/s0022-3476(79)80839-2. [DOI] [PubMed] [Google Scholar]
- 43.Sobajima T., Yoshimura S., Iwano T., Kunii M., Watanabe M., Atik N. Rab11a is required for apical protein localisation in the intestine. Biol Open. 2014;4:86–94. doi: 10.1242/bio.20148532. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.