Abstract
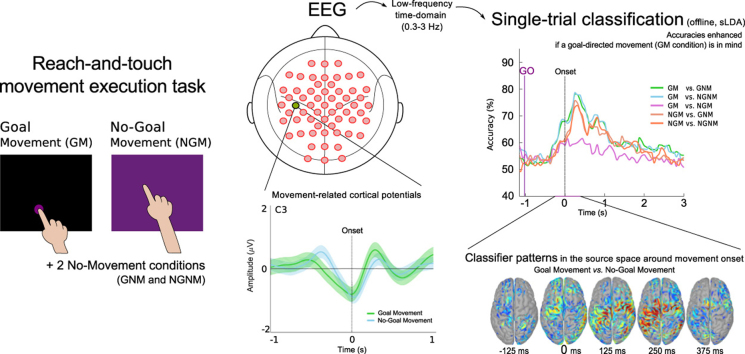
Using low-frequency time-domain electroencephalographic (EEG) signals we show, for the same type of upper limb movement, that goal-directed movements have different neural correlates than movements without a particular goal. In a reach-and-touch task, we explored the differences in the movement-related cortical potentials (MRCPs) between goal-directed and non-goal-directed movements. We evaluated if the detection of movement intention was influenced by the goal-directedness of the movement. In a single-trial classification procedure we found that classification accuracies are enhanced if there is a goal-directed movement in mind. Furthermore, by using the classifier patterns and estimating the corresponding brain sources, we show the importance of motor areas and the additional involvement of the posterior parietal lobule in the discrimination between goal-directed movements and non-goal-directed movements. We discuss next the potential contribution of our results on goal-directed movements to a more reliable brain-computer interface (BCI) control that facilitates recovery in spinal-cord injured or stroke end-users.
Keywords: Brain-computer interface (BCI), Electroencephalography (EEG), Goal-directed movement, Low-frequency, Movement-related cortical potential (MRCP), Brain sources
Graphical abstract
Introduction
The neural correlates of goal-directed actions and their differences from movements which do not result in an interaction with a particular goal (e.g. an object or visual target on a screen) have, over the past years, mainly been studied in movement observation tasks (Rizzolatti et al., 2014). These findings revealed the basis of action understanding and can have several implications in neurorehabilitation in general (Buccino et al., 2006). We believe some of the latest findings in this field can be of interest for brain-computer interface (BCI) research, since goal-directed movements are of utmost importance for BCIs which rely on the detection and decoding of movements. BCIs can be used to control devices such as functional electrical stimulation (FES) based neuroprosthesis (Müller-Putz et al., 2005) for the upper limb, offering the possibility to restore the hand and elbow function in tetraplegic end-users using thoughts (Rohm et al., 2013, Rupp et al., 2015).
Current state-of-the-art non-invasive BCIs use electroencephalography (EEG) to exploit sensorimotor rhythms (SMR) associated with the imagination of movements (Müller-Putz et al., 2016). Despite the developments of the past few years, SMR-based BCIs still lack natural and intuitive control for two main reasons. First, they solely rely on detecting the limb (usually both feet, right hand, left hand) subjected to the movement imagination (MI) (Müller-Putz et al., 2016), resulting in a low number of classes. The limited number of classes do not allow for a natural control since it can happen that a repetitive foot MI is assigned to a particular neuroprosthesis movement such as a hand-open command. This fails to reproduce the natural way in which one plans a movement. Second, the delay between the movement intention detection and the actual user’s intention is in the order of seconds (Müller-Putz et al., 2010), which is not short enough for the Hebbian principle to be applied (Mrachacz-Kersting et al., 2012). Reducing the temporal gap between the user’s intention and the feedback provided (e.g. electrical stimulation; robotic devices control) is fundamental, not just for fast and natural motor control, but also because it has the potential to promote motor recovery at the cortical level, inducing neural plasticity (Mrachacz-Kersting et al., 2012, Murphy and Corbett, 2009, Niazi et al., 2012). This last point is particularly important in robot-assisted therapy for stroke patients (Muralidharan et al., 2011).
To overcome these limitations, the BCI should be able to decode the way in which one plans and performs an action, providing timely and accurate feedback about the user’s intentions. This would be possible if the BCI could decode both the goal of the intended action and the movement characteristics (e.g. speed, force). Ideally, decoding at the goal level - describing the short-term goals necessary to achieve a certain action - and at the kinematic level - describing the arm kinematics (in space and time) - would be combined to achieve optimal and intuitive control (Müller-Putz et al., 2016). This two-level strategy was proposed by Grafton and Hamilton (Grafton and Hamilton, 2007) and describes the mechanism behind goal-directed actions, which are of great importance for BCI control since they imply interactions with targets.
Moreover, as an alternative to the power modulations in different frequency bands used in the SMR-based BCIs, time-domain amplitude modulations in the delta band can be used. These slow fluctuations, when associated with a motor task, are known as movement-related cortical potentials (MRCPs) and are EEG neural correlates of movement planning and execution (Birbaumer et al., 1990) which have been used for movement detection (Bhagat et al., 2016, Bhagat et al., 2014, Jochumsen et al., 2015a, Jochumsen et al., 2015b, Jochumsen et al., 2013, Kamavuako et al., 2015, Lew et al., 2012, López-Larraz et al., 2014, Xu et al., 2015, Xu et al., 2014). Concretely, the MRCP is characterized by a slow negative deflection before movement execution (ME), imagination (MI) or attempted ME, reaching the maximum negativity near the onset, which is followed by a positive rebound before returning to the baseline level (Jahanshahi and Hallett, 2003). MRCPs can provide a multifaceted and rich motor control signal for two main reasons. First, movement intention detection through MRCPs has been shown to have relatively short latencies, reducing the time between the actual intention and the system response. Second, for upper limb movements, the MRCP magnitude and slope are known to be modulated by movement-related parameters, like speed (Gu et al., 2009b, Gu et al., 2009c) and force (Jochumsen et al., 2013), as well as allowing for the discrimination of movement directions and trajectories (Bradberry et al., 2010, Ofner and Müller-Putz, 2015, Ofner and Müller-Putz, 2012); grasp types (Jochumsen et al., 2015a) or other movements of the upper limb (Vuckovic and Sepulveda, 2008). Since MRCPs reflect the cortical processes employed in movement planning and are known to be modulated by several movement-related parameters, it would be of interest to investigate whether the presence of a specific movement goal is also reflected in this neural correlate. If the presence of a goal is indeed reflected in the MRCPs, then one can ask which impact does this information have in movement detection for BCI control.
Most of the studies which investigate goal-directed actions are not in the context of BCIs. Instead, they try to find evidence for a mirror neuron network in humans and often involve movement observation tasks and techniques with higher spatial resolution than EEG, like functional magnetic resonance imaging (fMRI). Buccino et al. used fMRI to demonstrate that, during the observation of goal-directed actions, subjects showed greater activation in the posterior parietal cortex (PPC) than during the observation of the same actions without a goal (Buccino et al., 2001). Since action observation and action execution share similar neural correlates, the hypothesis is that the execution of goal-directed movements leads to different and more salient neural responses than equivalent, but non-goal-directed movements. In fact, differences have been found in EEG oscillations in movement observation (Muthukumaraswamy et al., 2004), imagination (Yong and Menon, 2015) and execution (Pereira et al., 2015) tasks. In these studies mu rhythm suppression was stronger in the goal-directed conditions. In consistency with previous electrocorticography (ECoG) studies (Caplan et al., 2003, Ekstrom et al., 2005), a magnetoencephalography (MEG) study additionally showed an increased theta power (4–8 Hz) in the hippocampus during a goal-directed navigation task (Cornwell et al., 2008).
Inspired by these studies, but having the ultimate goal of a natural and reliable BCI control in mind, we used EEG to investigate the neural correlates behind goal-directed movements in a reach-and-touch execution task. Due to the aforementioned MRCPs properties, we investigated the MRCPs during goal-directed and non-goal-directed movements. To our knowledge, these differences were never directly assessed with EEG and in BCI studies. We exploited the differences found in MRCPs between goal and non-goal-directed movements to successfully show that the detection of movement intention is improved every time a goal-directed movement is planned and executed. Our results suggest that goal-directed strategies can have a positive impact on future BCIs and neurorehabilitation in which an early and reliable detection of movement intention is fundamental for motor recovery of stroke and spinal cord injured patients.
Materials and methods
Participants
Ten healthy participants (age 26.3±5.4 years, 5 male) took part in this study. The study has been carried out in accordance with the Declaration of Helsinki and subjects gave their informed consent. Subjects had normal or corrected-to-normal vision and no history of neurological or psychiatric disorders. Subjects’ right-handedness was assessed by the Hand-Dominanz Test (Steingrüber and Lienert, 1971), which showed that all participants were right-hand dominant. Subjects sat in a comfortable chair, in a darkened and shielded room, facing the computer monitor that displayed the trial-based paradigm.
Conditions and paradigm
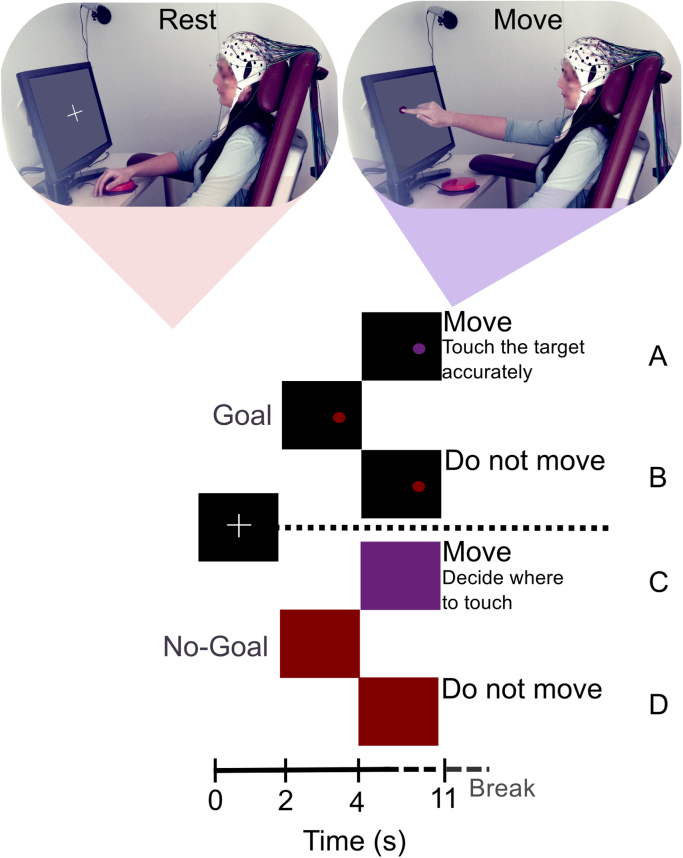
Subjects used their right index finger to perform a reach-and-touch task. Their left forearm was supported by an armrest. The right forearm was supported by the armrest and on a table of the same height. While at rest, the participants were asked to keep their hand still and relaxed, fully supported by a Joggle Switch button (Traxys Input Products, London, UK), which will be referred to as the home position. The body posture of the subjects during the experiment is exemplified in Fig. 1 (both at rest and during the reach-and-touch task).
Fig. 1.
Diagram of a trial and the four possible conditions. Each trial consisted of a two-second fixation cross, followed by one of the four conditions: (A) Goal Movement; (B) Goal No-Movement; (C) No-Goal Movement and (D) No-Goal No-Movement. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Participants performed four different conditions which were shown in a randomly alternated order but with the same frequency (i.e. 25% each). The four conditions in the study were: Goal Movement (GM); No-Goal Movement (NGM); Goal No-Movement (GNM) and No-Goal No-Movement (NGNM). A total of 72 trials were recorded per condition, separated into 12 runs. Trials were separated by breaks with a random duration between 1 and 2 s. Each run had a duration of 290 to 314 s and breaks between runs had a variable duration, depending on the subject.
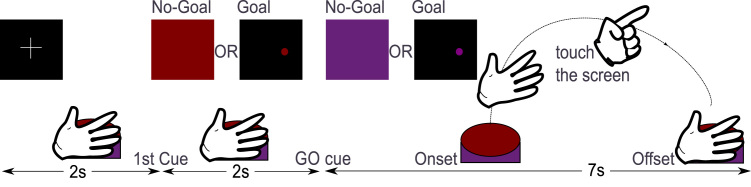
Each trial had a duration of 11 s and began with a two-second fixation cross, followed by one of the cues depicted in Fig. 1. At second 2, the subject observed either a Goal (small red ball in the black screen) or a red screen, which represented the No-Goal conditions. The ball position in the Goal conditions was changed randomly between trials. At second 4, in 50% of the trials, the color of the visual stimulus changed to purple (GO cue) instructing the subjects to perform a reach-and-touch movement with their right index finger (see Fig. 2).
Fig. 2.
Movement conditions. The change of color (GO cue) instructed the subjects to initiate the reach-and-touch task. Subjects touched the target as accurately as possible in the Goal Movement condition and decided where to touch in the No-Goal Movement condition. After touching the screen, they were instructed to move back to the home position. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
In case of the Goal Movement condition, the subjects were instructed to accurately touch the target. In case of the No-Goal Movement condition, subjects decided freely where to touch the screen. In both Movement conditions, subjects were instructed to start the movement as relaxed as possible and to touch the screen briefly. No further instructions were given in terms of kinematics (e.g. movement duration or movement trajectories). In this way, we ensured that participants’ movements were relatively natural. We checked the movement-related parameters by comparing the movement onsets and offsets recorded with the switch button, as well as by recording the touched positions and respective timing using the touchscreen monitor capabilities. Movements were observed by the experimenter during all measurements.
If there was no change in color, the subjects were instructed to remain at rest in the home position (Goal No-Movement and No-Goal No-Movement conditions). While at rest, we instructed the subjects to relax their hands, forearms, elbows and arms and to avoid inducing any other muscular tension that could affect the outcome of the experiment. They were also instructed to minimize eye movements and blinking during the trials. Three resting state runs were recorded, each lasting for 70s, in which subjects were instructed to remain at rest and maintain their gaze on the fixation cross displayed on the screen. These resting runs were used for source imaging.
EEG recordings
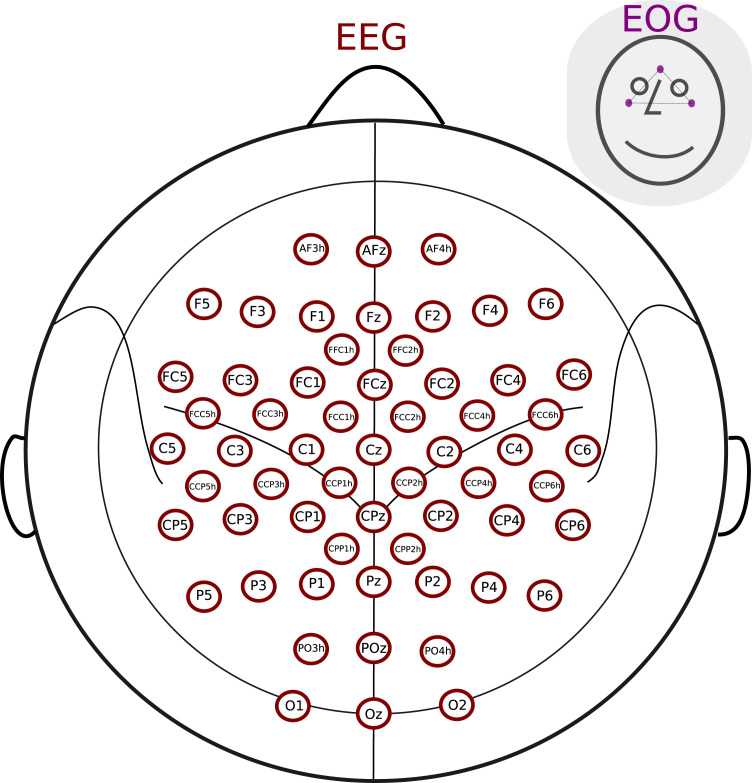
EEG signals were recorded via 60 passive Ag/AgCl electrodes covering frontal, sensorimotor, parietal and occipital areas (see Fig. 3). Three electrooculography (EOG) electrodes were used to monitor horizontal and vertical eye movements. These electrodes were positioned above the nasion and below the outer canthi of the eyes and as close to the eyes as possible to minimize the influence of non-EOG components, as shown in Fig. 3. The reference electrode was placed on the left earlobe and the ground on the right earlobe. The position of the EEG electrodes on the subject’s head was recorded before the start of the EEG measurements using ELPOS ultrasound-based system (Zebris Medical GmbH, Isny im Allgäu, Germany). We used the participants’ individual electrode positions in 3D coordinates for source imaging.
Fig. 3.
Electrode positions. Positions marked correspond to the sixty electrode sites used for the study, spread over frontal, sensorimotor, parietal and occipital areas symmetrically covering the head. EOG electrode positions: three EOG electrodes were positioned at the corners of a right-angled triangle.
We confirmed that the electrode impedances read less than 5 kOhm. The signals were sampled at 512 Hz using four commercial 16-channel amplifiers (g.tec, Graz, Austria). Signals were band-pass filtered from 0.01 Hz to 200 Hz with an 8th order Chebyshev filter and a notch filter with a null frequency of 50 Hz, to ensure rejection of the 50 Hz power supply frequency. Data was recorded in MATLAB R2012b and Simulink 8.0 (The MathWorks, Massachusetts, USA). TOBI SignalServer (Breitwieser et al., 2010) was used for data acquisition. The paradigm was displayed on a touchscreen monitor.
Behavioral analysis
We calculated the reaction time (RT) as the time when the participants left the home position with respect to the second cue (GO cue). We looked for temporal anticipation (RT <300 ms) and rejected those trials. We want to avoid temporal anticipation since it could evoke differences in movement planning and because we particularly instructed the subjects to initiate the movement as relaxed as possible. Moreover, we discarded trials where the subjects failed to follow the instructions (e.g. not touching the target in the Goal condition) or when the movement onset or touch position was not correctly determined due to a problem with the switch button.
We also analyzed the time points when participants touched the screen and finished the task (movement offset), both in relation to the movement onset. The time subjects took to reach and touch the target (i.e. touch relative to onset) is of particular importance, since previous studies showed that movement speed has an impact on the MRCP amplitude (Gu et al., 2009a, Gu et al., 2009b, Gu et al., 2009c). To control for this possible confound, we assessed whether there were significant differences in both touch and offset time points between Goal Movement and No-Goal Movement conditions using a paired t-test (α=0.05, Bonferroni corrected for multiple comparisons).
EEG processing
Offline processing of EEG and EOG data was performed using MATLAB R2012b (The MathWorks, Massachusetts, USA) with the BioSig toolbox (Schlögl and Brunner, 2008), the EEGLAB toolbox (Delorme and Makeig, 2004) and Brainstorm (Tadel et al., 2011).
We segmented the raw data and eliminated trials with incorrect responses from further data processing. All the remaining trials were visually inspected for corruption due to extreme amplitude values or abnormal trends (i.e. linear drifts that were present due to artifacts). Corrupted trials were removed from further analysis. We additionally removed noisy channels, leaving an average of 57 channels and 264 trials per subject. Thereafter we applied principal component analysis (PCA) for dimensionality reduction on the EEG data of all runs and conditions and retained components which explained 99% of the variance of the data. Subsequently, we performed independent component analysis (ICA) (Makeig et al., 1996) on the PCA-compressed data using the extended Infomax algorithm (Lee et al., 1999) implemented in the EEGLAB toolbox. We rejected the independent components which corresponded to muscle or eye movement and blink artifacts using visual inspection. Finally, we reconstructed the cleaned EEG by multiplying the non-artefactual components by the mixing matrix computed with the ICA algorithm.
Movement-related cortical potentials
MRCPs were analyzed as EEG neural correlates of movement intention. Runs were common average referenced and bandpass filtered from 0.3 to 3 Hz with a 4th order zero-phase Butterworth filter. Next, we time-locked the trials to the movement onset in the case of the Movement conditions and computed the grand-averaged MRCPs for each channel.
Feature extraction and classification
We performed single-trial offline classification between conditions on the MRCPs amplitude features. To extract the relevant MRCPs amplitude features, we applied a zero-phase anti-aliasing filter and downsampled the data to 16 Hz to reduce computational effort. Then, data were processed as described in Section - Movement-related cortical potentials.
We epoched the Movement conditions data from −4 to 4 s relative to the movement onset (second zero). For a fair comparison with the Movement conditions, we time-locked the No-Movement conditions to a “virtual onset”. We created this virtual onset by using the real onsets recorded by the switch button in the two Movement conditions. For each subject, we calculated the mean of the recorded onsets of both movement conditions and used those values to time-lock the No-Movement trials.
After extracting the MRCPs amplitude features according to the method described above, we classified the EEG with a two-class shrinkage regularized linear discriminant analysis (sLDA) (Blankertz et al., 2011). The sets of signal features were divided in training and testing: a classifier was trained for each time point on the training set and tested using the trials from the testing set on that same time point. All channels were used for classification, resulting in an average feature matrix size of 57 channels x 135 trials. To evaluate the classification we applied a 5x10 fold cross-validation and obtained a classification accuracy for each cross-validation fold at each time point. The accuracies reported correspond to the average over all cross-validation folds. We used 6 different binary classifiers since we had four different conditions and were interested in studying all 6 possible task pairs, which are described in Table 1.
Table 1.
Conditions and task pairs.
| Movement | Goal Movement vs. No-Goal Movement |
| No-Movement | Goal No-Movement vs. No-Goal No-Movement |
| Movement vs. No-Movement | Goal Movement vs. Goal No-Movement |
| Goal Movement vs. No-Goal No-Movement | |
| No-Goal Movement vs. Goal No-Movement | |
| No-Goal Movement vs. No-Goal No-Movement |
Discriminative spatial patterns
We were also interested in analyzing the sources used by the classifiers - i.e. the discriminative spatial patterns (DSPs). To obtain these DSPs we used the approach described by Liao et al. (2007). The DSP algorithm described by Liao et. al can be seen as a generalized eigenvalue problem, which solution is a set of eigenvectors and eigenvalues. Eigenvectors correspond to spatial filters, and the discriminative power of the filters is indicated by the associated eigenvalues. Because we use only one sample from each electrode, this method is in fact equivalent to the LDA problem: when we solve the problem for the eigenvector corresponding to the largest eigenvalue, we obtain the LDA weight vector. What DSP does is to solve the generalized eigenvalue problem for all the remaining eigenvectors, obtaining not a weight vector (like in LDA) but a weight matrix. When we sort this weight matrix by the eigenvalue, the first column of the DSP is in fact the LDA solution. The DSP is then obtained by inverting the weight matrix.
The projection of EEG channels, at each time point, in the LDA space can be written as:
| (1) |
where is the vector of EEG amplitudes at each channel (i.e. dimension is [channels x 1]) and is the weight matrix obtained by solving the generalized eigenvalue problem for all the eigenvectors.
As described in Liao et. al, the DSP corresponds to the inverse of the weight matrix, . The pattern corresponding to the LDA weights is then . This pattern shows the impact of each source (i.e. specific brain area) on each channel and is unitless. We want to have a pattern which has the same physical unit as the original channel space (i.e. Volt). In our classification, we have two classes in the channel space projected into an one dimensional LDA space, so we used the distance between the two class means (Goal Movement and No-Goal Movement) in the LDA space as a scaling factor for the LDA pattern:
| (2) |
Pattern corresponds to the differences between the two class means ( and ) which are being exploited by the LDA classifier, taking into account the covariance structure of the channels.
For each time point, we computed the absolute values to finally determine the spatial pattern corresponding to each classifier. We calculated the DSPs for the Goal Movement vs No-Goal Movement task pair, since we specifically wanted to investigate which brain areas were involved in the discrimination of these two movement conditions. We obtained a pattern per time point for each subject. We selected a time window of interest, −437 to 1313 ms, based on the classification results (i.e. accuracies above chance level) obtained for this task pair classification. We calculated the patterns in this time window relative to the movement onset and averaged the patterns per subject in non-overlapping 125 ms segments. We only then transformed each pattern from the channel space into the source space.
To estimate the brain sources associated to each DSP, we used the Brainstorm toolbox (Tadel et al., 2011). We computed boundary element head models using the Colin27 brain model template included in Brainstorm and the subject individual EEG electrode positions. The registration between the template and the digitized EEG electrode positions was done using the Brainstorm toolbox automatic alignment algorithm based on the three fiducial points (nasion, left ear and right ear) and a further iterative algorithm which finds a better fit between the two head shapes, using the remaining head points to improve the initial registration. We visually inspected this alignment.
We estimated full noise covariance matrices with shrinkage regularization (Schafer and Strimmer, 2005), based on the three resting runs which were preprocessed as described previously. Subsequently, we computed 5001 brain sources using standardized low-resolution brain electromagnetic tomography (sLORETA) (Pascual-Marqui, 2002) with dipole orientations normally constrained to the cortex. We determined the significantly different voxels in respect to a baseline, from –3.75 to −3.50 s relative to the movement onset using a paired t-test. Bonferroni correction was applied for the number of voxels and time points to the significance level of 0.05.
Results
Behavioral analysis
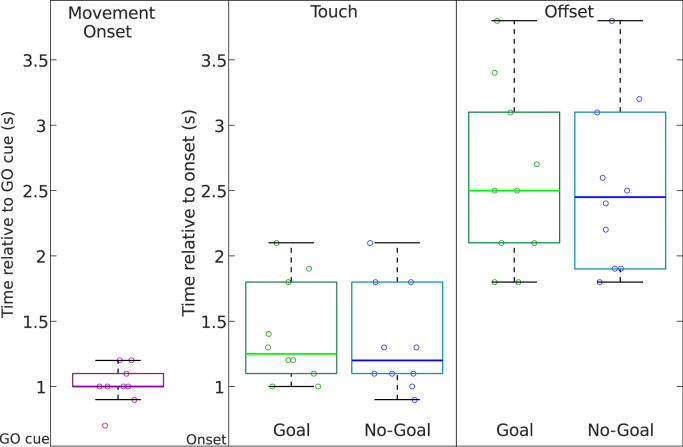
As a summary, Fig. 4 shows the boxplots correspondent to the reaction times (RTs) relative to the GO cue, as well as the times relative to the movement onset when the participants touched the screen and completed the movement (movement offset). We found no statistically significant differences between movement conditions (Goal and No-Goal) for the touch and offset. The grand-average RT in respect to the GO cue was 1.0 s (±0.2 standard deviation). Fig. 4 does not include the trials that were rejected due to temporal anticipation, which corresponded to an average of 1.8 trials per subject.
Fig. 4.
Behavioral analysis. We show the boxplots correspondent to the movement onset, as well as the touch and movement offset which are represented independently for each condition (Goal Movement and No-Goal Movement).
Movement-related cortical potentials
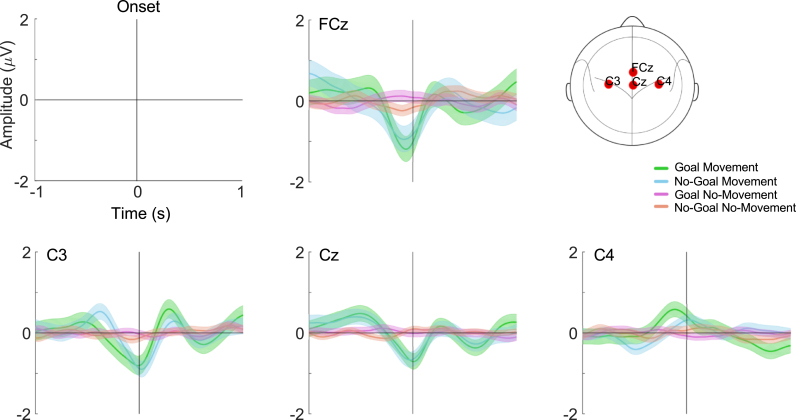
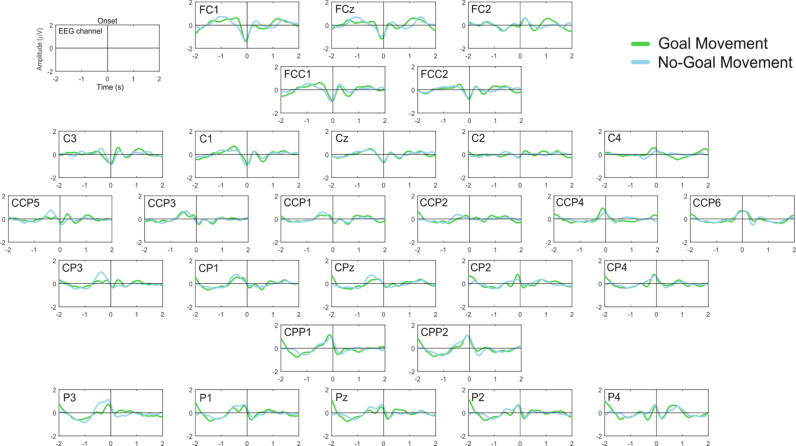
MRCPs were observed as a negative deflection (decrease in amplitude) in the activity recorded over the motor cortex, which started around 400 ms prior to the movement onset. Fig. 5 shows the grand-average MRCP over electrodes FCz, C3, Cz and C4 for the two Movement conditions as well as the No-Movement conditions, as a control. The MRCPs over the electrodes covering sensorimotor areas are in the Appendix (Fig. 1).
Fig. 5.
Grand-average MRCPs over electrodes FCz, C3, Cz and C4 with respect to movement onset. We show the 95% confidence interval for the mean. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
In the Movement conditions, the peak of maximal negativity was more pronounced in the Goal condition than in the No-Goal condition. After the negative deflection there is a rebound, also known as reafferent potential, which is stronger in the Goal condition. Generally, and as expected, the amplitude of the MRCP was stronger in the contralateral and central motor areas.
Single-trial offline classification
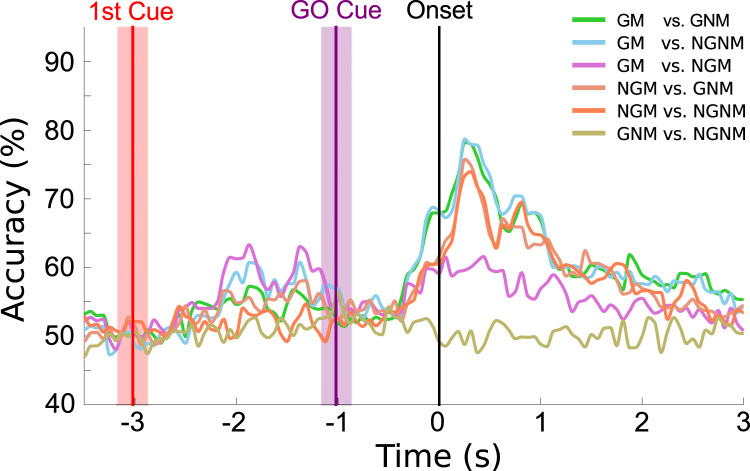
Fig. 6 shows the grand-average single-trial classification accuracies for the six different task pairs over time and Fig. 7 additionally shows the single-trial classification for each subject, separated per task pair. We calculated the classification accuracies in a time window from -4 to 4s, relative to the movement onset (second zero). The theoretical chance level is 50% and the threshold at a significance level, α=0.05, Bonferroni corrected for window length, is 64%. We calculated this threshold using the adjusted Wald interval (Müller-Putz et al., 2008, Billinger et al., 2012) and the average number of trials used for classification (i.e. 132).
Fig. 6.
Grand-average single-trial classification accuracies over time for all the 6 task pairs. The red vertical line corresponds to the average time point where the first cue was shown (± standard deviation). The purple vertical line corresponds to the average time point of the GO cue. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
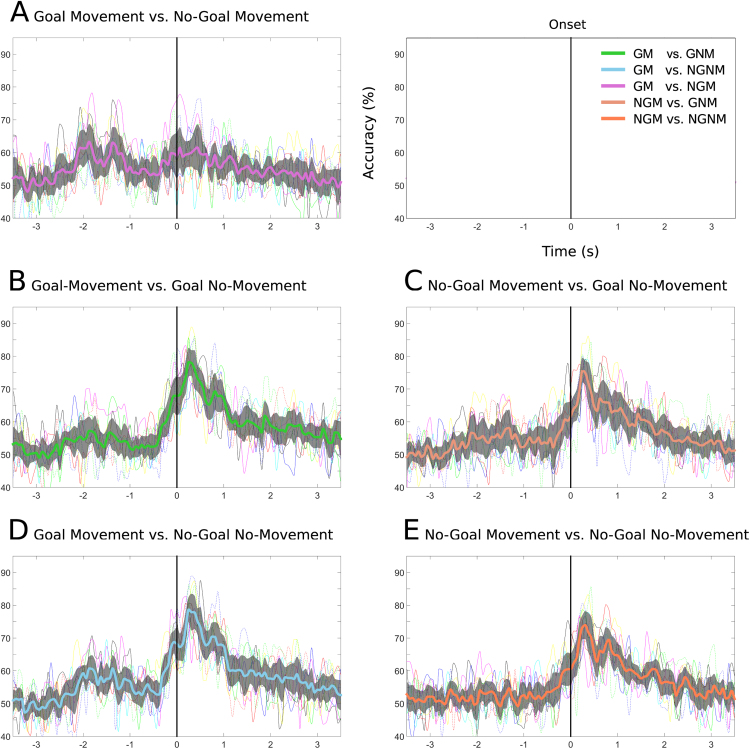
Fig. 7.
Single-trial classification accuracies over time. The thick colored lines (with the same color coding as in Fig. 6) correspond to the grand-average accuracies for the different task pairs. The 95% confidence interval for the mean is shadowed in grey. The thin colored lines correspond to the accuracies of each of the 10 subjects. The chance level for the individual accuracies is 64% (α = 0.05). In (A) we show the accuracies for Goal Movement vs. No-Goal Movement task pairs. (B),(C),(D),(E) shows the accuracies for the Movement vs. No-Movement task pairs. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
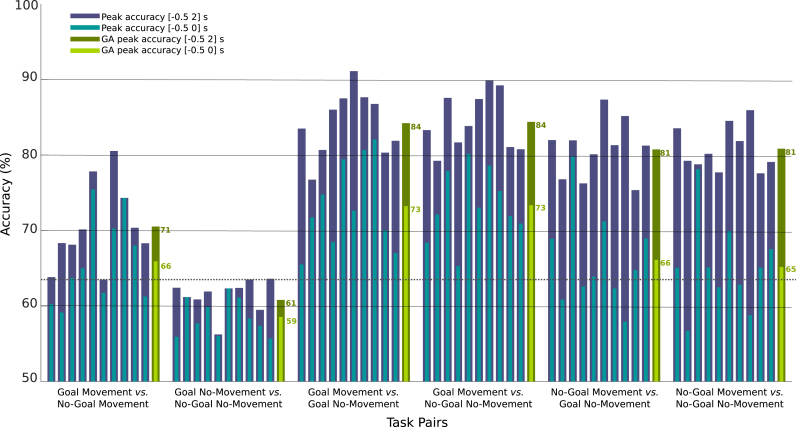
The peak average accuracies for each task pair are reported in Fig. 8. We determined the peak accuracy by finding the maximum accuracy over a time-window of −0.5 to 2 s. The peak accuracy is reached at different time points dependent on the subject (see Fig. 7). The maximum accuracies before the movement onset were evaluated in a time window from −0.5 to 0 s. These results are also presented in Fig. 8.
Fig. 8.
Peak accuracies in % relative to the six task pair comparisons. The average peak accuracies per subject over an interval of -0.5 to 0 seconds are plotted in light blue. The accuracies over a larger interval, from -0.5 to 2 seconds are plotted in dark blue bars. Grand-average peak accuracies per task pair are in light green for the [−0.5 0] s interval and in dark green for the [−0.5 2] s interval. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
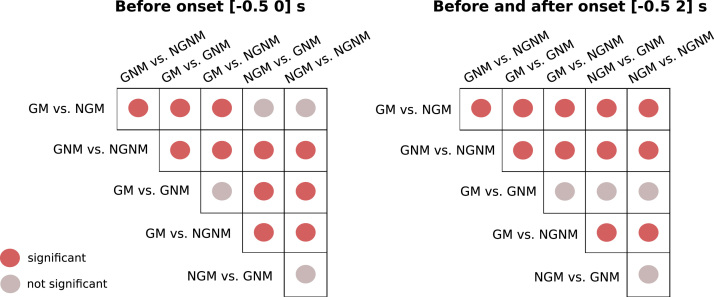
Statistically significant differences between the accuracies of the task pairs were assessed using paired t-tests (α=0.05). The results for all pairs comparisons are in Fig. 9, where statistically significant differences between the pairs are marked in red.
Fig. 9.
Statistically significant differences between the task pairs’ accuracies assessed using paired t-tests (α=0.05), for the [−0.5 0] s interval (left table) and for the [−0.5 2] s interval (right table). (For interpretation of the references to color in this figure, the reader is referred to the web version of this article.)
Goal vs. No-Goal
For the Movement conditions, the Goal vs. No-Goal grand-average classification accuracy over all subjects reached a maximum of 62% at movement onset, rising above the chance level at −336 ms and remaining significant until 1.3 s after movement onset (Fig. 6). The average peak accuracy was 71% (±5% std). Nine out of 10 subjects reached a significant peak accuracy, ranging from 65 to 81% after movement onset (Fig. 8). From −500 ms to movement onset, only 5 of the 10 subjects had accuracies above chance level and an average peak accuracy of 70% (±4%) was reached, ranging from 65 to 75% (Fig. 8). For the No-Movement conditions, the Goal vs. No-Goal grand-average classification accuracies were at chance level as were average peak accuracies (Figs. 6 and 8).
Movement detection
Movement detection was analyzed for the 4 Movement vs. No-Movement task pairs (see Table 1). For the Goal Movement task pairs (i.e. Goal Movement vs. Goal No-Movement / Goal Movement vs. No-Goal No-Movement), the grand-average classification accuracy over all subjects reached a maximum of 79/79% at 297/281 ms after movement onset, rising above the chance level at −375/−328 ms and staying significant until 3.2/3.0 s after movement onset (Figs. 7B and D, respectively). The average peak accuracy was 84(±4)/84%(±4). All subjects reached a significant peak accuracy, ranging from 77–91/79–90% after movement onset (Fig. 8). From –500 ms to movement onset, all subjects were above the chance level and an average peak accuracy of 73(±6)/73%(±5) was reached, ranging from 65–82/65–80% (Fig. 8).
For the No-Goal Movement task pairs (i.e. No-Goal Movement vs. Goal No-Movement / No-Goal Movement vs. No-Goal No-Movement), the grand-average classification accuracy over all subjects reached a maximum of 76/75% at 273/281 ms after movement onset, rising above chance level at −281/−297 ms and staying significant until 2.2/2.2 s after movement onset (Figs. 7C and E, respectively). The average peak accuracy was 81(±4)/81%(±3). All subjects reached significant classification accuracies, ranging from 75–87/78–86% after movement onset (Fig. 8). From -500 ms to movement onset, only 6 subjects had accuracies above chance level and an average peak accuracy of 71(±6)/68%(±5) was obtained, ranging from 65–80/65–78% (Fig. 8).
In the task pairs where the Goal Movement condition was present, the classification accuracies were higher than in the other two No-Goal Movement conditions in 8 out of 10 subjects. These differences are also evident before the movement onset. Fig. 9 shows that there are no statistically significant differences between the two Goal Movement task pairs before and after movement onset, however they do show statistically significant differences to both No-Goal Movement task pairs before movement onset. These differences implied that in 4 of the 10 subjects, movements were only detected before the onset if the Goal Movement condition was present. Good examples are subjects s5, s7 and s8, where a difference of 16, 19 and 23% in average peak accuracies before onset are registered when compared with the No-Goal Movement task pairs peak accuracies.
Discriminative spatial patterns
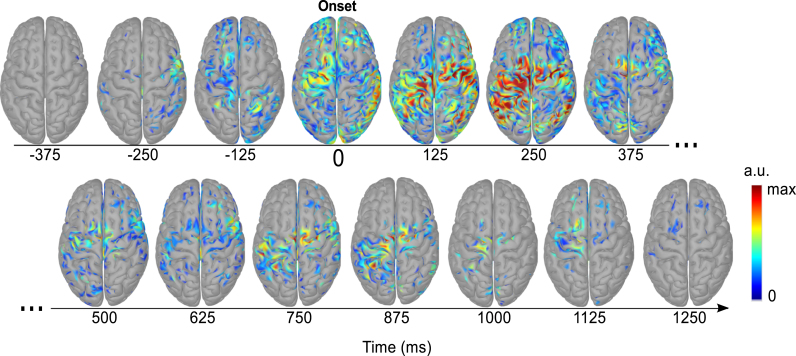
Fig. 10 shows the discriminantive spatial patterns sources on the cortex surface in a time window from −437 to 1313 ms in respect to movement onset for the Goal Movement vs No-Goal Movement task pair. This allowed us to investigate the DSPs within the window where accuracies above the chance level were reached (see Section - Goal vs. No-Goal). The patterns were averaged in 125 ms segments. In Fig. 10 we show the grand-average over the 10 subjects and only plot voxels which are significantly different from the baseline (paired t-test, α=0.05 Bonferroni corrected for the number of voxels and time points).
Fig. 10.
Discriminative spatial patterns in the source space relative to movement onset for the Goal Movement vs No-Goal Movement task pair. The patterns were averaged in 125 ms time segments (middle time point of the averaged window is indicated here). The sources correspond to the grand-average over the 10 subjects and only voxels which are significantly different from the baseline (paired t-test, p<0.05) are shown. The values are unitless (a.u.) since sLORETA yields standardized brain sources - blue corresponds to zero and red to the maximum value. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Before movement onset, the largest contributions come first from the ipsilateral inferior frontal cortex and then from the supplementary motor area (SMA), premotor cortex (PM) and superior parietal lobule. At movement onset, the patterns are widely spread over the right parietal lobule and premotor areas (both hemispheres). After movement onset these contributions expand also to the primary motor cortex (M1). From 500 ms and onwards, the information is more restricted to M1 and left PPC.
Discussion
In this paper we use low-frequency time-domain EEG signals to show for the first time the differences between goal-directed and non-goal-directed movements. We analyzed the movement-related cortical potentials associated with the Goal and No-Goal Movement conditions, which both required the execution of a reach-and-touch task with the same kinematics. We then performed single-trial offline classification to exploit the features associated to this particular EEG neural correlate of movement intention. Our classification results show that the detection of movement intention was higher when the movement was directed towards a goal. Furthermore, we show that the differences our classifier is exploiting come from premotor, primary motor areas and additionally the posterior parietal cortex.
Movement-related cortical potentials
Concerning the MRCP neurophysiology analysis, the maximal negativity over the central electrodes was more pronounced in the Goal condition than in the No-Goal condition. Furthermore, the reafferent potential of the MRCP after movement onset was stronger in the Goal condition. This potential has been previously associated with the precision of movement (Shibasaki et al., 1981). The amplitude of the MRCP was higher for contralateral and central channels located over the sensorimotor cortex than for the rest of the channels.
Since the magnitude and time course of MRCP are influenced by characteristics of the movement performed - like movement speed - we performed a behavioral analysis to eliminate possible confounds. The reach-and-touch movement had the same duration for both movement conditions and we can infer that the differences between conditions were not due to different movement kinematics.
Single-trial offline classification
The differences observed on the MRCPs were exploited in an offline classification procedure. Here, we studied all possible task pairs (i.e. condition combinations) in a binary classification. First, we show that both Goal Movement and No-Goal Movement tasks are discriminable before movement onset in 5 of the 10 subjects and after onset in 9 out of 10. In fact, accuracies rise above chance level after the first cue, returning to chance level around the GO cue. After the GO cue, despite the fact that the subjects already knew whether there was a goal or not, the accuracies were again raised above the chance level. A plausible explanation is that goal-related and movement planning/execution processes are in fact highly dependent on each other (Grafton and Hamilton, 2007). For the Goal No-Movement vs. No-Goal No-Movement task pair, classification accuracies were at chance level. This was expected, since at this time point the subjects already knew whether there was a goal or not and did not have to perform any movement. Second, movement detection was possible in all subjects and all task pairs but the highest classification accuracies were obtained when the Goal Movement condition was executed. Before movement onset, the differences in classification accuracies were more pronounced, again being higher for the Goal Movement task pairs. Notably, movement intention detection was possible in all subjects for the Goal Movement task pairs, but in only 6 out of 10 subjects for the No-Goal Movement task pairs. These results are of particular interest for BCI control where an early and accurate detection of the intended action (i.e. earlier than the execution/imagery/attempt onset) is fundamental.
The performance of our classification is within the range of what has been reported in previous state-of-the-art studies on upper limb movement intention detection: ~65-76% (Bhagat et al., 2016, Bhagat et al., 2014, Jochumsen et al., 2015a, Jochumsen et al., 2013, Kamavuako et al., 2015, Lew et al., 2012). However, a direct comparison is difficult, since not only the tasks (self-paced/cue-based; ME/MI or attempted ME and different types of upper limb movements) but also the methodology used (features type and offline/online/simulated online classification procedures) and the participants (healthy/stroke/SCI patients) are different.
It is important to note that in our paradigm, all four conditions involved the presentation of cues and, for this reason, the No-Movement trials cannot be seen as a real rest condition, as was the case in most of the previous studies. This factor might have an influence on our overall accuracies, but not on the goal-directed vs. non-goal-directed tasks comparison which was the main target of our study. We would also like to point out that in this paradigm the cues were different between the Goal and the No-Goal conditions (i.e. color changes in a smaller or bigger area of the screen, respectively). Future paradigms should avoid such differences since they can be seen as possible confounds. However, it is unlikely that the differences observed among conditions were due to the different quality of the cues. We are time-locking the trials to movement onset, which happened in average 1 s after the GO cue and 3 s relative to the first cue. Our classifier is exploiting movement-related cortical potentials, observed later (with respect to visually evoked potentials associated with the stimuli presentation), and distributed over the motor cortex. This is further supported by the discriminative spatial patterns observed around movement onset.
A further important point is that we used a single, virtual and abstract target. We did this to reduce the complexity of the movement itself, since complicated tasks could introduce several variables to our experiment. Now that we have tested our hypothesis, we believe that future studies would benefit from the presentation of real objects. Furthermore, MRCP differences between goal-directed and non-goal-directed movements should be assessed in patients during attempted ME or even MI tasks.
Regarding the features, we exclusively used time features but recent studies show that features from the time-domain combined with spectral features improve movement intention detection (Jochumsen et al., 2015a, Sburlea et al., 2015).
In this study, we directly addressed the neural correlates of goal-directed movements in a reach-and-touch task as a starting point to find out if goal-directed strategies should be preferred in further BCI contexts. We observed that movements with a goal improve the detection of movement intention in a movement execution task. If such results can be replicated in MI or attempted ME tasks, subjects should be instructed to perform the kinesthetic MI associated with a particular object. Recently, Yong and Menon showed that, while exploiting band-power features, classification accuracies of motor imagery of elbow flexion and extension were improved if a goal was associated with the MI (Yong and Menon, 2015). The two MI tasks were different in kinematics rather than just goal-directedness and the possible effect of these confounds on the results makes its interpretation less straightforward. In our study, we were able to control for these confounds by choosing two simple ME tasks which involved the same movement, and exploited MRCPs to show that movement intention (i.e. before movement onset) is better discriminated in goal-directed movements. Such performance improvements can lead to a reliable and natural response of the BCI-controlled system (e.g. neuroprosthesis or robotic arm) to the user’s intention, aiding the recovery process through neuroplastic changes (Grosse-Wentrup et al., 2011, Niazi et al., 2012).
Discriminative spatial patterns
Using the discriminative spatial patterns and their source estimation we were able to determine the brain areas relevant for the discrimination between the Goal Movement and the No-Goal Movement conditions. Before movement onset, the largest contributions come first from the ipsilateral inferior frontal cortex and then from the SMA, PM and superior parietal lobule. These areas, part of the frontoparietal network, are cortical regions with motor functions which have been previously associated to the goals of actions (Rizzolatti et al., 2014, Saxe et al., 2004). They are also known to be actively involved in the integration of neural signals from different sensory inputs. Specifically, the parietal lobule has been associated with visuomotor transformations before and during complex movements such as reaching movements (Andersen et al., 1997, Rizzolatti et al., 2014, Vingerhoets, 2014). During both Goal Movement and No-Goal Movement conditions subjects performed a center-out reach-and-touch task. However, it is fair to assume that, for the Goal Movement condition, specific information about the spatial location of the target had to be integrated for motor planning. This is a plausible explanation for the involvement of the fronto-parietal network in discriminating between goal and non-goal-directed conditions in our experiment.
Peak accuracies were reached at movement onset. Here, the patterns show a broader activation in the right parietal lobule and premotor areas. After movement onset, these contributions also expand to M1. This is expected, since this area is known to be responsible for the implementation of spatial characteristics of movement and is typically active only shortly before and during movement execution (Kandel et al., 2000). From 500 ms and onwards, the patterns are more restricted to M1 and left PPC.
To conclude, not only premotor and primary motor areas but also the posterior parietal lobule contributed to the discrimination between the goal-directed and non-goal-directed movement tasks in our experiment. Our results are consistent with an fMRI study that showed greater activation in the PPC during goal-directed action observation, when comparing to non-goal-directed action observation (Buccino et al., 2001), bounding this area to decoding movement goals. More recently, neuroprosthesis control was possible using spike activity exclusively from the PPC in a tetraplegic patient (Aflalo et al., 2015). In this study, Aflalo et al. showed for the first time that imagination of goals, trajectories and types of movement could successfully be decoded from the PPC.
Conclusion
In this study, we show that goal-directed movements differ from non-goal-directed movements with the same kinematics. We detected these differences in low-frequency time-domain EEG signals and further employed them to improve movement intention classification accuracies. Moreover, we show that the differences our classifier is exploiting come not only from premotor and primary motor areas but also from the posterior parietal cortex, which has been previously associated with decoding movement goals. Overall, our results show which brain areas are involved in the discrimination between goal-directed and non-goal-directed movements and suggest goal-directedness as a paradigm factor that improves the detection of movement intention in naturally-controlled BCI devices.
Acknowledgments
Funding: This work was supported by the EU ICT Programme Project H2020-643955, MoreGrasp, and the ERC Consolidator Grant ERC-681231, Feel Your Reach.
The authors acknowledge the fruitful discussions with David Steyrl and Filip Melinščak.
Appendix
See Appendix Fig. A1.
Fig. A1.
Grand-average MRCPs for the electrodes covering sensorimotor areas.
References
- Aflalo T., Kellis S., Klaes C., Lee B., Shi Y., Pejsa K., Shanfield K., Hayes-Jackson S., Aisen M., Heck C., Liu C., Andersen R.A. Neurophysiology. Decoding motor imagery from the posterior parietal cortex of a tetraplegic human. Science. 2015;348:906–910. doi: 10.1126/science.aaa5417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen R.A., Snyder L.H., Bradley D.C., Xing J. Multimodal representation of space in the posterior parietal cortex and its use in planning movements. Annu. Rev. Neurosci. 1997;20:303–330. doi: 10.1146/annurev.neuro.20.1.303. [DOI] [PubMed] [Google Scholar]
- Bhagat, N.A., French, J., Venkatakrishnan, A., Yozbatiran, N., Francisco, G.E., O’Malley, M.K., Contreras-Vidal, J.L., 2014. . Detecting movement intent from scalp EEG in a novel upper limb robotic rehabilitation system for stroke, In: Conference Proceedings IEEE Eng. Med. Biol. Soc. IEEE, pp. 4127–4130. doi:10.1109/EMBC.2014.6944532 [DOI] [PMC free article] [PubMed]
- Bhagat N.A., Venkatakrishnan A., Abibullaev B., Artz E.J., Yozbatiran N., Blank A.A., French J., Karmonik C., Grossman R.G., O’Malley M.K., Francisco G.E., Contreras-Vidal J.L. Design and optimization of an EEG-based brain machine interface (BMI) to an upper-limb exoskeleton for stroke survivors. Front. Neurosci. 2016;10:122. doi: 10.3389/fnins.2016.00122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billinger M., Daly I., Kaiser V., Jin J., Allison B.Z., Müller-Putz G.R., Brunner C. Is It significant? Guidelines for Reporting BCI Performance. In: Allison B.Z., Dunne S., Leeb R., Del R. Millán J., Nijholt A., editors. Towards Practical Brain-Computer Interfaces, Biological and Medical Physics, Biomedical Engineering. Springer Berlin Heidelberg; 2012. pp. 333–354. [Google Scholar]
- Birbaumer N., Elbert T., Canavan A.G., Rockstroh B. Slow potentials of the cerebral cortex and behavior. Physiol. Rev. 1990;70:1–41. doi: 10.1152/physrev.1990.70.1.1. [DOI] [PubMed] [Google Scholar]
- Blankertz B., Lemm S., Treder M., Haufe S., Müller K.-R. Single-trial analysis and classification of ERP components - a tutorial. Neuroimage. 2011;56:814–825. doi: 10.1016/j.neuroimage.2010.06.048. [DOI] [PubMed] [Google Scholar]
- Bradberry T.J., Gentili R.J., Contreras-Vidal J.L. Reconstructing three-dimensional hand movements from noninvasive electroencephalographic signals. J. Neurosci. 2010;30:3432–3437. doi: 10.1523/JNEUROSCI.6107-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breitwieser, C., Kreilinger, A., Neuper, C., Müller-Putz, G.R., 2010. . The TOBI hybrid BCI - the data acquisition module. In: Proceedings of the First TOBI Workshop.
- Buccino G., Binkofski F., Fink G.R., Fadiga L., Fogassi L., Gallese V., Seitz R.J., Zilles K., Rizzolatti G., Freund H.J. Action observation activates premotor and parietal areas in a somatotopic manner: an fMRI study. Eur. J. Neurosci. 2001;13:400–404. [PubMed] [Google Scholar]
- Buccino G., Solodkin A., Small S.L. Functions of the mirror neuron system: implications for neurorehabilitation. Cogn. Behav. Neurol. 2006;19:55–63. doi: 10.1097/00146965-200603000-00007. [DOI] [PubMed] [Google Scholar]
- Caplan J.B., Madsen J.R., Schulze-Bonhage A., Aschenbrenner-Scheibe R., Newman E.L., Kahana M.J. Human θ oscillations related to sensorimotor integration and spatial learning. J. Neurosci. 2003;23:4726–4736. doi: 10.1523/JNEUROSCI.23-11-04726.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornwell B.R., Johnson L.L., Holroyd T., Carver F.W., Grillon C. Human hippocampal and parahippocampal theta during goal-directed spatial navigation predicts performance on a virtual Morris water maze. J. Neurosci. 2008;28:5983–5990. doi: 10.1523/JNEUROSCI.5001-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delorme A., Makeig S. EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J. Neurosci. Methods. 2004;134:9–21. doi: 10.1016/j.jneumeth.2003.10.009. [DOI] [PubMed] [Google Scholar]
- Ekstrom A.D., Caplan J.B., Ho E., Shattuck K., Fried I., Kahana M.J. Human hippocampal theta activity during virtual navigation. Hippocampus. 2005;15:881–889. doi: 10.1002/hipo.20109. [DOI] [PubMed] [Google Scholar]
- Grafton S.T., Hamilton A.F. de C. Evidence for a distributed hierarchy of action representation in the brain. Hum. Mov. Sci. 2007;26:590–616. doi: 10.1016/j.humov.2007.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosse-Wentrup M., Mattia D., Oweiss K. Using brain-computer interfaces to induce neural plasticity and restore function. J. Neural Eng. 2011;8:025004. doi: 10.1088/1741-2560/8/2/025004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu Y., do Nascimento O.F., Lucas M.-F., Farina D. Identification of task parameters from movement-related cortical potentials. Med. Biol. Eng. Comput. 2009;47:1257–1264. doi: 10.1007/s11517-009-0523-3. [DOI] [PubMed] [Google Scholar]
- Gu Y., Dremstrup K., Farina D. Single-trial discrimination of type and speed of wrist movements from EEG recordings. Clin. Neurophysiol. 2009;120:1596–1600. doi: 10.1016/j.clinph.2009.05.006. [DOI] [PubMed] [Google Scholar]
- Gu Y., Farina D., Murguialday A.R., Dremstrup K., Montoya P., Birbaumer N. Offline identification of imagined speed of wrist movements in paralyzed ALS patients from single-trial EEG. Front. Neurosci. 2009;3:62. doi: 10.3389/neuro.20.003.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahanshahi M., Hallett M. Kluwer Academic/Plenum Publishers; New York: 2003. The Bereitschaftspotential: Movement-related Cortical Potentials. [Google Scholar]
- Jochumsen M., Niazi I.K., Dremstrup K., Kamavuako E.N. Detecting and classifying three different hand movement types through electroencephalography recordings for neurorehabilitation. Med. Biol. Eng. Comput. 2015 doi: 10.1007/s11517-015-1421-5. [DOI] [PubMed] [Google Scholar]
- Jochumsen M., Niazi I.K., Taylor D., Farina D., Dremstrup K. Detecting and classifying movement-related cortical potentials associated with hand movements in healthy subjects and stroke patients from single-electrode, single-trial EEG. J. Neural Eng. 2015;12:056013. doi: 10.1088/1741-2560/12/5/056013. [DOI] [PubMed] [Google Scholar]
- Jochumsen M., Niazi I.K., Mrachacz-Kersting N., Farina D., Dremstrup K. Detection and classification of movement-related cortical potentials associated with task force and speed. J. Neural Eng. 2013;10:056015. doi: 10.1088/1741-2560/10/5/056015. [DOI] [PubMed] [Google Scholar]
- Kamavuako E.N., Jochumsen M., Niazi I.K., Dremstrup K. Comparison of features for movement prediction from single-trial movement-related cortical potentials in healthy subjects and stroke patients. Comput. Intell. Neurosci. 2015;2015:858015. doi: 10.1155/2015/858015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandel E.R., Schwartz J.H., Jessell T.M., Siegelbaum S.A., Hudspeth A.J. McGraw-hill; New York: 2000. Principles of Neural Science. [Google Scholar]
- Lee T.W., Girolami M., Sejnowski T.J. Independent component analysis using an extended infomax algorithm for mixed subgaussian and supergaussian sources. Neural Comput. 1999;11:417–441. doi: 10.1162/089976699300016719. [DOI] [PubMed] [Google Scholar]
- Lew E., Chavarriaga R., Silvoni S., Del R., Millán J. Detection of self-paced reaching movement intention from EEG signals. Front. Neuroeng. 2012;5:13. doi: 10.3389/fneng.2012.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao X., Yao D., Wu D., Li C. Combining spatial filters for the classification of single-trial EEG in a finger movement task. IEEE Trans. Biomed. Eng. 2007;54:821–831. doi: 10.1109/TBME.2006.889206. [DOI] [PubMed] [Google Scholar]
- López-Larraz E., Montesano L., Gil-Agudo Á., Minguez J. Continuous decoding of movement intention of upper limb self-initiated analytic movements from pre-movement EEG correlates. J. Neuroeng. Rehabil. 2014;11:153. doi: 10.1186/1743-0003-11-153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makeig S., Bell A.J., Jung T.-P., Sejnowski T.J. Independent component analysis of electroencephalographic data. Adv. Neural Inf. Process. Syst. 1996:145–151. [Google Scholar]
- Mrachacz-Kersting N., Kristensen S.R., Niazi I.K., Farina D. Precise temporal association between cortical potentials evoked by motor imagination and afference induces cortical plasticity. J. Physiol. 2012;590:1669–1682. doi: 10.1113/jphysiol.2011.222851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller-Putz G.R., Kaiser V., Solis-Escalante T., Pfurtscheller G. Fast set-up asynchronous brain-switch based on detection of foot motor imagery in 1-channel EEG. Med. Biol. Eng. Comput. 2010;48:229–233. doi: 10.1007/s11517-009-0572-7. [DOI] [PubMed] [Google Scholar]
- Müller-Putz G.R., Scherer R., Brunner C., Leeb R., Pfurtscheller G. Better than random: a closer look on BCI results. Int. J. Bioelectromagn. 2008;10:52–55. [Google Scholar]
- Müller-Putz G.R., Scherer R., Pfurtscheller G., Rupp R. EEG-based neuroprosthesis control: a step towards clinical practice. Neurosci. Lett. 2005;382:169–174. doi: 10.1016/j.neulet.2005.03.021. [DOI] [PubMed] [Google Scholar]
- Müller-Putz G.R., Schwarz A., Pereira J., Ofner P. From classic motor imagery to complex movement intention decoding: the noninvasive Graz-BCI approach. Prog. Brain Res. 2016 doi: 10.1016/bs.pbr.2016.04.017. [DOI] [PubMed] [Google Scholar]
- Muralidharan A., Chae J., Taylor D.M. Extracting attempted hand movements from EEGs in people with complete hand paralysis following stroke. Front. Neurosci. 2011;5:39. doi: 10.3389/fnins.2011.00039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy T.H., Corbett D. Plasticity during stroke recovery: from synapse to behaviour. Nat. Rev. Neurosci. 2009;10:861–872. doi: 10.1038/nrn2735. [DOI] [PubMed] [Google Scholar]
- Muthukumaraswamy S.D., Johnson B.W., McNair N.A. Mu rhythm modulation during observation of an object-directed grasp. Cogn. Brain Res. 2004;19:195–201. doi: 10.1016/j.cogbrainres.2003.12.001. [DOI] [PubMed] [Google Scholar]
- Niazi I.K., Mrachacz-Kersting N., Jiang N., Dremstrup K., Farina D. Peripheral electrical stimulation triggered by self-paced detection of motor intention enhances motor evoked potentials. IEEE Trans. Neural Syst. Rehabil. Eng. 2012;20:595–604. doi: 10.1109/TNSRE.2012.2194309. [DOI] [PubMed] [Google Scholar]
- Ofner P., Müller-Putz G.R. Using a noninvasive decoding method to classify rhythmic movement imaginations of the arm in two planes. IEEE Trans. Biomed. Eng. 2015;62:972–981. doi: 10.1109/TBME.2014.2377023. [DOI] [PubMed] [Google Scholar]
- Ofner, P., Müller-Putz, G.R., 2012. . Decoding of velocities and positions of 3D arm movement from EEG,. Confer. Proc. IEEE Eng. Med. Biol. Soc. IEEE, pp. 6406–6409. doi:10.1109/EMBC.2012.6347460 [DOI] [PubMed]
- Pascual-Marqui R.D. Standardized low-resolution brain electromagnetic tomography (sLORETA): technical details. Methods Find. Exp. Clin. Pharmacol. 2002;24(Suppl D):S5–S12. [PubMed] [Google Scholar]
- Pereira, J., Ofner, P., Müller-Putz, G.R., 2015. . Goal-directed or aimless? EEG differences during the preparation of a reach-and-touch task. Confer. Proc. IEEE Eng. Med. Biol. Soc. IEEE, pp. 1488–1491. doi:10.1109/EMBC.2015.7318652 [DOI] [PubMed]
- Rizzolatti G., Cattaneo L., Fabbri-Destro M., Rozzi S. Cortical mechanisms underlying the organization of goal-directed actions and mirror neuron-based action understanding. Physiol. Rev. 2014;94:655–706. doi: 10.1152/physrev.00009.2013. [DOI] [PubMed] [Google Scholar]
- Rohm M., Schneiders M., Müller C., Kreilinger A., Kaiser V., Müller-Putz G.R., Rupp R. Hybrid brain–computer interfaces and hybrid neuroprostheses for restoration of upper limb functions in individuals with high-level spinal cord injury. Artif. Intelligence Med. 2013;59(2):133–142. doi: 10.1016/j.artmed.2013.07.004. [DOI] [PubMed] [Google Scholar]
- Rupp R., Rohm M., Schneiders M., Kreilinger A., Müller-Putz G.R. Functional rehabilitation of the paralyzed upper extremity after spinal cord injury by noninvasive hybrid neuroprostheses. Proc. IEEE. 2015;103(6):954–968. [Google Scholar]
- Saxe R., Xiao D.-K., Kovacs G., Perrett D.I., Kanwisher N. A region of right posterior superior temporal sulcus responds to observed intentional actions. Neuropsychologia. 2004;42:1435–1446. doi: 10.1016/j.neuropsychologia.2004.04.015. [DOI] [PubMed] [Google Scholar]
- Sburlea A.I., Montesano L., Minguez J. Continuous detection of the self-initiated walking pre-movement state from EEG correlates without session-to-session recalibration. J. Neural Eng. 2015;12:036007. doi: 10.1088/1741-2560/12/3/036007. [DOI] [PubMed] [Google Scholar]
- Schafer J., Strimmer K. A shrinkage approach to large-scale covariance matrix estimation and implications for functional genomics. Stat. Appl. Genet. Mol. Biol. 2005;4:32. doi: 10.2202/1544-6115.1175. [DOI] [PubMed] [Google Scholar]
- Schlögl A., Brunner C. BioSig: a free and open source software library for BCI research. Computer. 2008;41:44–50. [Google Scholar]
- Shibasaki H., Barrett G., Halliday E., Halliday A.M. Cortical potentials associated with voluntary foot movement in man. Electroencephalogr. Clin. Neurophysiol. 1981;52:507–516. doi: 10.1016/0013-4694(81)91426-7. [DOI] [PubMed] [Google Scholar]
- Steingrüber H.-J., Lienert G.A. Hogrefe; 1971. Hand-Dominanz-Test: hdt. Verlag für Psychologie. [Google Scholar]
- Tadel F., Baillet S., Mosher J.C., Pantazis D., Leahy R.M. Brainstorm: a user-friendly application for MEG/EEG analysis. Comput. Intell. Neurosci. 2011;2011:879716. doi: 10.1155/2011/879716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vingerhoets G. Contribution of the posterior parietal cortex in reaching, grasping, and using objects and tools. Front. Psychol. 2014;5:151. doi: 10.3389/fpsyg.2014.00151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuckovic A., Sepulveda F. Delta band contribution in cue based single trial classification of real and imaginary wrist movements. Med. Biol. Eng. Comput. 2008;46:529–539. doi: 10.1007/s11517-008-0345-8. [DOI] [PubMed] [Google Scholar]
- Xu R., Jiang N., Lin C., Mrachacz-Kersting N., Dremstrup K., Farina D. Enhanced low-latency detection of motor intention from EEG for closed-loop brain-computer interface applications. IEEE Trans. Biomed. Eng. 2014;61:288–296. doi: 10.1109/TBME.2013.2294203. [DOI] [PubMed] [Google Scholar]
- Xu R., Jiang N., Mrachacz-Kersting N., Dremstrup K., Farina D. Factors of influence on the performance of a short-latency non-invasive brain switch: evidence in healthy individuals and implication for motor function rehabilitation. Front. Neurosci. 2015;9:527. doi: 10.3389/fnins.2015.00527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yong X., Menon C. EEG classification of different imaginary movements within the same limb. PLoS One. 2015;10:e0121896. doi: 10.1371/journal.pone.0121896. [DOI] [PMC free article] [PubMed] [Google Scholar]