Abstract
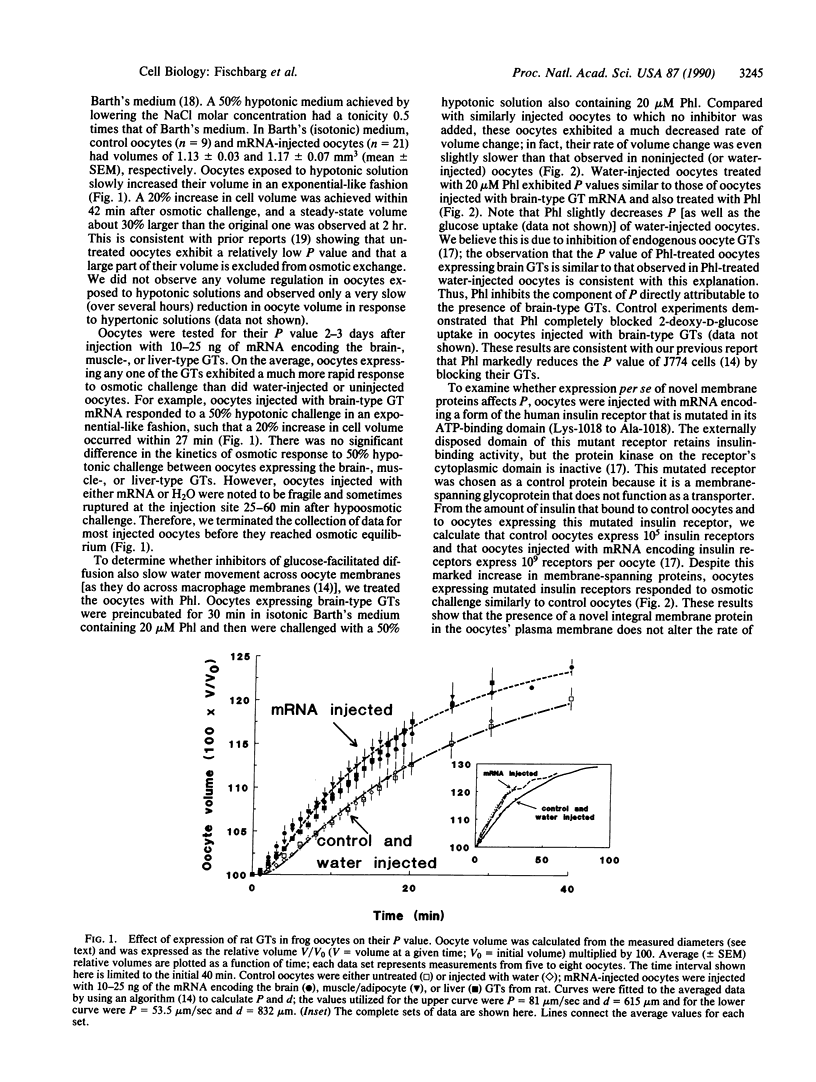
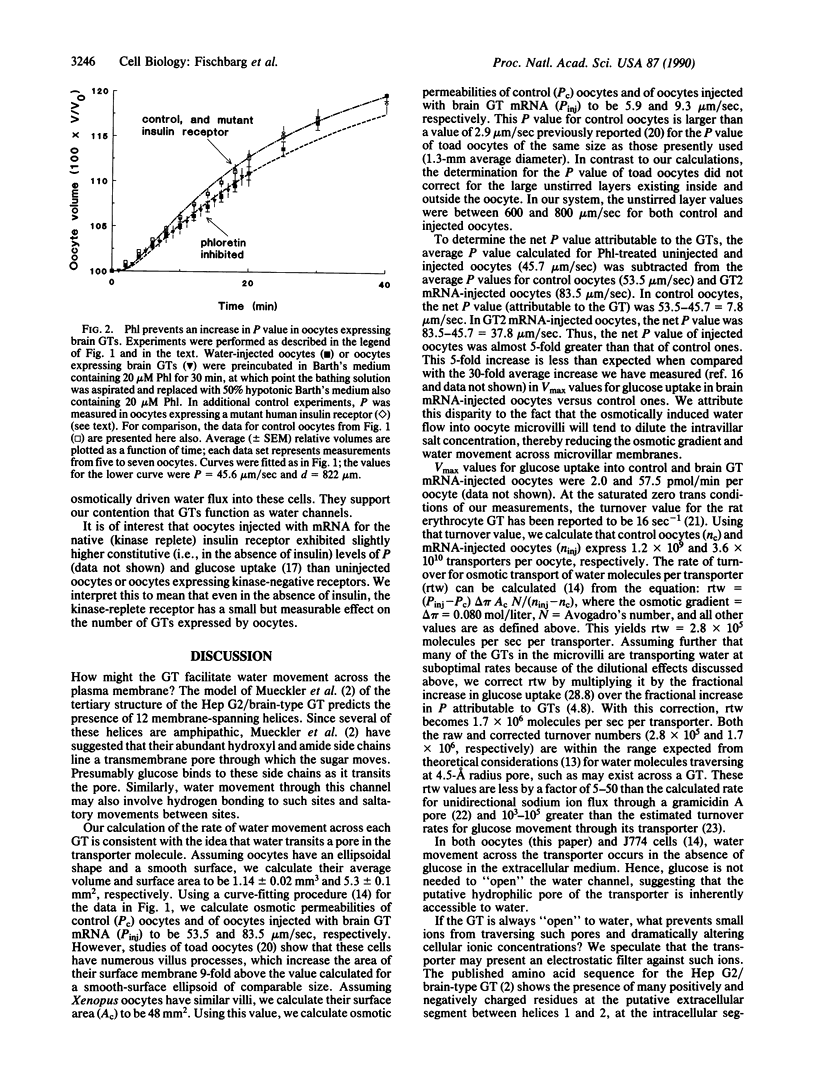
Water traverses the plasma membranes of some eukaryotic cells faster than can be explained by the water permeability of their lipid bilayers. This has led to a search for a water channel. Our previous work identified glucose transporters as candidates for such a channel. We report here that Xenopus laevis oocytes injected with mRNA encoding the brain/Hep G2, adult skeletal muscle/adipocyte, or liver forms of the glucose transporter exhibit an osmotic water permeability of their plasma membranes larger than that of untreated oocytes. The osmotic water permeability component attributable to glucose transporters increased an average of 4.8-fold in the injected oocytes. These studies provide direct evidence that the facilitative, sodium-independent mammalian glucose transporters serve as membrane water channels.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alvarez J., Lee D. C., Baldwin S. A., Chapman D. Fourier transform infrared spectroscopic study of the structure and conformational changes of the human erythrocyte glucose transporter. J Biol Chem. 1987 Mar 15;262(8):3502–3509. [PubMed] [Google Scholar]
- Birnbaum M. J., Haspel H. C., Rosen O. M. Cloning and characterization of a cDNA encoding the rat brain glucose-transporter protein. Proc Natl Acad Sci U S A. 1986 Aug;83(16):5784–5788. doi: 10.1073/pnas.83.16.5784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnbaum M. J. Identification of a novel gene encoding an insulin-responsive glucose transporter protein. Cell. 1989 Apr 21;57(2):305–315. doi: 10.1016/0092-8674(89)90968-9. [DOI] [PubMed] [Google Scholar]
- Brahm J. Kinetics of glucose transport in human erythrocytes. J Physiol. 1983 Jun;339:339–354. doi: 10.1113/jphysiol.1983.sp014720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dick E. G., Dick D. A., Bradbury S. The effect of surface microvilli on the water permeability of single toad oocytes. J Cell Sci. 1970 Mar;6(2):451–476. doi: 10.1242/jcs.6.2.451. [DOI] [PubMed] [Google Scholar]
- Fettiplace R. The influence of the lipid on the water permeability of artificial membranes. Biochim Biophys Acta. 1978 Oct 19;513(1):1–10. doi: 10.1016/0005-2736(78)90106-2. [DOI] [PubMed] [Google Scholar]
- Finkelstein A., Andersen O. S. The gramicidin A channel: a review of its permeability characteristics with special reference to the single-file aspect of transport. J Membr Biol. 1981 Apr 30;59(3):155–171. doi: 10.1007/BF01875422. [DOI] [PubMed] [Google Scholar]
- Fischbarg J., Kuang K. Y., Hirsch J., Lecuona S., Rogozinski L., Silverstein S. C., Loike J. Evidence that the glucose transporter serves as a water channel in J774 macrophages. Proc Natl Acad Sci U S A. 1989 Nov;86(21):8397–8401. doi: 10.1073/pnas.86.21.8397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischbarg J. On the possible permeation of water across the glucose transporter. Mol Cell Biochem. 1988 Jul-Aug;82(1-2):107–111. doi: 10.1007/BF00242524. [DOI] [PubMed] [Google Scholar]
- Fukumoto H., Kayano T., Buse J. B., Edwards Y., Pilch P. F., Bell G. I., Seino S. Cloning and characterization of the major insulin-responsive glucose transporter expressed in human skeletal muscle and other insulin-responsive tissues. J Biol Chem. 1989 May 15;264(14):7776–7779. [PubMed] [Google Scholar]
- Fukumoto H., Seino S., Imura H., Seino Y., Eddy R. L., Fukushima Y., Byers M. G., Shows T. B., Bell G. I. Sequence, tissue distribution, and chromosomal localization of mRNA encoding a human glucose transporter-like protein. Proc Natl Acad Sci U S A. 1988 Aug;85(15):5434–5438. doi: 10.1073/pnas.85.15.5434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurdon J. B. Methods for nuclear transplantation in amphibia. Methods Cell Biol. 1977;16:125–139. doi: 10.1016/s0091-679x(08)60096-5. [DOI] [PubMed] [Google Scholar]
- Helgerson A. L., Carruthers A. Analysis of protein-mediated 3-O-methylglucose transport in rat erythrocytes: rejection of the alternating conformation carrier model for sugar transport. Biochemistry. 1989 May 30;28(11):4580–4594. doi: 10.1021/bi00437a012. [DOI] [PubMed] [Google Scholar]
- James D. E., Strube M., Mueckler M. Molecular cloning and characterization of an insulin-regulatable glucose transporter. Nature. 1989 Mar 2;338(6210):83–87. doi: 10.1038/338083a0. [DOI] [PubMed] [Google Scholar]
- Jones M. N., Nickson J. K. Monosaccharide transport proteins of the human erythrocyte membrane. Biochim Biophys Acta. 1981 Jun 16;650(1):1–20. doi: 10.1016/0304-4157(81)90006-x. [DOI] [PubMed] [Google Scholar]
- Jung E. K., Chin J. J., Jung C. Y. Structural basis of human erythrocyte glucose transporter function in reconstituted system. Hydrogen exchange. J Biol Chem. 1986 Jul 15;261(20):9155–9160. [PubMed] [Google Scholar]
- Kaestner K. H., Christy R. J., McLenithan J. C., Braiterman L. T., Cornelius P., Pekala P. H., Lane M. D. Sequence, tissue distribution, and differential expression of mRNA for a putative insulin-responsive glucose transporter in mouse 3T3-L1 adipocytes. Proc Natl Acad Sci U S A. 1989 May;86(9):3150–3154. doi: 10.1073/pnas.86.9.3150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kayano T., Fukumoto H., Eddy R. L., Fan Y. S., Byers M. G., Shows T. B., Bell G. I. Evidence for a family of human glucose transporter-like proteins. Sequence and gene localization of a protein expressed in fetal skeletal muscle and other tissues. J Biol Chem. 1988 Oct 25;263(30):15245–15248. [PubMed] [Google Scholar]
- Mueckler M., Caruso C., Baldwin S. A., Panico M., Blench I., Morris H. R., Allard W. J., Lienhard G. E., Lodish H. F. Sequence and structure of a human glucose transporter. Science. 1985 Sep 6;229(4717):941–945. doi: 10.1126/science.3839598. [DOI] [PubMed] [Google Scholar]
- Sigler K., Janácek K. The effect of non-electrolyte osmolarity on frog oocytes. I. Volume changes. Biochim Biophys Acta. 1971 Aug 13;241(2):528–538. doi: 10.1016/0005-2736(71)90052-6. [DOI] [PubMed] [Google Scholar]
- Thorens B., Sarkar H. K., Kaback H. R., Lodish H. F. Cloning and functional expression in bacteria of a novel glucose transporter present in liver, intestine, kidney, and beta-pancreatic islet cells. Cell. 1988 Oct 21;55(2):281–290. doi: 10.1016/0092-8674(88)90051-7. [DOI] [PubMed] [Google Scholar]
- Vera J. C., Rosen O. M. Functional expression of mammalian glucose transporters in Xenopus laevis oocytes: evidence for cell-dependent insulin sensitivity. Mol Cell Biol. 1989 Oct;9(10):4187–4195. doi: 10.1128/mcb.9.10.4187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vera J. C., Rosen O. M. Reconstitution of an insulin signaling pathway in Xenopus laevis oocytes: coexpression of a mammalian insulin receptor and three different mammalian hexose transporters. Mol Cell Biol. 1990 Feb;10(2):743–751. doi: 10.1128/mcb.10.2.743. [DOI] [PMC free article] [PubMed] [Google Scholar]



