Abstract
Background
The barley stripe mosaic virus (BSMV) has become a popular vector to study gene function in cereals. However, studies have been limited to gene silencing in leaves of barley or wheat. In addition, the method produces high variability between different leaves and plants. To overcome these limitations, we explored the potential of modifying the inoculation protocol for BSMV gene overexpression. An improved light, oxygen or voltage-sensing (iLOV) domain-based fluorescent protein was used as a reporter of gene expression to monitor the infection and spread of BSMV. Tobacco (Nicotiana benthamiana) leaves were infected via agroinfiltration and the leaves were homogenized to extract the BSMV particles and inoculate wheat tissues using the traditional leaf abrasion method or by incubation during seed imbibition in a Petri dish.
Results
Compared to the leaf abrasion method, the seed imbibition method resulted in a high and uniform detection of iLOV in both roots and leaves of different wheat cultivars and other monocot and dicot species within 7 days after germination. The progression of viral infection via the imbibition method as measured by the expression of iLOV was more stable in different organs and tissues and is transmissible to the next generation.
Conclusion
Our results show that BSMV can be used as a vector for the expression of small genes such as iLOV in wheat roots and leaves. The inoculation by seed imbibition allows genes to be expressed rapidly and uniformly in wheat and different monocot and dicot species compared to the traditional leaf abrasion method. It also produces high successful transformation as early as 7 days post infection allowing gene function studies during the first generation of infected plants. Furthermore, the method is simple, rapid, and inexpensive compared to the production of transgenic plants.
Electronic supplementary material
The online version of this article (doi:10.1186/s13007-017-0175-5) contains supplementary material, which is available to authorized users.
Keywords: Barley stripe mosaic virus (BSMV), Gene overexpression, Wheat, Barley, Brachypodium, Maize, Arabidopsis, Nicotiana benthamiana
Background
As a result of rapid advances in genomics, the availability of powerful tools for gene function analysis has become a necessity, especially for important crops. In recent years, barley stripe mosaic virus (BSMV) vectors have been modified and showed important applications for high throughput assays due to its simplicity and ease of use [1]. The BSMV virus is a tripartite (α, β, γ) positive strand RNA virus with RNAs capped at the 5′ end [2]. These RNAs encodes all the genes necessary for viral RNA replication and viral propagation [3]. Recently, a BSMV vector system coupling a ligation independent cloning strategy with an Agrobacterium tumefaciens-mediated delivery system has been engineered and provides substantial advantages in expense, cloning efficiency and ability to apply virus-induced gene silencing (VIGS) for high throughput genomic studies [2]. However, the potential of BSMV vector for gene overexpression in wheat and the optimal inoculation protocol has been less studied [1, 4]. Virus-mediated overexpression (VOX) has been demonstrated for the first time in studies involving the green fluorescent protein (GFP) as reporter [1, 5, 6]. While GFP expression from BSMV was shown to be strong, studies reported that its overall level of expression in monocotyledonous plants is patchy [1, 4, 6]. It was suggested that this could be due to the relatively large size of GFP (720 bp), limiting local and systemic spread of BSMV, and causing instability in the viral genome [1]. Insert sizes lesser than 500 bp were shown to be relatively more stable than larger inserts [1]. An alternative strategy to verify the potential of BSMV vector for a more uniform and stable gene overexpression would be to use a smaller fluorescent protein to reduce gene size and increase stability. An improved light, oxygen or voltage-sensing (iLOV) domain of the plant blue light receptor, phototropin fluorescent protein has previously been demonstrated to be more stable compared to GFP in wheat leaves using BSMV [1]. However, several cells containing chloroplasts do not express iLOV, showing that the expression is not uniform. This is probably due to the use of the leaf abrasion method of virus inoculation [1].
Furthermore, most protocols of VIGS involving the use of BSMV in plants are so far established for gene silencing in vegetative tissues with very limited success in infection at an early stage resulting in difficulties to evaluate the impact of gene silencing since results vary from different tissue samples [2, 7–9]. Transmission of BSMV through seeds allows a more uniform gene expression in different tissues without showing any viral symptoms [6]. Based on this observation, we postulated that inoculation with BSMV particles at the earliest time possible may result in a more uniform gene expression. We have thus developed a new procedure of BSMV inoculation at the seed imbibition stage. This modification allows rapid and efficient gene overexpression in different wheat tissues, genotypes, and in different monocot and dicot species.
Methods
Plant material and growth conditions
Nicotiana benthamiana used for agroinfiltration was grown in a controlled environment chamber at 24 °C under 14 h photoperiod, 100 μmol m−2 s−1 (fluorescent and incandescent lighting) and 70% relative humidity. Different plant species and cultivars were used for BSMV inoculation: Triticum aestivum (wheat) cv. Atlas66, Norstar, Hordeum vulgare (barley) cv. Sophie, Zea mays (maize) cv. Pioneer Hybrid 3921, Brachypodium distachyon inbred line Bd21, N. benthamiana (tobacco) and Arabidopsis thaliana ecotype Columbia (Col-0).
Construction of Agrobacterium-mediated BSMV-iLOV vector
The three plasmids pCaBS-α, pCaBS-β and pCaBS-γ comprising respectively the tripartite genome (RNAα, RNAβ, and RNAγ) of the BSMV strain ND18 were selected for iLOV overexpression (Fig. 1a). The iLOV coding sequence (330 bp) was amplified by PCR from pGEX iLOV (Addgene, plasmid #26587) using the Q5 high fidelity DNA polymerase (New England Biolabs) and the following primers containing the LIC sites (underlined): AAGGAAGTTTAAATGATAGAGAAGAATTTCGTCATCACT (forward) and CAACCACCACCACCGTCTATACATGATCACTTCCATCGAGCTG (reverse). PCR products were purified and cloned into the pCaBS-γbLIC via ligation independent cloning (LIC) as described by Yuan et al. [2] (Fig. 1b; Additional file 1: Fig. S1). The construct was then introduced into Escherichia coli DH10B. Positive colonies were identified by colony PCR, endonuclease digestion, and confirmed by sequencing.
Fig. 1.
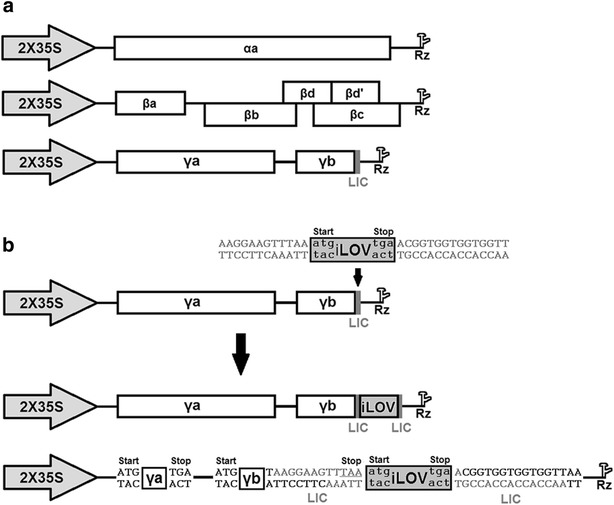
Construction of Agrobacterium-mediated BSMV:iLOV vector with the LIC cloning site. a Schematic representation of pCaBS-α, pCaBS-β and pCaBS-γ. BSMV α, β and γ cDNAs were inserted between the double 35S promoter and a ribozyme sequence (Rz). b Cloning of iLOV cDNA (330 bp) in pCaBS-γ via LIC cloning
Agroinfiltration of N. benthamiana and viral inoculation
The pCaBS-α, pCaBS-β and pCaBS-γb:iLOV were introduced into A. tumefaciens strain EHA105 as described previously by Yuan et al. [2]. Equal amounts of Agrobacterium harboring pCaBS-α, pCaBS-β and pCaBS-γb:iLOV were mixed (ratio 1:1:1) and incubated for 3–5 h at 28 °C. Infiltration of N. benthamiana leaves was performed using a 1-ml needleless syringe. The bacterial mixture was infiltrated into 3–6 spots (100 µl of resulting in approximately 3–4 cm2 infiltrated area) on each N. benthamiana leaf. After agro-infiltration, N. benthamiana plants were maintained in a controlled environment chamber. At 7 days post-infiltration (dpi), 0.5 g of spot-agroinfiltrated leaves were harvested and ground in 1 ml of 20 mM Na-phosphate buffer (pH 7.2) using a mortar and pestle. Homogenates can be directly used for virus inoculation or aliquoted in small volumes and stored at −20 °C for later use. Two methods of inoculation were tested in this study. Mechanical inoculation was performed using the traditional leaf abrasion method [2, 10]. Diatomaceous earth (1%, w/v) was added to the homogenate for mechanical inoculation onto 7-days old wheat leaves (first leaf). For the seed imbibition method, seeds of T. aestivum, H. vulgare, Z. mays, B. distachyon were germinated in a Petri dish containing N. benthamiana homogenate and distilled water. Different ratios of homogenate: distilled water were tested and a ratio of 1:100 (v/v) was used in this study, since this ratio did not lead to any alterations of seed germination and seedling development. Two different eudicot species (A. thaliana and N. benthamiana) were grown from seeds on 0.8% agarose and the diluted homogenate was directly spread on agarose after 3 days of seed stratification at 4 °C.
Quantitative RT-PCR analysis
Total RNA (including viral RNA) was isolated from different plant tissues using the RNeasy plant mini kit (Qiagen) and treated with on-column RNase free DNAase (Qiagen) before the reverse transcription step. Real-time quantitative RT-PCR was performed on a CFX96 Touch™ Thermal cycler (Bio-Rad) using the SsoFast EvaGreen Supermix (Bio-Rad). The iLOV RNA region was amplified by RT-PCR using the following primers ACAGATCAAGCGACTGTCCA (forward) and CACAGGTTGCAGGTGGAGTA (reverse). Amplification was performed with the following thermal cycling conditions: 5 min at 95 °C followed by 35 cycles of 95 °C for 15 s, 58 °C for 30 s. The copy number of BSMV:iLOV in infected tissue was normalized to the 18S rRNA and determined from a calibration curve using known amounts of iLOV cDNA as described previously [11].
Fluorescence imaging
iLOV fluorescence images were obtained using a confocal laser-scanning microscope (Nikon inverted microscope Eclipse Ti-E) or a NightOWL camera (Berthold LB 983 NC100) with an excitation wavelength of 488 and 450 nm, respectively. Fluorescence emission was detected at 550 nm. Z-stack images were generated through confocal microscopy to remove the out of focus signal collected within each individual image. In some experiments, bright field images were taken using transmitted light detection (TD) to show leaf and root structures.
Determination of growth parameters
Plants were harvested at 14, 21 and 31 days post inoculation (dpi). Entire plants were weighed after drying in a 70 °C oven for 3 days. Physiological variation in growth parameters were determined by total fresh and dry weight and by plant height.
Statistical analysis
qRT-PCR data are expressed as the mean of absolute quantification (copy number) ± standard deviation (SD) of 3 biological replicates. Comparisons of mean RNA abundance of BSMV:iLOV were conducted using one-way analysis of variance (ANOVA). Differences among means were analyzed using Tukey’s post hoc test at p values <.05. The significance test between treatments for physiological variation in growth parameters was determined by a Student’s t test. Significance was set at p values <0.05. Statistical analysis was performed using InStat 3.0. Graphs were made using GraphPad Prism 7.0.
Results and discussion
The efficiency of BSMV inoculation to silence gene expression in cereals has been documented and demonstrated in several studies [2, 6, 10, 12, 13]. Despite its substantial advantages in time, expense and efficiency, the system still has several limitations including an uneven distribution of gene silencing as shown using phytoene desaturase as reporter gene [2, 14]. To explore the potential use of BSMV for gene overexpression in different wheat tissues, a small gene (330 bp) encoding iLOV was cloned into a BSMV vector to inoculate N. benthamiana leaves via agro-infiltration.
After infiltration with an equal amount of A. tumefaciens harboring the three BSMV cDNA α, β and γ:iLOV, infected N. benthamiana plants started to develop yellow spots on infiltrated leaves and mild mottling on the upper uninfiltrated N. benthamiana leaves at 7 days post inoculation (dpi) (Additional file 2: Fig. S2). Since this phenotype indicates the presence of viral particles and systemic movement of BSMV from the infiltrated leaves, iLOV fluorescence in N. benthamiana leaves was visualized using a fluorescence camera (Berthold NightOWL) to confirm the infection with BSMV and iLOV expression (Additional file 2: Fig. S2). Infected leaves were ground to extract the BSMV particles. As mechanical transmission of BSMV via leaf abrasion is the recommended inoculation method to infect wheat plants, 7-day old wheat leaves were mechanically inoculated with the N. benthamiana leaf homogenate containing BSMV:iLOV. The presence of BSMV infection in inoculated wheat plants was observed from iLOV fluorescence using confocal microscopy 7 days after mechanical inoculation. Our result shows that iLOV is weakly expressed in both leaves and roots infected by mechanical inoculation but the signal distribution is very uneven with many cells showing no fluorescence signal (Fig. 2a panels 1 and 3, b panel 1). These results confirm the uneven distribution of BSMV particles throughout tissues as shown previously in different studies [1, 6, 10, 13, 15, 16]. Similar results were also observed in studies exploiting the potential of wheat streak mosaic virus-based vectors to overexpress GFP or beta-glucuronidase (GUS) using leaf abrasion [5, 17]. Ma et al. [18] reported that the effectiveness of BSMV expression in wheat was strongly related to the location of inoculation and the development stage of the plant. During seed imbibition, we expect that viral infection will be possible as soon as roots and leaves emerge. Although the mechanism of initial viral infection is still poorly understood [19], previous studies described that contact transmission is an important factor in the epidemiology of BSMV in the field [1, 20]. At the molecular level, the triple gene block proteins (TGB1, TGB2 and TGB3) present in most plant viruses including BSMV (encoded by the β genome) have been reported to play multiple functions in viral infection and propagation in the plant [1, 21, 22]. The interaction of the TGB1 protein with the TGB2 and TGB3 proteins is an important step to bind cell walls and cytoplasmic membranes and facilitate the movement of the viral nucleoprotein complex through the plasmodesmata to adjacent cells [22]. Furthermore, full systemic infection of plants by plant viruses can occur through roots by pouring infected sap on the soil or culture solutions in which they were growing indicating that simple contact may be sufficient for infection [20, 23]. We thus tested the possibility of incubating wheat seeds at the imbibition stage. Wheat seeds were germinated in distilled water with the N. benthamiana homogenate containing the viral particles carrying BSMV:iLOV. At 7 dpi, the distribution of iLOV fluorescence was observed throughout the leaf and root tissues and most if not all individual cells expressed iLOV (Fig. 2a panels 2 and 4, b panel 2). There is still some variation in signal intensity which may reflect the tissue structure and slight variation in the cell’s ability to replicate the viral RNAs or translate the different genes. However, most cells appear to translate the reporter gene efficiently. The progression of viral infection in the inoculated first leaf is presented in panels 5 and 6 after 21 dpi. The fluorescence signal has progressed throughout the leaf using the abrasion method but there is still an uneven signal with patches of stronger signal (Fig. 2a panel 1 vs 5). The iLOV signal for the imbibition method is similar to the one seen at 7 dpi (Fig. 2a panel 2 vs 6). The signal progression in the second leaf (21 dpi) and the emerging third leaf (25 dpi) is very weak throughout the leaf using the abrasion method (Fig. 2a panels 7 and 9) while the signal observed in the second and third leaf using the imbibition method is similar to the one observed in the first leaf (Fig. 2a panels 6, 8 and 10). BSMV:iLOV propagation in roots was also observed at 21 dpi. Unlike the roots infected via leaf abrasion (Fig. 2b panel 3), strong iLOV fluorescence is evenly emitted in roots infected via seed imbibition (Fig. 2b panel 4). These results demonstrate that the propagation of BSMV to different tissues through neighboring cells is relatively slow, suggesting that inoculation via leaf abrasion could require at least one generation to exhibit the phenotype of interest at whole plants level [1, 6], while the BSMV propagation via the imbibition method progressed at a faster rate possibly by exploiting the vascular tissues more efficiently.
Fig. 2.
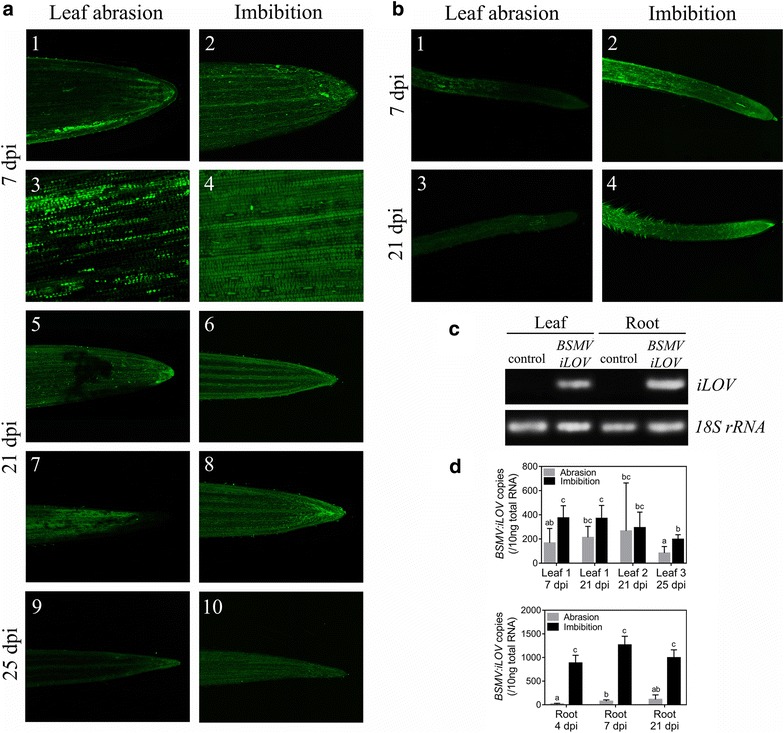
Expression of iLOV in wheat roots and leaves. a iLOV fluorescence images of leaves of BSMV:iLOV infected plants at 7 dpi (1–4), 21 dpi (5–8) and 25 dpi (9–10). A close-up is shown to see individual cells from the first leaves at 7 dpi using the scratching method (3) or the imbibition method (4). b iLOV fluorescence images of roots of BSMV:iLOV infected plants at 7 dpi (1–2) and 21 dpi (3–4). c Presence of iLOV region within the BSMV:iLOV RNA in leaves and roots at 7 dpi detected by RT-PCR. d Quantification of the iLOV region in leaves and roots at various days post infection by qRT-PCR. The copy number is calculated for the amount of RNA used in the qRT-PCR reaction using a calibration curve with known amounts of iLOV cDNA. Values represent mean ± SD (N = 3). Different letters indicate significant differences between groups (p < 0.05)
To compare the level of expression in the different tissues, the iLOV RNA region was analyzed by RT-PCR to confirm the specificity of iLOV amplification products (Fig. 2c) and quantified by qRT-PCR (Fig. 2d). While inoculation of BSMV:iLOV into wheat plants either via seed imbibition or leaf abrasion allows the overexpression of iLOV (Fig. 2d), quantification analysis revealed a higher abundance of BSMV:iLOV copies in the first leaf of plants infected via seed imbibition at 7 dpi (Fig. 2d, upper panel). The level of BSMV:iLOV RNA at 21 days in leaf 1 is similar to the one at 7 days. In leaf 2, the level of BSMV:iLOV RNA is similar at 21 dpi between the two methods. However, the large standard error in the leaf treated with the abrasion method reflects a large variation in RNA copy number suggesting that viral propagation is uneven between the three different leaves used for analysis. This result is in agreement with the confocal analysis (Fig. 2a). The BSMV:iLOV abundance in the emerging third leaf was also determined by qRT-PCR. Although the quantification of BSMV:iLOV copies in the third leaf shows less variability, the seed imbibition method allows for a higher abundance of BSMV:iLOV in the growing leaf at 25 dpi suggesting that viral propagation is more efficient with the imbibition method. Root tissues were also harvested to verify the infection and spread of BSMV:iLOV in plants infected either via leaf abrasion or seed imbibition. Analysis by qRT-PCR reveals that BSMV:iLOV RNA is significantly more abundant in roots infected via seed imbibition, in contrast to leaf abrasion as soon as 4 dpi. This suggests that the imbibition method could be particularly useful to study the physiological impact of gene overexpression or RNAi during the early stages of plant growth and as early as 4 dpi.
To confirm that the imbibition method does not cause a background fluorescence signal, two different controls were made (Fig. 3). Plants were either incubated with uninfected or BSMV wild type-infected (BSMV:00) N. benthamiana leaf homogenates. In both cases, no fluorescence signal was observed in the leaf (Fig. 3a, left and middle panels respectively) or root tissues (Fig. 3b, left and middle panels respectively), while a strong fluorescence signal was detected in both leaf and root tissues infected with BSMV:iLOV (right panels in Fig. 3a, b, respectively).
Fig. 3.
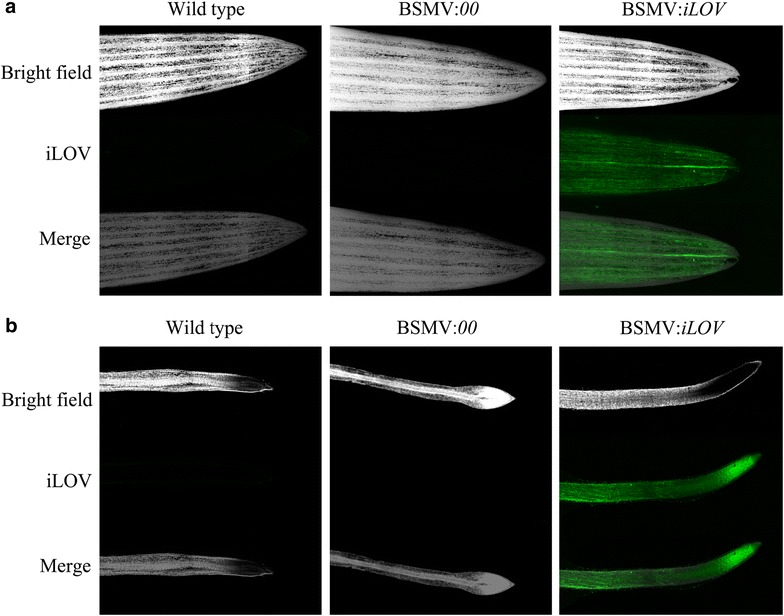
Expression of iLOV in leaves and roots of wheat plants. a leaves, b roots. Brightfield images (top); iLOV fluorescence images (middle) and merged images (bottom) are shown. Wheat seeds were imbibed for 3 days in extract of wild type-uninfected tobacco (Wild-type, left), tobacco infected with empty BSMV vector (BSMV:00, middle) or with BSMV:iLOV (right)
In previous studies performing virus-induced gene silencing (VIGS) with the scratching method, the peak effect of gene silencing was observed at 20 dpi indicating that the imbibition method allows gene expression and function studies at a much earlier growth development stage. Together, these results confirm the presence of iLOV in root and leaf tissues, and suggest that the seed imbibition method greatly improves the distribution of BSMV and expression of iLOV.
It has been reported that BSMV could mediate gene expression in different wheat organs [24]. However, a visually discernible signal throughout different organs/tissues is needed to support the use of BSMV in studies examining the effect of genes expressed in these organs. The progression of virus infection was measured over several weeks in different wheat tissues to verify the stability of the system and the expression in other tissues up to the flowering and seed maturation stages. Figure 4 shows a uniform fluorescence of iLOV in different organs or tissues (root tip, leaf tip, crown, flag leaf, anthers, immature seed and mature seed) of wheat infected via seed imbibition. Although a small level of auto fluorescence was observed on the coat of wild type mature seed (not shown), BSMV:iLOV infected mature seeds emitted a strong fluorescence on the coat and in the endosperm (Fig. 4, panel 8), indicating the presence of iLOV expression in mature seeds. This is in agreement with previous studies showing that gene silencing can be propagated to the next generation [1, 6].
Fig. 4.
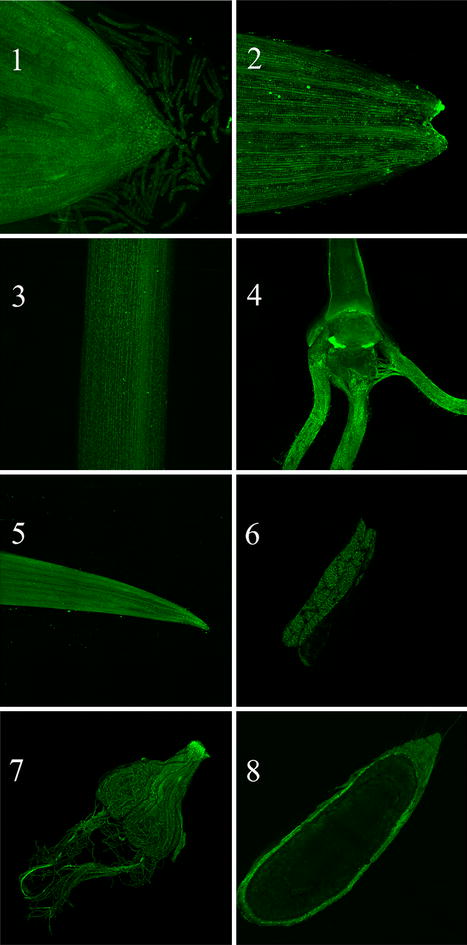
Expression of iLOV in different tissues/organs of BSMV:iLOV infected plants. Root tip (1); leaf tip (2); shoot (3); crown (4); flag leaf (5); anther (6); immature seed (7); mature seed (8)
Previous reports indicated that virus inoculation could alter photosynthesis in plants leading to a reduction of chlorophyll content and growth rate [14, 25, 26]. By inoculating barley plants via leaf abrasion, Liang et al. [14] stated that inoculation of BSMV reduced significantly the growth rate of barley plants and accelerated leaf senescence. Since the imbibition method did not cause any apparent symptoms (Fig. 5a, b) comparatively to the leaf abrasion method where necrotic lesions were apparent on infected tissues in wheat or tobacco (Fig. 5b; Additional file 2: Fig. S2), we evaluated the effects of BSMV inoculation by the imbibition method on the subsequent vegetative growth of plants at different growth periods (14, 21 and 31 dpi). The results show that inoculation of wheat plants by seed imbibition did not significantly affect the plant height and biomass as determined by fresh and dry weights (Fig. 5c). This suggests that seed imbibition could be a more appropriate method to investigate phenotypes of various genes related to development or stress responses. To illustrate the benefit of the imbibition method in dicot species, the expression of iLOV throughout leaf tissues of N. benthamiana and A. thaliana is illustrated in Additional file 3: Fig. S3.
Fig. 5.
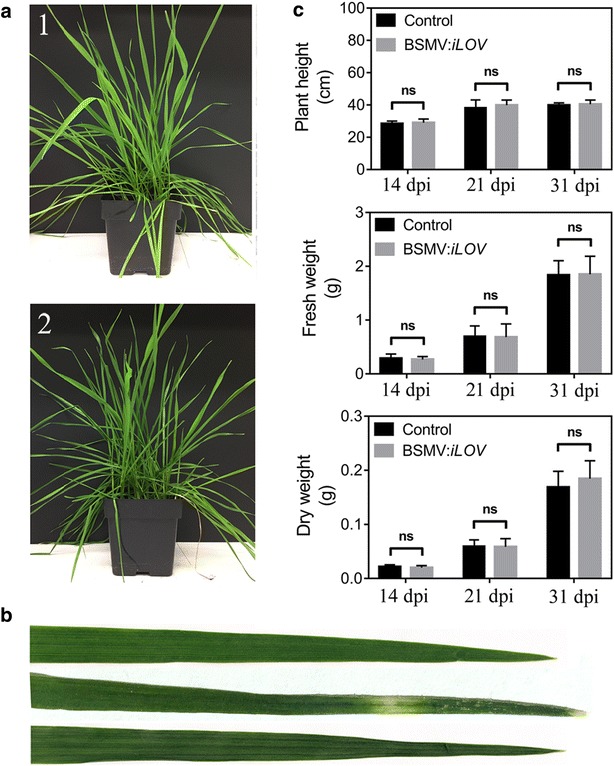
Effect of BSMV on wheat plant growth. a Phenotypes of uninfected (1) and BSMV:iLOV infected (2) wheat plants at 31 dpi. b Phenotype of uninfected (up) and BSMV:iLOV infected wheat leaves via leaf abrasion (middle) or seed imbibition (down) methods at 31 dpi. c Physiological variation in growth parameters. Height, fresh weight and dry weight of whole plants (n = 30) were measured at 14, 21 and 31 dpi. Control plants were inoculated with leaf homogenates of uninfected wild-type N. benthamiana. BSMV:iLOV plants were inoculated with leaf homogenates of BSMV:iLOV infected N. benthamiana. ns non significant difference
Since the presence of iLOV was detected in mature seeds (Fig. 4, panel 8), the expression of iLOV in the next generation was visualized at the seedling stage to confirm viral transmission. Confocal microscopy revealed the fluorescence of iLOV both in leaves and root tissues of infected plants (Fig. 6, panels 1 and 2). A close-up of iLOV fluorescence shows that BSMV:iLOV is uniformly spread throughout the leaf tissue, which is relatively similar to the results of inoculation obtained via imbibition method in the first plant generation (Fig. 2).
Fig. 6.
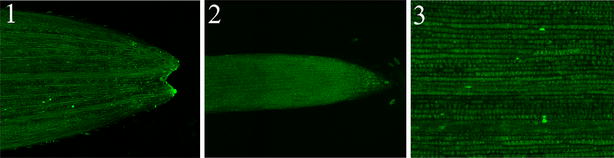
Expression of iLOV in leaf (panel 1) and root (panel 2) of the next generation of BSMV-infected wheat plants at the seedling stage (7 days). Close-up to see individual cells in the leaf tissue (panel 3)
Previous studies have reported that the ability of BSMV to infect host plant depends on genotypes [1, 6]. The potential of BSMV to infect different wheat genotypes via seed imbibition was examined. Different wheat cultivars (Atlas 66, Norstar) were inoculated via seed imbibition with N. benthamiana containing particles of BSMV:iLOV. Visualization of infected wheat plants by confocal microscopy reveals that iLOV was uniformly expressed in roots and leaves of both wheat cultivars (Fig. 7, panels 1–4). This suggests that inoculation of BSMV by seed imbibition can be used with different wheat cultivars. Mechanical transmission of BSMV to over 250 plant species including both monocot and dicots species has been reported [1, 27]. We thus investigated the ability of BSMV to infect different monocot and dicot species via seed imbibition. Seeds of B. distachyon, maize and barley were germinated on Petri dishes with water containing the N. benthamiana homogenate. Roots and leaves were imaged by confocal fluorescence microscopy at 7 dpi. The green fluorescence of iLOV was distributed uniformly in roots and leaves of all infected monocot plant species (Fig. 7, panels 1–10). By inoculating seeds of dicot species (N. benthamiana and A. thaliana) with the N. benthamiana homogenate containing BSMV:iLOV viral particles, we can observe that iLOV is expressed throughout the whole plant with a stronger signal in roots compared to leaves (Fig. 7, panels 11 and 12). However, the signal is uniform within a same tissue and in the different leaves either at the two leaf stage (Fig. 7, panels 11 and 12) or when additional leaves emerge (Additional file 3: Fig. S3). These results show that inoculation via seed imbibition allows uniform distribution of BSMV in different monocot and dicot species allowing gene function experiments to be performed with confidence.
Fig. 7.
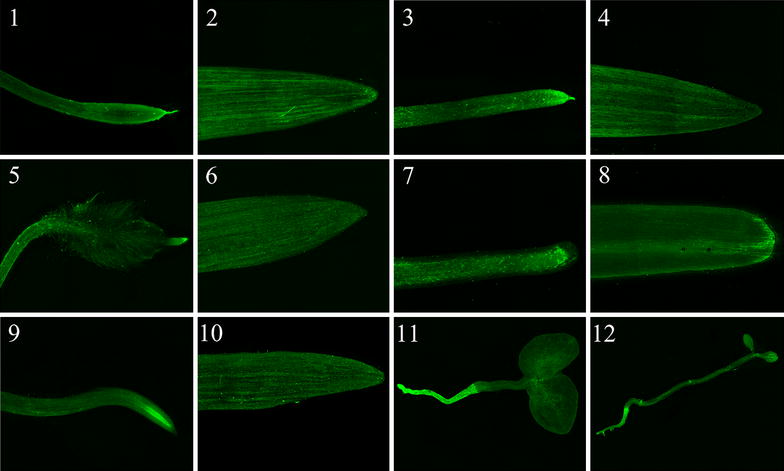
Expression of iLOV in leaves and roots of different wheat cultivars, monocot and dicot species at 7 dpi. Wheat cv. Atlas 66 root (1), leaf (2); wheat cv. Norstar root (3), leaf (4); B. distachyon root (5), leaf (6); Z. mays root (7), leaf (8); H. vulgare L. root (9), leaf (10); N. benthamiana (11); A. thaliana (12)
Conclusion
In this study, we have developed a low-cost high-throughput inoculation method allowing rapid and uniform gene expression in different plants based on the barley stripe mosaic virus. Using this new approach, we demonstrated that inoculation greatly improves transfection compared to the traditional leaf abrasion method, and allows efficient and stable viral propagation in different tissues during the first generation as evidence with the iLOV fluorescent reporter. Hence, this protocol could help researchers to take full advantage of the BSMV system which is a powerful functional genomics tool for gene function characterization using gene overexpression or gene silencing in different plant species, particularly during different stages of plant growth.
Additional files
Additional file 1. Schematic representation of BSMV:iLOV construct. Detail of LIC cloning is illustrated.
Additional file 2. (1) Uninfected N. benthamiana plant. (2) Agrobacterium mediated BSMV:iLOV infection in N. benthamiana plant. N. benthamiana leaves were inoculated by agro-infiltration with an equal amount of Agrobaterium mixtures harboring the pCaBS-α, pCaBS-β and pCaBS-γb:iLOV. (3) iLOV fluorescence imaged by a NightOWL camera in an uninfected leaf. (4) iLOV fluorescence imaged by a NightOWL camera in a BSMV:iLOV infected leaf.
Additional file 3. Photograph of BSMV-infected tobacco via seed imbibition. (2) Photograph of BSMV-infected A. thaliana via seed imbibition. (3) iLOV fluorescence imaged by a confocal microscopy in a BSMV:iLOV infected tobacco via seed imbibition. (4) iLOV fluorescence imaged by a confocal microscopy in a BSMV:iLOV-infected A. thaliana via seed imbibition.
Authors’ contributions
AC performed the experiments; AC and MH designed the experiments, and interpreted the data and wrote the manuscript. Both authors read and approved the final manuscript.
Acknowledgements
The authors thank Dawei Li (State Key Laboratory of Agro-biotechnology at China Agriculture University) and Shawn Clark (National Research Council Canada) for providing the BSMV pCaBS-α, pCaBS-β and pCaBS-γ. They also thank Dr. F. Ouellet for sharing the CFX96 TouchTM Thermal cycler (Bio-Rad), Mélanie Grondin and Denis Flipo for their technical assistance in performing the confocal microscopy.
Competing interests
The authors declare that they have no competing interests.
Availability of data and materials
There is no data sets for this manuscript. The material is available from Dawei Li (see acknowledgements). We will gladly provide the BSMV material in Canada upon written consent from Dawei Li allowing our laboratory to distribute the material.
Funding
A.C. acknowledges support from the Faculty of Science at UQAM for a Ph.D. scholarship and for a scholarship exempting international student tuition fees. This work was supported by a Natural Sciences and Engineering Research Council of Canada Grant (OGP0138557) to M.H.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Arnaud Cheuk, Phone: 1-514-987-3000, Email: cheuk.arnaud@courrier.uqam.ca.
Mario Houde, Phone: 1-514-987-3000, Email: houde.mario@uqam.ca.
References
- 1.Lee W-S, Hammond-Kosack KE, Kanyuka K. Barley stripe mosaic virus-mediated tools for investigating gene function in cereal plants and their pathogens: virus-induced gene silencing, host-mediated gene silencing, and virus-mediated overexpression of heterologous protein. Plant Physiol. 2012;160:582–590. doi: 10.1104/pp.112.203489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yuan C, Li C, Yan L, Jackson AO, Liu Z, Han C, Yu J, Li D. A high throughput barley stripe mosaic virus vector for virus induced gene silencing in monocots and dicots. PLoS ONE. 2011;6:e26468. doi: 10.1371/journal.pone.0026468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee W-S, Rudd JJ, Kanyuka K. Virus induced gene silencing (VIGS) for functional analysis of wheat genes involved in Zymoseptoria tritici susceptibility and resistance. Fungal Genet Biol. 2015;79:84–88. doi: 10.1016/j.fgb.2015.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lawrence DM, Jackson AO. Requirements for cell-to-cell movement of Barley stripe mosaic virus in monocot and dicot hosts. Mol Plant Pathol. 2001;2:65–75. doi: 10.1046/j.1364-3703.2001.00052.x. [DOI] [PubMed] [Google Scholar]
- 5.Tatineni S, McMechan AJ, Hein GL, French R. Efficient and stable expression of GFP through Wheat streak mosaic virus-based vectors in cereal hosts using a range of cleavage sites: formation of dense fluorescent aggregates for sensitive virus tracking. Virology. 2011;410:268–281. doi: 10.1016/j.virol.2010.10.043. [DOI] [PubMed] [Google Scholar]
- 6.Bennypaul HS, Mutti JS, Rustgi S, Kumar N, Okubara PA, Gill KS. Virus-induced gene silencing (VIGS) of genes expressed in root, leaf, and meiotic tissues of wheat. Funct Integr Genomics. 2012;12:143–156. doi: 10.1007/s10142-011-0245-0. [DOI] [PubMed] [Google Scholar]
- 7.Chen J-C, Jiang C-Z, Gookin T, Hunter D, Clark D, Reid M. Chalcone synthase as a reporter in virus-induced gene silencing studies of flower senescence. Plant Mol Biol. 2004;55:521–530. doi: 10.1007/s11103-004-0590-7. [DOI] [PubMed] [Google Scholar]
- 8.Lin Z, Hong Y, Yin M, Li C, Zhang K, Grierson D. A tomato HD-Zip homeobox protein, LeHB-1, plays an important role in floral organogenesis and ripening. Plant J. 2008;55:301–310. doi: 10.1111/j.1365-313X.2008.03505.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fu DQ, Zhu BZ, Zhu HL, Jiang WB, Luo YB. Virus-induced gene silencing in tomato fruit. Plant J. 2005;43:299–308. doi: 10.1111/j.1365-313X.2005.02441.x. [DOI] [PubMed] [Google Scholar]
- 10.Matthews REF. Transmission, movement, and host range. In: Fundamentals of plant virology. San Diego: Academic Press; 1992. p. 205–23.
- 11.Bustin SA. Absolute quantification of mRNA using real-time reverse transcription polymerase chain reaction assays. J Mol Endocrinol. 2000;25:169–193. doi: 10.1677/jme.0.0250169. [DOI] [PubMed] [Google Scholar]
- 12.Pacak A, Geisler K, Jørgensen B, Barciszewska-Pacak M, Nilsson L, Nielsen TH, Johansen E, Grønlund M, Jakobsen I, Albrechtsen M. Investigations of barley stripe mosaic virus as a gene silencing vector in barley roots and in Brachypodium distachyon and oat. Plant Methods. 2010;6:1–16. doi: 10.1186/1746-4811-6-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lin N-S, Langenberg W. Distribution of Barley stripe mosaic virus protein in infected wheat root and shoot tips. J Gen Virol. 1984;65:2217–2224. doi: 10.1099/0022-1317-65-12-2217. [DOI] [Google Scholar]
- 14.Liang J, Chen X, Zhao H, Yu S, Long H, Deng G, Pan Z, Yu M. The impacts of BSMV on vegetative growth and water status in hulless barley (Hordeum vulgare var. nudum) in VIGS study. Acta Soc Bot Pol. 2015;84:43–51. doi: 10.5586/asbp.2014.033. [DOI] [Google Scholar]
- 15.Huang C, Qian Y, Li Z, Zhou X. Virus-induced gene silencing and its application in plant functional genomics. Sci China Life Sci. 2012;55:99–108. doi: 10.1007/s11427-012-4280-4. [DOI] [PubMed] [Google Scholar]
- 16.Holzberg S, Brosio P, Gross C, Pogue GP. Barley stripe mosaic virus-induced gene silencing in a monocot plant. Plant J. 2002;30:315–327. doi: 10.1046/j.1365-313X.2002.01291.x. [DOI] [PubMed] [Google Scholar]
- 17.Choi IR, Stenger DC, Morris TJ, French R. A plant virus vector for systemic expression of foreign genes in cereals. Plant J. 2000;23:547–555. doi: 10.1046/j.1365-313x.2000.00820.x. [DOI] [PubMed] [Google Scholar]
- 18.Ma M, Yan Y, Huang L, Chen M, Zhao H. Virus-induced gene-silencing in wheat spikes and grains and its application in functional analysis of HMW-GS-encoding genes. BMC Plant Biol. 2012;12:1–13. doi: 10.1186/1471-2229-12-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rodrigo G, Zwart MP, Elena SF. Onset of virus systemic infection in plants is determined by speed of cell-to-cell movement and number of primary infection foci. J R Soc Interface. 2014;11:20140555. doi: 10.1098/rsif.2014.0555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Slack SA, Shepherd RJ, Hall DH. Spread of seed-borne stripe mosaic virus and the effect of the virus on barley in California. Phytopathology. 1975;65:1218–1223. doi: 10.1094/Phyto-65-1218. [DOI] [Google Scholar]
- 21.Hu Y, Li Z, Yuan C, Jin X, Yan L, Zhao X, Zhang Y, Jackson AO, Wang X, Han C. Phosphorylation of TGB1 by protein kinase CK2 promotes barley stripe mosaic virus movement in monocots and dicots. J Exp Bot. 2015;66:4733–4747. doi: 10.1093/jxb/erv237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lawrence DM, Jackson AO. Interactions of the TGB1 protein during cell-to-cell movement of barley stripe mosaic virus. J Virol. 2001;75:8712–8723. doi: 10.1128/JVI.75.18.8712-8723.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Roberts F. The infection of plants by viruses through roots. Ann Appl Biol. 1950;37:385–396. doi: 10.1111/j.1744-7348.1950.tb00964.x. [DOI] [Google Scholar]
- 24.Wang GF, Wei X, Fan R, Zhou H, Wang X, Yu C, Dong L, Dong Z, Wang X, Kang Z, et al. Molecular analysis of common wheat genes encoding three types of cytosolic heat shock protein 90 (Hsp90): functional involvement of cytosolic Hsp90s in the control of wheat seedling growth and disease resistance. New Phytol. 2011;191:418–431. doi: 10.1111/j.1469-8137.2011.03715.x. [DOI] [PubMed] [Google Scholar]
- 25.Funayama S, Sonoike K, Terashima I. Photosynthetic properties of leaves of Eupatorium makinoi infected by a geminivirus. Photosynth Res. 1997;53:253–261. doi: 10.1023/A:1005884007183. [DOI] [Google Scholar]
- 26.Funayama S, Terashima I. Effects of geminivirus infection and growth irradiance on the vegetative growth and photosynthetic production of Eupatorium makinoi. New Phytol. 1999;142:483–494. doi: 10.1046/j.1469-8137.1999.00418.x. [DOI] [Google Scholar]
- 27.Jackson AO, Lane LC. Hordeiviruses. In: Kurstak E, editor. Handbook of plant virus infections and comparative diagnosis. Amsterdam: Elsevier; 1981. pp. 565–625. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1. Schematic representation of BSMV:iLOV construct. Detail of LIC cloning is illustrated.
Additional file 2. (1) Uninfected N. benthamiana plant. (2) Agrobacterium mediated BSMV:iLOV infection in N. benthamiana plant. N. benthamiana leaves were inoculated by agro-infiltration with an equal amount of Agrobaterium mixtures harboring the pCaBS-α, pCaBS-β and pCaBS-γb:iLOV. (3) iLOV fluorescence imaged by a NightOWL camera in an uninfected leaf. (4) iLOV fluorescence imaged by a NightOWL camera in a BSMV:iLOV infected leaf.
Additional file 3. Photograph of BSMV-infected tobacco via seed imbibition. (2) Photograph of BSMV-infected A. thaliana via seed imbibition. (3) iLOV fluorescence imaged by a confocal microscopy in a BSMV:iLOV infected tobacco via seed imbibition. (4) iLOV fluorescence imaged by a confocal microscopy in a BSMV:iLOV-infected A. thaliana via seed imbibition.
Data Availability Statement
There is no data sets for this manuscript. The material is available from Dawei Li (see acknowledgements). We will gladly provide the BSMV material in Canada upon written consent from Dawei Li allowing our laboratory to distribute the material.


