Abstract
AIM
To investigate the relationship between 25-hydroxyvitamin D (25-OHD) deficiency and hepatic encephalopathy (HE) in patients with chronic liver disease (CLD).
METHODS
A retrospective analysis of the results of 392 adult patients with chronic liver disease who were assessed for liver transplantation between 2006 and 2010 was undertaken. HE, severity of CLD, nutritional status and 25-OHD were analysed in patients assessed for liver transplantation between 2006 and 2010. Patients who presented with acute, fulminant or subacute disease, with a primary diagnosis of liver cancer, were assessed for re-transplantation or who did not have a 25-OHD measurement were excluded from the analysis.
RESULTS
One hundred and sixty-five patients were included in this analysis. The mean age of all patients was 53 ± 8 years. Moderate to severe 25-OHD deficiency was identified in 49 patients of whom 36 had grade 2-3 HE compared with 13 patients who were not encephalopathic (P ≤ 0.0001). Mild 25-OHD deficiency was not associated with HE. There was a significant correlation between the severity of 25-OHD deficiency and the severity of liver disease (r = 0.39, P ≤ 0.0001) and disease severity and the presence of HE (P ≤ 0.0001). Importantly, individuals with 25-OHD deficiency were more likely to have a diagnosis of overt HE (OHE) at a significantly lower model for end stage liver disease (MELD) score than individuals without OHE (P ≤ 0.0001). This significant difference was observed with MELD scores from 10 to 38.
CONCLUSION
25-OHD deficiency was observed in the majority of patients with CLD and for the first time was found to be significantly worse in patients with OHE.
Keywords: Vitamin D, Chronic liver disease, Hepatic encephalopathy, Model For End Stage Liver Disease, Dementia, Malnutrition, Cognitive function
Core tip: A strong association between vitamin D deficiency and deteriorating liver disease is identified in this investigation which supports previous reported findings. The novel finding in this investigation is the relationship between vitamin D deficiency and overt hepatic encephalopathy (OHE) in patients with chronic liver disease (CLD) which is independent of renal impairment and nutritional status. As repeated episodes of OHE may result in some residual neuropsychiatric alterations, maintenance of vitamin D levels within normal range in patients with CLD should be considered in clinical management. These results provide a strong rationale for future intervention studies in this group.
INTRODUCTION
Hepatic encephalopathy (HE) describes a complex collection of neuropsychiatric symptoms ranging from sub-clinical neuropsychiatric changes to coma[1] and has been identified in up to 55% of patients with chronic liver disease[2]. Symptoms include impaired cognition and motor function and reduced energy levels[3] HE can be classified as covert or overt HE (OHE)[1]. Features of HE can be likened to symptoms seen in patients with dementia[4].
The aetiology of HE is complex and multifactorial, and includes abnormal ammonia metabolism, dysbiosis which promotes inflammation in the gut and liver[5], low levels of circulating branched chain amino acids[6], electrolyte abnormalities[7] and alterations in zinc and manganese levels[8]. Importantly, the features of HE can often be significantly reversed following treatment consistent with a largely functional not a structural cause of cognitive impairment[9].
The development of HE presents significant challenges to patients and their carers[10]. Until recently, lactulose was the major cornerstone in the management of HE and continues to be used as a first line management for the control of the symptoms of chronic HE and for the reversal of the symptoms of acute episodes of HE[11]. The introduction of Rifaximin has reduced the rate of OHE and the frequency of hospital admissions due to OHE and improved the quality of life for the patient and their carers[12].
Patients who experience repeated episodes of OHE can have persistent and cumulative deficits in working memory, response inhibition and learning[13]. There is growing evidence that some deficit in cognitive function remains in liver transplant recipients who were severely encephalopathic or who experienced multiple episodes of OHE prior to liver transplantation[9]. Therefore, the prevention of OHE is paramount to the preservation of mental integrity in patients with cirrhosis.
Vitamin D is a multifunctional steroid hormone with diverse actions that are only partially understood. It is increasingly apparent that vitamin D is not just involved in calcium homeostasis and bone metabolism but has multiple biological targets mediated by vitamin D receptors (VDR)[14] which are present in more than 30 tissues[15] including the brain, kidneys, intestine, parathyroid gland, pituitary, prostate, mammary glands, cardiac and skeletal muscle, non-parenchymal liver cells, endothelial cells and the immune system[16-20].
Vitamin D is obtained from dietary sources and ultraviolet light exposure. The first step in the activation of vitamin D is the hydroxylation of cholecalciferol to the active metabolite 25-hydroxyvitamin D (25-OHD) which occurs in the liver[21]. This is the major circulating metabolite of vitamin D, bound to the carrier protein vitamin D binding protein (DBP) with a half-life of 15-21 d[21,22]. The second activation process to 1,25 dihydroxy vitamin D occurs predominantly in the kidney[21] and to a lesser extent in a range of other tissues including bone, breast, brain, monocytes, parathyroid gland and placenta[21]. This active metabolite has a shorter half-life of 10-20 h[22]. Consequently, vitamin D status is commonly assessed by measuring circulating levels of 25-OHD[22].
Vitamin D has been identified as an immune modulator and anti-infective agent[23] and an association between vitamin D deficiency and the progression of liver disease has been identified in hepatitis C virus (HCV)[24], alcoholic liver disease (ALD)[25] and non-alcoholic fatty liver disease (NAFLD)[24].
There is growing evidence of clinical associations between vitamin D status and global and specific areas of cognitive function[26] and that vitamin D deficiency may be associated with both depression and schizophrenia[27]. Further, vitamin D deficiency is associated with low mood and impairment in some areas of cognitive functioning without any impairment in physical performance[28] and with an accelerated decline in cognitive function[29].
VDR protein is present in most neurons and the glia in the human brain[30]. The hypothalamus and the cortex of the human brain are key areas in cognition[31]. The presence of both VDR protein and vitamin D metabolites in these areas of the brain are an indication that the vitamin D system is involved in the normal functioning of the human brain[32].
As the first step in the hydroxylation of vitamin D occurs within the liver 25-OHD, levels decrease with progressive liver dysfunction. Vitamin D deficiency has been reported in up to 92% of patients with chronic liver disease and at least one third of these have severe 25-OHD deficiency[33]. 25-OHD deficiency is associated with increasing Child-Pugh classification rather disease aetiology[34] and is more prevalent in patients with cirrhosis than those who are not cirrhotic[33]. Increased all-cause mortality is associated with 25-OHD deficiency and specifically with increased mortality in patients with cirrhosis[35]. To date, an association between 25-OHD deficiency and HE has not been described. Therefore, we aimed to investigate the relationship between 25-OHD, cirrhosis and HE.
MATERIALS AND METHODS
Patient selection
All patients who present for assessment for liver transplantation routinely undergo a comprehensive series of tests and examinations prior to consideration of suitability for transplantation. A retrospective analysis of the results of 392 adult patients with chronic liver disease who were assessed for liver transplantation between 2006 and 2010 was undertaken. Approval to access medical records was granted by the Sydney Local Health District Ethics Review Committee (RPAH X15-0209).
Data collection
Data collated included primary diagnosis, demographic information, standard biochemical markers of liver function, disease severity scores model for end stage liver disease (MELD)[36] and Child Turcotte Pugh (CTP)[37], subjective nutritional assessment (SGA) scores[38], 25-OHD levels and the presence of HE assessed using the West Haven criteria[39]. Patients who presented with acute, fulminant or subacute disease were excluded from the analysis due to the acute nature of their illness. Patients with a primary diagnosis of liver cancer or who were undergoing assessment for re-transplantation or who did not have a 25-OHD measurement were also excluded from the analysis.
25-OH vitamin D status was defined as sufficient (> 75 nmol/L), insufficient (50-75 nmol/L), mild deficiency (25-50 nmol/L), moderate deficiency (12.5-25 nmol/L) and severe deficiency (< 12.5 nmol/L)[40,41].
During the study period, three different 25-OH D assay methods were used: (1) radioimmunoassay (RIA), referred to as the Diasorin-RIA® assay; (2) the electrochemiluminescence immunoassay, referred to as the Roche Elecys® vitamin D3 assay; and (3) the Liaison total automated direct competitive chemiluminescence immunoassay, referred to as the Diasorin Liaison® 25-OH vitamin D assay. 25-OHD levels are frequently overestimated with greater intra-assay variation when assessed by more recent methodologies[42]. As the Diasorin RIA® assay was regarded as the most accurate measure of 25-OHD[43] at the time of this investigation, the final analysis included only patients with 25-OHD levels measured using the Diasorin RIA® assay technique (Figure 1).
Figure 1.
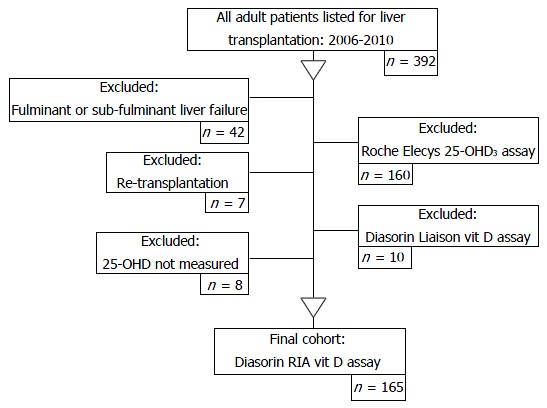
Study design. The selection and application of inclusion/exclusion criteria for inclusion in the final analysis. 25-OHD: 25-hydroxyvitamin D; RIA: Radioimmunoassay.
Statistical analysis
Results were analysed using Prism 6 for Mac (GraphPad Software Inc, La Jolla, CA, United States). Categorical values were analysed using χ2 and quantitative continuous results were compared using the Mann-Whitney U test. Relationships between quantitative variables were assessed using Spearman correlation analysis. Multiple comparisons were made using One-way ANOVA, Kruskal-Wallis test and Dunn’s multiple comparison test. The threshold for statistical significance is P < 0.05.
RESULTS
The patient selection process is outlined in Figure 1. After the exclusion criteria were applied 165 patients remained in the study. Table 1 identifies the primary disease aetiology for liver transplantation assessment and the patient physical and biochemical characteristics.
Table 1.
Population characteristics
| Total cohort (n) | Overt HE1 (n) | No HE1 (n) | |
| Demographics | 165 | 88 | 77 |
| Gender | |||
| Male | 119 | 68 | 51 |
| Female | 46 | 20 | 26 |
| Mean age (years ± SD) | 53 ± 8 | 52 ± 7 | 54 ± 8 |
| Primary indication for liver transplantation | |||
| Viral hepatitis | 91 | 52 | 39 |
| Alcoholic cirrhosis | 23 | 18 | 5 |
| Cholestatic disease (PBC, PSC, autoimmune) | 30 | 10 | 20 |
| Non-alcoholic steatohepatitis | 7 | 6 | 1 |
| Other | 14 | 2 | 12 |
| Ethnicity | |||
| Caucasian | 130 | 75 | 55 |
| Asian | 22 | 6 | 16 |
| Middle Eastern | 8 | 4 | 4 |
| Other | 5 | 3 | 2 |
| Clinical characteristics | |||
| CTP score mean ± SD | 9 ± 2.5 | 11 ± 1.7a | 7 ± 2a |
| CTP stage | |||
| A | 41 | 0a | 41a |
| B | 47 | 22 | 25 |
| C | 77 | 66a | 11a |
| Ascites | |||
| None | 51 | 7a | 44a |
| Medically controlled | 54 | 36a | 18a |
| Poorly controlled | 60 | 45a | 15a |
| BMI (kg/m2 ± SD) | 27.4 ± 5.2 | 28.7 ± 5.4a | 25.7 ± 4.2a |
| Nutritional status | 104 | 65 | 39 |
| SGA: A (well nourished) | 12 | 9 | 3 |
| SGA: B (moderately malnourished) | 65 | 40 | 25 |
| SGA: C (severely malnourished) | 27 | 16 | 11 |
| Biochemical characteristics | |||
| 25-OHD (vitamin D) (nmol/L) mean ± SD | 36 ± 15 | 30 ± 13a | 42 ± 16a |
| MELD score mean ± SD | 17.1 ± 6.8 | 19.9 ± 6. 5a | 13.9 ± 5.7a |
| Bilirubin (μmol/L) mean ± SD | 114 ± 152 | 141 ± 167a | 83 ± 128a |
| Creatinine (μmol/L) mean ± SD | 84 ± 50 | 85 ± 35a | 83 ± 63 |
| Albumin (g/L) mean ± SD | 33 ± 6 | 31 ± 5a | 35 ± 6a |
| INR mean ± SD | 1.6 ± 0.5 | 1.8 ± 0.6a | 1.3 ± 0.3a |
| Sodium (mmol/L) mean ± SD | 136 ± 5 | 135 ± 5a | 138 ± 4a |
| Zinc (μmol/L) mean ± SD | 8 ± 4 | 8 ± 3a | 10 ± 5a |
P < 0.05.
Unless otherwise indicated there is no significant difference between HE and no HE. 25-OHD: 25-hydroxyvitamin D; HE: Hepatic encephalopathy; CTP: Child Turcotte Pugh; BMI: Body mass index; SGA: Subjective nutritional assessment; MELD: Model for end stage liver disease; INR: International normalized ratio.
The group was predominantly male with a mean age of 53.0 ± 8 years. Seventy nine percent of the group was Caucasian and there was no significant difference in 25-OHD levels identified across the different ethnic groups. The major cause of listing for liver transplantation was decompensated cirrhosis secondary to viral hepatitis. All patients had advanced disease as demonstrated by the CTP and MELD scores. OHE was present in 53% of patients. The majority of the cohort (69%) had ascites which was defined as grade 3-4 in 53% of patients[44]. Patients with OHE had a higher body mass index (BMI) (P < 0.001) with an associated significantly increased incidence of medically controlled and poorly controlled ascites (P < 0.0001) and significantly lower serum albumin levels (P < 0.0005).
Our results showed a strongly negative correlation between MELD score and 25-OHD levels (P < 0.0001) in all patients (Figure 2). Patients with OHE had significantly worse liver disease with a MELD score of 19.9 ± 6.5 whilst those who were not encephalopathic had significantly lower MELD score of 13.9 ± 5.7 (P < 0.0001) (Figure 3A). 25-OHD levels were lower in patients with OHE (P < 0.0001) (Figure 3B).
Figure 2.
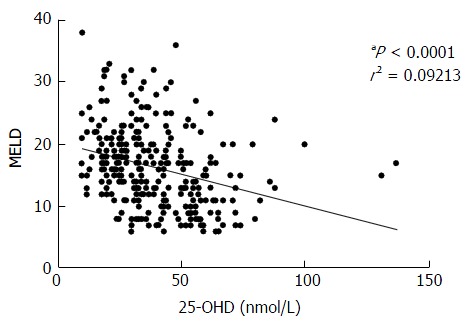
Vitamin D and severity of liver disease. 25-OHD levels fall with worsening liver disease. 25-OHD: 25-hydroxyvitamin D; MELD: Model for end stage liver disease.
Figure 3.
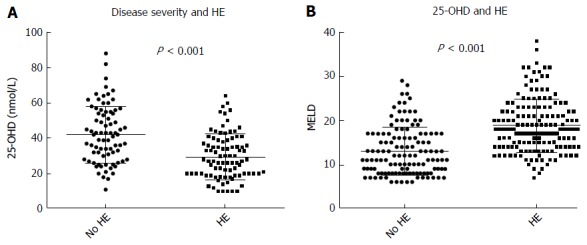
Disease severity, overt hepatic encephalopathy and vitamin D levels. A: Patients with overt HE have significantly higher MELD than patients without overt HE; B: 25-OHD levels measured by Diasorin-RIA and HE. Patients with overt HE have significantly lower 25-OHD levels than those without overt HE. 25-OHD: 25-hydroxyvitamin D; HE: Hepatic encephalopathy; MELD: Model for end stage liver disease.
SGA of nutritional status was available for 104 patients. The majority of patients were either moderately or significantly malnourished (88%) and there was no significant correlation between nutritional status and 25-OHD levels (Figure 4). There is trend towards a higher incidence and increased severity of malnutrition in patients with OHE but this did not reach statistical significance. The correlation between RIA 25-OHD and the biochemical and physical parameters of the group are further outlined in Table 2.
Figure 4.
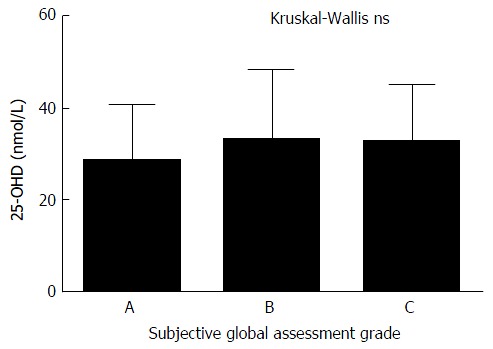
25-hydroxyvitamin D and nutritional status. 25-OHD levels are independent of nutritional status. 25-OHD: 25-hydroxyvitamin D; ns: Not significant.
Table 2.
25-hydroxyvitamin D categories in patients assessed for liver transplantation
| 25-OHD (nmol/L) | Total number | Overt HE1 (n = 88) | No overt HE1 (n = 77) | |
| Sufficient | > 75 | 2 | 0 | 2 |
| Insufficient | 50-75 | 27 | 6 | 21 |
| Mildly deficient | 25-50 | 87 | 46 | 41 |
| Moderately deficient | 12.5-25 | 42 | 30a | 12a |
| Severely deficient | < 12.5 | 7 | 6 | 1 |
P < 0.05.
Unless otherwise indicated there were no significant differences between the overt HE and no-overt HE groups. 25-OHD: 25-hydroxyvitamin D; HE: Hepatic encephalopathy.
Mild 25-OHD deficiency was not associated with an increase in OHE (Table 3). However, moderate and severe 25-OH D deficiency was significantly associated with the development of OHE (P < 0.0001). The relationship between 25-OH vitamin D and OHE is outlined in Figure 3B.
Table 3.
Univariate 25-hydroxyvitamin D correlations with physical and biochemical markers determined by Spearman correlation
| Spearman r | P value | Significance | |
| Age | 0.1110 | 0.16 | ns |
| Total bilirubin | -0.3493 | < 0.0001 | f |
| Albumin | 0.3153 | < 0.0001 | f |
| ALP | 0.0203 | 0.80 | ns |
| GGT | 0.2055 | 0.0081 | b |
| ALT | 0.0246 | 0.75 | ns |
| AST | -0.1741 | 0.0253 | a |
| Creatinine | -0.0687 | 0.38 | ns |
| Na+ | 0.2666 | 0.0005 | e |
| Zn+ | 0.2790 | 0.0004 | e |
| RBP | 0.2913 | 0.0002 | e |
| Transferrin | 0.3568 | < 0.0001 | f |
| INR | -0.4232 | < 0.0001 | f |
| Ca2+ | 0.2370 | 0.0022 | b |
| Ca2+ corrected | -0.1531 | 0.08 | ns |
| PTH | -0.1824 | 0.0205 | a |
| BMI | -0.2244 | 0.0055 | b |
P < 0.05;
P < 0.01;
P < 0.001;
P < 0.0001. ns: Not significant; ALP: Alkalinephosphatase; GGT: Glutamyl transpeptidase; ALT: Alanine transaminase; AST: Aspartate transaminase; RBP: Retinol binding protein; INR: International normalized ratio; PTH: Parathyroid hormone; BMI: Body mass index.
Using using χ2 analysis lower 25-OHD levels were associated with a significant trend towards increasing levels of OHE (P < 0.0001). A significant difference between 25-OHD levels in patients with OHE and those without OHE was identified (P < 0.0001) and is demonstrated in Figure 5. Furthermore, there was a significant correlation between increasing OHE in patients with lower 25-OHD levels at the same level of disease severity as measured by the MELD scores.
Figure 5.
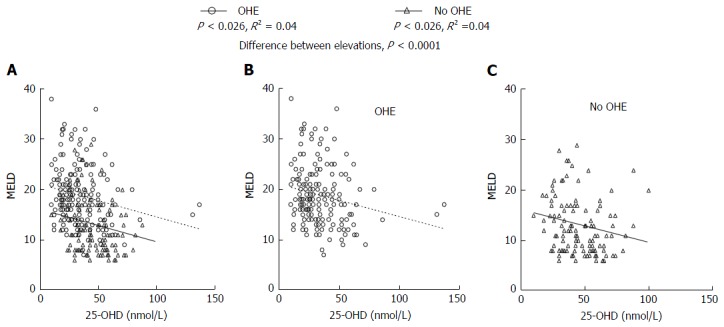
Overt hepatic encephalopathy, disease severity and 25-hydroxyvitamin D. Patients were stratified into those who had overt HE and those who did not. The combined data is presented in (A) and the sub groups of patients with OHE (B) and those without OHE (C). Vitamin D deficiency correlates with MELD score in both patients with and without OHE. Across the range of MELD scores from 10-30 patients with OHE (B) had significantly lower levels of 25-OHD than those who did not (C). 25-OHD: 25-hydroxyvitamin D; OHE: Overt hepatic encephalopathy; MELD: Model for end stage liver disease.
DISCUSSION
Our results demonstrate that at the same level of disease severity patients with OHE have significantly lower 25-OHD levels than those who do not have OHE. This is the first description of this association.
When stratified into patients who have OHE and those who do not, our results show that, in patients assessed for liver transplantation, there is a statistically significant relationship between low 25-OHD levels and OHE. Importantly, a significantly higher incidence of OHE was observed in individuals with low 25-OHD levels at similar levels of disease severity. This association raises the possibility that vitamin D deficiency has an effect on the manifestation of HE or is associated with other unrecognised factors. Importantly, OHE rarely occurred with normal Vitamin D levels but individuals could have low vitamin D and not have OHE. This is a similar relationship to the association of elevated serum ammonia with the development of OHE[45]. Consequently, the presence of moderate to severe 25-OHD deficiency means OHE is more likely in patients with ESLD.
It was beyond the scope of this investigation to explore the relationship between covert HE and 25-OHD deficiency. A large proportion of patients with insufficient (78%) or mild 25-OHD deficiency (47%) were not diagnosed with OHE. It possible that in a proportion of patients with insufficient or mild 25-OHD deficiency reduced levels of 25-OHD could be associated with the development or presence of covert HE. This is analogous to ammonia levels in ESLD which are elevated in HE but elevation does imply the presence of HE either OHE or covert HE.
There is a significant relationship between worsening liver function and 25-OHD deficiency. This is consistent with the growing awareness of the association between disease severity and reduced levels of 25-OHD in patients with cirrhosis[25]. Less than 2% of patients assessed for liver transplantation in this group had adequate levels of 25-OHD which is comparable to a previous study which identified vitamin D deficiency in 96% of patients waiting for liver transplantation[46].
As liver disease progresses, patients become increasingly malnourished with an associated increase in HE[8]. Alterations in macronutrient requirements and reductions in oral intake are a feature of decompensated cirrhosis[8]. However, our results did not show a significant association between malnutrition and 25-OHD levels. This is consistent with changes in 25-OHD metabolism being a determinant of deficiency in CLD.
Vitamin D supplementation is now recognised as an important component of the management of patients with cirrhosis. Routine vitamin D supplementation for patients with chronic liver disease and insufficient levels of 25-OHD has become the standard of care in hepatology clinics to treat or prevent osteoporosis in CLD[47]. These results suggest a role for vitamin D supplementation in patients with CLD and reduced levels of 25-OHD.
There is growing evidence for an association between 25-OHD deficiency and the development of all-cause dementia and a reduction in cognitive capacity[48] which is not confined to older populations. A linear relationship between 25-OHD deficiency and cognitive impairment has been identified in younger adults (30-60 years) as well as adults older than 60 years[49]. A systematic review of vitamin D and cognitive impairment concluded that “25-OHD insufficiency likely negatively affects specific cognitive functions, such as explicit episodic memory”[31] but there is a need for robust clinical investigation in this area.
Although it is plausible that 25-OHD deficiency could impact on cognitive function in CLD, neither a causative relationship nor a mechanism for this has been demonstrated. The level at which 25-OHD deficiency adversely affects brain function is unknown. 25-OHD is associated with verbal fluency, a marker of executive function and therefore a marker of cognitive function. Individuals with supratherapeutic 25-OHD levels of > 100 nmol/L scored significantly higher on verbal fluency tasks than those with inadequate 25-OHD levels[50] further supporting the role of vitamin D in the development of cognitive decline.
Neurobehavioral abnormalities are the major clinical component of HE and have shown to be associated with increased levels of inflammatory cytokines[51]. Systemic inflammation and changes in hepatic metabolism (i.e., increased ammonia levels) are increasingly recognised as a precipitants of HE and worsen existing HE[52]. Vitamin D has been shown to have anti-inflammatory properties[52]. It can be postulated that vitamin D deficiency is associated with an increase in systemic inflammation thereby giving rise to the development of HE.
It is unclear whether there is a steady decline in brain function as 25-OHD levels drop or whether there is a threshold from which point there is a significant reduction in brain function in patients with cirrhosis. To show a causative relationship of the vitamin D with HE it is now necessary to examine the effects of vitamin D supplementation on encephalopathy symptoms in patients with CLD.
To date, the literature suggests association not causality. There is sufficient association between cognitive and behavioral changes associated with 25-OHD deficiency to suggest a role for vitamin D deficiency in the development of HE in patients with chronic liver disease. Further studies are required to investigate the relationship between 25-OHD levels and the development of HE in patients with CLD.
COMMENTS
Background
Hepatic encephalopathy (HE) describes a complex range of neuropsychiatric symptoms and is associated with the development and progression of hepatic cirrhosis. HE has been described as a form of dementia which is largely reversible. Low vitamin D levels [25-hydroxyvitamin D (25-OHD)] are associated with dementia and impaired cognition in the general population. The association between low 25-OHD levels and HE has not been previously investigated.
Research frontiers
It is not known whether the association between low 25-OHD levels and HE is causative. The association described requires further investigation to determine the precise role of 25-OHD deficiency in the development of HE. Historically, the assays used to determine 25-OHD levels have varied significantly. Consistent assay methodology should be implemented to investigate this relationship further.
Innovations and breakthroughs
It has been established that patients with chronic liver disease (CLD) have low levels of 25-OHD which are associated with overt HE. Monitoring of 25-OHD vitamin D levels and regular and appropriate vitamin D supplementation in patients with cirrhosis may help prevent the development of HE.
Applications
Monitoring 25-OHD levels and replacement therapy is an important aspect of the overall management of patients with CLD.
Terminology
Automated immunoassays are used for routine analysis of serum 25-OHD levels and there is wide variation between the different assay techniques. At the time of this investigation, the Diasorin RIA assay was identified as the most reliable method of measuring serum 25-OHD levels.
Peer-review
This is very interesting paper about the relationship between 25-OH deficiency and HE. Author concluded that there is a significant association between low-25-OHD levels and the development of HE.
Footnotes
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Australia
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
Institutional review board statement: The study has approved by the Ethics Review Committee (RPAH Zone).
Informed consent statement: Informed consent was not necessary due to the retrospective nature of this investigation.
Conflict-of-interest statement: There were no conflicts of interests for any of the investigators.
Data sharing statement: Technical appendix, statistical code and dataset are available from the corresponding author at helen.vidot@sswahs.nsw.gov.au. Informed consent was not sought as all data was collected as part of standard care and all data is anonymised and risk of identification is low. No additional data is available.
Peer-review started: October 28, 2016
First decision: November 22, 2016
Article in press: March 13, 2017
P- Reviewer: Cao WK, Hashimoto N, Thompson JR S- Editor: Gong ZM L- Editor: A E- Editor: Li D
References
- 1.Vilstrup H, Amodio P, Bajaj J, Cordoba J, Ferenci P, Mullen KD, Weissenborn K, Wong P. Hepatic encephalopathy in chronic liver disease: 2014 Practice Guideline by the American Association for the Study of Liver Diseases and the European Association for the Study of the Liver. Hepatology. 2014;60:715–735. doi: 10.1002/hep.27210. [DOI] [PubMed] [Google Scholar]
- 2.Kalaitzakis E, Bjornsson E. Hepatic encephalopathy in patients with liver cirrhosis: is there a role of malnutrition? World J Gastroenterol. 2008;14:3438–3439. doi: 10.3748/wjg.14.3438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ferenci P, Lockwood A, Mullen K, Tarter R, Weissenborn K, Blei AT. Hepatic encephalopathy--definition, nomenclature, diagnosis, and quantification: final report of the working party at the 11th World Congresses of Gastroenterology, Vienna, 1998. Hepatology. 2002;35:716–721. doi: 10.1053/jhep.2002.31250. [DOI] [PubMed] [Google Scholar]
- 4.Córdoba J. New assessment of hepatic encephalopathy. J Hepatol. 2011;54:1030–1040. doi: 10.1016/j.jhep.2010.11.015. [DOI] [PubMed] [Google Scholar]
- 5.Rai R, Saraswat VA, Dhiman RK. Gut microbiota: its role in hepatic encephalopathy. J Clin Exp Hepatol. 2015;5:S29–S36. doi: 10.1016/j.jceh.2014.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Muto Y, Sato S, Watanabe A, Moriwaki H, Suzuki K, Kato A, Kato M, Nakamura T, Higuchi K, Nishiguchi S, et al. Effects of oral branched-chain amino acid granules on event-free survival in patients with liver cirrhosis. Clin Gastroenterol Hepatol. 2005;3:705–713. doi: 10.1016/s1542-3565(05)00017-0. [DOI] [PubMed] [Google Scholar]
- 7.Guevara M, Baccaro ME, Ríos J, Martín-Llahí M, Uriz J, Ruiz del Arbol L, Planas R, Monescillo A, Guarner C, Crespo J, et al. Risk factors for hepatic encephalopathy in patients with cirrhosis and refractory ascites: relevance of serum sodium concentration. Liver Int. 2010;30:1137–1142. doi: 10.1111/j.1478-3231.2010.02293.x. [DOI] [PubMed] [Google Scholar]
- 8.Bémeur C, Butterworth RF. Nutrition in the management of cirrhosis and its neurological complications. J Clin Exp Hepatol. 2014;4:141–150. doi: 10.1016/j.jceh.2013.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Teperman LW. Impact of pretransplant hepatic encephalopathy on liver posttransplantation outcomes. Int J Hepatol. 2013;2013:952828. doi: 10.1155/2013/952828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bajaj JS, Wade JB, Gibson DP, Heuman DM, Thacker LR, Sterling RK, Stravitz RT, Luketic V, Fuchs M, White MB, et al. The multi-dimensional burden of cirrhosis and hepatic encephalopathy on patients and caregivers. Am J Gastroenterol. 2011;106:1646–1653. doi: 10.1038/ajg.2011.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sanyal A, Younossi ZM, Bass NM, Mullen KD, Poordad F, Brown RS, Vemuru RP, Mazen Jamal M, Huang S, Merchant K, et al. Randomised clinical trial: rifaximin improves health-related quality of life in cirrhotic patients with hepatic encephalopathy - a double-blind placebo-controlled study. Aliment Pharmacol Ther. 2011;34:853–861. doi: 10.1111/j.1365-2036.2011.04808.x. [DOI] [PubMed] [Google Scholar]
- 12.Sturgeon JP, Shawcross DL. Recent insights into the pathogenesis of hepatic encephalopathy and treatments. Expert Rev Gastroenterol Hepatol. 2014;8:83–100. doi: 10.1586/17474124.2014.858598. [DOI] [PubMed] [Google Scholar]
- 13.Bajaj JS, Schubert CM, Heuman DM, Wade JB, Gibson DP, Topaz A, Saeian K, Hafeezullah M, Bell DE, Sterling RK, et al. Persistence of cognitive impairment after resolution of overt hepatic encephalopathy. Gastroenterology. 2010;138:2332–2340. doi: 10.1053/j.gastro.2010.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rosen CJ, Adams JS, Bikle DD, Black DM, Demay MB, Manson JE, Murad MH, Kovacs CS. The nonskeletal effects of vitamin D: an Endocrine Society scientific statement. Endocr Rev. 2012;33:456–492. doi: 10.1210/er.2012-1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Calvo MS, Whiting SJ, Barton CN. Vitamin D intake: a global perspective of current status. J Nutr. 2005;135:310–316. doi: 10.1093/jn/135.2.310. [DOI] [PubMed] [Google Scholar]
- 16.Merke J, Milde P, Lewicka S, Hügel U, Klaus G, Mangelsdorf DJ, Haussler MR, Rauterberg EW, Ritz E. Identification and regulation of 1,25-dihydroxyvitamin D3 receptor activity and biosynthesis of 1,25-dihydroxyvitamin D3. Studies in cultured bovine aortic endothelial cells and human dermal capillaries. J Clin Invest. 1989;83:1903–1915. doi: 10.1172/JCI114097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gascon-Barré M, Demers C, Mirshahi A, Néron S, Zalzal S, Nanci A. The normal liver harbors the vitamin D nuclear receptor in nonparenchymal and biliary epithelial cells. Hepatology. 2003;37:1034–1042. doi: 10.1053/jhep.2003.50176. [DOI] [PubMed] [Google Scholar]
- 18.Bischoff-Ferrari HA, Borchers M, Gudat F, Dürmüller U, Stähelin HB, Dick W. Vitamin D receptor expression in human muscle tissue decreases with age. J Bone Miner Res. 2004;19:265–269. doi: 10.1359/jbmr.2004.19.2.265. [DOI] [PubMed] [Google Scholar]
- 19.Eyles DW, Smith S, Kinobe R, Hewison M, McGrath JJ. Distribution of the vitamin D receptor and 1 alpha-hydroxylase in human brain. J Chem Neuroanat. 2005;29:21–30. doi: 10.1016/j.jchemneu.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 20.Wang Y, Zhu J, DeLuca HF. Where is the vitamin D receptor? Arch Biochem Biophys. 2012;523:123–133. doi: 10.1016/j.abb.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 21.Christakos S, Ajibade DV, Dhawan P, Fechner AJ, Mady LJ. Vitamin D: metabolism. Endocrinol Metab Clin North Am. 2010;39:243–253, table of contents. doi: 10.1016/j.ecl.2010.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Clemens TL, Adams JS, Nolan JM, Holick MF. Measurement of circulating vitamin D in man. Clin Chim Acta. 1982;121:301–308. doi: 10.1016/0009-8981(82)90239-x. [DOI] [PubMed] [Google Scholar]
- 23.Borella E, Nesher G, Israeli E, Shoenfeld Y. Vitamin D: a new anti-infective agent? Ann N Y Acad Sci. 2014;1317:76–83. doi: 10.1111/nyas.12321. [DOI] [PubMed] [Google Scholar]
- 24.Iruzubieta P, Terán Á, Crespo J, Fábrega E. Vitamin D deficiency in chronic liver disease. World J Hepatol. 2014;6:901–915. doi: 10.4254/wjh.v6.i12.901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Trépo E, Ouziel R, Pradat P, Momozawa Y, Quertinmont E, Gervy C, Gustot T, Degré D, Vercruysse V, Deltenre P, et al. Marked 25-hydroxyvitamin D deficiency is associated with poor prognosis in patients with alcoholic liver disease. J Hepatol. 2013;59:344–350. doi: 10.1016/j.jhep.2013.03.024. [DOI] [PubMed] [Google Scholar]
- 26.Buell JS, Dawson-Hughes B. Vitamin D and neurocognitive dysfunction: preventing “D”ecline? Mol Aspects Med. 2008;29:415–422. doi: 10.1016/j.mam.2008.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McCann JC, Ames BN. Is there convincing biological or behavioral evidence linking vitamin D deficiency to brain dysfunction? FASEB J. 2008;22:982–1001. doi: 10.1096/fj.07-9326rev. [DOI] [PubMed] [Google Scholar]
- 28.Wilkins CH, Sheline YI, Roe CM, Birge SJ, Morris JC. Vitamin D deficiency is associated with low mood and worse cognitive performance in older adults. Am J Geriatr Psychiatry. 2006;14:1032–1040. doi: 10.1097/01.JGP.0000240986.74642.7c. [DOI] [PubMed] [Google Scholar]
- 29.Moon JH, Lim S, Han JW, Kim KM, Choi SH, Kim KW, Jang HC. Serum 25-hydroxyvitamin D level and the risk of mild cognitive impairment and dementia: the Korean Longitudinal Study on Health and Aging (KLoSHA) Clin Endocrinol (Oxf) 2015;83:36–42. doi: 10.1111/cen.12733. [DOI] [PubMed] [Google Scholar]
- 30.Eyles DW, Burne TH, McGrath JJ. Vitamin D, effects on brain development, adult brain function and the links between low levels of vitamin D and neuropsychiatric disease. Front Neuroendocrinol. 2013;34:47–64. doi: 10.1016/j.yfrne.2012.07.001. [DOI] [PubMed] [Google Scholar]
- 31.Annweiler C, Allali G, Allain P, Bridenbaugh S, Schott AM, Kressig RW, Beauchet O. Vitamin D and cognitive performance in adults: a systematic review. Eur J Neurol. 2009;16:1083–1089. doi: 10.1111/j.1468-1331.2009.02755.x. [DOI] [PubMed] [Google Scholar]
- 32.Harms LR, Burne TH, Eyles DW, McGrath JJ. Vitamin D and the brain. Best Pract Res Clin Endocrinol Metab. 2011;25:657–669. doi: 10.1016/j.beem.2011.05.009. [DOI] [PubMed] [Google Scholar]
- 33.Arteh J, Narra S, Nair S. Prevalence of vitamin D deficiency in chronic liver disease. Dig Dis Sci. 2010;55:2624–2628. doi: 10.1007/s10620-009-1069-9. [DOI] [PubMed] [Google Scholar]
- 34.Malham M, Jørgensen SP, Ott P, Agnholt J, Vilstrup H, Borre M, Dahlerup JF. Vitamin D deficiency in cirrhosis relates to liver dysfunction rather than aetiology. World J Gastroenterol. 2011;17:922–925. doi: 10.3748/wjg.v17.i7.922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stokes CS, Krawczyk M, Reichel C, Lammert F, Grünhage F. Vitamin D deficiency is associated with mortality in patients with advanced liver cirrhosis. Eur J Clin Invest. 2014;44:176–183. doi: 10.1111/eci.12205. [DOI] [PubMed] [Google Scholar]
- 36.Kamath PS, Wiesner RH, Malinchoc M, Kremers W, Therneau TM, Kosberg CL, D’Amico G, Dickson ER, Kim WR. A model to predict survival in patients with end-stage liver disease. Hepatology. 2001;33:464–470. doi: 10.1053/jhep.2001.22172. [DOI] [PubMed] [Google Scholar]
- 37.Infante-Rivard C, Esnaola S, Villeneuve JP. Clinical and statistical validity of conventional prognostic factors in predicting short-term survival among cirrhotics. Hepatology. 1987;7:660–664. doi: 10.1002/hep.1840070408. [DOI] [PubMed] [Google Scholar]
- 38.Hasse J, Strong S, Gorman MA, Liepa G. Subjective global assessment: alternative nutrition-assessment technique for liver-transplant candidates. Nutrition. 1993;9:339–343. [PubMed] [Google Scholar]
- 39.Mullen KD. Review of the final report of the 1998 Working Party on definition, nomenclature and diagnosis of hepatic encephalopathy. Aliment Pharmacol Ther. 2007;25 Suppl 1:11–16. doi: 10.1111/j.1746-6342.2006.03216.x. [DOI] [PubMed] [Google Scholar]
- 40.Working Group of the Australian and New Zealand Bone and Mineral Society; Endocrine Society of Australia; Osteoporosis Australia. Vitamin D and health in adults in Australia and New Zealand: a position statement. Med J Aust. 2005;182:281–285. doi: 10.5694/j.1326-5377.2005.tb06701.x. [DOI] [PubMed] [Google Scholar]
- 41.Carter GD. Accuracy of 25-hydroxyvitamin D assays: confronting the issues. Curr Drug Targets. 2011;12:19–28. doi: 10.2174/138945011793591608. [DOI] [PubMed] [Google Scholar]
- 42.Wallace AM, Gibson S, de la Hunty A, Lamberg-Allardt C, Ashwell M. Measurement of 25-hydroxyvitamin D in the clinical laboratory: current procedures, performance characteristics and limitations. Steroids. 2010;75:477–488. doi: 10.1016/j.steroids.2010.02.012. [DOI] [PubMed] [Google Scholar]
- 43.Moore KP, Wong F, Gines P, Bernardi M, Ochs A, Salerno F, Angeli P, Porayko M, Moreau R, Garcia-Tsao G, et al. The management of ascites in cirrhosis: report on the consensus conference of the International Ascites Club. Hepatology. 2003;38:258–266. doi: 10.1053/jhep.2003.50315. [DOI] [PubMed] [Google Scholar]
- 44.Vierling JM, Mokhtarani M, Brown RS, Mantry P, Rockey DC, Ghabril M, Rowell R, Jurek M, Coakley DF, Scharschmidt BF. Fasting Blood Ammonia Predicts Risk and Frequency of Hepatic Encephalopathy Episodes in Patients With Cirrhosis. Clin Gastroenterol Hepatol. 2016;14:903–906.e1. doi: 10.1016/j.cgh.2015.11.018. [DOI] [PubMed] [Google Scholar]
- 45.Trautwein C, Possienke M, Schlitt HJ, Böker KH, Horn R, Raab R, Manns MP, Brabant G. Bone density and metabolism in patients with viral hepatitis and cholestatic liver diseases before and after liver transplantation. Am J Gastroenterol. 2000;95:2343–2351. doi: 10.1111/j.1572-0241.2000.02269.x. [DOI] [PubMed] [Google Scholar]
- 46.Nair S. Vitamin d deficiency and liver disease. Gastroenterol Hepatol (N Y) 2010;6:491–493. [PMC free article] [PubMed] [Google Scholar]
- 47.Littlejohns TJ, Henley WE, Lang IA, Annweiler C, Beauchet O, Chaves PH, Fried L, Kestenbaum BR, Kuller LH, Langa KM, et al. Vitamin D and the risk of dementia and Alzheimer disease. Neurology. 2014;83:920–928. doi: 10.1212/WNL.0000000000000755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Darwish H, Zeinoun P, Ghusn H, Khoury B, Tamim H, Khoury SJ. Serum 25-hydroxyvitamin D predicts cognitive performance in adults. Neuropsychiatr Dis Treat. 2015;11:2217–2223. doi: 10.2147/NDT.S87014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pettersen JA. Vitamin D and executive functioning: Are higher levels better? J Clin Exp Neuropsychol. 2016;38:467–477. doi: 10.1080/13803395.2015.1125452. [DOI] [PubMed] [Google Scholar]
- 50.Jayakumar AR, Rama Rao KV, Norenberg MD. Neuroinflammation in hepatic encephalopathy: mechanistic aspects. J Clin Exp Hepatol. 2015;5:S21–S28. doi: 10.1016/j.jceh.2014.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Aldridge DR, Tranah EJ, Shawcross DL. Pathogenesis of hepatic encephalopathy: role of ammonia and systemic inflammation. J Clin Exp Hepatol. 2015;5:S7–S20. doi: 10.1016/j.jceh.2014.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kitson MT, Roberts SK. D-livering the message: the importance of vitamin D status in chronic liver disease. J Hepatol. 2012;57:897–909. doi: 10.1016/j.jhep.2012.04.033. [DOI] [PubMed] [Google Scholar]


