Abstract
Background
A previous study reported a negative association between perfluorooctane sulfonamide (PFOSA) concentrations and fecundability.
Methods
We examined this association among women enrolled in the Norwegian Mother and Child Cohort Study (MoBa), in 2003–2004. This analysis was restricted to 451 primiparous women to avoid bias due to previous pregnancy. Self-reported time-to-pregnancy (TTP) and plasma were obtained around 18 weeks of gestation. Approximately half of the women had measurable PFOSA levels; missing values were multiply imputed. We used the logistic analogue of discrete-time survival analysis to examine the adjusted association between PFOSA, other perfluoroalkyl substances (PFAS), and TTP.
Results
The median measured PFOSA concentration was 0.03 ng/ml (interquartile range (IQR)=0.02, 0.07). The age and BMI-adjusted association between an interquartile distance increase in PFOSA and TTP was 0.91 (95% CI=0.71–1.17). Imputation of missing PFOSA resulted in similar estimates. No association was observed with other PFAS.
Conclusion
Based on a weakly decreased fecundability odds ratio, found only limited support for an association between plasma PFOSA concentrations and TTP among primiparous women.
Introduction
Perfluoroalkyl substances (PFAS) are persistent synthetic compounds used in industry and consumer products.1 Perfluorooctane sulfonamide (PFOSA) is a breakdown product of higher molecular weight PFAS that are manufactured; PFOSA can also be synthesized directly and has been used in various products.2 A previous investigation of time-trends of PFAS in Norway found the highest concentrations of PFOSA in the 1980’s and 1990’s and reported no significant correlation between serum concentrations of PFOSA with the more commonly studied PFAS: perfluorooctanoic acid (PFOA) or perfluoroctane sulfonic acid (PFOS).3 Although the use of some PFAS has been phased out in many countries, exposure is ongoing and potential risks, including decreased fecundability, continue to be assessed. In a recent prospective study (n = 501), serum concentration of PFOSA, but not other PFAS, was associated with decreased fecundability.4 In that study,4 the authors employed an exposome approach to examine 63 maternal chemical exposures in relation to time-to-pregnancy (TTP), and PFOSA was one of five that showed an association with TTP, suggesting that additional focus on this compound was warranted. However, in a second prospective study (n = 129) no association between PFOSA concentrations and fecundability was present.5 Other studies of PFAS and fecundability have not evaluated PFOSA.6–8
We conducted a retrospective study of PFAS and fecundability with primary focus on the PFOSA-fecundability association.
Methods
The Norwegian Mother and Child Cohort Study (MoBa) is a prospective pregnancy cohort study conducted by the Norwegian institute of Public Health.9,10 Participants were recruited from Norway from 1999–2008; 40.6% of invited women participated. MoBa was approved by the Regional Committee for Medical Research Ethics and the Norwegian Data Inspectorate and informed consent was obtained from each participant. The present study was based on MoBa data file v4.301.
This analysis is based on a previous case-base study of PFAS and subfecundity among MoBa women with a live birth, enrolled in 2003–2004 (refer to eTable 1).11 The base group of the previous study consisted of a random sample of women, regardless of time-to-pregnancy (TTP), and the case group was a random sample of subfecund (TTP>12 months) women. Following the drop in women’s PFAS concentrations during pregnancy and lactation, levels begin to return to baseline; thus, the time it takes for women’s next pregnancy to occur is positively related to PFAS in the absence of a causal relation.11–16 Because including parous women and may result in biased estimates (refer to the DAG in eFigure 1), the present analyses are restricted to primiparous women. Because some investigators support including parous women in the analysis, we have provided the results for all women in the supplementary material (see eTable 2). Four women missing pre-pregnancy body mass index (BMI) were excluded, leaving 451 women, of which 204 (45.2%) were originally in the base sample and 247 (54.8%) were originally in the case sample. All women in the analysis had planned pregnancies.
Women completed a questionnaire at enrollment, providing demographic information and reproductive history, including number of months of regular intercourse without contraception before becoming pregnant, from which TTP was derived. Women additionally provided plasma samples at enrollment.17 The median gestational age of the blood draw among the women included in the present analysis was 18 weeks (interquartile range (IQR) = 17, 20)). As described elsewhere, 13 PFAS compounds were measured in plasma using high performance liquid chromatography/tandem mass spectrometry.18 As previously mentioned, the focus of the present analysis is on PFOSA. Although 66 women (14.6%) had PFOSA concentrations >LOQ (0.05 ng/ml), an additional 160 (35.5%) had measured PFOSA concentrations <LOQ, resulting in 226 (50.1%) women with a non-missing PFOSA value (Table 1). (In the previous report by Buck-Louis et al.,4 10% of the sample had non-missing PFOSA values). To calculate the median PFAS concentrations among all women, we assigned missing PFAS concentrations a value equal to the LOQ divided by the square root of two.
Table 1.
Crude and adjusted fecundability odds ratios (FOR)a for the association between perfluoroalkyl substances (ng/ml) and time to pregnancy among 451 primiparous women from a case-base study among the Norwegian Mother and Child Cohort (MoBa) Study, 2003–2004
| n (%) >LOQb |
nc | Median A (IQR)d | Median B (IQR)e | Crude | Adjustede | |||||
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| FOR | Lower Limit |
Upper Limit |
FOR | Lower Limit |
Upper Limit |
|||||
|
|
|
|
||||||||
| Perfluorinated Sulfonamide | ||||||||||
| PFOSA | 66 (14.6) | 226 | 0.04 (0.03, 0.04) | 0.03 (0.02, 0.07) | 0.95 | 0.75 | 1.22 | 0.91 | 0.71 | 1.17 |
| Perfluorinated Carboxylates | ||||||||||
| PFBA | 0 (0.0) | – | – | – | — | — | — | — | — | — |
| PFHpA | 63 (14.0) | – | – | – | — | — | — | — | — | — |
| PFOA | 451 (100.0) | 451 | 2.82 (2.21, 3.54) | 2.82 (2.21, 3.54) | 1.06 | 0.92 | 1.22 | 1.04 | 0.90 | 1.20 |
| PFNA | 451 (100.0) | 451 | 0.43 (0.32, 0.57) | 0.43 (0.32, 0.57) | 1.12 | 0.96 | 1.31 | 1.08 | 0.92 | 1.27 |
| PFDA | 336 (74.5) | 429 | 0.11 (0.05, 0.16) | 0.11 (0.06, 0.17) | 1.04 | 0.89 | 1.22 | 1.00 | 0.85 | 1.18 |
| PFUnDA | 421 (93.3) | 447 | 0.23 (0.14, 0.34) | 0.23 (0.14, 0.34) | 1.01 | 0.85 | 1.19 | 0.93 | 0.78 | 1.11 |
| PFDoDA | 114 (25.3) | 410 | 0.04 (0.03, 0.05) | 0.04 (0.02, 0.06) | 0.96 | 0.84 | 1.09 | 0.91 | 0.77 | 1.08 |
| PFTrDA | 122 (27.0) | 353 | 0.04 (0.03, 0.06) | 0.04 (0.02, 0.07) | 1.05 | 0.89 | 1.24 | 1.00 | 0.85 | 1.19 |
| PFTeDA | 4 (0.01) | – | – | – | — | — | — | — | — | — |
| Perfluorinated Sulfonates | ||||||||||
| PFHxS | 450 (99.8) | 451 | 0.70 (0.53, 1.06) | 0.70 (0.53, 1.06) | 0.98 | 0.91 | 1.06 | 0.97 | 0.90 | 1.05 |
| PFHpS | 412 (91.4) | 446 | 0.15 (0.10, 0.22) | 0.16 (0.10, 0.22) | 1.03 | 0.88 | 1.20 | 1.02 | 0.87 | 1.19 |
| PFOS | 451 (100.0) | 451 | 14.56 (11.70, 18.45) | 14.56 (11.70, 18.45) | 1.01 | 0.89 | 1.14 | 1.00 | 0.88 | 1.13 |
PFOSA: perfluorooctane sulfonamide; PFBA: perfluorobutanoic acid; PFHpA: Perfluoroheptanoic acide; PFOA: perlurooctanoic acid; PFNA: perfluorononanoic acid; PFDA: perfluorodecanoic acid; PFUNDA: perfluoroundecanoic acid; PFDoDA: perfluordodecanoic acid; PFTrDA: perfluorotridecanoic acid; PFTeDA: perfluorotridecanoic acid; PFHxS: perfluorohexane sulfonate; PFHpS: perfluoroheptane sulfonate; PFOS: perfluorooctane sulfonate
FORs represent the odds of conception in a given month per interquartile increase in PFAS (ng/ml) concentration, based on the interquartile distance corresponding to Median B.
The LOQ for PFBA was 0.1 ng/ml; the LOQ for all other compounds was 0.05 ng/ml.
Indicates the number of observations included in the FOR analysis (i.e., this is the number of women with PFAS concentration >LOQ plus women with measured PFAS concentration <LOQ). We did not analyze PFBA, PFHpA, or PFTeDA for lack of observations.
Medians were calculated among all 451 women, assigning a value equal to the LOQ/sqrt(2) for non-measured PFAS concentrations
Medians were calculated among women included in the FOR analysis (i.e., women with PFAS concentration >LOQ plus women with measured PFAS concentration <LOQ).
Adjusted for maternal age at conception and pre-pregnancy BMI
We used the logistic analogue of discrete-time survival analysis in SAS v9.4 (Cary, NC) to estimate fecundability odds ratios (FOR) and 95% confidence intervals (CI) for the associations between plasma PFAS concentrations and TTP. PFAS concentrations were divided by the interquartile distance (IQD) of the distribution of values among women with a non-missing value. Therefore, the FORs represent the odds of conception in a given month per interquartile increase in PFAS concentration. To avoid medical intervention bias, TTP was censored at 13 months. Three women reported receiving infertility treatment for the index pregnancy at or before 12 months and were censored at TTP-1 month, such that they only contributed unsuccessful pregnancy attempts to analyses.
Maternal age at the pregnancy attempt (years) and pre-pregnancy BMI (kg/m2) were included in the model a priori. A DAG-informed approach was used to identify additional covariates (see eFigure 1). We chose the following potential confounders based on previous knowledge of their association with plasma PFAS concentration and potential to influence fecundity: maternal education, maternal annual income, maternal pre-pregnancy smoking, and maternal shellfish, lean fish, and oily fish consumption (g/day), menstrual cycle length, and frequency of intercourse during the month prior to pregnancy. The inclusion of any of these variables to age and BMI-adjusted models did not alter the effect estimates; the final models included only maternal age and BMI.
We implemented three multiple imputation (MI) models, assuming a missing-at-random pattern and using Markov Chain Monte Carlo methods to impute PFOSA values for 225 (49.9%) women missing this information. The three models differed according to predictors included in the model (see supplementary material).
Results
Women in this study were relatively young (75.4% < 30 years old), of normal weight (60.8% had BMI of 18.50–24.99), and well educated (64.1% had at least some college) (see eTable 3). The median PFOSA concentration among all 451 women was 0.04 (interquartile range (IQR) = 0.03, 0.04) and median concentration among the 226 women for which we had a measured value was 0.03 ng/ml (interquartile range (IQR) = 0.02, 0.07) (Table 1).
Among the 226 primiparous women with measured plasma PFOSA concentrations, the adjusted association between PFOSA and TTP was 0.91 (95% CI=0.71–1.17) (Table 1). Using imputed values of PFOSA concentrations resulted in similar estimates (Figure 1). We did not observe an association between any other PFAS and TTP among primiparous women (Table 1). The adjusted FOR for the association between PFOSA and TTP among all women was 0.85 (95% CI=0.83–1.09, supplementary Table 2).
Figure 1. Maternal age and pre-pregnancy BMI-adjusted fecundability odds ratios (FOR) for the association between PFOSA (ng/ml) and time to pregnancy among primiparous women from the Norwegian Mother and Child Cohort Study, 2003–2004.
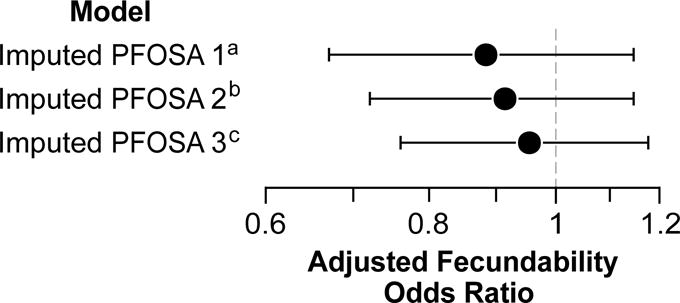
FORs represent the odds of conception in a given month per interquartile increase in PFOSA concentration. aImputation model 1 included the following covariates as predictors of PFOSA concentrations: maternal age at conception, maternal pre-pregnancy BMI, maternal education, maternal annual income, maternal pre-pregnancy smoking, maternal consumption of shellfish, lean fish, and oily fish during pregnancy, menstrual cycle regularity, oral contraceptive use in the previous 12 months, and serum albumin concentration (g/dL). bImputation model 2 included all of the model 1 covariates as well as concentrations of PFDA, PFDoDA, PFHpS, PFHxS, PFNA, PFOA, PFOS, PFTrDA, and PFUnDA. cImputation model 3 included the model 1 variables plus PFNA concentrations, which had the strongest correlation with PFOSA concentrations (r=0.27, p<0.001).
Discussion
Based on a weakly decreased FOR, we found only limited support for an association between plasma PFOSA concentrations and TTP among primiparous women in MoBa. Although nearly half of the women had missing PFOSA measurements, we employed MI techniques to overcome this limitation. Few previous studies have examined the association between PFOSA and fecundity. In a recent report from a prospective cohort which recruited couples during 2005–2007, reduced fecundity was associated with a standard-deviation increase in the log-transformed serum concentration of PFOSA (FOR=0.8, 95% CI=0.7, 0.9).4 In that study,MI techniques were employed to impute missing values for 90% of women with missing PFOSA levels.4 Results from a second prospective study5 indicated no association between PFOSA (as a log-transformed and continuous variable) and TTP (FOR=1.0; 95% CI=0.9, 1.2) and are consistent with the results from the present analysis. In both studies, the geometric mean or median PFOSA concentration was roughly 0.11 ng/ml.4,5
In the present study, women were recruited in 2003–2004; 14.6% of women had PFOSA levels >0.05 ng/ml. However, 35.5% of the women with values <LOQ had measured values which we utilized, imputing values for the remaining 49.9% of subjects. We explored three sets of variables to assess the robustness of the imputation model. PFAS concentrations measured in MoBa were lower than reported in either Vestergaard et al.5 or Buck-Louis et al.,4 where collection of biologic specimens occurred in the first trimester. In the present study, blood draws occurred around week 18 when plasma volume expansion (PVE) may have resulted in lower measured levels of PFAS.19 The correlation among PFAS concentration across different points in pregnancy, however, has been shown to be relatively high, e.g., 0.87 for PFOS and 0.88 for PFOA between measures in the first and second trimester, respectively.20 The present analysis employed a retrospective assessment of TTP. Previous studies of the validity of retrospectively assessed TTP revealed individual inaccuracies in self-reported TTP, with less accurate recall for longer TTP,21,22 although it has been noted that the overall distribution of retrospectively assessed TTP is valid.23 Although women were unaware of their exposure, PFAS could vary according to factors related to better TTP recall, such as education. Adjustment for education did not affect the results and we believe that differential misclassification of TTP by exposure was unlikely. The exclusion of women who are sterile or who have pregnancy losses from the present analysis may have limited the generalizability of our results or resulted in bias. Due to the influence of parity, we restricted our analysis to primiparous women to avoid the potential for reverse causality between PFAS concentrations and TTP among parous women.11–16 Due to the original selection of women for a case-base study of subfecundity, a large proportion (55%) of women in this analysis are subfecund. When analyses were restricted to women selected from the base sample, results were not meaningfully changed. Overall, our results do not support an association between plasma PFAS concentrations and decreased fecundity.
Supplementary Material
Acknowledgments
We are grateful to all the participating families in Norway who take part in this on-going cohort study. We would also like to thank Cathrine Carlson Bach, Penelope P. Howards, Katie M. O’Brien, and Alexandra J. White who provided helpful input on the causal graphs shown in the supplement.
Footnotes
Conflicts of Interest and Source of Funding: This research was supported in part by the Intramural Research Program of the National Institutes of Health, National Institute of Environmental Health Sciences. The Norwegian Mother and Child Cohort Study is supported by the Norwegian Ministry of Health, NIH/NIEHS (contract no N01-ES-85433), NIH/NINDS (grant no.1 UO1 NS 047537-01), and the Norwegian Research Council/FUGE (grant no. 151918/S10).
While this manuscript was being revised in response to reviewer comments, MPL began working part-time at Ramboll, with support from 3M. The work on the revision was done solely with NIEHS support (MPL as a government contractor). Each author certifies that their freedom to design, conduct, interpret, and publish research was not compromised by any sponsor.
References
- 1.Buck RC, Franklin J, Berger U, Conder JM, Cousins IT, de Voogt P, Jensen AA, Kannan K, Mabury SA, van Leeuwen SP. Perfluoroalkyl and polyfluoroalkyl substances in the environment: terminology, classification, and origins. Integr Environ Assess Manag. 2011;7(4):513–41. doi: 10.1002/ieam.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen M, Qiang L, Pan X, Fang S, Han Y, Zhu L. In vivo and in vitro isomer-specific biotransformation of perfluorooctane sulfonamide in common carp (Cyprinus carpio) Environ Sci Technol. 2015 doi: 10.1021/acs.est.5b00488. Just Accepted Manuscript. [DOI] [PubMed] [Google Scholar]
- 3.Haug LS, Thomsen C, Becher G. Time trends and the influence of age and gender on serum concentrations of perfluorinated compounds in archived human samples. Environ Sci Technol. 2009;43(6):2131–6. doi: 10.1021/es802827u. [DOI] [PubMed] [Google Scholar]
- 4.Buck Louis GM, Sundaram R, Schisterman EF, Sweeney AM, Lynch CD, Gore-Langton RE, Maisog J, Kim S, Chen Z, Barr DB. Persistent environmental pollutants and couple fecundity: the LIFE study. Environ Health Perspect. 2013;121(2):231–6. doi: 10.1289/ehp.1205301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vestergaard S, Nielsen F, Andersson AM, Hjollund NH, Grandjean P, Andersen HR, Jensen TK. Association between perfluorinated compounds and time to pregnancy in a prospective cohort of Danish couples attempting to conceive. Hum Reprod. 2012;27(3):873–80. doi: 10.1093/humrep/der450. [DOI] [PubMed] [Google Scholar]
- 6.Fei CY, McLaughlin JK, Lipworth L, Olsen J. Maternal levels of perfluorinated chemicals and subfecundity. Human Reproduction. 2009;24(5):1200–1205. doi: 10.1093/humrep/den490. [DOI] [PubMed] [Google Scholar]
- 7.Velez MP, Arbuckle TE, Fraser WD. Maternal exposure to perfluorinated chemicals and reduced fecundity: the MIREC study. Hum Reprod. 2015;30(3):701–9. doi: 10.1093/humrep/deu350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bach CC, Bech BH, Nohr EA, Olsen J, Matthiesen NB, Bossi R, Uldbjerg N, Bonefeld-Jorgensen EC, Henriksen TB. Serum perfluoroalkyl acids and time to pregnancy in nulliparous women. Environ Res. 2015;142:535–41. doi: 10.1016/j.envres.2015.08.007. [DOI] [PubMed] [Google Scholar]
- 9.Nilsen RM, Vollset SE, Gjessing HK, Skjærven R, Melve KK, Schreuder P, Alsaker ER, Haug K, Daltveit AK, Magnus P. Self-selection and bias in a large prospective pregnancy cohort in Norway. Paediatric and Perinatal Epidemiology. 2009;23(6):597–608. doi: 10.1111/j.1365-3016.2009.01062.x. [DOI] [PubMed] [Google Scholar]
- 10.Magnus P, Irgens LM, Haug K, Nystad W, Skjærven R, Stoltenberg C. Cohort profile: the Norwegian Mother and Child Cohort Study (MoBa) Int J Epidemiol. 2006;35(5):1146–50. doi: 10.1093/ije/dyl170. [DOI] [PubMed] [Google Scholar]
- 11.Whitworth KW, Haug LS, Baird DD, Becher G, Hoppin JA, Skjaerven R, Thomsen C, Eggesbo M, Travlos G, Wilson R, Longnecker MP. Perfluorinated compounds and subfecundity in pregnant women. Epidemiology. 2012;23(2):257–63. doi: 10.1097/EDE.0b013e31823b5031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Loccisano AE, Longnecker MP, Campbell JL, Jr, Andersen ME, Clewell HJ., 3rd Development of PBPK models for PFOA and PFOS for human pregnancy and lactation life stages. J Toxicol Environ Health A. 2013;76(1):25–57. doi: 10.1080/15287394.2012.722523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brantsaeter AL, Whitworth KW, Ydersbond TA, Haug LS, Haugen M, Knutsen HK, Thomsen C, Meltzer HM, Becher G, Sabaredzovic A, Hoppin JA, Eggesbo M, Longnecker MP. Determinants of plasma concentrations of perfluoroalkyl substances in pregnant Norwegian women. Environ Int. 2013;54:74–84. doi: 10.1016/j.envint.2012.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Olsen GW, Butenhoff JL, Zobel LR. Perfluoroalkyl chemicals and human fetal development: An epidemiologic review with clinical and toxicological perspectives. Reproductive Toxicology. 2009;27(3–4):212–230. doi: 10.1016/j.reprotox.2009.02.001. [DOI] [PubMed] [Google Scholar]
- 15.Gómez-Canela C, Fernández-Sanjuan M, Farrés M, Lacorte S. Factors affecting the accumulation of perfluoroalkyl substances in human blood. Environmental Science and Pollution Research. 2015;22(2):1480–1486. doi: 10.1007/s11356-014-3439-x. [DOI] [PubMed] [Google Scholar]
- 16.Berg V, Nost TH, Huber S, Rylander C, Hansen S, Veyhe AS, Fuskevag OM, Odland JO, Sandanger TM. Maternal serum concentrations of per- and polyfluoroalkyl substances and their predictors in years with reduced production and use. Environ Int. 2014;69:58–66. doi: 10.1016/j.envint.2014.04.010. [DOI] [PubMed] [Google Scholar]
- 17.Ronningen KS, Paltiel L, Meltzer HM, Nordhagen R, Lie KK, Hovengen R, Haugen M, Nystad W, Magnus P, Hoppin JA. The biobank of the Norwegian Mother and Child Cohort Study: a resource for the next 100 years. Eur J Epidemiol. 2006;21(8):619–25. doi: 10.1007/s10654-006-9041-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Haug LS, Thomsen C, Becher G. A sensitive method for determination of a broad range of perfluorinated compounds in serum suitable for large-scale human biomonitoring. Journal of Chromatography A. 2009;1216(3):385–393. doi: 10.1016/j.chroma.2008.10.113. [DOI] [PubMed] [Google Scholar]
- 19.Faupel-Badger JM, Hsieh CC, Troisi R, Lagiou P, Potischman N. Plasma volume expansion in pregnancy: implications for biomarkers in population studies. Cancer Epidemiol Biomarkers Prev. 2007;16(9):1720–3. doi: 10.1158/1055-9965.EPI-07-0311. [DOI] [PubMed] [Google Scholar]
- 20.Fei C, McLaughlin JK, Tarone RE, Olsen J. Perfluorinated chemicals and fetal growth: a study within the Danish National Birth Cohort. Environmental Health Perspectives. 2007;115(11):1677–1682. doi: 10.1289/ehp.10506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Joffe M, Villard L, Li Z, Plowman R, Vessey M. A time to pregnancy questionnaire designed for long term recall: Validity in Oxford, England. Journal of Epidemiology and Community Health. 1995;49(3):314–319. doi: 10.1136/jech.49.3.314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cooney MA, Buck Louis GM, Sundaram R, McGuiness BM, Lynch CD. Validity of self-reported time to pregnancy. Epidemiology. 2009;20(1):56–9. doi: 10.1097/EDE.0b013e31818ef47e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Joffe M. Validity of self-reported time to pregnancy. Epidemiology. 2010;21(1):160–161. doi: 10.1097/EDE.0b013e3181c1ec69. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


