Abstract
Recent investigations on the pathway of cell entry by polyomavirus (Py) and simian virus 40 (SV40) have defined specific gangliosides as functional receptors mediating virus binding and transport from the plasma membrane to the endoplasmic reticulum (B. Tsai et al., EMBO J. 22:4346-4355, 2003; Gilbert and Benjamin, in press). These studies were carried out with C6 rat glioma cells, a heterologous host chosen for its known deficiency in ganglioside biosynthesis. Here, a cell genetic approach was undertaken to identify components required for the early steps of infection using mouse cells as the natural host for Py. Receptor-negative (R−) mouse cells, screened based on resistance to Py infection, were shown to bind Py but failed to allow entry of the virus. R− cells were also found to be resistant to SV40. Infectibility was restored or enhanced by the addition of the same specific gangliosides found in earlier studies with C6 cells. In one R− line, overexpression of caveolin-1 also increased infectibility. These results support and extend findings on gangliosides in lipid rafts as functional receptors and mediators of internalization for Py and SV40.
Screening for R− cells.
Mouse cell lines were screened for resistance to polyomavirus (Py) at early stages of infection according to the following criteria: (i) failure to develop cytopathic effects following a high-multiplicity infection and failure to produce progeny virus and (ii) ability to produce a high yield of infectious virus following transfection with viral DNA. A dozen lines, chosen randomly from the American Type Culture Collection catalogue or derived in our laboratory, were screened. Three were found to meet these criteria. These have been denoted as R− cell lines for being functionally “receptor negative.” RAG-1 is a spontaneous renal adenocarcinoma cell line (purchased from the ATCC); A1-1 and A2855 were derived from Py-induced mammary tumors and are virus free. Besides failing to develop cytopathic effects following infection by small- and large-plaque Py strains, these R− cell lines were a hundred-fold less infectible than NIH 3T3 cells as judged by the lower single-cycle yields following infection at a low multiplicity of infection (MOI). Their resistance is not due to an intracellular block in replication since they produced levels of virus comparable to those in NIH 3T3 cells following transfection with viral DNA (Table 1).
TABLE 1.
Relative efficiencies of infection and transfection in R− cells
| Cell line | Virus yielda
|
Relative efficiencyb | |
|---|---|---|---|
| Infection | Transfection | ||
| NIH 3T3 | 3.7 × 106 | 6.2 × 105 | 1.00 |
| RAG-1 | 3.4 × 104 | 3.9 × 105 | 0.015 |
| A1-1 | 5.8 × 104 | 8.3 × 105 | 0.012 |
| A2855 | 2.0 × 104 | 5.6 × 105 | 0.006 |
Virus yields from each cell line were measured 48 h pi (infected at an MOI of 2 to 5 PFU/cell) or transfection as PFU per milliliter on UCI-B cells.
Relative efficiencies are calculated for each cell line as the ratio of virus yields (infection/transfection) normalized to the ratio on NIH 3T3 cells.
Ganglioside addition restores infectibility without increasing overall levels of virus binding.
Recent studies of the C6 rat glioma cell line defective in glycolipid biosynthesis (8) showed that preincubation with ganglioside GD1a substantially enhanced infectibility by Py while addition of the related ganglioside GM1 enhanced susceptibility to simian virus 40 (SV40) (3a, 11). Screened for resistance only to Py, R− cells also proved to be resistant to SV40. Though not selected based on any known defect in ganglioside biosynthesis, R− cells showed greatly enhanced susceptibility to viral infection following addition of the same specific gangliosides (Table 2). Addition of GD1a to R− cells had little or no effect on overall levels of binding of biotinylated Py (data not shown). These results indicate that GD1a provides specific functional binding sites amidst an abundance of nonspecific sites that either fail to mediate internalization and infection or do so inefficiently.
TABLE 2.
Effect of gangliosides on infection of R− cells by polyomavirus and SV40
| Cell line | % LT antigen positivea
|
|||
|---|---|---|---|---|
| Polyomavirus
|
SV40
|
|||
| −GD1a | +GD1a | −GM1 | +GM1 | |
| RAG-1 | 0 | 8 ± 1 | 0 | 1.4 ± 0.5 |
| A1-1 | 1.1 ± 1 | 24 ± 4 | 3.1 ± 0.5 | 38 ± 3.5 |
| A2855 | 0 | 12 ± 1.5 | 6 ± 1.2 | 30 ± 1 |
Cells were infected at an MOI of ∼200 and were fixed 32 h postinfection and analyzed for LT antigen expression by indirect immunofluorescence; values indicate the percentage of total cells that were LT antigen positive. Each experiment was performed three times. The data presented are an average of the three experiments.
Cholesterol and cytoskeletal requirements for Py infection of GD1a-supplemented R− cells.
Previous studies have given different results concerning the role of caveolae and dependence on cholesterol for infection by Py in different cells (2, 3, 4, 5, 10). Results in a recent study using C6 cells suggested the existence of two entry pathways that were at least partially distinct, a GD1a-mediated pathway exhibiting colocalization with caveolin-1 (Cav-1) and that was inhibitable by cholesterol-sequestering drugs and by microfilament-altering agents, and a less efficient pathway of basal infection in nonsupplemented cells that was not blocked by the same cholesterol-disrupting agents or agents affecting actin microfilaments (3, 3a). Py infection of GD1a-supplemented R− cells also required cholesterol and was dependent on intact microtubules (Colcemid sensitive and taxol resistant) as well as on a dynamic state of the microfilament system (Table 2). These results are essentially the same as found for GD1a-supplemented C6 cells (3a).
Bacterial toxins as a probe for cell surface gangliosides in R− cells.
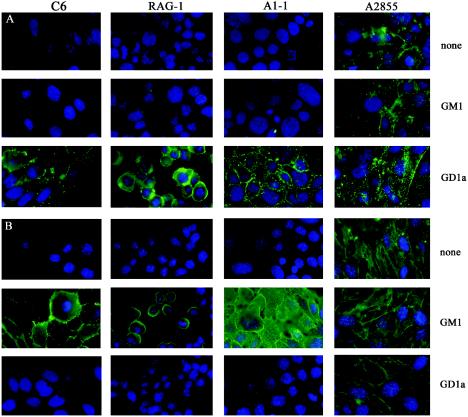
To determine whether R− cells are deficient in the cell surface expression of gangliosides, cells were exposed to fluorescent derivatives of the Escherichia coli heat-labile toxin LT-IIb that utilizes GD1a as its receptor (12) and of cholera toxin that utilizes GM1 (1, 13). Unsupplemented A1-1 and RAG-1 showed no detectible staining with LT-IIb, indicating absence of GD1a. In contrast, A2855 clearly stained. Addition of GD1a but not GM1 gave clear staining by LT-IIb in all three cells, as expected (Fig. 1A). Using labeled cholera toxin B subunit, A2855 cells were again positive while the other two lines were negative. Supplementation with GM1 but not GD1a led to positive staining by cholera toxin in all cells (Fig. 1B). These results support the conclusion that the absence or insufficiency of cell surface gangliosides accounts for virus resistance in at least two of the R− cells.
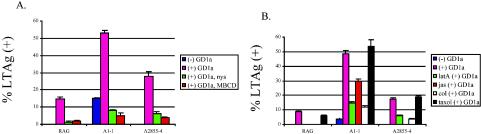
FIG. 1.
Compounds affecting Py infectivity on R− cells. R− cells supplemented with GD1a were treated with MBCD (methyl-β-cyclodextrin), nystatin, latrunculin A, jasplakinolide, demecolcine, or taxol for 1 h at 37°C prior to infection. Cells were infected at an MOI of ∼500 in the presence or absence of these compounds, as indicated. Infected cells were processed and analyzed for Py LT antigen (LTAg) by indirect immunofluorescence. Each individual experiment was performed three times in triplicate. The data presented are an average of the three experiments.
Caveolin is limiting in one R− cell line.
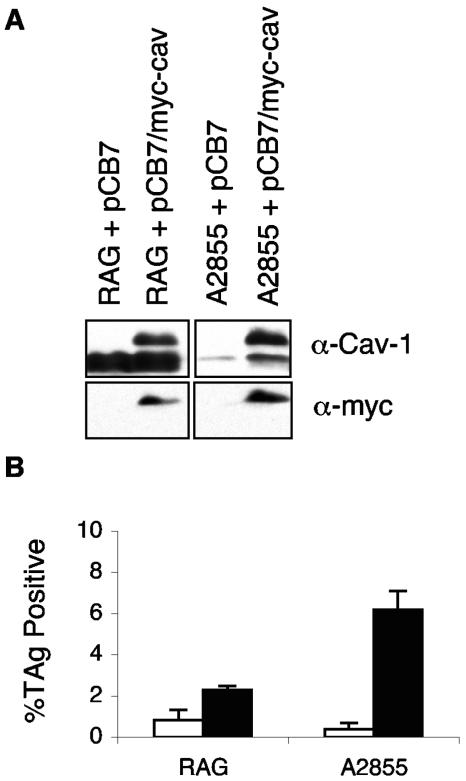
A2855 cells are of particular interest because they constitutively express gangliosides on the cell surface yet become highly infectible only after preincubation with exogenous gangliosides (Fig. 2 and Table 2). In intestinal epithelial cells, gangliosides must associate with lipid rafts to carry toxins from the plasma membrane to the endoplasmic reticulum where they unfold, rearrange, and translocate to the cytosol (1, 13). Gangliosides that do not associate with lipid rafts bind toxin, which is then conveyed to an endosomal compartment and fails to induce toxicity (1, 13). To investigate the failure of endogenous gangliosides in A2855 cells to mediate infection, we tested whether the gangliosides were dependent on caveolae for their function as virus receptors. Immunoblots of cell extracts with anti-Cav-1 showed that A2855 expressed very small amounts of Cav-1 compared to RAG-1 or NIH 3T3. Clones of A2855 and RAG-1 expressing exogenous myc-tagged Cav-1 (7) were isolated and tested to see if raising the levels of Cav-1 expression could rescue Py infectibility without addition of GD1a (Fig. 3). Increased expression of Cav-1 in A2855 cells was correlated with a roughly 15-fold increase in susceptibility to Py in the absence of added GD1a. A lesser enhancement (two- to threefold) was seen in RAG-1 cells that expressed a much higher level of endogenous Cav-1 along with the exogenous protein. Thus, in A2855 cells, the low level of Cav-1 appears to be a limiting factor along with GD1a for virus uptake. Overall, the results strongly suggest that the binding of virus to its ganglioside receptor in lipid rafts associated with caveolae leads to efficient virus uptake and infection.
FIG. 2.
Binding of toxins to R− cells. R− cells with or without GM1 or GD1a supplementation bind Alexa fluor 488-labeled LT-IIb (A) or Alexa fluor 488-labeled cholera toxin B (B) at 4°C for 1 h. Samples were washed, fixed, permeabilized, and examined by epifluorescence microscopy.
FIG. 3.
Cav-1 expression. (A) Cav-1 and myc-tagged Cav-1 expression in R− clones expressing pCB7 (vector alone) or pCB7/myc-cav (vector containing myc-tagged Cav). Western blots were probed with antibody to Cav (α-Cav-1) or antibody to the myc tag (α-myc). (B) Effect of expression of exogenous Cav on infectibility of R− clones by Py in the absence of added GD1a. Infected cells were processed and analyzed for Py LT antigen (LTAg) by indirect immunofluorescence.
Defects in R− cells.
In principle, R− cells could be defective at any stage of infection, from virus attachment, entry, intracellular transport to sites of uncoating, possible cofactors in disassembly per se, to nuclear entry and the initiation of viral gene expression. In the three R− lines examined thus far, however, the only defects observed were in expression of appropriate levels of gangliosides in association with caveolae. R− cells have an abundance of nonfunctional binding sites corresponding most likely to sialoglycoproteins. The low efficiency of basal infection in some R− cells as well as C6 cells (3a) may occur via glycoprotein receptors in a non-caveola-dependent pathway (2). Alternatively, uptake in the basal pathway may be mediated by gangliosides not present in cholesterol-rich membrane domains associated with caveolae.
The R− cells as well as C6 cells were all derived from tumors and may share common defects in the synthesis or distribution of gangliosides on the cell surface. For the Py tumor lines, loss of functional receptors could be a consequence of selection in vivo for virus resistance (10). The underlying basis of the defect(s) is unknown. Heterogeneity in the ceramide portion of the gangliosides may conceivably play a role in sorting virus-containing vesicles along the retrograde pathway to the endoplasmic reticulum. Thus, A2855 cells may express gangliosides lacking the proper ceramide structure to function efficiently as virus receptors. Loss of the enzyme GM2/GD2 synthase may also underlie the phenotype in some R− cells. This enzyme functions as an N-acetylgalactosaminyltransferase in an early step in ganglioside biosynthesis (4, 9) and is required to form the precursors of both GD1a and GM1.
Acknowledgments
We thank M. Lisanti for the caveolin expression vector. We also gratefully acknowledge the work of Aron Lukacher and John Carroll in the isolation of the mammary tumor cell lines and Roxanne Peterman in the characterization of Cav-1 expression.
This work has been supported by NIH grants to T.B. (RO1CA-082395 and PO1 CA50661; D. Livingston) and to R.H. (AI-31940) and by grants from the Harvard Digestive Disease Center to W.L. (DK48106 and DK34854). T.B. is a Virginia and D. K. Ludwig Professor of Cancer Research and Teaching.
REFERENCES
- 1.Fujinaga, Y., A. A. Wolf, C. Rodighiero, H. Wheeler, B. Tsai, L. Allen, M. G. Jobling, T. Rapoport, R. K. Holmes, and W. I. Lencer. 2003. Gangliosides that associate with lipid rafts mediate transport of cholera and related toxins from the plasma membrane to ER. Mol. Biol. Cell 14:4783-4793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gilbert, J. M., and T. L. Benjamin. 2000. Early steps of polyomavirus entry into cells. J. Virol. 74:8582-8588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gilbert, J. M., I. G. Goldberg, and T. L. Benjamin. 2003. Cell penetration and trafficking of polyomavirus. J. Virol. 77:2615-2622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3a.Gilbert, J. M., and T. L. Benjamin. 2004. Uptake pathway of polyomavirus via ganglioside GD1a. J. Virol. 78:12259-12267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kolter, T., R. L. Proia, and K. Sandhoff. 2002. Combinatorial ganglioside biosynthesis. J. Biol. Chem. 277:25859-25862. [DOI] [PubMed] [Google Scholar]
- 5.Mannová, P., and J. Forstová. 2003. Mouse polyomavirus utilizes recycling endosomes for a traffic pathway independent of COPI vesicle transport. J. Virol. 77:1672-1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Richterová, Z., D. Liebl, M. Horák, Z. Palková, J. Štokrová, P. Hozák, J. Korb, and J. Forstova. 2001. Caveolae are involved in the trafficking of mouse polyomavirus virions and artificial VP1 pseudocapsids toward cell nuclei. J. Virol. 75:10880-10891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Scherer, P. E., Z. Tang, M. Chun, M. Sargiacomo, H. F. Lodish, and M. P. Lisanti. 1995. Caveolin isoforms differ in their N-terminal protein sequence and subcellular distribution. J. Biol. Chem. 270:16395-16401. [DOI] [PubMed] [Google Scholar]
- 8.Sottocornola, E., I. Colombo, B. Vergani, G. Taraboletti, and B. Berra. 1999. Increase tumorigenicity and invasiveness of C6 rat glioma cells transfected with the human α-2,8 sialyltransferase cDNA. Invasion Metastasis 18:142-154. [DOI] [PubMed] [Google Scholar]
- 9.Takamiya, K., A. Yamamoto, K. Furukawa, S. Yamashiro, M. Shin, M. Okada, S. Fukumoto, M. Haraguchi, N. Takeda, K. Fujimura, M. Sakae, M. Kishikawa, H. Shiku, K. Furukawa, and S. Aizawa. 1996. Mice with disrupted GM2/GD2 synthase gene lack complex gangliosides but exhibit only subtle defects in their nervous system. Proc. Natl. Acad. Sci. USA 93:10662-10667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Talmage, D. A., R. Freund, T. Dubensky, M. Salcedo, P. Gariglio, L. M. Rangel, C. J. Dawe, and T. L. Benjamin. 1992. Heterogeneity in state and expression of viral DNA in polyoma virus-induced tumors of the mouse. Virology 187:734-747. [DOI] [PubMed] [Google Scholar]
- 11.Tsai, B., J. M. Gilbert, T. Stehle, W. Lencer, T. L. Benjamin, and T. Rapoport. 2003. Gangliosides are receptors for murine polyoma virus and SV40. EMBO J. 22:4346-4355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van den Akker, F., S. Sarfaty, E. M. Twiddy, T. D. Connell, R. K. Holmes, and W. G. Hol. 1996. Crystal structure of a new heat-labile enterotoxin, LT-IIb. Structure 4:665-678. [DOI] [PubMed] [Google Scholar]
- 13.Wolf, A. A., Y. Fujinaga, and W. I. Lencer. 2002. Uncoupling of the cholera toxin-GM1 ganglioside receptor complex from endocytosis, retrograde Golgi trafficking, and downstream signal transduction by depletion of membrane cholesterol. J. Biol. Chem. 277:16249-16256. [DOI] [PubMed] [Google Scholar]