Abstract
The poxviral RING protein p28 is a virulence factor whose molecular function is unknown. Many cellular RING-containing proteins act as ubiquitin ligases (RING-E3s) connecting selected substrate proteins to the ubiquitination machinery. Here we demonstrate that vaccinia virus p28 and its homologue in myxoma virus, M143R, can mediate the formation of polyubiquitin conjugates, while RING mutants of both p28 and M143R cannot. Furthermore, p28 is ubiquitinated in vivo and ubiquitin colocalizes with p28 to virus factories independently of an intact RING domain. These results implicate the ubiquitin system in poxviral virulence.
Poxviruses are large, complex viruses that replicate in the cytoplasm of infected cells (10). The poxviral RING (really interesting new gene) domain protein, p28, localizes to viral factories and is encoded by members of the leporipoxviruses and orthopoxviruses (12, 14). Notable exceptions include vaccinia virus (VV) strain WR, which contains a truncated copy of p28, and VV strain Copenhagen, which lacks p28 entirely (12, 14). Although the precise molecular function of p28 is presently unknown, p28 expression has been linked to apoptosis inhibition and a p28 knockout in ectromelia virus was strongly attenuated, resulting in reduced growth in macrophages and the complete clearance of virus from infected mice (2, 3, 11, 12).
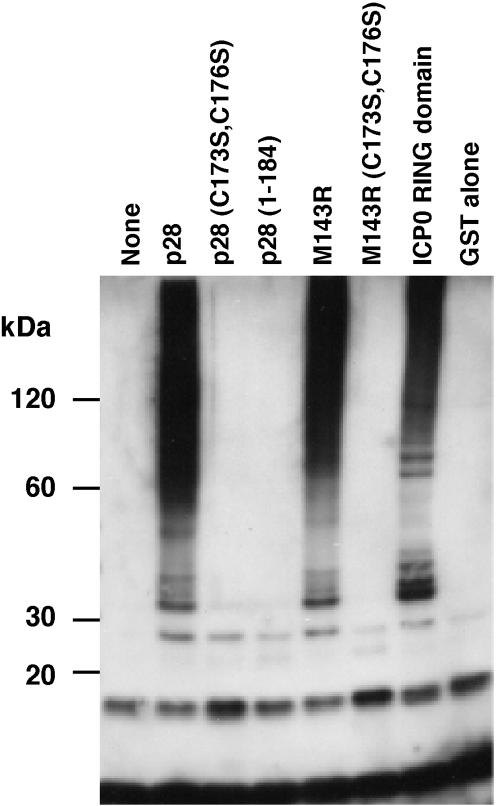
Recently, a number of RING-containing proteins have been shown to act as ubiquitin ligases (RING-E3s) which simultaneously interact with a specific substrate and a ubiquitin-conjugating enzyme (ubc), or E2, to mediate substrate-specific ubiquitination (7). In vitro, RING-E3s catalyze the formation of polyubiquitin in the presence of ubiquitin, ubiquitin-activating enzyme (E1), E2, and ATP (8). Given the highly conserved RING domain in p28, we examined whether VV p28 and the myxoma virus (MV) homologue, M143R, display ubiquitin ligase activity in vitro. VV p28, derived from strain IHD-W (14), and M143R, from MV strain Lausanne, were fused to glutathione S-transferase (GST) and purified from Escherichia coli as described previously (9). Mutations in the RING domains of M143R and p28 were constructed by replacing two conserved cysteines, C173 and C176, with serine, which disrupts the ability of RING domains to complex with zinc and abolishes E3 ligase activity (9). Furthermore, the RING domain of p28 was deleted by truncation at residue 184, resulting in a construct consistent with the p28 truncation in VV strain WR. At a concentration of 3 μM, each GST fusion was combined in vitro with 50 nM rabbit E1 (Boston Biochem), 0.5 μM human E2 UbcH5a (gift of R. Everett and purified as described previously [1]), 10 mM ATP, and 28 μM ubiquitin. Negative and positive controls consisted of GST and the RING domain of herpes simplex virus type 1 ICP0 (1), respectively. The in vitro ubiquitination reaction was performed in a solution of 20 μl of 50 mM Tris-HCl (pH 7.5), 200 mM NaCl, 1 mM dithiothreitol for 90 min at 30°C, and high-molecular-weight (HMW) ubiquitin conjugate formation was determined by immunoblotting with ubiquitin-specific antibody P4D1 (Santa Cruz Biotechnology). As expected, the RING domain of ICP0 catalyzed the formation of multiubiquitin adducts in the presence of UbcH5a, whereas GST was inactive (Fig. 1). HMW-ubiquitinated products were also observed in the presence of p28 and M143R, but not when the RING domain was truncated or lacked crucial cysteines (Fig. 1). These HMW bands likely consist of multiple ubiquitin adducts or ubiquitinated E1 or E2, because reprobing the blot with anti-GST did not reveal additional ubiquitinated bands of wild-type p28 compared to those of RING mutant p28 (data not shown). Thus, we conclude that p28 and M143R can function as RING-E3 ubiquitin ligases in vitro. To determine whether p28 affects ubiquitination in vivo, we examined the distribution of ubiquitin in VV-infected cells in the presence of wild-type or truncated p28 by using an infection/transfection strategy described previously (14). Under the control of the synthetic early-late poxviral promoter in pSC66 (4, 6), four versions of p28 with an N-terminal FLAG tag (DYKDDDDK) were expressed: wild-type p28, p28C173S/C176S, p28(1-204), and p28(1-184). Comparable expression levels and correct sizes of these constructs were verified by immunoblot (data not shown). To facilitate the detection of ubiquitination events occurring during poxviral infection, we inserted a hemagglutinin (HA) epitope-tagged form of ubiquitin (13) into pSC66 (4). HeLa cells were infected with VV-WR (multiplicity of infection [MOI], 5) and were cotransfected with HA-ubiquitin and either p28 or enhanced green fluorescent protein (EGFP) in pSC66 as a control (15). The distribution of both p28 and HA-ubiquitin was determined by confocal microscopy at 16 h postinfection. Cells were fixed with paraformaldehyde, permeabilized, and blocked with bovine serum albumin and fish gelatin as described previously (9). p28 was detected using fluorescein isothiocyanate (FITC)-conjugated anti-FLAG antibody (M2; Sigma), rabbit anti-I3L was used to detect virus factories (16), and mouse monoclonal anti-HA (HA-7; Sigma) was used to visualize ubiquitin. Primary antibodies were detected with goat anti-rabbit Alexa Fluor 350 (Molecular Probes) and goat anti-mouse Rhodamine B (Biosource, Camarillo, Calif.), respectively. Mouse Fc fragment (Cortex Biochem, San Leandro, Calif.) was used to block potential binding of the anti-FLAG to the anti-mouse secondary antibody. Absence of cross-reactivity was verified in control experiments (data not shown). Images for each fluorescence channel were obtained sequentially by confocal microscopy (Fig. 2).
FIG. 1.
In vitro ubiquitin ligase activity of p28 and M143R. Purified GST fusion proteins were combined in an in vitro ubiquitination reaction with E1, UbcH5a (E2), ATP, and ubiquitin. Reactions were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and were probed with anti-ubiquitin (P4D1; Santa Cruz Biotechnology). Western blotting with anti-ubiquitin shows the appearance of high-molecular-mass ubiquitin adducts in reactions containing either GST-p28, GST-M143R, or GST-ICP0 compared to reactions with no GST-fusion, GST alone, the C173S/C176S double mutants of both p28 and M143R, or p28(1-184).
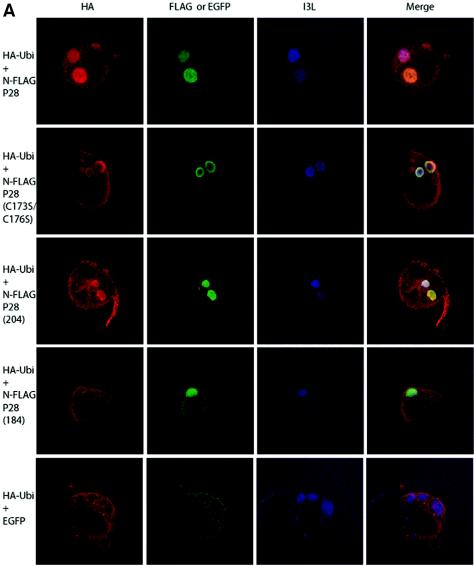
FIG. 2.
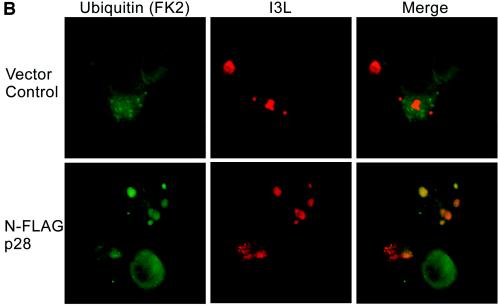
p28-dependent accumulation of ubiquitin in viral replication factories. (A) Colocalization with HA-ubiquitin. HeLa cells were infected with VV-WR, transfected with HA-ubiquitin, and cotransfected with either FLAG-p28, FLAG-p28(C173S/C176S), FLAG-p28(1-204), FLAG-p28(1-184), or EGFP. Cells were fixed and stained as described in the text to visualize HA-ubiquitin, FLAG constructs, EGFP, and I3L. All p28 constructs tested localized to virus factories, although FLAG-p28(1-184) was also cytoplasmic. EGFP was cytoplasmic and appeared to be excluded from the factories. HA-ubiquitin was excluded from viral factories in the absence of p28, while in the presence of FLAG-p28, FLAG-p28(C173S/C176S), or FLAG-p28(1-204), HA-ubiquitin was enriched in virus factories. In contrast, HA-ubiquitin was excluded from viral factories upon transfection with FLAG-p28(1-184). (B) Colocalization with endogenous ubiquitin. HeLa cells were infected with VV-WR FLAG-p28 or VV-WR for 16 h and stained with anti-ubiquitin antibody FK2 and anti-I3L. Ubi, ubiquitin.
As expected from previous studies, both full-length p28, p28(C173S/C176S), and p28(1-204) localized exclusively to virus factories and displayed similar staining patterns (Fig. 2), while p28(1-184) showed both cytoplasmic and viral factory staining (12, 14). In the presence of cotransfected EGFP, we observed a diffuse cytoplasmic distribution of HA-ubiquitin that was excluded from viral factories (Fig. 2). Similarly, HA-ubiquitin was excluded from viral factories when cotransfected with p28(1-184), despite a partial localization of p28(1-184) to viral factories. In stark contrast, cotransfection of both full-length p28 and p28(1-204) resulted in a strong enrichment of ubiquitin at viral factories. The same staining pattern was also seen using His-tagged ubiquitin (His6) (data not shown) (13), suggesting that ubiquitin is enriched in virus factories upon expression of p28. To determine if such ubiquitin enrichment could also be observed with endogenous ubiquitin, we used antibody FK-2 (Affiniti), which specifically detects conjugated ubiquitin. Cells infected with a recombinant VV-WR expressing FLAG-p28 showed colocalization of conjugated ubiquitin as determined by colocalization with the virus factory marker I3L (Fig. 2B). No colocalization was observed in VV-WR-infected cells (Fig. 2B). Colocalization was not observed in all cells but was most obvious in cells that displayed well-developed and large virus factories.
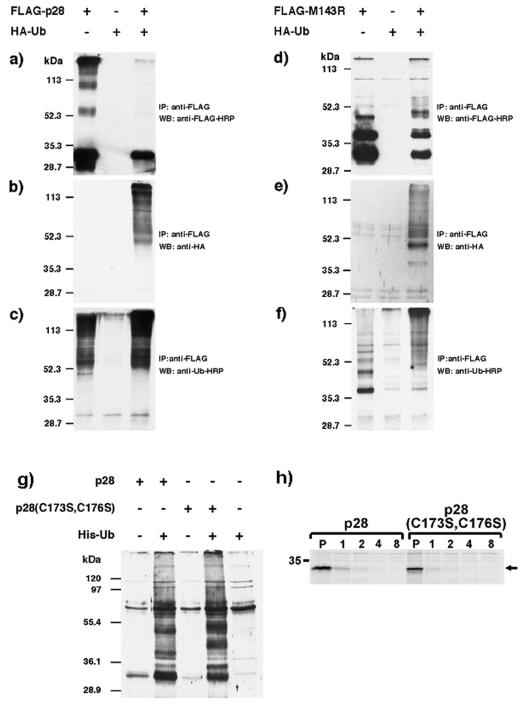
The colocalization of ubiquitin with p28 in virus factories could be the consequence of p28-mediated ubiquitination of poxviral or host proteins in this compartment; however, colocalization occurred independent of a functional RING domain, suggesting that colocalization could also be the result of ubiquitination of p28. To examine if p28 was ubiquitinated, we generated recombinant VV-WR expressing FLAG-p28, FLAG-M143R, HA-ubiquitin, or His-ubiquitin according to standard procedures (4). CV-1 cells were infected with VV-FLAG-p28 or VV-FLAG-M143R for 16 h at an MOI of 5 in the presence or absence of VV-HA-ubiquitin, and FLAG-tagged p28 or M143R was immunoprecipitated using immunoprecipitation (RIPA) buffer and anti-FLAG antibody (M2; Sigma). Immunoblotting the precipitates with anti-FLAG antibody revealed, as expected, a predominant band in the 28-kDa range in cells infected with VV-FLAG-p28 and VV-FLAG-M143R but not in VV-HA-ubiquitin (Fig. 3a and d). In addition, several HMW species were also observed which could represent ubiquitinated p28 or M143R. Coinfection with HA-ubiquitin slightly increased the size of HMW FLAG-tagged M143R (Fig. 3d), while generally lower amounts of all FLAG-tagged bands were observed as a result of coinfection. Immunoblotting the anti-FLAG precipitates with anti-HA revealed low background levels in VV-HA-ubiquitin-infected cells (Fig. 3b and e). However, coinfection of VV-HA-ubiquitin with either p28 or M143R, followed by immunoprecipitation with anti-FLAG and immunoblotting with anti-HA, resulted in the presence of HMW HA-tagged bands, including those that correspond to HMW FLAG-tagged forms of p28 or M143R (Fig. 3b and e). Importantly, only the HMW species were recognized with anti-HA, whereas the predominant 28-kDa protein detected by anti-Flag was not reactive with anti-HA. Similar observations were made using an antibody against endogenous ubiquitin (Fig. 3c and f), indicating that p28 and M143R are ubiquitinated during viral infection, which has been observed for other RING-E3 ligases (5). To determine if the ubiquitination of p28 was the result of autoubiquitination, we coinfected cells with VV expressing either FLAG-tagged wild-type or RING mutant p28 together with VV-His-ubiquitin. Ubiquitinated proteins were isolated by using nickel-chelate affinity chromatography and were probed for the presence of p28 by using anti-FLAG. HMW bands occurred in both wild-type and mutant p28-expressing cells that also expressed His-ubiquitin, but they did not occur in cells that were only infected with p28-expressing VV (Fig. 3g). Therefore, we conclude that autoubiquitination of p28 is unlikely to be the reason for ubiquitination of p28. Probably as a result of this ubiquitination, both wild-type and mutant p28 are short lived (Fig. 3 h).
FIG. 3.
Ubiquitination of p28 and M143R. CV-1 cells were infected with the indicated recombinant viruses at an MOI of 5. Sixteen hours postinfection, cells were treated with 25 μM MG132 for 4 h, lysed in RIPA buffer, and immunoprecipitated with anti-FLAG antibody (M2; Sigma). Immunoprecipitates were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and were blotted with either anti-FLAG-horseradish peroxidase (a and d), anti-HA (b and e), or anti-ubiquitin-horseradish peroxidase (c and f). (g) CV-1 cells were infected with the indicated viruses at an MOI of 2 for 16 h, treated with 25 μM MG132 for 4 h, and lysed in 1% NP-40. Cleared lysates were incubated with Ni2+ resin for 2 h at 4°C, and bound proteins were eluted using 1 M imidazole in 500 mM NaCl and 20 mM Tris (pH 7.9) and concentrated by acetone precipitation. Samples were separated by SDS-PAGE and probed with anti-FLAG-HRP (M2). (h) HeLa cells were infected with the indicated viruses at an MOI of 5 for 16 h and were metabolically labeled with [35S]methionine (0.1 mCi/ml) for 30 min, and the label was chased for the indicated hours. Cells were lysed, and p28 was immunoprecipitated using anti-FLAG. WB, Western blot; IP, immunoprecipitation; Ub, ubiquitin; HRP, horseradish peroxidase.
P28 is the first ubiquitin ligase identified in members of the orthopoxviruses. At present, the substrates of p28 and its myxoma virus homologue, M143R, are unknown, but they themselves seem to be targets for ubiquitination, which may contribute to the observed accumulation of ubiquitin in the replication factories. It remains to be demonstrated how the ubiquitin ligase function of p28 relates to its role as a virulence factor and its previously observed requirement for infection of macrophages and inhibition of apoptosis (2, 3, 12). This is the first evidence that p28 functions as a ubiquitin ligase. Recently, however, it was shown that myxoma virus encodes another ubiquitin ligase, M153R, which targets immune receptors for degradation (9). Our observations that p28/M143R and M153R function as ubiquitin ligases clearly suggest that regulation of ubiquitination during poxvirus infection is an important and recurring theme.
Acknowledgments
B.T.H.N. was supported by NIH training grant T32 AI007472. J.T. was supported by a postgraduate scholarship from NSERC Canada. Work in the laboratory of M.B. was supported by a grant from the Canadian Institutes for Health Research. M.B. is the recipient of an Alberta Heritage Foundation of Medical Research Scholar Award and a Canadian Institutes for Health Research New Investigator Award.
We thank Grant McFadden for helpful discussions and cDNA clones of VV-p28 and MV-M143R, Dirk Bohmann for tagged ubiquitin constructs, Jacomine Krijnse Locker for the generous gift of I3L antibody, and Anda Cornea for assistance with confocal microscopy.
REFERENCES
- 1.Boutell, C., S. Sadis, and R. D. Everett. 2002. Herpes simplex virus type 1 immediate-early protein ICP0 and its isolated RING finger domain act as ubiquitin E3 ligases in vitro. J. Virol. 76:841-850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brick, D. J., R. D. Burke, A. A. Minkley, and C. Upton. 2000. Ectromelia virus virulence factor p28 acts upstream of caspase-3 in response to UV light-induced apoptosis. J. Gen. Virol. 81:1087-1097. [DOI] [PubMed] [Google Scholar]
- 3.Brick, D. J., R. D. Burke, L. Schiff, and C. Upton. 1998. Shope fibroma virus RING finger protein N1R binds DNA and inhibits apoptosis. Virology 249:42-51. [DOI] [PubMed] [Google Scholar]
- 4.Chakrabarti, S., J. R. Sisler, and B. Moss. 1997. Compact, synthetic, vaccinia virus early/late promoter for protein expression. BioTechniques 23:1094-1097. [DOI] [PubMed] [Google Scholar]
- 5.Chen, A., F. E. Kleiman, J. L. Manley, T. Ouchi, and Z. Q. Pan. 2002. Autoubiquitination of the BRCA1*BARD1 RING ubiquitin ligase. J. Biol. Chem. 277:22085-22092. [DOI] [PubMed] [Google Scholar]
- 6.Davison, A. J., and B. Moss. 1990. New vaccinia virus recombination plasmids incorporating a synthetic late promoter for high level expression of foreign proteins. Nucleic Acids Res. 18:4285-4286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Joazeiro, C. A., and A. M. Weissman. 2000. RING finger proteins: mediators of ubiquitin ligase activity. Cell 102:549-552. [DOI] [PubMed] [Google Scholar]
- 8.Joazeiro, C. A., S. S. Wing, H. Huang, J. D. Leverson, T. Hunter, and Y. C. Liu. 1999. The tyrosine kinase negative regulator c-Cbl as a RING-type, E2-dependent ubiquitin-protein ligase. Science 286:309-312. [DOI] [PubMed] [Google Scholar]
- 9.Mansouri, M., E. Bartee, K. Gouveia, B. T. Hovey Nerenberg, J. Barrett, L. Thomas, G. Thomas, G. McFadden, and K. Fruh. 2003. The PHD/LAP-domain protein M153R of myxomavirus is a ubiquitin ligase that induces the rapid internalization and lysosomal destruction of CD4. J. Virol. 77:1427-1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moss, B. 1996. Poxviruses and their replication, p. 2637-2672. In B. N. Fields, D. M. Knipe, and P. M. Howley (ed.), Fields virology, 3rd ed., vol. 2. Lippincott-Raven, Philadelphia, Pa. [Google Scholar]
- 11.Senkevich, T. G., E. V. Koonin, and R. M. Buller. 1994. A poxvirus protein with a RING zinc finger motif is of crucial importance for virulence. Virology 198:118-128. [DOI] [PubMed] [Google Scholar]
- 12.Senkevich, T. G., E. J. Wolffe, and R. M. Buller. 1995. Ectromelia virus RING finger protein is localized in virus factories and is required for virus replication in macrophages. J. Virol. 69:4103-4111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Treier, M., L. M. Staszewski, and D. Bohmann. 1994. Ubiquitin-dependent c-Jun degradation in vivo is mediated by the delta domain. Cell 78:787-798. [DOI] [PubMed] [Google Scholar]
- 14.Upton, C., L. Schiff, S. A. Rice, T. Dowdeswell, X. Yang, and G. McFadden. 1994. A poxvirus protein with a RING finger motif binds zinc and localizes in virus factories. J. Virol. 68:4186-4195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wasilenko, S. T., T. L. Stewart, A. F. Meyers, and M. Barry. 2003. Vaccinia virus encodes a previously uncharacterized mitochondrial-associated inhibitor of apoptosis. Proc. Natl. Acad. Sci. USA 100:14345-14350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Welsch, S., L. Doglio, S. Schleich, and J. Krijnse Locker. 2003. The vaccinia virus I3L gene product is localized to a complex endoplasmic reticulum-associated structure that contains the viral parental DNA. J. Virol. 77:6014-6028. [DOI] [PMC free article] [PubMed] [Google Scholar]