Abstract
hTid-1, a human homolog of the Drosophila tumor suppressor l(2)Tid and a novel DnaJ protein, regulates the activity of nuclear factor κB (NF-κB), but its mechanism is not established. We report here that hTid-1 strongly associated with the cytoplasmic protein complex of NF-κB-IκB through direct interaction with IκBα/β and the IKKα/β subunits of the IκB kinase complex. These interactions resulted in suppression of the IKK activity in a J-domain-dependent fashion and led to the cytoplasmic retention and enhanced stability of IκB. Overexpression of hTid-1 by using recombinant baculovirus or adenovirus led to inhibition of cell proliferation and induction of apoptosis of human osteosarcoma cells regardless of the p53 expression status. Adherent cultured cells transduced with Ad.hTid-1 detached from the dish surface. Morphological changes consistent with apoptosis and cell death were evident 48 h after Ad.EGFP-hTid-1 transduction. In contrast, cells transduced with Ad.EGFP or Ad.EGFP-hTd-1ΔN100, a mutant that has the N-terminal J domain deletion and that lost suppressive activity on IKK, continued to proliferate. Similar data were obtained with A375 human melanoma cells. Ad.EGFP or Ad.EGFP-hTd-1ΔN100 ex vivo-transduced A375 cells injected subcutaneously into nude mice produced growing tumors, whereas Ad.EGFP-hTid-1-transduced cells did not. Collectively, the data suggest that hTid-1 represses the activity of NF-κB through physical and functional interactions with the IKK complex and IκB and, in doing so, it modulates cell growth and death.
hTid-1 is a human homologue of the Drosophila tumor suppressor Tid56 encoded by the lethal(2)tumorous imaginal discs, or l(2)tid, gene (26). Loss of the l(2)tid gene causes malignancy of the anlagen of the adult organs, the imaginal discs in Drosophila (26). hTid-1 was initially identified as a cellular target for the viral oncogenic protein E7 derived from human papillomavirus type 16 (HPV16) (36). hTid-1 also serves as the intracellular target for the viral transforming protein Tax from human T-cell leukemia virus type 1 (HTLV-1) and represses the Tax-induced transactivation of nuclear factor κB (NF-κB) (7, 8). Subsequent studies demonstrated that hTid-1 interacts with the viral nuclear protein UL9 from herpes simplex virus type 1 (HSV-1) in enhancing the binding of UL9 to the viral genome to facilitate viral replication (14) and that hTid-1 forms a protein complex with Jak2 tyrosine kinase, adversely affecting its kinase activity (35).
The htid1 gene encodes two spliced variants of hTid-1, hTid-1L, and hTid-1S (37). The full-length hTid-1L (long) comprises 480 amino acids, whereas hTid-1S (short), generated via alternative splicing, produces a predicted protein of 453 amino acids that lacks the C-terminal 33 amino acids of hTid-1L but contains an additional 6 amino acids (KRSTGN) (37). Aside from differences at the C termini, both hTid-1 variants share identical structural motifs, including an N-terminal mitochondria-processing signal peptide, an N-terminal conserved signature J domain, and a central cysteine-rich motif. hTid-1 was classified as a member of the DnaJ protein family and a molecular cochaperone based on its signature J domain and ability to interact with the heat shock protein 70 chaperone (Hsp70) (25, 27). Currently, the biological functions of these two forms of hTid-1 are unclear. The two variants were reported to exhibit opposing effects on induction of apoptosis in the human osteosarcoma cell line U2OS in response to tumor necrosis factor-alpha (TNF-α) and mitomycin C (37), but the mechanism by which hTid-1-mediated apoptosis and antiapoptosis is unknown. Whether hTid-1 functions as a human tumor suppressor is also unknown. The early reports that hTid-1 serves as an intracellular target for viral oncogenic proteins Tax and E7 suggest a role for hTid-1 as a tumor suppressor protein. Indeed, exogenous expression of hTid-1 in human lung adenocarcinoma cells suppressed their ability to form colonies in soft agar (7), supporting the assignment of hTid-1 as a suppressor of transformation. Moreover, a recent report shows that altered expression of hTid-1 is associated with primary human skin cancers and medulloblastoma (5).
To elucidate the intracellular mechanisms of hTid-1 in mediating suppression of transformation, we have assessed its activity on various signaling pathways and demonstrated that hTid-1 can repress NF-κB activity induced by TNF-α, Tax, and IκB kinase (IKKβ) by suppressing serine phosphorylation of IκBα, the inhibitor of NF-κB (8). NF-κB is a protein family of transcriptional factors expressed virtually in all tissues and is evolutionally conserved in humans and Drosophila. In complex with members of IκB family, NF-κB plays a key role in regulating the expression of genes involved in immunity, inflammation, the antiapoptotic response, and oncogenesis (16, 30). NF-κB is composed of homo- and heterodimers of NF-κB/Rel family proteins with a predominant form as a p65/p50 heterodimer. In resting cells, NF-κB is sequestered by its inhibitor IκB proteins, such as IκBα and IκBβ, in the cytoplasm through formation of an inactive NF-κB-IκB protein complex (16). Upon stimulation of cells by a number of extracellular inducers, IκB is rapidly phosphorylated, ubiquitinated, and degraded in the proteasome. Consequently, NF-κB is released from the protein complex, moves to the nucleus, and there activates the expression of various genes.
Regulation of NF-κB activity is controlled at multiple steps. The IκB kinase (IKK) complex and its substrate IκB proteins are central elements in regulating NF-κB activity (20, 24, 38, 44). IκB kinases are components of a 700-kDa protein complex that mediates specific serine phosphorylation of IκB (44). This kinase complex consists of three essential components: two catalytic subunits, IKKα and IKKβ, and one regulatory subunit, IKKγ (28, 34, 41, 44). In response to certain cytokines, growth factors, and viral proteins such as Tax, signals transmitted from particular upstream cascades converge at the IKK complex (9, 12, 15, 31, 33). The activated IKK phosphorylates IκBα and IκBβ at their N-terminal serine residues, targeting them for degradation in the proteasome. Recent studies showed that the IKK activity can be regulated by molecular chaperone proteins (1, 3, 6, 13, 43). In TNF-α-induced activation of NF-κB, recruitment of the IKK complex to the plasma membrane upon stimulation is a crucial step for the enhanced IKK activation. This recruitment process involves two additional components within the IKK complex: CDC37 and a molecular chaperone Hsp90 that are required for TNF-α-induced assembly, activation and trafficking of IKK in response to TNF receptor 1 (6). IKKγ, an essential modulator of the IKK complex, was found to associate with Hsp70 by a high-affinity interaction (1). Although hTid-1 is a cellular partner for Hsp70 and potentially functions as a cochaperone, it is not clear if hTid-1 physically interacts with components of the IKK complex to exert its repressive activity on NF-κB.
Constitutive NF-κB activity is found in a variety of human tumors, including prostate, ovarian, and melanoma tumors (4, 21-23, 42). The viral oncoprotein Tax-mediated transformation of primary T cells exclusively relies on its activation of NF-κB (32). Increased activity of NF-κB can stimulate tumor cell growth, angiogenesis and metastasis by enhancing the expressions of interleukin-8, matrix metalloproteinases 2 and 9, basic fibroblast growth factor, and vascular endothelial growth factor (4, 21-23, 42). Conversely, suppression of NF-κB activity has been shown to downregulate the expression of these proteins, resulting in growth arrest of tumors, increased apoptosis, and decreased metastatic potential (4, 21-23, 42). Some tumor suppressors can induce growth arrest and apoptosis through inhibition of NF-κB. For example, PTEN, one of the most frequently mutated and deleted genes in various human tumors, inhibits Akt activation and its downstream IKK activity and represses the nuclear transactivation potential of NF-κB (17). The tumor suppressor promyelocytic leukemia protein represses NF-κB activation by interacting with RelA/p65 to prevent its binding to the cognate enhancer (40). Promyelocytic leukemia protein potentiates apoptosis in the TNF-α-resistant cell line U2OS, and this proapoptotic activity is associated with its inhibition on NF-κB.
To address the molecular basis of hTid-1's regulation of NF-κB activity, we assessed the interaction of hTid-1 with components of the NF-κB signaling cascade. We found that hTid-1 regulated NF-κB activity through a strong physical and functional association with IκB and IKK complex. Overexpression of hTid-1 induced growth arrest and death in several tumor cell lines and suppressed tumor growth of human melanoma in nude mice. We conclude that hTid-1 may play a significant role in the regulation of cell growth and death, in part by regulating NF-κB activity.
MATERIALS AND METHODS
Cell cultures and antibodies.
HEK293 (human embryonic kidney cells), SAOS-2, U2OS, HOS (human osteosarcoma cells), and A375 cells (human melanoma cells) were grown in Dulbecco minimum essential medium (DMEM) supplemented with 10% fetal calf serum (FCS) and antibiotics. Jurkat T cells were cultured in RPMI medium with 10% FCS and antibiotics. SF9 insect cells were grown in Grace's insect supplemented medium containing 5% FCS and antibiotics at 28°C. Monoclonal antibody against hTid-1 was kindly provided by Karl Munger (Harvard Medical School, Boston, Mass.). Antibodies for IκBα, IκBβ, p65, IKKα/β, and hemagglutinin (HA) were purchased from Santa Cruz Biotechnology (Santa Cruz, Calif.). Anti-FLAG epitope antibody was purchased from Zymed (South San Francisco, Calif.).
Expression plasmids.
The methods constructing mammalian expression plasmids for FLAG-, HA-, glutathione S-transferase (GST), or green fluorescent protein (GFP)-tagged hTid-1L, IKKβ, dnIKKβ (dominant-negative mutant of IKKβ), dnJNK1 (dominant-negative mutant of JNK1), Hsp70, IκBα, IκBβ, and HTLV-1 Tax were described previously (7, 8). A cDNA construct (pCMV-hTid-1S) encoding hTid-1S was kindly provided by Karl Munger, and this cDNA was further modified by attaching a C-terminal FLAG tag (hTid-1S-FLAG). The cDNA fragments for the p65 subunit of NF-κB, IKKα, and IKKγ were obtained by high-fidelity PCR with human lymph node cDNAs as templates. They were subcloned in the pCEF vector backbone to generate epitope-tagged fusion gene constructs as indicated in the figures. Various hTid-1 mutants (ΔN50, ΔN100, ΔN150, ΔC140, ΔC180, and ΔCys) were created by PCR and constructed in the pCEF vector backbone containing the human elongation factor 1α promoter to drive expression of interest genes.
Recombinant baculo- and adenoviruses.
A modified version of the baculoviral and adenoviral expression systems was developed. This system combines the advantages of site-specific transposition technology from pFastBac1 (Invitrogen, Carlsbad, Calif.) and a tripromoter derived from pTriEX-1 (Novagen, San Diego, Calif.) for expression of a given gene in mammalian and insect cells and bacteria. Briefly, a BsmBI/SphI fragment from pTriEX-1 was blunt-ended and ligated with pFastBac1 pretreated with HindIII (blunt ended) and EcoRV to generate a new transfer vector named pBCAG that contained promoters for expression of foreign genes in both mammalian cells (driven by the CAG promoter, a hybrid promoter containing cytomegalovirus [CMV] enhancer and chicken β-actin promoter) and insect cells (controlled by the p10 promoter) in addition to transposase recognition sites (Tn7R and Tn7L). DNA fragments corresponding to hTid-1L-FLAG, hTid-1S-FLAG, p65-GST, IκBα-GST, IκBβ-GST, Tax-GST, and GST-tagged subunits of the IκB kinase complex, including GST-IKKα, GST-IKKβ, and GST-IKKγ, were constructed in pBCAG, and these transfer plasmids were used to transform DH10B-Bac competent bacteria (Invitrogen) containing a bacmid- and a plasmid-expressing transposase. Colonies containing recombinant bacmids were selected by Blue-gal and antibiotic screening, and the recombinant bacmids were prepared for transfection of SF9 cells by using Cellfectin (Invitrogen, Carlsbad, Calif.). At 4 days after transfection, the culture media were collected (P1 viral stock). P1 stocks were further amplified twice to generate P2 and P3 viral stocks. The high-titer P3 viral stocks were used for protein expression and in vitro protein purification.
Recombinant adenoviruses were generated by using the same strategy. The same pBCAG constructs were used to transform AD-294 competent bacteria (Qbiogene, Carlsbad, Calif.) containing preexisting Admid and transposase vector, and the recombinant adenoviruses including Ad.EGFP, Ad.EGFP-hTid-1 (the full-length of hTid-1) and Ad.EGFP-hTid-1ΔN100 were generated and purified according to the manufacturer's recommended protocol. For baculovirus-mediated transduction, 105 cells were transduced with 100 μl (∼108 viral particles) of the P3 viral stocks plus 900 μl of complete culture medium for 6 h at 37°C in a 5% CO2 incubator. The viruses were removed posttransduction, and fresh complete medium were added to the cell culture. Under these conditions, the transduction efficiency in SAOS-2 and U2OS cells was nearly 100% as assessed with Bac.EGFP to visualize GFP-positive cells. For adenovirus-mediated transduction, cells in log-phase growth in six-well plates or in 150-mm dishes were transduced with the purified recombinant adenoviruses at a multiplicity of infection of 25 to 50 at 37°C in a 5% CO2 incubator for 6 h. The adenoviruses were removed posttransduction, and complete medium was added to the culture.
Transfection, immunoprecipitation, and GST pull-down.
HEK293 cells (106 cells/well) of a six-well plate in log-phase growth were transiently transfected with expression plasmids as indicated in the figures by using PolyFect reagent (Qiagen, Valencia, Calif.). At 24 h later, the cells were harvested and lysed in 1 ml of the lysis buffer containing 1% Triton X-100, 20 mM Tris-Cl (pH 8.0), and 150 mM NaCl plus protease inhibitors (50 μg of aprotinin and 5 μg of leupeptin/ml) and Na3VO4 (1 mM). A total of 50 μl of the total cellular protein extracts was saved for measuring protein expression, and the rest was incubated with 30 μl of glutathione-Sepharose 4B beads (GSB; Amersham, Sunnyvale, Calif.) at room temperature for 2 h. After incubation, the beads were washed three times with the lysis buffer described above but without protease inhibitors, and the precipitates were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis and immunoblot analysis.
NF-κB reporter assay and in vitro kinase assay.
HEK293 cells (2 × 105) in log-phase growth in a 12-well plate were transiently transfected with different expression plasmids, and at 20 h after transfection the cells were analyzed for luciferase activity by using a kit purchased from Promega (Madison, Wis.). The in vitro kinase assay for IKK activity was performed as previously described (8).
MTT and soft-agar assays.
Methods for assessing the in vitro proliferation of cells by the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay and for examining anchorage-independent growth of tumor cells in soft agar were described previously (22, 23, 29).
In vivo tumorigenicity assay.
Male athymic nude mice (NCI-nu) were purchased from the Animal Production Area of the National Cancer Institute-Frederick Cancer Research Facility (Frederick, Md.). The mice were housed and maintained under specific-pathogen-free conditions. The facilities were approved by the American Association for Accreditation of Laboratory Animal Care and met all current regulations and standards of the U.S. Department of Agriculture, the U.S. Department of Health and Human Services, and the National Institutes of Health. The mice were used in accordance with institutional guidelines when they were 8 to 10 weeks old.
A375SM cells were transduced ex vivo with recombinant adenoviruses expressing enhanced GFP (EGFP) or EGFP-tagged fusion proteins, including the full length of hTid-1 and the ΔN100 mutant at a multiplicity of infection of 20. After overnight incubation at 37°C in a 5% CO2 incubator, cell viability (>95%) was determined by trypan blue dye exclusion. A375 melanoma cells (first set of experiments, 5 × 105 cells/mouse; second set of experiments, 106 cells/mouse) were injected subcutaneously into nude mice as described in detail previously (21).
Fluorescence imaging.
Fluorescent fusion constructs were transfected into HEK cells with PolyFect reagent (Qiagen). Expression and subcellular localization of the fluorescent fusion proteins in living cells were analyzed by conventional fluorescence microscopy.
hTid-1 RNAi.
The hTid1 interfering RNA (RNAi) construct was generated by using the lentivirus vector pLL3.7, kindly provided by Luk Van Parijs (Massachusetts Institute of Technology, Boston, Mass.). The target sense sequence of hTid-1 is 5′-GCGGCTCCCAGCATAGCTACT-3′, and the shRNA of hTid-1 was engineered in the pLL3.7 vector to express RNAi for knocking down the endogenous hTid-1. The recombinant lentivirus expressing hTid-1 RNAi was generated by cotransfection of pLL3.7/hTid-1 RNAi plasmid with the packaging plasmids (Invitrogen, Carlsbad, Calif.) into 293T packaging cells, and the viruses in the culture supernatants were harvested 48 h posttransfection and were used to transduce human melanoma cells A375SM and MeWo.
RESULTS
hTid-1 interacts with the NF-κB-IκB complex through direct binding to IκBα or IκBβ.
In the first set of experiments, we determined whether hTid-1 interacts with factors involved in the NF-κB signaling cascade by transient cotransfection of HEK293 cells with the FLAG-tagged hTid-1 and the GST fusion genes encoding IκBα, IκBβ, and the p65 subunit of NF-κB. In a GST pull-down assay, two spliced variants of FLAG-tagged hTid-1 proteins were reproducibly detected in the IκBα-GST and IκBβ-GST precipitates (Fig. 1A, lanes 2, 3, 6, and 7) but not in the GST precipitate (Fig. 1A, lanes 1 and 5). Coprecipitation of hTid-1S-FLAG with p65-GST was also detected but at a lower efficiency (Fig. 1A, lane 8).
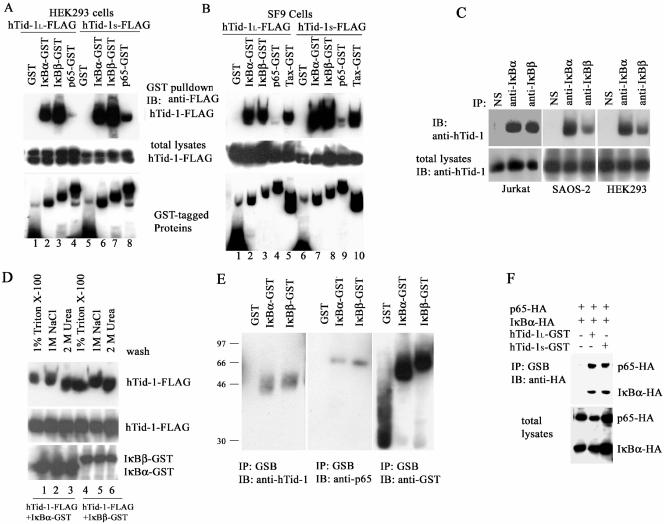
FIG. 1.
hTid-1 associates with the cytoplasmic NF-κB-IκB protein complex through direct binding to IκBα and IκBβ. (A) hTid-1 interacts with IκBα and IκBβ in cotransfected HEK293 cells. Transient cotransfections were performed as described in Materials and Methods with hTid-1-FLAG and GST fusion constructs including IκBα-GST, IκBβ-GST, and p65-GST (1 μg each). The GST pull-down precipitates were analyzed by anti-FLAG immunoblot (top panel). The membrane was then stripped and reblotted with anti-GST (bottom panel). Total cellular extracts were analyzed with anti-FLAG immunoblot to detect hTid-1-FLAG protein levels (middle panel). (B) hTid-1 coprecipitates with IκB proteins expressed in SF9 insect cells. In the top panel, a GST pull-down was analyzed by anti-FLAG immunoblotting. The GST fusion proteins in the GST pull-down were detected by using anti-GST antibody (bottom panel), and the total lysates were examined for hTid-1 expression levels with anti-FLAG (middle panel). (C) Protein complex formation of endogenous hTid-1 and IκB proteins. Endogenously expressed hTid-1 appeared in the anti-IκBα and anti-IκBβ immunoprecipitates but not in the nonspecific immunoprecipitates in Jurkat T, SAOS-2, and HEK293 cells. The immunoprecipitates were analyzed with anti-hTid-1 immunoblot (upper panel), and the total protein lysates were assessed for endogenous hTid-1 protein levels (lower panel). (D) hTid-1 binds to IκB proteins with high affinity. SF9 cells (2 × 106) were coinfected with recombinant baculoviruses coexpressing hTid-1S-FLAG and IκBα-GST or IκBβ-GST (100 μl of P3 virus stocks). The GST pull-down precipitates were washed extensively with 1% Triton X-100 lysis buffer (lanes 1 and 4), 1 M NaCl (lanes 2 and 5) or 2 M urea (lanes 3 and 6) in addition to 1% Triton X-100. The precipitates were then analyzed by immunoblotting with anti-FLAG antibody (top panel), and the membrane was stripped and reblotted with anti-GST (bottom panel). The hTid-1S-FLAG expression levels are indicated in the middle panel. (E) Endogenous hTid-1 and p65 proteins were coprecipitated with IκBα-GST and IκBβ-GST proteins. HEK cells (2 × 106 cells) were transfected with GST, IκBα-GST, or IκBβ-GST. The GST pull-down precipitates were evaluated with anti-hTid-1 antibody (left panel). The membrane was stripped and reblotted with anti-p65 antibody (middle panel); the GST fusion protein levels are indicated in the right panel. (F) hTid-1 is associated with the p65-IκBα protein complex. HA-tagged IκBα and p65, with or without hTid-1L-GST, were cotransfected into HEK293 cells. The GST pull-down was analyzed by anti-HA blotting (upper panel), and the IκBα-HA and p65-HA expression levels in total lysates were assessed by anti-HA blotting (lower panel). The results are representative ones of three independent experiments.
To confirm the direct interaction of hTid-1 with IκB, we generated recombinant baculoviruses that expressed FLAG-tagged hTid-1 and GST-fusion proteins in SF9 insect cells. Consistent with the data from cotransfected HEK293 cells, both hTid-1 variants associated with IκBα, IκBβ, and Tax proteins expressed and purified in SF9 cells (Fig. 1B, lanes 2, 3, 5, 7, 8, and 10). A weak interaction of hTid-1S-FLAG with p65 proteins expressed in SF9 cells was also visible (Fig. 1B, lane 9). These results indicate that hTid-1 preferentially binds to IκB in the absence of other mammalian cellular proteins. In addition to the protein interaction detected in transfected HEK293 cells, the interaction of endogenously expressed hTid-1 and IκB proteins in various cell lines was also detected. In the Jurkat T, SAOS-2, and HEK293 cells, endogenous hTid-1 proteins were present in the IκBα and IκBβ immunoprecipitates (Fig. 1C, top panel). The robust binding capacity of hTid-1 with IκB was comparable to that of hTid-1 with Hsp70. No significant loss in binding capacity was observed when the precipitates were washed in a buffer containing 1 M NaCl (Fig. 1D, lanes 2 and 5) or 2 M urea (Fig. 1D, lanes 3 and 6) in addition to 1% Triton X-100. These results support the notion that hTid-1 and IκB proteins form a strong and stable complex.
The finding that hTid-1 interacts with IκB proteins indicates that hTid-1 may be associated with the NF-κB-IκB complex. To examine this possibility, we assessed the coprecipitation of endogenously expressed hTid-1 with NF-κB and IκB. As shown in Fig. 1E, endogenous hTid-1 in HEK293 cells coprecipitated with exogenously expressed GST-tagged IκBα and IκBβ fusions, but not with GST (Fig. 1E, left panel). Endogenous p65 protein could also be detected (middle panel) in the same IκBα-GST and IκBβ-GST precipitates. In HEK293 cells cotransfected with p65-HA and IκBα-HA in the presence of hTid-1-GST, a GST pull-down assay showed that both the p65-HA and IκBα-HA proteins were coprecipitated by GST-tagged hTid-1 (Fig. 1F). Collectively, these results suggest that hTid-1 associates with the cytoplasmic protein complex of NF-κB-IκB through direct interaction with IκBα and IκBβ.
hTid-1 associates with the IKK complex and represses kinase activity.
To investigate the possibility that hTid-1 interacts with the IKK complex, HEK293 cells were cotransfected with FLAG-tagged hTid-1 and GST-tagged IKKα, IKKβ, and IKKγ. In a GST-IKKα pull-down assay, significant amounts of both variants of hTid-1 were detected (Fig. 2A, lanes 1, 4, 7, and 10). In addition, hTid-1S coprecipitated, though weakly, with IKKβ and IKKγ (Fig. 2A, lanes 8, 9, 11, and 12). When both hTid-1 and IKK subunits were overexpressed in HEK cells, the formation of an hTid-1-IKK complex did not appear to be affected by a 20-min treatment with 20 ng of TNF-α/ml (Fig. 2A); however, at lower expression levels of both hTid-1 and IKKα, the hTid-1-IKKα complex dissociated within 20 min of stimulation with 50 ng of TNF-α/ml; it began to regenerate 40 min later (Fig. 2B). This dynamic interaction could also be detected by using endogenously expressed proteins as seen in Fig. 2C. These results imply that the interaction of hTid-1 with IKK complex may be a dynamic response to TNF-α stimulation. To determine whether hTid-1 interacts directly with the IKK subunits, we expressed and purified the GST-tagged IKK proteins and FLAG-tagged hTid-1 in SF9 insect cells. As shown in Fig. 2D, both splicing variants of hTid-1 were coprecipitated by GST-IKKα and GST-IKKβ and less potently by GST-IKKγ. This result indicates that hTid-1 binds directly to the IKK complex.
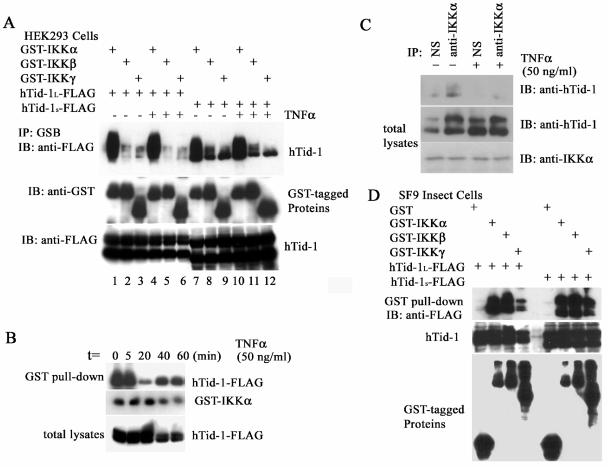
FIG. 2.
Both hTid-1 variants bind to the IKK complex and repress kinase activity. (A) hTid-1 also associates with IKKα/β. Transient cotransfection of FLAG-tagged hTid-1 with GST-tagged subunits of the IKK complex (1 μg each) was performed by using a method similar to that described in Fig. 1. The transfected cells were stimulated with or without TNF-α (20 ng/ml, 20 min). The GST pull-down precipitates were analyzed with anti-FLAG immunoblot (top panel). The amount of GST fusion proteins is indicated at the middle panel, whereas the hTid-1-FLAG protein levels are shown at the bottom panel. (B) Time course of the interaction of hTid-1 and IKKα upon cell stimulation with TNF-α. Cellular lysates from hTid-1-IKKα (0.2 μg each)-cotransfected HEK cells at different times of TNF-α stimulation (50 ng/ml) were assessed for the coprecipitation of both coexpressed proteins. In the top panel, hTid-1 proteins were detected by a GST pull-down assay with an anti-FLAG antibody. The expression levels of GST-IKKα and hTid-1-FLAG are shown in the middle and bottom panels, respectively. (C) Interaction of hTid-1 and IKKα with endogenously expressed proteins. Total lysates from HEK293 cells stimulated with or without TNF-α (50 ng/ml) were immunoprecipitated by nonspecific antibody (anti-FLAG) or by anti-IKKα. The immunoprecipitates were then analyzed by anti-hTid-1 blotting (top panel). The endogenous proteins of hTid-1 and IKKα from total lysates are shown in the middle and bottom panels, respectively. (D) hTid-1 binds directly to the subunits of IKKα and IKKβ. Coprecipitation of hTid-1-FLAG by GST tagged three subunits of the IKK complex expressed in SF9 insect cells. The top panel shows that the hTid-1-FLAG protein was coprecipitated by GST-tagged IKK subunits, the middle panel shows the hTid-1-FLAG expression level from the total lysates, and the GST-tagged IKK subunits from the GST pull-down is shown in the bottom panel.
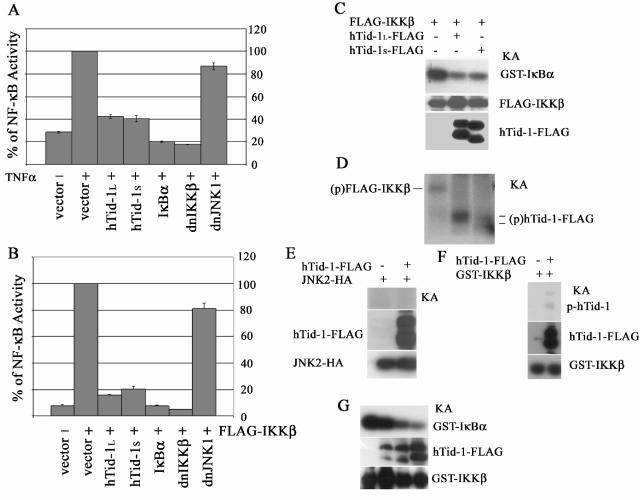
We next examined the role of hTid-1 in TNF-α-mediated activation of NF-κB by using an NF-κB-driven reporter assay. It was shown that both hTid-1L and hTid-1S, similar to the control proteins IκBα and the dominant-negative mutant of IKKβ, inhibited the luciferase activity induced by TNF-α in the HEK293 cells (Fig. 3A). IKKα and IKKβ form heterodimers in cells (24, 30). When overexpressed in HEK293 cells by transient transfection, IKKβ exhibits a high intrinsic kinase activity, whereas IKKα has much lower intrinsic kinase activity. We then utilized IKKβ as the activator of NF-κB. Transient overexpression of both variants of hTid-1, IκBα, or the dominant-negative mutant of IKKβ repressed NF-κB-driven luciferase activity induced by FLAG-IKKβ, whereas the control construct expressing the dominant-negative mutant of JNK1 had no such repressive activity (Fig. 3B). Further, an in vitro kinase assay showed that both hTid-1L and hTid-1S inhibited serine phosphorylation of GST-tagged IκBα fusion proteins by IKKβ (Fig. 3C). To determine whether the kinase-active IKKβ could phosphorylate hTid-1, IKKβ-, and hTid-1-cotransfected HEK293 cells were subjected to in vitro kinase assay. In the absence of hTid-1-FLAG, the FLAG-IKKβ kinase was autophosphorylated in anti-FLAG immunoprecipitates, whereas in the presence of hTid-1-FLAG, autophosphorylation of FLAG-IKKβ was diminished albeit the phosphorylated forms of the hTid-1 variants were detected (Fig. 3D). However, hTid-1 was not phosphorylated by JNK2 in the same kinase reaction condition (Fig. 3E). To investigate this further, we utilized recombinant proteins of GST-IKKβ and hTid-1-FLAG expressed in SF9 insect cells. As shown in Fig. 3F, the hTid-1L-FLAG protein was phosphorylated by GST-IKKβ. However, the phosphorylation of hTid-1 by IKKβ was much less efficient than the IκBα phosphorylation; it required extended exposure time (overnight) of autoradiography, whereas 30-min exposure was sufficient to detect the IκBα phosphorylation by the in vitro kinase assay. Further, hTid-1 was capable of repressing the IKKβ activity in the SF9 insect cells. The in vitro kinase assay showed that in the presence of the increasing amounts of hTid-1-FLAG, the phosphorylation of the GST-IκBα substrate was decreased in a dose-dependent manner (Fig. 3G). Thus, these data strongly suggest that hTid-1 protects IκBα from IKK-mediated phosphorylation by repressing the IKK activity.
FIG. 3.
hTid-1 downregulates TNF-α-mediated activation of NF-κB and represses the kinase activity of IKK. Both variants of hTid-1 suppress TNF-α (A)- and IKKβ (B)-induced activation of NF-κB by the NF-κB reporter assay, as described previously (8). The results shown are averages ± the SD; experiments were repeated three times with similar patterns. (C) Both hTid-1 variants inhibit serine phosphorylation of IκBα by IKKβ. The in vitro kinase assay was performed as described in Materials and Methods. The top panel shows the in vitro phosphorylated GST-IκBα; the protein levels of FLAG-IKKβ in the total cellular extracts (middle panel) were detected by anti-IKK immunoblotting, and the hTid-1 protein expression shown in the bottom panel was analyzed with anti-FLAG blot. KA, kinase assay. (D) Kinase-active IKKβ phosphorylates hTid-1. Transient cotransfection of FLAG-IKKβ and hTid-1-FLAG was performed in HEK293 cells. The FLAG-tagged proteins were immunoprecipitated with anti-FLAG antibody plus protein A beads. In vitro kinase assay was performed by using conditions similar to those described above with the exception that GST-IκBα protein was not added to the kinase reaction. The phosphorylated IKKβ and hTid-1 proteins are indicated (p, phosphorylated protein). (E) hTid-1 is not phosphorylated by JNK2. Transient cotransfection of hTid-1L-FLAG and JNK2-HA in HEK293 cells were performed, and the total lysates were coimmunoprecipitated by both anti-FLAG and anti-HA. The in vitro kinase assay was done by the same condition as the IKK kinase reaction. Expressions of hTid-1L-FLAG and JNK2 from total lysates are indicated. (F) Phosphorylation of hTid-1 by IKKβ by using recombinant proteins expressed in SF9 insect cells. (G) hTid-1 suppresses IKKβ activity in SF9 insect cells. The fixed amount of Bac.GST-IKKβ (100 μl) and various amounts of Bac.hTid-1L-FLAG (0, 10, 50, or 200 μl) were used to coinfect SF9 cells (5 × 106 cells). An in vitro kinase assay was performed to detect GST-IκBα phosphorylation (top panel). Protein expression levels of hTid-1-FLAG and GST-IKKβ are shown in the middle and bottom panels, respectively.
The N terminus of hTid-1 mediates interaction with IκB.
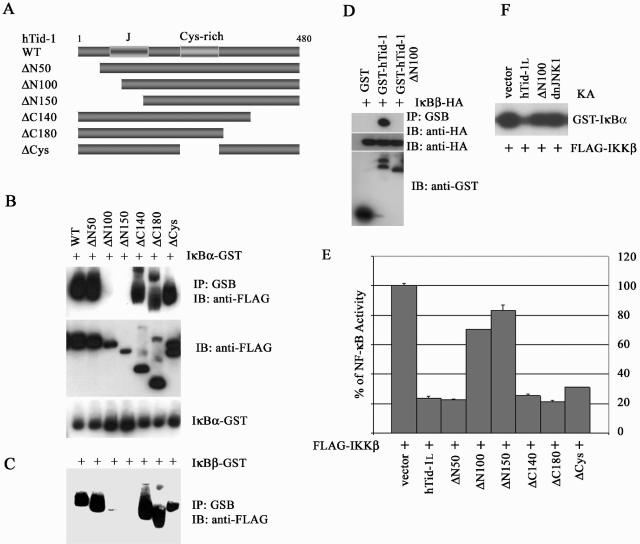
The precursors of hTid-1 are ca. 50 to 52 kDa in molecular mass, and the processed forms are in the range of 40 to 43 kDa. The putative mitochondria processing signal peptide is located at the N-terminal amino acids 63 to 67. After the processing signal sequence, there is a conserved J domain flanking the amino acids 89 to 169. Four CXXCXGXG motifs constitute the central cysteine-rich domain covering amino acids 236 to 300 (37). To determine the potential binding domains for IκB, we generated wild-type hTid-1 and sequential deletion mutants of N and C termini, and central regions of hTid-1, all tagged with a FLAG epitope (Fig. 4A). Using an approach similar to that described in Fig. 1A, we found that deletions of the N-terminal 50 (ΔN50), C-terminal 140 and 180 amino acids (ΔC140 and ΔC180, respectively), and the central Cys-rich domain (ΔCys) had no effect on the interaction of hTid-1 with IκBα and IκBβ (Fig. 4B and C). However, deletion of the N-terminal 100 and 150 amino acids (ΔN100 and ΔN150, respectively) abolished the in vivo association of hTid-1 with both IκB proteins (Fig. 4B and C). It was noted that in sodium dodecyl sulfate-12% polyacrylamide gel electrophoresis, the ΔC140 ran faster than the ΔN150 mutant due to the additional loss of N-terminal amino acids. In addition, lower expression levels of ΔN100 and ΔN150, compared to the full-length of hTid-1, were observed with the same inputs of the expression plasmids. However, reciprocal coprecipitation with GST-tagged hTid-1 and ΔN100 showed less variation in the expression levels, and interaction of GST-hTid-1ΔN100 with IκBβ was still not detectable (Fig. 4D). Thus, it appeared that the binding region of hTid-1 for IκB proteins was situated at the N terminus, spanning amino acids 50 to 100, which overlaps with the J domain of hTid-1, which is essential for interaction with Hsp70.
FIG. 4.
An N-terminal domain of hTid-1 is required for interaction with IκB and is important for hTid-1's repression of NF-κB activity. (A) Schematics of the wild-type and deletion mutants of hTid-1. The N-terminal conserved signature J domain and the central Cys-rich domain are indicated. The in vivo interaction of wild-type hTid-1 and its mutant proteins with GST-tagged IκBα (B) or IκBβ (C) were evaluated by using transient cotransfections in HEK cells as described in Fig. 1A. The presence of FLAG-tagged hTid-1 proteins in the GST pull-down was analyzed by anti-FLAG immunoblotting (B, top panel), and the membrane was stripped and reblotted with anti-GST (B, bottom panel). The expression of various hTid-1 proteins in whole-cell extracts was detected with anti-FLAG immunoblot (B, middle panel). Only hTid-1 proteins coprecipitated with IκBβ-GST are shown in panel C. (D) The in vivo binding of GST-tagged hTid-1 or the ΔN100 mutant with IκBβ-HA. In the top panel, proteins in the GST pull-down were analyzed with anti-HA antibody. The middle panel shows the protein levels of HA-tagged IκBβ; GST fusion proteins were detected by anti-GST immunoblotting (bottom panel). (E) The N-terminal domain of hTid-1 is important for the NF-κB repressive activity of the hTid-1. The repressive activity of the hTid-1 mutants was assessed by using the NF-κB reporter assay. The results shown are averages ± the SD; experiments were repeated three times with similar patterns. (F) In vitro kinase assay was performed by cotransfection of the kinase active IKKβ with hTid-1 or with the ΔN100 mutant in HEK cells, and the kinase activity was examined as previously described (8).
Next, we examined the ability of hTid-1 mutants to suppress activation of NF-κB by using an NF-κB reporter assay. hTid-1 mutants ΔN50, ΔC140, ΔC180, and ΔCys retained the suppressive activity on NF-κB activation induced by IKKβ, with efficiency comparable to that mediated by wild-type hTid-1 (Fig. 4E). These mutants possessed full IκB binding capacity, as demonstrated in Fig. 4B and C. In contrast, the ΔN100 and ΔN150 mutants, which failed to bind IκB, lost most of the hTid-1 suppressive activity. An in vitro kinase assay demonstrated that the suppressive activity of the ΔN100 mutant on IKKβ was also compromised, even when overexpressed (Fig. 4F). These results indicate that the interaction of hTid-1 with IκB is likely to be important for the repression of NF-κB activity.
hTid-1 retains IκBα in the cytoplasm and prevents IκBα degradation induced by the IKK activator.
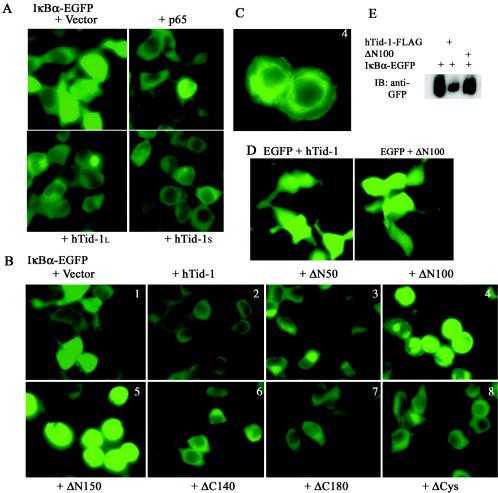
We used the fluorescence imaging technique to determine whether hTid-1 codistributes with free IκBα in resting cells. De novo-synthesized free IκBα can translocate from cytoplasm to the nucleus (2, 38). As shown in Fig. 5A, bright nuclear fluorescent signals were found in HEK293 cells expressing IκBα-EGFP (panel 1). This nucleus-imported, newly generated free IκBα was detected in roughly 30% of the transfected cells. Translocation of free IκBα proteins to the nucleus was blocked by coexpression of the p65 subunit of NF-κB (Fig. 5A). Likewise, hTid-1 also efficiently blocked the translocation of free IκBα to the nucleus by retaining IκBα in the cytoplasm. Virtually all green fluorescent signals of IκBα-EGFP were retained in the cytoplasm with coexpression of wild-type hTid-1 (Fig. 5A) and various mutants (Fig. 5B2, 3, 6, 7, and 8), the exceptions being ΔN100 and ΔN150. Cotransfection of either ΔN100 or ΔN150 with IκBα-EGFP resulted in redistribution of the fluorescent signals to the perinuclear clusters and plasma membrane, and it induced cytoplasmic aggregation of IκBα-EGFP (Fig. 5B4 and 5) (aggregation can be better seen in Fig. 5C). The rounding of cells depicted in panels 4 and 5 probably resulted from protein aggregation of IκBα-EGFP. The effects observed appeared to be specific for IκBα-EGFP, since the expression and subcellular distribution of EGFP was not altered by cotransfection with wild-type hTid-1 or the ΔN100 mutant (Fig. 5D). Very bright fluorescent signals (Fig. 5B and C) and accumulated proteins of IκBα-EGFP (Fig. 5E) were seen in the cells cotransfected with ΔN100, probably due to the protein aggregation, but not the increased expression levels of IκBα-EGFP. Although it is unclear how the ΔN100 and ΔN150 mutants induce IκBα redistribution and aggregation, our observations suggest that hTid-1 sequesters IκBα in the cytoplasm where it forms an inactive complex with NF-κB.
FIG. 5.
hTid-1 retains IκBα in the cytoplasm. (A) hTid-1 blocks the nuclear import of free IκBα. EGFP-tagged IκBα (1 μg) was cotransfected with empty vector, p65-HA, hTid-1L-FLAG, or hTid-1S-FLAG (1 μg each) in HEK293 cells. The fluorescent signal was examined with a fluorescence microscope 24 h after transfection. (B) Analysis of the subcellular distribution and expression of IκBα-EGFP-cotransfected wild-type hTid-1 and various hTid-1 mutants in HEK cells as indicated in the figure. (C) Panel B4 was enlarged for better viewing and showed protein aggregation and plasma and nuclear membrane attachment of IκBα-EGFP-cotransfected with the ΔN100 mutant. The results represent at least three independent experiments. (D) hTid-1 has no effect on EGFP subcellular distribution. EGFP was cotransfected with hTid-1-FLAG or the ΔN100 mutant in HEK cells. The EGFP fluorescence intensity and cellular distribution were not altered by coexpression with hTid-1. (E) Anti-GFP immunoblot to detect IκBα-EGFP levels from total lysates in the HEK293 cells that were cotransfected with hTid-1L-FLAG or ΔN100.
FIG. 6.
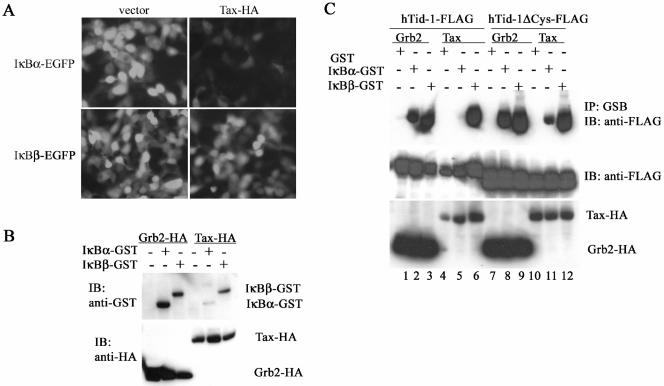
hTid-1 protects IκBα from degradation induced by an IKK activator, Tax. (A) Visualization of EGFP-tagged IκBα degradation induced by Tax. IκBα-EGFP or IκBβ-EGFP (1 μg) was cotransfected with an empty vector or with Tax1-HA (1 μg) in HEK293 cells. The fluorescence images were taken 24 h after transfection. (B) Immunoblot assay of IκBα-induced degradation by Tax. GST-tagged IκBα or IκBβ was cotransfected with Grb2-HA (a negative control) or with Tax-HA in HEK293 cells. The total protein extracts were analyzed with immunoblot with anti-GST (upper panel). Expression of the HA-tagged Grb2 and Tax is shown in the lower panel. (C) A Tax binding-minus mutant of hTid-1 (hTid-1ΔCys) protects the hTid-1-IκBα complex from dissociation induced by Tax. Wild-type hTid-1-FLAG or hTid-1ΔCys-FLAG was cotransfected with GST-tagged IκBα or IκBβ, together with Grb2-HA or Tax-HA, into HEK cells. Protein precipitates from a GST pull-down were analyzed by anti-FLAG immunoblotting (top panel), and the total cellular protein extracts were examined with anti-FLAG blot to determine FLAG-tagged hTid-1 expression (middle panel). Protein levels of HA-tagged Grb2 and Tax are shown in the bottom panel.
FIG. 7.
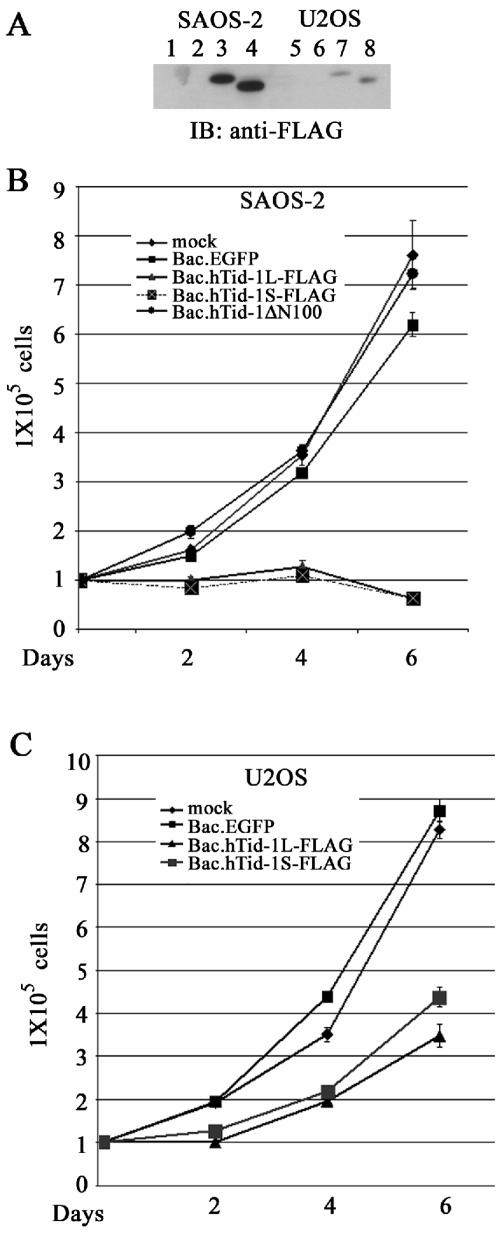
Overexpression of hTid-1 induces cell growth arrest and death. (A) Expression of the hTid-1 variants by using recombinant baculoviruses to transduce SAOS-2 and U2OS cells. The osteosarcoma cells were transduced with baculoviruses expressing EGFP (control), hTid-1L-FLAG, or hTid-1S-FLAG. At 40 h after transduction, the total protein lysates were collected for analysis of the protein expression of hTid-1 with anti-FLAG immunoblot. Lanes 1 to 4, SAOS-2 cells; lanes 5 to 8, U2OS cells. Lanes 1 and 5, mock treatment; lanes 2 and 6, Bac.EGFP; lanes 3 and 7, Bac.hTid-1L-FLAG; lanes 4 and 8, Bac.hTid-1S-FLAG. (B) Growth rate of SAOS-2 cells (105 cells) transduced with Bac.hTid-1L-FLAG, Bac.hTid-1S-FLAG, or Bac.hTid-1ΔN100 mutant at indicated times. (C) Growth rate of U2OS cells transduced with the recombinant baculoviruses expressing two spliced variants of hTid-1 at the indicated times. The results are representative of at least three independent experiments.
FIG. 8.
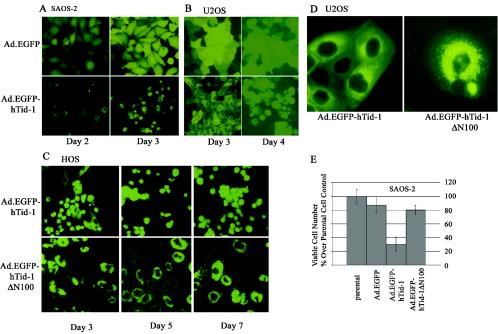
Adenovirus-mediated gene transfer of hTid-1 leads to cell death in a J domain-dependent manner. Recombinant adenoviruses expressing EGFP (control) and EGFP-tagged hTid-1 were used to transduce SAOS-2 (A) and U2OS (B) cells. At the indicated times, fluorescent images were recorded. (C) HOS cells were transduced with recombinant adenoviruses expressing EGFP-tagged full-length hTid-1 and the hTid-1 ΔN100 mutant. The fluorescent images were taken at different times, as indicated in the figure. The results represent at least three independent experiments. (D) Digitally enlarged fluorescent images show the subcellular distribution of EGFP-hTid-1 and EGFP-hTid-1ΔN100 in transduced U2OS cells. (E) In vitro cytotoxicity assay to assess the viability of SAOS-2 cells transduced with the recombinant adenoviruses expressing EGFP (control), EGFP-hTid-1, and EGFP-hTid-1ΔN100. At 3 days after transduction, viable cells were counted with a microscope, and dead cells were excluded by trypan blue staining.
To explore this possibility, we determined whether hTid-1 could protect IκBα degradation induced by IKK activator. Tax, a known activator of the IKK complex (9, 15), interacts with hTid-1, and its activation of NF-κB can be suppressed by overexpression of hTid-1 (8). Thus, Tax may also target hTid-1 to modulate NF-κB activity. We utilized the viral activator to assess whether Tax could disrupt the hTid-1-IκBα protein complex. As seen in Fig. 6A, Tax expression facilitated degradation of EGFP-tagged IκBα but had no significant effect on IκBβ-EGFP. Immunoblot assay showed that, consistent with the results shown in Fig. 6A, transient cotransfection of HA-tagged Tax with GST-tagged IκBα or IκBβ preferentially led to degradation of IκBα, whereas a control protein, Grb2, had no such activity (Fig. 6B). We next determined whether Tax affects the formation of the hTid-1-IκB complex. hTid-1-FLAG coprecipitated with either IκBα-GST or IκBβ-GST in the presence of Grb2-HA (Fig. 6C, lanes 2 and 3). In contrast, in the presence of Tax-HA, hTid-1-FLAG appeared only in the IκBβ-GST precipitate (lane 6), not the IκBα-GST precipitate (lane 5), presumably because Tax induced degradation of IκBα-GST. Interestingly, hTid-1ΔCys, a Tax-binding−/IκBα binding+ mutant of hTid-1 (7), was partially detected in the IκBα-GST precipitate even in the presence of Tax-HA (compare lane 5 with lane 11), suggesting that overexpression of hTid-1ΔCys could resist Tax-induced IκBα degradation by protecting the NF-κB-IκBα complex. These data indicate that Tax could displace hTid-1 from the NF-κB-IκBα complex, thereby enhancing IKK-mediated phosphorylation of IκBα. We therefore conclude that the NF-κB repressive activity of hTid-1 is probably mediated by two mechanisms: repression of IKK activity and enhancement of IκB stability.
Overexpression of hTid-1 induces cell growth arrest and death.
Since NF-κB regulates gene expression involved in oncogenesis, we next evaluated the effect of hTid-1 on tumor cell growth and survival. To avoid potential variation generated by clonal selection, we constructed recombinant baculoviruses expressing wild-type and mutant hTid-1. The human osteosarcoma cell lines SAOS-2 (p53−) and U2OS (p53+) were chosen because their p53 status differed. Nearly 100% of these cells were efficiently transduced by the recombinant baculoviruses as demonstrated by fluorescence microscopy of Bac.EGFP-transduced cells (data not shown). The expression levels were severalfold higher in SAOS-2 cells than in U2OS cells when equal virus titers were applied (Fig. 7A). The growth rate of the cells transduced with Bac.EGFP was near that of mock treated SAOS-2 cells (Fig. 7B), indicating that the baculoviruses alone did not induce significant cytotoxicity. However, the growth and viability of SAOS-2 cells transduced with the recombinant baculovirus overexpressing hTid-1L or hTid-1S was significantly impaired, whereas the ΔN100 mutant did not affect growth in vitro (Fig. 7B).
Transduction of U2OS cells with recombinant baculovirus expressing both hTid-1 variants reduced in vitro growth rate within 2 days. Thereafter, the cells continued to proliferate (Fig. 7C). This growth pattern is consistent with baculovirus-mediated gene expression in mammalian cells in that the peak expression is reached 48 to 72 h after transduction and then the expression level decreases gradually over time. The less dramatic effect of hTid-1 on cell growth in the transduced U2OS cells may be due to the reduced expression level of the transgene, as seen in Fig. 7A.
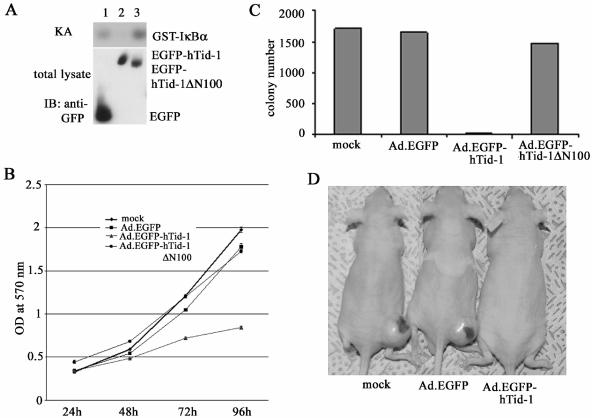
To confirm that tumor cell growth arrest and death is induced by recombinant baculoviruses expressing hTid-1, we constructed recombinant adenoviruses that expressed high levels of EGFP-tagged hTid-1. Ad.hTid-1L EGFP efficiently transduced SAOS-2 and U2OS cells and induced cell death 3 to 4 days after transduction (Fig. 8A and B). The transduced cells were condensed, detached, and floating from the bottom of tissue culture dishes, a finding consistent with apoptosis. In Ad.EGFP-hTid-1L-transduced HOS cells, apoptotic cells were seen at 3 days posttransduction. The cells transduced with Ad.EGFP-hTid-1ΔN100 did not exhibit growth arrest or cell death (Fig. 8C). These results clearly indicate that overexpression of hTid-1 can induce growth arrest and cell death in a p53-independent and J domain-dependent manner.
Full-length hTid-1 and ΔN100 had different subcellular distribution patterns. The former distributed evenly in the cytoplasm with a perinuclear cluster pattern, whereas the latter was heavily localized in perinuclear clusters (Fig. 8C). Similar patterns were seen in U2OS cells (Fig. 8D). The in vitro cytotoxicity assay showed that the viability of SAOS-2 cells transduced with Ad.EGFP-hTid-1L was reduced, whereas Ad.EGFP-hTid-1ΔN100 and the control Ad.EGFP did not significantly affect the viability of SAOS-2 cells (Fig. 8E). Cellular fractionation analysis showed that both the full-length and ΔN100 hTid-1 were present in the cytoplasm and mitochondria (data not shown). Whether the ΔN100 mutant that lacks the mitochondrial processing signal can translocate to the mitochondria remains unknown. One possibility is that this mutant could still form a protein complex with mitochondrial proteins or with its endogenous form of hTid-1 for targeting to the mitochondria.
Adenovirus-mediated gene transfer of hTid-1 into human melanoma cells inhibits proliferation in vitro and tumorigenicity in nude mice.
Altered expression of hTid-1 has been reported in human skin cancers (5). We examined the effect of hTid-1 on human melanoma in vitro proliferation and tumorigenicity in nude mice. Cells of the human melanoma line A375SM have a high basal level of IKK activity, probably because of constitutive activation of the IKK complex. Transduction of these cells with recombinant adenoviruses overexpressing hTid-1, but not ΔN100, led to reduced activity of IKK (Fig. 9A). MTT assay (22, 23, 29) of in vitro cell proliferation of transduced A375SM cells indicated overexpression of the full-length hTid-1, but not the ΔN100 mutant, inhibited cell proliferation in culture (Fig. 9B). Exogenous expression of hTid-1 but not ΔN100 suppressed the colony-forming ability of transduced melanoma cells in soft agar (Fig. 9C).
FIG. 9.
Adenovirus-mediated gene transfer of hTid-1 into human melanoma cells suppresses proliferation. (A) Endogenous IKK activity in A375SM cells transduced with recombinant adenoviruses expressing EGFP, EGFP-hTid-1, and EGFP-hTid-1ΔN100 was assessed by the in vitro kinase assay. (B) Overexpression of hTid-1, not the ΔN100 mutant, inhibits cell proliferation of A375 cells. Melanoma cells (3 × 103/per well) transduced with recombinant adenoviruses were plated into 96-well plates and incubated at 37°C. The number of viable cells at different times was determined by MTT assay. (C) Wild-type hTid-1, not the ΔN100 mutant, suppresses colony-forming ability of A375 cells in soft agar. The adenovirus-transduced cells (5 × 105/per well) were plated into six-well plates in 0.6% agar at 37°C in a 5% CO2 incubator (29). Ten days after plating, the cells were stained and recorded. The results are average ± the SD in triplicate. (D) The photo shows mice injected with ex vivo transduced human melanoma at the end of the 6-week observation period.
In the first set of in vivo experiments, nude mice (n = 5) were injected subcutaneously with 5 × 105 of Ad.EGFP-, Ad.EGFP-hTid-1-, or Ad.EGFP-hTid-1ΔN100-transduced A375SM cells, and the mice were killed 3 weeks later. The A375SM cells transduced with Ad.EGFP and Ad.EGFP-hTid-1ΔN100 produced tumors in four of five mice (0.14 ± 0.11 g) and five of five mice (0.15 ± 0.10 g), respectively, whereas the full-length hTid-1 transduced A375SM cells did not produce visible tumors (tumor incidence, 0 of 5). A second set of in vivo experiments with an extended observation period of up to 6 weeks and double the number (106 cells/mouse) of parental A375SM cells and Ad.EGFP-transduced cells produced tumors in all mice (n = 5), with median weights of 0.93 g (±0.36) and 1.07 g (±0.70), respectively. The A375SM cells transduced with the full-length hTid-1 only produced a small tumor (0.18 g) in one of the five mice injected. A typical appearance of mice in the three groups 6 weeks after tumor cell injection is shown in Fig. 9D.
Short interfering RNA directed against hTid-1 enhances TNF-α-induced activation of NF-κB.
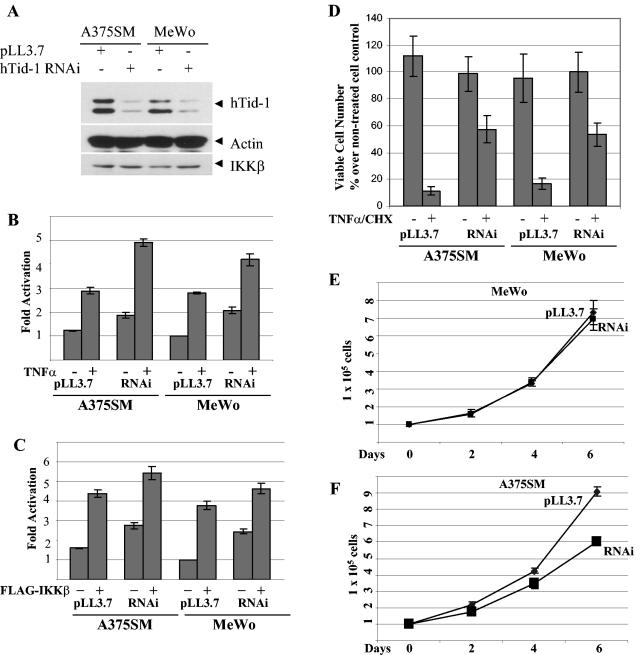
To determine the hTid-1 knockdown effect, we constructed recombinant lentivirus-mediated expression of hTid-1 RNAi in the lentivirus vector pLL3.7 (32a), in which the U6 promoter drives the expression of hTid-1 RNAi and the CMV promoter controls the expression of the fluorescent marker protein EGFP. The human melanoma cells were efficiently transduced with the lentivirus, and >95% of the cells were transduced by visualizing the green fluorescence by fluorescence imaging analysis (data not shown). The high transduction efficiency and long-term expression of the hTid-1 RNAi could be advantageous in reducing clonal selection variation. As shown in Fig. 10A, reduced levels of total endogenous hTid-1 in the A375SM and MeWo cells transduced with hTid-1 RNAi, but not with the control lentivirus pLL3.7, were observed. The protein levels of actin and IKKβ from the same total protein preparations were unchanged, indicating that the hTid-1 RNAi specifically suppressed the endogenous hTid-1 expression. We next evaluated the effect of hTid-1 knockdown on the NF-κB activity by using an NF-κB reporter assay. The NF-κB basal activity was higher in cells expressing hTid-1 RNAi than in the control cells (Fig. 10B). When stimulated with TNF-α or cotransfected with FLAG-IKKβ, the hTid-1 RNAi-expressing cells exhibited a higher level of the NF-κB activity than did the control cells at the same stimulation condition (Fig. 10B and C). We further observed an antiapoptotic effect of hTid-1RNAi-expressing melanoma cells in resisting TNF-α-cycloheximide-induced apoptosis (Fig. 10D), a finding consistent with results demonstrated in U2OS cells (13a). However, unlike the hTid-1 RNAi effect on U20S cells, the MeWo cells expressing Tid-1 RNAi exhibited a similar growth pattern as the control cells and the hTid-1 RNAi expressing A375SM cells showed a slightly retarded growth compared to the control cells (Fig. 10E and F). Taken together, these results support the role of hTid-1 as a negative regulator of the IKK complex.
FIG. 10.
Expression of hTid-1 RNAi enhances the NF-κB activity. (A) A375SM and MeWo cells were transduced with the lentivirus hTid-1 RNAi or with the control virus pLL3.7. At 3 days after transduction, the total lysates were analyzed with immunoblot with antibodies for hTid-1 (top panel), actin (middle panel), and IKKβ (bottom panel). (B) The stably transduced cells were transfected with NF-κB luciferase reporter plasmid (1 μg/each). At 2 days after transfection, the cells were stimulated with or without TNF-α (20 ng/ml) for 5 h. The reporter assay was performed as described in Materials and Methods. (C) The stably transduced cells were transfected with the NF-κB reporter (1 μg) alone or in combination with pCEF/FLAG-IKKβ (0.4 μg), and the reporter activity was determined 2 days posttransfection. (D) The transduced cells were treated with a combination of TNF-α (20 ng/ml) and cycloheximide (30 μg/ml) for 5 h. The viable cells were measured as described previously. The growth rates of the transduced A375SM and MeWo cells expressing hTid-1 RNAi or transduced with the control lentivirus pLL3.7 are shown in panels E and F, respectively.
DISCUSSION
We determined the mechanism for regulation of NF-κB activity and hence cell growth by hTid-1, a human DnaJ protein. The data show that suppression of NF-κB activity exhibited by overexpressed spliced variants of hTid-1 was dependent on their ability to interact with both the NF-κB-IκB and IKK complexes through direct binding to IκB and IKK. Consequently, hTid-1 enhanced the stability of the cytoplasmic NF-κB-IκB complex and repressed the kinase activity of IKK, culminating in an overall reduction of NF-κB activity. Further, overexpression of hTid-1 induced growth arrest and death in a p53-independent and J domain-dependent manner. Mutational analysis indicated that growth arrest by hTid-1 correlated with its suppression on NF-κB. Collectively, these data demonstrate an important role of hTid-1 in the regulation of NF-κB activity and cell growth.
The NF-κB regulatory activity of hTid-1 is dependent on the physical association of the hTid-1 proteins with the NF-κB-IκB and IKK complexes. Exogenous and endogenous hTid-1 and IκB coprecipitated in transfected cell lines. Fluorescence imaging analysis demonstrated that the nuclear translocation of newly synthesized IκBα was blocked by hTid-1. Significantly, two hTid-1 mutants, ΔN100 and ΔN150, which could not interact with IκB, lost the ability to repress NF-κB activity and instead facilitated IκBα protein aggregation and dislocation. It is unclear how these two mutants induce the protein aggregation of IκBα-EGFP. It is possible that they inhibit the function of the endogenous hTid-1 leading to inappropriate folding of IκBα, causing protein aggregation and dislocation. Regardless, hTid-1-mediated cytoplasmic retention and enhanced stability of IκBα could maximize the inhibitory activity of IκBα on NF-κB to stabilize the NF-κB-IκB complex by preventing its undesirable dissociation in resting cells.
We also demonstrated that hTid-1 associated with the IKK complex through an interaction with the IKKα/β subunit. These three subunits are integral components of the IKK complex in the forms of homodimers and heterodimers and are indispensable for intact activity of the complex (24, 34, 41, 44). Dominant-negative mutants of IKKγ and IKKα can diminish the kinase activity of IKKβ, a subunit of the IKK complex with a high intrinsic kinase activity. IKKβ was chosen to evaluate the effect of hTid-1 on the IKK complex due to its high intrinsic kinase activity in the absence of extracellular stimulation. Our results indicate that hTid-1 suppressed autophosphorylation of IKKβ. Further, by utilizing an RNA interference approach, we demonstrated that reduction of the endogenous protein level of both hTid-1 isoforms enhanced TNF-α and FLAG-IKKβ-mediated activation of NF-κB and protected the hTid-1 RNAi-expressing cells from TNF-α-induced apoptosis. These evidences are in support of a role for hTid-1 in the IKK complex.
How does hTid-1 stabilize the NF-κB-IκB protein complex? Based on the structural and functional analyses, hTid-1 may regulate NF-κB activity at several levels. First, through physical and functional interactions with the IKK complex, hTid-1 may help to maintain the stability of the kinase complex and, when overexpressed, suppress the activity of IKK. This action may be particularly important to ensure that transmission of activation signals passing through the complex is controlled, thus avoiding excessive stimulation of cells which can have an adverse effect on cell survival. Conversely, to transmit the activation signal through the IKK complex, the activated IKK could phosphorylate hTid-1 and modulate its activity. Indeed, we demonstrate that hTid-1 is phosphorylated by the kinase-active IKK, although this phosphorylation is less efficient than IKK-mediated phosphorylation of IκB. It remains to be determined how this phosphorylation is related to the hTid-1 modulation of NF-κB. Second, hTid-1 may block access of the IKK complex to IκBα. The binding of hTid-1 to IκBs could mask their serine phosphorylation sites, thus decreasing phosphorylation and slowing IκB turnover. Consistent with this, we found that hTid-1 inhibits in vitro phosphorylation of IκBα and prolongs its half-life (8). Third, by serving as an alternative substrate for activated IKK, overexpressed hTid-1 could compete for IKK phosphorylation, protecting IκB from phosphorylation and subsequent degradation. Fourth, the enhanced stability of IκB proteins may be related to hTid-1's cochaperone activity. The fluorescence imaging data implicate hTid-1 in maintaining proper protein folding and cytoplasmic distribution of IκBα (10, 11, 19). This potential chaperone activity of hTid-1 requires the J domain of hTid-1, indicating the possible involvement of Hsp70. In light of increasing evidence for molecular chaperones in the regulation of the NF-κB signaling complexes (1, 3, 6, 13, 43), it is tempting to speculate that the activity of hTid-1 is regulated in cooperation with its associated Hsp70.
The growth and death of cells is modulated by multiple signaling events. NF-κB is one of the key molecular switches, providing a survival mechanism in response to stress signals by upregulating antiapoptotic molecules such as Bcl-2. Altered expression and activity of NF-κB increase cell proliferation and even cellular transformation. In the present study, overexpression of wild-type hTid-1 in tumor cells induced cell growth arrest and death. This suppressive activity correlated with hTid-1 action on NF-κB; hTid-1 mutants that lost the NF-κB suppressive activity had no growth-inhibitory functions. However, the possibility that mitochondrial hTid-1 protein could also play a role in regulation of cell death and survival cannot be ruled out. It is noteworthy that we did not observe opposing effects of the two splicing variants of hTid-1 in induction of apoptosis. Both variants functioned similarly in the regulation of NF-κB activity, cell growth, and death, with the J domain playing an essential role. The C-terminal 180 amino acids could be deleted without significantly affecting the suppressive effects of hTid-1 on NF-κB. A recent report showed that by knocking down the expression of both spliced variants of hTid-1, U2OS cells exhibited a dramatic resistance to apoptosis induced by a variety of apoptotic stimuli and even enhanced anchorage-independent growth of the cells in soft agar (13a). These findings are consistent with our conclusion on the role of hTid-1, acting as a putative tumor suppressor to inhibit cell proliferation and tumor growth in nude mice. These findings also support, although indirectly, our observation that hTid-1 exhibits inhibitory activity on NF-κB, an antiapoptotic molecule.
The signals that lead to hTid-1's binding to the NF-κB-IκB complex in the cytoplasm and how this signal is negated upon appropriate stimulation are still unknown. Although originally described as a prominent mitochondrial protein, hTid-1 has subsequently been shown to distribute in the cytoplasm and the nucleus as well (7, 35). The interaction of hTid-1 with cytoplasmic, viral, and cellular factors supports a role for hTid-1 in the cytoplasm (7, 14, 35, 36). Notably, a recent report demonstrated that a cytoplasmic variant of the l(2)tid gene product Tid47 plays a major role in tumor suppression in Drosophila (5). Although Tid47 contains a mitochondrial processing signal, it is mainly localized in the cytoplasm. By extension, the cytoplasmic form of Tid47's human counterpart, hTid-1 could also play an important role in mediating growth suppressive activity, probably through physical and functional interaction with the components of the NF-κB signaling complexes in the cytoplasm. More study is required to further support the connection of NF-κB and tumor growth suppression mediated by the cytoplasmic form of hTid-1. Regardless, given the importance of NF-κB in cell growth and oncogenesis, additional studies on how the function of hTid-1 is modulated and on the identification of genetic mutations of hTid-1 in human primary tumors should provide critical insights into the process of tumor development and growth.
Acknowledgments
This study was supported in part by Cancer Center Support Core Grant CA16672 from the National Cancer Institute, National Institutes of Health.
We thank Karl Munger for anti-hTid-1 monoclonal antibody and pCMV-hTid-1S construct, Luk Van Parijs for pLL3.7 lentivirus vector, Mary Lloyd for technical assistance of animal studies, Walter Pagel for editorial review, Zhengxin Wang for critical comments, and Lola López for expert preparation of the manuscript.
REFERENCES
- 1.Agou, F., F. Ye, S. Goffinont, G. Courtois, S. Yamaoka, A. Israel, and M. Veron. 2002. NEMO trimerizes through its coiled-coil C-terminal domain. J. Biol. Chem. 277:17464-17475. [DOI] [PubMed] [Google Scholar]
- 2.Arenzana-Seisdedos, F., P. Turpin, M. Rodriguez, D. Thomas, R. T. Hay, J. L. Virelizier, and C. Dargemont. 1997. Nuclear localization of IκBα promotes active transport of NF-κB from the nucleus to the cytoplasm. J. Cell Sci. 110:369-378. [DOI] [PubMed] [Google Scholar]
- 3.Asea, A., M. Rehli, E. Kabingu, J. A. Boch, O. Bare, P. E. Auron, M. A. Stevenson, and S. K. Calderwood. 2002. Novel signal transduction pathway utilized by extracellular HSP70: role of Toll-like receptor (TLR) 2 and TLR4. J. Biol. Chem. 277:15028-15034. [DOI] [PubMed] [Google Scholar]
- 4.Bakker, T. R., D. Reed, T. Renno, and C. V. Jongeneel. 1999. Efficient adenoviral transfer of NF-κB inhibitor sensitizes melanoma to tumor necrosis factor-mediated apoptosis. Int. J. Cancer 80:320-323. [DOI] [PubMed] [Google Scholar]
- 5.Canamasas, I., A. Debes, P. G. Natali, and U. Kurzik-Dumke. 2003. Understanding human cancer using Drosophila: Tid47, a cytosolic product of the DnaJ-like tumor suppressor gene l2Tid, is a novel molecular partner of patched related to skin cancer. J. Biol. Chem. 278:30952-309060. [DOI] [PubMed] [Google Scholar]
- 6.Chen, G., P. Cao, and D. V. Goeddel. 2002. TNF-induced recruitment and activation of the IKK complex require Cdc37 and Hsp90. Mol. Cell 9:401-410. [DOI] [PubMed] [Google Scholar]
- 7.Cheng, H., C. Cenciarelli, Z. Shao, M. Vidal, W. P. Parks, M. Pagano, and C. Cheng-Mayer. 2001. Human T-cell leukemia virus type 1 Tax associates with a molecular chaperone complex containing hTid-1 and Hsp70. Curr. Biol. 11:1771-1775. [DOI] [PubMed] [Google Scholar]
- 8.Cheng, H., C. Cenciarelli, M. Tao, W. P. Parks, and C. Cheng-Mayer. 2002. HTLV-1 Tax-associated hTid-1, a human DnaJ protein, is a repressor of IκB kinase beta subunit. J. Biol. Chem. 277:20605-20610. [DOI] [PubMed] [Google Scholar]
- 9.Chu, Z. L., J. A. DiDonato, J. Hawiger, and D. W. Ballard. 1998. The tax oncoprotein of human T-cell leukemia virus type 1 associates with and persistently activates IκB kinases containing IKKα and IKKβ. J. Biol. Chem. 273:15891-15894. [DOI] [PubMed] [Google Scholar]
- 10.Cyr, D. M., X. Lu, and M. G. Douglas. 1992. Regulation of Hsp70 function by a eukaryotic DnaJ homolog. J. Biol. Chem. 267:20927-20931. [PubMed] [Google Scholar]
- 11.Devin, A., A. Cook, Y. Lin, Y. Rodriguez, M. Kelliher, and Z. Liu. 2000. The distinct roles of TRAF2 and RIP in IKK activation by TNF-R1: TRAF2 recruits IKK to TNF-R1 while RIP mediates IKK activation. Immunity 12:419-429. [DOI] [PubMed] [Google Scholar]
- 12.DiDonato, J. A., M. Hayakawa, D. M. Rothwarf, E. Zandi, and M. Karin. 1997. A cytokine-responsive IκB kinase that activates the transcription factor NF-κB. Nature 388:548-554. [DOI] [PubMed] [Google Scholar]
- 13.Dobbin, C. A., N. C. Smith, and A. M. Johnson. 2002. Heat shock protein 70 is a potential virulence factor in murine toxoplasma infection via immunomodulation of host NF-κB and nitric oxide. J. Immunol. 169:958-965. [DOI] [PubMed] [Google Scholar]
- 13a.Edwards, K. M., and K. Munger. 2004. Depletion of physiological levels of the human TID1 protein renders cancer cell lines resistant to apoptosis mediated by multiple exogenous stimuli. Oncogene 23:8419-8431. [DOI] [PubMed] [Google Scholar]
- 14.Eom, C. Y., and I. R. Lehman. 2002. The human DnaJ protein, hTid-1, enhances binding of a multimer of the herpes simplex virus type 1 UL9 protein to oris, an origin of viral DNA replication. Proc. Natl. Acad. Sci. USA 99:1894-1898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Geleziunas, R., S. Ferrell, X. Lin, Y. Mu, E. T. Cunningham, Jr., M. Grant, M. A. Connelly, J. E. Hambor, K. B. Marcu, and W. C. Greene. 1998. Human T-cell leukemia virus type 1 Tax induction of NF-κB involves activation of the IκB kinase alpha (IKKα) and IKKβ cellular kinases. Mol. Cell. Biol. 18:5157-5165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ghosh, S., M. J. May, and E. B. Kopp. 1998. NF-κB and Rel proteins: evolutionarily conserved mediators of immune responses. Annu. Rev. Immunol. 16:225-260. [DOI] [PubMed] [Google Scholar]
- 17.Gustin, J. A., T. Maehama, J. E. Dixon, and D. B. Donner. 2001. The PTEN tumor suppressor protein inhibits tumor necrosis factor-induced nuclear factor κB activity. J. Biol. Chem. 276:27740-27744. [DOI] [PubMed] [Google Scholar]
- 18.Hartl, F. U. 1996. Molecular chaperones in cellular protein folding. Nature 381:571-579. [DOI] [PubMed] [Google Scholar]
- 19.Hendrick, J. P., T. Langer, T. A. Davis, F. U. Hartl, and M. Wiedmann. 1993. Control of folding and membrane translocation by binding of the chaperone DnaJ to nascent polypeptides. Proc. Natl. Acad. Sci. USA 90:10216-10220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Henkel, T., T. Machleidt, I. Alkalay, M. Kronke, Y. Ben-Neriah, and P. A. Baeuerle. 1993. Rapid proteolysis of IκBα is necessary for activation of transcription factor NF-κB. Nature 365:182-185. [DOI] [PubMed] [Google Scholar]
- 21.Huang, S., A. DeGuzman, C. D. Bucana, and I. J. Fidler. 2000. Nuclear factor-κB activity correlates with growth, angiogenesis, and metastasis of human melanoma cells in nude mice. Clin. Cancer Res. 6:2573-2581. [PubMed] [Google Scholar]
- 22.Huang, S., C. A. Pettaway, H. Uehara, C. D. Bucana, and I. J. Fidler. 2001. Blockade of NF-κB activity in human prostate cancer cells is associated with suppression of angiogenesis, invasion, and metastasis. Oncogene 20:4188-4197. [DOI] [PubMed] [Google Scholar]
- 23.Huang, S., J. B. Robinson, A. DeGuzman, C. D. Bucana, and I. J. Fidler. 2000. Blockade of nuclear factor-κB signaling inhibits angiogenesis and tumorigenicity of human ovarian cancer cells by suppressing expression of vascular endothelial growth factor and interleukin 8. Cancer Res. 60:5334-5339. [PubMed] [Google Scholar]
- 24.Karin, M., and Y. Ben-Neriah. 2000. Phosphorylation meets ubiquitination: the control of NF-κB activity. Annu. Rev. Immunol. 18:621-633. [DOI] [PubMed] [Google Scholar]
- 25.Kelley, W. L. 1999. Molecular chaperones: how J. domains turn on Hsp70s. Curr. Biol. 9:R305-R308. [DOI] [PubMed] [Google Scholar]
- 26.Kurzik-Dumke, U., D. Gundacker, M. Renthrop, and E. Gateff. 1995. Tumor suppression in Drosophila is causally related to the function of the lethal(2) tumorous imaginal discs gene, a dnaJ homolog. Dev. Genet. 16:64-76. [DOI] [PubMed] [Google Scholar]
- 27.Laufen, T., M. P. Mayer, C. Beisel, D. Klostermeier, A. Mogk, J. Reinstein, and B. Bukau. 1999. Mechanism of regulation of hsp70 chaperones by DnaJ cochaperones. Proc. Natl. Acad. Sci. USA 96:5452-5457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li, J., G. W. Peet, S. S. Pullen, J. Schembri-King, T. C. Warren, K. B. Marcu, M. R. Kehry, R. Barton, and S. Jakes. 1998. Recombinant IκB kinases alpha and beta are direct kinases of IκBα. J. Biol. Chem. 273:30736-30741. [DOI] [PubMed] [Google Scholar]
- 29.Li, L., J. E. Price, D. Fan, R. D. Zhang, C. D. Bucana, and I. J. Fidler. 1989. Correlation of growth capacity of human tumor cells in hard agarose with their in vivo proliferative capacity at specific metastatic sites. J. Natl. Cancer Inst. 81:1406-1412. [DOI] [PubMed] [Google Scholar]
- 30.Li, Q., and I. M. Verma. 2002. NF-κB regulation in the immune system. Nat. Rev. Immunol. 2:725-734. [DOI] [PubMed] [Google Scholar]
- 31.Mercurio, F., H. Zhu, B. W. Murray, A. Shevchenko, B. L. Bennett, J. Li, D. B. Young, M. Barbosa, M. Mann, S. Manning, and A. Rao. 1997. IKK-1 and IKK-2: cytokine-activated IκB kinases essential for NF-κB activation. Science 278:860-866. [DOI] [PubMed] [Google Scholar]
- 32.Robek, M. D., and L. Ratner. 1999. Immortalization of CD4+ and CD8+ T lymphocytes by human T-cell leukemia virus type 1 Tax mutants expressed in a functional molecular clone. J. Virol. 73:4856-4865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32a.Robinson, D. A., C. P. Dillon, A. V. Kwiatkowski, C. Sievers, L. Yang, J. Kopinja, D. L. Rooney, N. M. Ihrig, M. T. McManus, F. B. Gertler, M. L. Scott, and L. Van Parijs. 2003. A lentivirus-based system to functionally silence genes in primary mammalian cells, stem cells, and transgenic mice by RNA interference. Nat. Genet. 33:401-406. [DOI] [PubMed] [Google Scholar]
- 33.Romashkova, J. A., and S. S. Makarov. 1999. NF-κB is a target of AKT in antiapoptotic PDGF signalling. Nature 401:86-90. [DOI] [PubMed] [Google Scholar]
- 34.Rothwarf, D. M., E. Zandi, G. Natoli, and M. Karin. 1998. IKKγ is an essential regulatory subunit of the IκB kinase complex. Nature 395:297-300. [DOI] [PubMed] [Google Scholar]
- 35.Sarkar, S., B. P. Pollack, K. T. Lin, S. V. Kotenko, J. R. Cook, A. Lewis, and S. Pestka. 2001. hTid-1, a human DnaJ protein, modulates the interferon signaling pathway. J. Biol. Chem. 276:49034-49042. [DOI] [PubMed] [Google Scholar]
- 36.Schilling, B., T. De-Medina, J. Syken, M. Vidal, and K. Munger. 1998. A novel human DnaJ protein, hTid-1, a homolog of the Drosophila tumor suppressor protein Tid56, can interact with the human papillomavirus type 16 E7 oncoprotein. Virology 247:74-85. [DOI] [PubMed] [Google Scholar]
- 37.Syken, J., T. De-Medina, and K. Munger. 1999. TID1, a human homolog of the Drosophila tumor suppressor l(2)tid, encodes two mitochondrial modulators of apoptosis with opposing functions. Proc. Natl. Acad. Sci. USA 96:8499-8504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Traenckner, E. B., H. L. Pahl, T. Henkel, K. N. Schmidt, S. Wilk, and P. A. Baeuerle. 1995. Phosphorylation of human IκBα on serines 32 and 36 controls IκBα proteolysis and NF-κB activation in response to diverse stimuli. EMBO J. 14:2876-2883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Turpin, P., R. T. Hay, and C. Dargemont. 1999. Characterization of IκBα nuclear import pathway. J. Biol. Chem. 274:6804-6812. [DOI] [PubMed] [Google Scholar]
- 40.Wu, W. S., Z. S. Xu, W. N. Hittelman, P. Salomoni, P. P. Pandolfi, and K. S. Chang. 2003. Promyelocytic leukemia protein sensitizes tumor necrosis factor alpha-induced apoptosis by inhibiting the NF-κB survival pathway. J. Biol. Chem. 278:12294-12304. [DOI] [PubMed] [Google Scholar]
- 41.Yamaoka, S., G. Courtois, C. Bessia, S. T. Whiteside, R. Weil, F. Agou, H. E. Kirk, R. J. Kay, and A. Israel. 1998. Complementation cloning of NEMO, a component of the IκB kinase complex essential for NF-κB activation. Cell 93:1231-1240. [DOI] [PubMed] [Google Scholar]
- 42.Yang, J., and A. Richmond. 2001. Constitutive IκB kinase activity correlates with nuclear factor-κB activation in human melanoma cells. Cancer Res. 61:4901-4909. [PubMed] [Google Scholar]
- 43.Yoo, C. G., S. Lee, C. T. Lee, Y. W. Kim, S. K. Han, and Y. S. Shim. 2000. Anti-inflammatory effect of heat shock protein induction is related to stabilization of IκBα through preventing IκB kinase activation in respiratory epithelial cells. J. Immunol. 164:5416-5423. [DOI] [PubMed] [Google Scholar]
- 44.Zandi, E., D. M. Rothwarf, M. Delhase, M. Hayakawa, and M. Karin. 1997. The IκB kinase complex (IKK) contains two kinase subunits, IKKα and IKKβ, necessary for IκB phosphorylation and NF-κB activation. Cell 91:243-252. [DOI] [PubMed] [Google Scholar]
- 45.Zhang, S. Q., A. Kovalenko, G. Cantarella, and D. Wallach. 2000. Recruitment of the IKK signalosome to the p55 TNF receptor: RIP and A20 bind to NEMO (IKKγ) upon receptor stimulation. Immunity 12:301-311. [DOI] [PubMed] [Google Scholar]