Abstract
Activins and other members of the transforming growth factor β family play a critical role in morphological changes of the epidermis that require epithelial cell movement. We investigated the molecular pathways in the transmission of activin signals that lead to actin reorganization and epithelial cell migration. We found that activins cause the activation of RhoA but not of Rac and CDC42, leading to MEKK1-dependent phosphorylation of JNK and transcription factor c-Jun. Through a RhoA-independent mechanism, the activins also induce p38 activity in keratinocytes from wild-type but not from MEKK1-deficient mice. Although neither pathway is dependent on Smad activation, the MEKK1-mediated JNK and p38 activities are both essential for activin-stimulated and transcription-dependent keratinocyte migration. Only JNK is involved in transcription-independent actin stress fiber formation, which needs also the activity of ROCK. Because ROCK is required for JNK activation by RhoA and its overexpression leads to MEKK1 activation, we propose a RhoA-ROCK-MEKK1-JNK pathway and a MEKK1-p38 pathway as Smad-independent mechanisms in the transmission of activin signals. Together, these pathways lead to the control of actin cytoskeleton reorganization and epithelial cell migration, contributing to the physiologic and pathological effects of activins on epithelial morphogenesis.
Like other transforming growth factor β (TGFβ) family factors, activin activities are mediated by their heteromeric receptor complexes, consisting of type I (ActRIA and ActRIB) and type II (ActRIIA and ActRIIB) receptors. Activins also bind to follistatins, which are soluble proteins that inhibit activin interaction with its receptors and prevent its biological effects (22). Both receptors are transmembrane proteins possessing an intracellular serine/threonine kinase domain (13), the activation of which by ligand binding leads to the phosphorylation of Smad2/3. The phosphorylated Smad2/3 form a heteromeric complex with Smad4, which in turn translocates to the nucleus, where it regulates target gene expression and biological responses (18). Activins are crucial for the regulation of epithelial cell function; their aberrant expression is associated with abnormal skin development and impaired wound healing (14, 16, 21, 24, 26). Some activin activities, however, are unlikely to be mediated through the Smad pathway, which has a known function opposite to that of activins in wound healing (1). Hence, activins might accomplish at least some of their effects in epithelial cells through signaling pathways other than Smad.
MEKK1 is a cytoplasmic MAPKKK, controlling the activation of mitogen-activated protein kinases (MAPKs) in a tissue- and stimulus-specific fashion (20). In previous work, we showed that MEKK1 knockout mice exhibit eye-open at birth (29, 30), a phenotype remarkably similar to that observed in activin βB knockout and follistatin transgenic mice, in which activin signals are blocked (24, 26). MEKK1-null keratinocytes are not responsive to activin in the induction of actin stress fibers, corresponding to lack of morphological changes and F-actin formation in the developing eyelid epithelium (30). Actin stress fiber formation is a critical cell activity underlying epithelial cell migration and the MEKK1-null keratinocytes are indeed defective in activin-induced migration. MEKK1 inactivation, however, has no effects on Smad activation by activins. Hence, MEKK1 might represent a Smad-independent mechanism in transmitting activin signals that lead to keratinocyte migration and eyelid morphogenesis.
We investigate here the molecular mechanisms by which the activin signals are transduced to MEKK1 and the downstream effectors of MEKK1 in the regulation of epithelial cell migration. We found a role for RhoA in mediating activin signals to MEKK1 and subsequent induction of JNK and c-Jun. This pathway is required for both the transcription-independent actin stress fiber formation and the transcription-dependent movement of keratinocytes. The activin signals are also transduced via a RhoA-independent MEKK1-p38 pathway, which is required only for the induction of keratinocyte migration and not for the formation of actin stress fibers.
MATERIALS AND METHODS
Mice, cells, and reagents.
The colonies for wild-type, Mekk1+/ΔKD, and Mekk1ΔKD/ΔKD mice were maintained as described previously (30). All experimental procedures with these mice were approved by the ethics committee of the University of Cincinnati. The mouse primary keratinocytes were isolated from newborn fetuses as described previously (30) and, unless otherwise indicated, all experiments were carried out with cells isolated from wild-type fetuses. Antibodies for RhoA, Rac, JNK, p38, ERK, p-c-Jun, MEKK1, and Smad4 were from Santa Cruz Biotechnology, and those for p-JNK and p-p38 were from Promega. Anti-p-ERK was from Cell Signaling, and rhodamine-phalloidin, fluorescein isothiocyanate (FITC)-phalloidin, and DAPI (4′,6′-diamidino-2-phenylindole) were from Sigma. The chemical inhibitors for JNK (SP600125), p38 (SB202190), ERK (PD98059), ROCK (Y27632), and protein synthesis (cycloheximide [CHX]) were from Calbiochem. Activin B and TGFα were from R&D Systems, Inc.
GTPase activity assay, MEKK1 kinase assay, and Western blot analyses.
Primary mouse keratinocytes were deprived of growth factor for 24 h, followed by treatment with TGFα, serum, or activin B (5 ng/ml) for 10 min. The cell lysates were assayed for GTP-bound RhoA and Rac by pull-down assay, and the total RhoA and Rac was determined by Western blotting as described previously (25). MEKK1 activities were measured as described previously (27). In brief, cell lysates were subjected to immunoprecipitation with anti-MEKK1, and kinase assay was performed in the presence of [γ-32P]ATP and bacterially expressed glutathione S-transferase-JNKK1 as a substrate. Protein phosphorylation was visualized after electrophoresis and autoradiography.
Virus production and infection of targeting cells.
Retrovirus expression vectors were used that directed the expression of green fluorescent protein (GFP) and one of the following proteins: RhoA mutants N19, L63, L63E40T, L63Y42C, L63F39V, and L63E40L; Rac mutants N17 and L61; Cdc42 mutants N17 and L61; and wild-type ROCK I. The specific features of each mutant were described previously (25) and are summarized in Fig. 3C. Recombinant retroviruses were produced by the Retroviral Core Facility at the University of Cincinnati. The respective retroviruses were incubated with primary keratinocytes for a 6-h period, and the infection was repeated three times. At 24 h postinfection, the cells were maintained in medium depleted of growth factors for 24 h before harvesting. In some experiments, the cells were pretreated with and were kept in 5 μM concentrations of SP600125, SB202190, Y27632, or CHX.
FIG. 3.
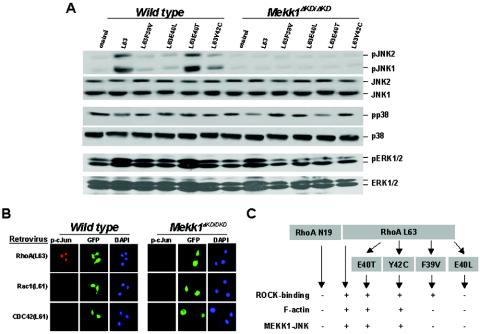
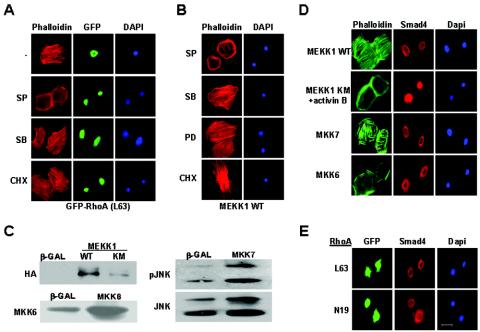
RhoA induces MEKK1-dependent activation of JNK and c-Jun. Wild-type and Mekk1ΔKD/ΔKD keratinocytes were infected by retroviruses for GFP and various mutants of RhoA, Rac1, or CDC42, as indicated. (A) Cell lysates were analyzed for the phosphorylated isoforms and total JNK, p38, and ERK by Western blotting. (B) Cells were subjected to immunostaining with a polyclonal antibody against p-c-Jun, followed by rhodamine-anti-rabbit antibody and DAPI. Phosphorylated c-Jun, GFP, and DAPI were visualized by fluorescence microscopy. (C) A summary of the RhoA effector mutants in ROCK binding (19), induction of the MEKK1-dependent JNK phosphorylation, and actin stress fiber formation.
Using the AdEasy system, we generated replication-deficient adenoviruses containing a gene for human MEKK1 [MEKK1(WT)] or its ATP-binding site mutant [MEKK1(KM)], with an N-terminal hemagglutinin (HA) tag (28), according to the manufacturer's specifications (Stratagene). Adenoviruses for β-galactosidase and for constitutive active forms of MKK6 and MKK7 were described previously (7). Viral infection was carried out by incubating adenoviruses with keratinocytes at 5 to 10 PFU for 1 h; excess virus was removed, and the cells were grown for 48 h before harvesting.
Immunofluorescence staining and in vitro wound-healing assay.
Cells grown on cover glasses were fixed, permeabilized, and stained with first antibody at 30°C for 1 h, followed by staining with the appropriate fluorescence-labeled second antibody, with or without FITC- or rhodamine-phalloidin and DAPI for 30 min. The staining was visualized by using a Zeiss fluorescence microscope. A total of five fields were evaluated for each treatment group.
Confluent mouse primary keratinocytes were used for the in vitro scratch wound-healing assay, and the wounds were photographed at 0, 12, 24, and 36 h after wounding as described before (30).
RESULTS
Activation of RhoA by activin leads to MEKK1-dependent actin stress fiber formation.
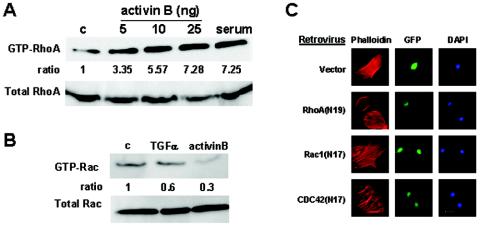
We first asked how the activin signals transduced through MEKK1 led to the reorganization of actin cytoskeleton. The Rho family of GTPases, including Rho, Rac, and CDC42, play unique roles in the dynamic processes of actin reorganization (2, 6, 8, 12). We therefore tested the hypothesis that activin-induced actin stress fiber formation might be mediated through the small GTPases. Induction of GTP-bound, active RhoA was detected in keratinocytes as early as 10 min after treatment with activin B; the levels of GTP-bound Rac, however, were unaltered by activin B or TGFα (Fig. 1A and B). RhoA activity was required for activin B to induce actin stress fibers because retroviruses bearing dominant-negative RhoA [RhoA(N19)], but not Rac [Rac(N17)] or CDC42 [CDC42(N17)], prevented activin B-induced actin stress fiber formation (Fig. 1C). Correspondingly, expression of active RhoA [RhoA(L63)], but not Rac or CDC42, was sufficient for the induction of actin stress fibers in the absence of activin B (Fig. 2A).
FIG. 1.
Activin-induced RhoA activation is required for actin stress fiber formation. GTP-bound and total RhoA (A) and Rac (B) were measured in keratinocytes treated with serum (10%), TGFα (10 ng/ml), or activin B (5 ng/ml) at the indicated doses for 10 min. Cell lysates were used to measure the levels of GTP-RhoA and GTP-Rac by GTP pull-down assay, and total RhoA and Rac1 was evaluated by Western blot analysis. The ratio of GTP-bound versus total GTPase is indicated. (C) Retroviruses carrying a GFP gene and genes for the enzyme-inactive mutant RhoA(N19), Rac(N17), and CDC42(N17) were used to infect wild-type keratinocytes. At 24 h after infection, the cells were treated with activin B (5 ng/ml) for 1 h. The cells were stained with rhodamine-phalloidin for F-actin and with DAPI for nuclei, and the virus-infected cells were identified by GFP fluorescence.
FIG. 2.
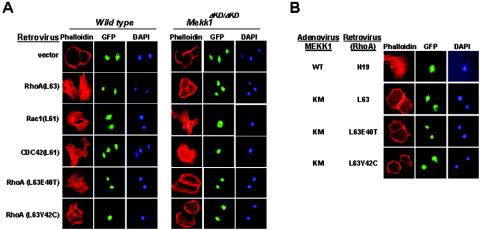
RhoA-stimulated actin stress fiber formation requires MEKK1. (A) Wild-type and Mekk1ΔKD/ΔKD keratinocytes were infected by retrovirus containing GFP and RhoA mutants as indicated. (B) Wild-type keratinocytes were infected with retroviruses containing the indicated RhoA mutants and adenoviruses that express constitutive-active (WT) or kinase-inactive (KM) MEKK1. The cells were stained for F-actin at 24 to 48 h after infection as described before.
These data, together with our previous findings (30), suggest that both RhoA and MEKK1 are required for activin to induce actin stress fiber formation in keratinocytes. We next investigated the connection between MEKK1 and RhoA in this process. We found that RhoA-induced actin stress fiber was completely abolished in cells deficient for MEKK1 (Mekk1ΔKD/ΔKD) or expressing an adenovirus-borne MEKK1 dominant-negative mutant [MEKK1(KM)] (Fig. 2A and B). Conversely, active MEKK1 [MEKK1(WT)] was sufficient to cause actin stress fiber, and this induction was unaffected by RhoA(N19) (Fig. 2B). Rho GTPases transmit signals through an interaction with effector molecules (10). Using RhoA mutants in which various effector binding abilities were altered, we observed that their role in keratinocyte actin stress fiber formation was similar to that described in NIH 3T3 fibroblasts (19). RhoA mutants L63, L63E40T, and L63Y42C stimulated actin stress fiber (Fig. 2A and 3C), whereas RhoA L63F39V and L63E40L failed to do so (data not shown). Importantly, all RhoA mutants required MEKK1 for induction of actin stress fibers, which were abolished in Mekk1ΔKD/ΔKD cells and in cells expressing MEKK1(KM) (Fig. 2A and B and 3C). From these data, we conclude that MEKK1 acts downstream of RhoA in a signaling pathway that leads to actin stress fiber formation.
Activin signals are transduced through a RhoA-dependent MEKK1-JNK pathway and a RhoA-independent MEKK1-p38 pathway.
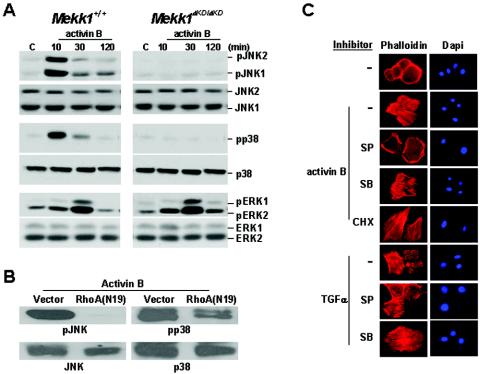
To search for the downstream events resulting from activation of the RhoA-MEKK1 pathway, we examined the phosphorylation status of JNK, p38, and ERK in wild-type and Mekk1ΔKD/ΔKD keratinocytes expressing various RhoA mutants. The same RhoA mutants that stimulated actin stress fiber formation also triggered the phosphorylation of JNK in a MEKK1-dependent fashion, but they caused only a marginal induction of ERK and were unable to stimulate p38 phosphorylation (Fig. 3A). RhoA (N19), L63F39V, and L63E40L mutants, which failed to induce actin stress fiber, also failed to activate JNK. In addition to actin stress fiber formation, nuclear c-Jun phosphorylation was observed in cells that express RhoA(L63) but not in cells expressing Rac(L61) and CDC42(L61), and c-Jun phosphorylation was also dependent on the presence of a functional MEKK1 protein (Fig. 3B). Hence, of the MAPKs examined, the RhoA-MEKK1 pathway appears to be specific for JNK, the activation of which correlates not only to actin stress fiber formation but also to nuclear factor phosphorylation in keratinocytes (Fig. 3C). In contrast to RhoA, activin B induced the activities of both JNK and p38 in a MEKK1-dependent manner; however, only its activity toward JNK was dependent on RhoA, and it was completely suppressed by the expression of RhoA(N19) (Fig. 4A and B). Activin B also stimulated ERK activity independently of MEKK1. These results suggest a RhoA-dependent MEKK1-JNK pathway and a RhoA-independent MEKK1-p38 pathway are both likely to be effective in the transmission of activin signals.
FIG. 4.
Activin B-induced, RhoA- and MEKK1-dependent JNK activity is essential, but p38 and protein syntheses are not required, for actin stress fiber formation. (A) Wild-type and Mekk1ΔKD/ΔKD keratinocytes were treated with activin B for the indicated times, and cell lysates were analyzed for the phosphorylated isoforms and total JNK, p38, and ERK by Western blotting. (B) Wild-type keratinocytes were infected with retroviruses containing empty vector or RhoA(N19). The cells were treated with activin B for 10 min before being harvested for Western blot analyses, as described above. (C) Wild-type keratinocytes were pretreated with chemical inhibitors as indicated, followed by stimulation with activin B and TGFα for 1 h. Polymerized actins were detected by rhodamine-phalloidin staining, and nuclei were identified by DAPI staining. SP, JNK inhibitor SP600125; SB, p38 inhibitor SB202190; CHX, protein synthesis inhibitor CHX.
Acitvation of JNK, but not p38 and Smad, is required for activin-induced, transcription-independent actin stress fiber formation.
To investigate the roles of JNK and p38 in activin-stimulated actin stress fiber formation, we used designated chemical inhibitors for each MAPK. We found that SP600125, an inhibitor of JNK, completely abolished activin B-stimulated actin stress fibers, whereas SB202190, an inhibitor of p38, had no such an effect (Fig. 4C). SP600125, but not SB202190, also prevented actin stress fibers induced by other activators of this pathway, namely, RhoA(L63) and MEKK1(WT) (Fig. 5A and B). In contrast, actin stress fibers induced by TGFα were MEKK1 independent and were unaffected by JNK inhibition (Fig. 4B). Next, we sought to determine whether JNK activation alone was sufficient to stimulate actin stress fibers. Keratinocytes were infected with adenoviruses expressing constitutively active mutants of MKK7 to activate JNK and MKK6 to activate p38 (Fig. 5C), and actin stress fiber formation was examined by staining with FITC-phalloidin (Fig. 5D). Activation of JNK was sufficient to induce actin stress fibers, whereas activation of p38 by MKK6 did not elicit such a response.
FIG. 5.
JNK, but not Smad, activation is essential and sufficient for inducing actin stress fiber formation. (A and B) Wild-type keratinocytes were pretreated with various chemical inhibitors as indicated, followed by infection with retroviruses for RhoA(L63) (A) or adenoviruses for MEKK1(WT) (B). F-actins were detected by rhodamine-phalloidin staining, RhoA-positive cells were identified by using GFP, and nuclei were visualized by DAPI staining. (C) Cells infected by adenoviruses for HA-MEKK1(WT), HA-MEKK1(KM), active MKK6, and MKK7 were lysed. Cell lysates were analyzed by Western blotting for the expression of MEKK1 with anti-HA, of MKK6 with anti-MKK6, and of MKK7 with anti-pJNK and anti-JNK. (D) Some of the adenovirus-infected cells were treated with activin B for 1 h before fixation and immunostaining with FITC-phalloidin and α-Smad4, which was recognized by rhodamine-anti-rabbit immunoglobulin G. (E) Cells infected by retroviruses of RhoA(L63) and RhoA(N19) were recognized by GFP and were subjected to immunostaining with α-Smad4, as described above.
We noticed that actin stress fibers were formed as early as 30 min after activin B treatment (data not shown), suggesting that they were the result of an immediate cell response, possibly independent of gene transcription. Indeed, pretreatment of cells with CHX, an inhibitor of de novo protein synthesis, had no effect on actin stress fibers induced by essentially all components of the activin B-RhoA-MEKK1 pathway (Fig. 4C and 5A and B).
A well-known downstream effect of activin signaling is the induction of Smad4 nuclear localization. We found that inhibition of MEKK1 activity by a kinase-inactive MEKK1 did not prevent induction of Smad4 nuclear translocation by activin B, whereas neither active RhoA nor active MEKK1 caused changes in the perinuclear localization of Smad4 (Fig. 5D and E). Activation of JNK and p38 by adenovirus-mediated expression of dominant-active mutants of MKK6 and MKK7 also failed to stimulate the nuclear translocation of Smad4 (Fig. 5D). Hence, Smad activation is clearly a distinct pathway independent of MEKK1 in activin signaling.
ROCK is required for JNK activation and actin stress fiber formation.
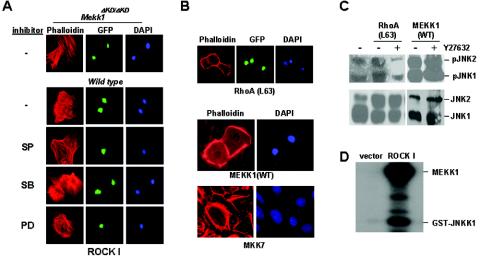
ROCK is a known downstream effector of RhoA that has been shown before to interact with the RhoA mutants L63E40T, L6342C, and L63F39V but not with L63E40L (Fig. 3C). Stimulation of actin stress fiber formation by wild-type ROCK (ROCK I) was unaffected by MEKK1 inhibition or by inhibitors of the MAPKs (Fig. 6A), whereas stimulation by active RhoA, MEKK1, or MKK7 was completely abolished by Y27632 (Y), a ROCK inhibitor (Fig. 6B). Although this result may suggest that ROCK acts downstream of the RhoA-MEKK1-JNK pathway, we found that Y27632 could also efficiently suppress RhoA-stimulated, but not MEKK1-stimulated JNK activity (Fig. 6C). Furthermore, expression of ROCK I caused a significant induction of MEKK1 activity, measured by its ability to phosphorylate itself and its substrate JNKK1 (Fig. 6D). We believe that ROCK might mediate RhoA signal to the MEKK1-JNK pathway, which in turn requires ROCK for the induction of actin stress fiber formation (Fig. 7B).
FIG. 6.
ROCK is required for actin stress fiber formation and the activation of the MEKK1-JNK pathway. (A) Wild-type andMekk1ΔKD/ΔKD keratinoyctes were infected by retroviruses of ROCK I in the presence or absence of the MAPK inhibitors, as indicated. (B) Wild-type keratinocytes were pretreated with Y27632 (10 μM), followed by infection with retroviruses expressing active RhoA(L63) or adenoviruses for active MEKK1(WT) and MKK7. Cells were analyzed for F-actin by using rhodamine-phalloidin, and nuclei were identified by DAPI staining. RhoA-positive cells were identified by using GFP as described before. (C) Cells with or without pretreatment with Y27632 were infected by retroviruses expressing active RhoA(L63) or adenoviruses for active MEKK1(WT). Cell lysates were analyzed by Western blotting for phosphorylated and total JNK. (D) Lysates from keratinocytes infected by ROCK I were subjected to immunocomplex kinase assay by using anti-MEKK1 for precipitation and GST-JNKK1 as a substrate in the presence of [γ-32P]ATP.
FIG. 7.
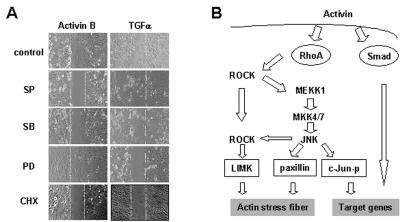
Both JNK and p38 are required for keratinocyte migration. (A) Primary mouse keratinocytes were used for an in vitro wound-healing assay. Cells were treated by activin B (10 ng/ml) or TGFα (5 ng/ml) in the presence of absence of various inhibitors at 5 μM. Pictures were taken at 0, 12, 24, and 36 h after wounding, and only the 24 h photos are shown. SP, JNK inhibitor SP600125; SB, p38 inhibitor SB202190; PD, ERK inhibitor PD98059; CHX, protein synthesis inhibitor CHX. (B) Model of molecular pathways by which activin signals induce actin stress fiber formation and keratinocyte migration. The activins cause activation of RhoA, which in turn induces the ROCK-dependent MEKK1-JNK pathway that leads to a transcription-independent actin stress fiber formation. ROCK, in regulating MLCK, might have a role independent of the MEKK1-JNK pathway in the control of actin cytoskeleton. The JNK might regulate actin stress fiber formation through phosphorylation of paxillin. Apart from actin stress fiber formation, this pathway also results in the induction of c-Jun phosphorylation, which might lead to transcription-dependent events. The activin signals transduce also through a RhoA-independent MEKK1-p38 pathway that is critical for the transcription-dependent migration. Neither pathway is connected to Smad activation.
Both JNK and p38 are critical for transcription-dependent keratinocyte migration.
Because actin stress fiber formation is a cell response characteristic of keratinocyte migration, we hypothesized that JNK activity was required for the induction of keratinocyte migration by activin B. We found that inhibition of JNK could prevent activin B-induced keratinocyte migration and healing of an in vitro wound, whereas it did not affect that induced by TGFα, which stimulated actin stress fiber formation through the activation of ERK (28). Inhibition of ERK limited only migration induced by TGFα but not by activin B. To our surprise, however, inhibition of p38 suppressed activin-induced cell migration and p38 activity was not needed for the induction of actin stress fibers (Fig. 7A). Unlike the formation of actin stress fibers, keratinocyte migration was a delayed response that required gene transcription, as demonstrated by the observation that CHX completely abolished in vitro wound healing of keratinocytes. We therefore propose that the induction of keratinocyte migration requires the transduction of activin B signals through both the RhoA-ROCK-MEKK1-JNK pathway and the MEKK1-p38 pathway, ultimately leading to gene expression reprogramming. The RhoA-to-JNK pathway is also essential and sufficient for the transcription-independent formation of actin stress fibers (Fig. 7B).
DISCUSSION
Members of the TGFβ family play diverse roles in the regulation of epithelial cell function, leading to inhibition of growth, stimulation of actin stress fiber formation, and induction of migration (11). Activation of Smad alone cannot explain all of the downstream events of TGFβ/activin signals reported. The Smad pathway is in fact mainly responsible for growth-inhibitory effects in the skin, as confirmed by the observation that Smad3 knockout mice show increased keratinocyte proliferation and accelerated wound healing (1). We present here evidence for two other pathways, one involving a RhoA-ROCK-MEKK1-JNK signaling cascade and the other involving a MEKK1-p38 cascade, that are also involved in the transduction of activin signals (Fig. 7B). Although both pathways are required for keratinocyte migration, only the RhoA-to-JNK pathway is involved in actin stress fiber formation. These are novel Smad-independent signaling mechanisms in keratinocytes that connect TGFβ/activin signals to cytoskeleton reorganization, mobility, and migration.
The involvement of small GTPases in TGFβ signaling has been observed in many cell types (2, 8, 12). Here, we show that in keratinocytes, RhoA, but not Rac1 or CDC42, acts downstream of activins in the control of actin stress fiber formation. The RhoA activity is in turn transmitted through MEKK1 to JNK. Rac1 and CDC42 are generally considered the small GTPases upstream of the JNK pathway (15) but, at least in regard to activin-induced actin stress fiber formation, it appears that RhoA is one of the major players. Not all activin signals, however, are transmitted through RhoA, because the activin-stimulated MEKK1-dependent p38 activation and MEKK1-independent Smad nuclear translocation are not RhoA dependent. We therefore suggest that there are a minimum of two Smad-independent pathways in activin signaling: one is the RhoA-dependent MEKK1-JNK pathway, and the other is the RhoA-independent MEKK1-p38 pathway. The former is essential and sufficient for actin stress fiber formation, which is an immediate cell response independent of gene transcription. This pathway also leads to the phosphorylation of transcription factor c-Jun, indicating that besides its cytoplasmic effects on actin cytoskeleton, the RhoA-to-JNK pathway could modulate transcription factors in the nucleus and possibly cause gene expression reprogramming. Keratinocyte migration, however, requires gene transcription and the participation of both JNK and p38. Through these designated pathways, the activin signals lead to diverse and specific cell functions.
Downstream transmission of the RhoA signal is accomplished through specific interactions between the GTP-bound RhoA and effector molecules. These interactions result in a conformational change of the effector and activation of the pathway (4). One downstream effector of RhoA is ROCK, a regulator of myosin light chain kinase (MLCK) that controls actin reorganization and focal adhesion (23). On the one hand, active ROCK induces, independently of MEKK1 and JNK, actin stress fiber formation, whereas a ROCK inhibitor prevents RhoA-, MEKK1-, and MKK7-stimulated actin stress fibers. These findings suggest that ROCK activity, possibly acting independently or downstream of the MEKK1-JNK pathway, is essential for actin cytoskeleton reorganization to take place. On the other hand, RhoA-induced JNK requires ROCK, whose activity in turn is sufficient for stimulating MEKK1, supporting the idea that ROCK plays a role in mediating RhoA activity to the MEKK1-JNK pathway. It appears that ROCK is involved in the control of actin cytoskeleton reorganization through at least two mechanisms: a RhoA-ROCK-MLCK pathway and a RhoA-ROCK-MEKK1-JNK pathway (Fig. 7B). A role for ROCK in JNK activation has been described also in fibroblasts (9), but the involvement of this pathway in actin stress fiber formation is unique for keratinocytes.
Recent findings that GTP-bound RhoA interacts with and activates MEKK1 (3) suggest that MEKK1 might act as a direct downstream effector of RhoA. If both ROCK and MEKK1 were downstream effectors, it would be of interest to find how they coordinate to mediate the RhoA signals that lead to actin reorganization. Studies in fibroblasts with various RhoA effector loop mutants indicate that interaction with ROCK correlates with the activity of RhoA in transformation but is not sufficient for the induction of actin stress fibers (19). In our hands, not all RhoA mutants that have been reported to interact with ROCK could stimulate actin stress fiber formation in keratinocytes. Instead, we found a good correlation between MEKK1-dependent JNK activity and induction of keratinocyte actin stress fiber formation by RhoA mutants. Perhaps, the specific RhoA activity requires both ROCK and MEKK1 as downstream effectors and together they lead to JNK activation. Several lines of evidence have suggested a role for JNK in the control of actin cytoskeleton reorganization. Earlier studies in Drosophila melanogaster have shown that JNK directly interacts with and phosphorylates the actin-associated Spir 150, a WASP family protein that might connect JNK activity to actin cytoskeleton (17). One such direct link between JNK and actin cytoskeleton has recently been found in mammals, with the observation that JNK phosphorylates paxillin, an actin-associated protein, and this phosphorylation appears to be essential for actin stress fiber formation in keratinocytes (5).
Dissecting the downstream pathways of TGFβ/activin is a productive approach to understanding the molecular basis underlying their promiscuous functions. Our studies establish that activation of the RhoA-MEKK1-JNK pathway and the MEKK1-p38 pathway are downstream effects of the activin signals that lead to actin stress fiber formation and keratinocyte migration, which do not appear to connect with Smad nuclear translocation. The modular specificity provided by these pathways is particularly intriguing because it presents the opportunity to manipulate only a subset of the cell functions controlled by TGFβ/activin without affecting others. Selective activation of the RhoA-MEKK1-JNK pathway or the MEKK1-p38 pathway would cause actin stress fiber formation and migration, but it would not induce Smad activation and growth inhibition of keratinocytes. The TGFβ/activin plays critical roles in physiological and pathological processes, such as tissue remodeling and wound healing, a specific modulation of which will possibly take place through controlling the RhoA-MEKK1-JNK and the MEKK1-p38 pathway.
Acknowledgments
This study was supported in part by Public Health Service grants P30 ES06096 and EY11798, and funding from Ohio Cancer Research Associates.
We thank Alvaro Puga for critical reading of the manuscript.
REFERENCES
- 1.Ashcroft, G. S., X. Yang, A. B. Glick, M. Weinstein, J. L. Letterio, D. E. Mizel, M. Anzano, T. Greenwell-Wild, S. M. Wahl, C. Deng, and A. B. Roberts. 1999. Mice lacking Smad3 show accelerated wound healing and an impaired local inflammatory response. Nat. Cell Biol. 1:260-266. [DOI] [PubMed] [Google Scholar]
- 2.Edlund, S., M. Landstrom, C. H. Heldin, and P. Aspenstrom. 2002. Transforming growth factor-beta-induced mobilization of actin cytoskeleton requires signaling by small GTPases Cdc42 and RhoA. Mol. Biol. Cell 13:902-914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gallagher, E. D., S. Gutowski, P. C. Sternweis, and M. H. Cobb. 2003. RhoA binds to the amino terminus of MEKK1 and regulates its kinase activity. J. Biol. Chem. [DOI] [PubMed]
- 4.Hall, A. 1998. Rho GTPases and the actin cytoskeleton. Science 279:509-514. [DOI] [PubMed] [Google Scholar]
- 5.Huang, C., Z. Rajfur, C. Borchers, M. D. Schaller, and K. Jacobson. 2003. JNK phosphorylates paxillin and regulates cell migration. Nature 424:219-223. [DOI] [PubMed] [Google Scholar]
- 6.Kaartinen, V., L. Haataja, A. Nagy, N. Heisterkamp, and J. Groffen. 2002. TGFbeta3-induced activation of RhoA/Rho-kinase pathway is necessary but not sufficient for epithelio-mesenchymal transdifferentiation: implications for palatogenesis. Int.J. Mol. Med. 9:563-570. [PubMed] [Google Scholar]
- 7.Liang, Q., R. J. Wiese, O. F. Bueno, Y. S. Dai, B. E. Markham, and J. D. Molkentin. 2001. The transcription factor gata4 is activated by extracellular signal-regulated kinase 1- and 2-mediated phosphorylation of serine 105 in cardiomyocytes. Mol. Cell. Biol. 21:7460-7469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maddala, R., V. N. Reddy, D. L. Epstein, and V. Rao. 2003. Growth factor induced activation of Rho and Rac GTPases and actin cytoskeletal reorganization in human lens epithelial cells. Mol. Vis. 9:329-336. [PubMed] [Google Scholar]
- 9.Marinissen, M. J., M. Chiariello, T. Tanos, O. Bernard, S. Narumiya, and J. S. Gutkind. 2004. The small GTP-binding protein RhoA regulates c-jun by a ROCK-JNK signaling axis. Mol. Cell 14:29-41. [DOI] [PubMed] [Google Scholar]
- 10.Marshall, C. J. 1996. Ras effectors. Curr. Opin. Cell Biol. 8:197-204. [DOI] [PubMed] [Google Scholar]
- 11.Massague, J. 1990. The transforming growth factor-beta family. Annu. Rev. Cell Biol. 6:597-641. [DOI] [PubMed] [Google Scholar]
- 12.Masszi, A., C. Di Ciano, G. Sirokmany, W. T. Arthur, O. D. Rotstein, J. Wang, C. A. McCulloch, L. Rosivall, I. Mucsi, and A. Kapus. 2003. Central role for Rho in TGF-β1-induced alpha-smooth muscle actin expression during epithelial-mesenchymal transition. Am. J. Physiol. Renal Physiol. 284:F911-F924. [DOI] [PubMed] [Google Scholar]
- 13.Mathews, L. S., and W. W. Vale. 1993. Molecular and functional characterization of activin receptors. Receptor 3:173-181. [PubMed] [Google Scholar]
- 14.Matzuk, M. M., N. Lu, H. Vogel, K. Sellheyer, D. R. Roop, and A. Bradley. 1995. Multiple defects and perinatal death in mice deficient in follistatin. Nature 374:360-363. [DOI] [PubMed] [Google Scholar]
- 15.Minden, A., A. Lin, F. X. Claret, A. Abo, and M. Karin. 1995. Selective activation of the JNK signaling cascade and c-Jun transcriptional activity by the small GTPases Rac and Cdc42Hs. Cell 81:1147-1157. [DOI] [PubMed] [Google Scholar]
- 16.Munz, B., H. Smola, F. Engelhardt, K. Bleuel, M. Brauchle, I. Lein, L. W. Evans, D. Huylebroeck, R. Balling, and S. Werner. 1999. Overexpression of activin A in the skin of transgenic mice reveals new activities of activin in epidermal morphogenesis, dermal fibrosis and wound repair. EMBO J. 18:5205-5215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Otto, I. M., T. Raabe, U. E. Rennefahrt, P. Bork, U. R. Rapp, and E. Kerkhoff. 2000. The p150-Spir protein provides a link between c-Jun N-terminal kinase function and actin reorganization. Curr. Biol. 10:345-348. [DOI] [PubMed] [Google Scholar]
- 18.Roberts, A. B., A. Russo, A. Felici, and K. C. Flanders. 2003. Smad3: a key player in pathogenetic mechanisms dependent on TGF-beta. Ann. N. Y. Acad. Sci. 995:1-10. [DOI] [PubMed] [Google Scholar]
- 19.Sahai, E., A. S. Alberts, and R. Treisman. 1998. RhoA effector mutants reveal distinct effector pathways for cytoskeletal reorganization, SRF activation and transformation. EMBO J. 17:1350-1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schlesinger, T. K., G. R. Fanger, T. Yujiri, and G. L. Johnson. 1998. The TAO of MEKK. Front. Biosci. 3:1181-1186. [DOI] [PubMed] [Google Scholar]
- 21.Schrewe, H., M. Gendron-Maguire, M. L. Harbison, and T. Gridley. 1994. Mice homozygous for a null mutation of activin beta B are viable and fertile. Mech. Dev. 47:43-51. [DOI] [PubMed] [Google Scholar]
- 22.Sugino, H., K. Sugino, O. Hashimoto, H. Shoji, and T. Nakamura. 1997. Follistatin and its role as an activin-binding protein. J. Med. Investig. 44:1-14. [PubMed] [Google Scholar]
- 23.Takaishi, K., T. Matozaki, K. Nakano, and Y. Takai. 2000. Multiple downstream signalling pathways from ROCK, a target molecule of Rho small G protein, in reorganization of the actin cytoskeleton in Madin-Darby canine kidney cells. Genes Cells 5:929-936. [DOI] [PubMed] [Google Scholar]
- 24.Vassalli, A., M. M. Matzuk, H. A. Gardner, K. F. Lee, and R. Jaenisch. 1994. Activin/inhibin beta B subunit gene disruption leads to defects in eyelid development and female reproduction. Genes Dev. 8:414-427. [DOI] [PubMed] [Google Scholar]
- 25.Wang, L., L. Yang, Y. Luo, and Y. Zheng. 2003. A novel strategy for specifically down-regulating individual Rho GTPase activity in tumor cells. J. Biol. Chem. 278:44617-44625. [DOI] [PubMed] [Google Scholar]
- 26.Wankell, M., B. Munz, G. Hubner, W. Hans, E. Wolf, A. Goppelt, and S. Werner. 2001. Impaired wound healing in transgenic mice overexpressing the activin antagonist follistatin in the epidermis. EMBO J. 20:5361-5372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xia, Y., C. Makris, B. Su, E. Li, J. Yang, G. R. Nemerow, and M. Karin. 2000. MEK kinase 1 is critically required for c-Jun N-terminal kinase activation by proinflammatory stimuli and growth factor-induced cell migration. Proc. Natl. Acad. Sci. USA 97:5243-5248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xia, Y., Z. Wu, B. Su, B. Murray, and M. Karin. 1998. JNKK1 organizes a MAP kinase module through specific and sequential interactions with upstream and downstream components mediated by its amino-terminal extension. Genes Dev. 12:3369-3381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yujiri, T., M. Ware, C. Widmann, R. Oyer, D. Russell, E. Chan, Y. Zaitsu, P. Clarke, K. Tyler, Y. Oka, G. R. Fanger, P. Henson, and G. L. Johnson. 2000. MEK kinase 1 gene disruption alters cell migration and c-Jun NH2-terminal kinase regulation but does not cause a measurable defect in NF-κB activation. Proc. Natl. Acad. Sci. USA 97:7272-7277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang, L., W. Wang, Y. Hayashi, J. V. Jester, D. E. Birk, M. Gao, C. Y. Liu, W. W. Kao, M. Karin, and Y. Xia. 2003. A role for MEK kinase 1 in TGF-beta/activin-induced epithelium movement and embryonic eyelid closure. EMBO J. 22:4443-4454. [DOI] [PMC free article] [PubMed] [Google Scholar]