Abstract
Mutations affecting the coding sequence of intermediate filament (IF) proteins account for >30 disorders, including numerous skin bullous diseases, myopathies, neuropathies, and even progeria. The manipulation of IF genes in mice has been widely successful for modeling key features of such clinically distinct disorders. A notable exception is pachyonychia congenita (PC), a disorder in which the nail and other epithelial appendages are profoundly aberrant. Most cases of PC are due to mutations in one of the following keratin-encoding genes: K6, K16, and K17. Yet null alleles obliterating the function of both K6 genes (K6α and K6β) or the K17 gene, as well as the targeted expression of a dominant-negative K6α mutant, elicit only a subset of PC-specific epithelial lesions (excluding that of the nail in mice). We show that newborn mice null for K6α, K6β, and K17 exhibit severe lysis restricted to the nail bed epithelium, where all three genes are robustly expressed, providing strong evidence that this region of the nail unit is initially targeted in PC. Our findings point to significant redundancy among the multiple keratins expressed in hair and nail, which can be related to the common ancestry, clustered organization, and sequence relatedness of specific keratin genes.
The complete or partial loss of intermediate filament (IF) function through mutation is responsible, at last count, for more than 30 genetically determined human disorders (33). In many but not all these instances, the pathogenesis of the lesions involved can be traced to the fragility of the cell(s) expressing the mutated defective IF protein (12, 33, 36), reflecting the important role of mechanical support fulfilled by IFs in the nucleus and cytoplasm. The large number of IF-encoding human genes (n > 67) (5, 16), their differentiation-related regulation in cells and tissues, and their unique properties among cytoskeletal polymers underlie additional roles, such as modulating the response to chemical stresses and pro-apoptotic signals (7, 34, 56, 58).
Cellular and molecular studies of the pathogenesis of IF disorders contributed immensely to our understanding of their properties, regulation, and function (12, 33, 36). In general, manipulating individual IF genes in mice has been very successful in mimicking key aspects of the corresponding disease seen in humans and providing key insight into IF function (1, 24, 42). Compared to gene inactivation via targeting and homologous recombination, however, the tissue-specific expression of mutated or wild-type IF genes has been more successful in phenocopying disease (20, 30, 32, 37). This in part reflects the occurrence of functional redundancy within the IF superfamily, especially since many cell types express more than one IF protein or network (6). Moreover, null alleles are less likely to interfere with a compensatory mechanism(s) than dominantly acting ones.
Keratins stand out among IF genes owing to their large number (>49) and their segregation into two distinct IF sequence types (I and II) clustered on separate chromosomes, along with their pairwise and differentiation-related regulation in epithelial tissues (5, 11, 16, 31, 41). Type I keratins (K9-K23, Ha1-Ha8) are smaller (40 to 64 kDa) and acidic, whereas type II keratins (K1-K6, Hb1-Hb6) are larger (52 to 70 kDa) and basic-neutral in charge (31). The significance of the dual characteristics of keratin proteins lies in their obligate heteropolymerization into 10- to 12-nm-long filaments. The significance of this polymerization requirement and of the differentiation-related regulation of keratin genes is not understood. There is evidence that “same-type keratins” are not interchangeable in vivo (17) even if highly related in primary structure (35). As many as two-thirds of all known keratin genes can be expressed in complex epithelial appendages such as hair and nails (48). Whereas such tissues are attractive experimental settings in which to carefully explore the significance of differential keratin gene regulation, a potentially significant limitation is functional redundancy.
Pachyonychia congenita (PC) is a rare and genetically determined ectodermal dysplasia in which several epithelial appendages show major alterations in their histology and appearance. As the name suggests, the most severe and consistent changes affect the nail, which is elevated, aberrantly shaped, and profoundly dyskeratotic, with the changes usually starting within a few months after birth (9). Type 1, or Jadahsson-Lewandowski, PC (Online Mendelian Inheritance in Man [OMIM] no. 167200) is further typified by oral leukoplakia and palmar-plantar keratoderma, which is debilitating for patients and is caused by mutations in either keratin 6a (K6a), a type II keratin, or keratin 16 (K16), a type I keratin (29). Type 2 (Jackson-Lawler) PC (OMIM no. 167210) is typified by the presence of multiple subepidermal cysts, the frequent occurrence of natal teeth, and generally milder nail lesions (29). Most cases of type 2 PC are caused by mutations in keratin 17 (K17) (www.interfil.org), a type I keratin related to K16 in sequence and regulation (27, 47). A single case of type 2 PC has been linked to a mutation in K6b (40), a K6 paralog that is poorly expressed in skin epithelia relative to K6a (43). In these disorders, as in most IF-based disorders, most mutations consist of missense alleles or, less commonly, small deletions affecting the coding sequence (33) (see www.interfil.org). In spite of various strategies applied to alter the function of K6, K16, or K17 in transgenic animals, PC is one among a few IF disorders that have not yet been fully reproduced in mice. Whereas modeling of the oral or hair component of PC disease in mouse has been straightforward, such has not been the case for the nail, a major component of PC's clinical presentation, or for the sebaceous gland or palmar-plantar epidermis.
We report here that in contrast to mice that are null for both K6α and K6β (54) or for K17 (28), mice that are triple null for K6α, K6β, and K17 show PC-like blistering of the nail bed shortly after birth, suggesting that this nail compartment is initially targeted in PC. Comparing and contrasting these triple-null mice with other relevant mouse models provide definitive evidence that functional redundancy acts as a powerful modulator of the expressivity of mutant keratin alleles in the nail bed and related stratified epithelia. The prevalence of redundancy among keratin genes expressed in epithelial appendages can be directly related to the common ancestry, clustered organization, and sequence relatedness of the keratin genes expressed in those complex tissues.
MATERIALS AND METHODS
Mouse lines.
All protocols involving mice were approved by the Johns Hopkins University Animal Care and Use Committee (Baltimore, Md.). C57BL/6 inbred mice hemizygous for a null allele at the K6α/K6β locus (54, 55) were crossed with C57BL/6 inbred mice homozygously null for the K17 allele (28). All controls used were age matched. Genotyping was done by PCR as described previously (28, 55).
Preparation of tissues for morphological analyses.
Protocols for these studies have been described before (28, 54). For routine histopathology, tissues were fixed in Bouin's and paraffin embedded and 5-μm-thick sections were cut and stained with hematoxylin and eosin (H&E). For epoxy-resin embedding, samples were fixed in 2% glutaraldehyde-1% paraformaldehyde-0.1 M sodium cacodylate solution overnight at 4°C, followed by postfixation in aqueous 1% osmium tetroxide (1 h at room temperature), and were infiltrated, embedded, cut, and stained with toluidine blue for semithin (0.5-μm-thick) sections or uranyl acetate and lead citrate staining for thin (70- to 90-nm thick) sections. Light micrographs were recorded on Kodak Ektachrome II film using a Zeiss Axioplan microscope equipped with a Contax 167MT camera. Electron micrographs were collected using a Philips CM120 transmission electron microscope. Recorded slides and negatives were scanned into the computer by use of Adobe Photoshop 5.5 and Epson Twain version 5.7.3A software.
Analyses conducted on newborn skin keratinocytes in primary culture.
Primary keratinocytes were harvested from 1- to 3-day-old pups as described previously (38). Final cell pellets were plated into wells containing glass coverslips and grown to ∼70% confluency. For indirect immunofluorescence, cells were fixed in 4% paraformaldehyde in phosphate-buffered saline (PBS) for 30 min and then permeabilized with 100% MeOH for 5 min (55). Final preparations were viewed using a Zeiss Axioplan microscope as described above and a 40× oil immersion objective.
Protein analyses.
Primary keratinocytes were scraped in ice-cold PBS supplemented with protease and phosphatase inhibitors (Sigma-Aldrich, St. Louis, Mo.) (1 mM EGTA, 20 μM Na3VO4, 10 mM NaF, 1 μg of leupeptin/ml, 2 μg of antipain/ml, 10 μg of aprotinin/ml, 10 μg of benzamidine/ml, 1 μg of cymostatin/ml, 1 μg of pepstatin-A/ml) and pelleted by centrifugation (16,000 × g for 10 min at 4°C). Cell pellets were lysed in buffer (1% deoxycholate, 1% Triton X-100, 0.1% sodium dodecyl sulfate, 150 mM NaCl, 50 mM Tris [pH 7.5], 0.5 mM EDTA, 1 mM EGTA) supplemented with inhibitors described above. Again, soluble proteins were separated from insoluble material by centrifugation at 16,000 × g for 10 min at 4°C. Insoluble proteins were resuspended in Tris-buffered 8 M urea with inhibitors (55). For each genotype, primary keratinocytes from three different mice were pooled and used. After protein quantitation (Bradford assay; Bio-Rad, Hercules, Calif.), 2 μg of protein was subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred onto nitrocellulose in the presence of 1 mM sodium orthovanadate. Membranes were blocked in 4% bovine serum albumin-PBS and subjected to Western immunoblotting (55). Bound primary antibodies were detected by chemiluminescence (Pierce Chemicals, Rockford, Ill.).
Antibodies.
Primary antibodies used include rabbit polyclonal antisera directed against K6 or K17 (27), K16 (2), K5 (Covance, Richmond, Calif.), and actin (Sigma). Secondary antibodies used were horseradish peroxidase (Sigma) or fluorophore (Kirkegaard & Perry Laboratories, Gaithersburg, Md.) conjugated.
RESULTS
K6α, K6β, and K17 triple-null mice are born but die early after birth.
The phenotypes of the K6α/K6β null and the K17 null mice amount to a partial phenocopy of the PC disorders caused by mutations in the corresponding human orthologs (28, 53, 54). In particular, the nail alterations that are a signature feature of PC are missing in these mice. A possible reason is functional redundancy, given that the nail bed epithelium, believed to be the initial target of nail alterations in PC (8, 26), also expresses two additional type II keratins (K5, K6hf) (48, 53) and three additional type I keratins (K14, K16, K17n) (46). The partitioning of type I and II keratin gene clusters to distinct chromosomes in the mouse genome (chromosomes 15 and 11, respectively; see http://www.ensembl.org) made it possible to directly test for functional redundancy by generating mice triple null for K6α, K6β, and K17 through selective crosses. To avoid strain effects, all experiments were carried out in the C57BL/6 inbred strain background, in which the K17 null phenotype is most severe (28).
Pups derived from K6α/K6β+/− K17−/− × K6α/K6b+/− K17−/− matings are found in the expected Mendelian ratio at embryonic day 18.5 (E18.5) and at birth (Table 1). However a subset of the pups die very shortly after birth and extending until day 10 postbirth (P10). Genotyping revealed that most of the pups dying at P1 to P4 are triple null (K6α/K6b−/− K17−/−), whereas pups dying later on, between P3 and P10, are K6α/K6β−/− K17+/+ and K6α/K6β−/− K17+/−. The latter was expected (54). Mice exhibiting other genotypes, including K6α/K6b+/− K17−/−, appeared normal (Table 1). The occurrence of severe epithelial lysis in the oral mucosa of K6α/K6b−/− null mice (54), the small size of the mice, and the absence of milk from their stomachs, together suggest that K6α/K6b−/− K17+/+, K6α/K6b−/− K17+/−, and K6α/K6b−/− K17−/− null mice die secondary to poor nutrition.
TABLE 1.
Genotypes resulting from K6α/K6β+/− K17−/− matingsa
| Time | No. (%) of mice of genotype:
|
||
|---|---|---|---|
| K6+/+K17−/− | K6+/−K17−/− | K6−/−K17−/− | |
| E18.5 | 15 (22) | 39 (58) | 13 (19) |
| Birth | 22 (24) | 42 (47) | 26 (29) |
| Total | 37 (23) | 81 (51) | 39 (25) |
K6α/K6β−/− K17−/− animals were born in the expected Mendelian ratio. Male and female K6α/K6β+/− K17−/− animals survived to adulthood and were subjected to timed matings. Those females that appeared pregnant at E18.5 were harvested, and embryos were collected for examination and genotyping. Other pregnant females were allowed to proceed to term so that newborn pups could be subjected to the same analyses.
Triple-null K6α/K6b−/− K17−/− mice show more severe oral lesions.
The dorsal tongue and palate are the most affected tissues in K6α/K6b−/− null mice at the time of their premature death (54). Given that K17 is also expressed in these tissues (27), this raises the possibility that the earlier lethality exhibited by most K6α/K6b−/− K17−/− triple-null mice would arise from an earlier onset of lethal oral lesions. Routine histology shows that this is indeed the case (Fig. 1). At birth (P0), at which time all mice are viable (Table 1), the dorsal tongue epithelium is literally destroyed in K6α/K6b−/− K17−/− triple-null mice, only mildly affected in K6α/K6b−/− K17+/+ null mice, and wild-type-like in all other genotypes (Fig. 1A to D). This tendency is maintained at P2, at which time the surviving K6α/K6b−/− K17−/− triple-null mice show the most severe lysis and inflammatory changes in the dorsal tongue epithelium (Fig. 1E to J) and upper palate (data not shown). Mice that are K6α/K6b+/− K17−/− do not exhibit any oral lesions and survive to adulthood.
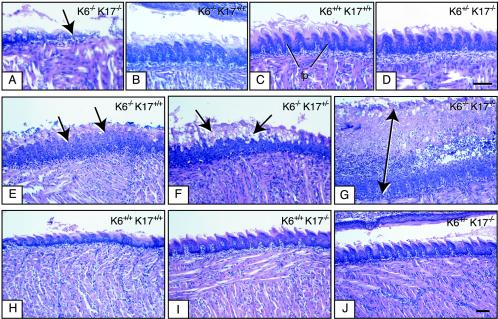
FIG. 1.
Earlier lysis of dorsal tongue epithelium in K6α/K6β−/− K17−/− null neonatal mice compared to other genotypes. Tongues from P0 (A to D), and P2 (E to J) mice were surgically removed and processed for routine histology. Micrographs shown are derived from H&E-stained preparations of longitudinally sectioned tongue tissue (rostrocaudal axis). The mouse genotype is indicated in the upper right corner. (A to D) Lysis and destruction of the dorsal tongue epithelium can be seen as early as P0 in K6α/K6β−/− K17−/− tongue (see arrow in panel A). At that time, K6α/K6β−/− K17+/+ mice show comparatively mild lesions in the same region (B) whereas wild-type mice (C) and even K6α/K6β+/− K17−/− mice (D) show normal histology with intact filiform papillae (fp). Bar, 100 μm (A to D). (E to J) At P2, obvious signs of oral lesions can be detected in K6α/K6β−/− K17+/+ mice (see arrows in panel E) as reported previously (54). These lesions are significantly more severe and inflamed in K6α/K6β−/− K17+/− (see arrows in panel F) and especially in K6α/K6β−/− K17−/− mice (see arrow in panel G). In contrast, the dorsal epithelial tongue appears normal in K6α/K6β+/+ K17+/+ (wild-type) mice (H), as expected, and in K6α/K6β+/+ K17−/− (I) and K6α/K6β+/− K17−/− (J) mice as well. Bar, 200 μm (E to J).
Normal morphogenesis of hair follicles in triple null K6α/K6b−/− K17−/− mice.
As is the case in oral mucosa, K6α/K6β and K17 proteins show a partially overlapping distribution in all major epithelial appendages of mature skin tissue (27, 44), raising the possibility that skin defects would be more pronounced in K6α/K6b−/− K17−/− triple-null mice than they are in K6α/K6b−/− null mice (28). Histological analyses conducted on back skin tissue samples harvested at P2 revealed, however, that they were similar in all genotypes. The overall characteristics with respect to thickness of skin and morphology of hair follicles and epidermis appeared indistinguishable between animals (data not shown). Footpad epidermis also appears to be histologically normal in P2 mice from all genotypes (data not shown). These results suggest that the compound loss of K6α, K6β, and K17 proteins does not alter the morphogenesis and differentiation of epidermis or hair follicles at least until P2 to P3. Due to the complications stemming from the oral mucosa phenotype, we could not determine whether hair cycling (28) was normal in the triple-null mice.
The periderm consists of a single layer of flattened epithelial cells that form early after the onset of ectoderm stratification at E10 to E11. Whereas their function is unknown (23), periderm cells participate in the formation of temporary epithelial fusions of eyelids, digits, and pinnae (ear) (10). This embryo-only cell type expresses K6α, K6β, and K17 mRNA and protein (25, 27). To test for possible alterations in periderm function in the absence of these keratins, mouse embryos were harvested at E16.5, E17.5, and E18.5 of embryonic development and subjected to a dye penetration assay that assesses barrier formation (13). Based on dye exclusion, there was no obvious alteration in barrier acquisition in any of the genotypes tested that was outside the range seen normally within control litters (data not shown). Likewise, temporary epithelial fusions occurred normally in these mice (data not shown). These observations add to the growing body of evidence showing that keratin proteins are not required for epithelial differentiation (6).
Triple-null K6α/K6b−/− K17−/− mice exhibit severe lysis of the nail bed epithelium.
We next examined nail histology in P0 (newborn) mice to look for alterations in either the morphogenesis or integrity of this tissue in K6α/K6b−/− K17−/− animals. Morphogenesis of the nail unit (Fig. 2A), including the nail plate, nail bed, matrix, proximal nail fold, and hyponychium, appeared to have occurred normally in all genotypes. Likewise, no macroscopic alterations were apparent in the nail plate in any transgenic mice (data not shown). In tissue sections prepared from K6α/K6b−/− K17−/− digits, however, obvious cell lysis typical of keratin-based blistering diseases was found to occur selectively in the nail bed (Fig. 2B). Follow-up studies using electron microscopy confirmed the presence of intracellular lysis affecting preferentially the lowermost suprabasal layers of the nail bed epithelium (Fig. 2F), where K6α, K6β, and K17 are known to be coexpressed (27, 28, 48). In particular, the thick bundles of densely packed filaments normally located in the cells layers immediately beneath the nail plate (Fig. 2C) are completely missing from triple-null nail bed epithelium (Fig. 2 F). In stark contrast, all other mice, including K6α/K6b−/− K17+/+ and K6α/K6b+/+ K17−/− mice, showed a nail bed ultrastructure indistinguishable from that of the wild type (Fig. 2D to E). In addition, we found no evidence for an upregulation of K14 or K6hf, which is also expressed in the nail bed epithelium (48). Likewise, the distribution of hard type I keratins appeared normal in the nail plate, as determined by immunohistochemistry (data not shown). Unlike hair follicle results, therefore, the presence of K6α, K6β, or K17 is essential to the maintenance of the nail bed integrity from a very early stage of postnatal development in mice.
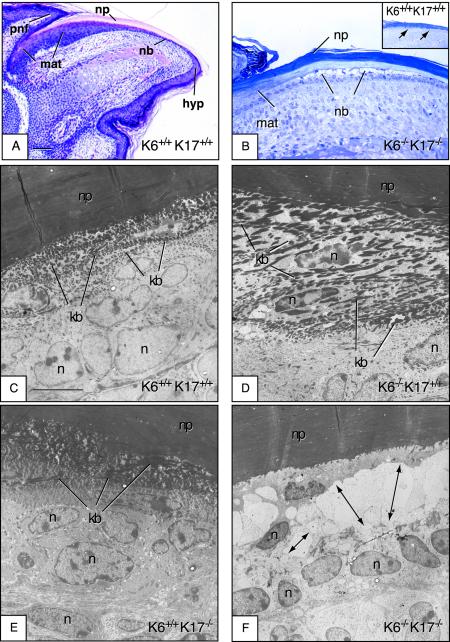
FIG.2.
Early lysis of the nail bed epithelium in K6α/K6β−/− K17−/− null newborn mice but not in other genotypes. Paws or digits from P0 mice (newborns) were surgically removed and processed for routine histology. Micrographs shown are derived from an H&E-stained section of paraffin-embedded tissue (A), toluidine-blue stained semithin sections (∼0.5 μm thick) prepared from epoxy-embedded tissues (B), and uranyl acetate- and lead citrate-counterstained stained ultrathin sections (∼50 to 70 nm thick) prepared from epoxy-embedded tissue (C to F). In all cases, the sectioning plane is along the anterior-posterior axis and the mouse genotype is indicated in the lower right corner. (A) Section of wild-type nail tissue showed for orientation. The proximal nail fold (pnf), matrix (mat), nail bed (nb), nail plate (np), and hyponychium (hyp) compartments are depicted. Bar, 300 μm. (B) Semithin section of epoxy-embedded K6α/K6β−/− K17−/− nail unit, depicting the nail bed area. There is striking lysis of the nail bed (nb) epithelium, while the matrix (mat) and nail plate (np) appear not affected. The inset shows an identical preparation from a wild-type mouse, which does not show any nail bed (nb) alteration. (C to F) Electron microscopy data taken from a smaller subset of the same general area (nail bed). In contrast to K6α/K6β+/+ K17+/+ (C), K6α/K6β−/− K17+/+ (D), and K6α/K6β+/+ K17+/+ (E) mice, the nail bed of K6α/K6β−/− K17−/− mice (F) shows obvious ballooning and lysis in the area immediate above the basal layer (see arrows). Likewise, the two to three layers of cells with dense keratin bundles (kb in panels C to E) are missing from the K6α/K6β−/− K17−/− sample (F). In contrast, the nail plate (np) and matrix appear normal in these mice. n, nucleus. Bar, 5 μm (C to F).
Assessing keratin IF organization and keratin protein levels in primary keratinocyte cultures.
Primary cultures of newborn mouse skin keratinocytes can be established (14) and offer an unparalleled means of assessing a combination of parameters including cellular morphology, intracellular organization of keratin IFs, and levels of K5, K6α/K6β, K14, K16, and K17, which are the main keratins expressed in this setting (49). This keratin profile is similar to that seen in the nail bed epithelium in vivo (46, 48). Analysis of the levels of these specific keratins confirmed that, as expected, K6α/K6β or K17 or both types of antigens are missing from the relevant genotypes (Fig. 3A). The levels of K6α/K6β antigens are slightly reduced in samples prepared from K6α/K6b+/− cells, and a similar outcome is seen for K17 antigens in K17+/− cells. No significant changes in K5 antigen levels are detected in any of the genotypes examined. In contrast, K16 levels are modestly decreased in samples prepared from K6α/K6b+/− cells and significantly so in samples from cells that are homozygously null for K6α/K6β and especially those triple null for K6α/K6β and K17 (Fig. 3A). These data extend the recent finding that the build-up in K16 protein levels depends upon the coinduction of K6 proteins in such a cell culture setting (55). They further show that the compound loss of K6α, K6β, and K17 has an even greater impact on K16 levels.
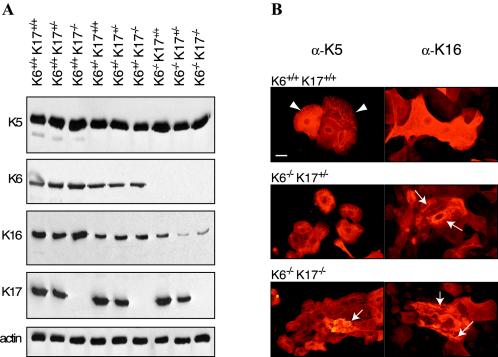
FIG. 3.
Analysis of the levels and distribution of specific keratin antigens in newborn skin keratinocytes in primary culture. (A) Western immunoblot analysis. The genotype is indicated on top, and the specificity of the antiserum used is given at the left of each blot. For each genotype, primary keratinocytes from three different mice were pooled and used. A total of 2 μg of proteins was loaded per lane. Bound primary antibodies were detected by chemiluminescence. Actin was used as a loading control (55). (B) Keratinocytes cultures from various genotypes, indicated at top of each pair of micrographs, were fixed and immunostained with antibodies directed towards K5 (left column) and K16 (right column). Wild-type keratinocytes exhibit pan-cytoplasmic IF networks (arrowheads). A subset of keratinocytes, identified with arrows, show altered and even collapsed keratin networks for the K6α/K6β−/− K17+/− and K6α/K6β−/− K17−/− genotypes. The number of cells affected and the severity of the disruption are clearly higher in the latter. There is no significant difference in the results obtained when staining for either K5 or K16 antigen, consistent with the known ability of several keratins to copolymerize into IFs. Bar, 50 μm.
We used antisera to K5 and K16 proteins to immunostain the corresponding antigen in these keratinocyte cultures. Similar to what has been reported for K6α/K6β−/− K17+/+ cells (55), a small subset of K6α/K6β−/− K17+/− primary keratinocytes exhibit altered keratin networks (Fig. 3B). We found that the frequency of keratinocytes exhibiting abnormal or collapsed filament networks is clearly higher for the K6α/K6β−/− K17−/− genotype (Fig. 3B and data not shown). These analyses establish that the concomitant build-up of K6α, K6β, and K17 is required for the elaboration of the pan-cytoplasmic keratin network in skin keratinocytes in primary culture. This outcome implies that expression of K5 and K14 is not enough to sustain IF network organization in a subset of keratinocytes under such conditions. These studies establish that the absence of K6α, K6β, and K17 can lead to IF disruption in keratinocytes in culture, even in the absence of significant trauma.
DISCUSSION
Eliciting PC-like lesions in mouse nail units requires overcoming functional redundancy.
We previously showed that mice null for K6α and K6β die between 3 and 10 days after birth, correlating with severe blistering in the oral mucosa (54). Until the onset of poor-nutrition-related complications at approximately P4, all skin epithelial appendages are histologically normal in these mice. Grafting of K6α/K6β null newborn trunk skin on immunocompromised mouse hosts revealed that the K6α/K6β null mutation does not significantly affect hair growth or ability to cycle (55) (see also reference 53). In striking contrast, mice with a single K6 null allele, namely K6α, do not shown any spontaneous lesions in the skin and oral mucosa (51). Mice null for K17, on the other hand, develop severe alopecia that is reversible and strain dependent, both correlating with K16 upregulation (28). These mice grow to adulthood, but histological analysis fails to reveal changes in the oral mucosa, glands, footpad epidermis, and nail (28, 46). In another study, Wojcik et al. (52) and colleagues found that the tissue-specific overexpression of dominantly acting K6α mutants elicited severe lesions in the hair but not in nail or footpad epidermis. Unlike hair follicles and oral filiform papillae, therefore, the nail appears unusually resistant to various keratin gene manipulations in mice.
Recent studies aimed at understanding the discrepancy between such null mouse models and PC diseases revealed that hitherto unknown keratins, the type II K6hf (48, 53) and newly discovered type I K17n (46), are expressed in the nail bed epithelium, where PC lesions are thought to initiate (see below). Altogether, the nail bed epithelium expresses at least four of each of type I (K14, K16, K17, K17n) and type II (K5, K6α, K6β, K6hf) keratins. Each of these two groups consists of highly homologous keratins (Fig. 4), creating a potential for significant functional redundancy. The studies reported here provide additional support to the concept that redundancy is a key player in this context (28, 51, 53), since three separate genes (K6α, K6β, K17) had to be inactivated, along with a potential decrease in the level of a fourth protein (K16) (Fig. 3), so that fragility typical of keratin-based disorders could be seen in nail bed epithelium. Our results do not exclude the possibility that other factors, such as species-related differences in nail biology or in the regulation of K6 genes (39, 44), also contribute to the discrepancy between previous mouse models and PC disease.
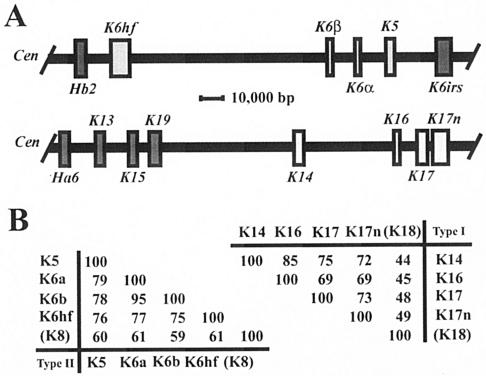
FIG. 4.
Clustering of related keratin genes on mouse chromosomes 15 and 11. (A) Organization of subsets of type II and type I keratin genes on mouse chromosomes 15 (top) and 11 (bottom), respectively. In both instances the centromere (Cen) is located on the left side. All keratin genes located in these two clusters, including the ones shown here, display the same transcriptional orientation towards the centromere. All type II keratin genes exhibit nine introns, whereas all type I genes exhibit eight introns, located in each case at identical positions within the coding sequence (data not shown; see reference 16). The genes of interest to this study are displayed in light-shaded boxes. This information was adapted from Wang et al. (48) and Tong and Coulombe (46). (B) Pairwise comparisons of the primary structures for type II (left) and type I (right) keratins expressed in the nail bed epithelium. Percent identity scores, as determined by the maximum matching routine in DNAsis version 3.5 software (Hitachi, Tokyo, Japan), are shown for the sequences encoding the nonhelical head and central rod domain of these keratins. K8 and K18, which represent the major keratin pair expressed in simple epithelia, have been included for reference purposes. The physical proximity, identical substructures, identical orientations of transcription, and obvious sequence relatedness of the type II keratin genes K5, K6α, K6β, and K6hf as well as the type I genes K14, K16, K17, and K17n strongly suggest that they were each generated through successive duplications from a common ancestral gene. The information needed to construct this figure is derived from the mouse genome browser available at http://www.ensembl.org/. The same principles apply to the human orthologs for these genes (4).
Functional redundancy provides an explanation for some unusual features of PC disease and suggests new avenues for therapy. First, PC disease is much less frequent than epidermolysis bullosa simplex, a condition most frequently caused by mutations in K5 or K14 (12, 33). A priori, there are no reasons why the human K6, K16, and K17 genes should not be as frequently mutated as K5 and K14, to which they are highly homologous and physically proximal in the genome (Fig. 4). Second, all the PC-causing mutations uncovered so far in K6a, K6b, or K17 are restricted to specific segments of the central rod domain, namely, subdomains 1A and 2B (see www.interfil.org), which are known to be especially critical for IF assembly in vitro (15) and which, when affecting other keratin genes (e.g., K5 and K14), cause very severe clinical phenotypes (12, 18, 33, 36). Third and finally, functional redundancy could be exploited for the purpose of therapy. For instance, siRNA-mediated reduction of mutant keratin expression by 50% suffices to normalize the organization of keratin IFs in cultured epithelial cells (50). Conversely, overexpression of a related wild-type keratin sequence in mutant keratin-expressing cells suffices to rescue the altered organization of cytoplasmic IFs in intestinal epithelia (59). Thus, any treatment capable of significantly and selectively elevating the levels of a related keratin already expressed in the nail bed epithelium or, conversely, of decreasing the expression of the mutated, disease-causing keratin allele can be predicted to significantly ameliorate key PC symptoms. In further support of this, there is direct in vivo evidence that K6α/K6β/K6hf (51, 53, 54), K14/K16 (35), and K17/K16 (28) are redundant to a significant extent in vivo. There appear to be enough fundamental differences in gene regulation between K5, K14, K6hf, and K17n and their PC-disease-causing homologs to make this a potentially successful endeavor.
The nail bed epithelium is initially targeted in PC.
Which compartment(s) of the nail unit account(s) for the pathogenesis of the nail defects typical of PC has been a long-debated question (9, 19, 26; for a different view, see reference 45). Yet resolving this issue is important when devising successful therapies aimed at curtailing the development of lesions. Examination of the distribution of K6α, K16, and K17, the main target genes in PC, showed that they overlap significantly only in the nail bed epithelium (26; see also reference 8). The studies reported here indicate that at least early on, it is the nail bed that shows cytolysis typical of keratin defect-based fragility. Moreover, loss of K6α, K6β, and K17 led to the disappearance of the thick bundles of keratin IFs that are normally found in the uppermost layers of the nail bed epithelium, underneath the nail plate, where all three genes are coexpressed (46, 48). Given their abundance and bundled organization, these filaments are poised to provide key structural support in these cells (22, 57).
The early lethality of the K6α/K6β/K17 triple-null mice precluded us from monitoring the subsequent evolution of these lesions and points to a significant limitation of this mouse model. Because each of the genes targeted by mutation in PC disease is strongly inducible in the context of skin tissue injury, it seems likely that the epithelial defects characteristic of PC would spread to the neighboring epithelial compartments, including the matrix (Fig. 2A). Therefore, the view that multiple epithelial compartments of the nail unit are eventually aberrant in PC is plausible, but as argued decades ago (19), the nail bed likely is the initial site of pathogenesis. Another limitation of the K6α/K6β/K17 triple-null mice as a model for PC is the lack of blistering within footpad epidermis. Patients report that painful palmar-plantar lesions are probably the most limiting element associated with a PC condition (9).
Additional evolutionary aspects.
There now exists virtually irrefutable evidence that the large numbers of functional type I and II keratin genes were each generated through successive duplications from a common ancestral gene (21). In strong support of this, the type II gene group K5, K6α, K6β, and K6hf and type I gene group K14, K16, K17, and K17n are each arranged in a compact subcluster in the genome and the two groups exhibit the same orientation of transcription, a physical mapping that is perfectly conserved in the mouse (Fig. 4) and human (4) genomes. Moreover, these type I and type II keratin genes are related in their transcriptional regulation in epithelia, as well as at the levels of their coding sequence (Fig. 4) and function (this study). Such subgroupings are prevalent throughout these two large gene clusters.
Mostly on the basis of nucleotide sequence homology, Blumenberg (3) postulated approximately 15 years ago that type I and II keratin genes had likely undergone concerted evolution in spite of their segregation to distinct chromosomes. The data emerging from the analysis of transgenic mouse models showing complex phenotypes, the large-scale sequencing of mammalian genomes, and the characterization of novel keratin genes (e.g., K6hf, K17n) showing highly specialized and similar regulation add support to this intriguing idea.
Acknowledgments
We are very grateful to Xuemei Tong for providing the K17 null mice, Angie Lebrun and Brad Harris for production to tissue sections, and members of the Coulombe laboratory for advice and support.
This work was supported by National Institutes of Health grants AR44232 and AR42047 to P.A.C.
REFERENCES
- 1.Arin, M. J., and D. R. Roop. 2001. Disease model: heritable skin blistering. Trends Mol. Med. 7:422-424. [DOI] [PubMed] [Google Scholar]
- 2.Bernot, K., K. McGowan, and P. A. Coulombe. 2002. Keratin 16 expression defines a subset of epithelial cells during skin morphogenesis and the hair cycle. J. Investig. Dermatol. 119:1137-1149. [DOI] [PubMed] [Google Scholar]
- 3.Blumenberg, M. 1988. Concerted gene duplications in the two keratin gene families. J. Mol. Evol. 27:203-211. [DOI] [PubMed] [Google Scholar]
- 4.Coulombe, P. A., and K. M. Bernot. 2004. Keratins and the skin, p. 497-504. In M. D. Lane, and W. Lennarz, (ed.), Encyclopedia of biological chemistry. Elsevier, Oxford, United Kingdom.
- 5.Coulombe, P. A., L. Ma, S. Yamada, and M. Wawersik. 2001. Intermediate filaments at a glance. J. Cell Sci. 114:4345-4347. [DOI] [PubMed] [Google Scholar]
- 6.Coulombe, P. A., and M. B. Omary. 2002. “Hard” and “soft” principles defining the structure, function and regulation of keratin intermediate filaments. Curr. Opin. Cell Biol. 14:110-122. [DOI] [PubMed] [Google Scholar]
- 7.Coulombe, P. A., and P. Wong. 2004. Cytoplasmic intermediate filaments revealed as dynamic and multipurpose scaffolds. Nat. Cell Biol. 6:699-706. [DOI] [PubMed] [Google Scholar]
- 8.De Berker, D., F. Wojnarowska, L. Sviland, G. E. Westgate, R. P. R. Dawber, and I. M. Leigh. 2000. Keratin expression in the normal nail unit: markers of regional differentiation. Br. J. Dermatol. 142:89-96. [DOI] [PubMed] [Google Scholar]
- 9.Feinstein, A., J. Friedman, and M. Schewach. 1988. Pachyonychia congenita. J. Am. Acad. Dermatol. 19:705-711. [DOI] [PubMed] [Google Scholar]
- 10.Findlater, G. S., R. D.Mcdougall, and M. H. Kaufman. 1993. Eyelid development, fusion and subsequent reopening in the mouse. J. Anat. 183:121-129. [PMC free article] [PubMed] [Google Scholar]
- 11.Fuchs, E. 1995. Keratins and the skin. Annu. Rev. Cell Dev. Biol. 11:123-153. [DOI] [PubMed] [Google Scholar]
- 12.Fuchs, E., and D. W. Cleveland. 1998. A structural scaffolding of intermediate filaments in health and disease. Science 279:514-519. [DOI] [PubMed] [Google Scholar]
- 13.Hardman, M. J., P. Sisi, D. N. Banbury, and C. Byrne. 1998. Patterned acquisition of skin barrier function during development. Development 125:1541-1552. [DOI] [PubMed] [Google Scholar]
- 14.Hennings, H., D. Michael, C. Cheng, P. Steinert, K. Holbrook, and S. H. Yuspa. 1980. Calcium regulation of growth and differentiation of mouse epidermal cells in culture. Cell 19:245-254. [DOI] [PubMed] [Google Scholar]
- 15.Herrmann, H., and U. Aebi. 2004. Intermediate filaments: molecular structure, assembly mechanism, and integration into functionally distinct intracellular scaffolds. Annu. Rev. Biochem. 73:749-789. [DOI] [PubMed] [Google Scholar]
- 16.Hesse, M., T. M. Magin, and K. Weber. 2001. Genes for intermediate filament proteins and the draft sequence of the human genome: novel keratin genes and a surprisingly high number of pseudogenes related to keratin genes 8 and 18. J. Cell Sci. 114:2569-2575. [DOI] [PubMed] [Google Scholar]
- 17.Hutton, E., R. D. Paladini, Q. C. Yu, M.-Y. Yen, P. A. Coulombe, and E. Fuchs. 1998. Functional differences between keratins of stratified and simple epithelia. J. Cell Biol. 143:1-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Irvine, A. D., and W. H. McLean. 1999. Human keratin diseases: the increasing spectrum of disease and subtlety of the phenotype-genotype correlation. Br. J. Dermatol. 140:815-828. [DOI] [PubMed] [Google Scholar]
- 19.Kelly, E. W., and H. Pinkus. 1958. Report of a case of pachyonychiaa congenita. Arch. Dermatol. 77:724-726. [DOI] [PubMed] [Google Scholar]
- 20.Ku, N. O., S. A. Michie, R. M. Soetikno, E. Z. Resurreccion, R. L. Broome, R. G. Oshima, and M. B. Omary. 1996. Susceptibility to hepatotoxicity in transgenic mice that express a dominant-negative human keratin 18 mutant. J. Clin. Investig. 98:1034-1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lewin, B. 1994. Genes V. Oxford University Press, New York, N.Y.
- 22.Ma, L., S. Yamada, D. Wirtz, and P. A. Coulombe. 2001. A “hot-spot” mutation alters the mechanical properties of keratin filament networks. Nat. Cell Biol. 3:503-506. [DOI] [PubMed] [Google Scholar]
- 23.M'Boneko, V., and H.-J. Merker. 1988. Development and morphology of the periderm of mouse embryos (Days 9-12 of gestation). Acta Anat. 133:325-336. [DOI] [PubMed] [Google Scholar]
- 24.Magin, T. M., M. Hesse, and Schroder. 2000. Novel insights into intermediate filament function from studies of transgenic and knockout mouse. Protoplasma 211:140-150. [Google Scholar]
- 25.Mazzalupo, S., and P. A. Coulombe. 2001. A reporter transgene based on a human keratin 6 gene promoter is specifically expressed in the periderm of mouse embryos. Mech. Dev. 100:65-69. [DOI] [PubMed] [Google Scholar]
- 26.McGowan, K. M., and P. A. Coulombe. 2000. Keratin 17 expression in the hard epithelial context of the hair and nail, and its relevance for the pachyonychia congenita phenotype. J. Investig. Dermatol. 114:1101-1107. [DOI] [PubMed] [Google Scholar]
- 27.McGowan, K. M., and P. A. Coulombe. 1998. Onset of keratin 17 expression coincides with the definition of major epithelial lineages during skin development. J. Cell Biol. 143:469-486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McGowan, K. M., X. Tong, E. Colucci-Guyon, F. Langa, C. Babinet, and P. A. Coulombe. 2002. Keratin 17 null mice exhibit age- and strain-dependent alopecia. Genes Dev. 16:1412-1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McLean, W. H., E. L. Rugg, D. P. Lunny, S. M. Morley, E. B. Lane, O. Swensson, P. J. Dopping-Hepenstal, W. A. Griffiths, R. A. Eady, C. Higgins, et al. 1995. Keratin 16 and keratin 17 mutations cause pachyonychia congenita. Nat. Genet. 9:273-278. [DOI] [PubMed] [Google Scholar]
- 30.Messing, A., M. W. Head, K. Galles, E. J. Galbreath, J. E. Goldman, and M. Brenner. 1998. Fatal encephalopathy with astrocyte inclusions in GFAP transgenic mice. Am. J. Pathol. 152:391-398. [PMC free article] [PubMed] [Google Scholar]
- 31.Moll, R., W. W. Franke, D. L. Schiller, B. Geiger, and R. Krepler. 1982. The catalog of human cytokeratins: patterns of expression in normal epithelia, tumors and cultured cells. Cell 31:11-24. [DOI] [PubMed] [Google Scholar]
- 32.Mounkes, L. C., S. Kozlov, L. Hernandez, T. Sullivan, and C. L. Stewart. 2003. A progeroid syndrome in mice is caused by defects in A-type lamins. Nature 423:298-301. [DOI] [PubMed] [Google Scholar]
- 33.Omary, M. B., P. A. Coulombe, and W. H. I. McLean. 2004. Intermediate filaments and their associated diseases. N. Engl. J. Med. 351:2087-2100. [DOI] [PubMed] [Google Scholar]
- 34.Oshima, R. G. 2002. Apoptosis and keratin intermediate filaments. Cell Death Differ. 9:486-492. [DOI] [PubMed] [Google Scholar]
- 35.Paladini, R. D., and P. A. Coulombe 1999. The functional diversity of epidermal keratins revealed by the partial rescue of the keratin 14 null phenotype by keratin 16. J. Cell Biol. 146:1185-1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Porter, R. M., and E. B. Lane. 2003. Phenotypes, genotypes and their contribution to understanding keratin function. Trends Genet. 19:278-285. [DOI] [PubMed] [Google Scholar]
- 37.Reichelt, J., H. Bussow, C. Grund, and T. M. Magin. 2001. Formation of a normal epidermis supported by increased stability of keratins 5 and 14 in keratin 10 null mice. Mol. Biol. Cell 12:1557-1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rhouabhia, M., L. Germain, F. Belanger, R. Guignard, and F. A. Auger. 1992. Optimization of murine keratinocyte culture for the production of graftable epidermal sheets. J. Dermatol. 19:325-334. [DOI] [PubMed] [Google Scholar]
- 39.Rothnagel, J. A., T. Seki, M. Ogo, M. A. Longley, S. M. Wojcik, D. S. Rundman, J. R. Bickenbach, and D. R. Roop. 1999. The mouse keratin 6 isoforms are differentially expressed in the hair follicle, footpad, tongue, and activated epidermis. Differentiation a 65:119-130. [DOI] [PubMed] [Google Scholar]
- 40.Smith, F. J., M. F. Jonkman, H. van Goor, C. M. Coleman, S. P. Covello, J. Uitto, and W. H. McLean. 1998. A mutation in human keratin K6b produces a phenocopy of the K17 disorder pachyonychia congenita type 2. Hum. Mol. Genet. 7:1143-1148. [DOI] [PubMed] [Google Scholar]
- 41.Steinert, P. M., and D. R. Roop. 1988. Molecular and cellular biology of intermediate filaments. Annu. Rev. Biochem. 57:593-625. [DOI] [PubMed] [Google Scholar]
- 42.Takahashi, K., P. A. Coulombe, and Y. Miyachi. 1999. Using transgenic models to study the pathogenesis of keratin-based inherited skin diseases. J. Dermatol. Sci. 21:73-95. [DOI] [PubMed] [Google Scholar]
- 43.Takahashi, K., R. Paladini, and P. A. Coulombe. 1995. Cloning and characterization of multiple human genes and cDNAs encoding highly related type II keratin 6 isoforms. J. Biol. Chem. 270:18581-18592. [DOI] [PubMed] [Google Scholar]
- 44.Takahashi, K., B. Yan, K. Yamanishi, S. Imamura, and P. A. Coulombe. 1998. The two functional keratin 6 genes of mouse are differentially regulated and evolved independently from their human orthologs. Genomics 53:170-183. [DOI] [PubMed] [Google Scholar]
- 45.Thomsen, R. J., R. L. Zuehlke, and B. I. Beckman. 1982. Pachyonychia congenita: surgical management of the nail changes. J. Dermatol. Surg. Oncol. 8:24-28. [DOI] [PubMed] [Google Scholar]
- 46.Tong, X., and P. A. Coulombe. 2004. A novel mouse type I intermediate filament gene, keratin 17n (K17n), exhibits preferred expression in nail tissue. J. Investig. Dermatol. 122:965-970. [DOI] [PubMed] [Google Scholar]
- 47.Troyanovsky, S. M., R. E. Leube, and W. W. Franke. 1992. Characterization of the human gene encoding cytokeratin 17 and its expression pattern. Eur. J. Cell Biol. 59:127-137. [PubMed] [Google Scholar]
- 48.Wang, Z., P. Wong, L. Langbein, J. Schweizer, and P. A. Coulombe. 2003. Type II epithelial keratin 6hf (K6hf) is expressed in the companion layer, matrix, and medulla in anagen-stage hair follicles. J. Investig. Dermatol. 121:1276-1282. [DOI] [PubMed] [Google Scholar]
- 49.Wawersik, M., and P. A. Coulombe. 2000. Forced expression of keratin 16 alters the adhesion, differentiation, and migration of mouse skin keratinocytes. Mol. Biol. Cell 11:3315-3327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Windoffer, R., S. Woll, P. Strnad, and R. E. Leube. 2004. Identification of novel principles of keratin filament network turnover in living cells. Mol. Biol. Cell 15:2436-2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wojcik, S. M., D. S. Bundman, and D. R. Roop. 2000. Delayed wound healing in keratin 6a knockout mice. Mol. Cell Biol. 20:5248-5255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wojcik, S. M., S. Imakado, T. Seki, M. A. Longley, L. Petherbridge, D. S. Bundman, J. R. Bickenbach, J. A. Rothnagel, and D. R. Roop. 1999. Expression of MK6a dominant-negative and C-terminal mutant transgenes in mice has distinct phenotypic consequences in the epidermis and hair follicles. Differentiation 65:97-112. [DOI] [PubMed] [Google Scholar]
- 53.Wojcik, S. M., M. A. Longley, and D. R. Roop. 2001. Discovery of a novel murine keratin 6 (K6) isoform explains the absence of hair and nail defects in mice deficient for K6a and K6b. J. Cell Biol. 154:619-630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wong, P., E. Colucci-Guyon, K. Takahashi, C. Gu, C. Babinet, and P. A. Coulombe. 2000. Introducing a null mutation in the mouse K6alpha and K6beta genes reveals their essential structural role in the oral mucosa. J. Cell Biol. 150:921-928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wong, P., and P. A. Coulombe. 2003. Loss of keratin 6 (K6) proteins reveals a function for intermediate filaments during wound repair. J. Cell Biol. 163:327-337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Worman, H. J., and J. C. Courvalin. 2004. How do mutations in lamins A and C cause disease? J. Clin. Investig. 113:349-351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yamada, S., D. Wirtz, and P. A. Coulombe. 2002. Pairwise assembly determines the intrinsic potential for self-organization and mechanical properties of keratin filaments. Mol. Biol. Cell 13:382-391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zastrow, M. S., S. Vlcek, and K. L. Wilson. 2004. Proteins that bind A-type lamins: integrating isolated clues. J. Cell Sci. 117:979-987. [DOI] [PubMed] [Google Scholar]
- 59.Zhou, Q., D. M. Toivola, N. Feng, H. B. Greenberg, W. W. Franke, and M. B. Omary. 2003. Keratin 20 helps maintain intermediate filament organization in intestinal epithelia. Mol. Biol. Cell 14:2959-2971. [DOI] [PMC free article] [PubMed] [Google Scholar]