Abstract
The anti-tumor effects associated with oncolytic virus therapy are mediated significantly through immune-mediated mechanisms which depends both on the type of virus and the route of delivery. Here, we show that intra-tumoral (i.t.) oncolysis by Reovirus induced the priming of a CD8+, Th1-type anti-tumor response. In contrast, systemically delivered VSV expressing a cDNA library of melanoma antigens (VSV-ASMEL) promoted a potent anti-tumor CD4+ Th17 response. Therefore, we hypothesised that combining the Reovirus-induced CD8+ T cell response, with the VSV-ASMEL CD4+ Th17 helper response, would produce enhanced anti-tumor activity. Consistent with this, priming with i.t. Reovirus, followed by an intra-venous VSV-ASMEL Th17 boost, significantly improved survival of mice bearing established subcutaneous (s.c.) B16 melanoma tumors. We also show that combination of either therapy alone with anti-PD-1 immune checkpoint blockade augmented both the Th1 response induced by systemically delivered Reovirus in combination with GM-CSF, and also the Th17 response induced by VSV-ASMEL. Significantly, anti-PD-1 also uncovered an anti-tumor Th1 response following VSV-ASMEL treatment that was not seen in the absence of checkpoint blockade. Finally, the combination of all three treatments (priming with systemically delivered Reovirus, followed by double boosting with systemic VSV-ASMEL and anti-PD-1) significantly enhanced survival, with long-term cures, compared to any individual, or double, combination therapies, associated with strong Th1 and Th17 responses to tumor antigens. Our data show that it is possible to generate fully systemic, highly effective anti-tumor immunovirotherapy by combining oncolytic viruses, along with immune checkpoint blockade, to induce complimentary mechanisms of anti-tumor immune responses.
INTRODUCTION
Oncolytic viruses (OV) are naturally occurring or genetically modified viruses that target tumor cells while largely sparing normal cells, dependent on a number of different mechanisms1–3. In this respect, it is now clear that the anti-tumor activity of these agents is, at least in part, dependent on immune responses raised to both the virus and tumor associated antigens released during the process of immunogenic tumor cell killing4–6. This concept is underscored by the recent FDA approval of talimogene laherparepvec (T-Vec, an HSV encoding GM-CSF), confirming the potential of OV as immunovirotherapeutic agents for cancer treatment.
The exact immune mechanisms through which OV induce anti-tumor responses depend upon multiple factors, including the type of virus used, the route of administration of the virus and the transgenes encoded. In this respect, we, and others, have shown that immune responses mediated by a range of OV encoding either tumor antigens (Ag), cytokines and/or co-stimulatory molecules, are effective in controlling tumor growth in pre-clinical models7–10, with several of these agents being tested in clinical trials11–13. For example, Reovirus replication occurs in tumor cells with defective anti-viral PKR signalling resulting in oncolysis14 but also generates potent anti-tumor immune responses, both innate and adaptive, which are highly important for tumor regression15–18. A number of Phase1/2 clinical trials of Reovirus serotype 3 Dearing (Oncolytics Biotech) have demonstrated it to be safe19–21. We have shown that, when delivered intra-tumorally (i.t.), Reovirus generates a Th1 anti-tumor response22, which also correlates with our previous observations that Reovirus activates CTL16, 17. However, when delivered systemically in combination with GM-CSF, we showed that the anti-tumor immune response is also heavily dependent on innate mechanisms23.
We have also developed an effective systemic immunovirotherapy against established tumors using Vesicular Stomatitis Virus (VSV) expressing either single, or multiple, tumor antigens. In particular, i.v. delivery of VSV expressing a cDNA library derived from either normal, or tumor, cells primed specific anti-tumor immune responses in models of melanoma, prostate cancer and brain tumors 10, 24, 25. Interestingly, in all of these models, the anti-tumor immune responses primed against tumor by expression of multiple tumor antigens encoded by the virally-expressed cDNA were dependent upon CD4+ Th17 cells10, 24.
Normal immune responses to infection or injury are modulated at checkpoints to prevent them leading to uncontrolled immune cell proliferation and auto-immune disease. For example, Programmed cell death-1 (PD-1) is a receptor found on immune cells including T cells, B cells and monocytes26 binding of which to one of its ligands, PD-L1 or PD-L2, inhibits immune cell activation. Expression of PD-L1 is found on many types of tumor27 resulting in the ability of tumor cells to evade immune responses against them. Checkpoint inhibitors are antibodies which target these negative immune regulators or their ligands, including PD1/PD-L1, and have shown great promise as immune therapy for the treatment of at least a proportion of patients with melanoma and other cancers28–30. These data clearly suggest that these checkpoint inhibitors relieve repression of (weak) T cell responses against self tumor associated antigens, as well as against pathogens associated with infection and injury. Therefore, given that OV can prime anti-tumor T cell responses, several groups have proposed that the combination of OV therapy and checkpoint inhibition will be of immunotherapeutic value 22, 25, 31, 32.
In the current study, we hypothesised that a combination of two different forms of oncolytic viroimmunotherapy, which stimulate alternative CD8+ Th1 and CD4 helper Th17 mechanisms of anti-tumor immunity, could combine co-operatively or synergistically, along with immune checkpoint blockade, to enhance anti-tumor therapy. We show here a Th1/Th17 prime-boost treatment with two different viruses, both delivered systemically, was significantly more effective in controlling tumors than either single immunovirotherapy treatment alone. Further addition of immune checkpoint blockade with anti-PD-1, generated long term cures in mice treated with the triple combination therapy under experimental conditions where double therapies alone did not.
RESULTS
Reovirus primes a Th1 response, while VSV-cDNA primes a Th17 response against B16 melanoma
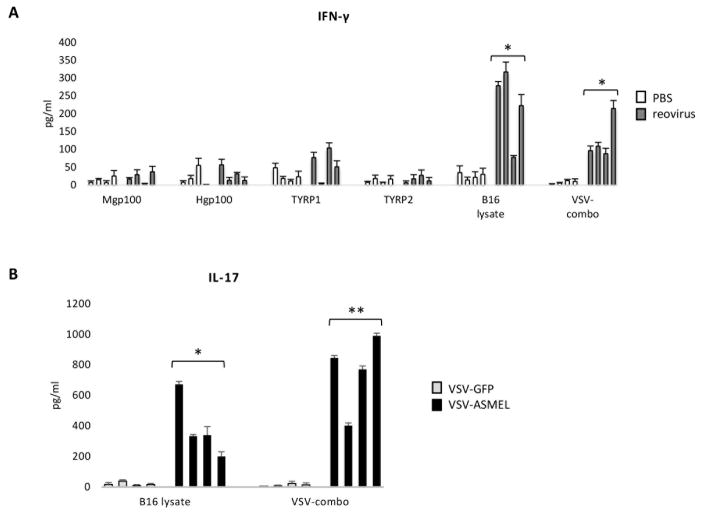
Pooled cultures of splenocytes and lymph node (S/LN) cells from mice treated intra-tumorally (i.t.) with Reovirus, but not with PBS, secreted IFN-γ in response to B16 tumor cell lysates (Fig. 1A). They also generated a Th1 recall response to a combination of the three VSV-expressed self antigens (VSV-NRAS, VSV-CYT-c, VSV-TYRP1), which we have previously described as rejection antigens for B16 tumors following treatment with a VSV-ASMEL cDNA library24 (Fig. 1A, VSV-combo). However, no IL-17 (< 50 pg/ml, data not shown) was detected as a result of i.t. Reovirus treatment indicating the absence of a Th17 immune response.
Figure 1. Reovirus primes a Th1 response, while VSV-cDNA primes a Th17 response against B16 melanoma.
A&B. C57Bl/6 mice (4 per group) bearing 10 day established B16 tumors, received 6 i.t. injections of either PBS or Reovirus on days 10,12,14,17,19,21 (A), and C57Bl/6 mice (4 per group) bearing 5 day established B16 tumors, received 6 i.v. injections of either VSV-GFP or VSV-ASMEL on days 5,7,9,12,14,16. (B). At day 25, mice were euthanised, spleens and LN dissociated into single cell suspensions and re-stimulated with either: B16 F/T lysate; VSV-NRAS + VSV-CYT-c + VSV-TYRP1 (VSV-combo, total MOI=1 per re-stimulation) or peptide as indicated (1 μg/ml per re-stimulation), every 24 h. Supernatants were harvested after 48 h and tested for IFN-γ and IL-17 by ELISA. Graphs show values +SD (triplicate wells) for individual mice. *p<0.05, **p<0.01 two-tailed t-test.
In this s.c. B16 model, we have shown that single agent Reovirus delivered i.t., but not intravenously (i.v.), was an effective anti-tumor therapy33. In contrast, established B16 tumors could be treated with a systemically delivered VSV-cDNA library (VSV-ASMEL – Altered Self Melanoma Eptiope Library)10. The anti-tumor response was dependent on CD4+ T cells and associated with a Th17 response against at least three dominant tumor Ag, NRAS, CYT-c and TYRP124. Consistent with those data, splenocyte/LN cells from VSV-ASMEL-treated mice secreted IL-17 in response to either B16 lysate or to the VSV-combo (Fig. 1B). In contrast, no IFN-γ was secreted on re-stimulation with B16 lysate or the VSV-combo (< 50 pg/ml, data not shown), indicating no significant detectable Th1-type response to this treatment. Therefore, i.t. Reovirus (Th1), and i.v. VSV-cDNA (Th17), prime different types of anti-tumor immune response.
Prime-boost using Reovirus and VSV-ASMEL improves anti-tumor therapy
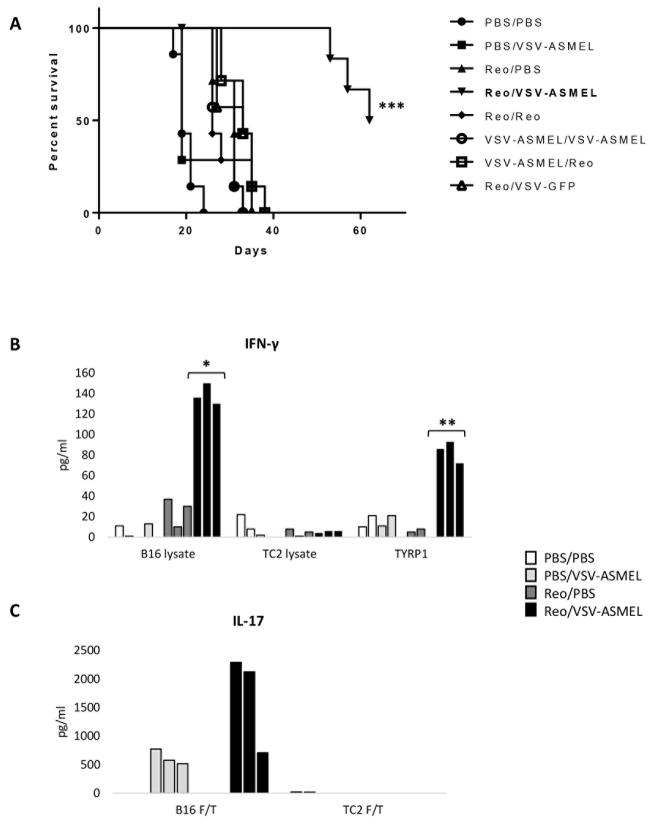
Therefore, we hypothesized that a combination of immunovirotherapies working through different immune mechanisms would enhance overall anti-tumor therapy in the context of a prime-boost strategy. Using sub-optimal individual treatments either alone, or in combination, to allow detection of improved efficacy, prime-boost with Reo/PBS, Reo/Reo, VSV-ASMEL/VSV-ASMEL, Reo/VSV-GFP and VSV-ASMEL/Reo all resulted in significantly improved survival compared to PBS/PBS treated controls (Fig. 2A, p<0.001 for all). However, prime-boost with Reo/VSV-ASMEL was a significantly better treatment than any of the other regimens (Fig. 2A, p<0.001 Reo/VSV-ASMEL vs any other treatment). Increased survival following Reo/VSV-ASMEL prime-boost was associated with a stronger Th1 recall response against B16 lysate, or the melanoma tumor antigen TYRP1, compared to that seen in mice treated with prime-boost Reo/PBS (Fig. 2B, p = 0.0140, B16 lysate; p = 0.0023, TYRP1). There was a trend towards increased Th17 responses following prime-boost Reo/VSV-ASMEL treatment compared to PBS/VSV-ASMEL although this did not reach statistical significance (Fig. 2C). IFN-γ or IL-17 recall responses to TC2 F/T lysate, a non-melanoma cell line, were minimal, indicating that the Th1 and Th17 responses were tumor-specific (Figs. 2B&C).
Figure 2. Prime-boost using Reovirus and VSV-ASMEL improves anti-tumor therapy.
A. C57Bl/6 mice (7 per group) bearing 10 day established B16 tumors, received 3 i.t. injections of either PBS, Reovirus or VSV-ASMEL on days 10,12,14 followed by 3 i.v. injections of either PBS/Reovirus/VSV-ASMEL on days 17,19,21 as indicated. Tumor measurements were taken 3x per week and mice euthanised when tumors reached 1.0 cm diameter. Graph shown is representative of n=2 individual experiments, ***p<0.001 Log-Rank test Reo/VSV-ASMEL compared to all other groups. B&C. At time of sacrifice due to tumor burden, S/LN were harvested from 3 mice per group. Single cell suspension cultures of S/LN were re-stimulated with either, B16 (relevant) or TC2 (irrelevant) F/T lysate, or TYRP1 peptide, every 24h. Supernatants were harvested after 72h and tested for IFN-γ and IL-17 by ELISA. Bars on graphs show values for individual mice. *p<0.05, **p<0.01 two-tailed t-test.
Enhancement of systemic Reovirus therapy by checkpoint blockade is dependent on CD8 cells
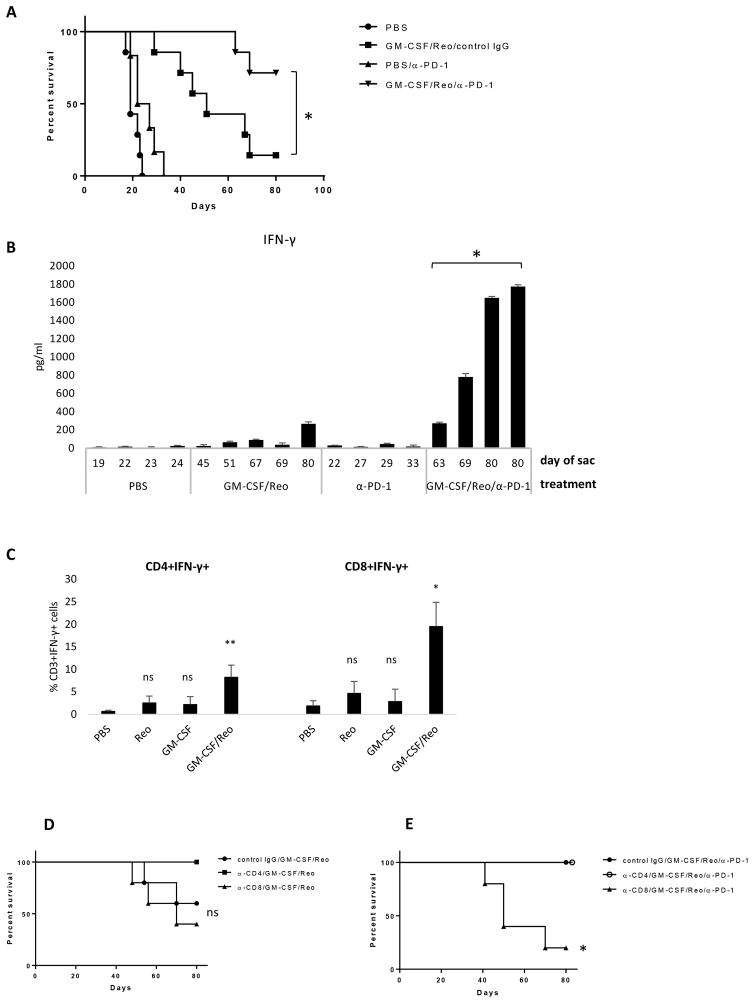
We have previously shown that systemically delivered Reovirus can be effective when used in combination with other agents such as GM-CSF, cyclophosphamide or VEGF23, 33, 34 or in the context of ex vivo loaded cell carriage18. In this respect, pre-conditioning with GM-CSF prior to systemic Reovirus delivery, effectively treated B16 tumors dependent on innate immune responses23. As before23, a suboptimal regimen of two cycles of GM-CSF/Reovirus significantly prolonged survival in C57Bl/6 mice bearing 5 day established B16 s.c. tumors (Fig. 3A). Combination with anti-PD-1 checkpoint blockade resulted in significantly improved survival (Fig. 3A, GM-CSF/Reovirus/anti-PD-1 vs GM-CSF/Reovirus alone, p = 0.0174). We observed a low level Th1 response to tumor Ag following GM-CSF/Reovirus treatment, both by an IFN-γ recall response to B16 tumor lysate (Fig. 3B), and by significantly increased numbers of IFN-γ secreting CD8+ T cells (p=0.012) and CD4+ T cells (p=0.009) infiltrating into GM-CSF/Reovirus-treated tumors compared to PBS-treated tumors (Fig. 3C). However, this Th1 response was significantly improved by the addition of anti-PD-1 (Fig. 3B, GM-CSF/Reovirus/anti-PD-1 vs GM-CSF/Reovirus, p = 0.0250). Previously we showed that GM-CSF/Reovirus therapy is largely mediated by innate effectors such as natural killer (NK) cells and monocytes23. Similarly, despite the increased numbers of CD8+ T cells in treated tumors, depletion of neither CD8, nor CD4, cells significantly affected survival after treatment with GM-CSF/Reovirus (Fig. 3D). Interestingly, we observed a trend for increased survival with depletion of CD4+ T cells (Fig. 3D). Although this was not statistically significant, we are currently testing the hypothesis that depletion of CD4+ T cells leads to removal of a suppressive population, which enhances the innate-immune mediated clearance of tumors induced by GM-CSF/Reovirus treatment. However, consistent with the improved Th1 response seen on addition of anti-PD1 (Fig. 3B), depletion of CD8, but not CD4, cells significantly reduced survival in mice treated with GM-CSF/Reovirus + anti-PD-1 (Fig. 3E, p = 0.0135). No Th17 response was detected following GM-CSF/Reovirus treatment, with, or without, addition of anti-PD-1 (IL-17 < 20 pg/ml, data not shown). These data suggest that, although the effect of GM-CSF/Reovirus is mainly mediated via innate effectors, a detectable, but low level Th1 response was also generated but did not contribute significantly to tumor control. However, in the presence of checkpoint blockade this weak Th1 response was significantly enhanced, which translated into improved overall survival.
Figure 3. Enhancement of systemic Reovirus therapy by checkpoint blockade is dependent on CD8 cells.
A&B. C57Bl/6 mice (7 per group) bearing 5 day established B16 tumors, were treated ± 2 cycles of GM-CSF/Reovirus beginning on days 5 and 12, then 3 injections of anti-PD-1 (250 μg) or control IgG on days 19,21,23. A. Tumors were measured 3x per week and mice euthanised when tumors reached 1.0 cm diameter. *p<0.05 Log-Rank test. B. S/LN were harvested at time of sacrifice (as indicated). Single cell suspension cultures of S/LN were re-stimulated with B16 F/T lysate every 24 h. Supernatants were harvested after 72 h and tested for IFN-γ by ELISA. Bars on graphs show values +SD (triplicate wells) for individual mice. *p<0.05 two-tailed t-test. C. Mice with 6 day established subcutaneous B16 tumors were treated with two rounds of PBS/PBS, PBS/Reo, GM-CSF/PBS or GM-CSF/Reovirus (days 6–10 and 13–17). On day 22 tumors were excised and analysed by intracellular staining for CD3+ CD8+ IFN-γ+, and CD3+ CD4+ IFN-γ+, T cells. The mean percentage of CD3+ CD4+ or CD3+ CD8+ cells, which were also IFN-γ positive, in tumors from each group is shown. Standard deviations represent values from 4 mice per group (except GM-CSF/PBS where n=3). *p<0.05, **p<0.01 two-tailed t-test. D&E. C57Bl/6 mice (5 per group) bearing 5 day established B16 tumors, received 3 cycles of GM-CSF/Reovirus with co-injection of anti-CD4 or anti-CD8 depleting antibodies along with the GM-CSF, begining on days 5,12,19. Anti-PD-1 (250 μg) or control IgG was administered on days 19,21,23. Tumors were measured 3x per week and mice euthanised when tumors reached 1.0 cm diameter. D. Depletion of CD4 or CD8 cells on GM-CSF/Reovirus therapy; E. Depletion of CD4 or CD8 cells on GM-CSF/Reo/anti-PD-1 therapy. *p<0.05 Log-Rank test. D&E are results from the same experiment.
Checkpoint inhibition improves VSV-ASMEL therapy and uncovers a Th1 anti-tumor response
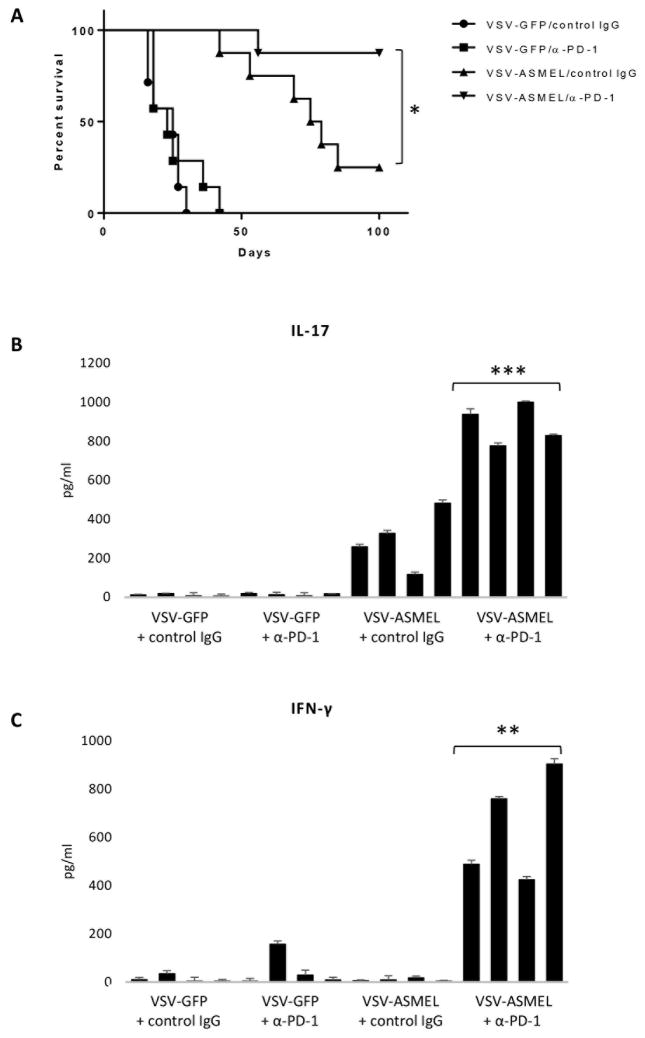
The addition of anti-PD-1 significantly prolonged survival of mice with established s.c. B16 tumors treated with VSV-ASMEL alone (Fig. 4A, VSV-ASMEL + anti-PD-1 vs VSV-ASMEL + control IgG, p = 0.018). Improved survival following VSV-ASMEL + anti-PD-1 was associated with a significantly stronger Th17 recall response against B16 lysate compared to VSV-ASMEL alone (Fig. 4B, p = 0.001). Furthermore, anti-PD-1 treatment uncovered a Th1 response to tumor as evidenced by production of IFN-γ from splenocyte/LN cells in response to B16 lysate (Fig. 4C, p = 0.0014), which was not detectable in the absence of anti-PD-1.
Figure 4. Checkpoint inhibition improves VSV-ASMEL therapy and uncovers a Th1 anti-tumor response.
C57Bl/6 mice (7–8 per group) bearing 5 day established B16 tumors, received 6 injections of either VSV-GFP or VSV-ASMEL on days 5,7,9,12,14,16, followed by 6 injections of anti-PD-1 (250 μg) or control Ig on days 19,21,23,26,28,30. A. Tumor measurements were taken 3x per week and mice euthanised when tumors reached 1.0 cm diameter. Graph shown is representative of n=3 individual experiments, *p<0.05 Log-Rank test. B&C. S/LN were harvested from 4 mice/group at time of sacrifice. Single cell suspension cultures of S/LN were re-stimulated with B16 F/T lysate every 24 h. Supernatants were harvested after 72 h and tested for IL-17 (B) and IFN-γ (C) by ELISA. Bars on graphs show values +SD (triplicate wells) for individual mice. **p<0.01, ***p<0.001 two-tailed t-test.
Combined Th1/Th17 therapy, together with checkpoint inhibition, cures B16 melanoma
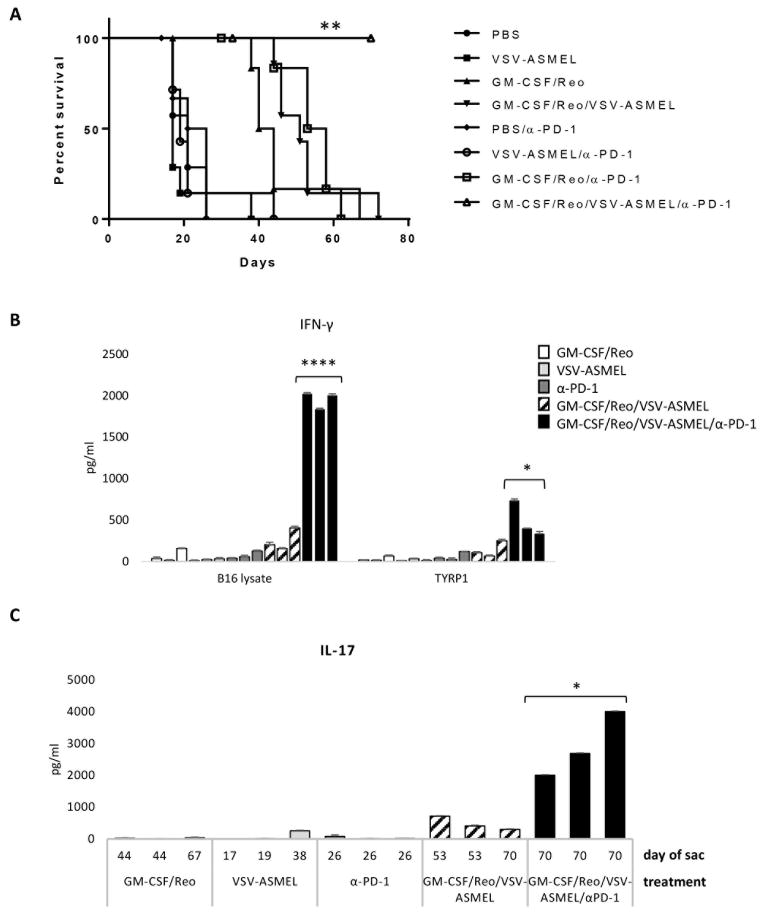
Finally, we hypothesized that combining an innate-driven/Th1 Reovirus-induced anti-tumor response, with a Th17 VSV-ASMEL-induced response, both of which were enhanced with anti-PD-1 blockade, would generate more effective anti-tumor therapy than either alone. As before, GM-CSF/Reovirus was effective in treating s.c. B16 tumors (Fig. 5A, p = 0.0004 vs PBS), while combination with anti-PD-1 further improved survival (Figs. 3A&5A). As with i.t. Reovirus + VSV-ASMEL (Fig. 2A), prime-boost with systemic GM-CSF/Reovirus followed by VSV-ASMEL, was superior to GM-CSF/Reovirus alone (Fig. 5A). However, addition of anti-PD-1 to the GM-CSF/Reovirus/VSV-ASMEL prime-boost treatment was the only therapy able to generate long-term cures under these experimental conditions (Fig. 5A, p < 0.01 vs GM-CSF/Reo, GM-CSF/Reo/anti-PD-1, GM-CSF/VSV-ASMEL). Splenocyte/LN cultures from the long-term cured mice produced significantly higher levels of IFN-γ in response to B16 lysate than mice from any other treatment group which had been euthanised earlier due to tumor burden, (Fig. 5B, p = 0.00006). This Th1 recall response included a specific component against the melanoma Ag TYRP1 (Fig. 5B, p = 0.0216 vs control group). In addition, mice treated with GM-CSF/Reovirus/VSV-ASMEL + anti-PD-1had a significantly improved Th17 recall response compared to those treated with the prime-boost regimen without checkpoint blockade (Fig. 5C, p = 0.0156). These data show that two separate oncolytic immunovirotherapies, working through different immune effector mechanisms, and combined with checkpoint blockade, can be effectively combined to eradicate established disease.
Figure 5. Combined Th1/Th17 therapy, together with checkpoint inhibition, is effective in curing B16 melanoma.
C57Bl/6 mice (7 per group) bearing 10 day established B16 tumors, received 2 ‘prime’ cycles of either PBS or GM-CSF/Reovirus starting at days 10 and 17, then 3 ‘boost’ injections of PBS or VSV-ASMEL on days 24,26,28. Anti-PD-1 (225 μg) or control IgG was given on days 24,26,28,31,33,35. A. Tumor measurements were taken 3x per week and mice euthanised when tumors reached 1.0 cm diameter. Graph shown is representative of n=2 individual experiments, **p<0.01 Log-Rank test. B&C. S/LN were harvested from 3 mice/group at time of sacrifice (as indicated in C). Single cell suspension cultures of S/LN were re-stimulated with B16 F/T lysate or peptide as indicated, every 24 h. Supernatants were harvested after 72 h and tested for IFN-γ (B) and IL-17 (C) by ELISA. Bars on graphs show values +SD (triplicate wells) for individual mice. *p<0.05, ***p<0.001 two-tailed t-test.
DISCUSSION
It is now clear that the efficacy of many oncolytic virus regimens depends upon an immune component. Thus, Reovirus is effective against B16OVA tumors which are not susceptible to direct oncolysis17, and systemic VSV did not generate significant anti-tumor therapy in nude mice35. However, the immunological mechanisms of such effects will vary between virus types, routes of administration and transgenes encoded by the viruses. In this respect, we show here that, whereas i.t. injection of oncolytic Reovirus primed a Th1-type response to B16 s.c. tumors, systemic administration of the VSV-ASMEL cDNA library primed a Th17 response to tumor-specific Ag. Therefore, we hypothesized that combining complementary immunological effector pathways, induced by different oncolytic viruses, would generate improved immune-mediated anti-tumor therapy.
Repeated treatment with the same type of immunovirotherapy (Reo/Reo (Th1) or VSV-ASMEL/VSV-ASMEL (Th17)) resulted in prolonged survival compared to PBS-treated controls (Fig. 2A). However, combination Reovirus/VSV-ASMEL (Th1/Th17) prime-boost treatment significantly improved survival compared to repeated single therapies (Fig. 2A), associated with enhanced Th1, and, to a lesser extent, Th17 anti-tumor Ag responses, (Figs. 2B&C). Interestingly, reversing the order of the prime-boost from Th1/Th17 to Th17/Th1 still significantly improved survival compared to controls. However, this improvement was only comparable to single repeated immunovirotherapies and was significantly less effective than the Th1/Th17 prime-boost (Fig. 2A). These data show that two different oncolytic viruses, each priming a different type of immune response, can be combined to produce significantly better therapy than either virus alone. Furthermore, the order in which the responses were induced was important (Th1 followed by Th17).
As part of our long term goal to develop delivery regimens for oncolytic immunovirotherapy which do not necessitate direct i.t. injection, we developed an effective systemic Reovirus therapy by pre-conditioning tumor-bearing mice with GM-CSF prior to i.v. Reovirus injection, which is mediated by NK cells and CD11b+ monocytes23. We have also shown that Reovirus-mediated NK cell activation following i.t. Reovirus injection was augmented by anti-PD-1 leading to improved tumor therapy22. Therefore, we investigated whether anti-PD-1 could improve our systemic Reovirus treatment. Fig. 3A shows that addition of anti-PD-1 treatment significantly enhanced survival of mice compared to GM-CSF/Reovirus alone. Significantly, this improvement in therapy was associated with an enhanced Th1 response to B16 tumor Ag, which was only minimally detected in the absence of anti-PD-1 (Fig. 3B). The improved therapy was also dependent upon CD8+ T cells (Figs. 3B&E), consistent with the mechanism of checkpoint blockade as acting predominantly via release of inhibition on T cells36–38. These data show that checkpoint blockade mechanistically enhanced systemic GM-CSF/Reovirus therapy by significantly augmenting an otherwise very weak CD8+ T cell dependent component which was associated with significantly better anti-tumor therapy.
Similarly, although therapy associated with systemic delivery of VSV-ASMEL was dependent upon CD4+ T cells and a Th17 response (Fig. 4B), with no detectable Th1 response (Fig. 4C), addition of anti-PD-1 uncovered a Th1 response to tumor Ag that was not detectable in the absence of checkpoint blockade (Fig. 4C). As for the addition of anti-PD-1 to the GM-CSF/Reovirus regimen, uncovering of this anti-tumor Th1 response was associated with extended survival, and increased tumor cures, in vivo (Fig. 4A). Anti-PD-1 also moderately enhanced the anti-tumor Th17 response against B16 tumor Ag (Fig. 4B). We are currently investigating the possibility that anti-PD-1 therapy acts so effectively to augment these otherwise undetectable Th1 T cell responses (for both GM-CSF/Reovirus and VSV-ASMEL treatments), through direct activity on suppressive cells such as MDSC or Treg induced in response to virotherapy.
Since the combination of GM-CSF/Reovirus and VSV-ASMEL therapy enhanced therapy compared to either alone (Fig. 2), and since both mono-immunovirotherapies were significantly enhanced by anti-PD-1 checkpoint inhibition (Figs. 3&4), we tested the combination of all three therapies. As seen in Fig. 5, the triple therapy (GM-CSF/Reovirus (innate immune mediated, C8+T Th1lo) + VSV-ASMEL boost (CD4+ Th17, Th1lo) + anti-PD-1 (Th1 and Th17 enhancement) was significantly more effective than any of the double combinations, resulted in tumor regression with 100% of the mice cured long term at day 70, and was associated with very strong Th1 and Th17 responses to tumor antigens, including TYRP-1 (Fig 5). The data of Figs 4B&C and 5B&C, show that long term survival and tumor cure correlated with the development of both anti-tumor Th1 and Th17 recall responses. In contrast, development of either alone, or neither response, was associated with significantly shorter long term survival. These assays were performed on splenocytes from mice at the time of sacrifice due to tumor burden or at day 100 (Fig. 4) or 70 (Fig. 5), following tumor seeding for the long term survivors. We did not perform similar assays on mice at a defined time point following treatment because we believe that the multiple components of the innate and adaptive immune responses that are operative with the full combination therapy would not have developed fully by the early time points at which control treated mice started to die due to tumor burden.
Our data are consistent with a model in which primary treatment with GM-CSF/Reovirus leads to initial tumor killing through virus delivery and innate immune activation23. This therapy induced detectable, but very low level, Th1 responses against tumor antigens (Fig. 3B). We hypothesise that, critically, initial tumor killing releases a very broad range of tumor Ag, against which only very weak anti-self T cell responses can be primed. Subsequent delivery of VSV-ASMEL provides a similarly broad range of tumor Ag in the form of the cDNA library. These stimulate CD4+ Th17 responses which can, therefore, provide additional help to the T cell responses stimulated by the primary GM-CSF/Reovirus treatment (Fig. 2B&C). Finally, late boosting with anti-PD-1 further augments both the already enhanced Th1 and Th17 responses against this broad range of tumor antigens leading to the potent and sustained therapy observed in Fig. 5.
Several other groups have also successfully used combinations of oncolytic viruses for tumor therapy consistent with a heterologous prime-boost strategy to generate efficient anti-tumor Ag-specific therapy. For example, rhabdoviruses, such as VSV or Maraba virus, expressing a defined melanoma associated antigen, provided an effective immunological boost against the antigen in mice previously vaccinated with an adenoviral vector to prime the response 39, 40. Tysome et al. showed that sequential treatment with oncolytic adenovirus and vaccinia viruses cured about 60% of tumor bearing Syrian Hamsters. Efficacy was dependent upon the sequence of the virus treatments, and, significantly, upon CD3+ T cells, indicating that the combination of viruses was acting through an immunological prime-boost-like mechanism 41. The combination of adenovirus and vaccinia virus was also successful in slowing anti-viral, and innate cellular, immune responses leading to better anti-tumor therapy 42. Similarly, a combination of Semliki Forest Virus and Vaccinia virus was effective at boosting anti-tumor immune responses in a murine ovarian cancer model and generated improved therapy through both oncolysis and enhanced anti-tumor immunity 43. Our approach here moves beyond the use of different vectors encoding specific antigens and uses the release of multiple antigens through oncolysis as the basis of the priming step, which is then boosted by the use of the cDNA library. We believe that raising T cell responses against multiple tumor antigens simultaneously reduces the ability of tumor cells to escape immune pressure by developing antigen loss variants. Our approach here is also novel in that it specifically exploits the complementary immunological mechanisms by which two oncolytic viruses (Reovirus and VSV) stimulate anti-tumor immunity through different immune effectors.
In summary, we show here that it is possible to combine oncolytic viruses, which induce complimentary mechanisms of anti-tumor immune responses, along with immune checkpoint blockade, to generate fully systemic, highly effective anti-tumor immunovirotherapy.
MATERIALS AND METHODS
Cell lines
Murine B16 melanoma and TRAMP-C2 (TC2) prostate tumor cells were grown in DMEM (Life Technologies) supplemented with 10% (v/v) FCS (Life Technologies) and L-glutamine (Life Technologies). Cell lines were monitored routinely and found to be free of Mycoplasma infection.
Viruses
Wild type Reovirus type 3 (Dearing strain, REOLYSIN®) was obtained from Oncolytics Biotech (Calgary, Canada). Stock titers were measured by plaque assays on L929 cells. The ASMEL VSV-cDNA library was generated as previously reported10, 24, 44. Individual viral clones were isolated by limiting dilution as previously described24, 44, expanded in BHK cells and purified by sucrose gradient centrifugation. VSV-GFP was manufactured as described45.
In vivo experiments
6–8 week old female C57Bl/6 mice were purchased from Jackson Laboratories (Bar Harbor, Maine). All in vivo studies were approved by the Mayo IACUC. Mice were challenged subcutaneously with 2x105 B16 melanoma cells in 100 μL PBS (HyClone). Tumors were measured 3 times per week, and mice were euthanized when tumors reached 1.0 cm diameter. Reovirus was administered i.v. at 5x107 or i.t. at 1x108 TCID50 per injection; VSV-GFP and VSV-ASMEL were administered i.v. at 1x107 pfu per injection. GM-CSF was administered i.p. at 300 ng/injection, as described previously23, 1 cycle of GM-CSF/Reo = GM-CSF i.p. on 3 consecutive days followed by Reovirus (5x107TCID50) i.v. on the following 2 days. Anti-PD-1 (BioXcell, West Lebanon, NH) or control IgG (BioXcell) was given i.v. at either 225 or 250 μg per injection as detailed in the figure legends. Anti-CD4 (GK1.5, BioXcell) or anti-CD8 antibodies (Lyt2.43, BioXcell) for cell depletions were administered i.p. at 100 μl per injection.
Definitions and Dosing Regimens Used in these Studies
VSV-ASMEL
A VSV-cDNA library expressing cDNA from human melanoma cell lines which, therefore encode altered self epitopes in the mouse (VSV-ASMEL – Altered Self Melanoma Epitope Library). Previously, we have shown that 9 i.v. injections of the VSV-cDNA library (VSV-ASMEL) can cure ~80% of mice with 5 day established, subcutaneous B16 tumors 24.
GM-CSF/Reovirus
A treatment schedule in which a single cycle consists of GM-CSF administered i.p. on days 1&2 followed by intravenous Reovirus on days 3,4&5. Previously, we have shown that three of these cycles of GM-CSF/Reovirus, over a period of three weeks, cured 60–80% of mice with 5d-established s.c. tumors 23.
In the studies reported here, we required models in which either VSV-ASMEL or GM-CSF/Reovirus alone would have diminished therapy, in order to investigate whether combination with each other, and/or checkpoint inhibition would enhance therapy. To achieve this, we reduced the starting tumor burden and/or the number of injections of VSV-ASMEL or GM-CSF/Reovrus depending upon the experimental situations as follows:
Figure 2: Tumor burden was increased at the time of treatment from 5 day- to 10 day-established tumor and the number of systemic injections of VSV-ASMEL was reduced from 9 (optimally therapeutic) to 3. Under these conditions, i.v. VSV-ASMEL was significantly better than PBS but led to no cures.
Figure 3: Tumor burden was kept at 5 day established s.c. B16 but therapy with GM-CSF/Reovirus was reduced relative to the optimal protocol by administering only two cycles of GM-CSF/Reovirus instead of 3. Under these circumstances only ~15% of mice were cured but all mice had significant prolongation of survival before tumors reached 1.0 cm diameter. This condition allowed a significant improvement in therapy with GM-CSF/Reovirus to be shown with the addition of anti-PD-1 therapy.
Figure 4: Tumor burden was kept at 5 day established s.c. B16. However, the number of systemic injections of VSV-ASMEL was reduced from 9 (optimally therapeutic) to 6. Under these conditions, i.v. VSV-ASMEL was significantly better than i.v. VSV-GFP but led to significantly fewer cures (~25%) than with 9 i.v. injections of VSV-ASMEL. This condition allowed a significant improvement in therapy with VSV-ASMEL to be shown with the addition of anti-PD-1 therapy.
Figure 5: Tumor burden was increased at the time of treatment from 5 day- to 10 day-established tumor. Priming therapy with GM-CSF/Reovirus was reduced relative to the optimal protocol by administering only two cycles of GM-CSF/Reovirus instead of the optimal 3. The increased tumor burden made the therapy of GM-CSF/Reovirus+anti-PD-1 less effective here (all mice with tumor by d70) than the same therapy in Figure 3 (~80% cured of tumor by day 80). The number of systemic injections of VSV-ASMEL was reduced from 9 (optimally therapeutic) to just 3, which, along with the larger tumor burden, made VSV-ASMEL therapy completely ineffective, which could not be rescued by the addition of anti-PD-1 (VSV-ASMEL/anti-PD-1). This lack of therapy was in contrast to Figure 4 where a smaller starting tumor burden, and more i.v. injections of VSV-ASMEL, generated better single agent therapy which was enhanced by anti-PD-1. Under these conditions, significant improvement in therapy with either VSV-ASMEL+anti-PD-1 or GM-CSF/Reovirus+anti-PD-1 could be shown with the triple prime-boost combination of GM-CSF/Reovirus+VSV-ASMEL+anti-PD-1.
In vitro splenic re-stimulation of splenocytes/lymph nodes and enzyme-linked immunosorbent assay for IFN-γ/TNF-α
Spleen and lymph nodes (S/LN) were immediately excised from euthanized mice and dissociated in vitro to achieve single-cell suspensions. S/LN cells were pooled for each individual mouse. Red blood cells were lysed with ACK lysis buffer for 2 min. Cells were re-suspended in Iscove’s modified Dulbecco’s medium (Gibco, Grand Island, NY) + 5% FBS + 1% Pen-Strep + 40 μM 2-ME. Supernatants were harvested from 1 x 106 S/LN stimulated with one of the following: VSV-combination (VSV-NRAS, VSV-CYT-c, VSV-TYRP1) at MOI=1 per stimulation; 1 μg/ml synthetic H2-b-restricted peptides murine TRP-2180–188 SVYDFFVWL (H2Kb), murine TRP-1222–229 TAYRYHLL (H2Kb), human gp10025–33 (Hgp100) KVPRNQDWL (H2Db), murine gp10025–33 (Mgp100) EGSRNQDWL (H2Db) or with freeze-thaw lysates (equivalent to 1 x 106 tumor cells), from B16 (relevant) or TC2 (irrelevant) tumor cells every 24 h. Cell-free supernatants were collected at 48 or 72 h and tested by enzyme-linked immunosorbent assay for murine IFN-γ or murine IL-17 (BD Biosciences, San Jose, CA). The peptides were synthesized at Mayo Foundation Core Facility (Rochester, MN).
Statistics
Survival data from the animal studies were analyzed by the log-rank test using GraphPad Prism 6 Software. A Student’s t-test analysis was applied for in vitro data. Statistical significance was determined at the level of P < 0.05.
Acknowledgments
This work was supported by NIH Grants CA175386-01A1 and CA108961-10P3; by the University of Minnesota/Mayo Foundation Partnership Grant; by Oncolytics Biotech (Calgary); The European Research Council Advanced Grant (ONCOVIRAX); Vyriad Pharmaceuticals; and the Mayo Foundation. RV is in receipt of research funding from Oncolytics.
We thank Toni Higgins for expert secretarial assistance.
Footnotes
There is no conflict of interest.
References
- 1.Russell SJ, Peng KW. Measles virus for cancer therapy. Curr Top Microbiol Immunol. 2009;330:213–41. doi: 10.1007/978-3-540-70617-5_11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stojdl DF, Lichty BD, tenOever BR, Paterson JM, Power AT, Knowles S, et al. VSV strains with defects in their ability to shutdown innate immunity are potent systemic anti-cancer agents. Cancer Cell. 2003;4(4):263–75. doi: 10.1016/s1535-6108(03)00241-1. [DOI] [PubMed] [Google Scholar]
- 3.Martin TA, Watkins G, Jiang WG. The Coxsackie-adenovirus receptor has elevated expression in human breast cancer. Clin Exp Med. 2005;5(3):122–8. doi: 10.1007/s10238-005-0076-1. [DOI] [PubMed] [Google Scholar]
- 4.Andtbacka RH, Kaufman HL, Collichio F, Amatruda T, Senzer N, Chesney J, et al. Talimogene Laherparepvec improves durable response rate in patients with advanced melanoma. J Clin Oncol. 2015;33:2780–2788. doi: 10.1200/JCO.2014.58.3377. [DOI] [PubMed] [Google Scholar]
- 5.Chiocca EA, Rabkin SD. Oncolytic viruses and their application to cancer immunotherapy. Cancer Immunol Res. 2014;2(4):295–300. doi: 10.1158/2326-6066.CIR-14-0015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kaufman HL, Kohlhapp FJ, Zloza A. Oncolytic viruses: a new class of immunotherapy drugs. Nat Rev Drug Discov. 2015;14(9):642–62. doi: 10.1038/nrd4663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Choi IK, Lee JS, Zhang SN, Park J, Sonn CH, Lee KM, et al. Oncolytic adenovirus co-expressing IL-12 and IL-18 improves tumor-specific immunity via differentiation of T cells expressing IL-12Rb2 or IL-18Ra. Gene Ther. 2011;18(9):898–909. doi: 10.1038/gt.2011.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Diaconu I, Cerullo V, Hirvinen ML, Escutenaire S, Ugolini M, Pesonen SK, et al. Immune response is an important aspect of the antitumor effect produced by a CD40L-encoding oncolytic adenovirus. Cancer Res. 2012;72(9):2327–38. doi: 10.1158/0008-5472.CAN-11-2975. [DOI] [PubMed] [Google Scholar]
- 9.Kim JH, Oh JY, Park BH, Lee DE, Kim JS, Park HE, et al. Systemic armed oncolytic and immunologic therapy for cancer with JX-594, a targeted poxvirus expressing GM-CSF. Mol Ther. 2006;14(3):361–70. doi: 10.1016/j.ymthe.2006.05.008. [DOI] [PubMed] [Google Scholar]
- 10.Kottke T, Errington F, Pulido J, Galivo F, Thompson J, Wongthida P, et al. Broad antigenic coverage induced by vaccination with virus-based cDNA libraries cures established tumors. Nat Med. 2011;17(7):854–9. doi: 10.1038/nm.2390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pesonen S, Diaconu I, Kangasniemi L, Ranki T, Kanerva A, Pesonen SK, et al. Oncolytic immunotherapy of advanced solid tumors with a CD40L-expressing replicating adenovirus: assessment of safety and immunologic responses in patients. Cancer Res. 2012;72(7):1621–31. doi: 10.1158/0008-5472.CAN-11-3001. [DOI] [PubMed] [Google Scholar]
- 12.Cerullo V, Pesonen S, Diaconu I, Escutenaire S, Arstila PT, Ugolini M, et al. Oncolytic adenovirus coding for granulocyte macrophage colony-stimulating factor induces antitumoral immunity in cancer patients. Cancer Res. 2010;70(11):4297–309. doi: 10.1158/0008-5472.CAN-09-3567. [DOI] [PubMed] [Google Scholar]
- 13.Heo J, Reid T, Ruo L, Breitbach CJ, Rose S, Bloomston M, et al. Randomized dose-finding clinical trial of oncolytic immunotherapeutic vaccinia JX-594 in liver cancer. Nat Med. 2013;19(3):329–36. doi: 10.1038/nm.3089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Coffey MC, Strong JE, Forsyth PA, Lee PW. Reovirus therapy of tumors with activated Ras pathway. Science. 1998;282(5392):1332–4. doi: 10.1126/science.282.5392.1332. [DOI] [PubMed] [Google Scholar]
- 15.Errington F, White CL, Twigger KR, Rose A, Scott K, Steele L, et al. Inflammatory tumour cell killing by oncolytic reovirus for the treatment of melanoma. Gene Ther. 2008;15(18):1257–70. doi: 10.1038/gt.2008.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Prestwich RJ, Errington F, Ilett EJ, Morgan RS, Scott KJ, Kottke T, et al. Tumor infection by oncolytic reovirus primes adaptive antitumor immunity. Clin Cancer Res. 2008;14(22):7358–66. doi: 10.1158/1078-0432.CCR-08-0831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Prestwich RJ, Ilett EJ, Errington F, Diaz RM, Steele LP, Kottke T, et al. Immune-mediated antitumor activity of reovirus is required for therapy and is independent of direct viral oncolysis and replication. Clin Cancer Res. 2009;15(13):4374–81. doi: 10.1158/1078-0432.CCR-09-0334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ilett EJ, Prestwich RJ, Kottke T, Errington F, Thompson JM, Harrington KJ, et al. Dendritic cells and T cells deliver oncolytic reovirus for tumour killing despite pre-existing anti-viral immunity. Gene Ther. 2009;16(5):689–99. doi: 10.1038/gt.2009.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vidal L, Pandha HS, Yap TA, White CL, Twigger K, Vile RG, et al. A phase I study of intravenous oncolytic reovirus type 3 dearing in patients with advanced cancer. Clin Cancer Res. 2008;14(21):7127–37. doi: 10.1158/1078-0432.CCR-08-0524. [DOI] [PubMed] [Google Scholar]
- 20.Karapanagiotou EM, Roulstone V, Twigger K, Ball M, Tanay M, Nutting C, et al. Phase I/II trial of carboplatin and paclitaxel chemotherapy in combination with intravenous oncolytic reovirus in patients with advanced malignancies. Clin Cancer Res. 2012;18(7):2080–9. doi: 10.1158/1078-0432.CCR-11-2181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Adair RA, Roulstone V, Scott KJ, Morgan R, Nuovo GJ, Fuller M, et al. Cell carriage, delivery, and selective replication of an oncolytic virus in tumor in patients. Sci Transl Med. 2012;4(138):138ra77. doi: 10.1126/scitranslmed.3003578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rajani K, Parrish C, Kottke T, Thompson J, Zaidi S, Ilett L, et al. Combination therapy with reovirus and anti-PD-1 blockade controls tumor growth through innate and adaptive immune responses. Mol Ther. 2015;24:166–174. doi: 10.1038/mt.2015.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ilett E, Kottke T, Donnelly O, Thompson J, Willmon C, Diaz R, et al. Cytokine conditioning enhances systemic delivery and therapy of an oncolytic virus. Mol Ther. 2014;22:1851–1863. doi: 10.1038/mt.2014.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pulido J, Kottke T, Thompson J, Galivo F, Wongthida P, Diaz RM, et al. Using virally expressed melanoma cDNA libraries to identify tumor-associated antigens that cure melanoma. Nat Biotechnol. 2012;30(4):337–43. doi: 10.1038/nbt.2157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cockle JV, Rajani K, Zaidi S, Kottke T, Thompson J, Diaz RM, et al. Combination viroimmunotherapy with checkpoint inhibition to treat glioma, based on location-specific tumor profiling. Neuro Oncol. 2015;18(4):518–527. doi: 10.1093/neuonc/nov173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Francisco LM, Sage PT, Sharpe AH. The PD-1 pathway in tolerance and autoimmunity. Immunol Rev. 2010;236:219–42. doi: 10.1111/j.1600-065X.2010.00923.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dong H, Strome SE, Salomao DR, Tamura H, Hirano F, Flies DB, et al. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat Med. 2002;8(8):793–800. doi: 10.1038/nm730. [DOI] [PubMed] [Google Scholar]
- 28.Larkin J, Chiarion-Sileni V, Gonzalez R, Grob JJ, Cowey CL, Lao CD, et al. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med. 2015;373(1):23–34. doi: 10.1056/NEJMoa1504030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Powles T, Eder JP, Fine GD, Braiteh FS, Loriot Y, Cruz C, et al. MPDL3280A (anti-PD-L1) treatment leads to clinical activity in metastatic bladder cancer. Nature. 2014;515(7528):558–62. doi: 10.1038/nature13904. [DOI] [PubMed] [Google Scholar]
- 30.Borghaei H, Paz-Ares L, Horn L, Spigel DR, Steins M, Ready NE, et al. Nivolumab versus Docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med. 2015;373(17):1627–39. doi: 10.1056/NEJMoa1507643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zamarin D, Holmgaard RB, Subudhi SK, Park JS, Mansour M, Palese P, et al. Localized oncolytic virotherapy overcomes systemic tumor resistance to immune checkpoint blockade immunotherapy. Sci Transl Med. 2014;6(226):226ra32. doi: 10.1126/scitranslmed.3008095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Engeland CE, Grossardt C, Veinalde R, Bossow S, Lutz D, Kaufmann JK, et al. CTLA-4 and PD-L1 checkpoint blockade enhances oncolytic measles virus therapy. Mol Ther. 2014;22(11):1949–59. doi: 10.1038/mt.2014.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Qiao J, Wang H, Kottke T, White C, Twigger K, Diaz RM, et al. Cyclophosphamide facilitates antitumor efficacy against subcutaneous tumors following intravenous delivery of reovirus. Clin Cancer Res. 2008;14(1):259–69. doi: 10.1158/1078-0432.CCR-07-1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kottke T, Hall G, Pulido J, Diaz RM, Thompson J, Chong H, et al. Antiangiogenic cancer therapy combined with oncolytic virotherapy leads to regression of established tumors in mice. J Clin Invest. 2010;120(5):1551–60. doi: 10.1172/JCI41431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Qiao J, Wang H, Kottke T, Diaz RM, Willmon C, Hudacek A, et al. Loading of oncolytic vesicular stomatitis virus onto antigen-specific T cells enhances the efficacy of adoptive T-cell therapy of tumors. Gene Ther. 2008;15:604–616. doi: 10.1038/sj.gt.3303098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brahmer JR, Tykodi SS, Chow LQ, Hwu WJ, Topalian SL, Hwu P, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med. 2012;366(26):2455–65. doi: 10.1056/NEJMoa1200694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tumeh PC, Harview CL, Yearley JH, Shintaku IP, Taylor EJ, Robert L, et al. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature. 2014;515(7528):568–71. doi: 10.1038/nature13954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Karyampudi L, Lamichhane P, Scheid AD, Kalli KR, Shreeder B, Krempski JW, et al. Accumulation of memory precursor CD8 T cells in regressing tumors following combination therapy with vaccine and anti-PD-1 antibody. Cancer Res. 2014;74(11):2974–85. doi: 10.1158/0008-5472.CAN-13-2564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bridle BW, Clouthier D, Zhang L, Pol J, Chen L, Lichty BD, et al. Oncolytic vesicular stomatitis virus quantitatively and qualitatively improves primary CD8 T-cell responses to anticancer vaccines. Oncoimmunol. 2013;2(8):e26013. doi: 10.4161/onci.26013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pol JG, Zhang L, Bridle BW, Stephenson KB, Resseguier J, Hanson S, et al. Maraba virus as a potent oncolytic vaccine vector. Mol Ther. 2014;22(2):420–9. doi: 10.1038/mt.2013.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tysome JR, Li X, Wang S, Wang P, Gao D, Du P, et al. A novel therapeutic regime to eradicate established solid tumors with an effective induction of tumor-specific immunity. Clin Cancer Res. 2012;18:6679–6689. doi: 10.1158/1078-0432.CCR-12-0979. [DOI] [PubMed] [Google Scholar]
- 42.Vaha-Koskela M, Tahtinen S, Gronberg-Vaha-Koskela S, Taipale K, Saha D, Merisalo-Soikkeli M, et al. Overcoming tumor resistance by heterologous adeno-poxvirus combination therapy. Mol Ther Oncolytics. 2015;1:14006. doi: 10.1038/mto.2014.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang YQ, Tsai YC, Monie A, Wu TC, Hung CF. Enhancing the therapeutic effect against ovarian cancer through a combination of viral oncolysis and antigen-specific immunotherapy. Mol Ther. 2010;18(4):692–9. doi: 10.1038/mt.2009.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Alonso-Camino V, Rajani K, Kottke T, Rommelfanger-Konkol D, Zaidi S, Thompson J, et al. The profile of tumor antigens which can be targeted by immunotherapy depends upon the tumor’s anatomical site. Mol Ther. 2014;22(11):1936–48. doi: 10.1038/mt.2014.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fernandez M, Porosnicu M, Markovic D, Barber GN. Genetically engineered vesicular stomatitis virus in gene therapy: application for treatment of malignant disease. J Virol. 2002;76(2):895–904. doi: 10.1128/JVI.76.2.895-904.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]