Abstract
Biogenic amine defects constitute a complex and expanding group of neurotransmitter disorders affecting cognitive, motor and autonomic system development, mostly in the pediatric age. In recent years different enzymatic defects have been identified impairing the tetrahydrobiopterin cofactor pathway and/or biogenic amine synthesis, catabolism and transport, with subsequent new disease entities described. The lumbar puncture, with subsequent withdrawal of cerebrospinal fluid (CSF), remains a key step in the diagnostic procedure. Due to the specific nature of CSF, timing of analysis, sample collection and storage, technical issues of the analytic process are still crucial for the diagnosis and follow-up of patients. A progressive approach to the diagnosis of biogenic amine defects is presented, pointing out criticalities and difficulties concerning sample collection and results interpretation, especially due to the increasing reports of secondary neurotransmitter alterations that, at present, constitute a challenge.
Key words: monoamine neurotransmitter deficiencies, cerebrospinal fluid, tetrahydrobiopterin defects
INTRODUCTION
Monoamine neurotransmitter defects are included in the group of neurometabolic syndromes attributable to disturbances of neurotransmitter metabolism/transport and cofactors (i.e. tetrahydrobiopterin) synthesis/regeneration. Biogenic amines, serotonin and all catecholamines (dopamine, epinephrine and norepinephrine) are major neurotransmitters within the central nervous system (CNS). Their regulation is tuned and governed by the rate of neurotransmitter (NT) synthesis, packaging, release, re-uptake, degradation, and by receptor status. Clinically they are mainly characterized by a range of extrapyramidal manifestations including dystonia, hyperkinesia, chorea and oculogyric crisis (1,2). These defects are characterized by an elevation of phenylalanine, the presence of abnormal monoamines and deranged monoamine synthesis, degradation and transport (3) (Figure 1). Recently, abnormal neurotransmitter profiles have been reported in association with other non-metabolic and genetic diseases, and defined as secondary neurotransmitter abnormalities (4,5) (Table 1). Biochemical distinction between the two groups is difficult due to a considerable overlap in the concentrations of HVA and 5HIAA. Hence, a careful analysis of the pattern of all metabolites may be necessary to reach the correct diagnosis. Recent discoveries emphasizing the role of NTs in brain development have led to the possibility of treating these defects. Therefore an early and accurate diagnosis of biogenic amine disorder is paramount to an efficient therapeutic intervention (7).
Figure 1.
Biochemical pathways involving dopamine, serotonin, epinephrine, norepinephrine, and the cofactor BH4

Legend Figure 1: Biochemical pathways (p. 66 above)
The monoamines consist of catecholamines (for instance dopamine, norepinephrine and epinephrine) and serotonin. The amines are synthesized throughout a complex multienzymatic pathway which converts, tryptophan and tyrosine into serotonin and dopamine respectively, through reactions catalysed by tryptophan hydroxylase (TPH, EC 1.14.16.4), tyrosine hydroxylase (TH, EC 1.14.16.2) and aromatic L-aminoacid decarboxylase (AADC, EC 4.1.1.28). This latter enzyme acts as a common converging decarboxylating system for active neurotransmitter biosynthesis. In the case of AADC deficiency, the dopamine precursor (L-dihydroxyphenylalanine, DOPA) is metabolized into 3-Orthomethyldihydroxy-phenylalanine (3-OMD) and vanillactic acid (VLA). Both TPH and TH require tetrahydrobiopterin (BH4) as cofactor, while AADC needs vitamin B6 (pyridoxine). In noradrenergic neurons, dopamine is further converted by dopamine beta hydroxylase (DBH) into norepinephrine and epinephrine by phenylethanolamine N-methyltransferase (PNMT). Since BH4 is crucial in serotonin and dopamine biosynthesis, a large subset of monoamine defects is to be referred to pterins build up and regeneration mostly presenting hyperphenylalaninemia as a characterizing hallmark. The biosynthesis and regeneration of BH4 is carried out by a complex system of enzymes starting from guanosine triphosphate cyclohydrolase 1 (GTPCH1), the rate limiting enzyme for BH4 biosynthesis, which is responsible for the hydrolysis of guanosine triphosphate into 7,8-dihydroneopterin triphosphate (H2NP3), thus releasing neopterin. H2NP3 is further metabolized into 6-pyruvoyltetrahydropterin (6-PTP) by 6-pyruvoyltetrahydropterin synthase (PTPS), the second critical enzyme for BH4 build up. 6-PTP is used to form BH4 through sepiapterin (SPT) by a two step enzymatic pathway of aldose reductase (AR) and sepiapterin reductase (SR). BH4 is the basic cofactor of tyrosine hydroxylase (TH) and tryptophan hydroxylase (TPH) and once it links with this enzyme it is then released as tetrahydrobiopterin-4a-carbinolamine. Tetrahydrobiopterin-4a-carbinolamine is used to recycle BH4 through the biosynthesis of quinonoid-dihydrobiopterin (qBH2) by pterin-4a-carbinolamin dehydratase (PCD) and dihydropteridin reductase (DHPR), which is also linked to folate metabolism via methylenetetrahydrofolate reductase (MTHFR) through an incompletely defined mechanism. This two step enzymatic regeneration (through PCD and DHPR) can be partly enzymatic partly not. BH4 is also related to nitric oxide (NO) metabolism through arginine, cytrulline and nicotinamide adenine dinucleotide phosphate (NADPH). This finding may explain the effect of BH4 in vasogenic control. The catabolism of the monoamine is then carried out by a two step pathway involving monoamine oxidase (MAO) and cathecol-O-methyltransferase (COMT) and major metabolites are represented by homovanillic acid (HVA), 5-hydroxyindolacetic acid (5-HIAA) and 3-methoxy-4-hydroxyphenylglycol (MHPG) and also vanillylmandelic acid (VMA). The three major end products are measured in the CSF reflecting overall dopaminergic, serotoninergic and noradrenergic activity. The process of monoamine neurotransmission requires, in summary, the biosynthesis of monoamines in the nerve terminal, their upload in the synaptic vesicles through the vesicular monoamine transporter (VMAT2) with the subsequent excytotic release, action at specific receptors in the postsynaptic interface and the termination of the effect either by degradation or by reuptake by dopamine transporter (DAT).
Table 1.
Acquired and genetic neurological diseases with abnormal biogenic amine values (data from literature and cases presentation)
| Low HVA and low 5-HIAA |
| epileptic encephalopathies (SCN2A, SCN8A), Rett syndrome (FOXG1), organic acidurias (ACSF3), disorders of cholesterol synthesis (Smith-Lemli-Opitz Syndrome), brain tumours, leukemia (del6q21), perinatal hypoxia/ischaemia, preterm haemorrhagic injuries, thiamine metabolism disorders (SLC19A3), oligosaccharidoses, diseases of copper metabolism (Occipital Horn Syndrome), lysosomal disorders (Niemann-Pick type C), Lesch-Nyhan syndrome, pontocerebellar hypoplasia type 2, Steinert disease, stroke, dysautonomia, leukodystrophies, eye disorders (PITX3), acute necrotizing encephalopathy (RanBP2), intellectual disability (CASK), mitochondrial diseases (SDH), lysinuric protein intolerance (SLC7A2) |
| Low or normal HVA and normal 5-HIAA |
| Aicardi-Goutiéres syndrome (ADAR1, RNASEH2A, RNASEH2B), pontocerebellar hypoplasia (i.e. EXOSC3), seizure/epileptic encephalopathies (KCNQ2), chromosomal abnormalities (dup17p13.3), organic acidurias (ACSF3), alternating hemiplegia (ATP1A3), folate metabolism disorders (FOLR1, MTHFR, MTHFD1), mitochondrial diseases (NFU1, POLG, KSS, congenital myopathies (MTM1),, meningitis/encephalitis, malformative syndromes, astrocytoma |
| Normal HVA and low 5-HIAA |
| Hartnup disease (SLC6A19), chromosomal abnormalities (del8p23, tris12p23), post vaccine |
| High HVA high 5-HIAA |
| mitochondrial diseases (POLG) |
In this review, we provide an overview of primary neurotransmitter diseases (PNDs) by cerebrospinal fluid (CSF) investigations. Sample management, analytical methodology, and diagnostic interpretation are described.
CLINICAL AND BIOCHEMICAL FEATURES OF MONOAMINE NEUROTRANSMITTER DISEASES
In all monoamine metabolic disorders, the clinical symptoms are strictly related to the effects of dopamine and serotonin deficiency. Signs of dopamine deficiency include Parkinsonism, dystonia, chorea, oculogyric crisis, ptosis, hypersalivation, and myoclonic epilepsy. The manifestations of serotonin deficiency are less well defined, and include temperature instability, sweating, aggressive behaviour, irritability and sleeping disturbance. Non-specific symptoms include epileptic encephalopathy, mental retardation, microcephaly, swallowing difficulties, and pyramidal tract features mimicking cerebral palsy. A potential defect in biogenic amine should be considered in an infant presenting with any of the above symptoms, which may appear in isolation or together (8). Furthermore clinical monitoring of these patients could be challenging. Firstly, symptoms can fluctuate according to last medication with on-off effects. Secondly, high doses of L-Dopa inhibit postsynaptic receptors, resulting in symptoms (e.g., involuntary movements, dyskinesia, irritability, insomnia and opisthotonus) that are indistinguishable from the one resulting from under-treatment and/or dopamine deficiency.
Defect of tetrahydrobiopterin (BH4) with consequent dopamine and serotonin deficiency are associated with an increased plasma phenylalanine (Phe) concentration (8). BH4 acts as a cofactor or co-substrate in a range of biochemical reactions including the hydroxylation of aromatic amino acids (Phe, tyrosine and tryptophan) by the corresponding hydroxylases. De novo biosynthesis of BH4 from GTP requires 3 enzymes (GTPCH, PTPS and SR), while dihydropteridine reductase (DHPR) regenerates BH4 from q-dihydrobiopterin (qBH2). Overall BH4 synthesis, as such, is thus complex, and not completely understood as yet. In autosomal recessive and autosomal dominant GTP cyclohydrolase 1 (GTPCH1), 6-pyruvoyltetrahydropterin synthase (PTPS), and DHPR, the measurement of blood and CSF pterins facilitates the diagnosis. Noteworthy, all disorders that present hyperphenylalaninemia are diagnosed by neonatal screening.
If all metabolic investigations are normal in plasma and urine and the patient is suggestive for a possible PNDs, a CSF puncture should be performed for determination of HVA and 5HIAA (9). These metabolites allow the diagnosis of the following defects: tyrosine hydroxylase (TH), aromatic L-aminoacid decarboxylase(AADC), sepiapterine reductase (SR), dopamine transport deficiency including 3-hydroxylase deficiencies(DβH) and vesicular monoamine transporters defects (VMAT2).
MONOAMINE NEUROTRANSMITTER ANALYSIS: KEY FEATURES
Analysis of the CSF neurotransmitter metabolites includes the measurement of homovanillic acid (HVA), 5-hydroxyindolacetic acid (5-HIAA) and their ratio. The concentrations of 3-O-methyldopa (3-OMD), L-3,4-dihydroxyphenylalanine (DOPA), 3,4-dihydroxyphenylacetic acid (DOPA-C), 5-hydroxytryptophan (5-HTP), and 3-methoxy-4-hydroxyphenylglycol (MHPG) may be highly variable due to possible drug interactions.
SAMPLE COLLECTION AND STORAGE OF CSF
The measurement of biogenic amines can be misleading and hard to interpret if sample collection and handling do not follow strict procedures (10). There are several factors that can affect metabolite concentration, some playing a minor effect that are difficult to monitor, while others require careful and detailed instructions to minimize their impact. In should be mentioned that there is a rostrocaudal concentration gradient of monoamines in CSF. Therefore, CSF withdrawal should always be performed at the same spinal level (usually from a lumbar spinal tap). The first 0.5 mL aliquot is used for biogenic amine metabolite; the second can be stored at -70°C for further future analysis; the third 1 mL is either immediately frozen with dry ice at the bedside or filled with an antioxidant mixture ensuring BH4 stability to measure pterins. The fourth 1 mL aliquot is used as a back-up sample. Finally, as blood contamination of CSF can cause a rapid metabolite oxidation (degradation of HVA and 5HIAA), the contaminated samples must be rapidly centrifuged and the supernatant transferred in new tubes before freezing. Therefore red blood cell count and proteins should always be measured, to exclude blood-brain barrier damage, and found normal for proper interpretation.
ANALYTICAL METHODS
The biogenic amine metabolites are tested using several different techniques including capillary electrophoresis (CE), GC-MS, HPLC with electrochemical (EC) detection or fluorescent detection (FD) and liquid chromatography coupled with tandem mass spectrometry (LC-MS/MS) (11-17).
HPLC with Electrochemical detection (HPLC-EC)
HPLC-EC has been considered the gold standard for biogenic amine metabolite analysis because of its relatively low costs, and technical feasibility and simplicity, demonstrated in many procedures in which CSF was directly injected into the HPLC system without prior derivatization of the sample.
Chromatographic separation of analytes is in general performed by reverse-phase column in an isocratic condition. The elution order of biogenic amines is in order of increasing hydrophobicity. Acidic pH and ion pairing modifiers are commonly used in the mobile phase in order to suppress the positive charge of the catechol ring and optimize interaction with the stationary phase. Adapting the percentage of organic solvent (acetonitrile or methanol) retention times can be changed to obtain an accurate separation of analytes from other compound contents in CSF that can affect an accurate measurement. This is important especially for MPHG, DOPAC and DOPA that, as compare to HVA or 5-HIAA, are present in small amounts.
Since then, HPLC with electrochemical and/or fluorescence analysis has represented the gold standard in biogenic amine metabolite analysis and in defining biogenic amine metabolites age-related reference values. The fluorimetric method allows rapid sample preparation and the simultaneous determination of up to 7 biogenic amine metabolites in CSF. HPLC has also been largely tested in series of biological samples proving to be highly sensitive despite different concentration levels of biogenic amine metabolites in CSF. Nevertheless some limitations occur concerning the inability to pinpoint the interference of closely eluting metabolites regardless of the use of ion-pairing reagents to improve retention.
Liquid chromatography tandem mass spectrometry (LC-MS/MS)
In the past ten years the application of LC-MS/MS has increased in routine clinical chemistry and inborn error of metabolism diagnostic laboratories. Detection by LC-MS/MS is based on structural characterization of small molecules. The application of this technique to biogenic amines overcomes the requirement of a highly resolved column separation necessary for other types of detectors (i.e. EC). Several LC-MS/MS methods have been recently introduced and standardized for the analysis of biogenic amine metabolites in bodily fluids (11-17). The high sensitivity acquired through positive ESI LC-MS/MS overcome the need of derivatization while improved separation and chromatographic resolution permit unequivocal identification of closely related compounds in a short time run, which is still a limit in HPLC analysis. The possibility to quantitate BH4, BH2, sepiapterin and neopterin simultaneously and the ability to detect conjugated neurotransmitters (such as glucuronides and sulfates) represents on one hand a clear advantage in differentiating pterin defects (most of all for DHPR and SR deficiency) and on the other hand the ability to speculate the role of conjugates in monoamine metabolism and brain neurotransmission.
QUALITY CONTROL
Since 2016, an established External Quality Control (EQA) scheme by ERNDIM is available that includes HVA, 5-HIAA, 3OMD and 5-HTP (http://www.erndim.org/home/start.asp). The scheme evaluates the quantitative performance and assesses the ability of laboratories to diagnose inborn errors of neurotransmitter metabolism. The scheme consists of 8 spiked samples, paired two by two, of lyophilised pooled CSF (4 samples) and lyophilised artificial CSF (4 samples). Accuracy, recovery, precision, linearity and inter-laboratory CV are evaluated by ERNDIM. A high degree of harmonization between laboratories is important for the use of common reference values.
During the two-year pilot study period (2014-2015), a general agreement was observed in 5HIAA and HVA concentrations while a significant discrepancy was detected concerning 3OMD and 5HTP between different laboratories. Overall there was improvement of laboratory performance during these two years.
For internal quality control, commercial quality control samples are not available, but they can be easily prepared in-house. Aliquots of pooled “positive controls” CSF or spiked CSF with biogenic amine standards can be stored at -70°C and used to monitor the performance of the method (19).
AGE-RELATED REFERENCE RANGE
Despite precise internal collecting protocols, the recent increase in CSF biogenic amines measurement for the diagnosis of neurologically compromised children has resulted in challenging monoamine profiles difficult to interpret. As such, a critical role in the reliability of CSF biogenic amines interpretation is represented by the issue of reference values that can help to recognize misleading alterations or milder/moderate forms of deviations outside primary biogenic amine defects. Age-related reference ranges are important for differential diagnosis, management and monitoring (see Table 2) (20).
Table 2.
CSF HVA and 5HIAA age specific reference ranges. Reference ranges are established by our laboratory based on data from 100 individuals from our geographical area
| Age | HVA (nmol/L) | 5HIAA (nmol/L) |
|---|---|---|
| 0-30 d | 601-1397 | 382-949 |
| 1 m – 5 m | 345-1111 | 206-922 |
| 6 m – 1 yr | 302-797 | 120-345 |
| 2 yrs – 4 yrs | 242-684 | 95-329 |
| 5 yrs – 10 yrs | 130-573 | 80-183 |
| 11 yrs – 16 yrs | 122-515 | 68-187 |
| > 16 yrs | 111-371 | 55-163 |
Biogenic amines have been tested, to build up reference range values, both in CSF of healthy neonates and infants, and in mixed samples of healthy infants and infants with medical complications, and, most recently, in series of patients with several neurologic diseases. Metabolite concentration shows a decrement with age over the first few years, with peak values found during the first three months and with concentrations reaching a sort of plateau around five years of age. No correlation has been found between length of the child and age, i.e., for craniocaudal gradient (20). In adults a seasonal variation has been anecdotally described, but never confirmed in children. Reference intervals are critical. The available reference intervals in the literature are quite similar, but the differences may be related to the age of patients. This reinforces the concept that establishment of reference intervals specific to laboratories that perform neurotransmitter analysis is mandatory (18).
DIAGNOSTIC PATTERNS OF CSF MONOAMINE NEUROTRANSMITTER DISEASES
Table 3a summarizes the biochemical features of each monoamine neurotransmitter disorder with and without plasma hyperPhe. The biochemical classification of the disease is based on plasma phenylalanine as reported in the introduction section. In GTPCH1, DHPR and PTPS the determination of HVA and 5HIAA are reduced and pterin is abnormal. In SR, CSF analysis reveals decreased levels of HVA and 5-HIAA, normal to slightly increased neopterin, and elevated total biopterin, dihydrobiopterin (BH2) and sepiapterin. Minimal abnormalities in random collections of plasma or urinary pterins are occasionally found but are not consistent. Oral phenylalanine-loading test (100 mg/kg) demonstrates abnormal and prolonged increase of phenylalanine levels due to impaired phenylalanine hydroxylation under loading conditions.
Table 3a.
Disorders of pterin metabolism with and without hyperphenylalaninemia
| HVA | 5-HIAA | 3-OMD | 5-HTP | MHPG | MTHF | BH4 | BH2 | Neo | Sep | Prim (U) | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| with hyperphenylalaninemia | |||||||||||
| AR-GTPCH1 |  |
 |
↓ |  |
|||||||
| PTPS |  |
 |
↓ | ↓ | n/↓ |  |
|||||
| PTPS mild | ↓ |  |
|||||||||
| DHPR | ↓ | ↓ |  |
n↓ |  |
||||||
| PCD | ↓ | ↑ |  |
||||||||
| with hyperphenylalaninemia | |||||||||||
| AD-GTPCH1* | n/↓ | n/↓ | ↓ |  |
|||||||
| compound heterozygotes AR-GTPCH1 |  |
 |
↓ |  |
|||||||
| SR | ↓ | ↓ | n/↓ | ↑ |  |
||||||
AD-GTPCH1: autosomal dominant guanosin triphosphate cyclohydrolase 1; AR-GTPCH1: autosomal recessive guanosin triphosphate cyclohydrolase 1; PTPS: 6-pyruvoyltetrahydropterin synthase; SR: sepiapterin reductase; PCD: pterin-4a-carbinolamin dehydratase; DHPR: dihydropteridin reductase; HVA: homovanillic acid; 5-HIAA: 5-hydroxyindolacetic acid; 3-OMD: 3 orthomethyldihydroxyphenylalanine; 5-http: 5-hydroxytryptophan; MHPG: 3-methoxy-4-hydroxyphenylglycol; BH4: tetrahydrobiopterin; BH2: dihydrobiopterin; Neo: neopterin; Sep: sepiapterin; Prim: primapterin; n: normal.
Note: Empty cells should be considered as normal values.
*AD-GTPCH1 present compromised Phe catabolism at Phe oral loading test.
Figure 2 shows the CSF neurotransmitter chromatograms.
Figure 2.
Example chromatograms
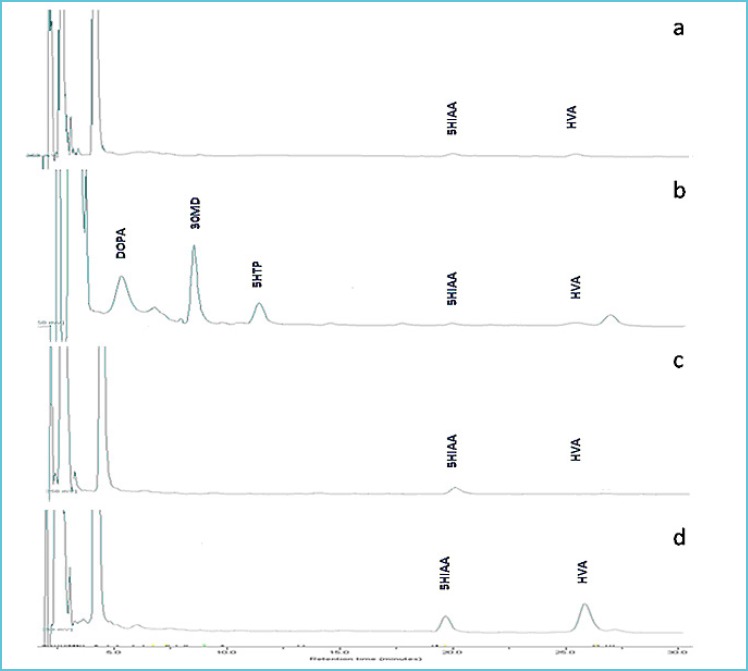
Patient with GTPCH1 deficiency; note the decrease in concentration of HVA and 5HIAA
Patient with AADC deficiency; note the decrease in concentration of HVA and 5HIAA and the elevations of DOPA, 3OMD and 5HTP
Patient with TH deficiency; note the decrease in concentration of HVA and the normal 5HIAA
Healthy control with normal concentrations of 5HIAA and HVA. Chromatographic conditions: 50 μl CSF diluted with 50 μl of mobile phase was injected onto a 250 x 4.6 mm, 5 μm Spherisorb ODS1 column (Waters). Compounds were eluted under isocratic conditions at flow rate of 1.0 mL/min and mobile phase consisting of Acetate buffer (pH 3.5) containing sodium 1-eptasuphonate, EDTA and 10% methanol. Compounds were detected using a ESA Coularray 5600 electrochemical detector.
Table 3b includes monoamine neurotransmitter disorder due to enzyme deficiencies (TH, AADC) and defects of monoamine transport recently reported (Dopamine transporter deficiency syndrome, DTDS, and vesicular monoamine transporter 2 deficiency, VMAT2).
Table 3b.
Disorders of biogenic amine biosynthesis and transport
| HVA | 5-HIAA | HVA/5-HIAA | 3-OMD | 5-HTP | MHPG | |
|---|---|---|---|---|---|---|
| TH |  |
 |
↓ | |||
| TPH |  |
↑ | ↓ | |||
| AADC |  |
 |
 |
↑ | ↓ | |
| VMAT2 | ||||||
| DAT |  |
↑ |
TH: tyrosine hydroxylase; TPH: Tryptophan hydroxylase; AADC: aromatic L-aminoacid decarboxylase;
VMAT2: vesicular monoamine transporter; DAT: dopamine transporter; HVA: homovanillic acid;
5-HIAA: 5-hydroxyindolacetic acid; 3-OMD: 3 orthomethyldihydroxyphenylalanine; 5-http: 5-hydroxytryptophan;
MHPG: 3-methoxy-4-hydroxyphenylglycol; BH4: tetrahydrobiopterin; BH2: dihydrobiopterin; Neo: neopterin; Sep: sepiapterin; Prim: primapterin; n: normal.
Note: Empty cells should be considered as normal values
DTDS is associated with an increase of HVA while 5HIAA is normal leading to a raised HVA:5HIAA ratio. In VMAT2 both HVA and 5HIAA are normal.
TH affected patients have very low concentrations of HVA in CSF, and the HVA/5HIAA ratio is the most sensitive marker. In AADC deficiency, levels of HVA and 5HIAA are both low, but 3-OMD is highly increased.
CONCLUSIONS
The utility of CSF analyses for the investigation of monoamine metabolism abnormalities has been proposed in patients with progressive extrapyramidal movement disorders, especially parkinsonism-dystonia, chorea or mixed movement disorders combined with truncal hypotonia or in patients presenting epileptic encephalopathies of unknown origin. With the analysis of CSF, it is possible to appreciate the global functioning of monoaminergic neurotransmission. Homovanillic acid is a stable end product of dopamine and can be used as a marker of dopamine metabolism, whereas 5-hydroxyindoleacetic acid is a stable end product of serotonin and is a marker of serotonin turnover. Therefore, their quantification allows for identification of either defects of neurotransmitter biosynthesis (e.g. TH or AADC deficiencies) or defects of tetrahydrobiopterin biosythesis and regeneration (e.g. GTPCH1, PTPS, SR and DHPR deficiencies). However, abnormalities of biogenic amines are not only observed in PNDs but were recently reported in several acquired and genetic neurological diseases (see Table 1). Furthermore, HVA and 5-HIAA measurement can also be influenced by drugs that are frequently used in neurological patients presenting hypokinetic movement disorders and impaired mood associated with dopaminergic/serotoninergic dysfunction. Therefore a complete documentation is mandatory. The clinical history, including neurological examination, should enclose all special features such as any deviation from the protocol, sample color, all medications and current clinical conditions such as fever. In fact, monoamine profile can be incorrectly interpreted due to the lack of information about pharmacological concomitant therapy that is not fulfilled in patient information schedules and the referring center is not completely aware about its effect on metabolite measurements. It is to be considered that, besides common drugs that acts upon dopamine and serotonin pathways, several others have been reported to possibly present biasing effects (see Table 4 for a detailed list of drugs that can impair monoamine CSF values). In the view of this, a drug-free wash out period is recommended for at least 1 week and up to 4 weeks before executing the lumbar puncture (21).
Table 4.
Drugs that may interfere with monoamine measurement
| Metabolites precursors or monoamines |
| 3,4-dihydroxyphanylalanine (L-DOPA)/carbidopa, 5-hydroxytryptophan (5-HTP), dopamine |
| Monoamine oxydase (MAO) inhibitors |
| hydrazines, nialamide, isocarboxazid, bifemelane, pirlindole, toloxatone, rasagiline, selegiline, moclobemide, tranylcypromine, phenelzine, safinamide |
| Cathecol-O-methyltransferase (COMT) inhibitors |
| entacapone, tolcapone |
| Reuptake inhibitors |
| fluoxetine, citalopram, fluvoxamine, paroxetine, sertraline, venlafaxine, amantadine |
| Others |
| diazepam, chlorpromazine, pyridoxine, pyridoxal-5-phosphate, sapropterin, physostigmine, propranolol, phenotiazine, valproate, vigabatrin, 4-hydroxybutirrate, anticholinergic drugs |
In some cases, drug interference is based on experimental models or mechanism of drug action.
No data are available on dopamine agonist drugs.
NT levels are age dependent with particular attention to the neonatal period. Preterm neurologically compromised neonates represent, in this sense, the most striking example of the difficulties of results interpretation. In this case, samples refer more frequently to severely affected patients. Patients sometimes present hypoxic/ischaemic encephalopathies or anoxia that can be a presenting symptom of several primary neurotransmitter disorders, but monoamine measurement in CSF can be impaired by brain atrophy itself. Furthermore, preterm neonates can be hemodynamically unstable thus requiring inotrope and vasopressor support, which is frequently obtained using dopamine administration. The diagnostic process is further complicated by the fact that a marked and non-linear age dependency as well as a craniocaudal gradient of the CSF concentrations must be taken into account.
Nevertheless, laboratories have different age-related reference ranges requiring variations in the technique of CSF sampling and the precise aliquots used for analysis. As it is for other biochemical parameters, internal age-related reference ranges are thus recommended in laboratories that perform CSF monoamine metabolite measurements.
There may also be diurnal or catamenial changes, which are probably subtle (except in patients with Segawa syndrome) and not totally investigated.
Regardless of the growing interest in PNDs, the incidence of this cluster of disorders remains uncertain, with controversial results even among patients presenting with the most typical features of the disorders (i.e. movement disorders and/or epilepsy). This finding raises the question about the possibility of unrecognized patients presenting parkinsonian-like features even in adulthood. CSF neurotransmitter analysis, one of the few available direct measurements of brain neurotransmission, can help us to better understand brain pathophysiology and its potential connection with functional neuroimaging techniques.
In conclusion, a close collaboration between laboratory experts and clinicians is mandatory for the interpretation of CSF neurotransmitters levels.
Abbreviations
- CSF:
cerebrospinal fluid
- PNDs:
primary neurotransmitters diseases
- Phe:
phenylalanine
- AD-GTPCH1:
autosomal dominant guanosin triphosphate cyclohydrolase 1
- AR-GTPCH1:
autosomal recessive guanosin triphosphate cyclohydrolase 1
- PTPS:
6-pyruvoyltetrahydropterin synthase
- SR:
sepiapterin reductase
- PCD:
pterin-4a-carbinolamin dehydratase
- DHPR:
dihydropteridin reductase
- TH:
tyrosine hydroxylase
- TPH:
Tryptophan hydroxylase
- AADC:
aromatic L-aminoacid decarboxylase
- MAO:
monoamine oxidase
- DβH:
dopamine beta hydroxylase
- VMAT2:
vesicular monoamine transporter
- DAT:
dopamine transporter
- HVA:
homovanillic acid
- 5-HIAA:
5-hydroxyindolacetic acid
- 3-OMD:
3 orthomethyldihydroxyphenylalanine
- 5-HTP:
5-hydroxytryptophan
- MHPG:
3-methoxy-4-hydroxyphenylglycol
- BH4:
tetrahydrobiopterin
- BH2:
dihydrobiopterin
- Neo:
neopterin
- Sep:
sepiapterin
- Prim:
primapterin
REFERENCES
- 1.Hyland K. (1999) Presentation, diagnosis, and treatment of the disorders of monoamine neurotransmitter metabolism, Sem Perinatol 23,2:194-203. [DOI] [PubMed] [Google Scholar]
- 2.Hyland K. (2008) Clinical utility of monoamine neurotransmitter metabolite analysis in cerebrospinal fluid, Clin Chem 54(4):633-641. [DOI] [PubMed] [Google Scholar]
- 3.Ng J, Papandreou A, Heales SJ, Kurian MA. (2015) Monoamine neurotransmitter disorders – clinical advances and future perspectives, Nat Rev Neurol 11(10):567-584 [DOI] [PubMed] [Google Scholar]
- 4.Ormazabal A, García-Cazorla A, Fernández Y, Fernández-Álvarez E, Campistol J, Artuch R. (2005) HPLC with electrochemical and fluorescence detection procedures for the diagnosis of inborn errors of biogenic amines and pterins, J Neurosci Method 142:153-158. [DOI] [PubMed] [Google Scholar]
- 5.García-Cazorla A, Serrano M, Pérez-Dueñas B, González V, Ormazábal A, Pineda M, Fernándz-Alvarez E, Campistol JMD, Artuch RMD. (2007) Secondary abnormalities of neurotransmitters in infants with neurological disorders, Dev Med Child Neurol 49:740-744 [DOI] [PubMed] [Google Scholar]
- 6.Marín-Valencia I, Serrano M, Ormazabal A, Pérez-Dueñas B, García-Cazorla A, Campistol J, Artuch R. (2008) Biochemical diagnosis of dopaminergic disturbances in paediatric patients: analysis of cerebrospinal fluid homovanillic acid and other biogenic amines. Clin Biochem. 41(16-17):1306-1315. [DOI] [PubMed] [Google Scholar]
- 7.Ng J, Heales SJR, Kurian MA. (2014) Clinical features and pharmacotherapy of childhood monoamine neurotransmitter disorders, Ped Drugs 16:275-291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burlina A, Blau N, (2014) Tetrahydrobiopterin disorders presenting with hyperphenylalaninemia, Congenital Neurotransmitter disorders: a Clinical approach. Hoffman G, Blau N, eds Nova. [Google Scholar]
- 9.Rodan LH, Gibson KM, Pearl PL. (2015) Clinical use of CSF neurotransmitters, Ped Neurol 53(4):277-286. [DOI] [PubMed] [Google Scholar]
- 10.Hyland K. (2003) The lumbar puncture for diagnosis of pediatric neurotransmitter diseases, Ann Neurol 54 Suppl 6):S13-S17. [DOI] [PubMed] [Google Scholar]
- 11.Bertilsson L, Asberg M. (1984) Amine metabolites in the cerebrospinal fluid as a measure of central neurotransmitter function: methodological aspects, Adv Biochem Psychopharmacol 39:27-34. [PubMed] [Google Scholar]
- 12.Manini P, Andreoli R, Cavazzini S, Bergamaschi E, Mutti A, Niessen WMA. (2000) Liquid chromatography-electrospray tandem mass spectrometry of acidic monoamine metabolites, J Chromatograph 744(2):423-431. [DOI] [PubMed] [Google Scholar]
- 13.Bourcier S, Benoist J.-F, Clerc F, Rigal O, Taghi M, Hoppilliard Y. (2006), Detection of 28 neurotransmitters and related compounds in biological fluids by liquid chromatography/tandem mass spectrometry. Rapid Commun. Mass Spectrom, 20:1405-1421. [DOI] [PubMed] [Google Scholar]
- 14.Ormazabal A, García-Cazorla A, Fernández Y, Fernández-Álvarez E, Campistol J, Artuch R. (2005) HPLC with electrochemical and fluorescent detection procedure for the diagnosis of inborn erros of biogenic amines and pterins. J Neurosci Methods, 142:153-158. [DOI] [PubMed] [Google Scholar]
- 15.Cox JM, Butler JP, Lutzke BS, Jones BA, Buckholz JE, Raymond Biondolillo R, Jayne A, Talbot JA, Eyassu Chernet E, Svensson KA, Ackermann BL. (2015) A validated LC–MS/MS method for neurotransmitter metabolite analysis in human cerebrospinal fluid using benzoyl chloride derivatization, Bioanalysis, 7, 19, 2461. [DOI] [PubMed] [Google Scholar]
- 16.Arning E, Bottiglieri T. (2014) LC-MS/MS analysis of cerebrospinal fluid metabolites in the pterin biosynthetic pathway. JIMD Rep, 29:1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Suominen T, Uutela P, Ketola RA, Bergquist J, Hillered L, Finel M, Zhang H, Laakso A, Kostiainen R. (2013) Determination of serotonin and dopamine metabolites in human brain microdialysis and cerebrospinal fluid samples by UPLC-MS/MS: discovery of intact glucuronide and sulfate conjugates. PLoS One, 8(6):e68007. doi:10.1371/journal.pone.0068007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hyland Surtees AH, Heales SJR, Bowron A, Howells DW, Smith I. (1993) Cerebrospinal fluid concentrations of pterins and metabolites of serotonin and dopamine in a pediatric reference population, Ped Res 34(1):10-14. [DOI] [PubMed] [Google Scholar]
- 19.Bräutigam C, Weykamp C, Hoffmann GF, Wevers RA. (2002) Neurotransmitters metabolites in CSF: an external quality control scheme, J Inherit Metab Dis 25:287-298. [DOI] [PubMed] [Google Scholar]
- 20.Chotai J, Murphy DL, Costantino JN. (2006) Cerebrospinal fluid monoamine metabolite levels in human newborn infants born in winter differ from those born in summer, Psychiatry Res 145:189-197. [DOI] [PubMed] [Google Scholar]
- 21.King DJ, Cooper SJ, Liddle J. (1983) The effect of propanolol on CSF amine metabolites in psychiatric patients. Br J Clin Pharmacol 15:331-337. [DOI] [PMC free article] [PubMed] [Google Scholar]


