Abstract
Rationale: The predominant cause of chronic lung allograft failure is small airway obstruction arising from bronchiolitis obliterans. However, clinical methodologies for evaluating presence and degree of small airway disease are lacking.
Objectives: To determine if parametric response mapping (PRM), a novel computed tomography voxel-wise methodology, can offer insight into chronic allograft failure phenotypes and provide prognostic information following spirometric decline.
Methods: PRM-based computed tomography metrics quantifying functional small airways disease (PRMfSAD) and parenchymal disease (PRMPD) were compared between bilateral lung transplant recipients with irreversible spirometric decline and control subjects matched by time post-transplant (n = 22). PRMfSAD at spirometric decline was evaluated as a prognostic marker for mortality in a cohort study via multivariable restricted mean models (n = 52).
Measurements and Main Results: Patients presenting with an isolated decline in FEV1 (FEV1 First) had significantly higher PRMfSAD than control subjects (28% vs. 15%; P = 0.005), whereas patients with concurrent decline in FEV1 and FVC had significantly higher PRMPD than control subjects (39% vs. 20%; P = 0.02). Over 8.3 years of follow-up, FEV1 First patients with PRMfSAD greater than or equal to 30% at spirometric decline lived on average 2.6 years less than those with PRMfSAD less than 30% (P = 0.004). In this group, PRMfSAD greater than or equal to 30% was the strongest predictor of survival in a multivariable model including bronchiolitis obliterans syndrome grade and baseline FEV1% predicted (P = 0.04).
Conclusions: PRM is a novel imaging tool for lung transplant recipients presenting with spirometric decline. Quantifying underlying small airway obstruction via PRMfSAD helps further stratify the risk of death in patients with diverse spirometric decline patterns.
Keywords: bronchiolitis obliterans syndrome, chronic lung allograft dysfunction, parametric response mapping, computed tomography of the chest, prognosis
At a Glance Commentary
Scientific Knowledge on the Subject
Bronchiolitis obliterans syndrome is diagnosed by spirometry, and clinical tools are lacking to identify the degree of small airway obstruction and gauge prognosis for individual patients. Recent data suggest that lung allograft rejection has multiple pathophysiologic origins with different clinical phenotypes.
What This Study Adds to the Field
Quantification of functional small airways disease by parametric response mapping, a novel computed tomography–based biomarker, in lung transplant recipients with spirometric decline offers pathophysiologic information of chronic rejection phenotype. Functional small airways disease levels greater than or equal to 30% as measured by parametric response mapping identify patients with significantly shorter survival.
Chronic allograft failure, the major cause of poor long-term survival in patients undergoing lung transplantation, presents predominantly as obstructive airflow limitation by spirometry, termed bronchiolitis obliterans syndrome (BOS). Functional small airways disease (fSAD) secondary to fibrotic obliteration or bronchiolitis obliterans (BO) is the primary driver of this clinical spirometric decline (1). However, there is growing awareness that chronic lung allograft dysfunction is a heterogeneous disorder with pathologic processes including BO and interstitial or parenchymal fibrosis in varying combinations and severity within an individual (2–4). Diverse physiologic decline patterns have been shown to provide prognostic information; isolated decline in FEV1 as compared with concurrent FEV1 and FVC fall on spirometry is associated with superior survival suggesting that patients with fSAD versus parenchymal disease have heterogeneous outcomes (5, 6). In patients with BOS, the degree of airflow obstruction by spirometry is also linked to survival (7, 8). Hence, a clinical methodology to quantitate fSAD and to differentiate underlying phenotypes would have significant impact on care of lung transplant recipients.
Small airways, the predominant histologic target of fibrotic remodeling in a chronically rejecting lung allograft, have been termed the “silent zone” of the lung secondary to difficulty in evaluating small airway pathology (9). Transbronchial biopsies fail to adequately sample small airways and have limited sensitivity in diagnosing BO in lung transplant recipients (10, 11). Similarly, routine radiographic imaging techniques cannot easily visualize small airways, although some direct and indirect signs of bronchiolar disease have been proposed on high-resolution computed tomography (HRCT) chest imaging. These findings, including air trapping and bronchiectasis, have limited sensitivity and specificity for a diagnosis of BOS and provide no prognostic information (12–14).
Parametric response mapping (PRM) is a voxel-based methodology for image analysis that has been demonstrated to be effective in improving the diagnostic ability of radiographic methodologies, such as magnetic resonance imaging and CT scans in various organs and disease states (15–19). In the lung, comparison of inspiratory and expiratory images on a voxel-by-voxel basis by image coregistration has been shown to provide a quantitative measure of fSAD in patients with chronic obstructive pulmonary disease (16, 20–22). This unique ability to quantitate fSAD on CT chest images suggests that PRM can potentially be used as an imaging biomarker for evaluation of BO in lung allografts. In this study, we investigate the PRM signature of lung transplant recipients with spirometric decline and demonstrate the utility of this methodology to evaluate disease phenotype and survival.
Methods
Patient Population
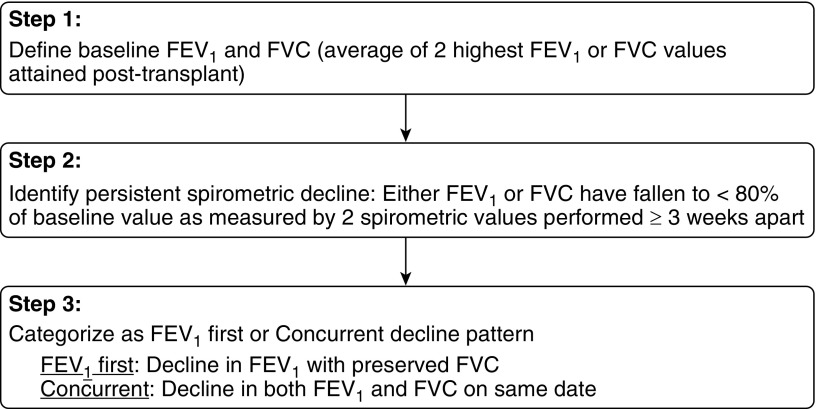
Bilateral lung transplant recipients at the University of Michigan Health System between 1991 and 2014 were included in the study group. Many patients included in our previous analysis of spirometric function following lung transplantation (6) were also included in this study, now with a longer follow-up period. Patients were managed in line with a standard clinical algorithm (23). Baseline FEV1 was defined according to International Society for Heart and Lung Transplantation (ISHLT) guidelines as the average of the two highest FEV1 values obtained greater than or equal to 3 weeks apart post-transplant (24). FVC baseline was similarly defined as the average of the two highest FVC values obtained greater than or equal to 3 weeks apart post-transplant (6). Persistent spirometric decline was defined as a decline in FEV1 and/or FVC to less than 80% of the respective post-transplant baseline as measured by two consecutive FEV1 and/or FVC values greater than or equal to 3 weeks apart. Patients were assigned to the two spirometric decline pattern groups, FEV1 First or Concurrent, as previously described (6). Briefly, FEV1 First was defined as a persistent fall in FEV1 with a preserved FVC at the time of spirometric decline. Concurrent decline pattern was defined as a fall in both FEV1 and FVC to less than 80% of the respective post-transplant baselines on the same date (6). A simple algorithm outlining the criteria for development of persistent spirometric decline and its various patterns is provided in Figure 1. BOS grade was defined per ISHLT guidelines (25). Time of death was assessed up to October 21, 2015; all other data were censored.
Figure 1.
An algorithm outlining the definitions of baseline spirometric function, persistent spirometric decline, and categorization of decline type.
Computed Tomography
CT data were obtained as whole-lung volumetric CT scans at full inspiration (total lung capacity) and incremental scans at relaxed expiration (functional residual capacity) on General Electric 64-detector scanners (General Electric, Boston, MA) and reconstructed using a bone reconstruction kernel. Slice thicknesses were 1.25 mm for all scans, with incremental scans acquired with 1-cm gaps. All CT scans were checked for Hounsfield unit (HU) drift and if necessary corrected based on aortic blood (50 HU) and central air (−1,000 HU) as previously described (26).
Parametric Response Mapping
PRM was applied to all paired CT scans and has been previously described (17). Briefly, lungs from both paired CT scans were segmented from the thoracic cavity using an in-house algorithm written in Matlab (The MathWorks, Inc., Natick, MA). The whole-lung inspiratory CT scan was spatially aligned to the incremental expiratory CT scan using Elastix, an open source image registration algorithm (27, 28). This process allows the paired images to share the same geometric space, where each voxel, the smallest unit of volume in a three-dimensional image dataset, consists of HU values at inspiration and expiration. Each voxel was classified based on a scheme of three predetermined thresholds as previously described (17). In brief, voxels with values greater than or equal to 950 HU and less than −810 HU at inspiration and greater than or equal to −856 HU at expiration were classified normal (PRMNormal, green voxels), greater than or equal to 950 HU and less than −810 HU at inspiration and less than −856 at expiration were functional small airways disease (PRMfSAD, yellow voxels), and greater than or equal to −810 HU at inspiration were parenchymal disease (PRMPD, purple voxels). The relative lung volumes, calculated as the sum of all voxels within a class normalized to the sum of all voxels within the expiratory lungs multiplied by 100, were used as global measures.
Statistical Analysis
Statistical analyses were performed using R version 3.2.2 (R Foundation for Statistical Computing, Vienna, Austria). In the case-control analysis, continuous variables were compared using linear mixed models with a random effect for matched pair (29), which corresponds to a matched pair Student’s t test when case/control status is the only predictor in the model. Logistic and multinomial random effects models, with a random effect for matched pair (30), were used to compare binary and categorical predictors between matched cases and control subjects. For matched pair comparisons of PRM values, Wilcoxon signed rank tests were done (31). Multivariable linear mixed models (29), stratified by spirometric decline group of the case and including a random effect for matched pair, were used to describe associations between PRM values and case status adjusted for age, baseline FEV1% predicted, and baseline FVC % predicted.
Demographics of the retrospectively followed lung transplant recipients with persistent FEV1 decline are reported as mean ± SD for continuous predictors or count (percent) for categorical predictors, stratified by availability of PRM data. Demographic differences between patients with versus without available PRM data were compared using chi-square (32) or Fisher exact (33) tests for categorical descriptors, as appropriate, or two-sample Student’s t tests for continuous descriptors (34). Associations between clinical risk factors for BOS occurring before spirometric decline and PRMfSAD values at spirometric decline (≥ or <30%), adjusted for time to spirometric decline, were assessed using negative binomial regression for count-oriented outcomes or logistic regression for binary outcomes (35). Survival curves were estimated using the Kaplan-Meier method (36) with two-sample comparisons conducted using both restricted mean (RM) tests (37) and log-rank tests (38–40). Multivariable censored survival regression analysis was conducted using the RM model framework (41).
Results
Spirometric Decline Patterns Are Associated with Distinct PRM Signatures
We first investigated the application of PRM to patients with spirometric decline in a case-control fashion. Cases were defined as bilateral lung transplant recipients experiencing a persistent spirometric decline (Figure 1) in the absence of obvious confounders in concordance with ISHLT guidelines (25). Cases were restricted to patients with irreversible lung function decline as defined by no improvement in FEV1 to greater than or equal to 80% of the post-transplant baseline during the entire study period (n = 22). Control subjects (CT scans obtained from patients with stable pulmonary function) were matched to cases by time from transplantation to CT scan. The average time from spirometric decline to CT scan was 4 days (range, −59 to 73 d) in the FEV1 First group and −7 (range, −49 to 37 d) in the Concurrent group.
Sixty-eight percent (15 of 22) of cases had an FEV1 First decline pattern, whereas 32% (7 of 22) had a Concurrent spirometric decline pattern. Demographics for the cases in each group and their respective control groups are shown in Table 1. FEV1 First patients were younger than their respective control subjects (39 ± 18 yr vs. 50 ± 9 yr; P = 0.04) and had a lower baseline FVC % predicted post-transplant (82 ± 14% vs. 93 ± 12%; P = 0.03). Concurrent group patients were not significantly different from their matched control subjects.
Table 1.
Demographics of FEV1 First and Concurrent Decline Groups as Compared with their Matched Control Subjects*
| FEV1 First Group (n = 15) | Control Group (n = 15) | P Value | Concurrent Group (n = 7) | Control Group (n = 7) | P Value | |
|---|---|---|---|---|---|---|
| Age at transplant, yr | 39 ± 18 | 50 ± 9 | 0.04 | 46 ± 12 | 46 ± 13 | 0.93 |
| Male | 10 (67) | 11 (73) | 0.66 | 4 (57) | 4 (57) | 1 |
| Baseline FEV1, % predicted | 90 ± 20 | 103 ± 19 | 0.08 | 93 ± 13 | 87 ± 13 | 0.41 |
| Baseline FVC, % predicted | 82 ± 14 | 93 ± 12 | 0.03 | 86 ± 14 | 82 ± 15 | 0.64 |
| LAS score | 44 ± 15 | 47 ± 16 | 0.69 | 44 ± 13 | 36 ± 4 | 0.20 |
| LAS | 1.00† | 1.00† | ||||
| Group A | 5 (33) | 2 (13) | 2 (29) | 5 (71) | ||
| Group B | 0 (0) | 0 (0) | 1 (14) | 0 (0) | ||
| Group C | 8 (53) | 2 (13) | 1 (14) | 2 (29) | ||
| Group D | 2 (13) | 11 (73) | 3 (43) | 0 (0) |
Definition of abbreviation: LAS = lung allocation score.
Continuous predictors reported as mean ± SD; categorical as n (%).
Negligible number of patients in LAS group B, so no comparisons were performed for that group. Otherwise, mixed model analysis was used for overall LAS diagnosis group comparisons for groups A, C, and D.
Quantification of fSAD (PRMfSAD, yellow) and parenchymal disease (PRMPD, purple) obtained by PRM was compared between spirometric decline groups and their respective control subjects (Figure 2). PRM measurements revealed significantly higher fSAD in the FEV1 First group as compared with stable matched control subjects (Wilcoxon signed rank test, P = 0.005). No difference was seen in PRMPD between FEV1 First cases and control subjects. In contrast, Concurrent group patients were found to have significantly more PRMPD than their control group (Wilcoxon signed rank test, P = 0.02) and as a group had PRMfSAD values comparable with those of control subjects.
Figure 2.
Bar plots demonstrating distribution of parametric response mapping (PRM) of functional small airways disease (PRMfSAD) and PRM of parenchymal disease (PRMPD) in lung transplant recipients with irreversible spirometric decline by decline group status and respective control subjects. (A) Patients presenting with isolated decline in FEV1 (FEV1 First group) had significantly more PRMfSAD compared with their matched control subjects (28 ± 3% vs. 15 ± 3%; P = 0.005). No significant difference was noted in PRMPD values (n = 15 per group). (B) Patients presenting with simultaneous decline in both FEV1 and FVC (Concurrent group) had significantly higher PRMPD compared with their matched control subjects (39 ± 6% vs. 20 ± 2%; P = 0.02). No significant difference was noted in PRMfSAD values between Concurrent patients and their matched control subjects (n = 7 per group). *P < 0.05. Data are presented as mean ± SEM.
In a model that controlled for age, baseline FEV1% predicted, and baseline FVC % predicted, FEV1 First patients continued to have significantly more PRMfSAD than their respective control group (30% vs. 18%; P = 0.03) (Table 2). Similarly, Concurrent patients had significantly more PRMPD than their respective control subjects after adjusting for these variables (34% vs. 15%; P = 0.02) (Table 3). Representative cross-sectional HRCT images and associated PRM images from a control, FEV1 First, and Concurrent patient, respectively, are shown in Figure 3.
Table 2.
Multivariate Analysis to Investigate Clinical Predictors of PRM Values in Case/Control Pairs where Case has FEV1 First Pattern of Spirometric Decline
| PRMfSAD |
PRMPD |
|||||
|---|---|---|---|---|---|---|
| Estimate* (%) | SE | P Value | Estimate* (%) | SE | P Value | |
| Intercept | 17.9 | 3.9 | <0.001 | 15.9 | 2.7 | <0.001 |
| FEV1 First group (vs. control) | 11.7 | 5.1 | 0.03 | 1.0 | 3.7 | 0.80 |
| Age (per 10-yr increase) | −0.8 | 1.8 | 0.64 | 0.5 | 1.2 | 0.72 |
| Baseline FEV1% predicted (per 10% change) | −2.2 | 2.1 | 0.32 | −0.1 | 1.5 | 0.95 |
| Baseline FVC % predicted (per 10% change) | 2.1 | 3.2 | 0.51 | −1.8 | 2.3 | 0.45 |
Definition of abbreviations: FEV1 First pattern = patients presenting with isolated decline in FEV1; fSAD = functional small airways disease; PD = parenchymal disease; PRM = parametric response mapping.
Estimate for intercept reflects average PRMfSAD or PRMPD for a control patient who is 45 years of age and has a baseline FEV1 and FVC of 95% predicted. All other estimates reflect the change in PRMfSAD and PRMPD based on unit change of the predictor.
Table 3.
Multivariate Analysis to Investigate Clinical Predictors of PRM Values in Case/Control Pairs where Case has Concurrent Pattern of Spirometric Decline
| PRMfSAD |
PRMPD |
|||||
|---|---|---|---|---|---|---|
| Estimate* (%) | SE | P Value | Estimate* (%) | SE | P Value | |
| Intercept | 22.0 | 8.3 | 0.03 | 14.7 | 6.0 | 0.04 |
| Concurrent group (vs. control) | −5.3 | 9.7 | 0.60 | 19.2 | 4.8 | 0.02 |
| Age (per 10-yr increase) | 3.2 | 4.4 | 0.48 | 3.6 | 2.6 | 0.22 |
| Baseline FEV1% predicted (per 10% change) | −1.3 | 6.2 | 0.84 | 4.7 | 4.1 | 0.27 |
| Baseline FVC % predicted (per 10% change) | 3.5 | 5.5 | 0.54 | −6.5 | 4.4 | 0.18 |
Definition of abbreviations: Concurrent pattern = patients presenting with simultaneous decline in both FEV1 and FVC; fSAD = functional small airways disease; PD = parenchymal disease; PRM = parametric response mapping.
Estimate for intercept reflects average PRMfSAD or PRMPD for a control patient who is 45 years of age and has a baseline FEV1 and FVC of 95% predicted. All other estimates reflect the change in PRMfSAD and PRMPD based on unit change of the predictor.
Figure 3.
Inspiratory and expiratory high-resolution computed tomography images with associated parametric response mapping (PRM) images from representative scans are shown for a control, FEV1 First (presenting with isolated decline in FEV1), and Concurrent (presenting with simultaneous decline in both FEV1 and FVC) patient, respectively. Normal lung tissue is shown in green, functional small airways disease is yellow, and parenchymal disease is purple. HRCT = high-resolution computed tomography.
Quantitative Assessment of fSAD by PRM Predicts Survival in Patients with Spirometric Decline
To evaluate if PRM values at the onset of spirometric decline can provide prognostic information, a retrospective analysis of the prospective cohort of 287 bilateral lung transplant recipients at the University of Michigan from 1991 to 2014 was performed (Figure 4). The study group included all patients with persistent spirometric decline who had a CT chest in the time range of 30 days before to 60 days after spirometric decline that was available for PRM analysis (n = 64). Demographics of patients with and without PRM data available were not significantly different (Table 4). The clinical information at the time of each patient’s spirometric decline event was closely reviewed. Patients with confounding factors responsible for the spirometric decline event were removed from the cohort (n = 12). Confounding conditions included acute abdominal process (n = 1), acute rejection that eventually improved (n = 1), bronchial stenosis (n = 1), drug toxicity (n = 2), flare of connective tissue disease (n = 1), neuromuscular weakness (n = 2), organizing pneumonia (n = 1), and volume overload (n = 3). Thus, 52 patients comprised the final study group with persistent spirometric decline. Of those, 75% (39 of 52) had an FEV1 First decline pattern, and 25% (13/52) had a Concurrent decline pattern.
Figure 4.
CONSORT (Consolidated Standards of Reporting Trials) diagram delineating study cohort. Concurrent decline = simultaneous decline in both FEV1 and FVC; FEV1 First = isolated decline in FEV1; PRM = parametric response mapping.
Table 4.
Demographics of Lung Transplant Recipients with Persistent Spirometric Decline stratified by Availability of PRM Data*
| PRM Data Available (n = 64) | PRM Data Unavailable (n = 55) | P Value | |
|---|---|---|---|
| Age at transplant, yr | 44 ± 15 | 45 ± 10 | 0.52 |
| Male | 37 (58) | 24 (44) | 0.17 |
| Baseline FEV1, % predicted | 86 ± 19 | 89 ± 28 | 0.59 |
| Baseline FVC, % predicted | 81 ± 16 | 85 ± 22 | 0.26 |
Definition of abbreviation: PRM = parametric response mapping.
Continuous predictors reported as mean ± SD; categorical as n (%).
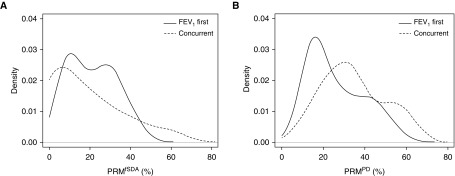
The distributions of PRMfSAD and PRMPD obtained at the time of spirometric decline are shown in Figure 5. FEV1 First patients had an average PRMfSAD value of 21% (95% confidence interval [CI], 18–25%). Concurrent group patients had an average PRMPD of 35% (95% CI, 27–42%). To investigate quantitative fSAD as a survival predictor, a threshold PRMfSAD value of 30% was selected because it marked the first quartile and hence identified the 25% of patients with the highest PRMfSAD values. Sex, recipient age, baseline FEV1 and FVC % predicted, and BOS grade at spirometric decline were not significantly different between patients with PRMfSAD greater than or equal to 30% versus those with PRMfSAD less than 30% (data not shown). However, patients with PRMfSAD greater than or equal to 30% had more episodes of acute rejection (average 1.77 vs. 0.9 episodes; P = 0.02) and were more likely to have a history of lymphocytic bronchitis (54% vs. 23%; P = 0.04) as compared with patients with low PRMfSAD (Table 5). There was no significant difference in history of bronchoalveolar lavage neutrophilia, history of Pseudomonas colonization or infection, history of Aspergillus fumigatus colonization or infection, history of cytomegalovirus pneumonitis, or history of community-acquired respiratory virus infection between patients with low versus high PRMfSAD.
Figure 5.
Distribution of parametric response mapping (PRM) of functional small airways disease (PRMfSAD) (A) and PRM of parenchymal disease (PRMPD) (B) at the time of spirometric decline stratified by FEV1 First (presenting with isolated decline in FEV1) and Concurrent (presenting with simultaneous decline in both FEV1 and FVC) (n = 13) group status.
Table 5.
Presence of Key Clinical Characteristics Stratified by PRMfSAD
| PRMfSAD ≥30% (n = 13) | PRMfSAD <30% (n = 39) | P Value* | |
|---|---|---|---|
| BAL neutrophilia >20% (at least 3 mo post-transplant), n (%) | 10 (77) | 25 (64) | 0.40 |
| Number of acute rejection episodes before persistent spirometric decline† | 1.77 (1.24) | 0.9 (1.12) | 0.02 |
| Any history of acute cellular rejection, n (%) | 11 (85) | 21 (54) | 0.06 |
| Any history of lymphocytic bronchitis, n (%) | 7 (54) | 9 (23) | 0.04 |
| History of Pseudomonas colonization or infection, n (%) | 6 (46) | 14 (36) | 0.54 |
| History of Aspergillus fumigatus colonization or infection, n (%) | 5 (39) | 12 (31) | 0.60 |
| History of CMV pneumonitis, n (%) | 3 (23) | 9 (23) | 0.97 |
| History of community-acquired respiratory viral infection, n (%) | 3 (23) | 3 (8) | 0.15 |
| Azithromycin use before persistent spirometric decline, n (%) | 4 (31) | 7 (18) | 0.35 |
Definition of abbreviations: BAL = bronchoalveolar lavage; CMV = cytomegalovirus; fSAD = functional small airways disease; PRM = parametric response mapping.
Adjusted for time from transplant to spirometric decline.
Mean (SD).
Kaplan-Meier survival curves based on spirometric decline group status and PRMfSAD values are shown in Figure 6. PRMfSAD was found to be a significant predictor of survival in FEV1 First patients (RM, P = 0.004; log-rank, P = 0.038). Over 8.3 years of follow-up, FEV1 First patients with PRMfSAD greater than or equal to 30% (10 of 39 patients; 26%) at the time of spirometric decline lived an average of 2.50 years as compared with 5.12 years in patients with PRMfSAD less than 30% (average difference, 2.6 yr; 95% CI, 0.83–4.41 yr). A multivariable analysis was performed using RM regression as shown in Table 6. In a model adjusted for age, baseline FEV1% predicted, baseline FVC % predicted, and BOS grade at spirometric decline, PRMfSAD greater than or equal to 30% in FEV1 First patients was the only significant predictor of survival; those with PRMfSAD greater than or equal to 30% lived lifetimes that were 48% shorter over the follow-up period (95% CI, 23–97% shorter; P = 0.04).,
Figure 6.
Survival following spirometric decline in various patient cohorts stratified by parametric response mapping (PRM) of functional small airways disease (PRMfSAD). (A) FEV1 First patients (presenting with isolated decline in FEV1) with PRMfSAD greater than or equal to 30% have significantly shorter average survival than patients with PRMfSAD less than 30% (2.50 ± 0.6 vs. 5.12 ± 0.7 yr; restricted mean [RM], P = 0.004; log-rank, P = 0.038). (B) Concurrent patients (presenting with simultaneous decline in both FEV1 and FVC) with PRMfSAD greater than or equal to 30% similarly had significantly shorter average survival than those with PRMfSAD less than 30% (0.27 ± 0.03 vs. 2.31 ± 0.7 yr; RM, P = 0.005; log-rank, P = 0.029). (C) PRMfSAD greater than or equal to 30% is a key prognostic indicator in all patients regardless of spirometric decline pattern (RM, P = 0.001; log-rank, P = 0.018).
Table 6.
Multivariable Restricted Mean Analysis Evaluating Impact of Clinical Variables on Survival in the FEV1 First Group (n = 39)
| Estimate* | Lower Bound | Upper Bound | P Value | |
|---|---|---|---|---|
| Intercept | 5.24 | 3.91 | 7.02 | <0.001 |
| PRMfSAD ≥30% | 0.48 | 0.23 | 0.97 | 0.04 |
| Age (per 10-yr interval) | 1.06 | 0.91 | 1.25 | 0.47 |
| Baseline FEV1% predicted (per 10% increase) | 0.90 | 0.75 | 1.07 | 0.21 |
| Baseline FVC % predicted (per 10% increase) | 1.12 | 0.91 | 1.37 | 0.30 |
| BOS grade at spirometric decline | 0.82 | 0.54 | 1.25 | 0.35 |
Definition of abbreviations: BOS = bronchiolitis obliterans syndrome; FEV1 First = patients presenting with isolated decline in FEV1; fSAD = functional small airways disease; PRM = parametric response mapping.
Estimate for intercept reflects the average time lived following spirometric decline for a 45-year-old lung transplant recipient with FEV1 and FVC baseline % predicted of 95%, who at the time of spirometric decline has BOS grade 1 and PRMfSAD less than 30%.
PRMfSAD was also evaluated as a predictor of survival in Concurrent patients and subsequently in all patients regardless of spirometric decline pattern. Interestingly, even in Concurrent patients, PRMfSAD continued to be a significant predictor of survival (RM P = 0.005; log-rank, P = 0.029). Over 5.1 years of follow-up, Concurrent patients with PRMfSAD greater than or equal to 30% lived an average of only 0.27 years as compared with Concurrent patients with PRMfSAD less than 30% who lived an average of 2.31 years (Figure 6B; average difference 2.04 yr; 95% CI, 0.61–3.47 yr). All three patients in the Concurrent spirometric decline group with PRMfSAD greater than or equal to 30% died within 4 months of their spirometric decline (range, 82–119 d).
In the entire cohort including both FEV1 First and Concurrent patients, those with PRMfSAD greater than or equal to 30% had a significantly shorter average survival following spirometric decline (RM, P = 0.001; log-rank, P = 0.018) (Figure 6C). Those with PRMfSAD greater than or equal to 30% lived an average of 1.98 of 8.3 follow-up years compared with those with PRMfSAD less than 30% who lived on average 4.63 years in the same follow-up period (average difference, 2.65 yr; 95% CI, 1.03–4.27 yr).
To determine if spirometric and radiographic parameters can be combined to provide prognostic information, we compared survival of patients categorized by their spirometric decline pattern and further by PRMfSAD in the FEV1 First group. Figure 7 demonstrates survival in patients with an FEV1 First pattern of decline with PRMfSAD less than 30%, FEV1 First pattern of decline with PRMfSAD greater than or equal to 30%, and Concurrent patients. Although FEV1 First patients with PRMfSAD values of less than 30% had longer survival than both patients with PRMfSAD greater than or equal to 30% in the FEV1 First group (P = 0.004) and the Concurrent group (P = 0.0005), no significant difference in survival was noted between Concurrent and FEV1 First patients with high PRMfSAD (P = 0.42). Thus, PRMfSAD greater than or equal to 30% was found to identify patients in the FEV1 First group who had a prognosis as poor as those presenting with Concurrent spirometric decline. Figure 8 outlines a useful clinical algorithm to predict survival following spirometric decline with the application of PRM data.
Figure 7.
Survival stratified by spirometric decline pattern and parametric response mapping (PRM) of functional small airways disease (PRMfSAD). FEV1 First patients (presenting with isolated decline in FEV1) with PRMfSAD greater than or equal to 30% have an average survival similar to Concurrent patients (presenting with simultaneous decline in both FEV1 and FVC) (2.5 ± 0.6 vs. 1.8 ± 0.6 yr; average difference, 0.67 yr; P = 0.42). FEV1 First patients with PRMfSAD less than 30% on average live longer than both Concurrent patients (P = 0.0005) and FEV1 First patients with PRMfSAD greater than or equal to 30% (P = 0.004). Open circles are censoring events (at the last patient visit).
Figure 8.
Prognostic stratification by spirometric decline pattern and computed tomography–based parametric response mapping of functional small airways disease (PRMfSAD) in lung transplant recipients. Concurrent pattern = simultaneous decline in both FEV1 and FVC; FEV1 First pattern = isolated decline in FEV1.
Discussion
In this study, we evaluate the application of PRM, a voxel-wise image analysis technique, in a lung transplant population to quantify small airway obstruction and offer prognostic information. We demonstrate that PRM-based evaluation of chest CT images provides a unique signature in patients with diverse spirometric decline patterns after lung transplantation. Patients who presented with isolated FEV1 decline have significantly higher PRMfSAD than their control subjects matched by time post-transplant, whereas patients with Concurrent decline have higher PRMPD. Furthermore, we demonstrate that degree of fSAD as assessed by PRM is a robust predictor of survival with significantly poorer survival noted in patients with PRMfSAD values greater than or equal to 30% at the onset of spirometric decline. We have shown that PRM can offer unique rejection signatures and predict survival after onset of chronic rejection in lung transplant recipients.
This study is the first to demonstrate the utility of PRM as a prognostic measure in a lung transplant population with spirometric decline. We demonstrate that higher PRMfSAD at the time of spirometric decline onset predicts significantly worse survival. In the FEV1 First cohort, patients with PRMfSAD greater than or equal to 30% had an average survival of only 2.5 years compared with 5.12 years in patients with PRMfSAD less than 30%. Significant variability has been described in the rate of spirometric decline and survival in patients presenting with chronic allograft failure, underscoring the need to identify prognostic markers that can be used in guiding therapeutic interventions and in time consideration of retransplantation (8, 42, 43). In patients with BOS, clinical factors, such as early versus late and rapid versus gradual onset, have been associated with abrupt decline and mortality (8, 43). Similarly, spirometric variables, such as higher BOS grade at onset and progression to higher BOS grades, have been shown to portend a poor prognosis (7, 8). PRMfSAD represents the first quantitative radiographic measure of small airway obstruction, which has been demonstrated to correlate with survival in lung allograft recipients presenting with spirometric decline. In a recent investigation, PRMfSAD was shown to increase over time after BOS onset, thus further demonstrating the correlation of this measure with disease severity (44).
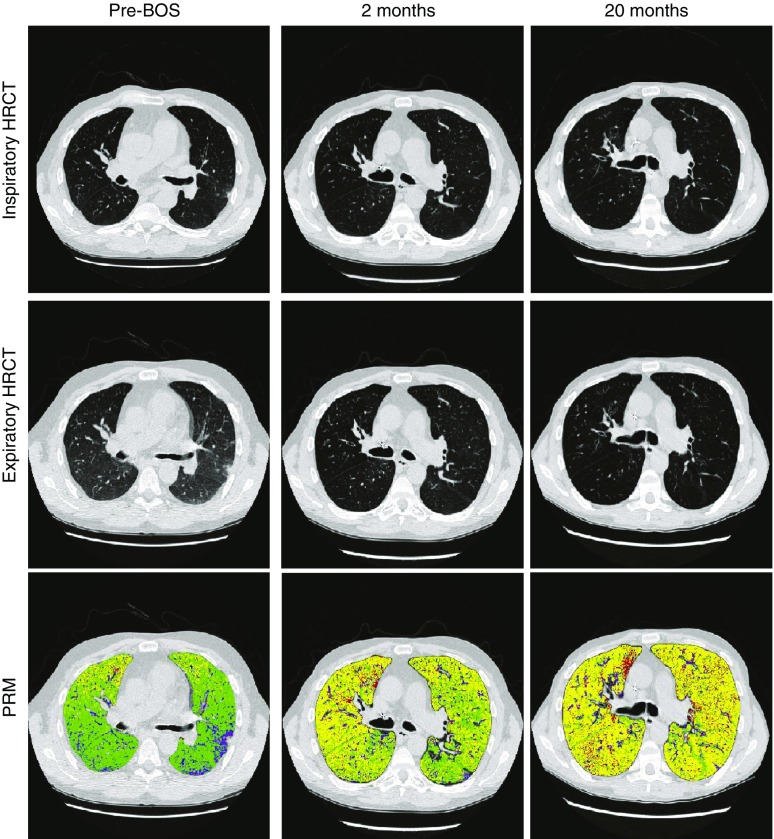
Illustrated in Figure 9 are representative HRCT images and PRM analyses from a patient before and after BOS onset demonstrating progressive increase in PRMfSAD values over time. It is also important to note that in patients with isolated FEV1 decline pattern, PRMfSAD greater than or equal to 30% was found to be the only significant predictor of survival in a model adjusting for age, baseline FEV1% predicted, baseline FVC % predicted, and BOS grade at spirometric decline. Thus PRM methodology offers a simple radiographic biomarker of disease severity with a predictive ability that is more robust than physiology alone. Furthermore, because chest CT scans are routinely performed in lung transplant recipients when spirometry declines to exclude causes of decline other than chronic rejection, PRM can have wide clinical applicability.
Figure 9.
Serial parametric response mapping (PRM) images from a patient with bronchiolitis obliterans syndrome (BOS). The left column shows high-resolution computed tomography (HRCT) and PRM images from a time point approximately 1 year before BOS onset. At this time point, the patient had low PRM of functional small airways disease (PRMfSAD) of 12%. Images in the middle column were obtained approximately 2 months following BOS onset; PRMfSAD has increased to 48%. In the right column, a scan obtained 20 months after the onset of BOS, PRMfSAD demonstrated further increase at 54%. Normal lung tissue is shown in green, and functional small airway disease is yellow.
A significant contribution of this study is that it provides a novel approach of combining physiologic and radiographic phenotyping for prognostication and patient stratification. Spirometry is the most commonly used modality for routine graft function monitoring after lung transplantation, and recent investigations have highlighted diverse patterns of spirometric decline that correlate with survival (5, 6). Two specific physiologic patterns based on spirometry have emerged: patients who present with an initial fall in FEV1 alone, and those who suffer concomitant decline in FEV1 and FVC. Although patients with concomitant decline in FEV1 and FVC comprise the lesser proportion (∼ 30%), this decline pattern has been shown to portend a very poor long-term outcome (6). The more predominant decline pattern is that of FEV1 alone, and we now demonstrate that assessment of fSAD by PRM can provide additional important prognostic information in these patients at the time of disease onset. Patients with FEV1 First decline pattern and PRMfSAD greater than or equal to 30% had very poor survival, similar to that noted in patients with a Concurrent spirometric decline pattern. This ability to predict prognosis by spirometry and PRM as shown in Figure 8 has relevance in strategizing therapeutic options, such as retransplantation, and in patient stratification for clinical trials.
Distinct PRM signatures were noted in patients with FEV1 First versus Concurrent spirometric decline patterns. Patients who presented with Concurrent decline in FEV1 and FVC had significantly more PRMPD than their matched control group. This finding similarly fits well with what is known regarding pathophysiology of restrictive chronic lung allograft dysfunction, which has not yet been rigorously defined (2, 3, 5, 25). These patients are generally described to have radiographic interstitial or alveolar changes and histologic evidence of parenchymal and pleural fibrosis (2, 3, 5). It is important to note that PRMPD does not imply the presence of restrictive chronic lung allograft dysfunction with certainty; PRMPD reflects any process that results in hyperattenuation or increased parenchymal density, which could include infection, pulmonary edema, organizing pneumonia, and so forth. These various processes must be excluded before an elevated PRMPD can be attributed to restrictive chronic lung allograft dysfunction. It has also been postulated that the Concurrent group may also include patients with very severe obstruction leading to premature airway closure and a resultant drop in FVC (45); fibrotic airway narrowing and obstruction has been demonstrated in the lungs of patients with restrictive chronic lung allograft dysfunction (4).
Interestingly, although patients with Concurrent spirometric decline typically have higher PRMPD, 23% (3 of 13) of the Concurrent patients in our larger cohort had PRMfSAD values greater than or equal to 30%, suggesting significant fSAD. Furthermore, in the Concurrent patients in our cohort, PRMfSAD greater than or equal to 30% predicted death within 4 months suggesting that even in patients with a Concurrent spirometric decline pattern, PRMfSAD may prove very useful to the clinician in identifying rejection phenotype. However, because of the small sample size in the Concurrent group, these findings must be confirmed in a larger cohort.
Our study is limited by its retrospective study design and evaluation of PRM at a single time point. However, the demonstration of utility of PRM in predicting prognosis and evaluating disease phenotype in lung transplant recipients provides the foundation for future prospective multicentric longitudinal studies to investigate use of this novel radiographic methodology in monitoring graft health in lung transplant recipients. Assessment of the amount of fSAD present in a healthy lung transplant patient population with baseline graft function also requires further study. In a prior study evaluating the utility of PRM to quantify fSAD and aid in BOS diagnosis in a hematopoietic stem cell transplant population, nontransplanted healthy nonsmoking control subjects had an average PRMfSAD value of 8.4% (17). In a cohort of current or previous smokers with normal FEV1 and Global Initiative for Chronic Obstructive Lung Disease stage 0, Bhatt and colleagues (20) found average PRMfSAD values of 12.4%. The average PRMfSAD of our control groups were 15.3% and 18.8%, respectively, for our FEV1 First and Concurrent control groups. Interestingly, similar findings were noted in the evaluation of a European cohort where mean PRMfSAD values in a normal lung transplant cohort were noted to be higher than those expected for nontransplanted normal subjects (44). Because a lung allograft is subjected to various insults unique to transplantation, such as ischemia reperfusion injury, immune insults, denervation, and lack of bronchial circulation, it is possible that perhaps a higher threshold for normal PRMfSAD will have to be considered in this population. A prospective analysis of serial PRM data over time in a transplant population would be better able to address this finding.
In summary, we have offered a view of the PRM landscape in a group of lung transplant recipients with persistent spirometric decline. We demonstrate that PRM, a quantitative imaging application, can offer insight into chronic lung allograft dysfunction phenotypes and provide prognostic information that has relevance in clinical care and future clinical trials.
Footnotes
Supported by National Institutes of Health research grants R01 HL118017 (V.N.L.), R01 HL094622 (V.N.L.), R01HL122438 (C.J.G.), and R44HL008837 (C.J.G.).
Author Contributions: E.A.B., C.J.G., B.D.R., and V.N.L. were involved in conception and hypotheses delineation. E.A.B., C.J.G., L.J.S., and V.N.L. were involved in data collection. C.J.G. performed all parametric response mapping analyses. E.A.B., I.D., X.W., S.M., C.J.G., and V.N.L. were involved in analysis and interpretation of data. E.A.B., I.D., S.M., C.J.G., and V.N.L. were involved in the writing of the manuscript. E.A.B., I.D., B.D.R., S.E.V., E.A.K., G.A.Y., S.M., C.J.G., and V.N.L. were involved in review of the final manuscript.
Originally Published in Press as DOI: 10.1164/rccm.201604-0732OC on October 25, 2016
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Yousem SA, Berry GJ, Cagle PT, Chamberlain D, Husain AN, Hruban RH, Marchevsky A, Ohori NP, Ritter J, Stewart S, et al. Revision of the 1990 working formulation for the classification of pulmonary allograft rejection: Lung Rejection Study Group. J Heart Lung Transplant. 1996;15:1–15. [PubMed] [Google Scholar]
- 2.Sato M, Waddell TK, Wagnetz U, Roberts HC, Hwang DM, Haroon A, Wagnetz D, Chaparro C, Singer LG, Hutcheon MA, et al. Restrictive allograft syndrome (RAS): a novel form of chronic lung allograft dysfunction. J Heart Lung Transplant. 2011;30:735–742. doi: 10.1016/j.healun.2011.01.712. [DOI] [PubMed] [Google Scholar]
- 3.Verleden GM, Raghu G, Meyer KC, Glanville AR, Corris P. A new classification system for chronic lung allograft dysfunction. J Heart Lung Transplant. 2014;33:127–133. doi: 10.1016/j.healun.2013.10.022. [DOI] [PubMed] [Google Scholar]
- 4.Verleden SE, Vasilescu DM, McDonough JE, Ruttens D, Vos R, Vandermeulen E, Bellon H, Geenens R, Verbeken EK, Verschakelen J, et al. Linking clinical phenotypes of chronic lung allograft dysfunction to changes in lung structure. Eur Respir J. 2015;46:1430–1439. doi: 10.1183/09031936.00010615. [DOI] [PubMed] [Google Scholar]
- 5.Todd JL, Jain R, Pavlisko EN, Finlen Copeland CA, Reynolds JM, Snyder LD, Palmer SM. Impact of forced vital capacity loss on survival after the onset of chronic lung allograft dysfunction. Am J Respir Crit Care Med. 2014;189:159–166. doi: 10.1164/rccm.201306-1155OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Belloli EA, Wang X, Murray S, Forrester G, Weyhing A, Lin J, Ojo T, Lama VN. Longitudinal forced vital capacity monitoring as a prognostic adjunct after lung transplantation. Am J Respir Crit Care Med. 2015;192:209–218. doi: 10.1164/rccm.201501-0174OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Burton CM, Carlsen J, Mortensen J, Andersen CB, Milman N, Iversen M. Long-term survival after lung transplantation depends on development and severity of bronchiolitis obliterans syndrome. J Heart Lung Transplant. 2007;26:681–686. doi: 10.1016/j.healun.2007.04.004. [DOI] [PubMed] [Google Scholar]
- 8.Finlen Copeland CA, Snyder LD, Zaas DW, Turbyfill WJ, Davis WA, Palmer SM. Survival after bronchiolitis obliterans syndrome among bilateral lung transplant recipients. Am J Respir Crit Care Med. 2010;182:784–789. doi: 10.1164/rccm.201002-0211OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Burgel PR, Bergeron A, de Blic J, Bonniaud P, Bourdin A, Chanez P, Chinet T, Dalphin JC, Devillier P, Deschildre A, et al. Small airways diseases, excluding asthma and COPD: an overview. Eur Respir Rev. 2013;22:131–147. doi: 10.1183/09059180.00001313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kramer MR, Stoehr C, Whang JL, Berry GJ, Sibley R, Marshall SE, Patterson GM, Starnes VA, Theodore J. The diagnosis of obliterative bronchiolitis after heart-lung and lung transplantation: low yield of transbronchial lung biopsy. J Heart Lung Transplant. 1993;12:675–681. [PubMed] [Google Scholar]
- 11.Chamberlain D, Maurer J, Chaparro C, Idolor L. Evaluation of transbronchial lung biopsy specimens in the diagnosis of bronchiolitis obliterans after lung transplantation. J Heart Lung Transplant. 1994;13:963–971. [PubMed] [Google Scholar]
- 12.Leung AN, Fisher K, Valentine V, Girgis RE, Berry GJ, Robbins RC, Theodore J. Bronchiolitis obliterans after lung transplantation: detection using expiratory HRCT. Chest. 1998;113:365–370. doi: 10.1378/chest.113.2.365. [DOI] [PubMed] [Google Scholar]
- 13.Worthy SA, Park CS, Kim JS, Müller NL. Bronchiolitis obliterans after lung transplantation: high-resolution CT findings in 15 patients. AJR Am J Roentgenol. 1997;169:673–677. doi: 10.2214/ajr.169.3.9275875. [DOI] [PubMed] [Google Scholar]
- 14.Bankier AA, Van Muylem A, Knoop C, Estenne M, Gevenois PA. Bronchiolitis obliterans syndrome in heart-lung transplant recipients: diagnosis with expiratory CT. Radiology. 2001;218:533–539. doi: 10.1148/radiology.218.2.r01fe09533. [DOI] [PubMed] [Google Scholar]
- 15.Galbán CJ, Chenevert TL, Meyer CR, Tsien C, Lawrence TS, Hamstra DA, Junck L, Sundgren PC, Johnson TD, Ross DJ, et al. The parametric response map is an imaging biomarker for early cancer treatment outcome. Nat Med. 2009;15:572–576. doi: 10.1038/nm.1919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Galbán CJ, Han MK, Boes JL, Chughtai KA, Meyer CR, Johnson TD, Galbán S, Rehemtulla A, Kazerooni EA, Martinez FJ, et al. Computed tomography-based biomarker provides unique signature for diagnosis of COPD phenotypes and disease progression. Nat Med. 2012;18:1711–1715. doi: 10.1038/nm.2971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Galban CJ, Boes JL, Bule M, Kitko CL, Couriel DR, Johnson TD, Lama V, Telenga ED, van den Berge M, Rehemtulla A, et al. Parametric response mapping as an indicator of bronchiolitis obliterans syndrome after hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2014;20:1592–1598. doi: 10.1016/j.bbmt.2014.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hoff BA, Toole M, Yablon C, Ross BD, Luker GD, VanPoznak C, Galban CJ. Potential for early fracture risk assessment in patients with metastatic bone disease using parametric response mapping of CT images. Tomography. 2015;1:98–104. doi: 10.18383/j.tom.2015.00154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Galban CJ, Lemasson B, Hoff BA, Johnson TD, Sundgren PC, Tsien C, Chenevert TL, Ross BD. Development of a multiparametric voxel-based magnetic resonance imaging biomarker for early cancer therapeutic response assessment. Tomography. 2015;1:44–52. doi: 10.18383/j.tom.2015.00124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bhatt SP, Soler X, Wang X, Murray S, Anzueto AR, Beaty TH, Boriek AM, Casaburi R, Criner GJ, Diaz AA, et al. Association between functional small airways disease and FEV decline in COPD Am J Respir Crit Care Med 2016194178–184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Boudewijn IM, Postma DS, Telenga ED, Ten Hacken NH, Timens W, Oudkerk M, Ross BD, Galbán CJ, van den Berge M. Effects of ageing and smoking on pulmonary computed tomography scans using parametric response mapping. Eur Respir J. 2015;46:1193–1196. doi: 10.1183/09031936.00009415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Boes JL, Bule M, Hoff BA, Chamberlain R, Lynch DA, Stojanovska J, Martinez FJ, Han MK, Kazerooni EA, Ross BD, Galban CJ. The impact of sources of variability on parametric response mapping of lung CT scans. Tomography. 2015;1:69–77. doi: 10.18383/j.tom.2015.00148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.DiGiovine B, Lynch JP, III, Martinez FJ, Flint A, Whyte RI, Iannettoni MD, Arenberg DA, Burdick MD, Glass MC, Wilke CA, et al. Bronchoalveolar lavage neutrophilia is associated with obliterative bronchiolitis after lung transplantation: role of IL-8. J Immunol. 1996;157:4194–4202. [PubMed] [Google Scholar]
- 24.Cooper JD, Billingham M, Egan T, Hertz MI, Higenbottam T, Lynch J, Mauer J, Paradis I, Patterson GA, Smith C, et al. A working formulation for the standardization of nomenclature and for clinical staging of chronic dysfunction in lung allografts: International Society for Heart and Lung Transplantation. J Heart Lung Transplant. 1993;12:713–716. [PubMed] [Google Scholar]
- 25.Meyer KC, Raghu G, Verleden GM, Corris PA, Aurora P, Wilson KC, Brozek J, Glanville AR ISHLT/ATS/ERS BOS Task Force Committee; ISHLT/ATS/ERS BOS Task Force Committee. An international ISHLT/ATS/ERS clinical practice guideline: diagnosis and management of bronchiolitis obliterans syndrome. Eur Respir J. 2014;44:1479–1503. doi: 10.1183/09031936.00107514. [DOI] [PubMed] [Google Scholar]
- 26.Stoel BC, Stolk J. Optimization and standardization of lung densitometry in the assessment of pulmonary emphysema. Invest Radiol. 2004;39:681–688. doi: 10.1097/00004424-200411000-00006. [DOI] [PubMed] [Google Scholar]
- 27.Klein S, Staring M, Murphy K, Viergever MA, Pluim JP. Elastix: a toolbox for intensity-based medical image registration. IEEE Trans Med Imaging. 2010;29:196–205. doi: 10.1109/TMI.2009.2035616. [DOI] [PubMed] [Google Scholar]
- 28.Shamonin DP, Bron EE, Lelieveldt BP, Smits M, Klein S, Staring M Alzheimer’s Disease Neuroimaging Initiative. Fast parallel image registration on CPU and GPU for diagnostic classification of Alzheimer’s disease. Front Neuroinform. 2014;7:50. doi: 10.3389/fninf.2013.00050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Laird NM, Ware JH. Random-effects models for longitudinal data. Biometrics. 1982;38:963–974. [PubMed] [Google Scholar]
- 30.Papageorgiou G, Hinde J. Multivariate generalized linear mixed models with semi-nonparametric and smooth nonparametric random effects densities. Stat Comput. 2012;22:79–92. [Google Scholar]
- 31.Wilcoxon F. Individual comparisons by ranking methods. Biometrics Bulletin. 1945;1:80–83. [Google Scholar]
- 32.Pearson K. On the criterion that a given system of deviations from the probable in the case of a correlated system of variables is such that it can be reasonably supposed to have arisen from random sampling. London: 1900. [Google Scholar]
- 33.Fisher RA. The logic of inductive inference. Journal of the Royal Statistical Society. 1935;98:39–82. [Google Scholar]
- 34.Gosset WS. The probable error of a mean. Biometrika. 1908;6:1–25. [Google Scholar]
- 35.Nelder JA, Wedderburn RWM. Generalized linear models. J R Stat Soc [Ser A] 1972;135:370–384. [Google Scholar]
- 36.Kaplan EL, Meier P. Nonparametric-estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 37.Pepe MS, Fleming TR. Weighted Kaplan-Meier statistics: a class of distance tests for censored survival data. Biometrics. 1989;45:497–507. [PubMed] [Google Scholar]
- 38.Harrington DP, Fleming TR. A class of rank test procedures for censored survival-data. Biometrika. 1982;69:553–566. [Google Scholar]
- 39.Savage IR. Contributions to the theory of rank order-statistics - the 2-sample case. The Annals of Mathematical Statistics. 1956;27:590–615. [Google Scholar]
- 40.Mantel N. Evaluation of survival data and two new rank order statistics arising in its consideration. Cancer Chemother Rep. 1966;50:163–170. [PubMed] [Google Scholar]
- 41.Andersen PK, Hansen MG, Klein JP. Regression analysis of restricted mean survival time based on pseudo-observations. Lifetime Data Anal. 2004;10:335–350. doi: 10.1007/s10985-004-4771-0. [DOI] [PubMed] [Google Scholar]
- 42.Nathan SD, Ross DJ, Belman MJ, Shain S, Elashoff JD, Kass RM, Koerner SK. Bronchiolitis obliterans in single-lung transplant recipients. Chest. 1995;107:967–972. doi: 10.1378/chest.107.4.967. [DOI] [PubMed] [Google Scholar]
- 43.Lama VN, Murray S, Lonigro RJ, Toews GB, Chang A, Lau C, Flint A, Chan KM, Martinez FJ. Course of FEV(1) after onset of bronchiolitis obliterans syndrome in lung transplant recipients. Am J Respir Crit Care Med. 2007;175:1192–1198. doi: 10.1164/rccm.200609-1344OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Verleden SE, Vos R, Vandermeulen E, Ruttens D, Bellon H, Heigl T, Van Raemdonck DE, Verleden GM, Lama V, Ross BD, et al. Parametric response mapping of bronchiolitis obliterans syndrome progression after lung transplantation. Am J Transplant. 2016;16:3262–3269. doi: 10.1111/ajt.13945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Woodrow JP, Shlobin OA, Barnett SD, Burton N, Nathan SD. Comparison of bronchiolitis obliterans syndrome to other forms of chronic lung allograft dysfunction after lung transplantation. J Heart Lung Transplant. 2010;29:1159–1164. doi: 10.1016/j.healun.2010.05.012. [DOI] [PubMed] [Google Scholar]