Abstract
A hallmark characteristic of epithelial tumor progression as well as some processes of normal development is the loss of the epithelial phenotype and acquisition of a motile or mesenchymal phenotype. Such epithelial to mesenchymal transitions are accompanied by the loss of E-cadherin function by either transcriptional or posttranscriptional mechanisms. Here we demonstrate that, upon v-Src expression, a potent trigger of epithelial to mesenchymal transitions, E-cadherin is internalized and then shuttled to the lysosome instead of being recycled back to the lateral membrane. Thus, while E-cadherin internalization facilitates the dissolution of adherens junctions, its subsequent traffic to the lysosome serves as a means to ensure that cells do not reform their cell-cell contacts and remain motile. We also show that ubiquitin tagging of E-cadherin is essential for its sorting to the lysosome. The lysosomal targeting of E-cadherin is mediated by hepatocyte growth factor-regulated tyrosine kinase substrate (Hrs) and v-Src-induced activation of the Rab5 and Rab7 GTPases. Our studies reveal that the lysosomal targeting of E-cadherin is an important posttranscriptional mechanism to deplete cellular E-cadherin during Src-induced epithelial to mesenchymal transitions.
Epithelial cell sheets, an integral part of diverse tissues in multicellular organisms, are largely a product of the varied functions of the adherens junctions (33). The adherens junctions are specialized forms of cadherin-based adhesive contacts. E-cadherin, a type I transmembrane protein of the adherens junctions, complexes with cytosolic proteins, the catenins, which in turn provide anchorage to the actin cytoskeleton to form stable cell-cell contacts (12). The down-regulation of the adherens junctions is a hallmark characteristic of an epithelial to mesenchymal transition (EMT), a process by which cells lose their polarized epithelial phenotype and concomitantly acquire a migratory or mesenchymal phenotype (39). EMTs have been shown to occur during normal embryonic development. For example, neural crest cells of the dorsal neural epithelium undergo an EMT prior to extensive migration and differentiation (28). EMTs also play a critical role in the formation of the mesoderm during gastrulation (9). In the adult, EMTs occur unnaturally, and tumor progression is often a consequence (3, 40). The occurrence of mutated forms of adherens junction proteins in several epithelium-derived invasive carcinomas affirms the inverse relationship between the establishment of these junctions and the acquisition of a motile phenotype (2, 3, 14). Thus, the presence of E-cadherin at the cell surface is a key determinant in distinguishing epithelial cells from mesenchymal cells and in establishing epithelial cell polarity within tissues.
EMTs are always associated with cell scattering, defined by the loss of intercellular junctions leading to cell-cell dissociation and the acquisition of cell motility. These processes are driven by the rearrangement of the cytoskeleton and the formation of new cell-substratum contacts. Alterations in gene transcription also accompany EMTs. The synthesis of proteins such as those involved in cell-cell adhesion is turned off, and the expression of new genes, such as those encoding matrix-degrading enzymes, is turned on. The down-regulation of E-cadherin expression during an EMT is well documented. Silencing mutations in E-cadherin or its transcriptional repression have principally been attributed to the decrease in cellular levels of E-cadherin during EMTs (2, 4, 8, 24, 29). However, in a number of experimental model systems and in a significant percentage of invasive tumors, the genes encoding E-cadherin as well as the associated catenins are normal, suggesting that posttranscriptional processes which regulate adherens junction stability may account for cell-cell dissociation and acquisition of migratory potential (39). Furthermore, activation of growth factor receptors or infection of cells with the Rous sarcoma virus has been shown to trigger EMTs even when transcription of E-cadherin is normal (25, 37). In fact, oncogenic v-Src, a Rous sarcoma virus tyrosine kinase, has been shown to be a potent inducer of EMTs when expressed in epithelial cells (1, 6, 38). The Src family of tyrosine kinases has a pivotal role in the regulation of a variety of biological functions which are associated with changes in cell morphology, including malignant transformation, cell plasticity, and modulation of intercellular adhesion during EMTs (10). The activity and/or expression level of the mammalian Src family of nonreceptor tyrosine kinases is upregulated in a variety of epithelial cancers, and there appears to be a direct correlation between the levels of Src activity and malignant potential (13, 17, 18, 26). More recently, Src-mediated phosphorylation of E-cadherin has been shown to be required for tagging of the protein with ubiquitin and its subsequent endocytosis (11). Importantly, inhibition of Src kinase activity reverses EMT, therefore underlining the importance of this kinase in dictating the cellular phenotype (5, 27).
The endosomal trafficking of E-cadherin represents a cellular process that can impinge on the stability of the adherens junctions. Although such cycling is minimal in confluent and poorly motile epithelial monolayers, it is markedly enhanced as cells disassemble their intercellular contacts (23, 31). It has been speculated that this cycling of E-cadherin is responsible for maintaining the dynamics of the epithelial monolayer. Previous studies by others and us have shown that E-cadherin is constitutively internalized into early endosomes and recycled back to the basolateral plasma membrane of polarized epithelial cells (19, 23, 31). In this regard, our work has also shown that activation of the ARF6 GTPase facilitates the recruitment of the nucleoside diphosphate kinase Nm23-H1 at the basolateral cell surface, resulting in the disassembly of cell-cell contacts (32). This recruitment of Nm23-H1 to cell junctions has a dual role: (i) to promote the endocytosis of E-cadherin and (ii) to induce a reduction in cellular levels of Rac1-GTP, processes that facilitate the loss of cell-cell adhesion. This ARF6-mediated internalization of E-cadherin is essential for adherens junction disassembly and increased cell migration induced by Src activation as well as extracellular agonists such as hepatocyte growth factor/scatter factor (30, 31). Taken together, the above findings suggest that endosomal trafficking of E-cadherin plays an important role in determining the availability of E-cadherin at the adherens junctions.
In this study we have examined the fate of E-cadherin during EMTs. We show that, upon Src activation, intracellular E-cadherin is shuttled to the lysosome for degradation, instead of following its normal route of recycling back to the lateral plasma membrane of MDCK cells. The modification of E-cadherin by ubiquitin is essential for its sorting to the lysosome, which occurs by a process mediated by hepatocyte growth factor receptor substrate (Hrs) and the activation of specific Rab GTPases. Thus, we propose a novel mechanism for the down-regulation of E-cadherin during EMTs.
MATERIALS AND METHODS
Plasmids.
The retroviral expression plasmids (pLZRS-IRES-neo) encoding hemagglutinin (HA)-tagged ARF6 and ARF6(T27N) have been previously described (31). Plasmids encoding Myc-tagged E-cadherin(Y755F-Y756F) have been described previously (11). The plasmid encoding Eps15(Ed95/295)-green fluorescent protein (GFP) was a kind gift from Alexander Benmerah (INSERM, Paris, France). The GFP-tagged E-cadherin construct was a kind gift from James Nelson (Stanford University School of Medicine). The Myc-tagged Hrs constructs were generously provided by Harald Stenmark (Norwegian Radium Hospital, Oslo, Norway), and the Rab5 and Rab7 constructs were provided by Guangpu Li (University of Oklahoma Health Science Center), Phil Stahl (Washington University, St. Louis, Mo.), Marino Zerial (Max-Planck Institute, Dresden, Germany), Angela Wandinger-Ness (University of New Mexico, Santa Fe), and Jeff Schorey (University of Notre Dame).
Cell culture and transfection.
MDCKpp60v-Src cells were cultured at the nonpermissive temperature of 41°C in Dulbecco modified Eagle medium (DMEM) supplemented with 10% fetal bovine serum and antibiotics. Cell transfections were carried out at 37°C with the Lipofectamine transfection reagent (Invitrogen, San Diego, Calif.) according to the manufacturer's protocol. After transfection, cells were briefly washed with serum-free medium and incubated with complete medium prewarmed to 41°C for 24 h. To allow expression of v-Src, cells were washed with phosphate-buffered saline and incubated with complete medium prewarmed to 35°C for various time periods as indicated. The MDCKpp60v-Src cell lines stably expressing HA-tagged ARF6 proteins were generated as previously described using the retroviral transfection protocol (31). In separate experiments, MDCKpp60v-Src cells were incubated at 41°C for 1 h in the presence of 50 μM chloroquine or 10 μM herbimycin A and then switched to 35°C for 4 h. In addition, MDCK cells were switched to 35°C for 1 h prior to treatment with lactacystin for an additional 3 h. The cells were then lysed, and the total levels of E-cadherin were analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) with mouse monoclonal anti-E-cadherin antibodies (Transduction Labs).
Antibodies and immunofluorescence labeling.
Immunofluorescence staining and microscopy were conducted as previously described (31, 32). In brief, cells on coverslips were fixed, permeabilized, and incubated according to the experimental design with anti-E-cadherin mouse monoclonal antibodies (rr1 hybridoma bank, University of Iowa), anti-HA polyclonal antibodies (Covance, Berkeley, Calif.), anti-transferrin receptor mouse monoclonal antibodies (Zymed, San Francisco, Calif.), and anti-lysosome-associated membrane protein 1 (anti-LAMP-1) monoclonal antibodies (AC-17) kindly provided by Andre LeBivic, Marseille, France. For double and triple labeling procedures, primary antibody staining was visualized by incubation with fluorescein isothiocyanate-conjugated anti-rabbit immunoglobulin G (IgG), Texas red-conjugated anti-mouse IgG (Molecular Probes, Eugene, Oreg.), or Cy5-conjugated anti-rabbit IgG (Amersham-Pharmacia, Chicago, Ill.) as indicated. In cells expressing Myc-tagged proteins, a fluorescently labeled fluorescein isothiocyanate-anti-Myc antibody was used (Invitrogen). The nuclear stain Draq5 (Alexis Biochemicals) was used to visualize nuclei. For labeling of lysosomes with LysoTracker, MDCKpp60v-Src cells transfected with GFP-tagged E-cadherin were incubated in serum-free DMEM at 41°C for 1 h followed by an incubation with a 1:1,000 dilution of LysoTracker-red DND-99 reagent (Molecular Probes) for 3 h at 41°C according to the manufacturer's protocol. Cells were then washed and incubated in complete medium at 41°C for 2 h to allow the LysoTracker reagent to reach the lysosomes. The cells were then switched to 35°C for 6 h to allow expression of v-Src prior to being fixed and processed for immunofluorescence microscopy.
Immunoprecipitation.
MDCKpp60v-Src cells were grown at 41°C at medium confluency in 100-mm-diameter plates to allow the formation of small cell colonies. Cells were then switched to 35°C for different time periods prior to being lysed in RIPA lysis buffer as previously described (32). The medium was collected, and E-cadherin was immunoprecipitated from the growth medium with monoclonal antibody directed against the extracellular domain of the protein. As a positive control, in experiments done in parallel, cells were treated with 0.01% trypsin (type IV; Sigma Chemical Co.) for 20 min followed by addition of 100-fold excess soybean trypsin inhibitor, prior to collection of growth medium. Immunoprecipitates were collected using agarose-linked protein A beads, and the supernatant was resolved by SDS-PAGE. Proteins were transferred to polyvinylidene difluoride membranes and probed using mouse monoclonal anti-E-cadherin antibodies (Covance).
For coimmunoprecipitation experiments, 106 cells transiently expressing Myc-tagged wild-type Hrs or mutant Hrs(S270E) in six-well plastic culture dishes were incubated with 500 μl of RIPA lysis buffer in the presence of protease inhibitors for 30 min on ice with gentle rocking. Cells were scraped, and the lysates were then centrifuged at 10,000 × g for 10 min at 4°C. Cell lysates were transferred to a 1.5-ml Eppendorf tube and incubated with anti-Myc monoclonal antibody for 1 h on ice. Immunoprecipitates were collected using agarose-linked protein A beads, and the supernatant was resolved by SDS-PAGE. Proteins were transferred to polyvinylidene difluoride membranes and probed for E-cadherin.
Activation assays for Rab5 and Rab7.
MDCKpp60v-Src cells expressing either GFP-tagged wild-type Rab5 or GFP-tagged wild-type Rab7 were incubated at 41°C in phosphate-free DMEM for 3 h with 300 μCi of 32Pi (Perkin-Elmer, Billerica, Mass.). The cells were then switched to 35°C for 4 h and immediately lysed using RIPA lysis buffer containing phosphatases and protease inhibitors. Rab5 and Rab7 were immunoprecipitated using mouse monoclonal anti-GFP antibodies (Roche, Indianapolis, Ind.), and the immunoprecipitates were collected by centrifugation with agarose-linked protein A beads. The beads were washed three times in wash buffer (20 mM Tris-HCl, 0.1% NP-40, 500 mM NaCl, 5 mM MgCl2, 1 mM EGTA, 1 mM dithiothreitol, 1 mg of bovine serum albumin/ml, 10 mM NaF, and 1 mM Na3VO4 plus a protease inhibitor cocktail), and the bound nucleotides were eluted in 10 μl of elution buffer (10 mM EDTA, 1 mM dithiothreitol, 0.1% SDS, 5 mM GDP, and 5 mM GTP) for 5 min in boiling water. Five microliters of the eluted samples was spotted onto polyethyleneimine-cellulose thin-layer chromatography plates (Merck) and developed for 1 h in 0.75 M phosphate buffer (pH 3.4). The plates were dried, placed in autoradiography cassettes, and exposed at −70°C for 24 to 48 h.
RESULTS
Activation of Src promotes the shuttling of E-cadherin to the lysosome.
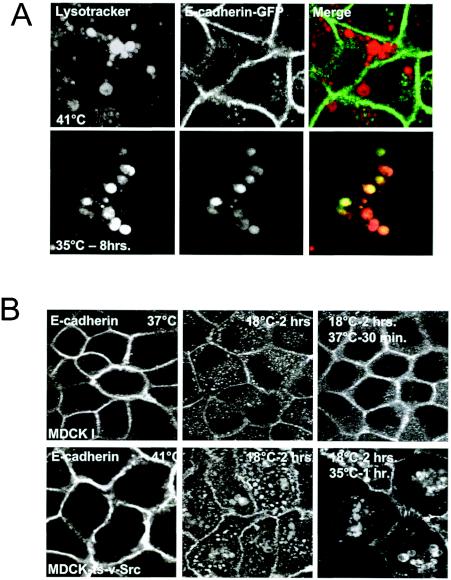
To investigate the role of membrane traffic and the fate of E-cadherin during EMTs, we made use of an MDCK cell line expressing a temperature-sensitive mutant of v-Src, MDCKpp60v-Src. The MDCKpp60v-Src cell line serves as a good cell model system to study EMTs and has been used in a wide variety of investigations of EMTs. At the nonpermissive temperature of 41°C, these cells form compact polarized cell colonies, but when shifted to the permissive temperature of 35°C they undergo the dissolution of cell-cell contacts within a few hours and acquire a migratory or fibroblast-like phenotype (1, 30). We initiated these investigations by first examining the distribution of E-cadherin in MDCKpp60v-Src cells before and after Src expression. When cells were shifted to permissive temperatures, we found that the majority of E-cadherin labeling was in cytoplasmic vesicles. These vesicles were larger than E-cadherin-positive vesicles that we had previously observed in parental MDCK cells expressing an activated GTPase-defective mutant of ARF6, ARF6(Q67L) (31) (Fig. 1A). However, the formation of Src-induced vesicles was completely blocked by expression of ARF6(T27N), the dominant-negative ARF6 mutant (Fig. 1B). This suggested that the Src-induced vesicles were derived from the cell surface via endocytosis. With more prolonged periods of Src activation, we found that E-cadherin labeling in cells was significantly diminished. These observations led us to hypothesize that E-cadherin is actively degraded upon Src activation and that E-cadherin is trafficked to lysosomes via the endocytic pathway for degradation. To test this hypothesis, MDCKpp60v-Src cells grown at 41°C were transfected with E-cadherin-GFP and then switched to the permissive temperature of 35°C to allow expression of v-Src. Cells were incubated with serum-free medium containing LysoTracker, a fluorescently labeled marker for lysosomes. As seen in Fig. 2A, a significant fraction of E-cadherin-GFP colocalized with LysoTracker, indicating that E-cadherin was indeed shuttled to the lysosome upon Src activation. These E-cadherin-positive vesicles were also labeled for LAMP-1, confirming their identity as lysosomes (data not shown).
FIG. 1.
v-Src expression promotes the formation of enlarged vesicles that contain E-cadherin. (A) MDCKpp60v-Src cells at nonpermissive or permissive temperatures for 4 h and parental MDCK I cells stably expressing ARF6(Q67L) were fixed and labeled for E-cadherin. Expression of v-Src in MDCK cells induces the formation of enlarged vesicles that contain E-cadherin that are morphologically distinct from those observed in cells expressing the ARF6-GTP mutant. The overall levels of E-cadherin in v-Src-expressing cells appear to be reduced compared to those in untransfected MDCK cells. (B) MDCKpp60v-Src cells stably expressing HA-tagged ARF6(T27N) were maintained at the permissive temperature of 35°C for 4 h and then fixed and labeled for E-cadherin (red) and HA (green). Expression of ARF6(T27N) blocks E-cadherin internalization and the formation of Src-induced enlarged vesicles.
FIG. 2.
Src activation induces the shuttling of E-cadherin to the lysosome and prevents its recycling to the basolateral plasma membrane. (A) MDCKpp60v-Src cells transiently expressing GFP-tagged E-cadherin at the nonpermissive temperature of 41°C (top panels) or at the permissive temperature of 35°C (bottom panels) were incubated with LysoTracker to label lysosomes. v-Src activation induces the shuttling of E-cadherin to the lysosome. (B) Parental MDCK I cells or MDCKpp60v-Src cells were incubated at 37 and 41°C, respectively (left panels), followed by incubation at 18°C for 2 h (middle panels) and then for 30 min at 37 or 35°C as indicated (right panels). Cells were fixed at each temperature and labeled for E-cadherin. In normal cells, E-cadherin gets internalized into the cytoplasm and then recycles back to the plasma membrane. Src activation blocks the recycling of E-cadherin to the plasma membrane and instead shuttles E-cadherin to lysosomes.
If Src facilitates the shuttling of E-cadherin to the lysosome, one would expect that its recycling to the plasma membrane would be concomitantly inhibited. Thus, we examined the fate of internalized E-cadherin in MDCKpp60v-Src cells and in parental MDCK cells. For these studies, parental MDCK cells or MDCKpp60v-Src cells were incubated for 2 h at 18°C to allow protein internalization and prevent further transport and recycling back to the cell surface. Cells were then shifted to permissive temperatures to facilitate the recycling of E-cadherin. As seen in Fig. 2B (upper panels), in parental MDCK cells at 37°C, E-cadherin is recycled back to the cell surface. In contrast, in MDCKpp60v-Src cells at 35°C, E-cadherin accumulates in cytoplasmic vesicles (Fig. 2B, lower panels). Furthermore, under the same experimental conditions in MDCKpp60v-Src cells, other ligands such as Tfn-Rs were efficiently recycled back to the basolateral membrane at permissive temperatures (data not shown). Thus, E-cadherin is specifically routed to lysosomes upon Src activation.
Transport of E-cadherin to lysosomes correlates with its degradation.
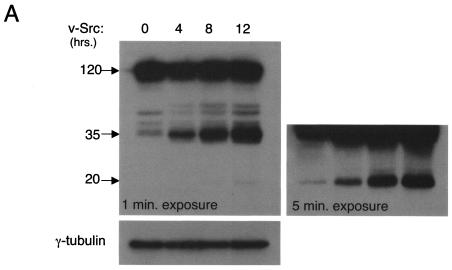
We next determined whether we could visualize E-cadherin degradation products by conventional Western blotting techniques in response to Src activation. Cell lysates of MDCKpp60v-Src cells maintained at permissive temperatures were resolved by SDS-PAGE and probed with an anti-E-cadherin antibody. As seen in Fig. 3A, upon v-Src expression, degradation products of E-cadherin of lower molecular weight than the full-length protein were observed. A small amount of degradation products was observed in the absence of Src activation, suggesting that this pathway likely operates to a minor extent under normal conditions as well and may represent a pathway for the turnover of endogenous E-cadherin. We confirmed that the degradation of E-cadherin was mediated by lysosomal proteolysis by incubating the cells with chloroquine, a known inhibitor of lysosomal proteases. Under the latter experimental conditions E-cadherin degradation was blocked (Fig. 3B). The bright band observed at approximately 35 kDa was quantitated as a measure of E-cadherin degradation. To test whether proteosome-mediated degradation also contributes to Src-induced proteolysis of E-cadherin, we tested the effect of lactacystin, an inhibitor of the 26S proteosome machinery, on E-cadherin degradation in MDCKpp60v-Src cells at permissive temperatures. As seen in Fig. 3B, the addition of lactacystin had no effect on the degradation of E-cadherin. Finally, in the presence of herbimycin A, a tyrosine kinase inhibitor expected to block Src-mediated tyrosine phosphorylation, E-cadherin degradation was blocked, confirming that enhanced E-cadherin degradation in cells at permissive temperatures was indeed due to Src activation (Fig. 3B).
FIG. 3.
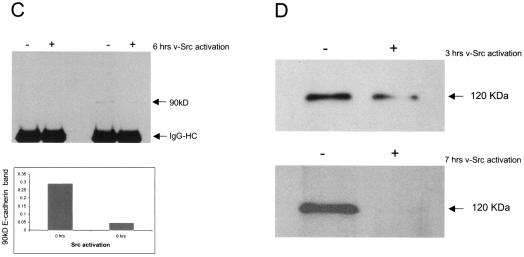
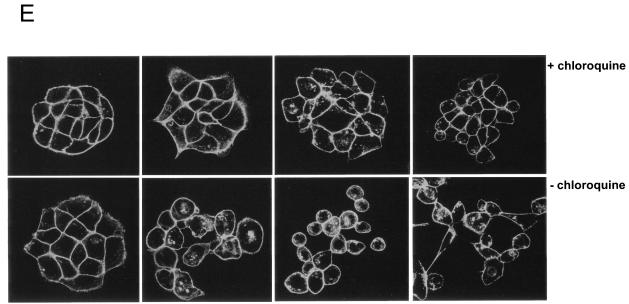
Src activation promotes the lysosome-mediated degradation of E-cadherin. (A) MDCKpp60v-Src cells grown at nonpermissive temperatures were switched to 35°C for times indicated to allow expression of v-Src. Cells were then lysed in RIPA buffer and examined for E-cadherin degradation by resolving proteins by SDS-PAGE and then labeling with anti-E-cadherin antibodies by Western blotting procedures followed by autoradiography. At low exposure a major degradation product of 35 kDa was observed. At an increased time (5 min) of autoradiography another degradation product of approximately 20 kDa became apparent. Cell lysates were probed for γ-tubulin expression as a control for equal loading of protein on SDS gels. Numbers at left are molecular masses in kilodaltons. (B) From left to right in each panel, respectively, MDCKpp60v-Src cells were grown at the nonpermissive temperature of 41°C and then switched to 35°C for 4 h to allow expression of v-Src in medium alone or medium containing herbimycin A, chloroquine, or lactacystin. E-cadherin degradation was monitored as described above. The major degradation product of 35 kDa was quantitated by densitometry as a measure of E-cadherin degradation (right). Herbimycin A and chloroquine blocked the degradation of E-cadherin, but lactacystin had no effect. Numbers at left are molecular masses in kilodaltons. (C) MDCKpp60v-Src cells were grown at the nonpermissive temperature of 41°C (lane 1) and then switched to 35°C for 6 h to allow expression of v-Src. In experiments done in parallel, cells were treated with type IV trypsin prior to collection of growth medium. The 90-kDa fragment released by trypsin treatment was quantitated by densitometry. E-cadherin fragments were not detected in the growth medium upon Src activation; however, 90-kDa fragments of the E-cadherin extracellular domain were observed in the growth medium upon trypsin treatment. (D) MDCKpp60v-Src cells were grown at the nonpermissive temperature of 41°C (lane 1) and then switched to 35°C as indicated to allow expression of v-Src. The cell surface was biotinylated, and surface levels of biotinylated E-cadherin were determined as previously described (32). Surface E-cadherin is significantly decreased upon v-Src expression. (E) Epithelial cell colonies at nonpermissive temperatures, with and without treatment with chloroquine for 1 h, were switched to permissive temperatures for times indicated. Cells were fixed, labeled with phalloidin and examined by confocal immunofluorescence microscopy. Chloroquine-treated cells were significantly hampered in their ability to scatter relative to untreated cells.
Based on the decreased localization of E-cadherin at the cell surface in Src-expressing cells, it is likely that the full-length E-cadherin observed on SDS gels represents E-cadherin that is present on cytoplasmic early and late endosomes that has not yet reached the lysosome. In support of this contention a significant fraction of E-cadherin label correlates with internalized transferrin in cells expressing v-Src (see Fig. S1 in the supplemental material). While the 120-kDa E-cadherin band is recognized by an anti-E-cadherin monoclonal antibody directed against the extracellular domain of E-cadherin, the degradation products are not recognized by this antibody, likely due to loss of the epitope upon degradation. Other E-cadherin antibodies directed against the cytoplasmic domain react more easily with the smaller E-cadherin fragments (see Fig. S2 in the supplemental material). However, in light of the above observations, we also investigated whether the proteolytic cleavage of the E-cadherin extracellular domain might occur upon v-Src signaling.
Proteolytic cleavage of the extracellular region of E-cadherin has been linked to the destabilization of the adherens junction during the metastatic progression of some cancers, and the released fragments have been used as a biomarker for disease progression (22). Thus, we examined the extracellular medium for the presence of E-cadherin fragments upon Src activation. As a positive control, cells were subjected to mild trypsin treatment, a protocol that has been shown to release a 90-kDa fragment from the extracellular portion of the E-cadherin protein (23). As shown in Fig. 3C, E-cadherin fragments were not detected in the medium upon v-Src expression. However, these fragments were detectable upon trypsin treatment. Importantly, the amount of cleaved E-cadherin released was consistently six- to sevenfold lower at 6 h post-Src expression, relative to that obtained from cells at nonpermissive temperatures, suggesting that the surface levels of E-cadherin were decreased upon Src signaling due to endocytosis of surface E-cadherin molecules. Furthermore, examination of the surface levels of E-cadherin with increasing times of Src expression revealed that E-cadherin at the plasma membrane was significantly decreased upon v-Src activation (Fig. 3D). Taken together, the above observations indicate that the degradation of E-cadherin during Src-induced EMTs is not mediated by proteolytic cleavage of the extracellular domain but is mediated by lysosomal proteolysis upon Src-induced endocytosis of E-cadherin.
Next, we examined the effect of blocking lysosomal degradation on the ability of epithelial cell scattering, an essential feature of most EMTs. As shown in Fig. 3E, chloroquine treatment of cells significantly attenuated the ability of epithelial colonies to scatter upon Src activation, relative to nontreated cells. After prolonged periods of v-Src expression (greater than 16 h), some cells eventually do break away from the colony and scatter. When switched back to nonpermissive temperatures, chloroquine-treated cells regain the epithelial phenotype faster than control nontreated cells do (data not shown). Thus, from the above set of investigations we conclude that, while the endocytosis of E-cadherin facilitates the disassembly of adherens junctions, its targeting to the lysosome serves as a mechanism to ensure that cells do not reform their adherens junctions and thereby acquire a “mesenchymal ” phenotype.
The ubiquitination of E-cadherin is required for its transport to the lysosome.
A previous study by Y. Fujita et al. (11) showed that, upon Src activation, E-cadherin is tyrosine phosphorylated, a step required for its subsequent ubiquitination. The ubiquitination of E-cadherin is mediated by Hakai, a c-Cbl-like ubiquitin ligase (11). The study also suggested that ubiquitination might serve as a signal to trigger the internalization of E-cadherin into early endosomes, since a tyrosine phosphorylation-defective mutant of E-cadherin, E-cadherin(Y755F-Y756F), which was not a substrate for Hakai and therefore unable to be tagged by ubiquitin, appeared to be localized to the plasma membrane and prevented cell scattering in response to Src activation (11). When we immunoprecipitated E-cadherin from MDCKpp60v-Src cell lysates and probed them with antiubiquitin antibodies, our results confirmed earlier findings that E-cadherin gets ubiquitinated upon Src activation (data not shown). Here, we have examined the role of E-cadherin ubiquitination and find that, rather than serving as an endocytosis signal, ubiquitination is essential for the shuttling of E-cadherin to the lysosome.
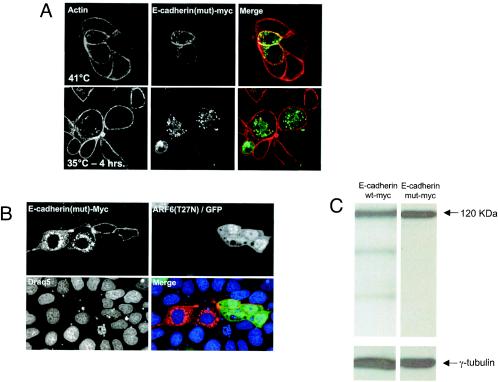
To further investigate the requirement for ubiquitin modification of E-cadherin during its traffic to the lysosome, we examined the trafficking of a ubiquitination-deficient E-cadherin mutant, E-cadherin(Y755F-Y756F), to the lysosome. These studies revealed that, while a fraction of the mutant protein was at sites of cell-cell contact, it was also in endosomal compartments, at both permissive and nonpermissive temperatures (Fig. 4A). In addition, the E-cadherin(Y755F-Y756F)-positive structures were not labeled for LAMP-1 (data not shown). As stated above, previous work in our laboratory has shown that expression of the dominant-negative ARF6 mutant, ARF6(T27N), prevents Src-induced scattering by inhibiting the endocytosis of E-cadherin (31). Thus, we transfected Myc-tagged E-cadherin(Y755F-Y756F) into cells expressing ARF6(T27N). As seen in Fig. 4B, in the presence of ARF6(T27N) the majority of E-cadherin(Y755F-Y756F) was detected at the plasma membrane. These data suggest that the endosomal localization of E-cadherin(Y755F-Y756F) results from its internalization into endosomal compartments. The staining of mutant E-cadherin in intracellular compartments appeared significantly higher relative to the intracellular distribution of endogenous E-cadherin, which led us to question whether the mutant was not being recycled back to the cell surface. However, we found that Myc-tagged wild-type E-cadherin exhibited a similar distribution at intracellular endosomal compartments at nonpermissive temperatures (data not shown). Thus, the increased cytoplasmic distribution is likely due to an accumulation of exogenous E-cadherin in the cytoplasm because of protein overexpression.
FIG. 4.
Ubiquitination-deficient E-cadherin is internalized but not trafficked to the lysosomes for degradation. (A) MDCKpp60v-Src cells transiently expressing a Myc-tagged ubiquitination-deficient mutant of E-cadherin, E-cadherin(Y755F-Y756F), at nonpermissive temperatures or at 35°C for 4 h were labeled for actin (red) and Myc (green). The ubiquitination-deficient mutant of E-cadherin localizes to cell-cell contacts and to cytoplasmic vesicles. (B) Cells were transiently transfected with retrovirus encoding ARF6(T27N) and GFP for 24 h followed by transient transfection with the Myc-tagged ubiquitination-deficient mutant of E-cadherin. Cells were fixed and labeled for Myc and with Draq5, a nuclear stain. Expression of ARF6(T27N) prevents the cytoplasmic distribution of mutant E-cadherin, suggesting that internalized mutant E-cadherin was derived by endocytosis from the cell surface. (C) MDCKpp60v-Src cells transiently expressing Myc-tagged wild-type E-cadherin or E-cadherin(Y755F-Y756F) at the permissive temperature of 35°C for 4 h were lysed in RIPA buffer, and equal amounts of the cell lysates were probed with antibodies to Myc, by Western blotting procedures. Myc-tagged E-cadherin(Y755F-Y756F) is not degraded upon expression of v-Src.
We also examined the degradation profile of the E-cadherin mutant upon Src activation. For these latter studies, cell lysates were resolved on SDS gels and probed with anti-Myc antibodies by Western blotting procedures. As seen in Fig. 4C, the E-cadherin mutant was not degraded upon Src activation. Wild-type E-cadherin, however, was efficiently degraded (data not shown). Taken together, the studies described here indicate that Src-induced tyrosine phosphorylation of E-cadherin and its subsequent ubiquitination are indeed signals for its transport to the lysosome and that ubiquitin tagging of E-cadherin is not essential for its internalization.
Hrs, Rab5, and Rab7 regulate the shuttling of E-cadherin from endosomes to lysosomes.
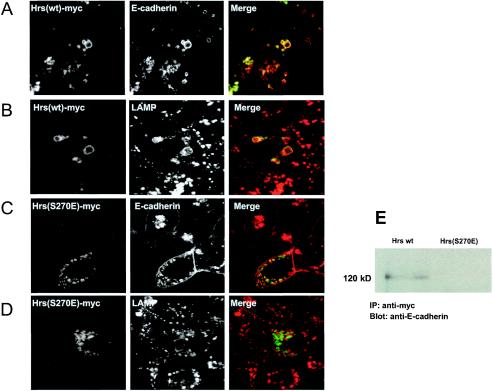
Since our studies indicate that E-cadherin is shuttled to the lysosome upon Src activation, we investigated whether the lysosomal targeting of E-cadherin would be regulated by proteins known to control membrane traffic to the lysosome. Recent studies have demonstrated a regulatory role for the hepatocyte growth factor receptor substrate (Hrs) in the sorting and shuttling of ubiquitinated endosomal cargo to the lysosome. Hrs is an endosomal scaffolding protein containing clathrin and ubiquitin-interacting motifs (UIM) and serves to sort and recruit cargo destined for lysosomal degradation (21, 35, 36, 41). Thus, we tested the effects of wild-type Hrs and a mutant of Hrs, Hrs(S270E), which lacks the UIM of Hrs and is therefore unable to bind to ubiquitinated proteins (35), on the shuttling of E-cadherin to the lysosome. MDCKpp60v-Src cells at 41°C were transfected with Myc epitope-tagged wild-type Hrs and switched to the permissive temperature to induce expression of v-Src. Cells were then labeled with antibodies against Myc and E-cadherin. As expected, at 41°C the majority of E-cadherin was localized to the cell junctions (data not shown). At the permissive temperature of 35°C, when v-Src is expressed, most of the E-cadherin was localized to enlarged vesicles that also contained Hrs (Fig. 5A). Further labeling studies showed that many of these compartments were labeled with antibodies directed against LAMP-1 (Fig. 5B). On the other hand, expression of Hrs(S270E) at permissive temperatures stabilized the levels of E-cadherin at the cell junctions and retained E-cadherin in early endosomes (Fig. 5C). Moreover, lack of labeling of these vesicles with anti-LAMP antibodies demonstrated that, in cells expressing Hrs(S270E), E-cadherin was not targeted to the lysosome (Fig. 5D). Furthermore, in coimmunoprecipitation experiments, we found that only wild-type Hrs and not mutant Hrs(S270E) coprecipitated with E-cadherin (Fig. 5E). Thus, the Src-induced shuttling of E-cadherin to the lysosome is dependent on an endosomal sorting event mediated by Hrs.
FIG. 5.
Hrs regulates the traffic of ubiquitinated E-cadherin to the lysosome. (A to D) MDCKpp60v-Src cells transiently transfected with Myc-tagged wild-type Hrs or Hrs(S270E) as indicated were grown at the nonpermissive temperature of 41°C and then switched to 35°C for 8 h. Cells were fixed and labeled for Myc (green) and E-cadherin (A and C, red) or LAMP-1 (B and D, red). Coincident staining appears yellow in the merged images. At nonpermissive temperatures, wild-type Hrs colocalizes with E-cadherin on vesicles that do not label for LAMP-1. Upon expression of v-Src, wild-type Hrs and E-cadherin are shuttled toward the late endosomal-lysosomal compartments as seen by the colocalization with LAMP-1 antibodies. However, Hrs(S270E) and E-cadherin are retained at vesicular compartments that do not colocalize with LAMP-1. Thus, expression of Hrs(S270E) blocks the shuttling of E-cadherin to the lysosome upon Src activation. (E) Lysates of MDCKpp60v-Src cells expressing Myc-tagged wild-type Hrs or Hrs(S270E) at permissive temperatures were immunoprecipitated with anti-Myc monoclonal antibody. Immunoprecipitates were resolved on SDS gels and probed with anti-E-cadherin antibody. Only wild-type Hrs, not the Hrs mutant lacking a functional UIM domain, coprecipitates with E-cadherin upon Src activation.
We also investigated the effect of the Rab GTPases on Src-induced transport of internalized E-cadherin to the lysosome for degradation. The Rab GTPases are key regulators of membrane traffic along the endocytic pathway, and two Rab proteins in particular play important roles in regulating the traffic of cell-surface membrane proteins to the lysosome. Rab5 activation has been shown to promote the fusion of early endosomes, whereas the activation of the Rab7 GTPase promotes the transport of proteins from early endosomes to late endosomes and lysosomes (34). In this regard, the “swollen” morphology of endosomes observed upon v-Src expression was reminiscent of that observed in cells expressing activated Rab5. Thus, we examined whether Src activation facilitated the shuttling of E-cadherin to the lysosome in part by modulating the activities of Rab5 and Rab7.
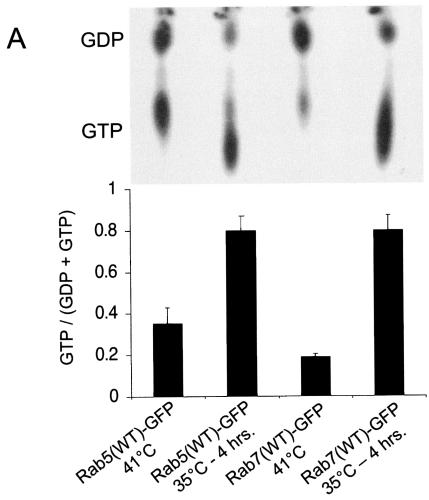
First, we determined the activation profiles of Rab5 and Rab7 upon expression of v-Src by measuring the GTP/GDP ratios of the Rab GTPases in cells. To this end, MDCKpp60v-Src cells at 41°C were transfected with GFP-tagged forms of either wild-type Rab5 or wild-type Rab7 and incubated with radioactively labeled phosphorus (32P). Cells were then switched to 35°C to allow expression of v-Src followed by immunoprecipitation of Rab5 and Rab7 with anti-GFP antibodies. The immunoprecipitates were then eluted, and the GTP/GDP ratios were analyzed by thin-layer chromatography. As seen in Fig. 6A, a significant fraction of Rab5 was present in its GTP-bound state in cells at nonpermissive temperatures, but this pool was doubled upon Src activation. On the other hand, Rab7-GTP was not as abundant in resting MDCK cells, and its activation was significantly enhanced at temperatures permissive for Src expression.
FIG. 6.
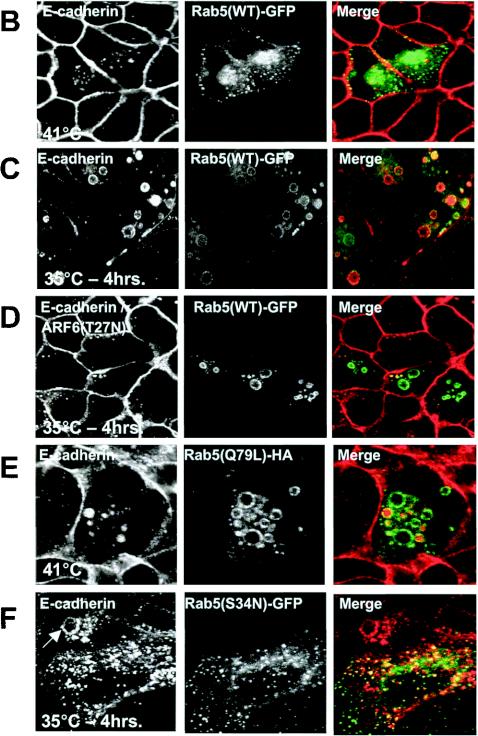
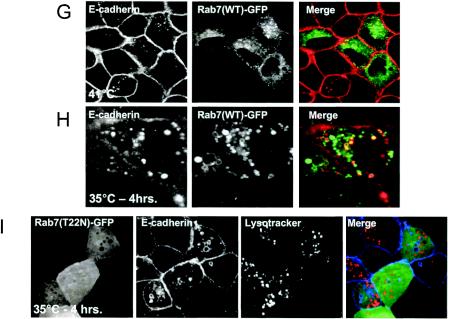
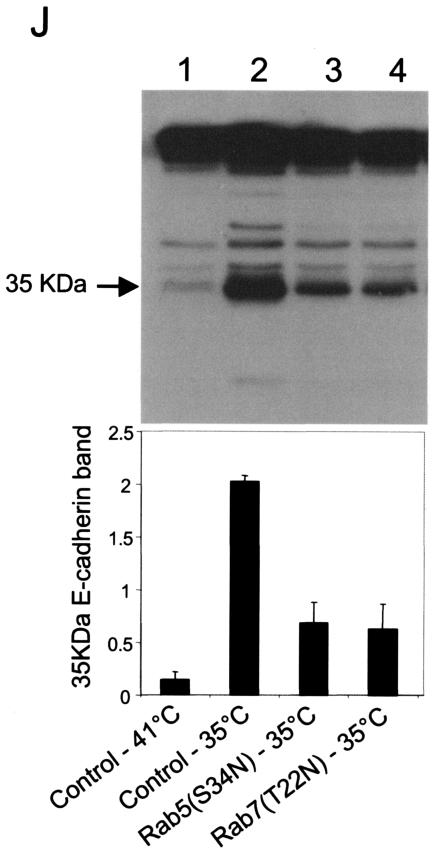
Expression of v-Src induces the activation of Rab5 and Rab7 to facilitate the shuttling of E-cadherin to the lysosome. (A) MDCKpp60v-Src cells transiently transfected with GFP-tagged wild-type Rab5 (lanes 1 and 2) and GFP-tagged wild-type Rab7 (lanes 3 and 4) were incubated at either 41 or 35°C for 4 h as indicated, and the nucleotide bound to Rab5 and Rab7 was determined. Upon Src activation the cellular levels of GTP-bound Rab5 and Rab7 are markedly enhanced. (B and C) MDCKpp60v-Src cells transiently transfected with GFP-tagged wild-type Rab5 at the nonpermissive temperature of 41°C (B) or at the permissive temperature of 35°C (C) were fixed and labeled for E-cadherin (red). Coincident staining appears yellow in the merged images. Upon Src activation, wild-type Rab5 localizes to enlarged endosomes that also contain E-cadherin. Some Rab5-positive endosomes did not contain E-cadherin; these vesicles could represent endosomes that are derived from the apical membrane, which also contain Rab5 (7). (D) MDCKpp60v-Src cells expressing ARF6(T27N) were transiently transfected with GFP-tagged wild-type Rab5, incubated at 35°C for 4 h, and labeled for E-cadherin (red). Coexpression of dominant-negative ARF6, ARF6(T27N), with wild-type Rab5 blocked the internalization of E-cadherin but did not prevent the fusion of Rab5-positive endosomes in response to Src activation. (E) Parental MDCK cells transiently transfected with HA-tagged Rab5(Q79L) were labeled for E-cadherin. Activated Rab5 expression had no effect on the adherens junctions. (F) MDCKpp60v-Src cells transiently transfected with GFP-tagged Rab5(S34N) were grown at 35°C for 4 h and labeled for E-cadherin (red). Expression of dominant-negative Rab5, Rab5(S34N), blocks the formation of enlarged endosomes upon Src activation. The arrow points to an E-cadherin-positive enlarged endosome formed by expression of v-Src in an untransfected cell adjacent to a Rab5(S34N)-transfected cell where enlarged endosomes are not observed. (G and H) MDCKpp60v-Src cells transiently transfected with GFP-tagged wild-type Rab7 at 41 or 35°C were fixed and labeled for E-cadherin (red). In the absence of Src activation, wild-type Rab7 localizes to late endosomes. Upon Src activation, wild-type Rab7 localizes to enlarged vesicles that also contain E-cadherin and are likely formed by the fusion of early and late endosomes. (I) MDCKpp60v-Src cells transiently transfected with GFP-tagged Rab7(T22N) at 41°C were incubated at the permissive temperature of 35°C for 4 h and labeled for E-cadherin (blue) and LysoTracker (red) to visualize lysosomes. Expression of dominant-negative Rab7, Rab7(T22N), prevents the trafficking of E-cadherin to the lysosome. (J) Untransfected MDCKpp60v-Src cells (lanes 1 and 2) and those transiently transfected with GFP-tagged Rab5(S34N) (lane 3) and GFP-tagged Rab7(T22N) (lane 4) were maintained at 41 or 35°C for 4 h as indicated. Cells were then lysed in RIPA buffer, and the cell lysates were analyzed by SDS-PAGE and blotted with antibodies against E-cadherin. The 35-kDa E-cadherin degradation product was quantified using a densitometer and plotted in the graph shown below. Transient expression of Rab5(S34N) and Rab7(T22N) blocked the degradation of E-cadherin in response to Src activation.
We also examined the distribution of Rab5-GFP in MDCKpp60v-Src cells at permissive and nonpermissive temperatures. At 41°C, as expected Rab5 was distributed in punctate early endosomes (Fig. 6B). When cells were switched to 35°C to allow expression of v-Src, wild-type Rab5 was redistributed to swollen vesicles which also contained E-cadherin (Fig. 6C), likely due to increased fusion mediated by enhanced levels of Rab5-GTP. Previous work in our laboratory has shown that ARF6 is activated upon Src expression, a step that facilitates the internalization of E-cadherin and the dissolution of cell-cell contacts. Hence, we investigated whether Rab5 activation in v-Src-expressing cells was dependent on prior activation of ARF6. To this end we coexpressed Rab5 and ARF6(T27N), the dominant-negative ARF6 mutant, in MDCKpp60v-Src cells. As seen in Fig. 6D, expression of ARF6(T27N) blocked E-cadherin internalization, but it did not prevent Rab5-mediated endosome fusion. This suggests that Rab5 activation occurs in parallel with ARF6 activation upon expression of v-Src. In support of this contention, we found that, in contrast to the effect of activated ARF6 on cell-cell junctions, expression of activated Rab5, Rab5(Q79L), had no significant effect on the stability of the adherens junctions in parental MDCK cells (Fig. 6E). Finally we tested the effect of Rab5(S34N), a dominant-negative mutant of Rab5, on v-Src-induced shuttling of E-cadherin to the lysosome. Expression of Rab5(S34N) completely inhibited the formation of E-cadherin-positive enlarged endosomes and prevented the shuttling of E-cadherin to the lysosome (Fig. 6F). These data indicate that Rab5-mediated fusion of E-cadherin-containing endosomes is an obligate step prior to its shuttling to the lysosome upon Src activation.
Since expression of v-Src promoted the activation of Rab7, we also tested the effects of Rab7 expression on Src-induced shuttling of E-cadherin to the lysosome. As seen in Fig. 6G, GFP-tagged wild-type Rab7 exhibited a punctate distribution at the perinuclear area in MDCKpp60v-Src cells at nonpermissive temperatures. When cells were switched to the permissive temperature of 35°C, wild-type Rab7 localized to vesicles that were labeled for E-cadherin (Fig. 6H) and LAMP (data not shown). Additionally, expression of a dominant-negative mutant of Rab7, Rab7(T22N), resulted in the accumulation of E-cadherin in large cytoplasmic vesicles, indicating that Src-induced activation of Rab7 is required for the shuttling of E-cadherin to the lysosome. However, these vesicles did not contain lysosomal markers (Fig. 6I), suggesting that Rab7(T22N) expression blocked the shuttling of E-cadherin from early endosomes to lysosomes. The swollen morphology of the vesicles is likely due to the increased level of Rab5-GTP in cells. Thus, the introduction of dominant-negative mutants of Rab5 and Rab7 produced stage-specific blocks in the shuttling of E-cadherin to the lysosome.
We also examined the effects of expressing the dominant-negative mutants of Rab5 and Rab7 on Src-induced degradation of E-cadherin. Lysates of MDCKpp60v-Src cells and those transiently expressing Rab5(S34N) and Rab7(T22N) at 35°C were examined for E-cadherin degradation by Western blotting procedures described above. Expression of Rab5(S34N) or Rab7(T22N) prevented the degradation of E-cadherin in response to Src activation, as seen by the decrease in E-cadherin degradation products in transfected cell lysates compared to untransfected cells (Fig. 6J). Taken together, these results suggest that expression of v-Src induces the activation of Rab5 and Rab7 to facilitate the shuttling of E-cadherin to the lysosome for degradation.
DISCUSSION
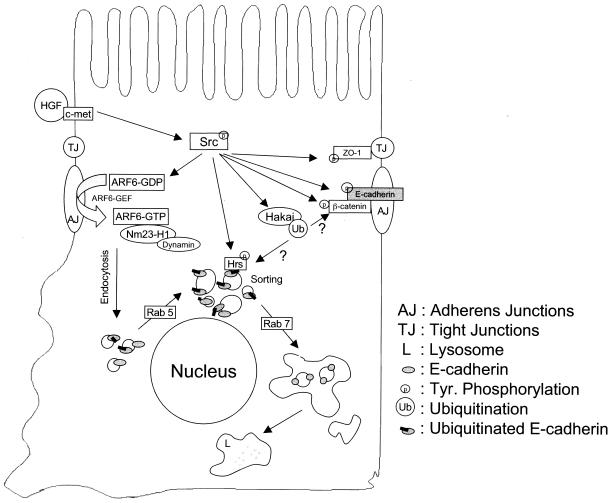
In this study we have elucidated a novel posttranscriptional mechanism for cell-cell dissociation during EMTs (Fig. 7). We show that, upon v-Src expression, E-cadherin is ubiquitinated and then shuttled to the lysosome instead of following its normal trafficking route of recycling back to the lateral plasma membrane. We also show that the sorting of ubiquitinated E-cadherin is mediated by Hrs (hepatocyte growth factor-regulated tyrosine kinase substrate), an endosomal scaffolding protein that regulates the sorting of ubiquitinated cargo to the lysosome. An Hrs mutant lacking the UIM completely blocks the trafficking of E-cadherin to the lysosome. Furthermore, an E-cadherin mutant that is not a substrate for the ubiquitin ligase is retained in endosomal compartments, indicating that the ubiquitin tagging of E-cadherin is a critical signal for its lysosomal targeting. Finally we demonstrate that expression of v-Src also induces the activation of the GTPases Rab5 and Rab7, and expression of the dominant-negative mutants of these GTPases introduces stage-specific blocks in the trafficking of E-cadherin to the lysosome.
FIG. 7.
Working model for the regulation of the E-cadherin turnover rate in response to Src activation. As previously described, the activation of ARF6 in response to extracellular stimuli and/or Src activation promotes the internalization of E-cadherin to endosomes vis a process mediated by Nm23-H1 and dynamin (32). Src also promotes the ubiquitination of E-cadherin (11), although it is unclear whether this occurs at the plasma membrane or on internal vesicular compartments. Src-mediated activation of Rab5 promotes early-endosome fusion to induce the formation of E-cadherin-positive enlarged endosomes and perhaps accelerates the rate of E-cadherin internalization. Ubiquitinated E-cadherin is sorted in early endosomes via its recognition by Hrs. Thus, while nonubiquitinated E-cadherin is recycled back to the plasma membrane, ubiquitinated E-cadherin is trafficked to the lysosome for degradation. The latter step is facilitated by Src-induced activation of Rab7. It is unclear at this time if Src induces the phosphorylation of Hrs to promote the lysosomal targeting of E-cadherin. HGF, hepatocyte growth factor.
There is accumulating evidence that, to maintain the dynamics of epithelial monolayers, E-cadherin is rapidly removed from the plasma membrane and then subsequently recycled back to the cell surface to reform new cell-cell contacts. Thus, the recycling of E-cadherin through the endosomal recycling pathway represents an effective mechanism for the remodeling of adhesive contacts in dynamic situations where cell-cell contacts must be dissolved and reformed. EMTs, however, are accompanied by a loss of cell surface E-cadherin. This loss of surface E-cadherin has often been linked to repression of E-cadherin expression by factors such as Snail/Slug/SIP/E12/47 that bind to the E-box of the E-cadherin promoter to suppress gene expression (24, 29, 33). However, excessive internalization and/or degradation of E-cadherin will also facilitate the down-regulation of surface E-cadherin, raising the possibility that such posttranscriptional mechanisms could potentially be operational during tumor progression and metastases, as well as during some processes of normal development.
A more recent study has shown that Hakai, a c-Cbl-like E3 ubiquitin ligase, induces the monoubiquitination of E-cadherin in response to Src activation (11). It is well established that polyubiquitination of cytosolic proteins is an essential posttranslational modification that regulates the degradation of cytosolic proteins via the 26S proteosome (15). Monoubiquitination of proteins, on the other hand, has been shown to act as a signal for targeting of membrane-bound proteins to the lysosome for degradation (16). We believe that the monoubiquitination of E-cadherin is a strategic cellular sorting signal for its traffic to the lysosome. This sorting step is mediated by Hrs, since expression of an Hrs mutant lacking its UIM blocks the traffic of E-cadherin to the lysosome. Hrs likely interacts with ubiquitinated E-cadherin in endosomes along with subunits of the mammalian ESCRT (endosomal sorting complex required for transport) (20). At this time, it is still unclear whether the ubiquitination of E-cadherin by Hakai occurs at the plasma membrane prior to internalization or at early endocytic compartments.
The investigations described here are particularly germane to the understanding of the role of Src during EMT. The Src-induced tyrosine phosphorylation of E-cadherin (which leads to its ubiquitination) likely represents a critical step for the lysosomal targeting and subsequent down-regulation of E-cadherin during EMTs. Furthermore, our data have shown that activation of Src initiates a series of signaling pathways at almost every step of the endocytic pathway that leads to the shuttling of E-cadherin to the lysosome for degradation (Fig. 7). In addition to the tyrosine phosphorylation of E-cadherin that leads to its ubiquitination and recognition by Hrs, Src signaling also stimulates the activation of the GTPases Rab5 and Rab7, indicating that the endocytic machinery is subject to active regulation during EMTs. Rab5-GTP promotes the fusion of early endosomes containing endocytosed molecules, whereas Rab7-GTP promotes the transport of endosomal ligands from early endosomes toward the lysosome (34). Thus, while the activation of Rab5 may serve to enhance the rate of E-cadherin transport, Rab7 activation may serve to shift the balance of endocytic traffic toward the lysosome instead of its recycling back to the cell surface. It would be of interest to determine the mechanism by which Src signaling induces the activation of the Rab5 and Rab7 GTPases. One possibility is that Src might phosphorylate a guanine nucleotide exchange factor or some other upstream regulator of the Rab GTPases. Irrespective of the mechanism by which Src promotes the buildup of Rab5-GTP and Rab7-GTP, the data presented in this study demonstrate that the activities of these GTPases are a requisite for the transport of E-cadherin to the lysosome.
Given the physiological as well as the therapeutic implications of understanding the EMT process, considerable effort has been made over the past several years to understand mechanisms favoring cell dispersion. The studies presented here have demonstrated the involvement of regulatory molecules that function in normal cells as important participants in Src-induced loss of cell-cell contacts. It is possible that in normal cells the activation of individual molecules produces a response that is not sufficient to trigger the array of cellular changes observed in EMTs. However, the summation of individual signals elicited by critical signaling molecules such as Src or the activation or excessive expression of other proto-oncogenes in cancer cells may be required to trigger an EMT response. Thus, EMTs are highly regulated, and it is likely that more than one cellular response may be required to bring about decisive changes that lead to this phenomenon. The studies described here show that the lysosomal trafficking and degradation of E-cadherin can lead to cell-cell dissociation, an obligate step in EMTs. Investigating this regulated degradation of E-cadherin pathway during embryonic development or tumor progression in the adult is an important area for future investigation.
Supplementary Material
Acknowledgments
We thank Harald Stenmark, Philippe Chavrier, Jill Schweitzer, and Jeff Schorey for critical reading of the manuscript and helpful suggestions. We also thank Walter Birchmeier, Andre LeBivic, Harald Stenmark, Marino Zerial, Guangpu Li, Jeff Schorey, Philip Stahl, and Angela Wandinger-Ness for reagents used in these investigations.
This work was supported by a Research Scholar Grant from the American Cancer Society (RSG03-023-01) to C.D.-S.
Footnotes
Supplemental material for this article may be found at http://mcb.asm.org/.
REFERENCES
- 1.Behrens, J., L. Vakaet, R. Friis, E. Winterhager, F. Van Roy, M. M. Mareel, and W. Birchmeier. 1993. Loss of epithelial differentiation and gain of invasiveness correlates with tyrosine phosphorylation of the E-cadherin/beta-catenin complex in cells transformed with a temperature-sensitive v-SRC gene. J. Cell Biol. 120:757-766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Berx, G., A. M. Cleton-Jansen, F. Nollet, W. J. de Leeuw, M. van de Vijver, C. Cornelisse, and F. van Roy. 1995. E-cadherin is a tumour/invasion suppressor gene mutated in human lobular breast cancers. EMBO J. 14:6107-6115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Birchmeier, W., J. Behrens, K. M. Weidner, J. Hulsken, and C. Birchmeier. 1996. Epithelial differentiation and the control of metastasis in carcinomas. Curr. Top. Microbiol. Immunol. 213:117-135. [DOI] [PubMed] [Google Scholar]
- 4.Bolos, V., H. Peinado, M. A. Perez-Moreno, M. F. Fraga, M. Esteller, and A. Cano. 2003. The transcription factor Slug represses E-cadherin expression and induces epithelial to mesenchymal transitions: a comparison with Snail and E47 repressors. J. Cell Sci. 116:499-511. [DOI] [PubMed] [Google Scholar]
- 5.Boyer, B., Y. Bourgeois, and M. F. Poupon. 2002. Src kinase contributes to the metastatic spread of carcinoma cells. Oncogene 21:2347-2356. [DOI] [PubMed] [Google Scholar]
- 6.Boyer, B., A. M. Valles, and N. Edme. 2000. Induction and regulation of epithelial-mesenchymal transitions. Biochem. Pharmacol. 60:1091-1099. [DOI] [PubMed] [Google Scholar]
- 7.Bucci, C., A. Wandinger-Ness, A. Lutcke, M. Chiariello, C. B. Bruni, and M. Zerial. 1994. Rab5a is a common component of the apical and basolateral endocytic machinery in polarized epithelial cells. Proc. Natl. Acad. Sci. USA 91:5061-5065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cheng, C. W., P. E. Wu, J. C. Yu, C. S. Huang, C. T. Yue, C. W. Wu, and C. Y. Shen. 2001. Mechanisms of inactivation of E-cadherin in breast carcinoma: modification of the two-hit hypothesis of tumor suppressor gene. Oncogene 20:3814-3823. [DOI] [PubMed] [Google Scholar]
- 9.Ciruna, B., and J. Rossant. 2001. FGF signaling regulates mesoderm cell fate specification and morphogenetic movement at the primitive streak. Dev. Cell 1:37-49. [DOI] [PubMed] [Google Scholar]
- 10.Frame, M. C., V. J. Fincham, N. O. Carragher, and J. A. Wyke. 2002. v-Src's hold over actin and cell adhesions. Nat. Rev. Mol. Cell Biol. 3:233-245. [DOI] [PubMed] [Google Scholar]
- 11.Fujita, Y., G. Krause, M. Scheffner, D. Zechner, H. E. Leddy, J. Behrens, T. Sommer, and W. Birchmeier. 2002. Hakai, a c-Cbl-like protein, ubiquitinates and induces endocytosis of the E-cadherin complex. Nat. Cell Biol. 4:222-231. [DOI] [PubMed] [Google Scholar]
- 12.Gumbiner, B. M. 1996. Cell adhesion: the molecular basis of tissue architecture and morphogenesis. Cell 84:345-357. [DOI] [PubMed] [Google Scholar]
- 13.Guy, C. T., S. K. Muthuswamy, R. D. Cardiff, P. Soriano, and W. J. Muller. 1994. Activation of the c-Src tyrosine kinase is required for the induction of mammary tumors in transgenic mice. Genes Dev. 8:23-32. [DOI] [PubMed] [Google Scholar]
- 14.Handschuh, G., S. Candidus, B. Luber, U. Reich, C. Schott, S. Oswald, H. Becke, P. Hutzler, W. Birchmeier, H. Hofler, and K. F. Becker. 1999. Tumour-associated E-cadherin mutations alter cellular morphology, decrease cellular adhesion and increase cellular motility. Oncogene 18:4301-4312. [DOI] [PubMed] [Google Scholar]
- 15.Hershko, A., and A. Ciechanover. 1998. The ubiquitin system. Annu. Rev. Biochem. 67:425-479. [DOI] [PubMed] [Google Scholar]
- 16.Hicke, L., and R. Dunn. 2003. Regulation of membrane protein transport by ubiquitin and ubiquitin-binding proteins. Annu. Rev. Cell Dev. Biol. 19:141-172. [DOI] [PubMed] [Google Scholar]
- 17.Irby, R. B., and T. J. Yeatman. 2002. Increased Src activity disrupts cadherin/catenin-mediated homotypic adhesion in human colon cancer and transformed rodent cells. Cancer Res. 62:2669-2674. [PubMed] [Google Scholar]
- 18.Jacobs, C., and H. Rubsamen. 1983. Expression of pp60c-src protein kinase in adult and fetal human tissue: high activities in some sarcomas and mammary carcinomas. Cancer Res. 43:1696-1702. [PubMed] [Google Scholar]
- 19.Kamei, T., T. Matozaki, T. Sakisaka, A. Kodama, S. Yokoyama, Y. F. Peng, K. Nakano, K. Takaishi, and Y. Takai. 1999. Coendocytosis of cadherin and c-Met coupled to disruption of cell-cell adhesion in MDCK cells—regulation by Rho, Rac and Rab small G proteins. Oncogene 18:6776-6784. [DOI] [PubMed] [Google Scholar]
- 20.Katzmann, D. J., M. Babst, and S. D. Emr. 2001. Ubiquitin-dependent sorting into the multivesicular body pathway requires the function of a conserved endosomal protein sorting complex, ESCRT-I. Cell 106:145-155. [DOI] [PubMed] [Google Scholar]
- 21.Komada, M., R. Masaki, A. Yamamoto, and N. Kitamura. 1997. Hrs, a tyrosine kinase substrate with a conserved double zinc finger domain, is localized to the cytoplasmic surface of early endosomes. J. Biol. Chem. 272:20538-20544. [DOI] [PubMed] [Google Scholar]
- 22.Kuefer, R., M. D. Hofer, J. E. Gschwend, K. J. Pienta, M. G. Sanda, A. M. Chinnaiyan, M. A. Rubin, and M. L. Day. 2003. The role of an 80 kDa fragment of E-cadherin in the metastatic progression of prostate cancer. Clin. Cancer Res. 9:6447-6452. [PubMed] [Google Scholar]
- 23.Le, T. L., A. S. Yap, and J. L. Stow. 1999. Recycling of E-cadherin: a potential mechanism for regulating cadherin dynamics. J. Cell Biol. 146:219-232. [PMC free article] [PubMed] [Google Scholar]
- 24.Leptin, M. 1991. twist and snail as positive and negative regulators during Drosophila mesoderm development. Genes Dev. 5:1568-1576. [DOI] [PubMed] [Google Scholar]
- 25.Maina, F., G. Pante, F. Helmbacher, R. Andres, A. Porthin, A. M. Davies, C. Ponzetto, and R. Klein. 2001. Coupling Met to specific pathways results in distinct developmental outcomes. Mol. Cell 7:1293-1306. [DOI] [PubMed] [Google Scholar]
- 26.Muthuswamy, S. K., and W. J. Muller. 1995. Activation of Src family kinases in Neu-induced mammary tumors correlates with their association with distinct sets of tyrosine phosphorylated proteins in vivo. Oncogene 11:1801-1810. [PubMed] [Google Scholar]
- 27.Nam, J. S., Y. Ino, M. Sakamoto, and S. Hirohashi. 2002. Src family kinase inhibitor PP2 restores the E-cadherin/catenin cell adhesion system in human cancer cells and reduces cancer metastasis. Clin. Cancer Res. 8:2430-2436. [PubMed] [Google Scholar]
- 28.Nieto, M. A. 2001. The early steps of neural crest development. Mech. Dev. 105:27-35. [DOI] [PubMed] [Google Scholar]
- 29.Nieto, M. A. 2002. The snail superfamily of zinc-finger transcription factors. Nat. Rev. Mol. Cell Biol. 3:155-166. [DOI] [PubMed] [Google Scholar]
- 30.Palacios, F., and C. D'Souza-Schorey. 2003. Modulation of Rac1 and ARF6 activation during epithelial cell scattering. J. Biol. Chem. 278:17395-17400. [DOI] [PubMed] [Google Scholar]
- 31.Palacios, F., L. Price, J. Schweitzer, J. G. Collard, and C. D'Souza-Schorey. 2001. An essential role for ARF6-regulated membrane traffic in adherens junction turnover and epithelial cell migration. EMBO J. 20:4973-4986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Palacios, F., J. K. Schweitzer, R. L. Boshans, and C. D'Souza-Schorey. 2002. ARF6-GTP recruits Nm23-H1 to facilitate dynamin-mediated endocytosis during adherens junctions disassembly. Nat. Cell Biol. 4:929-936. [DOI] [PubMed] [Google Scholar]
- 33.Perez-Moreno, M., C. Jamora, and E. Fuchs. 2003. Sticky business: orchestrating cellular signals at adherens junctions. Cell 112:535-548. [DOI] [PubMed] [Google Scholar]
- 34.Pfeffer, S. R. 2001. Rab GTPases: specifying and deciphering organelle identity and function. Trends Cell Biol. 11:487-491. [DOI] [PubMed] [Google Scholar]
- 35.Raiborg, C., K. G. Bache, D. J. Gillooly, I. H. Madshus, E. Stang, and H. Stenmark. 2002. Hrs sorts ubiquitinated proteins into clathrin-coated microdomains of early endosomes. Nat. Cell Biol. 4:394-398. [DOI] [PubMed] [Google Scholar]
- 36.Raiborg, C., and H. Stenmark. 2002. Hrs and endocytic sorting of ubiquitinated membrane proteins. Cell Struct. Funct. 27:403-408. [DOI] [PubMed] [Google Scholar]
- 37.Shimizu, Y., N. Yamamichi, K. Saitoh, A. Watanabe, T. Ito, M. Yamamichi-Nishina, M. Mizutani, N. Yahagi, T. Suzuki, C. Sasakawa, S. Yasugi, M. Ichinose, and H. Iba. 2003. Kinetics of v-src-induced epithelial-mesenchymal transition in developing glandular stomach. Oncogene 22:884-893. [DOI] [PubMed] [Google Scholar]
- 38.Takeda, H., A. Nagafuchi, S. Yonemura, S. Tsukita, J. Behrens, and W. Birchmeier. 1995. V-src kinase shifts the cadherin-based cell adhesion from the strong to the weak state and beta catenin is not required for the shift. J. Cell Biol. 131:1839-1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Thiery, J. P. 2003. Epithelial-mesenchymal transitions in development and pathologies. Curr. Opin. Cell Biol. 15:740-746. [DOI] [PubMed] [Google Scholar]
- 40.Thiery, J. P. 2002. Epithelial-mesenchymal transitions in tumour progression. Nat. Rev. Cancer 2:442-454. [DOI] [PubMed] [Google Scholar]
- 41.Urbe, S., I. G. Mills, H. Stenmark, N. Kitamura, and M. J. Clague. 2000. Endosomal localization and receptor dynamics determine tyrosine phosphorylation of hepatocyte growth factor-regulated tyrosine kinase substrate. Mol. Cell. Biol. 20:7685-7692. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.