Abstract
Vascular endothelial growth factor (VEGF) regulates vasculogenesis and angiogenesis by using two tyrosine kinase receptors, VEGFR1 and VEGFR2. VEGFR1 null mutant mice die on embryonic day 8.5 (E8.5) to E9.0 due to an overgrowth of endothelial cells and vascular disorganization, suggesting that VEGFR1 plays a negative role in angiogenesis. We previously showed that the tyrosine kinase (TK) domain of VEGFR1 is dispensable for embryogenesis, since VEGFR1 TK-deficient mice survived and were basically healthy. However, the molecular basis for this is not yet clearly understood. To test the hypothesis that the specific role of VEGFR1 during early embryogenesis is to recruit its ligand to the cell membrane, we deleted the transmembrane (TM) domain in TK-deficient VEGFR1 mice. Surprisingly, about half of the VEGFR1(TM-TK)-deficient mice succumbed to embryonic lethality due to a poor development of blood vessels, whereas other mice were healthy. In VEGFR1(TM-TK)−/− mice with growth arrest, membrane-targeted VEGF was reduced, resulting in the suppression of VEGFR2 phosphorylation. Furthermore, the embryonic lethality in VEGFR1(TM-TK)−/− mice was significantly increased to 80 to 90% when the genotype of VEGFR2 was changed from homozygous (+/+) to heterozygous (+/−) in 129/C57BL6 mice. These results strongly suggest that the membrane-fixed ligand-binding region of VEGFR1 traps VEGF for the appropriate regulation of VEGF signaling in vascular endothelial cells during early embryogenesis.
Growth factors mediate signals for a variety of cellular processes, including proliferation, differentiation, migration, survival, and apoptosis. The primary mediators of such physiological cell responses are receptor tyrosine kinases (RTKs) that couple ligand binding to downstream signaling cascades via the catalytic tyrosine kinase domain. All RTKs consist of a single transmembrane domain that separates the intracellular tyrosine kinase region from the extracellular portion (27, 28).
The vascular endothelial growth factor (VEGF) receptor family, including VEGFR1 (Flt-1), VEGFR2 (KDR/Flk-1), and VEGFR3 (Flt-4), belongs to the RTKs. Among them, VEGFR1 is expressed as a full-length RTK and as a soluble form which carries only the extracellular domain (12, 15, 23, 26).
The VEGF system is a crucial regulatory system for angiogenesis (6, 17, 20, 22) and is proven to have roles in embryonic vasculogenesis (3, 5, 7, 21). VEGFR2 is expressed in mesodermal progenitor cells that are destined to differentiate into hemangioblasts and angioblasts (4, 18), and VEGF and VEGFR2 are required for the generation and further differentiation of hemangioblasts (3, 5, 21).
VEGFR1 null mutant mice die on embryonic day 8.0 (E8.0) to E8.5. They contain differentiated endothelial cells, but these cells are disorganized and abnormally overgrow in blood vessels, suggesting an inhibitory role of VEGFR1 at this stage (7). Interestingly, as previously reported, VEGFR1(TK)−/− mice are healthy and have basically normal development, indicating that the tyrosine kinase domain is not required for this role (10). VEGFR1 and VEGFR2 are located close to each other on the endothelial cell membrane (1). The extracellular domain of VEGFR1, including soluble VEGFR1, has about a 10-fold stronger binding affinity for VEGF than that of VEGFR2 (8, 10, 19). Thus, we hypothesized that the extracellular domain of VEGFR1 acts to absorb excessive VEGF and that this membrane-bound VEGFR1 plays an important role in the delivery of VEGF to the membrane, thereby enabling an appropriate quantitative regulation of the VEGF that binds to VEGFR2. In this model, the TK activity of VEGFR1 itself is not required, but the VEGF ligand family should be recruited to the cell membrane via the transmembrane (TM) domain of VEGFR1. To test this hypothesis, we generated VEGFR1(TM-TK)-deficient mice, leaving only the extracellular domain, which can absorb VEGF but cannot induce the recruitment of VEGF to the cell membrane. We found that about one-half of 129/C57BL6 mice lacking the TM-TK region of VEGFR1 died as embryos with abnormal blood vessel formation.
MATERIALS AND METHODS
Mice.
To generate VEGFR1(TM) mutant mice, we first obtained a 24-kb mouse VEGFR1 genomic DNA clone, including a cDNA carrying exons 16 to 19, from a genomic library of the 129Sv strain (Stratagene, La Jolla, Calif.). The targeting vector contains a neomycin resistance gene which replaces exon 16, encoding the transmembrane domain, and also encodes diphtheria toxin A. Targeted CCE embryonic stem (ES) cell clones were injected into C57BL6/J blastocysts, and male chimeric mice were crossed with female C57B6L/J mice to yield mice that were heterozygous for the VEGFR1(TM-TK) mutation. The genotypes of the pups obtained by crosses between VEGFR1(TM-TK)+/− mice were determined by Southern blot hybridization and PCR analyses. The generation of VEGFR1(TK) mutant mice with a genetic background of 50% 129Sv and 50% C57BL/6 was reported previously (10). VEGFR2 null mutant mice were purchased from Jackson Laboratory (Bar Harbor, Maine).
Preparation of proteins.
Lung tissues derived from 10 wild-type or VEGFR1(TM-TK)−/− mice were lysed in an ice-cold buffer containing 150 mM NaCl, 50 mM HEPES (pH 7.4), 10 mM EDTA, 1% Triton X-100, 10% glycerol, 2% aprotinin, and 1 mM phenylmethylsulfonyl fluoride and were applied to a 1-ml HiTrap heparin affinity column (Pharmacia, Uppsala, Sweden). Bound proteins were eluted with a series of buffers from 0.3 to 2.0 M NaCl containing 50 mM HEPES (pH 7.4), 10 mM EDTA, 1% Triton X-100, 10% glycerol, 2% aprotinin, and 1 mM phenylmethylsulfonyl fluoride. The fractionated samples were analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and Western blotting.
Immunohistochemistry and TUNEL staining.
The anti-mouse VEGF antibody used for this study is an affinity-purified rabbit polyclonal antibody raised against the carboxy-terminal 20 amino acids of mouse VEGF (14). Dissected embryos were stained essentially as previously described (25). In brief, the specimens were dehydrated, treated with 0.3% H2O2 in methanol to eliminate endogenous peroxidase activity, and rehydrated. After the samples were blocked with a mixture containing 2% skim milk, 0.2% bovine serum albumin, and 0.3% Triton X-100, anti-mouse VEGFR2 (Pharmingen) was added and incubated at 4°C overnight for whole-mount staining. Washed specimens were incubated with secondary antibodies conjugated to horseradish peroxidase or alkaline phosphatase. For LacZ staining, whole embryos were fixed and incubated in a 0.1% X-Gal (5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside) solution at 4°C overnight. For examinations of the localization of VEGF and phosphorylated VEGFR2 (Tyr 1173) (24), paraffin-embedded sections were deparaffinized and incubated with each specific antibody at 4°C overnight. To detect apoptosis, we used an in situ cell death detection kit (Takara, Kyoto, Japan) to perform a terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end labeling (TUNEL) assay.
Reverse transcription-PCR (RT-PCR) analysis.
Total RNAs from embryo tissue samples were extracted and reverse transcribed with specific primers. Specific primers for VEGFR1, VEGF, and β-actin were designed as previously described (11, 14). In addition, appropriate primers for PlGF, VEGF-B, VEGF-C, VEGF-D, neuropilin, and VEGFR2 were designed with the following sequences: for PlGF, 5′-ATGCTGGTCATGAAGCTGTTCA-3′ and 5′-GGACTGAATATGTGAGACACCT-3′; for VEGF-B, 5′-ATGAGCCCCCTGCTCCGT-3′ and 5′-CTACAGGTGTCTGGGTTGAG-3′; for VEGF-C, 5′-ATGCACTTGCTGTGCTTCTTG-3′ and 5′-TGTCCTGGTATTGAGGGTGG-3′; for VEGF-D, 5′-ATGTATGGAGAATGGGGAATG-3′ and 5′-TTTACACAGGGGGGCTTGAA-3′; for neuropilin, 5′-ATGGAGAGGGGGCTGCCGTT-3′ and 5′-GAAGAGAAAGGGCCCTGAAG-3′; and for VEGFR2, 5′-AAGTGATTGAGGCAGACGCT-3′ and 5′-TGATGCCAAGAACTCCAT-3′.
Statistical analysis.
For statistical analyses of endothelial cell numbers in the anterior and middle portions of embryos, the data were expressed as means ± standard deviations and were analyzed by Student's t test. P values of <0.05 were considered significant.
RESULTS
Severe disturbance of embryonic development in about one-half of VEGFR1(TM-TK) mutant mice.
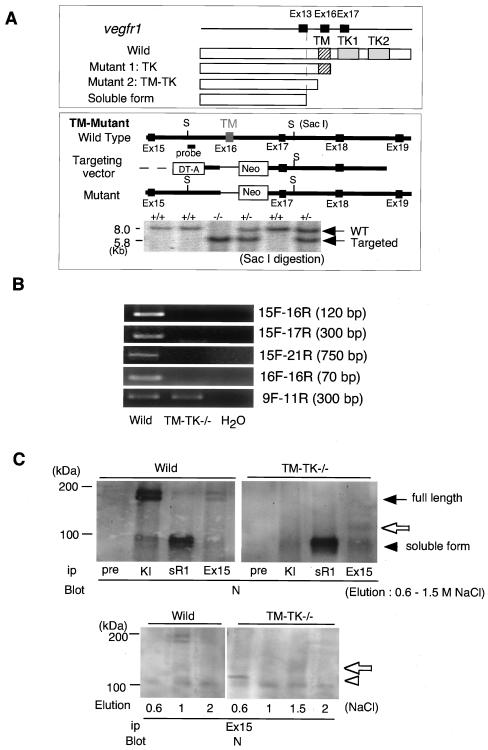
To generate VEGFR1(TM-TK)-deficient mice, we disrupted exon 16, encoding the transmembrane domain, in mouse ES cells by homologous recombination and used the ES cells for the preparation of mice (Fig. 1A). We examined whether any aberrant splicing might occur to generate an aberrant mRNA in the TM-TK−/− mice. However, we did not see clear RT-PCR bands between exon 15 and downstream exons such as 17 and 21 at detectable levels (Fig. 1B). Therefore, we concluded that the major mutant protein in TM-TK−/− mice was truncated at exon 15 and terminated at a termination codon in intron 15.
FIG. 1.
Targeted inactivation of the transmembrane domain of VEGFR1 in mice. (A) Scheme of full-length, tyrosine kinase (TK)-deficient, and transmembrane (TM)-deficient VEGFR1 and endogenous soluble VEGFR1 (sVEGFR1). Wild-type mice express full-length VEGFR1 and sVEGFR1, whereas VEGFR1(TK)−/− mutant mice express the TK truncated form and sVEGFR1 and VEGFR1(TM-TK)−/− mice express the TM-TK truncated form and sVEGFR1. Exon 16, encoding the TM domain, was replaced with the neomycin-resistant gene to generate the TM-TK mutant. VEGFR1(TM-TK)+/− or VEGFR1(TM-TK)−/− mice were confirmed by Southern blot analysis. (B) VEGFR1 mRNA expression of wild-type and VEGFR1(TM-TK)−/− mice. No splicing variants were detected for the region downstream of exon 15 of the VEGFR1 gene in VEGFR1(TM-TK)−/− mice by RT-PCR. (C) Immunoblot analysis of proteins obtained from mouse lungs with various antibodies (Ab). Pre, preimmune rabbit serum; sR1, Ab against soluble VEGFR1, specific for the carboxyl-terminal 31 amino acids; KI, Ab against the VEGFR1 kinase insert which is encoded by exon 21; N, Ab against the amino-terminal region of the VEGFR1 extracellular domain; Ex15, Ab against the upstream exon of the transmembrane domain (Ex16). The closed arrow and arrowhead indicate the full-length and soluble forms of VEGFR1, respectively (upper panel). The open arrows and arrowhead indicate truncated VEGFR1 (upper and lower panels).
For examinations of VEGFR1 proteins, lung tissues from wild-type and homozygous mice were lysed in buffer and partially purified by heparin affinity chromatography. As shown in Fig. 1C (top panels), wild-type mice contained both the 190-kDa full-length molecule and the 110-kDa endogenous soluble form of VEGFR1. This soluble form of VEGFR1 carries about 85% of the extracellular domain and results from a termination within intron 13 associated with 31 amino acids derived from this intron (12, 13). In contrast, VEGFR1(TM-TK)−/− mice completely lost full-length VEGFR1, although the endogenous soluble form was expressed at a level almost equal to that in wild-type mice. Furthermore, VEGFR1(TM-TK)−/− mice expressed a new truncated form of VEGFR1 with a size of approximately 120 kDa which was detected with an antiserum raised against the amino acid sequence of exon 15 (Fig. 1C, top panels). This form was eluted from a heparin column with 1.5 M NaCl. A band eluted with 0.6 M NaCl may have been a premature form of this 120-kDa protein with less glycosylation (Fig. 1C, bottom panel). Therefore, VEGFR1(TM-TK)−/− mice expressed both a novel truncated form of VEGFR1 lacking the transmembrane-kinase region and the endogenous soluble VEGFR1.
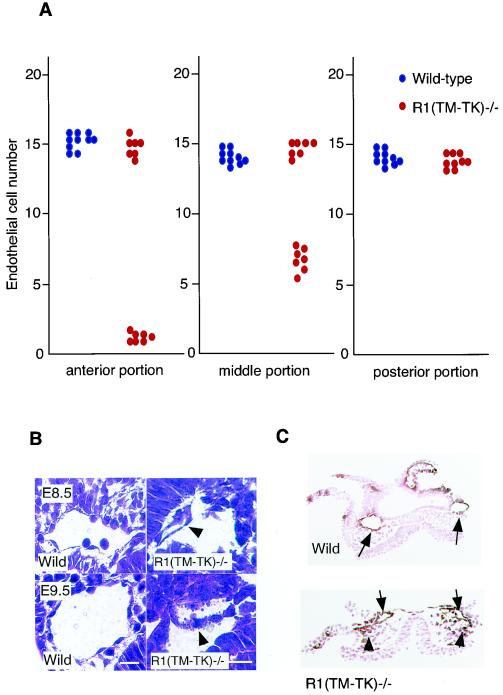
Remarkably, about 50% of the VEGFR1(TM-TK)−/− mice showed growth arrest with a lack of “turning” before E8.5. On the other hand, half of the VEGFR1(TM-TK)−/− mice developed basically normally and grew into adults (Table 1). VEGFR2-positive cells were abnormally distributed in half of the embryos with growth retardation, and the numbers of these cells significantly decreased in the anterior and middle portions of the embryos (Fig. 2A).
TABLE 1.
Survival rates of R1(TM-TK) mutant mice with background of 50 or 100% C57BL6/Ja
| Mating | Genotype | No. of embryos (no. of embryos without turning)
|
|||
|---|---|---|---|---|---|
| E8.5 | E9.5 | E10.5 | Adult | ||
| R1(TM-TK)+/− × R1(TM-TK)+/− | R1(TM-TK)+/+b | 16 | 20 | 15 | 48 |
| R1(TM-TK)+/−b | 35 (17) | 49 (24) | 17 | 50 | |
| R1(TM-TK)−/−b | 12 (7) | 23 (12) | 6 | 29 | |
| R1(TM-TK)+/− × R1(TM-TK)+/− | R1(TM-TK)+/+c | 20 | |||
| R1(TM-TK)+/−c | 48 | ||||
| R1(TM-TK)−/−c | 19 | ||||
One-half of VEGFR1(TM-TK)+/− and R1(TM-TK)−/− mice revealed no turning phenotype even at E9.5, resulting in a ratio of adult mice with a genetic background of 50% C57BL6/J of 1:1:0.5 [wild type to VEGFR1(TM-TK)+/− to R1(TM-TK)−/−]. This embryonically lethal phenotype was recovered by a genetic background of >99% C57BL6/J.
Genetic background, 50% C57BL6/J and 50% 129.
Genetic background, >99% C57BL6/J.
FIG. 2.
Disturbed endothelial cell differentiation in VEGFR1(TM-TK) mutant mice. (A) Numbers of endothelial cells lining the dorsal aorta in the anterior, middle, and posterior portions of wild-type or VEGFR1(TM-TK)−/− mice (stained with anti-VEGFR2 antibody) Each single dot is the average cell number for five sections from one embryo. (B) Small-vessel formation of dorsal aorta in hematoxylin- and eosin-stained transverse sections of mutant mice at E8.5 or E9.5. Arrowheads indicate small and fragmented endothelial cells. (C) Structural abnormality of the dorsal aorta (arrows) and mismigrated VEGFR2-positive cells (arrowheads) in the middle portion of the embryo at E8.0 were detected in VEGFR1(TM-TK)−/− mice (bottom) compared to wild-type mice (top) by immunohistochemistry with an anti-VEGFR2 antibody. The sections were counterstained with eosin.
Histologically, the dorsal aortas in the mid-portion of half of the VEGFR1(TM-TK)−/− embryos with growth retardation were smaller than those in wild-type embryos at E8.5 (Fig. 2B, top panel, and C). In the VEGFR1(TM-TK)−/− embryos with growth arrest at E9.5, fragmented endothelial cells were observed (Fig. 2B, bottom panel, arrowhead). Nearly half of the VEGFR1(TM-TK)+/− mice also showed severe developmental defects similar to those of VEGFR1(TM-TK)−/− mice.
Decrease in VEGF receptor activation and increase in endothelial apoptosis in VEGFR1(TM-TK)−/− embryos with severe developmental defects.
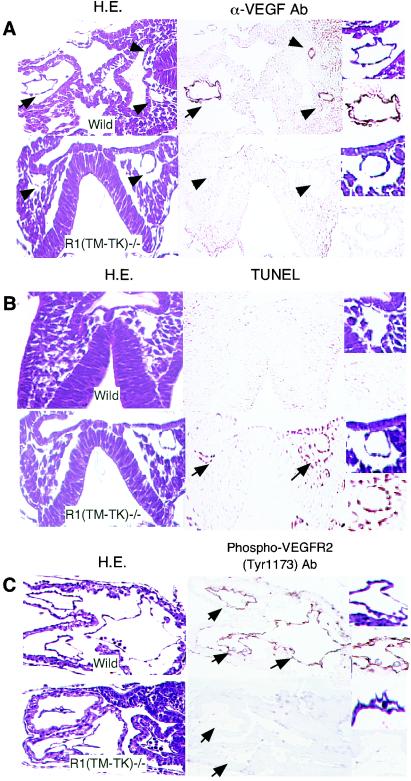
VEGF ligands and receptors work in a paracrine manner (22), and in the case of VEGF deprivation, endothelial cells that are highly dependent on the VEGF system enter apoptosis (16, 24). To compare the membrane-targeted VEGF proteins of wild-type and VEGFR1(TM-TK)−/− mice, we immunohistochemically examined the localization of the VEGF protein by use of an anti-mouse VEGF antibody that is able to detect even the receptor-bound form (14). The VEGF protein was clearly localized on the endothelial cells of the dorsal aorta and primitive heart and on the surrounding cells in wild-type mice. In contrast, only a small amount of VEGF was observed on these cells in TM-TK−/− mice with developmental defects (Fig. 3A). Furthermore, these endothelial cells often became apoptotic in mutant embryos, as seen by TUNEL staining (Fig. 3B, arrows). Tyrosine phosphorylation of VEGFR2 is known to induce endothelial cell proliferation (24). Phosphorylation of VEGFR2 at the critical site was apparent in wild-type but not VEGFR1(TM-TK)−/− mice (Fig. 3C). Thus, a proper amount of VEGF did not appear to reside on the cell surface, resulting in a severe reduction in VEGFR2 signaling in half of the VEGFR1(TM-TK)-deficient mice. This reduction may induce dysfunction and disturbance of the differentiation, proliferation, and survival of endothelial cells.
FIG. 3.
Reduced membrane-targeted VEGF in VEGFR1(TM-TK)−/− mice. (A) Transverse sections of wild-type and (TM-TK)−/− embryos at E8.0 stained with hematoxylin and eosin (H.E.; left panels) or an anti-mouse VEGF antibody (right panels). Arrows and arrowheads point to endothelial cells of the primitive heart and dorsal aorta, respectively. (B) Apoptosis of endothelial cells in the dorsal aorta (arrows) of a VEGFR1(TM-TK)−/− embryo at E8.0, as evidenced by TUNEL staining (right panels). (C) Phosphorylated VEGFR2 on endothelial cells was immunohistochemically detected by use of an antibody against phosphorylated Y1173 on VEGFR2, which is the major VEGF-dependent autophosphorylation site. Images of parts of blood vessels and primitive canals at a higher magnification are depicted to the right.
Screening for a second molecule with an additive effect on developmental defects.
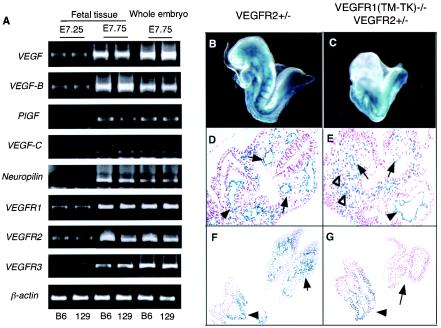
One important question was why half of the VEGFR(TM-TK)−/− mice showed embryonic lethality with poor blood vessel formation while the other half were basically normal. To investigate this issue, we changed the mouse genetic background because the background sometimes strongly affects the phenotypes of mutant mice. We generated VEGFR1(TM-TK)−/− mice with a genetic background of >99% C57BL6/J (TM-TK−/− B6) and compared their rate of survival with that of the original 50% C57BL6/J-50% 129 (TM-TK−/− 129/B6) mice. The Mendelian ratio of TM-TK +/+, +/−, and −/− B6 mice was about 1:2:1 (Table 1), indicating that most of the TM-TK−/− B6 mice could survive, in contrast to TM-TK−/− 129/B6 mice. Thus, we thought it was possible that a second molecule existed to overcome the lethal phenotype seen for the VEGFR1(TM-TK)−/− 129/B6 embryos. We screened molecules related to the VEGF system by RT-PCR, using C57BL6/J and 129 mouse embryos. A clear difference was seen in VEGFR2 expression, which was higher in C57BL6 mice than in 129 mice (Fig. 4A), but other genes such as VEGFR1, VEGFR3, VEGF family, and neuropilin-1 did not show a clear difference between these two strain of mice. These results suggest that the difference in VEGFR2 expression between the two genetic backgrounds may contribute at least in part to the lethal phenotype.
FIG. 4.
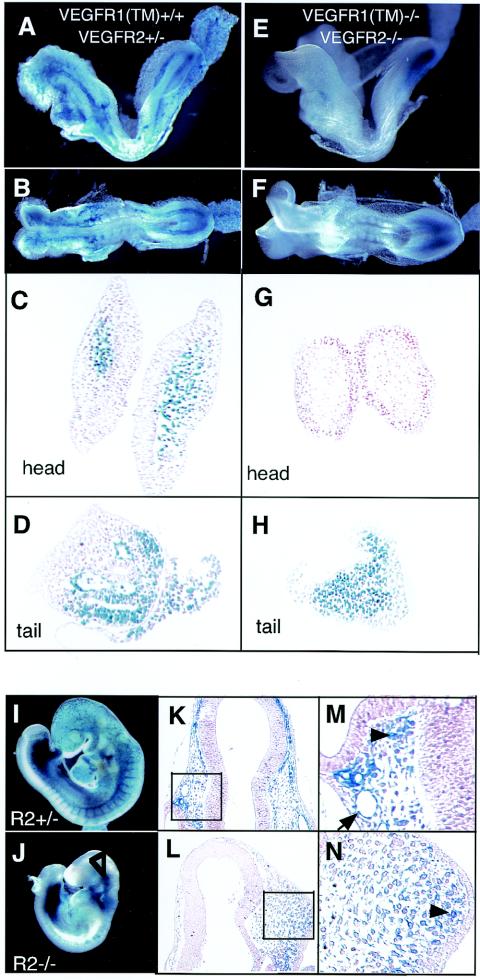
Severe endothelial cell differentiation and accumulation-migration defects in VEGFR1(TM-TK)−/− VEGFR2+/− mice. (A) mRNA expression of genes encoding various VEGF ligands and receptors in fetal tissues at E7.25 and E7.75 and in whole embryos including the fetus, yolk sac, and ectoplacental cone at E7.75 derived from C57BL6/J (B6) and 129 strains, as estimated by semiquantitative RT-PCR. (B to G) Anatomical features of LacZ-positive VEGFR2-expressing cells in VEGFR2+/− (B, D, and F) and VEGFR1(TM-TK)−/− VEGFR2+/− mice (C, E, and G) at E8.5. There were very few VEGFR2-positive cells in the anterior portion of the embryos, and growth arrest occurred at E8.0 in VEGFR1(TM-TK)−/− VEGFR2+/− mice (C) compared to VEGFR2+/− mice (B). The organization of the dorsal aorta in the middle portion (arrows) and of endothelial cells of the primitive heart (arrowheads) and cell accumulation (open arrowheads) were disturbed in VEGFR1(TM-TK)−/− VEGFR2+/− mice (E) compared to VEGFR2+/− mice (D). VEGFR2-positive cell accumulation in the heads of wild-type mice (arrow in panel F) was absent from the same region of VEGFR1(TM-TK)−/− VEGFR2+/− mice (arrow in panel G). However, these cells surrounding the gut (arrowheads) in the posterior region were seen in VEGFR1(TM-TK)−/− VEGFR2+/− mice (G), similar to VEGFR2+/− mice (F).
To reduce the signal for VEGFR2, we generated VEGFR1(TM-TK)−/− 129/B6 mice lacking a single VEGFR2 allele. About 90% of the VEGFR1(TM-TK)−/− VEGFR2+/− mice died as embryos (Table 2 and Fig. 4C) with a disturbed differentiation of endothelial cells and an abnormal accumulation of VEGFR2-positive cells at the middle to posterior portion, as detected by LacZ staining (Fig. 4E and G). These results support the idea that the degree of VEGFR2 signaling is important for the normal development of blood vessels.
TABLE 2.
Embryonal turning rates for various mutant micea
| Mating | Genotypeb | No. of embryos at E8.5 (no. of embryos without turning) |
|---|---|---|
| R1(TM-TK)+/− R2+/− × R1(TM-TK)+/− | R1(TM-TK)+/+ R2+/+ | 12 |
| R1(TM-TK)+/− R2+/+ | 28 (13) | |
| R1(TM-TK)−/− R2+/+ | 12 (6) | |
| R1(TM-TK)+/+ R2+/− | 12 | |
| R1(TM-TK)+/− R2+/− | 24 (11) | |
| R1(TM-TK)−/− R2+/− | 14 (12) |
The deletion of one allele of VEGFR2 significantly affected the incidence of embryonic lethality in VEGFR1(TM-TK)−/− mice with a genetic background of 50% C57BL6/J. Twelve of 14 VEGFR1(TM-TK)−/− VEGFR2+/− embryos showed no turning at E8.5.
Genetic background, 50% C57BL6/J.
Strong disturbance of VEGFR2-positive cell accumulation in the anterior portion of embryos in VEGFR1 and -2 kinase doubly deficient mice.
Distinct features of VEGFR1(TM-TK)-deficient embryos with developmental defects are a loss of turning in the embryo and a severe disturbance of VEGFR2-positive cell accumulation and migration to the anterior portion (Table 1 and Fig. 4C and G). Therefore, we suggested that VEGFR signaling is crucial for the proper accumulation and migration of the VEGFR2-positive cells in the whole embryo, particularly at the anterior end, and that VEGFR1(TM-TK)−/− mice may frequently fail to achieve sufficient VEGF signaling for this process. Thus, finally we examined whether the accumulation and migration of VEGFR2-positive cells in the anterior portion are really dependent on VEGFR tyrosine kinase activity.
VEGFR2-positive cells were traced by staining for lacZ, which was inserted into the VEGFR2 gene (21). As a positive control for staining, we used VEGFR2+/− mice that were already known to be basically normal (21). LacZ-positive cells were well distributed and accumulated in the anterior portion of embryos in VEGFR1+/+ VEGFR2+/− mice (Fig. 5A, C, and D). However, VEGFR1-R2 tyrosine kinase doubly deficient mice [VEGFR1(TM-TK)−/− VEGFR2−/− mice] all showed embryonic lethality, and LacZ-positive cells were observed only in the posterior portion (Fig. 5E, F, and H). The accumulation and migration of VEGFR2-positive cells to the anterior portion were strongly suppressed in these VEGFR1/2 kinase-deficient mice (Fig. 5G).
FIG. 5.
Complete loss of VEGFR-positive cell accumulation and migration to the anterior in VEGFR1(TM-TK)−/− VEGFR2−/− mice. (A to F) Whole-mounted LacZ-stained embryos at E.8.0 lost signals in the anterior portion in VEGFR1(TM-TK)−/− VEGFR2−/− mice (E and F) compared with VEGFR2+/− mice (A and B). The histology of LacZ-positive cells in the head region (C and G) and tail region (D and H) derived from VEGFR2+/− (C and D) and VEGFR1(TM-TK)−/− VEGFR2−/− (G and H) mice is shown. (I and J) VEGFR2-promoter-driven LacZ-positive cells in VEGFR2+/− (I) and VEGFR2−/− (J) mice at E8.5. LacZ-positive cells were seen in the cranial region of VEGFR2−/− mice (J, open arrowhead). (K to N) Histology of LacZ-positive cells surrounding neural tubes in VEGFR2+/− (K and M) and VEGFR2−/− (L and N) mice. The higher magnification shows endothelial cells lining head vessels (arrow) and migrated LacZ-positive cells (arrowheads).
In the case of VEGFR1+/+ VEGFR2−/− mice, LacZ-positive cells were distributed near the anterior portion, but no blood vessels were developed, suggesting that VEGFR1 tyrosine kinase can partially stimulate the migration of endothelial progenitor cells without supporting the differentiation of these cells into mature endothelial cells (Fig. 5J, L, and N).
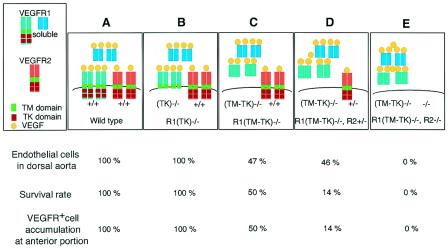
In summary, endothelial cell differentiation early in embryogenesis depends essentially on the strength of VEGFR2 signaling, which is regulated by the proper delivery of VEGF via ligand binding and the TM domains of VEGFR1 (Fig. 6). Consistent with these results, fewer endothelial cells were observed lining the smaller lumen of the dorsal aorta in the anterior than the posterior part of VEGF+/− embryos (3, 5). In contrast, VEGFR2-positive cell accumulation and migration depend on VEGFR tyrosine kinases (Fig. 6).
FIG. 6.
Hypothesized model of the VEGF system regulated by the transmembrane (TM) or tyrosine kinase (TK) domain of VEGFR1. The percentages of endothelial cells lining the dorsal aorta in embryos (top), of embryos that showed normal development (middle), and of embryos successfully showing the accumulation and migration of VEGFR-positive cells to the anterior portion (bottom) are expressed for each genotype. The percentages of endothelial cells in VEGFR1(TM-TK)−/− and VEGFR1(TM-TK)−/− VEGFR2+/− mice are shown for abnormal embryos. The VEGFR1(TM-TK) domain-dependent regulation of the strength of VEGFR2 signaling affects endothelial cell generation and differentiation (A to E). The extent of the disturbance of migration depends on the VEGFR2 gene dosage in VEGFR1(TM-TK)−/− mice (C and D). VEGFR1 also contributes to this migration (C).
DISCUSSION
The VEGF system is implicated in blood vessel and lymphatic development (6, 17, 20, 22). If mouse embryos lack the gene encoding either VEGF or VEGFR2, yolk sac blood islands fail to appear, and vasculogenesis does not take place (3, 5, 21). On the other hand, a null mutation of VEGFR1 resulted in the disorganization of differentiated endothelial cells and blood cells, with an increase in the numbers of these cells (7). Therefore, VEGFR1 and VEGFR2 have distinct functions for endothelial cells and blood cells in embryogenesis. In brief, VEGFR2 generates endothelial and blood cells from progenitor cells, while VEGFR1 suppresses oversignaling of the VEGF system and organizes the cells that have differentiated.
It is an attractive idea that in mice there is a crucial point in the development of blood vessels early in embryogenesis at which a proper degree of VEGFR2 signaling via the VEGFR1 extracellular TM domain is required. Supporting this idea, in the case of oversignaling in VEGFR1 null mutant mice (7) or reduced signaling in half of the VEGFR1(TM-TK)+/− and VEGFR1(TM-TK)−/− mice shown here, the closed circulatory system was frequently not completed, leading to embryonic lethality.
An important question to be answered is why half of the VEGFR1(TM-TK)−/− mice showed embyronic lethality and half were basically healthy. Although a simple explanation is difficult, we suggest the following two reasons. The first is that the embryo may have a critical short period around E7.0 to E8.0 for normal vasculogenesis and angiogenesis which may be overcome by a balanced VEGFR1 and VEGFR2 interaction. Once this period had passed, angiogenesis proceeds smoothly with some flexibility. The second is that in VEGFR1(TM-TK)−/− mice, the truncated VEGFR-1 ligand-binding domain is not fixed to the cell membrane, so these molecules randomly work either (i) properly near the endothelial cell surface in some embryos or (ii) not properly far from the cell surface in other embryos. In the former case, the mice undergo vasculogenesis and angiogenesis almost normally, but in the latter case, the embryos die due to a severe disturbance in vasculogenesis. This randomness model may be supported by the finding that about half of the offspring of the surviving VEGFR1(TM-TK)−/− mice again showed embryonic lethality due to a strong vasculogenesis-angiogenesis defect (Table 1).
In addition, it should be pointed out that the deficiency in VEGFR1(TM-TK)−/− mice appears to be closely related to the degree of VEGFR2 signaling, since the survival rate of mice was dose dependent at the VEGFR2 locus. Heterozygous VEGFR2 [VEGFR2+/− VEGFR1(TM-TK)−/−] mice showed about 90% embryonic lethality in the 129/B6 genetic background.
Another striking feature of VEGFR1(TM-TK)−/− mice was a significant difference in mortality rates for different genetic backgrounds. In the 50% 129-50% C57BL6 background, about half of the mice died as embryos, whereas in the 99% C57BL6 background, most of the mice survived. The expression level of the VEGFR2 gene was about threefold higher in C57BL6 embryos than in 129 embryos (Fig. 4A), suggesting that C57BL6 mice with a higher level of VEGFR2 easily overcome the critical period for vasculogenesis and angiogenesis. However, differences in other factors between these two strains of mice, such as the cell-to-cell or cell-to-matrix interaction system and the intracellular signaling system, cannot be ruled out at this point.
RTKs elicit their biological responses mainly by the sequential steps of ligand binding to the extracellular domain inducing receptor dimerization followed by increased kinase activity and autophosphorylation (9, 27, 28). The role of the TM domain has been thought to keep the RTK stable as a cell surface transmembrane protein. In addition, mutations within the TM domain have been shown to induce ligand-independent kinase activation in RTK HER2/neu (2). Our findings in this study suggest that the VEGFR1 extracellular TM domain has an important role in fixing the ligand-binding domain to the cell membrane and directly regulating the levels of ligands near the cell surface.
Acknowledgments
We thank Tetsuo Noda and Satoshi Tanaka for the preparation of VEGFR1(TK)−/− mice and for critical advice.
This work was supported by Special Project Research on Cancer-Bioscience grant-in-aid 12215024 from the Ministry of Education, Culture, Sports, Science and Technology of Japan, by the program Research for the Future of the Japan Society for the Promotion of Science, and by the program of the Organization for Pharmaceutical Safety and Research.
REFERENCES
- 1.Autiero, M., J. Waltenberger, D. Communi, A. Kranz, L. Moons, D. Lambrechts, J. Kroll, S. Plaisance, M. De Mol, F. Bono, S. Kliche, G. Fellbrich, K. Ballmer-Hofer, D. Maglione, U. Mayr-Beyrle, M. Dewerchin, S. Dombrowski, D. Stanimirovic, P. Van Hummelen, C. Dehio, D. J. Hicklin, G. Persico, J. M. Herbert, D. Communi, M. Shibuya, D. Collen, E. M. Conway, and P. Carmeliet. 2003. Role of PlGF in the intra- and intermolecular cross talk between the VEGF receptors Flt1 and Flk1. Nat. Med. 7:936-943. [DOI] [PubMed] [Google Scholar]
- 2.Bargmann, C. I., M. C. Hung, and R. A. Weinberg. 1986. The neu oncogene encodes an epidermal growth factor receptor-related protein. Cell 45:649-657. [DOI] [PubMed] [Google Scholar]
- 3.Carmeliet, P., V. Ferreira, G. Breier, S. Pollefeyt, L. Kieckens, M. Gertsenstein, M. Fahrig, A. Vandenhoeck, K. Harpal, C. Eberhardt, C. Declercq, J. Pawling, L. Moons, D. Collen, W. Risau, and A. Nagy. 1996. Abnormal blood vessel development and lethality in embryos lacking a single VEGF allele. Nature 380:435-439. [DOI] [PubMed] [Google Scholar]
- 4.Ema, M., P. Faloon, W. J. Zhang, M. Hirashima, T. Reid, W. L. Stanford, S. Orkin, K. Choi, and J. Rossant. 2003. Combinatorial effects of Flk1 and Tal1 on vascular and hematopoietic development in the mouse. Genes Dev. 17:380-393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ferrara, N., K. Carver-Moore, H. Chen, M. Dowd, L. Lu, K. S. O'Shea, L. Powell-Braxton, K. J. Hillan, and M. W. Moore. 1996. Heterozygous embryonic lethality induced by targeted inactivation of the VEGF gene. Nature 380:439-442. [DOI] [PubMed] [Google Scholar]
- 6.Ferrara, N., and T. Davis-Smyth. 1997. The biology of vascular endothelial growth factor. Endocr. Rev. 18:4-25. [DOI] [PubMed] [Google Scholar]
- 7.Fong, G. H., J. Rossant, M. Gertsenstein, and M. L. Breitman. 1995. Role of the Flt-1 receptor tyrosine kinase in regulating the assembly of vascular endothelium. Nature 376:66-70. [DOI] [PubMed] [Google Scholar]
- 8.He, Y., S. K. Smith, K. A. Day, D. E. Clark, D. R. Licence, and D. S. Charnock-Jones. 1999. Alternative splicing of vascular endothelial growth factor (VEGF)-R1 (FLT-1) pre-mRNA is important for the regulation of VEGF activity. Mol. Endocrinol. 13:537-545. [DOI] [PubMed] [Google Scholar]
- 9.Heldin, C. H. 1995. Dimerization of cell surface receptors in signal transduction. Cell 80:213-223. [DOI] [PubMed] [Google Scholar]
- 10.Hiratsuka, S., O. Minowa, J. Kuno, T. Noda, and M. Shibuya. 1998. Flt-1 lacking the tyrosine kinase domain is sufficient for normal development and angiogenesis in mice. Proc. Natl. Acad. Sci. USA 95:9349-9354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hiratsuka, S., K. Nakamura, S. Iwai, M. Murakami, T. Itoh, H. Kijima, J. M. Shipley, R. M. Senior, and M. Shibuya. 2002. MMP9 induction by vascular endothelial growth factor receptor-1 is involved in lung-specific metastasis. Cancer Cell 2:289-300. [DOI] [PubMed] [Google Scholar]
- 12.Kendall, R. L., and K. A. Thomas. 1993. Inhibition of vascular endothelial cell growth factor activity by an endogenously encoded soluble receptor. Proc. Natl. Acad. Sci. USA 90:10705-10709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kondo, K., S. Hiratsuka, E. Subbalakshmi, H. Matsushime, and M. Shibuya. 1998. Genomic organization of the flt-1 gene encoding for vascular endothelial growth factor (VEGF) receptor-1 suggests an intimate evolutionary relationship between the 7-Ig and the 5-Ig tyrosine kinase receptors. Gene 208:297-305. [DOI] [PubMed] [Google Scholar]
- 14.Luo, J. C., S. Yamaguchi, A. Shinkai, K. Shitara, and M. Shibuya. 1998. Significant expression of vascular endothelial growth factor/vascular permeability factor in mouse ascites tumors. Cancer Res. 58:2652-2660. [PubMed] [Google Scholar]
- 15.Matthews, W., C. T. Jordan, M. Gavin, N. A. Jenkins, N. G. Copeland, and I. R. Lemischka. 1991. A receptor tyrosine kinase cDNA isolated from a population of enriched primitive hematopoietic cells and exhibiting close genetic linkage to c-kit. Proc. Natl. Acad. Sci. USA 88:9026-9030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Meeson, A. P., M. Argilla, K. Ko, L. Witte, and R. A. Lang. 1999. VEGF deprivation-induced apoptosis is a component of programmed capillary regression. Development 126:1407-1415. [DOI] [PubMed] [Google Scholar]
- 17.Mustonen, T., and K. Alitalo. 1995. Endothelial receptor tyrosine kinases involved in angiogenesis. J. Cell Biol. 129:895-898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nishikawa, S. I., S. Nishikawa, M. Hirashima, N. Matsuyoshi, and H. Kodama. 1998. Progressive lineage analysis by cell sorting and culture identifies FLK1+VE-cadherin+ cells at a diverging point of endothelial and hemopoietic lineages. Development 125:1747-1757. [DOI] [PubMed] [Google Scholar]
- 19.Park, J. E., H. H. Chen, J. Winer, K. A. Houck, and N. Ferrara. 1994. Placenta growth factor. Potentiation of vascular endothelial growth factor bioactivity, in vitro and in vivo, and high affinity binding to Flt-1 but not to Flk-1/KDR. J. Biol. Chem. 269:25646-25654. [PubMed] [Google Scholar]
- 20.Risau, W. 1997. Mechanisms of angiogenesis. Nature 386:671-674. [DOI] [PubMed] [Google Scholar]
- 21.Shalaby, F., J. Rossant, T. P. Yamaguchi, M. Gertsenstein, X. F. Wu, M. L. Breitman, and A. C. Schuh. 1995. Failure of blood-island formation and vasculogenesis in Flk-1-deficient mice. Nature 376:62-66. [DOI] [PubMed] [Google Scholar]
- 22.Shibuya, M. 2001. Structure and function of VEGF/VEGF-receptor system involved in angiogenesis. Cell Struct. Funct. 26:25-35. [DOI] [PubMed] [Google Scholar]
- 23.Shibuya, M., S. Yamaguchi, A. Yamane, T. Ikeda, A. Tojo, H. Matsushime, and M. Sato. 1990. Nucleotide sequence and expression of a novel human receptor-type tyrosine kinase gene (flt) closely related to the fms family. Oncogene 5:519-524. [PubMed] [Google Scholar]
- 24.Takahashi, T., S. Yamaguchi, K. Chida, and M. Shibuya. 2001. A single autophosphorylation site on KDR/Flk-1 is essential for VEGF-A-dependent activation of PLC-gamma and DNA synthesis in vascular endothelial cells. EMBO J. 20:2768-2778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Takakura, N., T. Watanabe, S. Suenobu, Y. Yamada, T. Noda, Y. Ito, M. Satake, and T. Suda. 2000. A role for hematopoietic stem cells in promoting angiogenesis. Cell 102:199-209. [DOI] [PubMed] [Google Scholar]
- 26.Terman, B. I., M. Dougher-Vermazen, M. E. Carrion, D. Dimitrov, D. C. Armellino, D. Gospodarowicz, and P. Bohlen. 1992. Identification of the KDR tyrosine kinase as a receptor for vascular endothelial cell growth factor. Biochem. Biophys. Res. Commun. 187:1579-1586. [DOI] [PubMed] [Google Scholar]
- 27.Ulrich, A., and J. Schlessinger. 1990. Signal transduction by receptors with tyrosine kinase activity. Cell 61:203-212. [DOI] [PubMed] [Google Scholar]
- 28.Zwick, E., J. Bange, and A. Ullrich. 2001. Receptor tyrosine kinase signalling as a target for cancer intervention strategies. Endocr. Relat. Cancer 8:161-173. [DOI] [PubMed] [Google Scholar]