Abstract
Marine pollution caused by frequent oil spill accidents has brought about tremendous damages to marine ecological environment. Therefore, the facile large-scale preparation of three-dimensional (3D) porous functional materials with special wettability is in urgent demand. In this study, we report a low-cost and salt-tolerant superoleophobic aerogel for efficient oil/seawater separation. The aerogel is prepared through incorporating graphene oxide (GO) into alginate (ALG) matrix by using a facile combined freeze-drying and ionic cross-linking method. The 3D structure interconnected by ALG and GO ensures the high mechanical strength and good flexibility of the developed aerogel. The rough microstructure combined with the hydrophilicity of the aerogel ensures its excellent underwater superoleophobic and antifouling properties. High-content polysaccharides contained in the aerogel guarantees its excellent salt-tolerant property. More impressively, the developed aerogel can retain its underwater superoleophobicity even after 30 days of immersion in seawater, indicating its good stability in marine environments. Furthermore, the aerogel could separate various oil/water mixtures with high separation efficiency (>99%) and good reusability (at least 40 cycles). The facile fabrication process combined with the excellent separation performance makes it promising for practical applications in marine environments.
In the past decades, the marine pollution caused by frequent oil spill accidents has brought about tremendous damages to marine ecological environment1,2,3. How to efficiently and effectively realize oil/water separation in marine environments has become a subject of global concern. Since oil/water separation is an interfacial issue, applying functional materials with special wetting properties is considered an effective and facile strategy4,5,6,7,8. Up until now, most in-air superoleophobic materials are based on the modification of fluorination reagents9,10,11. However, fluoride-modified materials will lose their superoleophobicity in seawater. Encouragingly, the discovery of unique underwater oil-repellent property of fish scale opened a new window for fabricating underwater superoleophobic materials such as hydrogels coatings12,13,14,15 and inorganic metal oxide coatings16,17,18,19. However, the biggest challenge of using these materials is their poor environmental adaptability. They may fall off from their substrates or suffer from swelling deformation and result in a gradual loss of superoleophobicity after a long-term seawater immersion. Furthermore, current methods are complicated, expensive, and difficult to realize a large-scale production. Therefore, it is imperative to develop function materials that are cost-effective, salt-tolerant and scalable to achieve the oil/water separation in marine environments.
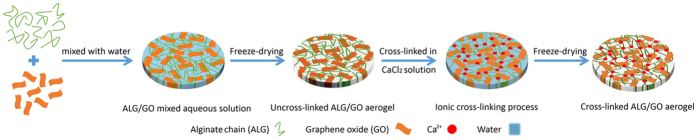
Aerogel, a kind of highly porous solid material with three-dimensional (3D) network structure and low density, has been considered to be a promising candidate for achieving an efficient oil/water separation in a gravity-driven process with high-flux20,21,22. Alginate (ALG) is a linear, unbranched polysaccharide consisting of (1, 4)-linked α-L-guluronic acid units (G-blocks) and β-D-mannuronic acid units (M-blocks)23. It is naturally present in the cell walls of brown seaweeds and commercially available as a sodium salt24. Recent studies have indicated that this low-cost natural biomaterial possesses excellent salt-tolerant and underwater superoleophobic properties25. Thus, alginate aerogel, combining the advantages of both, has great potentials to achieve oil/water separation in marine environments. However, there are still two obstacles to overcome, namely (i) the low mechanical strength of pure alginate aerogel and (ii) complicated ionic cross-linking process. To overcome the first obstacle, an effective approach is the incorporation of reinforcing fillers into the alginate matrix and here graphene oxide (GO) is chosen as reinforcing filler owing to its excellent mechanical property and remarkable flexibility26,27,28, which can effectively resist the load transferred from the alginate matrix. It is well known that directly introducing divalent ions into alginate solution is more likely to produce anisotropic gel particles instead of a homogeneous gel, which attributes to the rapid cross-linking reaction between the carboxyl groups of the alginate and divalent ions. To tackle this problem, the CaCO3-GDL (Gluconolactone) system developed by Kuo et al. was usually introduced to reduce the release rate of calcium ions29,30,31,32. Although this system is available to produce homogenous alginate gel, it is more complex in practical operation. Therefore, in response to the second obstacle, this study presents a more facile method by which the ALG/GO mixture was firstly freeze-dried to obtain uncross-linked aerogel scaffold and then immersed in CaCl2 aqueous solution to achieve the rapid ionic cross-linking reaction. In other words, the method takes full advantage of the rapid reaction between alginate and calcium ions to quickly harden the porous ALG/GO scaffold.
Herein, a low-cost and robust alginate (ALG)/graphene oxide (GO) hybrid aerogel was fabricated via a facile combined freeze-drying and ionic cross-linking method (Fig. 1). The developed ALG/GO aerogel without any further chemical modification was, to our knowledge, used for oil/seawater separation for the first time. The characteristics of the ALG/GO aerogel, including wetting behaviours in air and seawater, salt tolerance, mechanical performance, separation efficiency and recyclability for oil/seawater mixtures were investigated in detail. As expected, the developed hybrid aerogel exhibited high separation efficiency and good recyclability for oil/seawater mixtures, making it promising for practical applications in marine environments.
Figure 1. The preparation of cross-linked alginate (ALG)/graphene oxide (GO) aerogel.
Results and Discussion
The microstructural morphology of the ALG/GO aerogel was studied using a scanning electron microscopy (SEM). As illustrated in Fig. 2, the aerogel exhibits a fine pore structure, similar to a sponge or crumb of bread. There is no obvious difference between structures on the cross sections (Fig. 2a) and vertical sections (Fig. 2b). Quite evidently, the aerogel is highly porous and has well-connected 3D network structures. The pore size of the aerogel is randomly distributed in the range of 50–150 μm. The ideal pore size and network-distribution of the as-prepared aerogel allow oil/water separation to be achieved in a gravity-driven process with high-flux. The highly-magnified SEM image (Fig. 2c) shows the micropores in aerogel are irregular in shape and have a rough surface. The surface roughness combined with its high affinity to water is in fact essential for achieving underwater superoleophobic property of the aerogel.
Figure 2.
FE-SEM images of cross sections (a), vertical sections (b), and high magnification (c) of the ALG/GO aerogel.
To investigate the wetting behaviour of ALG/GO aerogel towards water and oil in air, a high-resolution camera was used to record the spreading of water and oil droplets. When a water droplet (5 μL) contacts the aerogel surface in air, it spreads out and permeats into the aerogel quickly, resulting in a contact angle of approximately 0° (Fig. 3a). A similar phenomenon is also observed when an oil droplet (5 μL) contacts the aerogel surface (Fig. 3b). The superamphiphilic property of the aerogel in air may be attributed to the cooperative effect of its surface rough microstructure and large amounts of hydroxyl groups on the aerogel surface.
Figure 3.
Water wettability (a) and oil wettability (b) of ALG/GO aerogel in air.
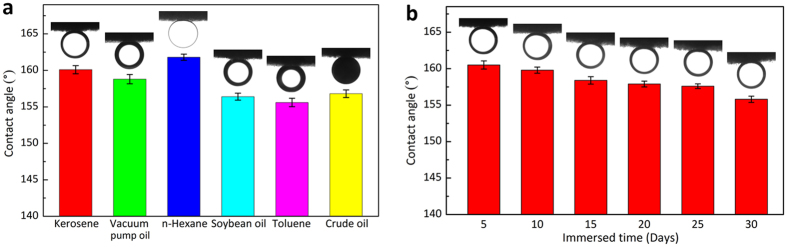
The underwater superoleophobicity of the ALG/GO aerogel was investigated by immersing the aerogel into seawater. In the three-phase system of oil/water/solid, the water trapped in the micro-roughness structures of ALG/GO aerogel gives rise to a strong repulsive force to oil, resulting in a large contact angle. Therefore, without any chemical modification, the as-prepared ALG/GO aerogel possesses underwater superoleophobic property. The measuring process of underwater oil contact angle was recorded in Supplementary Movie S1. As shown in the movie, the underwater OCA of ALG/GO aerogel is as large as 160.1°, indicating the aerogel has excellent underwater superoleophobic property. Meanwhile, the oil droplet (kerosene) would shake and quickly slide off the aerogel surface when it was disturbed slightly. The low affinity of the water-wetted aerogel for oil can effectively prevent ALG/GO aerogel to be polluted or blocked up by oil during oil/water separation process. In addition, the aerogel also shows extraordinary underwater superoleophobic property for other oils and organic solvents (Fig. 4a). The oil contact angles for crude oil, kerosene, n-hexane, toluene and soybean oil are all larger than 155° in seawater.
Figure 4.
(a) the contact angles of various oil on ALG/GO aerogel under seawater; (b) the underwater contact angles of kerosene on ALG/GO aerogel during 30 days of immersion in seawater.
To evaluate the environmental stability, the contact angle of aerogel was measured after being exposed to seawater for a prolonged period of time (Fig. 4b). It is apparent that the aerogel retains its underwater superoleophobic property (OCA > 150°) after soaking for 30 days, indicating an excellent durability in high ionic strength environments, which ascribes to its main content of polysaccharides and robust 3D interconnected network structure.
From the viewpoint of practical applications, the mechanical strength and flexibility of the ALG/GO aerogel also play important role in the separation of oil/seawater mixture. As shown in Supplementary Movie S2, the aerogel can be crumpled into a ball and recovered immediately to its original shape without any damage, which mainly due to its mechanical stability of 3D cross-linked structures.
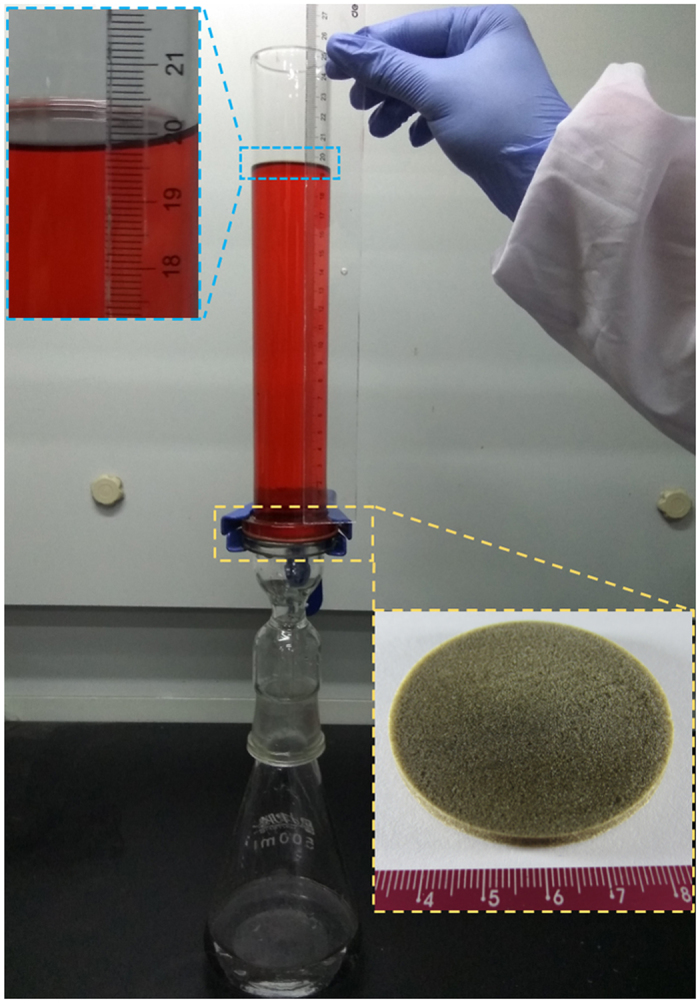
To take full advantage of the above properties, the ALG/GO aerogel was used to perform gravity-driven oil/seawater separation. The experiment was carried out as illustrated in Supplementary Movie S3. The ALG/GO aerogel was fixed between two glass tubes by stainless steel spring clamps and then a mixture of oil and seawater (1:1, v/v) was poured into the upper tube. The seawater quickly penetrated through the pre-wetted aerogel by gravity, while the oil was selectively blocked. There was no visible oil observed in the filtered water, indicating an efficient separation of the oil/seawater mixture.
The separation efficiency η of the aerogel was also confirmed by the following eqn (1):
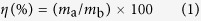 |
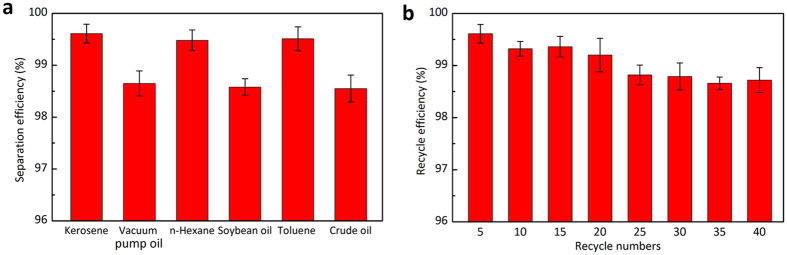
where, mb and ma represent the mass of the oil before and after filtration, respectively. The separation efficiency was calculated to be above 99.6% for the kerosene-seawater mixture and above 98.5% for other oils and organic solvents (Fig. 5a). The efficiency of the separation may be attributed to the high porosity and underwater superoleophobicity of the aerogel. Moreover, the reusability of ALG/GO aerogel has been studied. It has been found that the separation efficiency for the kerosene-seawater mixture remains above 98.5% after the aerogel has been reused for 40 separation cycles (Fig. 5b), confirming the excellent reusability of the as-prepared aerogel.
Figure 5.
(a) the separation efficiency of oil/seawater mixtures; (b) the separation efficiency versus the recycle numbers by taking the kerosene/seawater mixture as an example.
To further study the separation ability of the ALG/GO aerogel, Oil intrusion pressure and water flux were also investigated. Oil intrusion pressure, also called oil breakthrough, essentially caused by repulsive force between the water-wetted aerogel and oil in the oil/water/solid three-phase system, macroscopically reflected the maximum height (hmax) of oil that the as-prepared aerogel can support.
The oil intrusion pressure (Po) was calculated using eqn (2):
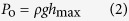 |
where, ρ is the density of oil, g is the acceleration of gravity, and hmax represents the maximum height of oil that the aerogel can support33. As displayed in Fig. 6, the value of hmax can reach 19.8 cm for kerosene and the oil intrusion pressure is 1.55 kPa. Under this pressure, the aerogel can achieve the separation of kerosene/seawater mixture.
Figure 6. The intrusion pressure of oil taking kerosene as an example.
The water flux (F), defined as the volume of water that passes through the aerogel per unit time, was calculated according to eqn (3):
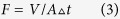 |
where, V is the volume of seawater that permeates through the aerogel, A is the effective filtration area of the aerogel and ▵t represents the time required for the permeation. Here, the water flux calculated from eqn (3) was as high as 3.8 L m−2 s−1.
To illustrate the water/oil separation mechanism of the ALG/GO aerogel, the equation to calculate theoretical intrusion pressure was introduced34,35,36.
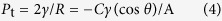 |
where γ is the surface tension, θ is the liquid contact angle on the material, R is the radius of the meniscus, C is the circumference of the pore in the material, and A is the cross-sectional area of the pore. According to eqn (4), the intrusion pressure Pt is >0 (negative capillary effect) when θ > 90°. Under this condition, the material can sustain a certain liquid pressure. In other words, liquid cannot permeate the aerogel spontaneously without an external pressure. In contrast, the intrusion pressure Pt is <0 (capillary effect) when θ < 90°. In this case, materials cannot support any pressure and liquid would permeate the aerogel spontaneously by self-gravity. From the discussion in the previous sections, it is apparent that the superamphiphilicity (θ is nearly 0°) of the aerogel allows liquid (water or oil) to permeate through the aerogel quickly by self-gravity. When the aerogel was immersed in water, water would trap in the rough microstructure of the aerogel that then formed an oil/water/solid three-phase system in the presence of oil. The repulsive force between the trapped water and oil resulted in a significant reduction in the contact area between the oil and surface of the aerogel, leading to θ > 150° (underwater superoleophobicity) and Pt > 0, hence oil/water mixtures could be selectively separated efficiently using the developed underwater superoleophobic ALG/GO aerogel.
In conclusion, a robust salt-tolerant superoleophobic aerogel has been developed through incorporating graphene oxide (GO) into alginate (ALG) matrix by combining freeze-drying and ionic cross-linking process. The 3D interconnected network structure of the ALG/GO aerogel was obtained, which ensured its high mechanical strength and good flexibility. The developed aerogel showed excellent underwater superoleophobicity, salt-tolerance and antifouling properties. The aerogel can be adapted to perform different types of oil/seawater separation and demonstrated both highly efficient and reusable in high-salinity environments. We believe that this kind of aerogel is a promising candidate material for oil/water separation in marine environments.
Methods
Materials
Graphene oxide (GO) suspensions (1 wt%) were obtained from Shanghai Ashine Technology Development Co., Ltd. Sodium alginate was purchased from Shanghai Macklin Biochemical Co., Ltd. Calcium chloride (CaCl2) was purchased from Xilong Chemical Co., Ltd. Crude oil was provided by Befar Group Co., Ltd. Toluene and n-hexane were purchased from Shanghai Aladdin Bio-Chem Technology Co., Ltd. Kerosene was obtained from SINOPEC Fujian Refining & Chemical Co., Ltd. Vacuum pump oil was purchased from ExxonMobil (China) Investment Co., Ltd. Soybean oil was obtained from Shandong Luhua Group Co., Ltd. Sea salts were purchased from Sigma Aldrich, which is an artificial salt mixture closely resembling the composition of the dissolved salts of ocean water. The seawater was prepared by dissolving 17.2 g sea salts in 500 mL of deionized water.
Preparation of alginate (ALG)/graphene oxide (GO) aerogel
The ALG/GO aerogel was fabricated through incorporating graphene oxide (GO) into alginate (ALG) matrix by combining freeze-drying and ionic cross-linking process. Firstly, the GO suspension (1 wt%) was ultrasonic-dispersed for 15 min to obtain a homogeneous dispersion. Meanwhile, sodium alginate was dissolved in deionized water and constantly stirred for 2 h to form a transparent solution (2 wt%). Then, GO suspensions were added into the above sodium alginate solution in a volume ratio of 1: 12 (GO suspensions: sodium alginate solution), with continuous magnetic stirring, until a homogeneous dispersion was obtained. The sodium alginate/graphene oxide mixture was poured into a Teflon Petri dish, frozen at −18 °C, and lyophilized at 600 mbar (absolute pressure) for 40 h in a laboratory-scale freeze dryer (LyoBeta 3PS, Telstar, Spain) to produce the aerogel. After that, the aerogel was immerged in 5 wt% CaCl2 solution for 10 h to achieve the calcium ion induced cross-linking process. After cross-linked by calcium ions, the aerogel was washed with deionized water several times to remove the unbound calcium ions and freeze-dried again to obtain the cross-linked ALG/GO aerogel.
Oil/seawater separation experiment
Six types of oils and organic solvents, including kerosene, toluene, n-hexane, vacuum pump oil, soybean oil and crude oil were dyed red and respectively mixed with seawater to prepare different kinds of oil/water mixtures. The ALG/GO aerogel with 40 mm diameter and 6 mm thickness was carefully fixed between two glass tubes by stainless steel spring clamps. Then, a mixture of oil and seawater (1:1, v/v) was poured into the upper tube. Finally, the separation process was achieved by the driving force of gravity.
Characterization methods
The surface morphologies of the aerogel were assessed using field emission scanning electron microscopy (FE-SEM, Hitachi SU8000). The water and oil contact angles were measured using a drop shape analysis system (Kruss DSA30) by injecting 5 μL of water or oil droplets onto the ALG/GO aerogel surfaces. Five different locations of the surface were examined to obtain the average value.
Additional Information
How to cite this article: Li, Y. et al. A robust salt-tolerant superoleophobic alginate/graphene oxide aerogel for efficient oil/water separation in marine environments. Sci. Rep. 7, 46379; doi: 10.1038/srep46379 (2017).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Material
Acknowledgments
We gratefully acknowledge the financial support from the National Natural Science Foundation of China (Grant No. 21506031), the Natural Science Foundation of Fujian Province (Grant No. 2016J01207) and the Outstanding Youth Fund of Fujian Agriculture and Forestry University (Grant No. XJQ201419).
Footnotes
The authors declare no competing financial interests.
Author Contributions L.Y. and Z.H. conceived the idea and designed the experiments. L.Y. performed the major experiments, analyzed the data and wrote the manuscript. Z.H. and Z.P. contributed to the corrections and amendments of the manuscript. Z.J. performed parts of the FE-SEM characterization. C.L. and F.M. supervised the whole project.
References
- Pezeshki S. R., Hester M. W., Lin Q. & Nyman J. A. The effects of oil spill and clean-up on dominant US Gulf coast marsh macrophytes: A review. Environ. Pollut. 108, 129–139 (2000). [DOI] [PubMed] [Google Scholar]
- Hong S. et al. Environmental and ecological effects and recoveries after five years of the Hebei Spirit oil spill, Taean, Korea. Ocean Coast. Manage. 102, 522–532 (2014). [Google Scholar]
- Li P., Cai Q., Lin W., Chen B. & Zhang B. Offshore oil spill response practices and emerging challenges. Mar. Pollut. Bull. 110, 6–27 (2016). [DOI] [PubMed] [Google Scholar]
- Gao X. et al. Dual-Scaled porous nitrocellulose membranes with underwater superoleophobicity for highly efficient Oil/Water separation. Adv. Mater. 26, 1771–1775 (2014). [DOI] [PubMed] [Google Scholar]
- Xu L. et al. An Ion-Induced Low-Oil-Adhesion Organic/Inorganic hybrid film for stable superoleophobicity in seawater. Adv. Mater. 25, 606–611 (2013). [DOI] [PubMed] [Google Scholar]
- Chen K., Zhou S. & Wu L. Self-Healing underwater superoleophobic and antibiofouling coatings based on the assembly of hierarchical microgel spheres. ACS Nano. 10, 1386–1394 (2016). [DOI] [PubMed] [Google Scholar]
- Chen K., Zhou S., Yang S. & Wu L. Fabrication of All-Water-Based Self-Repairing Superhydrophobic Coatings Based on UV-Responsive Microcapsules. Adv. Funct. Mater. 25, 1035–1041 (2015). [Google Scholar]
- Han K., Heng L. & Jiang L. Multiphase media antiadhesive coatings: Hierarchical Self-Assembled porous materials generated using breath figure patterns. ACS Nano. 10, 11087–11095 (2016). [DOI] [PubMed] [Google Scholar]
- Bellanger H., Darmanin T. & Guittard F. Surface Structuration (Micro and/or Nano) Governed by the Fluorinated Tail Lengths toward Superoleophobic Surfaces. Langmuir. 28, 186–192 (2012). [DOI] [PubMed] [Google Scholar]
- Zhang W. et al. Micro/nano hierarchical poly(acrylic acid)-grafted-poly(vinylidene fluoride) layer coated foam membrane for temperature-controlled separation of heavy oil/water. Sep. Purif. Technol. 156, 207–214 (2015). [Google Scholar]
- Darmanin T. & Guittard F. Enhancement of the superoleophobic properties of fluorinated PEDOP using polar glycol spacers. J. Phys. Chem. C. 118, 26912–26920 (2014). [Google Scholar]
- Lin L. et al. Bio-Inspired hierarchical Macromolecule-Nanoclay hydrogels for robust underwater superoleophobicity. Adv. Mater. 22, 4826 (2010). [DOI] [PubMed] [Google Scholar]
- Teng C. et al. Underwater Self-Cleaning PEDOT-PSS hydrogel mesh for effective separation of corrosive and hot Oil/Water mixtures. Advanced Materials Interfaces 1 (2014). [Google Scholar]
- Xue Z. et al. A novel superhydrophilic and underwater superoleophobic Hydrogel-Coated mesh for Oil/Water separation. Adv. Mater. 23, 4270–4273 (2011). [DOI] [PubMed] [Google Scholar]
- Zhang W., Cao Y., Liu N., Chen Y. & Feng L. A novel solution-controlled hydrogel coated mesh for oil/water separation based on monolayer electrostatic self-assembly. RSC Adv. 4, 51404–51410 (2014). [Google Scholar]
- Liu X. et al. Clam’s shell inspired High-Energy inorganic coatings with underwater low adhesive superoleophobicity. Adv. Mater. 24, 3401–3405 (2012). [DOI] [PubMed] [Google Scholar]
- Li J. et al. Superhydrophilic-underwater superoleophobic ZnO-based coated mesh for highly efficient oil and water separation. Mater. Lett. 153, 62–65 (2015). [Google Scholar]
- Wang C., Tzeng F., Chen H. & Chang C. Ultraviolet-Durable superhydrophobic zinc Oxide-Coated mesh films for surface and Underwater-Oil capture and transportation. Langmuir. 28, 10015–10019 (2012). [DOI] [PubMed] [Google Scholar]
- Li J. et al. Facile fabrication of underwater superoleophobic TiO2 coated mesh for highly efficient oil/water separation. Colloid. Surface. A. 489, 441–446 (2016). [Google Scholar]
- Li Y., Zhang H., Fan M., Zhuang J. & Chen L. A robust salt-tolerant superoleophobic aerogel inspired by seaweed for efficient oil-water separation in marine environments. Phys. Chem. Chem. Phys. 18, 25394–25400 (2016). [DOI] [PubMed] [Google Scholar]
- Si Y. et al. Superelastic and superhydrophobic Nanofiber-Assembled cellular aerogels for effective separation of Oil/Water emulsions. ACS Nano. 9, 3791–3799 (2015). [DOI] [PubMed] [Google Scholar]
- Chen X., Liang Y. N., Tang X., Shen W. & Hu X. Additive-free poly (vinylidene fluoride) aerogel for oil/water separation and rapid oil absorption. Chem. Eng. J. 308, 18–26 (2017). [Google Scholar]
- Wan W. et al. BMSCs laden injectable amino-diethoxypropane modified alginate-chitosan hydrogel for hyaline cartilage reconstruction. J. Mater. Chem. B. 3, 1990–2005 (2015). [DOI] [PubMed] [Google Scholar]
- Sarker B. et al. Fabrication of alginate-gelatin crosslinked hydrogel microcapsules and evaluation of the microstructure and physico-chemical properties. J. Mater. Chem. B. 2, 1470–1482 (2014). [DOI] [PubMed] [Google Scholar]
- Cai Y. et al. Salt-Tolerant Superoleophobicity on Alginate Gel Surfaces Inspired by Seaweed (Saccharina japonica). Adv. Mater. 27, 4162–4168 (2015). [DOI] [PubMed] [Google Scholar]
- Hu K., Kulkarni D. D., Choi I. & Tsukruk V. V. Graphene-polymer nanocomposites for structural and functional applications. Prog. Polym. Sci. 39, 1934–1972 (2014). [Google Scholar]
- Liu J. et al. Graphene oxide and graphene nanosheet reinforced aluminium matrix composites: Powder synthesis and prepared composite characteristics. Mater. Design. 94, 87–94 (2016). [Google Scholar]
- Zuo P. et al. Fabrication of biocompatible and mechanically reinforced graphene oxide-chitosan nanocomposite films. Chem. Cent. J. 7 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo C. K. & Ma P. X. Ionically crosslinked alginate hydrogels as scaffolds for tissue engineering: Part 1. Structure, gelation rate and mechanical properties. Biomaterials. 22, 511–521 (2001). [DOI] [PubMed] [Google Scholar]
- Bruchet M. & Melman A. Fabrication of patterned calcium cross-linked alginate hydrogel films and coatings through reductive cation exchange. Carbohyd. Polym. 131, 57–64 (2015). [DOI] [PubMed] [Google Scholar]
- Chen H., Wang Y., Sanchez-Soto M. & Schiraldi D. A. Low flammability, foam-like materials based on ammonium alginate and sodium montmorillonite clay. Polymer. 53, 5825–5831 (2012). [Google Scholar]
- Fan L. et al. Sodium alginate conjugated graphene oxide as a new carrier for drug delivery system. Int. J. Biol. Macromol. 93, Part A, 582–590 (2016). [DOI] [PubMed] [Google Scholar]
- Li J. et al. Underwater superoleophobic palygorskite coated meshes for efficient oil/water separation. J. Mater. Chem. A. 3, 14696–14702 (2015). [Google Scholar]
- Li J. et al. A prewetting induced underwater superoleophobic or underoil (super) hydrophobic waste potato residue-coated mesh for selective efficient oil/water separation. Green Chem. 18, 541–549 (2016). [Google Scholar]
- Guo Z. et al. Photoelectric cooperative patterning of liquid permeation on the micro/nano hierarchically structured mesh film with low adhesion. Nanoscale. 6, 12822–12827 (2014). [DOI] [PubMed] [Google Scholar]
- Tian D. et al. Photo-induced water–oil separation based on switchable superhydrophobicity–superhydrophilicity and underwater superoleophobicity of the aligned ZnO nanorod array-coated mesh films. Journal of Materials Chemistry. 22, 19652 (2012). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.