Abstract
The hemangioblast in the mesoderm gives rise to both angioblasts and hematopoietic stem cells. The movement of hemangioblast precursor cells in the fetal trunk is a critical event in early embryogenesis. Vascular endothelial growth factor (VEGF) signaling is likely involved in this migration given the partial disturbance of VEGF receptor (VEGFR)-positive cell accumulation and migration in VEGFR2 null mice or mice with a truncated VEGFR1. However, it is not clear how the VEGF system regulates this migration or its direction. We show here that the expression of VEGF-A is dominant in the anterior portion of the embryo, whereas VEGFR1 and VEGFR2 are expressed in the posterior portion of the embryo. An inhibitor of VEGFR kinase blocked the migration of VEGFR-positive cells in a whole-embryo culture system. In addition, VEGFR-positive cells migrated toward a VEGFR1- or VEGFR2-specific ligand in vitro. Furthermore, VEGFR-positive cells derived from wild-type or VEGFR2+/− mice moved rapidly anteriorly, whereas cells derived from VEGFR2+/− mice carrying a truncated VEGFR1 [VEGFR1(TM-TK)−/−] migrated little when injected into wild-type mice. These results suggest that the VEGF-A protein concentrated in the anterior region plays an important role in the guidance of VEGFR-positive cells from the posterior portion to the head region by interacting with VEGFR in the mouse embryo.
Blood vessels are constructed by two processes, namely, vasculogenesis and angiogenesis. In vasculogenesis, blood vessels and blood cells share a common progenitor, the hemangioblast (38, 39). Hemangioblast precursor cells, which differentiate into hemangioblasts, cells of the smooth muscle cell lineage, and others, are derived from moving mesodermal cells and are dependent on growth factors such as bone morphogenetic protein, basic fibroblast growth factor 2 (FGF2), and vascular endothelial growth factor (VEGF). At first, the mesoderm appears between the endoderm and the ectoderm at the embryonic-extraembryonic junction, which marks the future posterior portion of the embryo. The nascent mesoderm moves in two directions. The most posterior mesoderm pushes its way into the extraembryonic region. It also moves predominantly anteriorly in the fetal tissue (20, 32, 42, 56).
VEGF and its receptor family, including VEGFR1 (Flt-1), VEGFR2 (KDR/Flk-1), and VEGFR3 (Flt-4), form a crucial regulatory system for normal and pathological angiogenesis (14, 37, 45, 50). VEGFR1, VEGFR2, and VEGFR3 are structurally related to the Fms/Kit/PDGFR family and contain an extracellular domain carrying seven immunoglobulin (Ig)-like sequences, a transmembrane (TM) domain, and a cytoplasmic tyrosine kinase (TK) domain with a long kinase insert. VEGFR1 is expressed as a full-length tyrosine kinase receptor and, in some cases, as a soluble form which carries only the extracellular domain (29, 36, 51, 58).
In VEGF-A gene knockout studies, even VEGF-A heterozygous (+/−) mice showed embryonic lethality due to multiple defects in vascular structure formation (6, 13). Both VEGFR1 null mutant mice and VEGFR2 null mutant mice were embryonically lethal at very similar stages, from embryonic day 8.0 (E8.0) to E8.5, but their phenotypes were different from each other (15, 49). VEGFR2 homozygous (−/−) mice died with no development of a vascular system or hematopoiesis, whereas VEGFR1 homozygotes (−/−) died due to an overgrowth of vascular endothelial cells and the disorganization of blood vessels.
In adults, VEGFR1 and VEGFR2 are specifically expressed on vascular endothelial cells (12, 25, 27, 44, 59). As an exception, the VEGFR1 mRNA has been shown to be expressed on monocytes/macrophages (3, 9). In addition, other cell types were also recently reported to express VEGFR1 (7, 18, 21). In contrast to VEGFR2, which is a major positive signal transducer for angiogenesis through its strong tyrosine kinase activity, VEGFR1 has a unique biochemical activity with a 10-fold higher affinity for VEGF-A than VEGFR2 but with a much weaker tyrosine kinase activity (37, 43, 47, 52).
The VEGF-A system has been proven to have roles in the embryonic development of vessels and blood cells by the use of mice deprived of the genes encoding its ligand or receptors (6, 13, 15, 49). VEGF-A and VEGFR2 contribute to the generation and regulation of hemangioblasts (5, 6, 49), and VEGFR1 acts as a negative regulator of the proliferation of differentiated endothelial cells in vessels (15, 16, 22). In addition, the migration of hemangioblast precursor cells toward fetal embryos is a critical step in the formation of the capillary network. Importantly, VEGFR1-TM-TK-deficient mice very often show a partial disturbance of VEGFR-positive cell accumulation and migration in the anterior portion of the embryo (see the companion to this paper [21a]). This is likely brought about by a loss of VEGFR1 signaling and reduced VEGFR2 signaling. Consistent with this idea, the major functional receptor concerning endothelial cell migration toward VEGF-A is believed to be VEGFR2 rather than VEGFR1 (19), whereas macrophage migration toward VEGFs was proven to be controlled by VEGFR1 because of its dominant expression (3, 9, 19, 47). However, it is not known which of the VEGF family members guides the precursor cells that express VEGF receptors.
We examined the locations where VEGF ligand receptors are expressed in early developmental fetal tissue and found the unique expression of a ligand and receptors in restricted regions. Detailed functional analyses performed with specific inhibitors and VEGFR-positive cells derived from VEGFR1/2-deficient mice strongly suggested that the interaction between VEGF-A and VEGF receptors is involved in hemangioblast precursor cell migration in the early embryo.
MATERIALS AND METHODS
Mice.
To generate VEGFR1(TM-TK) mutant mice, we first obtained a 24-kb mouse VEGFR1 genomic DNA clone, including a cDNA carrying exons 16 to 19, from a genomic library of the 129Sv strain (Stratagene, La Jolla, Calif.). The targeting vector contains a neomycin resistance gene which replaces exon 16, encoding the transmembrane domain, and it also encodes diphtheria toxin A. Targeted CCE embryonic stem cell clones were injected into C57BL6/J blastocysts, and male chimeric mice were crossed with female C57B6L/J mice to yield mice that were heterozygous for the VEGFR1(TM-TK) mutation (accompanying paper). The genotypes of the pups obtained by crosses between VEGFR1(TM-TK)+/− mice were determined by Southern blot hybridization and PCR analyses. VEGFR2 null mutant mice were purchased from Jackson Laboratory (Bar Harbor, Maine).
Whole embryo culture.
Pregnant mice were dissected at 7.25 to 7.75 days postcoitum, and embryos were roller cultured in DR75 medium (75% rat serum and 25% Dulbecco's modified Eagle's medium) (57) with dimethyl sulfoxide (DMSO; 0.01%) or 10 μM SU5416 in 0.01% DMSO at 37°C in 5% CO2 for 24 h. The wild-type and VEGFR2+/− embryos were then fixed for 10 min in 4% paraformaldehyde and stained with X-Gal (5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside) overnight at room temperature. Stained embryos were refixed in 4% paraformaldehyde, photographed, and processed for wax histology. Five-micrometer-thick sections were counterstained with eosin.
Reverse transcription-PCR (RT-PCR) analysis.
Total RNAs from embryo tissue samples were extracted and reverse transcribed with specific primers. Specific primers for VEGFR1, VEGF-A, brachyury, and β-actin were designed as previously described (23, 34). In addition, appropriate primers for PlGF, VEGF-B, VEGF-C, VEGF-D, neuropilin, VEGFR2, and FGF8 were designed with the following sequences: for PlGF, 5′-ATGCTGGTCATGAAGCTGTTCA-3′ and 5′-GGACTGAATATGTGAGACACCT-3′; for VEGF-B, 5′-ATGAGCCCCCTGCTCCGT-3′ and 5′-CTACAGGTGTCTGGGTTGAG-3′; for VEGF-C, 5′-ATGCACTTGCTGTGCTTCTTG-3′ and 5′-TGTCCTGGTATTGAGGGTGG-3′; for VEGF-D, 5′-ATGTATGGAGAATGGGGAATG-3′ and 5′-TTTACACAGGGGGGCTTGAA-3′; for neuropilin, 5′-ATGGAGAGGGGGCTGCCGTT-3′ and 5′-GAAGAGAAAGGGCCCTGAAG-3′; for VEGFR2, 5′-AAGTGATTGAGGCAGACGCT-3′ and 5′-TGATGCCAAGAACTCCAT-3′; and for FGF8, 5′-GGTGACGGATCAGCTCAGCC-3′ and 5′-TGTAGAGACCTGTCTCTGCG-3′.
Whole-mount immunohistochemistry.
Dissected embryos were stained essentially as previously described (54). In brief, the specimens were dehydrated, treated with 0.3% H2O2 in methanol to eliminate endogenous peroxidase activity, and rehydrated. After the samples were blocked with a mixture containing 2% skim milk, 0.2% bovine serum albumin, and 0.3% Triton X-100, an anti-mouse VEGF-A antibody (34), an anti-mouse VEGFR1 antibody for the N-terminal amino acid sequence (22), or an anti-mouse VEGFR2 antibody (Pharmingen, San Diego, Calif.) was added and incubated at 4°C overnight. Washed specimens were incubated with secondary antibodies conjugated to horseradish peroxidase, alkaline phosphatase, rhodamine, or fluorescein isothiocyanate. Immunofluorescence was scanned by confocal microscopy.
Isolation of VEGFR-positive cells and cell labeling.
Mesoderm cells were collected according to a protocol described in a previous report (23), with some modifications. In brief, fetal tissues from E7.5 to E7.75 that had been deprived of the allantois and head were digested in collagenase at 37°C for 60 min and then filtered through a sterile 58-μm-pore-size nylon mesh. The collected cells were incubated with a rat anti-mouse VEGFR2 antibody (Pharmingen), followed by anti-rat IgG microbeads (Miltenyi Biotec, Bergish Gladbach, Germany). These cells were labeled with rhodamine by use of a PKH26 fluorescent staining kit (Zynaxis, Malvern, Pa.).
In vitro migration assay.
Soft agar gels (3%) containing 0, 10, 40, and 100 ng of VEGF-E, mouse VEGF-A164 (R&D, Minneapolis, Minn.), or mouse PlGF (R&D)/ml were placed in each corner of collagen I-coated eight-well culture slides (Becton Dickinson, Bedford, Mass.), and collected mesoderm cells sorted by use of an anti-VEGFR2 antibody (as indicated above) were applied to the center of each well. After incubating the cells in 2% fetal calf serum-Dulbecco's modified Eagle's medium, with or without SU5416, we counted the cells that had migrated to the VEGF-containing gels.
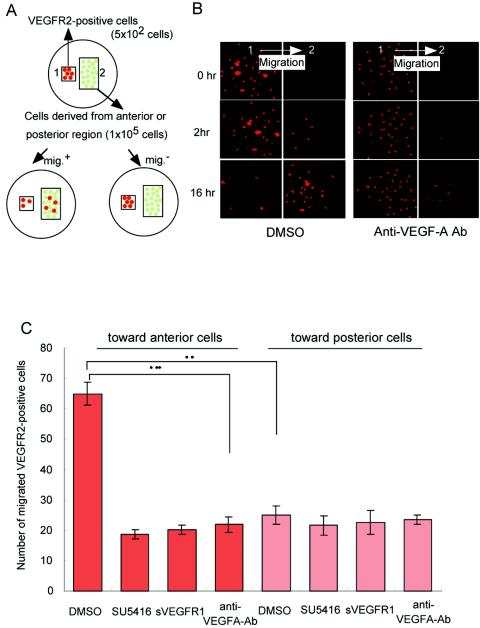
The isolation of VEGFR2-positive cells from 8 to 10 embryos without heads at E7.5 was performed as described above. We then labeled these cells with rhodamine and seeded them on cover glass (2 mm2) coated with collagen I for 90 min. We also obtained cells derived from the anterior or posterior portion of the embryos, which were treated with collagenase solution and were seeded on the center part of each eight-well slide chamber. After attachment of the cells to the slide chamber, we placed a piece of cover glass with VEGFR-positive cells on parts of the slide chamber adjacent to those cells. We counted the numbers of VEGFR-positive cells that migrated toward the cells derived from the anterior or posterior portion of the embryos by dark-field microscopy.
Transplantation and colonization.
About 10 rhodamine-labeled mesoderm cells were grafted orthotopically into the ventral mid-portion of a recipient embryo at an equivalent stage to the donor embryo by use of a micromanipulator under a dissecting microscope (30). The micromanipulated embryos were roller cultured and examined after 24 h. Migrated cells were detected with a Leica microscope. Six to 10 embryos of each genotype were examined.
Statistical analysis.
For statistical analysis, the data were expressed as means ± standard deviations and were analyzed by Student's t test. P values of <0.05 were considered significant.
RESULTS
An inhibitor of VEGF receptor kinases blocks VEGFR-positive cell accumulation and migration.
To examine whether the cell accumulation and migration of embryonic VEGFR-positive cells are regulated by the VEGFR tyrosine kinases, we cultured wild-type embryos from E7.25 to E7.75 with SU5416 (17, 24), a tyrosine kinase inhibitor specific to VEGFR1 and VEGFR2, for 24 h. We similarly cultured VEGFR2+/− embryos, in which the lacZ gene is driven under the control of the endogenous VEGFR2 promoter, at an equivalent stage to trace VEGFR2-positive cells. After 24 h, >70% of control DMSO-treated wild-type embryos showed embryonal “turning” (data not shown). However, all wild-type embryos cultured with SU5416 showed growth arrest before turning.
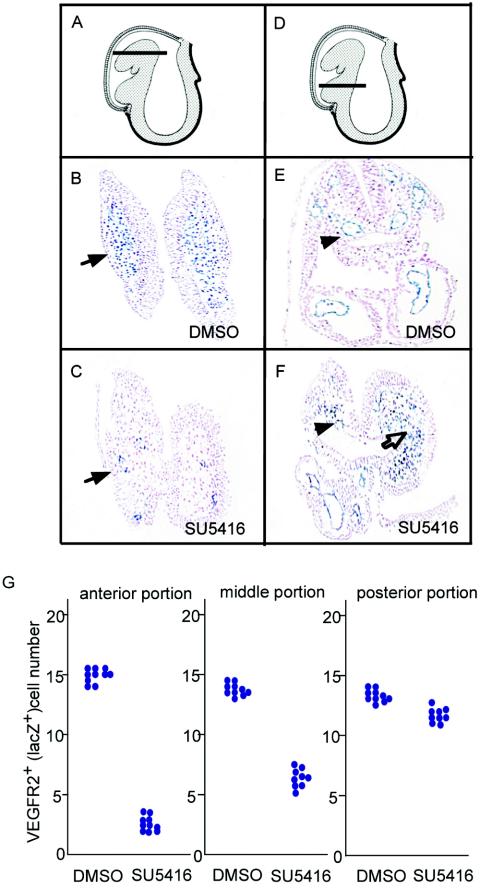
We also observed the same phenotype in the background of VEGFR2+/− embryos. VEGFR2-positive lacZ-stained cells were not apparent in embryonic heads after the treatment with SU5416. Histologically, SU5416 inhibited the formation of vessels in the dorsal aorta (arrowheads in Fig. 1F), in contrast with DMSO (arrowhead in Fig. 1E), in a transverse section of the middle portion of the embryo (Fig. 1D and G). Notably, very few VEGFR-positive cells were seen in the cranial region of cultured embryos due to the effect of SU5416 compared to the control embryos (arrow in Fig. 1C) at the cephalic level (Fig. 1A). The majority of these cells accumulated in the middle ventral or posterior region of the embryo (open arrowhead in Fig. 1F). These results suggest that the migration of posterior VEGFR-positive cells is regulated by VEGFR tyrosine kinases.
FIG. 1.
Blockade of the VEGF system inhibits VEGFR-positive cell migration. (A to F) Whole embryos were cultured with DMSO (B and E) or SU5416 (C and F), and VEGFR-positive cells in transverse sections at the levels schematized in panels A and D were identified by lacZ staining. Fewer LacZ-positive cells were found in the dorsal aorta (arrowheads) and head region (arrows) in embryos that were cultured with SU5416 (C and F). The open arrow indicates mismigrated cells surrounding the gut (F). (G) Numbers of endothelial cells lining the dorsal aorta in the anterior, middle, and posterior portions of cultured embryos with DMSO or SU5416 (each single dot is the average cell number for five sections per embryo).
Distinct expression patterns of VEGF-A and VEGFRs in embryos.
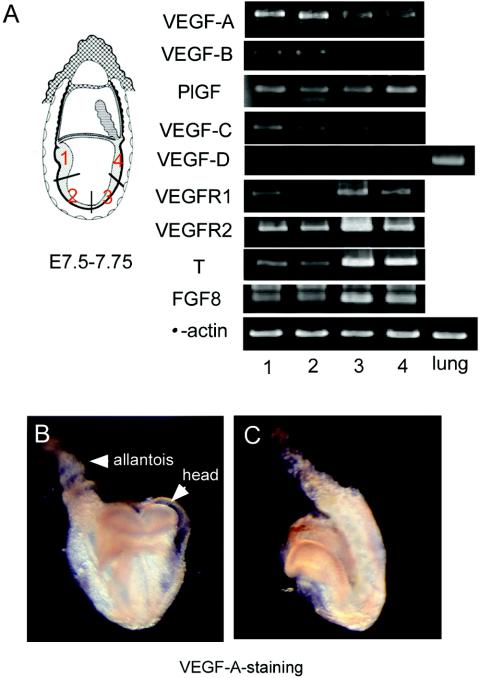
We examined the sites of expression of VEGF family members and VEGF receptors. We divided the embryos from E7.5 to E7.75 into four parts along the anterior-posterior axis and obtained RNAs from each part (left panel in Fig. 2A). Of the VEGF ligand family members, including VEGF-A, VEGF-B (40, 41), placenta growth factor (PlGF) (35), VEGF-C (26, 33), and VEGF-D (2), VEGF-A was the most apparent gene whose expression level was higher in the anterior than in the posterior region by RT-PCR analysis. In contrast, the cell population expressing both VEGFR1 and VEGFR2 existed mainly in the posterior portion. By using real-time PCR, we confirmed that the levels of VEGF-A were higher in the anterior than the posterior portion, whereas VEGFR1 and VEGFR2 levels were higher in the posterior portion. The ratio of VEGF-A mRNAs from the posterior to anterior four sections was 1:0.95:1.5:1.5, and the ratios of VEGFR2 and VEGFR1 mRNAs from the posterior to anterior four sections were 1:1:0.55:0.6 and 1:1:0.75:0.75, respectively. At this time, the brachyury (T) and FGF8 genes (46), which are mesoderm-specific markers, were expressed predominantly in the posterior rather than the anterior portion (right panel in Fig. 2A).
FIG. 2.
Spatially distinct expression pattern of genes encoding the VEGF ligand and receptors in embryos from E7.5 to E7.75. (A) Embryos were divided into four parts (left), and the mRNA level in each part was semiquantitatively estimated by RT-PCR (right). RNA derived from the lung was a positive control for VEGF-D. (B and C) Immunohistochemistry showing the distribution of VEGF-A protein in front (B) and side (C) views of a whole embryo. The VEGF-A signal was stronger in the anterior than the posterior portion of the trunk.
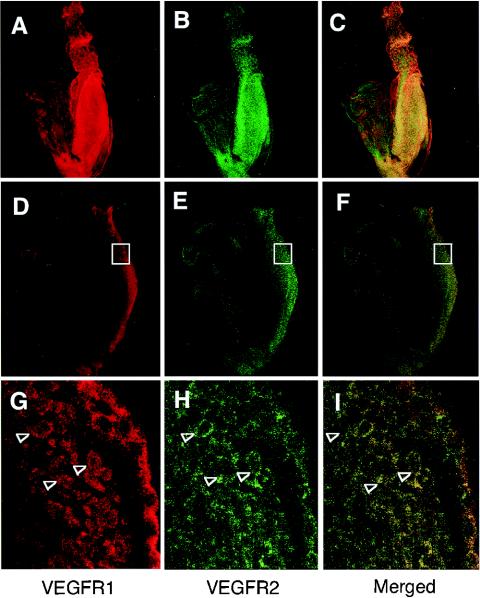
Next, we immunohistochemically examined the localization of the VEGF-A protein. Consistent with the RT-PCR analysis, the VEGF-A protein was found in the entire embryo, most remarkably in the anterior portion and the allantois (Fig. 2B and C). Previously, it was reported that VEGFR1 and VEGFR2 are initially expressed in the mesoderm at an early developmental stage when VEGFR1 or VEGFR2 null knockout fetuses do not show any apparent phenotype (11, 16, 49). When we doubly stained whole-mounted embryos with both anti-mouse VEGFR1 and VEGFR2 antibodies, the VEGFR1 and VEGFR2 proteins were found to colocalize on the same cells (open arrowheads) in the posterior region (Fig. 3D to L). The contrast in the locations of VEGF-A and VEGFRs permitted us to examine the possibility that VEGF-A may guide the receptor-expressing cells.
FIG. 3.
Posterior cells dominantly express both VEGFR1 and VEGFR2. Sagittal sections of embryos at E7.75 were stained with anti-mouse VEGFR1 (A, D, and J) or anti-mouse VEGFR2 (B, E, and H), and the images were merged (C, F, and I). The embryo was scanned by confocal microscopy, and a three-dimensional image was reconstructed to represent the whole embryo (A to C). The colocalization of VEGFR1 and VEGFR2 in posterior cells (open boxes in panels D to F) was apparent at a higher magnification (arrowheads in panels G to I).
VEGFR1- and VEGFR2-specific ligands guide VEGFR-positive cells in an in vitro system.
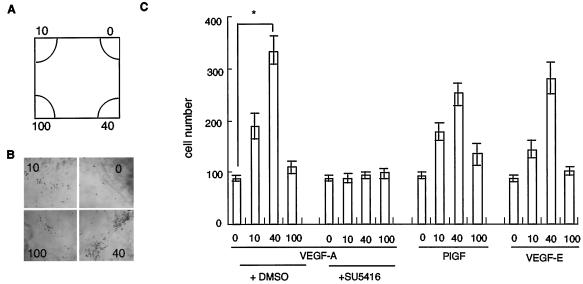
Since SU5416 inhibited VEGFR-positive migration in whole embryo cultures in vitro, we next examined which kinase of VEGFRs is involved in this phenotype. We collected VEGFR2-positive cells at E7.5 by using an anti-mouse VEGFR2 antibody and examined the migration toward VEGF-A in vitro. These cells also expressed VEGFR1 (data not shown). Because only a limited number of VEGFR2-positive cells can be obtained from embryos at E7.5 without a yolk sac and allantois, we developed a novel system to assess migration in vitro (see Materials and Methods) (Fig. 4A).
FIG. 4.
VEGF-A guides VEGFR-positive cells in vitro. (A) Scheme of a well in which several concentrations of ligand-containing gel were placed in the corners. (B) VEGFR-positive cells migrated to gels containing 0, 10, 40, and 100 ng of VEGF-A/ml after 16 h of culture. (C) Numbers of cells that migrated to VEGF-A in medium containing DMSO as a negative control or 10 μM SU5416, a VEGFR kinase-specific inhibitor. VEGF-A at 40 ng/ml was most effective, and SU5416 completely suppressed this migration. PlGF or VEGF-E at 40 ng/ml also induced maximum migration. *, P < 0.05 by a t test.
VEGFR2-positive cells derived from 10 wild-type embryos at E7.5 were applied to the center of a well and cultured for 16 h to allow migration toward ligand-containing gels. The most appropriate concentration for the migration was 40 ng of VEGF-A/ml (Fig. 4B and C), and SU5416 strongly suppressed VEGFR-positive cell migration at all VEGF-A concentrations (Fig. 4C). Next, we examined whether PlGF and VEGF-E, which are VEGFR1- and VEGFR2-specific ligands, respectively, stimulate the migration of VEGFR-positive cells. At 40 ng/ml, PlGF elicited 71% and VEGF-E elicited 80% of the migration stimulated by VEGF. When we applied both PlGF and VEGF-E, the migration capacity was comparable to that obtained with VEGF-A (data not shown). Thus, a specific ligand for VEGFR1 or -2 can independently induce migration in vitro.
In addition, we used mixtures of ligands, such as 10 ng of PlGF/ml, with various concentrations of VEGF-A. At a low concentration (10 ng/ml) of VEGF-A, the scores were partially additive (30% increase). At higher doses of VEGF-A (40 and 100 ng/ml), additive effects were hardly detected (data not shown). We also tested PlGF with VEGF-E (only with high doses of VEGF-E) and found that the effect was only minor. Since PlGF null mutant mice were reported to have no clear abnormalities during the early embryonic stage and since the VEGF-E gene is not present in the human and mouse genomes, we suggest that the major ligand for the stimulation of VEGFR-positive cell migration is VEGF-A.
VEGFR-positive cells migrate anteriorly in the VEGF-A-VEGFR system.
To determine in which direction VEGFR-positive cells move in vivo, we performed the following experiment. We collected VEGFR2-positive cells from several embryos at E7.5 and seeded them on cover glass. We then placed them (square 1 in Fig. 5A) next to a spot on the cover glass where cells from the anterior or posterior region of the embryos had been seeded (square 2 in Fig. 5A). After 16 h, the VEGFR-positive cells had migrated to cells obtained from the anterior portion of embryos, and this migration was eliminated by a neutralizing anti-mouse VEGF-A antibody (Fig. 5B). It was also blocked by the application of SU5416 or soluble VEGFR1 (Fig. 5C). VEGFR-positive cells did not migrate toward cells obtained from the posterior region of embryos. Thus, abundant VEGF-A in the anterior rather than posterior portion may induce VEGFR-positive cells to migrate anteriorly.
FIG. 5.
VEGFR-positive cells move toward the anterior portion of embryos with concentrated VEGF in vitro. (A) Scheme of the assay system enabling the migration of VEGFR-positive cells toward embryonic cells derived from the anterior or posterior portion of several wild-type embryos at E7.5. (B) Rhodamine-labeled VEGFR-positive embryonic cells migrated toward cells from the anterior region after 0, 2, and 6 h (left panels). A neutralizing anti-mouse VEGF-A antibody strongly suppressed the migration of the cells derived from the anterior portion (right panels). The direction of migration was from box 1 to box 2. The images were made by dark-field microscopy. (C) Numbers of migrated VEGFR2-positive cells in medium containing DMSO as a negative control, 10 μM SU5416, 1 μg of sVEGFR1/ml, or 1 μg of neutralizing anti-mouse VEGF-A antibody/ml. *, P < 0.05 by a t test.
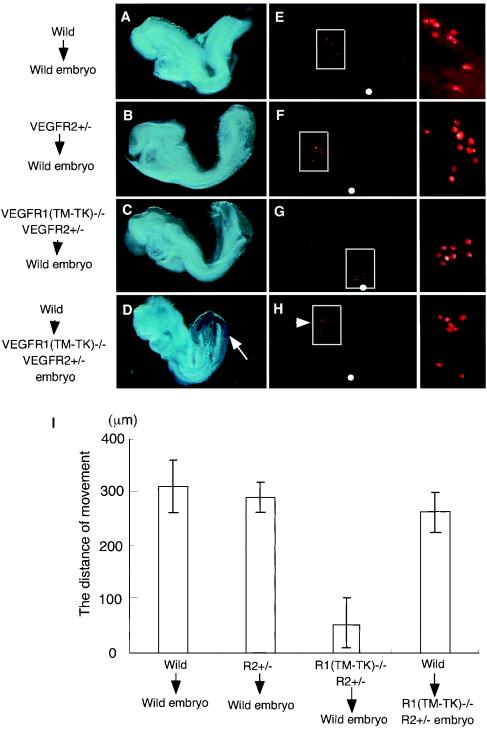
To assess whether purified VEGFR-positive cells from E7.5 to E7.75 move in the anterior direction in vivo, we injected rhodamine-labeled cells derived from wild-type, VEGFR2+/−, or VEGFR1(TM-TK)−/− VEGFR2+/− embryos into the rostral mid-portion of wild-type or VEGFR1(TM-TK)−/− VEGFR2+/− embryos at an equivalent stage and cultured the whole embryos for 24 h. As expected from the results obtained with the VEGF-VEGFR paracrine system, when VEGFR-positive cells collected from wild-type or VEGFR2+/− embryos were injected into wild-type mice, they migrated to the cranial region and the caudal part of the heart (Fig. 6E and F). On the other hand, VEGFR1(TM-TK)−/− VEGFR2+/− cells did not significantly migrate from the injection site (Fig. 6G).
FIG. 6.
VEGFR-positive cells migrate in the anterior direction in a manner dependent on VEGFRs in vivo. (A to H) Colonization of rhodamine-labeled VEGFR-positive cells derived from wild-type (A and E), VEGFR2+/− (B and F), and VEGFR1(TM-TK)−/− VEGFR2+/− (C and G) mice in wild-type mice. (D and H) Transplanted cells from wild-type mice migrated to the cranial region (arrowhead) compared to host VEGFR2-positive cells (arrow) in VEGFR1(TM-TK)−/− VEGFR2+/− embryos. The left panels show a bright field view, and the middle panels show a dark field view. The boxed part of the dark field view is magnified in the right panels. (I) Distances migrated from the injection site (white circle in panels E to H). More than 90% of the VEGFR-positive cells derived from VEGFR1(TM-TK)−/− VEGFR2+/− mice remained around the injection site.
Notably, VEGFR2-positive cells from wild-type embryos efficiently moved to the hindbrain and the caudal part of the heart in VEGFR1(TM-TK)−/− VEGFR2+/− embryos (arrowhead in Fig. 6H). However, many lacZ-stained VEGFR2-positive cells of host VEGFR1(TM-TK)−/− VEGFR2+/− embryos still remained in the posterior-to-tail portion (arrow in Fig. 6D). Thus, the lack of migration of VEGFR-positive cells to the anterior portion in VEGFR1(TM-TK)−/− VEGFR2+/− embryos is due to a loss of migration activity instead of to environmental factors.
DISCUSSION
VEGFR-positive cell migration is regulated by the VEGF-A-VEGFR system.
The movement of hemangioblast precursor cells as well as their differentiation into endothelial cells is a critical event in early embryogenesis. The mesodermal progenitors initially appear in the posterior portion of the embryo and move anteriorly toward the trunk. The mesoderm can be divided into five subgroups, the chordamesoderm, the paraxial mesoderm, the intermediate mesoderm, the lateral mesoderm, and the head mesenchyme (20, 32, 42, 55, 56). The various mesoderm precursor cells migrate to different locations along the embryonic anteroposterior axis. For the mesodermal derivatives in the embryo, those destined for rostral structures such as the heart and forebrain mesoderm ingress through the early primitive streak. They are followed by those for the rest of the cranial mesoderm and, lastly, the paraxial and lateral mesoderm (30, 42, 56). The lateral plate mesoderm gives rise to the heart, blood vessels, and blood cells, and the head mesenchyme will contribute to cephalic connective tissues.
In this study, we examined which ligand of the VEGF family is most involved in the migration of VEGFR-expressing mesodermal cells and showed that VEGF-A is expressed at a high concentration in the anterior portion of the embryo and that VEGFR1- and VEGFR2-expressing cells are located in the posterior region from E7.5 to 7.75 (Fig. 2 and 3). By using an embryo culture assay (Fig. 1), we showed that the VEGFR tyrosine kinase inhibitor SU5416 significantly suppressed the migration and accumulation of VEGFR-positive cells at the anterior region. In an vitro cell migration assay, the majority of VEGFR-positive cells migrated toward the VEGF family, including VEGF-A, PlGF, and VEGF-E, and the cell population was derived from the anterior but not the posterior part (Fig. 4 and 5). This migration was blocked by the VEGFR tyrosine kinase inhibitor SU5416, soluble VEGFR1, and an anti-VEGF-A antibody. SU5416 was reported to inhibit not only VEGFR, but also the platelet-derived growth factor receptor (PDGFR) (17, 24). Therefore, a partial involvement of PDGFR cannot be completely ruled out for these experiments using SU5416. To rule out the possible effects of SU5416 on the tyrosine kinases of other receptors such as PDGFR, we performed a semi-in vivo migration assay by injecting VEGFR2-positive cells into the fetal trunk. Cells derived from wild-type mice moved to the anterior but not the posterior portion of embryos, while those from VEGFR1(TM-TK)−/− VEGFR2+/− mice lost almost all of their ability to migrate (Fig. 6). Thus, among the VEGF family, VEGF-A concentrated in the anterior portion most likely provides the route for VEGFR-positive cells. Consistent with this view, it was reported that fewer endothelial cells lined the smaller lumen of the dorsal aorta in the anterior than the posterior region of VEGF-A+/− embryos (6), indicating that VEGFR-positive cells may be suppressed during migration to the anterior portion.
Other VEGF family members such as PlGF, VEGF-B, and VEGF-C are also expressed to some extent from E7.5 to E7.75 (Fig. 2). However, they are not the major inducers of cell migration since their expression was similar between the anterior and posterior regions and because mice in which these genes were knocked out were reported to have basically normal vasculogenesis (1, 4, 5, 28).
By which mechanism do the cells in the anterior portion of the embryo express VEGF-A early in embryogenesis? It is well known that the hypoxia-inducible factor (HIF) system plays a crucial role in the upregulation of VEGF-A under hypoxic conditions. However, just before vasculogenesis in early embryogenesis (about E7.0 to E7.5), O2 is distributed by diffusion in utero, and the level of O2 seems to be almost the same throughout the embryo. Therefore, a transcriptional regulatory system other than HIF appears to be responsible for the upregulation of VEGF-A.
Another important question is which VEGF receptor, R1, R2, or both, is involved in the accumulation and migration of VEGFR-expressing cells in early embryogenesis. VEGFR2 has a relatively strong kinase activity compared to VEGFR1 (48) and induces a major endothelial mitotic and migration signal (19, 49, 53). Thus, VEGFR2 is a major candidate for this migration signal. However, we consider VEGFR1 tyrosine kinase to also be involved in this cell migration for the following reasons: (i) the VEGFR1-specific ligand PlGF induced the migration of VEGFR-positive cells in vitro (Fig. 4), and (ii) VEGFR2−/− mice carrying wild-type VEGFR1 showed turning of the embryo and migration of VEGFR-positive cells to the middle and anterior portions, although to a lesser degree than normal (3, 21a). The migration of injected VEGFR-positive cells derived from VEGFR1(TM-TK)−/− VEGFR2+/− mice, but not from VEGFR2+/− mice, was severely disturbed in wild-type embryos (Fig. 6).
Physiological meaning of VEGFR-positive cell movement in early embryogenesis.
What are the roles of VEGFR-positive cell migration during embryogenesis? VEGFR-positive cell movement may have at least two roles. Firstly, it may act as a supplier of progenitor cells to rigidly form blood vessels starting from the posterior region, because a more severe structural abnormality of the dorsal aorta was observed in the middle portion than in the posterior portion in VEGFR1(TM-TK)-deficient mice. The second role might be to support the formation of the head and other tissues since VEGFR-positive cells were shown to differentiate not only into endothelial cells and hematopoietic cells but also into the smooth muscle cell lineage (60).
In mammals, three VEGF receptors, VEGFR1, VEGFR2, and VEGFR3, and five VEGF family members, VEGF-A, PlGF, VEGF-B, VEGF-C, and VEGF-D, have been identified (50). An early function of the VEGF system has been shown to be the generation of hemangioblasts in embryos. In this study, we presented evidence that the mammalian VEGF system plays another important role, that is, it has a role in the migration of VEGFR-positive mesodermal cells during early embryogenesis. Recently, it was reported that the VEGFR homolog of Drosophila is expressed in hemocytes and border cells, and VEGF homologs are expressed along hemocyte and border cell migration routes (8, 10). The interactions of ligands and receptors control the migration of these cells. Thus, the ancestral function of the VEGF-VEGFR pathway is considered to be the guidance of target cells. The single VEGFR gene of Drosophila may be functionally abolished and divided into three mammalian VEGFRs to regulate more complicated mechanisms in the formation and maintenance of the blood vessel system. However, the fundamental role of the VEGF-VEGFR system to ensure that VEGFR-expressing cells are guided to an appropriate region by a ligand during early embryogenesis may be conserved in mammals and Drosophila.
Acknowledgments
We thank Tetsuo Noda (Cancer Institute) for the preparation of VEGFR1(TK)−/− mice and Nobuaki Yoshida (University of Tokyo) for support with the transplantation technique.
This work was supported by Special Project Research on Cancer-Bioscience grant-in-aid 12215024 from the Ministry of Education, Culture, Sports, Science and Technology in Japan, by the program Research for the Future of the Japan Society for Promotion of Science, and by the program Promotion of Fundamental Research in Health Sciences from the Organization for Pharmaceutical Safety and Research.
REFERENCES
- 1.Aase, K., G. von Euler, X. Li, A. Ponten, P. Thoren, R. Cao, Y. Cao, B. Olofsson, S. Gebre-Medhin, M. Pekny, K. Alitalo, C. Betsholtz, and U. Eriksson. 2001. Vascular endothelial growth factor-B-deficient mice display an atrial conduction defect. Circulation 104:358-364. [DOI] [PubMed] [Google Scholar]
- 2.Achen, M. G., M. Jeltsch, E. Kukk, T. Makinen, A. Vitali, A. F. Wilks, K. Alitalo, and S. A. Stacker. 1998. Vascular endothelial growth factor D (VEGF-D) is a ligand for the tyrosine kinases VEGF receptor 2 (Flk1) and VEGF receptor 3 (Flt4). Proc. Natl. Acad. Sci. USA 95:548-553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barleon, B., S. Sozzani, D. Zhou, H. A. Weich, A. Mantovani, and D. Marme. 1996. Migration of human monocytes in response to vascular endothelial growth factor (VEGF) is mediated via the VEGF receptor flt-1. Blood 87:3336-3343. [PubMed] [Google Scholar]
- 4.Bellomo, D., J. P. Headrick, G. U. Silins, C. A. Paterson, P. S. Thomas, M. Gartside, A. Mould, M. M. Cahill, I. D. Tonks, S. M. Grimmond, S. Townson, C. Wells, M. Little, M. C. Cummings, N. K. Hayward, and G. F. Kay. 2000. Mice lacking the vascular endothelial growth factor-B gene (Vegfb) have smaller hearts, dysfunctional coronary vasculature, and impaired recovery from cardiac ischemia. Circ. Res. 86:29-35. [DOI] [PubMed] [Google Scholar]
- 5.Carmeliet, P., L. Moons, A. Luttun, V. Vincenti, V. Compernolle, M. De Mol, Y. Wu, F. Bono, L. Devy, H. Beck, D. Scholz, T. Acker, T. DiPalma, M. Dewerchin, A. Noel, I. Stalmans, A. Barra, S. Blacher, T. Vandendriessche, A. Ponten, U. Eriksson, K. H. Plate, J. M. Foidart, W. Schaper, D. S. Charnock-Jones, D. J. Hicklin, J. M. Herbert, D. Collen, and M. G. Persico. 2001. Synergism between vascular endothelial growth factor and placental growth factor contributes to angiogenesis and plasma extravasation in pathological conditions. Nat. Med. 7:575-583. [DOI] [PubMed] [Google Scholar]
- 6.Carmeliet, P., V. Ferreira, G. Breier, S. Pollefeyt, L. Kieckens, M. Gertsenstein, M. Fahrig, A. Vandenhoeck, K. Harpal, C. Eberhardt, C. Declercq, J. Pawling, L. Moons, D. Collen, W. Risau, and A. Nagy. 1996. Abnormal blood vessel development and lethality in embryos lacking a single VEGF allele. Nature 380:435-439. [DOI] [PubMed] [Google Scholar]
- 7.Casella, I., T. Feccia, C. Chelucci, P. Samoggia, G. Castelli, R. Guerriero, I. Parolini, E. Petrucci, E. Pelosi, O. Morsilli, M. Gabbianelli, U. Testa, and C. Peschle. 2003. Autocrine-paracrine VEGF loops potentiate the maturation of megakaryocytic precursors through Flt1 receptor. Blood 101:1316-1323. [DOI] [PubMed] [Google Scholar]
- 8.Cho, N. K., L. Keyes, E. Johnson, J. Heller, L. Ryner, F. Karim, and M. A. Krasnow. 2002. Developmental control of blood cell migration by the Drosophila VEGF pathway. Cell 108:865-876. [DOI] [PubMed] [Google Scholar]
- 9.Clauss, M., H. Weich, G. Breier, U. Knies, W. Rockl, J. Waltenberger, and W. Risau. 1996. The vascular endothelial growth factor receptor Flt-1 mediates biological activities. Implications for a functional role of placenta growth factor in monocyte activation and chemotaxis. J. Biol. Chem. 271:17629-17634. [DOI] [PubMed] [Google Scholar]
- 10.Duchek, P., K. Somogyi, G. Jekely, S. Beccari, and P. Rorth. 2001. Guidance of cell migration by the Drosophila PDGF/VEGF receptor. Cell 107:17-26. [DOI] [PubMed] [Google Scholar]
- 11.Dumont, D. J., G. H. Fong, M. C. Puri, G. Gradwohl, K. Alitalo, and M. L. Breitman. 1995. Vascularization of the mouse embryo: a study of flk-1, tek, tie, and vascular endothelial growth factor expression during development. Dev. Dyn. 203:80-92. [DOI] [PubMed] [Google Scholar]
- 12.Eichmann, A., C. Marcelle, C. Breant, and N. M. Le Douarin. 1993. Two molecules related to the VEGF receptor are expressed in early endothelial cells during avian embryonic development. Mech. Dev. 42:33-48. [DOI] [PubMed] [Google Scholar]
- 13.Ferrara, N., K. Carver-Moore, H. Chen, M. Dowd, L. Lu, K. S. O'Shea, L. Powell-Braxton, K. J. Hillan, and M. W. Moore. 1996. Heterozygous embryonic lethality induced by targeted inactivation of the VEGF gene. Nature 380:439-442. [DOI] [PubMed] [Google Scholar]
- 14.Ferrara, N., and T. Davis-Smyth. 1997. The biology of vascular endothelial growth factor. Endocr. Rev. 18:4-25. [DOI] [PubMed] [Google Scholar]
- 15.Fong, G. H., J. Rossant, M. Gertsenstein, and M. L. Breitman. 1995. Role of the Flt-1 receptor tyrosine kinase in regulating the assembly of vascular endothelium. Nature 376:66-70. [DOI] [PubMed] [Google Scholar]
- 16.Fong, G. H., L. Zhang, D. M. Bryce, and J. Peng. 1999. Increased hemangioblast commitment, not vascular disorganization, is the primary defect in flt-1 knock-out mice. Development 126:3015-3025. [DOI] [PubMed] [Google Scholar]
- 17.Fong, T. A., L. K. Shawver, L. Sun, C. Tang, H. App, T. J. Powell, Y. H. Kim, R. Schreck, X. Wang, W. Risau, A. Ullrich, K. P. Hirth, and G. McMahon. 1999. SU5416 is a potent and selective inhibitor of the vascular endothelial growth factor receptor (Flk-1/KDR) that inhibits tyrosine kinase catalysis, tumor vascularization, and growth of multiple tumor types. Cancer Res. 59:99-106. [PubMed] [Google Scholar]
- 18.Gerber, H. P., and N. Ferrara. 2003. The role of VEGF in normal and neoplastic hematopoiesis. J. Mol. Med. 81:20-31. [DOI] [PubMed] [Google Scholar]
- 19.Gille, H., J. Kowalski, L. Yu, H. Chen, M. T. Pisabarro, T. Davis-Smyth, and N. Ferrara. 2000. A repressor sequence in the juxtamembrane domain of Flt-1 (VEGFR-1) constitutively inhibits vascular endothelial growth factor-dependent phosphatidylinositol 3′-kinase activation and endothelial cell migration. EMBO J. 19:4064-4073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hashimoto, K., H. Fujimoto, and N. Nakatsuji. 1987. An ECM substratum allows mouse mesodermal cells isolated from the primitive streak to exhibit motility similar to that inside the embryo and reveals a deficiency in the T/T mutant cells. Development 100:587-598. [DOI] [PubMed] [Google Scholar]
- 21.Hattori, K., B. Heissig, Y. Wu, S. Dias, R. Tejada, B. Ferris, D. J. Hicklin, Z. Zhu, P. Bohlen, L. Witte, J. Hendrikx, N. R. Hackett, R. G. Crystal, M. A. Moore, Z. Werb, D. Lyden, and S. Rafii. 2002. Placental growth factor reconstitutes hematopoiesis by recruiting VEGFR1(+) stem cells from bone-marrow microenvironment. Nat. Med. 8:841-849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21a.Hiratsuka, S., K. Nakao, K. Nakamura, M. Katsuki, Y. Maru, and M. Shibuya. 2005. Membrane fixation of vascular endothelial growth factor receptor 1 ligand-binding domain is important for vasculogenesis and angiogenesis in mice. Mol. Cell. Biol. 25:346-354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hiratsuka, S., O. Minowa, J. Kuno, T. Noda, and M. Shibuya. 1998. Flt-1 lacking the tyrosine kinase domain is sufficient for normal development and angiogenesis in mice. Proc. Natl. Acad. Sci. USA 95:9349-9354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hiratsuka, S., K. Nakamura, S. Iwai, M. Murakami, T. Itoh, H. Kijima, J. M. Shipley, R. M. Senior, and M. Shibuya. 2002. MMP9 induction by vascular endothelial growth factor receptor-1 is involved in lung-specific metastasis. Cancer Cell 2:289-300. [DOI] [PubMed] [Google Scholar]
- 24.Itokawa, T., H. Nokihara, Y. Nishioka, S. Sone, Y. Iwamoto, Y. Yamada, J. Cherrington, G. McMahon, M. Shibuya, M. Kuwano, and M. Ono. 2002. Antiangiogenic effect by SU5416 is partly attributable to inhibition of FIt-1 receptor signaling. Mol. Cancer Ther. 1:295-302. [PubMed] [Google Scholar]
- 25.Jakeman, L. B., J. Winer, G. L. Bennett, C. A. Altar, and N. Ferrara. 1992. Binding sites for vascular endothelial growth factor are localized on endothelial cells in adult rat tissues. J. Clin. Investig. 89:244-253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Joukov, V., K. Pajusola, A. Kaipainen, D. Chilov, I. Lahtinen, E. Kukk, O. Saksela, N. Kalkkinen, and K. Alitalo. 1996. A novel vascular endothelial growth factor, VEGF-C, is a ligand for the Flt4 (VEGFR-3) and KDR (VEGFR-2) receptor tyrosine kinases. EMBO J. 15:290-298. [PMC free article] [PubMed] [Google Scholar]
- 27.Kaipainen, A., J. Korhonen, K. Pajusola, O. Aprelikova, M. G. Persico, B. I. Terman, and K. Alitalo. 1993. The related FLT4, FLT1, and KDR receptor tyrosine kinases show distinct expression patterns in human fetal endothelial cells. J. Exp. Med. 178:2077-2088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Karkkainen, M. J., P. Haiko, K. Sainio, J. Partanen, J. Taipale, T. V. Petrova, M. Jeltsch, D. G. Jackson, M. Talikka, H. Rauvala, C. Betsholtz, and K. Alitalo. 2004. Vascular endothelial growth factor C is required for sprouting of the first lymphatic vessels from embryonic veins. Nat. Immunol. 5:74-80. [DOI] [PubMed] [Google Scholar]
- 29.Kendall, R. L., and K. A. Thomas. 1993. Inhibition of vascular endothelial cell growth factor activity by an endogenously encoded soluble receptor. Proc. Natl. Acad. Sci. USA 90:10705-10709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kinder, S. J., T. E. Tsang, G. A. Quinlan, A. K. Hadjantonakis, A. Nagy, and P. P. Tam. 1999. The orderly allocation of mesodermal cells to the extraembryonic structures and the anteroposterior axis during gastrulation of the mouse embryo. Development 126:4691-4701. [DOI] [PubMed] [Google Scholar]
- 31.Kondo, K., S. Hiratsuka, E. Subbalakshmi, H. Matsushime, and M. Shibuya. 1998. Genomic organization of the flt-1 gene encoding for vascular endothelial growth factor (VEGF) receptor-1 suggests an intimate evolutionary relationship between the 7-Ig and the 5-Ig tyrosine kinase receptors. Gene 208:297-305. [DOI] [PubMed] [Google Scholar]
- 32.Lawson, K. A., J. J. Meneses, and R. A. Pedersen. 1991. Clonal analysis of epiblast fate during germ layer formation in the mouse embryo. Development 113:891-911. [DOI] [PubMed] [Google Scholar]
- 33.Lee, J., A. Gray, J. Yuan, S. M. Luoh, H. Avraham, and W. I. Wood. 1996. Vascular endothelial growth factor-related protein: a ligand and specific activator of the tyrosine kinase receptor Flt4. Proc. Natl. Acad. Sci. USA 93:1988-1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Luo, J. C., S. Yamaguchi, A. Shinkai, K. Shitara, and M. Shibuya. 1998. Significant expression of vascular endothelial growth factor/vascular permeability factor in mouse ascites tumors. Cancer Res. 58:2652-2660. [PubMed] [Google Scholar]
- 35.Maglione, D., V. Guerriero, G. Viglietto, P. Delli-Bovi, and M. G. Persico. 1991. Isolation of a human placenta cDNA coding for a protein related to the vascular permeability factor. Proc. Natl. Acad. Sci. USA 88:9267-9271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Matthews, W., C. T. Jordan, M. Gavin, N. A. Jenkins, N. G. Copeland, and I. R. Lemischka. 1991. A receptor tyrosine kinase cDNA isolated from a population of enriched primitive hematopoietic cells and exhibiting close genetic linkage to c-kit. Proc. Natl. Acad. Sci. USA 88:9026-9030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mustonen, T., and K. Alitalo. 1995. Endothelial receptor tyrosine kinases involved in angiogenesis. J. Cell Biol. 129:895-898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nishikawa, S. I., S. Nishikawa, M. Hirashima, N. Matsuyoshi, and H. Kodama. 1998. Progressive lineage analysis by cell sorting and culture identifies FLK1+VE-cadherin+ cells at a diverging point of endothelial and hemopoietic lineages. Development 125:1747-1757. [DOI] [PubMed] [Google Scholar]
- 39.Ogawa, M., S. Fraser, T. Fujimoto, M. Endoh, S. Nishikawa, and S. I. Nishikawa. 2001. Origin of hematopoietic progenitors during embryogenesis. Int. Rev. Immunol. 20:21-44. [DOI] [PubMed] [Google Scholar]
- 40.Olofsson, B., E. Korpelainen, M. S. Pepper, S. J. Mandriota, K. Aase, V. Kumar, Y. Gunji, M. M. Jeltsch, M. Shibuya, K. Alitalo, and U. Eriksson. 1998. Vascular endothelial growth factor B (VEGF-B) binds to VEGF receptor-1 and regulates plasminogen activator activity in endothelial cells. Proc. Natl. Acad. Sci. USA 95:11709-11714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Olofsson, B., K. Pajusola, A. Kaipainen, G. von Euler, V. Joukov, O. Saksela, A. Orpana, R. F. Pettersson, K. Alitalo, and U. Eriksson. 1996. Vascular endothelial growth factor B, a novel growth factor for endothelial cells. Proc. Natl. Acad. Sci. USA 93:2576-2581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Parameswaran, M., and P. P. Tam. 1995. Regionalisation of cell fate and morphogenetic movement of the mesoderm during mouse gastrulation. Dev. Genet. 17:16-28. [DOI] [PubMed] [Google Scholar]
- 43.Park, J. E., H. H. Chen, J. Winer, K. A. Houck, and N. Ferrara. 1994. Placenta growth factor. Potentiation of vascular endothelial growth factor bioactivity, in vitro and in vivo, and high affinity binding to Flt-1 but not to Flk-1/KDR. J. Biol. Chem. 269:25646-25654. [PubMed] [Google Scholar]
- 44.Quinn, T. P., K. G. Peters, C. De Vries, N. Ferrara, and L. T. Williams. 1993. Fetal liver kinase 1 is a receptor for vascular endothelial growth factor and is selectively expressed in vascular endothelium. Proc. Natl. Acad. Sci. USA 90:7533-7537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Risau, W. 1997. Mechanisms of angiogenesis. Nature 386:671-674. [DOI] [PubMed] [Google Scholar]
- 46.Russ, A. P., S. Wattler, W. H. Colledge, S. A. Aparicio, M. B. Carlton, J. J. Pearce, S. C. Barton, M. A. Surani, K. Ryan, M. C. Nehls, V. Wilson, and M. J. Evans. 2000. Eomesodermin is required for mouse trophoblast development and mesoderm formation. Nature 404:95-99. [DOI] [PubMed] [Google Scholar]
- 47.Sawano, A., T. Takahashi, S. Yamaguchi, and M. Shibuya. 1997. The phosphorylated 1169-tyrosine containing region of flt-1 kinase (VEGFR-1) is a major binding site for PLCgamma. Biochem. Biophys. Res. Commun. 238:487-491. [DOI] [PubMed] [Google Scholar]
- 48.Seetharam, L., N. Gotoh, Y. Maru, G. Neufeld, S. Yamaguchi, and M. Shibuya. 1995. A unique signal transduction from FLT tyrosine kinase, a receptor for vascular endothelial growth factor VEGF. Oncogene 10:135-147. [PubMed] [Google Scholar]
- 49.Shalaby, F., J. Rossant, T. P. Yamaguchi, M. Gertsenstein, X. F. Wu, M. L. Breitman, and A. C. Schuh. 1995. Failure of blood-island formation and vasculogenesis in Flk-1-deficient mice. Nature 376:62-66. [DOI] [PubMed] [Google Scholar]
- 50.Shibuya, M. 2001. Structure and function of VEGF/VEGF-receptor system involved in angiogenesis. Cell Struct. Funct. 26:25-35. [DOI] [PubMed] [Google Scholar]
- 51.Shibuya, M., S. Yamaguchi, A. Yamane, T. Ikeda, A. Tojo, H. Matsushime, and M. Sato. 1990. Nucleotide sequence and expression of a novel human receptor-type tyrosine kinase gene (flt) closely related to the fms family. Oncogene 5:519-524. [PubMed] [Google Scholar]
- 52.Takahashi, T., H. Ueno, and M. Shibuya. 1999. VEGF activates protein kinase C-dependent, but Ras-independent Raf-MEK-MAP kinase pathway for DNA synthesis in primary endothelial cells. Oncogene 18:2221-2230. [DOI] [PubMed] [Google Scholar]
- 53.Takahashi, T., S. Yamaguchi, K. Chida, and M. Shibuya. 2001. A single autophosphorylation site on KDR/Flk-1 is essential for VEGF-A-dependent activation of PLC-gamma and DNA synthesis in vascular endothelial cells. EMBO J. 20:2768-2778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Takakura, N., T. Watanabe, S. Suenobu, Y. Yamada, T. Noda, Y. Ito, M. Satake, and T. Suda. 2000. A role for hematopoietic stem cells in promoting angiogenesis. Cell 102:199-209. [DOI] [PubMed] [Google Scholar]
- 55.Tam, P. P., and R. S. Beddington. 1987. The formation of mesodermal tissues in the mouse embryo during gastrulation and early organogenesis. Development 99:109-126. [DOI] [PubMed] [Google Scholar]
- 56.Tam, P. P., and R. R. Behringer. 1997. Mouse gastrulation: the formation of a mammalian body plan. Mech. Dev. 68:3-25. [DOI] [PubMed] [Google Scholar]
- 57.Tam, P. P., and S. S. Tan. 1992. The somitogenetic potential of cells in the primitive streak and the tail bud of the organogenesis-stage mouse embryo. Development 115:703-715. [DOI] [PubMed] [Google Scholar]
- 58.Terman, B. I., M. Dougher-Vermazen, M. E. Carrion, D. Dimitrov, D. C. Armellino, D. Gospodarowicz, and P. Bohlen. 1992. Identification of the KDR tyrosine kinase as a receptor for vascular endothelial cell growth factor. Biochem. Biophys. Res. Commun. 187:1579-1586. [DOI] [PubMed] [Google Scholar]
- 59.Yamane, A., L. Seetharam, S. Yamaguchi, N. Gotoh, T. Takahashi, G. Neufeld, and M. Shibuya. 1994. A new communication system between hepatocytes and sinusoidal endothelial cells in liver through vascular endothelial growth factor and Flt tyrosine kinase receptor family (Flt-1 and KDR/Flk-1). Oncogene 9:2683-2690. [PubMed] [Google Scholar]
- 60.Yamashita, J., H. Itoh, M. Hirashima, M. Ogawa, S. Nishikawa, T. Yurugi, M. Naito, K. Nakao, and S. I. Nishikawa. 2000. Flk1-positive cells derived from embryonic stem cells serve as vascular progenitors. Nature 408:92-96. [DOI] [PubMed] [Google Scholar]