Abstract
Objectives:
Although endoscopic submucosal dissection (ESD) is an efficient treatment for superficial esophageal cancer, it is associated with stricture formation after wide-circumference resection that leads to a low quality of life. Although locoregional steroid injections prevent stricture formation, a randomized comparative study did not report any advantages associated with steroid injection. We evaluated the prophylactic efficacy of a single locoregional triamcinolone injection for stricture formation after esophageal ESD.
Methods:
This was a retrospective matched case-control study using propensity score matching (PSM). Between April 2006 and July 2015, a total of 602 patients with superficial esophageal neoplasia underwent ESD. Among them, 189 patients with mucosal defects that spanned more than 2/3 of the esophageal circumference were included. After exclusion, 150 patients were enrolled. Triamcinolone acetonide (80 mg) was injected into the residual submucosal layer of the resected region immediately after ESD. PSM was performed to reduce the effects of selection bias for steroid injection. The primary outcome was the incidence of stricture formation. The secondary outcome was the number of balloon dilatation procedures required to resolve the stricture formation.
Results:
Thirty-seven patients, with and without triamcinolone injection each, were matched after PSM. The incidence of stricture formation decreased from 45.9% (17/37) without triamcinolone injection to 18.9% (7/37) with triamcinolone injection (p=0.016). After matching, the mean number of balloon dilatation procedures required also decreased from 2.8±4.6 to 0.6±1.5 times (P<0.01).
Conclusions:
A single locoregional triamcinolone injection efficiently prevented stricture formation after esophageal ESD.
Introduction
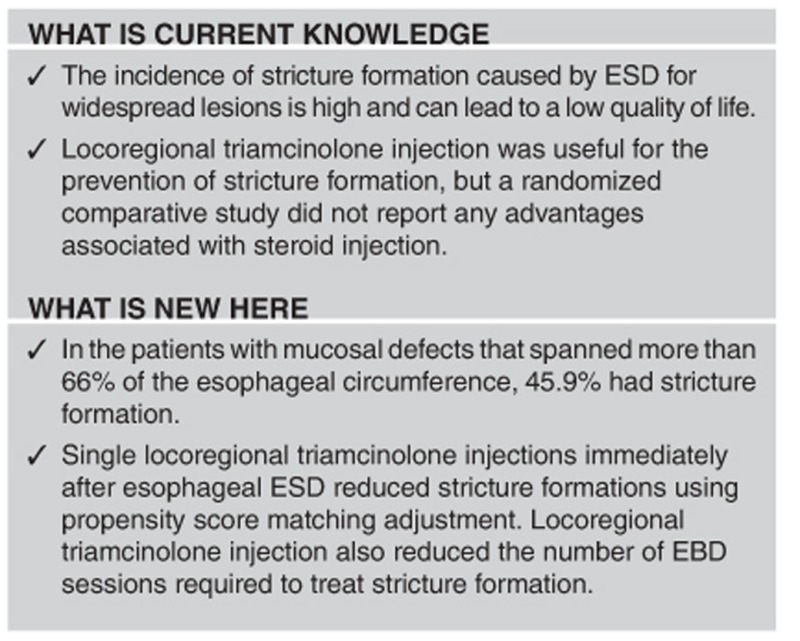
Esophageal cancer has a poor prognosis; this is because most patients are diagnosed at an advanced stage.1, 2 However, new optical imaging procedures, such as narrow-band imaging endoscopy, make it possible to detect esophageal cancer at an early stage,3, 4 improving the survival rates associated with curable endoscopic resection.5 Endoscopic submucosal dissection (ESD) can utilize an en bloc resection procedure for superficial esophageal neoplasia, regardless of tumor size.5, 6, 7 However, the incidence of stricture formations caused by ESD for widespread lesions is high in patients with a circumferential mucosal defect size of over 71%.5, 8, 9 Stricture formation can result in lower quality of life and multiple endoscopic balloon dilatation (EBD) sessions may be required.5, 9
Previous studies reported that locoregional steroid injections were useful for the prevention of such strictures.10, 11, 12, 13 Hanaoka et al. reported that a single triamcinolone injection immediately after ESD prevented stricture formation.11 However, this single arm prospective study was compared against historical controls, creating a potential selection bias, particularly with regard to circumferential mucosal defects that can influence stricture formation. A randomized comparative study (RCT) did not report any advantages associated with steroid injection, because of the small sample size and the inclusion of many whole-circumference defect cases.14 This higher rate of stricture formation might be due to baseline expansion of the circumference of the mucosal defect into a whole-circumference defect.
Using propensity score matching, we evaluated the prophylactic efficacy of a single session of locoregional triamcinolone injection immediately after ESD for superficial esophageal neoplasia to determine if it could prevent stricture formation in patients at high risk for stricture formation.
MethodS
Patients
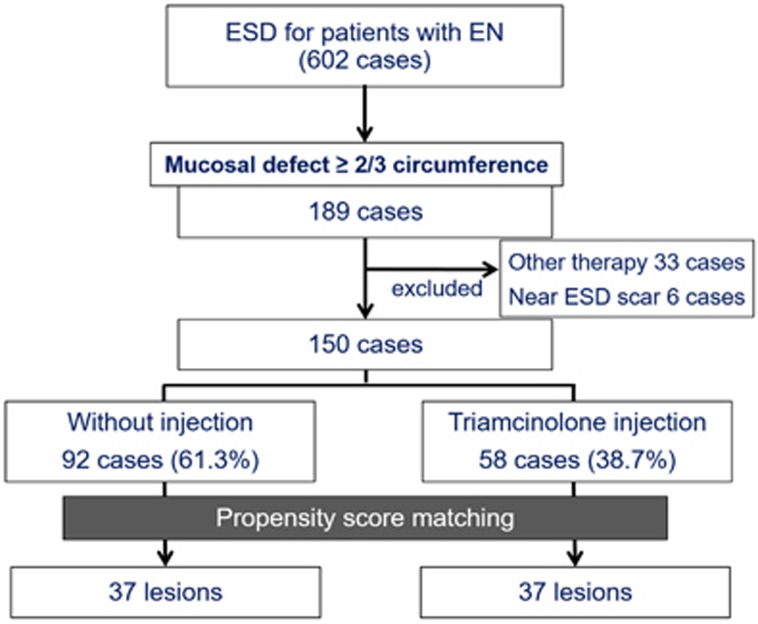
This was a retrospective, matched case-control study from a single referral center in Japan. Between April 2006 and July 2015, a total of 602 patients with superficial esophageal neoplasia underwent ESD in our hospital (Figure 1). Among them, 189 patients had mucosal defects that covered more than 2/3 (66%) of the esophageal circumference; it is reported that a circumferential mucosal defect size of over 71% is associated with a high risk of stricture formation.8 Patients who underwent other forms of prophylactic therapy for stricture formation or those who received ESD near a previous ESD scar were excluded because these therapies and conditions could affect stricture formation.
Figure 1.
Diagram of the study design. EN, esophageal neoplasia; ESD, endoscopic submucosal dissection.
The lesions with the largest circumference were considered the target lesions in patients who were treated for multiple synchronous or metachronous lesions and in patients who underwent en bloc resection of multiple lesions. A propensity score matching analysis was performed to reduce the effects of a selection bias for the triamcinolone injections, such as circumferential mucosal defects and potential confounding factors.15
The protocol of this study was approved by the ethics committee of the Osaka City University Graduate School of Medicine (number 3339). Written informed consent was obtained from each patient who underwent ESD or locoregional triamcinolone injections.
Outcomes
The primary outcome of this study was the incidence of stricture formation in patients who did or did not receive single locoregional triamcinolone injections. For the purpose of evaluating these outcomes, we evaluated the risk of stricture formation caused by ESD. The secondary outcome was the number of balloon dilatation sessions required to resolve the stricture formation.
ESD procedure
Six experienced endoscopists conducted all the endoscopic procedures. Intravenous midazolam or propofol with pethidine hydrochloride was used to place patients under deep sedation during endoscopic procedures.16 A single-channel upper gastrointestinal endoscope (GIF-Q260J; Olympus, Tokyo, Japan) and a standard electrosurgical generator (ICC 200 or VIO300D; ERBE Elektromedizin GmbH, Tübingen, Germany) were used. The main electrosurgical knives utilized were a bipolar needle knife (B knife; XEMEX, Tokyo, Japan) in the earlier study period and a monopolar needle knife (Flush knife, DK2618JN; Fujifilm Medical, Tokyo, Japan) in the later period. The procedure was carried out as previously reported.7, 13 Briefly, marking dots were placed around the margin and the procedure was performed as followed: (1) A hyaluronic acid solution was injected into the submucosal layer, (2) a circumferential mucosal incision was made, and (3) the submucosal dissection was performed. The total procedure time was defined as the period from the start of the circumferential mucosal incision to the removal of the tumor.
Locoregional triamcinolone injection
The indication for locoregional triamcinolone injection at our institution was defined as a mucosal defect encompassing over 2/3 of the esophageal circumference, as mentioned above. We did not administer triamcinolone injection therapy alone for cases with whole-circumference mucosal defects because it has been previously reported that locoregional steroid injections have little effect on such cases.14, 17, 18 We injected a single session of triamcinolone acetonide (Kenacort; Bristol-Myers Squibb, Tokyo, Japan) into the residual submucosal layer of the resected region immediately after ESD.11, 13 Regardless of the size of the resected specimens, 40 sequential injections of 0.5 ml (2 mg) triamcinolone acetonide were performed for a total of 80 mg.
Follow-up and stricture formation
Endoscopic stricture evaluation was performed every 4 weeks after ESD until scarring was confirmed. Stricture formation was determined when a standard upper gastrointestinal endoscope with a 9.2 mm diameter (GIF-Q260; Olympus Medical, Tokyo, Japan) could not be passed through the treatment site.13 When the patient presented with a chief complaint of dysphagia to semi-solid foods (dysphagia score 2) before a scheduled examination, endoscopic evaluation was performed. EBD was repeated when a stricture persisted, either endoscopically or symptomatically.
Statistical analyses
Data are presented as the mean±standard deviation for continuous variables and as numbers for categorical variables. For categorical data, comparisons between groups were performed using the chi-squared test (or Fisher's exact test when necessary because of small sample sizes), whereas continuous data were compared using Student's t-test. A propensity score matching analysis was performed to reduce the effects of selection bias for triamcinolone injections.15 The propensity score matching method was proposed to evaluate statistically causal effects free from confounding effects by mathematically refashioning an observational study into a randomized study—a so-called pseudo-randomized study.19 We used our clinical experience and knowledge to select the possible confounding factors that may be associated with outcome. Logistic regression analysis was used to generate a model to calculate the propensity scores using 12 variables (described in Table 1). The reliability of the model was evaluated using the Hosmer-Lemeshow goodness-of-fit test. We used the standardized difference to measure covariate balance, whereby an absolute standardized difference above 10% represented a meaningful imbalance. We created a propensity score-matched cohort by attempting to match each patient who received triamcinolone injections with one who did not receive triamcinolone injections (a 1:1 match) without replacement by using a greedy matching technique. The validity of the model was assessed by estimating the area under the receiver operating characteristics curve using c-statistics. After matching, crude comparisons of the matched cohorts were performed using the Mantel–Haenszel chi-square test (using McNemar's test for binary data) and paired t-tests. The relationship between triamcinolone injection and the risk of stricture formation was estimated by calculating the odds ratio (OR) and 95% confidence interval (CI) using logistic regression analysis.
Table 1. Baseline characteristics before and after propensity score matching.
|
Before matching (n=150) |
After matching (n=74) |
|||||||
|---|---|---|---|---|---|---|---|---|
| Triamcinolone injection (no) (n=92) | Triamcinolone injection (yes) (n=58) | P-value | ASD | Triamcinolone injection (no) (n=37) | Triamcinolone injection (yes) (n=37) | P-value | ASD | |
| Age | 66.1±7.72 | 70.6±6.59 | <0.01 | 0.63 | 69.6±7.19 | 69.5±6.84 | 0.93 | 0.01 |
| Gender | ||||||||
| Male | 73 (79.3) | 49 (84.5) | 0.52 | 0.14 | 29 (78.4) | 30 (81.1) | 1.00 | 0.07 |
| Female | 19 (20.7) | 9 (15.5) | 8 (21.6) | 7 (18.9) | ||||
| ASA-PS | ||||||||
| 1 | 16 (17.4) | 3 (5.2) | 0.04 | 0.39 | 3 (8.1) | 3 (8.1) | 1.00 | 0.00 |
| 2 | 68 (73.9) | 46 (79.3) | 0.13 | 30 (81.1) | 30 (81.1) | 0.00 | ||
| 3 | 8 (8.7) | 9 (15.5) | 0.21 | 4 (10.8) | 4 (10.8) | 0.00 | ||
| Location | ||||||||
| Ce, Ut | 15 (16.3) | 13 (22.4) | 0.57 | 0.16 | 5 (13.5) | 8 (21.6) | 0.66 | 0.21 |
| Mt | 46 (50.0) | 25 (43.1) | 0.14 | 18 (48.6) | 16 (43.2) | 0.11 | ||
| Lt, Ae | 31 (33.7) | 20 (34.5) | 0.02 | 14 (37.8) | 13 (35.1) | 0.06 | ||
| Endoscopic appearance | ||||||||
| Elevated | 3 (3.3) | 3 (5.2) | 0.41 | 0.09 | 0 (0.0) | 3 (8.1) | 0.22 | 0.42 |
| Flat | 7 (7.6) | 8 (13.8) | 0.20 | 3 (8.1) | 3 (8.1) | 0.00 | ||
| Depressed | 82 (89.1) | 47 (81.0) | 0.23 | 34 (91.9) | 31 (83.8) | 0.25 | ||
| Tumor diameter (mm) | 35.3±12.7 | 39.7±16.7 | 0.07 | 0.30 | 38.3±11.7 | 37.3±12.2 | 0.37 | 0.08 |
| Treatment time | 123.2±65.3 | 121.0±45.3 | 0.75 | 0.04 | 120.5±54.1 | 119.7±44.6 | 0.71 | 0.02 |
| Cutting diameter | 50.0±12.5 | 55.9±16.7 | 0.02 | 0.40 | 53.5±12.8 | 51.4±11.9 | 0.46 | 0.17 |
| Circumferential mucosal defect (%) | 75.3±8.6 | 78.4±9.9 | 0.04 | 0.33 | 77.6±8.3 | 76.8±10.2 | 0.72 | 0.09 |
| Histology | ||||||||
| SCC | 90 (97.8) | 56 (96.6) | 0.78 | 0.19 | 37 (100) | 36 (97.3) | 1.00 | 0.00 |
| Adenocarcinoma | 2 (2.2) | 2 (3.4) | 0.18 | 0 (0.0) | 1 (2.7) | 0.00 | ||
| Invasion depth | ||||||||
| EP/LPM | 55 (59.8) | 42 (72.4) | 0.31 | 0.27 | 20 (54.1) | 25 (67.6) | 0.31 | 0.24 |
| MM/SM1 | 26 (28.3) | 11 (19.0) | 0.22 | 14 (37.8) | 8 (21.6) | 0.36 | ||
| SM2 | 11 (12.0) | 5 (8.6) | 0.11 | 3 (8.1) | 4 (10.8) | 0.09 | ||
| Previous CRT | ||||||||
| Yes | 5 (5.4) | 4 (6.9) | 0.74 | 0.06 | 2 (5.4) | 1 (2.7) | 1.00 | 0.14 |
| No | 87 (94.6) | 54 (93.1) | 35 (94.6) | 36 (97.3) | ||||
Ae, abdominal esophagus; ASA-PS, American Society of Anaesthesiologist Physical Status classification; ASD, absolute standardized difference; Ce, cervical esophagus; CRT, chemoradiotherapy; EP, epithelium; Lt, lower thoracic esophagus; LPM, lamina propria; MM, muscularis mucosa; Mt, middle thoracic esophagus; SCC, squamous cell carcinoma; SM1, submucosal invasion <200 μm; SM2, submucosal invasion ≥200 μm; Ut, upper thoracic esophagus.
Statistical analyses were performed using SPSS software version 23.0 for Windows (SPSS, IBM Corporation, Armonk, NY, USA) and the R statistical package V.3.0.2 (R Core Team, Vienna, Austria). All statistical tests were two-sided, and P<0.05 was considered significant.
Results
Clinicopathological characteristics of the study subjects
Among 602 patients, 189 had mucosal defects that covered more than 2/3 of the esophageal circumference (Figure 1). After exclusion of 33 patients who received other prophylactic therapy and 6 who underwent ESD near a previous ESD scar, 150 patients were enrolled. Of those 150, 58 (38.7%) underwent locoregional triamcinolone injection immediately after ESD. The clinicopathological characteristics of the study subjects are shown in Table 1.
Compared with patients who did not receive locoregional triamcinolone injections, patients who did receive these injections were significantly older, had a higher incidence of an American Society of Anesthesiologists physical status classification system (ASA-PS) score of 3, had resected lesions with a significantly larger diameter, and had mucosal defects with a wider circumference, before propensity score matching. The two groups were similar with respect to sex, location, endoscopic appearance, tumor diameter, treatment time, histology, tumor invasion depth, and history of chemoradiotherapy
After propensity score matching, there were 37 matched pairs of patients who did and did not receive locoregional triamcinolone injections. The baseline characteristics of the two groups were comparable (Table 1).
Effect of locoregional triamcinolone injection on stricture formation
Esophageal stricture formation occurred in 37 (24.7%) of 150 patients. Before propensity score matching, 12 of 58 patients (20.7%) who received locoregional triamcinolone injections had stricture formations, whereas 25 of 92 patients (27.2%) who did not receive steroid injections had stricture formations (P=0.37; Table 2). After matching, the incidence of stricture formation decreased from 45.9% (17/37) without triamcinolone injection to 18.9% with triamcinolone injection (7/37) (P=0.016).
Table 2. The incidence of stricture formation and the number of EBD dilatation.
| Triamcinolone injection (no) | Triamcinolone injection (yes) | P-value | |
|---|---|---|---|
| Stricture | |||
| Before matching | 25/92 (27.2) | 12/58 (20.7) | 0.37 |
| After matching | 17/37 (45.9) | 7/37 (18.9) | 0.016 |
| EBD numbers (mean±s.d., range) | |||
| Before matching | 1.7±3.7, 0–20 | 0.5±1.2, 0–8 | 0.02 |
| After matching | 2.8±4.6, 0–20 | 0.6±1.5, 0–8 | 0.006 |
EBD, endoscopic balloon dilatation.
Before matching, tumor location (upper esophageal), tumor diameter, and circumference of the mucosal defect were considered risk factors for stricture formation caused by ESD (Table 3). After propensity score matching, locoregional triamcinolone injection reduced the incidence of stricture formation (OR 6.00, 95% CI 1.34–26.8). No serious adverse events associated with locoregional steroid injections, such as bleeding, infection and perforation, were encountered.
Table 3. The risk factors for esophageal stricture formation by crude logistic regression before and after propensity score matching.
|
Before matching (n=150) |
After matching (n=74) |
|||||||
|---|---|---|---|---|---|---|---|---|
| n | case (%) | Crude OR (95%CI) | P-value | n | case (%) | Crude OR (95%CI) | P-value | |
| Age | 150 | 37 (24.7) | 0.97 (0.93–1.02) | 0.29 | 74 | 24 (32.4) | 0.95 (0.85–1.06) | 0.37 |
| Gender | ||||||||
| Male | 122 | 30 (24.6) | 1.00 | 59 | 19 (32.2) | 1.00 | ||
| Female | 28 | 7 (25.0) | 1.02 (0.40–2.64) | 0.96 | 15 | 5 (33.3) | 1.54 (0.34–7.08) | 0.58 |
| ASA-PS | ||||||||
| 1 | 19 | 7 (36.8) | 1.00 | 6 | 4 (66.7) | 1.00 | ||
| 2 | 114 | 27 (23.7) | 0.53 (0.19–1.49) | 0.23 | 60 | 19 (31.7) | — | 0.99 |
| 3 | 17 | 3 (17.6) | 0.37 (0.08–1.74) | 0.21 | 8 | 1 (12.5) | — | 0.99 |
| Location | ||||||||
| Ce, Ut | 28 | 11 (39.3) | 1.00 | 13 | 8 (61.5) | 1.00 | ||
| Mt | 71 | 12 (16.9) | 0.31 (0.12–0.84) | 0.02 | 34 | 8 (23.5) | 0.25 (0.03–2.24) | 0.22 |
| Lt, Ae | 51 | 14 (27.5) | 0.59 (0.22–1.55) | 0.28 | 27 | 8 (29.6) | — | 0.99 |
| Endoscopic appearance | ||||||||
| Elevated | 6 | 1 (16.7) | 1.00 | 3 | 1 (33.3) | 1.00 | ||
| Flat | 15 | 2 (13.3) | 0.77 (0.06–10.49) | 0.84 | 6 | 1 (16.7) | — | 0.99 |
| Depressed | 129 | 34 (26.4) | 1.79 (0.20–15.87) | 0.60 | 65 | 22 (33.8) | 0.99 | |
| Tumor diameter (mm) | 150 | 37 (24.7) | 1.04 (1.01–1.07) | <0.01 | 74 | 24 (32.4) | 1.04 (0.96–1.12) | 0.36 |
| Steroid injection | ||||||||
| Yes | 58 | 12 (20.7) | 0.70 (0.32–1.53) | 0.37 | 37 | 7 (18.9) | 1.00 | |
| No | 92 | 25 (27.2) | 1.00 | 37 | 17 (45.9) | 6.00 (1.34–26.8) | 0.02 | |
| Cutting diameter | 150 | 37 (24.7) | 1.02 (0.99–1.05) | 0.07 | 74 | 24 (32.4) | 1.02 (0.97–1.07) | 0.54 |
| Treatment Time | 150 | 37 (24.7) | 1.01 (0.99–1.01) | 0.11 | 74 | 24 (32.4) | 0.99 (0.97–1.03) | 0.93 |
| Circumferential mucosal defect (%) | 150 | 37 (24.7) | 1.12 (1.01–1.17) | <0.01 | 74 | 24 (32.4) | 1.08 (0.98–1.19) | 0.14 |
| Histology | ||||||||
| SCC | 146 | 36 (24.8) | 1.00 | 73 | 24 (32.9) | 1.00 | ||
| Adenocarcinoma | 4 | 1 (25.0) | 1.01 (0.10–10.01) | 0.99 | 1 | 0 (0.0) | — | 0.99 |
| Invaion depth | ||||||||
| EP/LPM | 97 | 24 (24.7) | 1.00 | 45 | 14 (31.1) | 1.00 | ||
| MM/SM1 | 37 | 10 (27.0) | 1.13 (0.48–2.66) | 0.79 | 22 | 8 (36.4) | 1.67 (0.40–6.97) | 0.48 |
| SM2 | 16 | 3 (18.8) | 0.70 (0.18–2.67) | 0.60 | 7 | 2 (28.6) | — | 0.99 |
| Previous CRT | ||||||||
| Yes | 9 | 0 (0.0) | — | — | 3 | 0 (0.0) | — | — |
| No | 141 | 37 (26.2) | 1.00 | 71 | 24 (33.8) | 1.00 | ||
Ae, abdominal esophagus; ASA-PS, American Society of Anaesthesiologist Physical Status classification; Ce, cervical esophagus; CI, confidence interval; CRT, chemoradiotherapy; EP, epithelium; LPM, lamina propria; Lt, lower thoracic esophagus; MM, muscularis mucosa; Mt, middle thoracic esophagus; OR, odds ratio; SCC, squamous cell carcinoma; SM1, submucosal invasion <200 μm; SM2, submucosal invasion ≥200 μm; Ut, upper thoracic esophagus.
The effect of locoregional triamcinolone injection on the incidence of stricture formation remained after adjustment for the propensity score, the circumference of the mucosal defect, age, sex, macroscopic findings, ASA-PS, and summation of these variables (Table 4).
Table 4. Univariate and multivariate Odds Ratios of esophageal stricture formation without steroid injection compared with steroid injection after propensity score matching.
|
After matching |
||
|---|---|---|
| OR (95% CI) | P-value | |
| Unadjusted | 6.00 (1.34–26.8) | 0.019 |
| Adjusted for PS | 5.64 (1.20–26.6) | 0.029 |
| Adjusted for Circumferential mucosal defect | 7.68 (1.06–55.4) | 0.043 |
| Adjusted for age | 5.88 (1.28–27.0) | 0.023 |
| Adjusted for gender | 9.44 (1.26–70.8) | 0.029 |
| Adjusted for macroscopic appearance | 13.7 (1.54–122.0) | 0.019 |
| Adjusted for ASA-PS | 8.91 (1.24–64.2) | 0.03 |
| Adjusted for age, gender, macroscopic appearance, ASA-PS, circumferential mucosal defect | 11.4 (1.15–112.0) | 0.038 |
ASA-PS, American Society of Anaesthesiologist Physical Status classification; CI, confidence interval; OR, odds ratio; PS, propensity score.
The number of endoscopic balloon dilatations
Locoregional triamcinolone injection also reduced the mean number of EBD sessions from 1.7±3.7 (without triamcinolone injection) to 0.5±1.2 times (with triamcinolone injection; P=0.02) before matching, and from 2.8±4.6 (without triamcinolone injection) to 0.6±1.5 times (with triamcinolone injection; P<0.01) after matching.
Propensity score estimation
The propensity score model was well calibrated (Hosmer-Lemeshow test, P=0.38) and it discriminated well between patients who did or did not receive triamcinolone injection (c statistic=0.77). Therefore, it is probable that the most likely possible confounders were identified in our study. However, the median absolute standardized difference after matching was 0.08 (interquartile range, 0–0.17). Therefore, the absolute standardized difference values noted in this study indicate that our propensity score matching could not completely remove the imbalance of variables.
Discussion
In the present study, a single locoregional triamcinolone injection immediately after ESD for superficial esophageal neoplasia reduced the incidence of stricture formation compared with that in the control group. The results also showed that locoregional triamcinolone injection reduced the number of EBD sessions required to treat stricture formation.
The present study has three important strengths. First, we conducted the study using a propensity-matched analysis. Second, we included patients with mucosal defects that covered more than 2/3 of the circumference of the esophagus. Third, compared with previous studies, a lower single dose of locoregional triamcinolone was administered to every patient, regardless of the size of the resected specimens.
A number of previous studies have reported that locoregional steroid injection may prevent stricture formation after esophageal ESD.10, 11, 12, 13 However, selection bias may have been an issue in these retrospective observation studies; the relationship between stricture formation and locoregional triamcinolone injection could be affected by confounding factors in particular, circumferential mucosal defect after ESD. Previous studies did not report the differences in the circumferences of the lesions or in the circumferential mucosal defects between the analyzed groups.10, 11, 12 A tumor larger than 75% of the esophageal circumference was reported as a risk factor for refractory stricture formation,17 even with steroid injection, and a whole-circumference mucosal defect after ESD was difficult to prevent with steroid injection.14, 17, 18 Therefore, the incidence of stricture formation could be affected by circumference of the lesion or the circumference of the mucosal defect. Although a well-designed, randomized control study would be helpful, it would be difficult to conduct because most patients in the control group would not accept the low quality-of-life associated with dysphagia due to the high incidence of stricture formation.5, 9, 11 In addition, it would be difficult to enroll and randomize the patients after removing the lesion because the patients would be under sedation at that point. Therefore, the present study retrospectively selected patients who underwent resection and who had a circumferential mucosal defect of more than 2/3; these patients were then pseudo-randomized using propensity score matching. It is often difficult to conduct an RCT because of budgetary and time constraints or ethical concerns,20 but retrospective observational studies have potential selection bias. Propensity score matching analysis resolves such bias in observational studies and the effects of treatment are considered to be approximately randomized.19, 21, 22
Most previous studies have determined that patients with circumferential mucosal defects greater than 75% are at risk for stricture formation.11, 12, 14 Our study demonstrated similar results for these types of lesions: 17 of 28 lesions (60.7%) without locoregional triamcinolone injections had stricture formations, whereas, only 6 of 23 lesions (26.1%) treated with locoregional triamcinolone injections had stricture formations (P=0.02). However, the risk of stricture formation was determined by using two-armed categorical data showing whether the circumferential mucosal defect was less than or greater than/equal to 75%.9 The cutoff for the risk of stricture formation was a circumferential mucosal defect of over 71%.8 Although we had previously evaluated the effect of steroid injection on stricture formation in patients with circumferential mucosal defects of any size, who had received two types of steroid injection, there were too many associated limitations.13 In addition, the indication for locoregional triamcinolone injection in our institution is resection of a circumferential mucosal defect of more than 2/3; entire circumferential resections are excluded. Therefore, in the present study, we selected patients with circumferential mucosal defects of more than 2/3. Regardless of the low incidence of stricture formation in the control group (45.9%), our study demonstrated that locoregional steroid injections reduced stricture formation.
Although several methods of steroid injections have been reported,10, 11, 12, 13, 14, 18 a single triamcinolone injection immediately after ESD was as effective as the other methods.11 In addition, it may be easier to recognize, and inject into, the submucosal layer immediately after ESD; therefore, adverse events such as perforation could be avoided. Furthermore, a single session could reduce not only the effort of the patients and the medical staff, but also the administration period and medical costs. The present study showed that a single triamcinolone injection of 80 mg offered sufficient protection against stricture formation, even though this dose was lower than that used in previous studies.11, 14 However, a prospective study evaluating the optimal dose of triamcinolone injection is required.
Intralesional corticosteroids may prevent stricture formation by inhibiting not only collagen synthesis, but also fibrosis and inflammation.22, 23, 24, 25 Although oral prednisolone also prevents stricture formation after esophageal ESD and has the potential to prevent more extensive lesions,26 long treatment duration and high total steroid dosage lead to systemic adverse events, such as infection or worsening of diabetes mellitus.27 In addition, oral prednisolone may delay additional surgery or chemoradiotherapy in cases with a deeper histological depth of invasion, due to possible infection or failure of the sutures.13 Conversely, a single triamcinolone injection immediately after ESD is considered to be easier, with fewer adverse events. A multicenter RCT comparing the 2 methods is ongoing.28 However, it was difficult to prevent stricture formation in wholly circumferential cases, even with the use of local and systemic steroids.18, 26, 29, 30 Hybrid therapy using steroid injection and polyglycolic acid sheets may be more efficient at preventing stricture formation than steroid injections alone, even in wholly circumferential cases.31, 32 Compared with several therapies,26, 33, 34 a single session of locoregional triamcinolone injection immediately after ESD may be easier, less expensive, and may also demonstrate similar efficacy and safety profiles.
Our study had some limitations. First, this was a retrospective study using a propensity score analysis in a single center15, 21 Second, the sample size decreased after propensity score matching. However, even in small studies, propensity score matching can yield unbiased estimations of treatment effect, unless the true confounders and the variables related only to the outcome are not included in the propensity model.35 In addition, the present study had a larger sample size than previous similar studies regardless of matching. Third, the lesion located at the cervical esophagus, which was considered at risk of stricture, was not evaluated, because all patients received a triamcinolone injection, but only 1 patient (16.7%) developed a stricture. In addition, post-CRT status was also considered a risk factor for stricture, but the present study found no stricture cases in post-CRT patients, similar to a previous report.17
In conclusion, a single session of locoregional triamcinolone injection immediately after ESD for superficial esophageal neoplasia efficiently prevented stricture formation.
Study Highlights
Footnotes
Meeting presentation: DDW2016.
Guarantor of the article: Yasuaki Nagami, MD, PhD.
Specific author contributions: Each author approved the final draft submitted. Conducting the study endoscopy, manuscript production: Yasuaki Nagami; statistical analysis, manuscript production: Masatsugu Shiba; endoscopy, management of patient's enrollment, data collection, manuscript review: Masaki Ominami, Taishi Sakai, Hiroaki Minamino, Shusei Fukunaga, and Satoshi Sugimori; management of patient's enrollment, production of the figure and tables, review of the manuscript: Fumio Tanaka, Noriko Kamata, Tetsuya Tanigawa, Hirokazu Yamagami, Toshio Watanabe, and Kazunari Tominaga; adviser for manuscript production, management of patient's enrollment, review of the manuscript: Yasuhiro Fujiwara; management of patient's enrollment, overall director for the present study: Tetsuo Arakawa.
Financial support: None.
Potential competing interests: Dr Arakawa received lecture fees from Otsuka and Eisai and research grants from Otsuka, Eisai, Astellas, Abbott Japan, Takeda, Dainippon Sumitomo, and Daiichi Sankyo. Dr Fujiwara received lecture fees from Takeda and research grants from Ono. The remaining authors declare no conflict of interest.
References
- Enzinger PC, Mayer RJ. Esophageal cancer. N Engl J Med 2003; 349: 2241–2252. [DOI] [PubMed] [Google Scholar]
- Jemal A, Siegel R, Ward E et al. Cancer statistics, 2008. CA Cancer J Clin 2008; 58: 71–96. [DOI] [PubMed] [Google Scholar]
- Muto M, Minashi K, Yano T et al. Early detection of superficial squamous cell carcinoma in the head and neck region and esophagus by narrow band imaging: a multicenter randomized controlled trial. J Clin Oncol 2010; 28: 1566–1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagami Y, Tominaga K, Machida H et al. Usefulness of non-magnifying narrow-band imaging in screening of early esophageal squamous cell carcinoma: a prospective comparative study using propensity score matching. Am J Gastroenterol 2014; 109: 845–854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono S, Fujishiro M, Niimi K et al. Long-term outcomes of endoscopic submucosal dissection for superficial esophageal squamous cell neoplasms. Gastrointest Endosc 2009; 70: 860–866. [DOI] [PubMed] [Google Scholar]
- Kim JS, Kim BW, Shin IS. Efficacy and safety of endoscopic submucosal dissection for superficial squamous esophageal neoplasia: a meta-analysis. Dig Dis Sci 2014; 59: 1862–1869. [DOI] [PubMed] [Google Scholar]
- Nagami Y, Machida H, Shiba M et al. Clinical Efficacy of Endoscopic Submucosal Dissection for Adenocarcinomas of the Esophagogastric Junction. Endosc Int Open 2014; 2: E15–E20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuta H, Nishimori I, Kuratani Y et al. Predictive factors for esophageal stenosis after endoscopic submucosal dissection for superficial esophageal cancer. Dis Esophagus 2009; 22: 626–631. [DOI] [PubMed] [Google Scholar]
- Ono S, Fujishiro M, Niimi K et al. Predictors of postoperative stricture after esophageal endoscopic submucosal dissection for superficial squamous cell neoplasms. Endoscopy 2009; 41: 661–665. [DOI] [PubMed] [Google Scholar]
- Barret M, Beye B, Leblanc S et al. Systematic review: the prevention of oesophageal stricture after endoscopic resection. Aliment Pharmacol Ther 2015; 42: 20–39. [DOI] [PubMed] [Google Scholar]
- Hanaoka N, Ishihara R, Takeuchi Y et al. Intralesional steroid injection to prevent stricture after endoscopic submucosal dissection for esophageal cancer: a controlled prospective study. Endoscopy 2012; 44: 1007–1011. [DOI] [PubMed] [Google Scholar]
- Hashimoto S, Kobayashi M, Takeuchi M et al. The efficacy of endoscopic triamcinolone injection for the prevention of esophageal stricture after endoscopic submucosal dissection. Gastrointest Endosc 2011; 74: 1389–1393. [DOI] [PubMed] [Google Scholar]
- Nagami Y, Shiba M, Tominaga K et al. Locoregional steroid injection prevents stricture formation after endoscopic submucosal dissection for esophageal cancer: a propensity score matching analysis. Surg Endosc 2015; 30: 1441–1449. [DOI] [PubMed] [Google Scholar]
- Takahashi H, Arimura Y, Okahara S et al. A randomized controlled trial of endoscopic steroid injection for prophylaxis of esophageal stenoses after extensive endoscopic submucosal dissection. BMC Gastroenterol 2015; 15: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenbaum PR, Rubin DB. The bias due to incomplete matching. Biometrics 1985; 41: 103–116. [PubMed] [Google Scholar]
- Ominami M, Nagami Y, Shiba M et al. Prediction of poor response to modified neuroleptanalgesia with midazolam for endoscopic submucosal dissection for esophageal squamous cell carcinoma. Digestion 2016; 94: 73–81. [DOI] [PubMed] [Google Scholar]
- Hanaoka N, Ishihara R, Uedo N et al. Refractory strictures despite steroid injection after esophageal endoscopic resection. Endosc Int Open 2016; 4: E354–E359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miwata T, Oka S, Tanaka S et al. Risk factors for esophageal stenosis after entire circumferential endoscopic submucosal dissection for superficial esophageal squamous cell carcinoma. Surg Endosc 2015; 30: 4049–4056. [DOI] [PubMed] [Google Scholar]
- Rubin DB. Estimating causal effects from large data sets using propensity scores. Ann Intern Med 1997; 127: 757–763. [DOI] [PubMed] [Google Scholar]
- Suzuki S, Gotoda T, Kusano C et al. The efficacy and tolerability of a triple therapy containing a potassium-competitive acid blocker compared with a 7-day PPI-based low-dose clarithromycin triple therapy. Am J Gastroenterol 2016; 111: 949–956. [DOI] [PubMed] [Google Scholar]
- D'Agostino RB Jr.. Propensity score methods for bias reduction in the comparison of a treatment to a non-randomized control group. Stat Med 1998; 17: 2265–2281. [DOI] [PubMed] [Google Scholar]
- Ramage JI Jr., Rumalla A, Baron TH et al. A prospective, randomized, double-blind, placebo-controlled trial of endoscopic steroid injection therapy for recalcitrant esophageal peptic strictures. Am J Gastroenterol 2005; 100: 2419–2425. [DOI] [PubMed] [Google Scholar]
- Nonaka K, Miyazawa M, Ban S et al. Different healing process of esophageal large mucosal defects by endoscopic mucosal dissection between with and without steroid injection in an animal model. BMC Gastroenterol 2013; 13: 72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochhar R, Makharia GK. Usefulness of intralesional triamcinolone in treatment of benign esophageal strictures. Gastrointest Endosc 2002; 56: 829–834. [DOI] [PubMed] [Google Scholar]
- Miyashita M, Onda M, Okawa K et al. Endoscopic dexamethasone injection following balloon dilatation of anastomotic stricture after esophagogastrostomy. Am J Surg 1997; 174: 442–444. [DOI] [PubMed] [Google Scholar]
- Yamaguchi N, Isomoto H, Nakayama T et al. Usefulness of oral prednisolone in the treatment of esophageal stricture after endoscopic submucosal dissection for superficial esophageal squamous cell carcinoma. Gastrointest Endosc 2011; 73: 1115–1121. [DOI] [PubMed] [Google Scholar]
- Ishida T, Morita Y, Hoshi N et al. Disseminated nocardiosis during systemic steroid therapy for the prevention of esophageal stricture after endoscopic submucosal dissection. Dig Endosc 2014; 27: 388–391. [DOI] [PubMed] [Google Scholar]
- Mizutani T, Tanaka M, Eba J et al. A Phase III study of oral steroid administration versus local steroid injection therapy for the prevention of esophageal stricture after endoscopic submucosal dissection (JCOG1217, Steroid EESD P3). Jpn J Clin Oncol 2015; 45: 1087–1090. [DOI] [PubMed] [Google Scholar]
- Isomoto H, Yamaguchi N, Nakayama T et al. Management of esophageal stricture after complete circular endoscopic submucosal dissection for superficial esophageal squamous cell carcinoma. BMC Gastroenterol 2011; 11: 46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato H, Inoue H, Kobayashi Y et al. Control of severe strictures after circumferential endoscopic submucosal dissection for esophageal carcinoma: oral steroid therapy with balloon dilation or balloon dilation alone. Gastrointest Endosc 2013; 78: 250–257. [DOI] [PubMed] [Google Scholar]
- Sakaguchi Y, Tsuji Y, Fujishiro M et al. Triamcinolone injection and shielding with polyglycolic acid sheets and fibrin glue for postoperative stricture prevention after esophageal endoscopic resection: A Pilot Study. Am J Gastroenterol 2016; 111: 581–583. [DOI] [PubMed] [Google Scholar]
- Nagami Y, Shiba M, Tominaga K et al. Hybrid therapy with locoregional steroid injection and polyglycolic acid sheets to prevent stricture after esophageal endoscopic submucosal dissection. Endosc Int Open 2016; 4: E1017–E1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ezoe Y, Muto M, Horimatsu T et al. Efficacy of preventive endoscopic balloon dilation for esophageal stricture after endoscopic resection. J Clin Gastroenterol 2011; 45: 222–227. [DOI] [PubMed] [Google Scholar]
- Ohki T, Yamato M, Ota M et al. Prevention of esophageal stricture after endoscopic submucosal dissection using tissue-engineered cell sheets. Gastroenterology 2012; 143: 582–8 e1-2. [DOI] [PubMed] [Google Scholar]
- Pirracchio R, Resche-Rigon M, Chevret S. Evaluation of the propensity score methods for estimating marginal odds ratios in case of small sample size. BMC Med Res Methodol 2012; 12: 70. [DOI] [PMC free article] [PubMed] [Google Scholar]