Abstract
Objectives:
A defect in bicarbonate secretion contributes to the pathophysiology of gastrointestinal complications in patients with cystic fibrosis (CF). We measured gastrointestinal pH, clinical outcomes, and intestinal transit profiles in patients with the G551D mutation before and after treatment with ivacaftor, a CF transmembrane regulator channel (CFTR) potentiator.
Methods:
Observational studies of ivacaftor effectiveness were conducted in the United States and Canada. A subset of subjects ingested a wireless motility capsule (n=10) that measures in vivo pH, both before therapy with ivacaftor and 1 month after treatment; values obtained were compared for mean pH and area under the pH curve, and regional intestinal motility. We also queried subjects about abdominal pain and recorded body weight before and after treatment.
Results:
One month after administering ivacaftor, a significant increase in mean pH was observed after gastric emptying (P<0.05). Area under the pH curve analyses indicate increased bicarbonate mass (P<0.05 for select 5 min intervals and all segments >30 min); mean weight gain was 1.1 kg (P=0.08). No difference in abdominal pain or regional transit times was seen.
Conclusions:
CFTR modulation improves the proximal small intestinal pH profile in patients with the G551D CFTR mutation and we observed clinically relevant, contemporaneous weight gain, although it did not reach statistical significance. These data provide in vivo evidence that CFTR is an important regulator of bicarbonate secretion, which may be a translational link between CFTR function and clinical improvement.
Introduction
Cystic fibrosis (CF) is caused by dysfunction of the cystic fibrosis transmembrane conductance regulator (CFTR), an ion channel on the apical surface of epithelia. Dysfunction of CFTR as a result of mutations in the gene leads to obstruction in the airways, pancreatic and hepatobiliary ducts, and intestine, which causes the clinical signs and symptoms of CF. There is strong evidence that duodenal bicarbonate is secreted via CFTR.1, 2 Bicarbonate drives pancreatic and other epithelial fluid secretion in a wide range of animals, including humans.3, 4 It has been proposed that luminal bicarbonate is critical in facilitating normal unfolding and hydration of mucus.5 Lack of bicarbonate leads to excessively adhesive mucus in mouse models of the CF intestine6, 7 and excised airways of swine.8, 9 and thus may be central to the pathophysiology of CF. The proximal small intestine has a major bicarbonate-secreting function to rapidly neutralize gastric acid from a pH of 1–3 to a physiologic level of 5.5–7. These large swings in pH make it an ideal site in which to demonstrate abnormalities in pH modulation.
The majority of patients with CF have severe pancreatic insufficiency (PI) as a result of decreased flow of pancreatic bicarbonate and fluid, obstruction of the pancreatic ducts, and subsequent severe reduction of pancreatic enzyme secretion.10, 11 The pancreatic parenchyma is destroyed and replaced by fat; however, the duct remains present. Bicarbonate secretion in the gastrointestinal lumen is primarily via the ductal cells of pancreas, but the biliary tree and duodenal epithelial cells also contribute to CFTR-mediated bicarbonate secretion.12
Inadequate acid neutralization in the proximal small intestine likely interferes with nutrient absorption by diminishing pancreatic enzyme activity and by causing intraluminal precipitation of bile acids with impaired micelle formation.13 Poor growth is closely correlated with poor lung function, decreased forced expiratory volume in 1 s in adults and pediatric patients with CF.14 Strategies to improve nutrition and decrease malabsorption by optimizing intestinal pH and increasing luminal bicarbonate are likely to benefit both pulmonary and gastrointestinal symptoms in patients with CF.
Modulation of CFTR activity is now possible for some patients with CF. The CFTR potentiator ivacaftor has marked effects on clinical outcomes in patients with the CFTR G551D gating mutation15 and other related alleles. We hypothesized that these benefits may be as a result of increased bicarbonate secretion. We aimed to study the effect of CFTR function as a mediator of in vivo bicarbonate secretion by measuring intestinal pH along with GI transit profiles and clinical parameters in patients with the G551D mutation before and after treatment with ivacaftor.
Methods
The data presented here are from two substudies of longitudinal observational cohort studies of the effectiveness of ivacaftor conducted in ambulatory subjects in tertiary care settings at CF Centers in the United States and Canada and expand upon preliminary results reported from the US cohort.16 For these substudies, subjects >18 years of age with CF and at least one G551D CFTR mutation were enrolled at four US and one Canadian site. Demographic information was collected in the longitudinal studies along with clinical outcomes and quality of life data for all subjects as described previously.16 GI pH and regional transit times were measured using a wireless motility capsule (WMC) before and 1 month after subjects started treatment with ivacaftor based on their physician's prescription. Study protocols and informed consent forms were approved by the Institutional Review Board at each US site and by the Regulatory Ethics Board in Canada; all subjects provided written consent.
CF was diagnosed by sweat confidence interval (Cl) >60 mmol/l or the presence of two CF-causing mutations (http://www.cftr2.org). PI was based on fecal elastase <100 μg/g, or serum immunoreactive trypsinogen <7 μg/l (measured when the subject was >8 years of age). Exclusion criteria included history of fibrosing colonopathy, intestinal resection, lack of daily bowel movements, CF-related diabetes, liver disease, or severe obstructive lung disease with forced expiratory volume in 1 s <25% predicted. Subjects with CF-related diabetes were not excluded from pH pill study participation. No subjects had been hospitalized in the prior 2 months and all subjects were evaluated and in stable health at the time of the study. Subjects were instructed to withhold acid-suppressing medicines (proton pump inhibitors or histamine-2 blockers) during the week before capsule ingestion.
The WMC system (SmartPill Medtronic GI Solutions, Sunnyvale, CA) includes an ingestible capsule (cylindrical, 26.8 mm long by 11.7 mm in diameter), a receiver that is worn outside the body by a subject, and dedicated display and analysis software. The WMC houses sensors for pH, temperature, and pressure, and transmits data at 434 MHz to the receiver for up to 5 days. The frequency of pH measurements is maintained at every 5 s (12 values every minute) for the first 24 h, then reduced to once every 20 s (3 values every minute) for the next 24 h, and finally reduced further to once every 40 s until the end of the test to conserve battery power. WMC technology has been approved in United States and Canada in adults >18 years of age.
Following an overnight fast, subjects ate a standardized low fat meal bar (255 kcal; 16 g of protein, 40 g of carbohydrates, 0.5 g of fiber, 1.83 g of total fat, and 1.51 g of saturated fat) with half their usual enzyme dose for PI subjects and no enzymes in PS subjects and swallowed the WMC with water. One subject ingested an oral glucose substrate (75 g) instead of the meal bar, which has been shown to be equivalent to the meal bar as a substrate for measuring GI pH.17 Subjects were observed for 1 h before discharge. Subjects were instructed on recognition of capsule passage via an indicator of a signal loss on the receiver. Once the receiver indicated loss of signal coinciding with capsule evacuation, the WMC was discarded and only the receiver was mailed back to the research coordinator. Data from the receiver was downloaded and analyzed at a central site (University at Buffalo, Buffalor, NY).
Data analysis and statistical methods
pH profile data were extracted using the Gastrointestinal Motility Software (GIMS) (Medtronic GI Solutions, Sunnyvale, CA). Gastric emptying was defined as a sustained rise in the pH by ≥3 units above the gastric pH. Ileocolonic transit was defined as an abrupt drop in the intestinal pH≥1 unit without recovering to the prior mean intestinal pH. Completion of the study was defined as an acute temperature drop corresponding to capsule evacuation along with permanent loss of signal on the receiver.
Mean pH values over 1 min increments from gastric emptying were calculated for the duration of 2 h using the GIMS Data Viewer 2.2.1 (Medtronic GI Solutions). The area under the curve (AUC) of the pH profile in the small intestine was calculated for the entire 120 min, for 30 and 60 min intervals, and for each 5 min increment using the trapezoidal rule. P values are two-sided and 95% CIs are reported. If subjects were more than 5 ft from the receiver, data signal from the capsule was not captured by the receiver and would be labeled as data gaps. These data gaps were excluded from the pH analysis. Paired t-tests were used to compare clinical and quality of life outcomes, means of 1 min pH profiles, and AUC calculations before and after ivacaftor therapy. The time required to reach and maintain pH 5.5 was calculated for each test and compared before and 1 month after ivacaftor exposure with a paired t-test. This level was chosen because it is the minimum pH for dissolution of the enteric coating of proprietary pancreatic enzymes18, 19 and pancreatic enzymatic activity.20 Similarly, gastric emptying time, small intestinal transit, and colonic transit intervals were calculated for each subject before and after ivacaftor. The study was designed to have 80% power with 15 subjects to conduct a two-sided paired t-test to detect a change of 1 pH unit with a type-I error 0.05. This calculation assumed a variance estimate from preliminary single-center paired data and a 10% drop-out rate. Statistical analyses were performed using R (R Development Core Team, version 2.15, Vienna, Austria), SPSS (IBM Corporation, version 9.3, Cary NC), and Prism GraphPad (GraphPad Software Inc, version 6.0e for Mac OS X, La Jolla, CA).
Results
Subject clinical characteristics are shown in Table 1. The mean age was 35.7 years (range: 25–50 years) and 50% were female. Seventy percent had F508del as their second CFTR mutation. Eight out of 10 subjects had PI at baseline. Mean weight gain after 1 month of ivacaftor was 1.1 kg (P=0.08). Five of 10 subjects had clinically relevant weight gain (defined as >1 kg over 1 month); three subjects lost weight during the month. Mean sweat chloride values before and after ivacaftor were 95.4 and 43.4 mmol/l, respectively (P<0.001). Seven of 10 subjects reported abdominal pain at baseline; there was no change in self-reported abdominal pain based on analysis of the GI subscale of the quality of life instrument used in the longitudinal cohort studies.16
Table 1. Demographics and clinical characteristics.
| Second mutation | PI | Abdominal pain at screening | Abdominal pain after ivacaftor | Weight at screening (kg) | Weight changea(kg) | Sweat Cl before ivacaftor (mmol/l) | Sweat Cl after ivacaftor (mmol/l) |
|---|---|---|---|---|---|---|---|
| F508del | Y | Y | Y | 74.5 | 2.0 | 102 | 67 |
| F508del | Y | Y | Y | 72.1 | 3.6 | 91 | 31 |
| F508del | Y | Y | Y | 87.1 | 0.4 | 102 | 37 |
| Unidentified | Y | Y | Y | 72 | 0.1 | 110 | 65 |
| F508del | Y | Y | Y | 52.5 | −1.2 | 102 | 25 |
| F508del | Y | Y | Y | 56.6 | −1.1 | 87 | 36 |
| F508del | Y | Y | Y | 57.9 | −0.1 | 114 | 47 |
| Unidentified | N | N | N | 104.0 | 3.5 | 79 | 48 |
| F508del | Y | N | N | 53.6 | 2.2 | 86 | 35 |
| 5T | N | N | N | 93.9 | 1.6 | 81 | 43 |
CI, confidence interval; PI, pancreatic insufficiency.
Weight at the time of the second WMC study one month on Ivacaftor therapy.
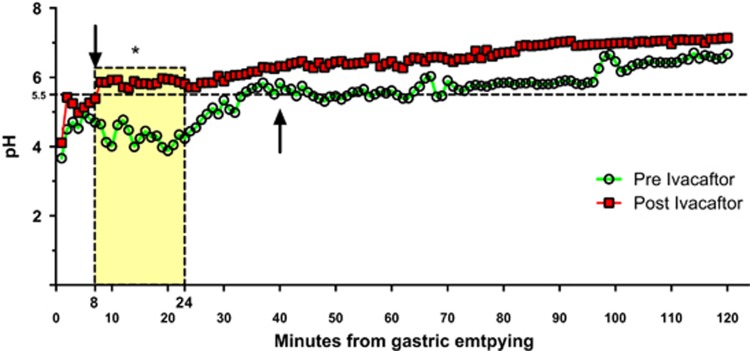
A statistically significant increase was observed in mean pH values between 8 and 24 min after gastric emptying (P<0.05) (Figure 1). The mean time required to reach and sustain pH of 5.5, before and after taking ivacaftor was 40 vs. 8 min respectively (Figure 1) (P<0.02; difference 32.4 min, 95% CI: 7.5, 57.1). These findings remained significant when pancreatic sufficient subjects were removed from the analysis.
Figure 1.
pH changes in 1 min increments after gastric emptying. Average pH profiles at 1 min increments starting from gastric emptying (time=0) in the same cohort of subjects before ivacaftor (green circles) and 1 month after treatment with ivacaftor (red squares). Mean time interval to reach and sustain pH of 5.5 preivacaftor (up arrow)—40 min and postivacaftor (down arrow)—8 min. *Time interval between 8 and 24 min after gastric emptying with paired t-test differences. P<0.05.
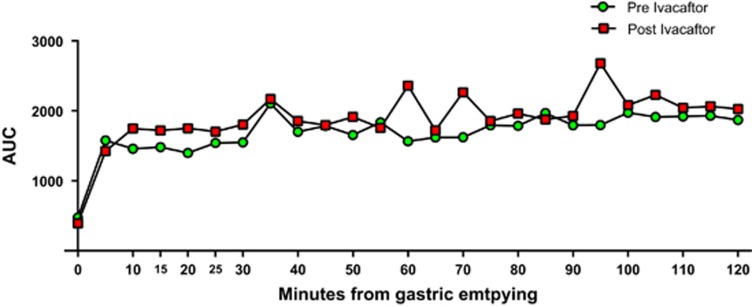
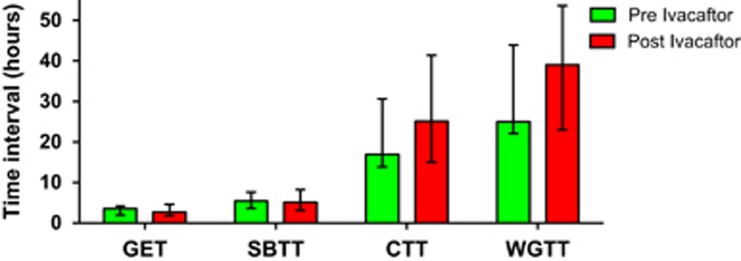
The pH profile AUC was calculated to analyze the relative change in base required to buffer acid. Bicarbonate is the predominant source of base used in luminal homeostasis and pH balance. Consequently, AUC of the pH curve can indirectly represent bicarbonate mass.21 AUC analysis among all subjects (PI and PS) showed significant improvement between 0 and 30 min (AUC= 2,072.9; 95% CI=950.5, 3,195.3; P=0.00238), 0–60 min (AUC=4,355.1; 95% CI=1,576.2, 7,134.0; P=0.0062) and 0–120 min (AUC=9,529.8; 95% CI=2,442.0, 1,6617.6; P=0.01398), suggesting an overall increase in luminal bicarbonate within the small intestine in response to ivacaftor. Further, paired t-test of the mean 5 min increments of pH AUC comparing subjects before and after taking ivacaftor showed significant differences between 15 and 20 min (P=0.02) and 25 and 30 min (P=0.03). Repeating this analysis only on PI subjects demonstrated significance at each 5-min interval between 5 and 25 min (5–10 min: P=0.024; 10–15 min: P=0.018; 15–20 min P=0.009, 20–15 min: P=0.032) (Figure 2). Use of ivacaftor did not lead to significant differences in gastric emptying, small intestinal transit time, colonic transit, or whole gut transit compared with preivacaftor values (Figure 3).
Figure 2.
Area under the curve (AUC) analysis of the pH profile. Average AUC of the pH profile was calculated at 5 min intervals using the trapezoidal rule. AUC (integral of the pH curve) is suggestive of increased bicarbonate flux in subjects after ivacaftor (red squares) compared with same group before ivacaftor (green circles).
Figure 3.
Gastrointestinal transit profiles before and after ivacaftor. Transit profile with interquartile range error bars. No change in transit profiles after 1 month of treatment with ivacaftor. CTT, colonic transit time; GET, gastric emptying time; SBTT, small bowel transit time; WGTT, whole gut transit time.
Discussion
Ivacaftor potentiates ion transport via CFTR. The subjects enrolled in these two effectiveness studies had the same reduction in sweat chloride concentrations as in the pivotal phase III study of ivacaftor in CF patients with the G551D mutation.15 Noticeable changes of sweat chloride and forced expiratory volume in 1 s were observed within 2 weeks of starting therapy in this pivotal study, which supported our study design to repeat the pH pill study 1 month after starting therapy. In addition to improved chloride resorption in sweat ducts, we provide evidence of the increased bicarbonate secretion anticipated with improved CFTR activity, as shown by a significant increase in proximal small intestinal pH and a decreased time to reach a stable pH>5.5. The pH profile seen in this group after taking ivacaftor for 1 month appeared to be similar to that seen in healthy individuals without CF.21 This difference was seen even though we predicted that we would need a larger sample size. The statistically significant difference in the pH profile after treatment with ivacaftor was seen despite the recruitment of fewer subjects because of the large effect size, pointing to the potency of ivacaftor to increase CFTR-mediated bicarbonate secretion. In addition, two subjects were pancreatic sufficient and had a higher baseline pH, which might have limited our ability to see a change from baseline, thus strengthening our findings.
There is strong evidence that CFTR's role as a bicarbonate channel and a modulator of chloride and bicarbonate transport is at the heart of the disruptions in normal physiology that directly contribute to the clinical manifestations of CF.22, 23 Abnormal CFTR-mediated bicarbonate secretion affects the airways,8 but is easier to study in the GI tract than in the airways because of the immediate and marked increase in pH that follows gastric emptying and the ability to measure pH directly in vivo, as we have shown with this substudy cohort. In the GI tract, perturbations in CFTR-mediated bicarbonate secretion contribute to dehydration, increased viscosity, and adhesion of intraluminal secretions and to dysbiosis in both the small and large intestine. Improving these perturbations by improving CFTR-associated bicarbonate regulation has been hypothesized to contribute to the marked weight gain seen in the pivotal studies of ivacaftor.24 Weight gain is a positive outcome in this population where malnutrition is closely associated with adverse pulmonary outcomes.
This study reports clinical outcomes in additional to regional transit calculations. This cohort had an average weight over 1 month of 1.1 kg, which may be clinically relevant in adults with CF even though it did not reach statistical significance (P=0.08). We have demonstrated for the first time that increased weight in patients taking ivacaftor occurs contemporaneously with increased proximal small intestinal pH in some subjects, although we cannot prove cause and effect. Although present in 70% of subjects, reported abdominal pain was minimal at baseline and did not change with treatment. We did not identify improvement or changes in the gastric emptying, small intestinal, or whole gut transit times. Lack of measurable transit profile differences might be attributed to the small number of subjects enrolled or the limited time of exposure to ivacaftor (1 month). Although small bowel transit was noted to be slower in CF subjects compared with healthy controls,25 we did not observe changes in small bowel motility in the subjects in this substudy after treatment with ivacaftor. It is possible that studying a larger cohort over a longer period of time would identify improved small intestinal transit as noted in comparing CF subjects to healthy age-matched control subjects.21 There is evidence of intestinal dysbiosis seen in the GI tract of patients with CF.26 Ivacaftor appears to improve airway dysbiosis,16 possibly via improved function of defensins.25 Increasing luminal bicarbonate might change the microbiome of the gastrointestinal tract, but such change might occur over a longer time period and the consequent impact on motility might be equally delayed.
This study has several limitations, including the small number of subjects. The rapid dissemination of ivacaftor use in the United States shortly after its approval interfered with our ability to achieve a target enrollment of 15 subjects. Approval took a longer time in Canada and we were able to increase the size of our cohort somewhat; however, we were only able to add one additional study site. In both the United States and Canada, all subjects were over 18 years of age because the WMC has been approved only for use in adults; however, the underlying pathophysiology in patients with CF is likely the same in children and adults. Generalizability is also limited because subjects with severe obstructive lung disease (forced expiratory volume in 1 s <25% of predicted) were excluded. Detailed analyses of AUC at frequent, 5 min intervals were post hoc and intended as exploratory; therefore, no adjustments for multiple comparisons were made and P values should be interpreted as such. Although we did not measure bicarbonate directly, in health, the changes in pH in the lumen of the proximal small intestine are predominantly due to bicarbonate secretion.10, 12 The AUC measurements of the pH curve identify a significant influx of base that we have proposed reflects bicarbonate mass. Presuming this assumption is correct, another limitation of this study is that the WMC cannot identify the site of proximal small intestinal bicarbonate secretion, which may be from the residual pancreatic duct, from the hepatobiliary tree or from Brunner's glands.
We have demonstrated that CFTR modulation improves the proximal small intestinal pH profile in patients with the G551D CFTR mutation, and we observed clinically relevant, contemporaneous weight gain, although it did not reach statistical significance. These data provide in vivo evidence that CFTR is an important regulator of bicarbonate secretion, which may be a translational link between CFTR function and clinical improvement.
Study Highlights
Footnotes
Guarantor of the article: Daniel Gelfond, MD.
Specific author contributions: Daniel Gelfond—assisted in protocol design, data extraction and analysis, and writing and revision of the manuscript. Sonya Heltshe—assisted with protocol design, data analysis and writing and revision of this manuscript, and approved the final draft. Changxing Ma—assisted with data analysis and review and revision of this manuscript, and approved the final draft. Steven M. Rowe—assisted with protocol design, data analysis and review and revision of this manuscript, and approved the final draft. Carla Frederick—assisted with protocol conduct and review and revision of this manuscript, and approved the final draft. Ahmet Uluer—assisted with protocol conduct and review and revision of this manuscript, and approved the final draft. Leonard Sicilian—assisted with protocol conduct and review and revision of this manuscript, and approved the final draft. Michael Konstan—assisted with protocol conduct and review and revision of this manuscript, and approved the final draft. Elizabeth Tullis—assisted with protocol design and review and revision of this manuscript, and approved the final draft. Christine Roach—assisted with protocol design and conduct and review of this manuscript, and approved the final draft. Katherine Griffin—assisted with protocol design and conduct and review of this manuscript, and approved the final draft. Elizabeth Joseloff—assisted with protocol design and review and revision of this manuscript, and approved the final draft. Drucy Borowitz—assisted in protocol design, data analysis, and writing and revision of the manuscript, and approved the final draft.
Financial support: This work was supported by grant support: CFFT BOROWI03CS0, GOAL13K1, NIH: DK072482, P30 DK089507/DK/NIDDK NIH HHS.
Potential competing interests: Dr Gelfond—received consultancy fees from Vertex, Abbvie, and Chiesi. Dr Rowe—UAB—receives institutional funding for the design and conduct of CF clinical trials. Dr Uluer—Boston Children's Hospital—receives institutional funding for design and conduct of CF clinical trials. Dr Konstan—receives consultancy fees from Vertex. Dr Sicilian—Massachusetts General Hospital, receives institutional funding for design and conduct of CF clinical trials. Drs Borowitz, Christine Roach, Changxing Ma—University of Buffalo Jacobs School of Medicine—receives institutional funding for the design and conduct of CF clinical trials. After this study was completed but during the writing of this manuscript, Dr Borowitz became a paid employee of the CF Foundation. Dr Tullis—St Michael's Hospital—receives institutional funding for design and conduct of CF clinical trials, and also received consultancy and speaking fees from Vertex.
Supplementary Material
References
- Ishiguro H, Steward MC, Naruse S et al. CFTR functions as a bicarbonate channel in pancreatic duct cells. J Gen Physiol 2009; 133: 315–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogan DL, Crombie DL, Isenberg JI et al. CFTR mediates cAMP- and Ca2+-activated duodenal epithelial HCO3− secretion. Am J Physiol 1997; 272 (Part 1): G872–G878. [DOI] [PubMed] [Google Scholar]
- Novak I, Wang J, Henriksen KL et al. Pancreatic bicarbonate secretion involves two proton pumps. J Biol Chem 2011; 286: 280–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaunitz JD, Akiba Y. Review article: duodenal bicarbonate—mucosal protection, luminal chemosensing and acid-base balance. Aliment Pharmacol Ther 2006; 24 (Suppl 4): 169–176. [DOI] [PubMed] [Google Scholar]
- Quinton PM. Cystic fibrosis: impaired bicarbonate secretion and mucoviscidosis. Lancet 2008; 372: 415–417. [DOI] [PubMed] [Google Scholar]
- Gustafsson JK, Ermund A, Ambort D et al. Bicarbonate and functional CFTR channel are required for proper mucin secretion and link cystic fibrosis with its mucus phenotype. J Exp Med 2012; 209: 1263–1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia MA, Yang N, Quinton PM. Normal mouse intestinal mucus release requires cystic fibrosis transmembrane regulator-dependent bicarbonate secretion. J Clin Invest 2009; 119: 2613–2622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birket SE, Chu KK, Liu L et al. A functional anatomic defect of the cystic fibrosis airway. Am J Respir Crit Care Med 2014; 190: 421–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoegger MJ, Fischer AJ, McMenimen JD et al. Impaired mucus detachment disrupts mucociliary transport in a piglet model of cystic fibrosis. Science 2014; 345: 818–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed N, Corey M, Forstner G et al. Molecular consequences of cystic fibrosis transmembrane regulator (CFTR) gene mutations in the exocrine pancreas. Gut 2003; 52: 1159–1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadorn B, Johansen PG, Anderson CM. Pancreozymin secretin test of exocrine pancreatic funtion in cystic fribrosis and the significance of the result for the pathogenesis of the disease. Can Med Assoc J 1968; 98: 377–385. [PMC free article] [PubMed] [Google Scholar]
- Pratha VS, Hogan DL, Martensson BA et al. Identification of transport abnormalities in duodenal mucosa and duodenal enterocytes from patients with cystic fibrosis. Gastroenterology 2000; 118: 1051–1060. [DOI] [PubMed] [Google Scholar]
- Borowitz D, Lubarsky B, Wilschanski M et al. Nutritional status improved in cystic fibrosis patients with the G551D mutation after treatment with ivacaftor. Dig Dis Sci 2015; 61: 198–207. [DOI] [PubMed] [Google Scholar]
- Stallings VA, Stark LJ, Robinson KA et al. Evidence-based practice recommendations for nutrition-related management of children and adults with cystic fibrosis and pancreatic insufficiency: results of a systematic review. J Am Diet Assoc 2008; 108: 832–839. [DOI] [PubMed] [Google Scholar]
- Ramsey BW, Davies J, McElvaney NG et al. A CFTR potentiator in patients with cystic fibrosis and the G551D mutation. N Engl J Med 2011; 365: 1663–1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe SM, Heltshe SL, Gonska T et al. Clinical mechanism of the cystic fibrosis transmembrane conductance regulator potentiator ivacaftor in G551D-mediated cystic fibrosis. Am J Respir Crit Care Med 2014; 190: 175–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borowitz D, Gelfond D. Equivalent substrates enable simultaneous study of gastrointestinal pH and CF-related diabetes. J Cyst Fibros 2015; 14: e6–e8. [DOI] [PubMed] [Google Scholar]
- Ferrone M, Raimondo M, Scolapio JS. Pancreatic enzyme pharmacotherapy. Pharmacotherapy 2007; 27: 910–920. [DOI] [PubMed] [Google Scholar]
- Kraisinger M, Hochhaus G, Stecenko A et al. Clinical pharmacology of pancreatic enzymes in patients with cystic fibrosis and in vitro performance of microencapsulated formulations. J Clin Pharmacol 1994; 34: 158–166. [DOI] [PubMed] [Google Scholar]
- Balasubramanian K, Zentler-Munro PL, Batten JC et al. Increased intragastric acid-resistant lipase activity and lipolysis in pancreatic steatorrhoea due to cystic fibrosis. Pancreas 1992; 7: 305–310. [DOI] [PubMed] [Google Scholar]
- Gelfond D, Ma C, Semler J et al. Intestinal pH and gastrointestinal transit profiles in cystic fibrosis patients measured by wireless motility capsule. Dig Dis Sci 2013; 58: 2275–2281. [DOI] [PubMed] [Google Scholar]
- Borowitz D. CFTR, bicarbonate, and the pathophysiology of cystic fibrosis. Pediatr Pulmonol 2015; 50 (Suppl 40): S24–S30. [DOI] [PubMed] [Google Scholar]
- Quinton PM. Role of epithelial HCO3(−) transport in mucin secretion: lessons from cystic fibrosis. Am J Physiol Cell Physiol 2010; 299: C1222–C1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borowitz D. CFTR, bicarbonate, and the pathophysiology of cystic fibrosis. Pediatr Pulmonol 2015; 50 (Suppl 40): S24–S30. [DOI] [PubMed] [Google Scholar]
- Gelfond D, Ma C, Semler J et al. Intestinal pH and gastrointestinal transit profiles in cystic fibrosis patients measured by wireless motility capsule. Dig Dis Sci 2012; 58: 2275–2281. [DOI] [PubMed] [Google Scholar]
- Hoffman LR, Pope CE, Hayden HS et al. Escherichia coli dysbiosis correlates with gastrointestinal dysfunction in children with cystic fibrosis. Clin Infect Dis 2014; 58: 396–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.