Abstract
Detection and complete removal of precancerous neoplastic polyps are central to effective colorectal cancer screening. The prevalence of neoplastic polyps in the screening population in the United States is likely >50%. However, most persons with neoplastic polyps are never destined to develop cancer, and do not benefit for finding and removing polyps, and may only be harmed by the procedure. Further 70–80% of polyps are diminutive (≤5 mm) and such polyps almost never contain cancer. Given the questionable benefit, the high-cost and the potential risk changing our approach to the management of diminutive polyps is currently debated. Deemphasizing diminutive polyps and shifting our efforts to detection and complete removal of larger and higher-risk polyps deserves discussion and study. This article explores three controversies, and emerging concepts related to endoscopic polyp resection. First, we discuss challenges of optical resect-and-discard strategy and possible alternatives. Second, we review recent studies that support the use of cold snare resection for ≥5 mm polyps. Thirdly, we examine current evidence for prophylactic clipping after resection of large polyps.
INTRODUCTION
Endoscopic colorectal cancer (CRC) prevention reduces CRC mortality by ~50%.1,2,3 The benefit of colonoscopy on cancer prevention is dependent on effective polyp detection and removal.1 Three important aspects of our approach to managing colon polyps are in evolution:
The first aspect is related to the management of diminutive polyps (polyps up to 5 mm in size). Because diminutive polyps are very common and almost never contain cancer, new management strategies to improve cost-effectiveness have been proposed.4, 5 The “resect-and-discard” strategy that uses real-time polyp diagnosis of diminutive polyps has been recently endorsed by endoscopy societies.5 However, adoption of this strategy into clinical practice faces several challenges.
The second topic is related to resection of mid-size polyps.6 While “hot” electrocautery snare resection has been the standard-of-care for several decades, recent studies suggest that “cold” snare resection without electrocautery may be as safe and effective as hot snare resection.6, 7
Finally, several studies have focused on the management of large ≥20 mm polyps, particularly with respect to lowering the risk of bleeding complications.8, 9 Prophylactic clip closure of the mucosal defect has become a common practice; however, whether clipping truly decreases bleeding risk remains unclear.
In this article, we discuss recent research developments and controversies with regards to the management of diminutive polyps, cold snare resection of mid-size polyps, and bleeding prophylaxis after resection of large polyps. The discussed observations call for refocusing our cancer prevention efforts in practice and research from removing diminutive polyps to the detection and safe resection of higher-risk polyps.
Should we adopt a resect-and-discard strategy for diminutive polyps?
Current colonoscopy practice guidelines recommend to remove, whenever possible, all polypoid lesions for histopathology assessment irrespective of the size or appearance. Of all detected polyps, 70–80% are diminutive, and ~50% of diminutive polyps are non-neoplastic. Cancer is exceedingly rare, and previous studies have described cancer prevalence between 0 and 0.08% for diminutive polyps and cancer prevalence between 0 and 1.5% in polyps up to 10 mm.10, 11, 12, 13, 14, 15, 16, 17 A recent and largest cross-sectional study to date included >42,000 polypectomies of up to 9 mm polyps did not find any cancer in any of these diminutive or small polyps.18 Resection of diminutive polyps increases patient risk and cost, yet the benefit on cancer prevention by removing diminutive polyps is questionable.15, 19, 20 However, histopathology evaluation of diminutive polyps remains important because presence of adenoma may determine low- or high-risk status of the patient and affect the colonoscopy surveillance interval.21
One avenue to reduce colonoscopy related cost would be to replace histopathology assessment by using endoscopic image, enhancing modalities to distinguish neoplastic from non-neoplastic polyps (Figure 1).5, 22 Novel image modalities have shown to predict neoplastic polyps with high accuracy and thus allow to determine the interval for the subsequent surveillance colonoscopy.2324 This new ability has inspired the concept of the “resect-and-discard” strategy. According to this strategy, diminutive polyps are diagnosed real time as adenomas or non-adenomatous polyps by using digital chromoendoscopy, like narrow band imaging (NBI). Polyps that are diagnosed with high confidence are resected and discarded, while others are sent for pathology evaluation. Calculated cost savings of this approach have been estimated to be 33,000,000$ per year in the United States.19 A >90% agreement between the optical and the pathology-based surveillance recommendations has been set as the required quality benchmark in order to adopt the resect-and-discard strategy.5 In recent years, multiple studies have shown that this benchmark can be accomplished, but only if optical diagnosis is done with high confidence by experienced endoscopists in an academic setting.25, 26 On the basis of these results, the resect-and-discard strategy has been endorsed by the European and American Societies for Gastrointestinal Endoscopy (ESGE and ASGE).27, 28, 29, 30 Both societies are well aware of challenges with successful implementation of resect-and-discard. It requires training, credentialing and monitoring of quality. Legal aspects of discarding tissue may also be a concern. Further, the resect-and-discard approach requires additional efforts by the endoscopist during the examination, added photo and text documentation, and the need to combine optical with pathology based diagnoses in a large proportion of patients.31 This added complexity to everyday practice might further hinder widespread adoption. Alternative concepts to simplify the resect-and-discard strategy and minimize or eliminate the need for optical and histopathology assessment have recently been proposed.32 In a post hoc analysis, a non-optical resect-and-discard strategy was examined, in which all rectosigmoid diminutive polyps were considered as hyperplastic and all polyps proximal to the rectosigmoid as neoplastic.33 The non-optical strategy agreed with the pathology-based surveillance recommendations in 89% of patients, just shy of the 90% benchmark, but not significantly different from the optical strategy. The non-optical strategy also reduced the number of required pathology examinations and provided more patients with surveillance recommendations immediately following the colonoscopy compared to the optical resect-and-discard strategy. While resect-and-discard is a promising idea to reduce colonoscopy associated cost, further research on how to make the concept feasible for community practice is warranted.
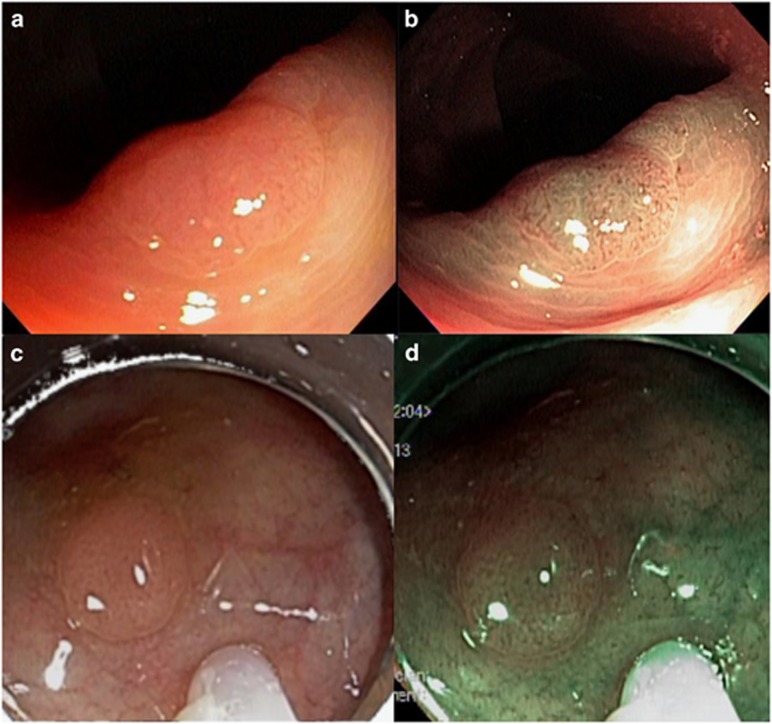
Figure 1.
Diminutive polyps examined with white light and optical chromoendoscopy (a) adenomatous polyp in white light imaging (b) adenomatous polyp in optical chromoendoscopy (c) hyperplastic polyp in white light imaging (d) hyperplastic polyp in in optical chromoendoscopy.
The low risk of diminutive polyps to develop cancer might support an even more radical approach to polyp management, namely to defer resection of diminutive polyp, and to only remove those that have grown to higher-risk polyps during the surveillance interval. Ignoring diminutive polyps is already an accepted CRC screening practice with CT colonography.34, 35, 36, 37 According to CT colonography guidelines, polyps ≤5 mm are not reported and exams repeated every 5 years.35, 37 Available studies on natural history of polyps, albeit few support this approach. The risk of transition to cancer increases with size, and cost-effectiveness studies have only considered that polyps larger than 5 mm would transition to cancer.38 In two follow-up CT colonography studies, the majority of 6-9 mm polyps (65–78%) did not grow within 2–3 years.39, 40 Interestingly, approximately one quarter decreased in size. Only a small proportion grew and none progressed to cancer among a total of 401 polyps. When considering even smaller ≤5 mm polyps, it is plausible that their resection does not sufficiently contribute to CRC prevention to justify their removal, and its associated risk and cost.41, 42 Instead, overdiagnosis and overtreatment may be a concern.
Although leaving diminutive polyps in place would constitute a paradigm shift, in a recent survey 72% of gastroenterologists would be agreeable to leave diminutive polyps in place if such an approach was endorsed by governing societies.43, 44 Also, in daily practice, gastroenterologists may not resect diminutive polyps when their appearance suggests non-adenomatous tissue.43 It has been estimated that deferring resection of diminutive polyps would result in a 64% reduction of therapeutic interventions during colonoscopies.44 Prospective studies will have to show the safety and efficacy of this approach and whether it truly does not affect overall effectiveness of screening. In addition, patients' expectations and fears with regard to perceived cancer risk of deferring polyp resection would have to be evaluated.
Should we use cold snare resection for all polyps?
Polyps are removed either by forceps or by snare. While both are comparable for ≤3 mm polyps, larger polyps are insufficiently removed with a forceps and should be resected with a snare.45, 46 In current practice, the most common approach to removing medium and large sized polyps ≥5 mm is to use electrocautery or “hot” snare resection.43, 47, 48 Added cautery ablates marginal tissue and may therefore improve completeness of resection (Figure 2). Further, it might lower the risk of immediate bleeding. However, there is little proof that these assertions are true.
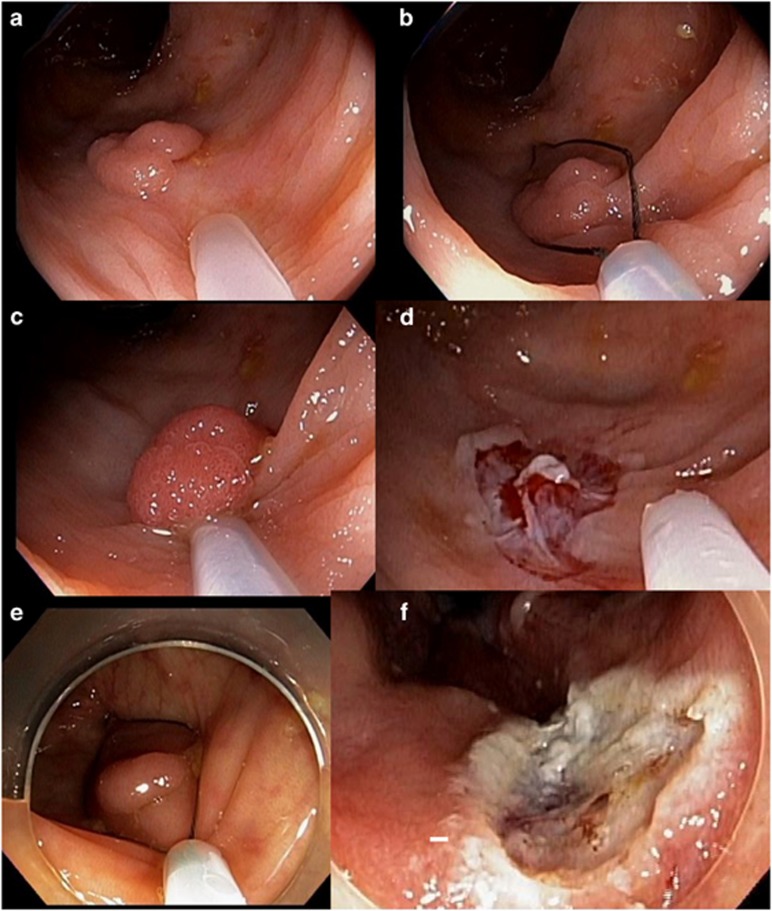
Figure 2.
Polyps removed with hot and cold snare. 7 mm polyp removed with cold snare (a–c) and resection site after cold snare polypectomy (d). 8 mm polyp removed with hot snare (e) and resection site after hot snare polypectomy (f).
Hot snare resection is often incomplete. In the complete adenoma resection (CARE), study 10% of 5–20 mm neoplastic polyps were not completely removed. Incomplete resection increased with size and varied broadly across endoscopists.6 A recent smaller study from Australia presented in abstract form reported an only 5% incomplete resection of up to 20 mm polyps when using a cold snare suggesting that resection may be at least be similarly complete when using a cold snare than a hot snare.49
With respect to bleeding risk a randomized trial among patients on anticoagulation with up to 10 mm polyps suggests that cold snare resection may actually lower the risk of bleeding.50 Cold snare resection resulted in a lower-immediate (6 vs. 23%) and delayed bleeding risk (0 vs. 14%) when compared to hot snare resection. Two other randomized trials compared cold to hot snare resection for up to 8 mm polyps. In one study, no immediate or delayed bleeding occured.46 The other study reported immediate bleeding in 9% with cold snare resection; however, all resolved spontaneously, and none required an intervention.51 These studies are small and bleeding is not well defined, however, the results question the assumed benefit of cautery on bleeding risk. Aside from randomized trials, an increasing number of uncontrolled cohort studies suggest that cold snare polypectomy is safe and effective for up to 10 mm polyps.46, 50, 52
Current commonly used snares have been designed to be used with electrocautery and may not easily cut through the polyp base without cautery. Specialized fine wire snares to facilitate cold resection have been introduced.52, 53 A first randomized trial compared the use of a dedicated cold snare with a standard snare for cold resection of up to 10 mm polyps and found a lower incomplete resection rate with using a dedicated cold snare (9 vs. 21%).52
While there are increasing number of studies on the safety and efficacy of cold snare resection for polyps up to 10 mm, the data on cold snare resection for larger polyps are limited to feasibility. Case series have reported on piecemeal resection of up to >20 mm colorectal polyps.7, 49, 54 Among those three studies, immediate bleeding requiring intervention only occurred in one patient who was on anticoagulation treatment.
Finally, it should be noted, that cold snare polypectomy has no risk of cautery damage to the colonic wall or the resected polyp. This absence of electrocautery may decrease complications (perforation, post-polypectomy syndrome) and allow for better histopathology evaluation of the polyp and examination of the resection margins after polyp removal. Remnant polyp tissue may be more readily visible, which may improve completeness of resection.
Although the data on cold snare resection of larger polyps is still emerging, cold snare resection appears to be at least as safe and effective as hot snare resection for polyps up to 10 mm in size. Future studies should systematically examine efficacy and safety of cold snare resection; particularly determine the upper limit for en-bloc and piecemeal resection, the need for submucosal injection, and associated bleeding risks.15
Should we use prophylactic clipping after large polyp resection?
The risk of advanced histology of transition to cancer increases with polyp size. The prevalence of cancer in polyps equal or larger than 10 mm has been reported to be between 2.4 and 10.2%.9, 10, 11, 12, 14, 15, 16 Therefore, it is of vital importance to assure complete resection particularly of large lesions. The standard-of-care for large polyps used to be surgical resection. A growing number of studies have demonstrated that resection can effectively and safely remove 85–90% of these polyps and has therefore become the preferred treatment.9, 55, 56, 57
For non-pedunculated polyps, endoscopic mucosal resection (EMR) is the current standard-of-care in Western countries. En bloc resection is the goal because the risk of recurrence is lower when compared to piecemeal resection.58 However, in the majority of these polyps en bloc resection cannot be achieved and are therefore removed piece-meal. EMR typically includes submucosal injection with a contrasting agent (methylene blue or indigo carmine) to provide a submucosal safety cushion and to better delineate the submucosal layer. With the application of electrocautery to a larger area of the colonic wall during resection and the resultant large mucosal defect, the risk for perforation, post-polypectomy syndrome, and bleeding increases. It does therefore not surprise that the risk for complications increases with lesion size. It ranges overall from 8 to 26% in prospective studies.8, 9, 55, 56, 59, 60
The most common complication, delayed bleeding, is observed in 3–10% of patients.9, 55, 61, 62, 63, 64 Bleeding typically occurs within 7–10 days after EMR and may require hospital admission, endoscopic intervention, and blood transfusion. Proximal polyp location, size, and bleeding during the resection have been identified as risk factors for delayed bleeding.9, 55, 62, 63, 64, 65 Age, comorbidities, use of anticoagulation, and electrocautery setting have been reported risks in some but not other studies.63, 65, 66
Recent publications suggest that closing the mucosal defect with clips may reduce the bleeding risk (Figure 3).63, 65, 67 A large retrospective single endoscopist study found that complete clip closure after removal of ≥20 mm polyps among 225 patients was associated with a 2% bleeding risk, significantly lower than the 10% observed in 247 historical controls, who did not undergo prophylactic clipping.63 Similarly, clip closure was associated with lower bleeding risk in a recent prospective multicenter cohort study from Spain.65 Although both studies support the use of clips, a major concern is an uncontrolled study design with the possibility of patient selection bias and unmeasured factors that may affect bleeding risk. Although the study from Spain was large (1214 patients), the lack of a standardized resection protocol, a clear definition of outcomes and assurance of complete outcome assessment are some of the limitations. For instance, clip closure may have been more frequently applied to lower-risk lesions because they may have been easier to clip (preferential clipping), which may have confounded risk assessment. A randomized trial from China might have overcome these limitations. Prophylactic clip closure was compared to no clip closure after endoscopic resection of ≥10 mm sessile polyps among 348 patients.67 Polyps were removed either by EMR, endoscopic submucosal dissection (ESD) or hybrid ESD (combination of EMR and ESD). Delayed bleeding occurred less frequently after clip closure compared to no clipping (1 vs. 7%). However, the study included smaller polyps, allowed different endoscopic resection techniques, observed a higher than expected rate of complications in the control group, and used an unclear definition of bleeding events.67 Therefore, the results are not sufficient to inform current EMR practice.
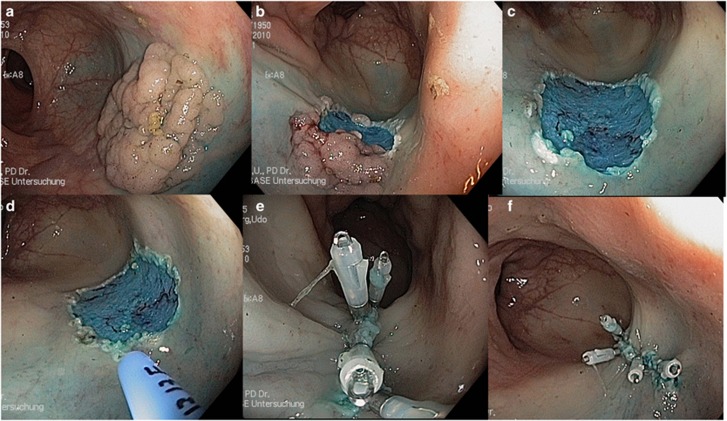
Figure 3.
EMR of a 40 mm lateral spreading granular type colon polyp (a–c). Treatment of the boarders with APC to prevent recurrence (d) and clip closure of the mucosal defect after resection (e,f).
Despite the lack of good evidence, several observational studies indicated that clip closure is increasingly applied. Additional studies are under way and will hopefully provide a more definitive answer in the near future. At present stage, however, there is no adequate evidence to support prophylactic use of clips after EMR.
SUMMARY
Adequate polyp management is key for effective endoscopic CRC screening. While we spent most of our time and resources on the detection and removal of diminutive polyps, it is unclear that this effort is worthwhile. The proposed resect-and-discard strategy is an approach to shift this balance. However, training, monitoring, auditing requirements, challenges in implementation, and the added complexity may further hinder adoption into clinical practice. Alternative strategies include a simplified resect-and-discard strategy or deferring removal of diminutive polyps until they grow to higher-risk polyps. New strategies need to be studied, particularly with a focus on trade-offs of safety compared to cost-savings.
As we may de-emphasize the importance of removing diminutive polyps, our effort should have a renewed focus on the detection and complete resection of higher-risk polyps. Cold snare resection may be at least as safe and effective as hot snare resection for polyps up to 10 mm in size. Future comparative effectiveness studies should be encouraged to define best practice. The larger the polyp the higher the risk for complications. Although clipping of the mucosal defect after resection seems to be increasingly performed, there is currently insufficient evidence to support this practice for all non-pedunculated ≥20 mm polyps. Results of ongoing studies are awaited to understand if this approach is justified.
Study Highlights
✓ Adequate polyp management is key for colonoscopy practitioners. New developments like the resect-anddiscard strategy, deferring removal of diminutive polyps and considerations how polypectomy techniques can be improved are discussed in this article.
Acknowledgments
The findings, statements, and views expressed are those of the authors and do not necessarily represent those of the Commission, the Department of Veterans Affairs or the United States Government.
Footnotes
Guarantor of the article: Daniel von Renteln, MD.
Specific author contributions: Daniel von Renteln and Heiko Pohl: drafting and revision of the manuscript. Daniel von Renteln and Heiko Pohl have approved the final draft submitted.
Financial support: None.
Potential competing interests: Heiko Pohl is a consultant for Interscope, Inc, Daniel von Renteln is a consultant for Boston Scientific. The remaining authors declare no conflict of interest.
References
- Adachi M, Ryan P, Collopy B et al. Adenoma-carcinoma sequence of the large bowel. Aust N Z J Surg 1991; 61: 409–414. [DOI] [PubMed] [Google Scholar]
- Mandel JS, Bond JH, Church TR et al. Reducing mortality from colorectal cancer by screening for fecal occult blood. Minnesota Colon Cancer Control Study. N Engl J Med 1993; 328: 1365–1371. [DOI] [PubMed] [Google Scholar]
- Zauber AG, Winawer SJ, O'Brien MJ et al. Colonoscopic polypectomy and long-term prevention of colorectal-cancer deaths. N Engl J Med 2012; 366: 687–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner H, Altenhofen L, Stock C et al. Natural history of colorectal adenomas: birth cohort analysis among 3.6 million participants of screening colonoscopy. Cancer Epidemiol Biomarkers Prev 2013; 22: 1043–1051. [DOI] [PubMed] [Google Scholar]
- Rex DK, Kahi C, O'Brien M et al. The American Society for Gastrointestinal Endoscopy PIVI (Preservation and Incorporation of Valuable Endoscopic Innovations) on real-time endoscopic assessment of the histology of diminutive colorectal polyps. Gastrointest Endosc 2011; 73: 419–422. [DOI] [PubMed] [Google Scholar]
- Pohl H, Srivastava A, Bensen SP et al. Incomplete polyp resection during colonoscopy-results of the complete adenoma resection (CARE) study. Gastroenterology 2013; 144: 74–80 e1. [DOI] [PubMed] [Google Scholar]
- Muniraj T, Sahakian A, Ciarleglio MM et al. Cold snare polypectomy for large sessile colonic polyps: a single-center experience. Gastroenterol Res Pract 2015; 2015: 175959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrara F, Luigiano C, Ghersi S et al. Efficacy, safety and outcomes of 'inject and cut' endoscopic mucosal resection for large sessile and flat colorectal polyps. Digestion 2010; 82: 213–220. [DOI] [PubMed] [Google Scholar]
- Moss A, Bourke MJ, Williams SJ et al. Endoscopic mucosal resection outcomes and prediction of submucosal cancer from advanced colonic mucosal neoplasia. Gastroenterology 2011; 140: 1909–1918. [DOI] [PubMed] [Google Scholar]
- Odom SR, Duffy SD, Barone JE et al. The rate of adenocarcinoma in endoscopically removed colorectal polyps. Am Surg 2005; 71: 1024–1026. [PubMed] [Google Scholar]
- Church JM. Clinical significance of small colorectal polyps. Dis Colon Rectum 2004; 47: 481–485. [DOI] [PubMed] [Google Scholar]
- Aldridge AJ, Simson JN. Histological assessment of colorectal adenomas by size. Are polyps less than 10 mm in size clinically important? Eur J Surg 2001; 167: 777–781. [DOI] [PubMed] [Google Scholar]
- Butterly LF, Chase MP, Pohl H et al. Prevalence of clinically important histology in small adenomas. Clin Gastroenterol Hepatol 2006; 4: 343–348. [DOI] [PubMed] [Google Scholar]
- Gschwantler M, Kriwanek S, Langner E et al. High-grade dysplasia and invasive carcinoma in colorectal adenomas: a multivariate analysis of the impact of adenoma and patient characteristics. Eur J Gastroenterol Hepatol 2002; 14: 183–188. [DOI] [PubMed] [Google Scholar]
- Lieberman D, Moravec M, Holub J et al. Polyp size and advanced histology in patients undergoing colonoscopy screening: implications for CT colonography. Gastroenterology 2008; 135: 1100–1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickhardt PJ, Choi JR, Hwang I et al. Computed tomographic virtual colonoscopy to screen for colorectal neoplasia in asymptomatic adults. N Engl J Med 2003; 349: 2191–2200. [DOI] [PubMed] [Google Scholar]
- Rex DK, Overhiser AJ, Chen SC et al. Estimation of impact of American College of Radiology recommendations on CT colonography reporting for resection of high-risk adenoma findings. Am J Gastroenterol 2009; 104: 149–153. [DOI] [PubMed] [Google Scholar]
- Ponugoti PL, Cummings OW, Rex DK. Risk of cancer in small and diminutive colorectal polyps. Dig Liver Dis 2017; 49: 34–37. [DOI] [PubMed] [Google Scholar]
- Hassan C, Pickhardt PJ, Rex DK. A resect and discard strategy would improve cost-effectiveness of colorectal cancer screening. Clin Gastroenterol Hepatol 2010; 8: 869 e1–3. [DOI] [PubMed] [Google Scholar]
- Moon HS, Park SW, Kim DH et al. Only the size of resected polyps is an independent risk factor for delayed postpolypectomy hemorrhage: a 10-year Single-Center Case-Control Study. Ann Coloproctol 2014; 30: 182–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman DA, Rex DK, Winawer SJ et al. Guidelines for colonoscopy surveillance after screening and polypectomy: a consensus update by the US Multi-Society Task Force on Colorectal Cancer. Gastroenterology 2012; 143: 844–857. [DOI] [PubMed] [Google Scholar]
- Rees CJ, Rajasekhar PT, Wilson A et al. Narrow band imaging optical diagnosis of small colorectal polyps in routine clinical practice: the Detect Inspect Characterise Resect and Discard 2 (DISCARD 2) study. Gut 2016; doi: 10.1136/gutjnl-2015-310584 [e-pub ahead of print]. [DOI] [PMC free article] [PubMed]
- Iwatate M, Ikumoto T, Hattori S et al. NBI and NBI combined with magnifying colonoscopy. Diagn Ther Endosc 2012; 2012: 173269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rex DK. Narrow-band imaging without optical magnification for histologic analysis of colorectal polyps. Gastroenterology 136: 1174–1181. [DOI] [PubMed] [Google Scholar]
- Kaltenbach T, Rastogi A, Rouse RV et al. Real-time optical diagnosis for diminutive colorectal polyps using narrow-band imaging: the VALID randomised clinical trial. Gut 2015; 64: 1569–1577. [DOI] [PubMed] [Google Scholar]
- Kaminski MF, Regula J, Kraszewska E et al. Quality indicators for colonoscopy and the risk of interval cancer. N Engl J Med 2010; 362: 1795–1803. [DOI] [PubMed] [Google Scholar]
- Kaminski MF, Hassan C, Bisschops R et al. Advanced imaging for detection and differentiation of colorectal neoplasia: European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy 2014; 46: 435–449. [DOI] [PubMed] [Google Scholar]
- Abu Dayyeh BK, Thosani N, Konda V et al. ASGE Technology Committee systematic review and meta-analysis assessing the ASGE PIVI thresholds for adopting real-time endoscopic assessment of the histology of diminutive colorectal polyps. Gastrointestinal Endoscopy 2015; 81: 502.e1–502.e16. [DOI] [PubMed] [Google Scholar]
- Schachschal G, Mayr M, Treszl A et al. Endoscopic versus histological characterisation of polyps during screening colonoscopy. Gut 2014; 63: 458–465. [DOI] [PubMed] [Google Scholar]
- Ladabaum U, Fioritto A, Mitani A et al. Real-time optical biopsy of colon polyps with narrow band imaging in community practice does not yet meet key thresholds for clinical decisions. Gastroenterology 2013; 144: 81–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang LM, East JE. Diminutive polyp cancers and the DISCARD strategy: much ado about nothing or the end of the affair? Gastrointest Endosc 2015; 82: 385–388. [DOI] [PubMed] [Google Scholar]
- Atkinson NS, East JE. Optical biopsy and sessile serrated polyps: Is DISCARD dead? Long live DISCARD-lite! Gastrointest Endosc 2015; 82: 118–121. [DOI] [PubMed] [Google Scholar]
- von Renteln D, Anderson JC, Pohl H A Non-optical resect-and-discard strategy achieves a simi- lar colonoscopy surveillance agreement compared to the optical resect-and-discard strategy. American College of Gastroenterology. Annual scientific meeting, 2016;OP37.
- Kim DH, Pickhardt PJ, Taylor AJ et al. CT colonography versus colonoscopy for the detection of advanced neoplasia. N Engl J Med 2007; 357: 1403–1412. [DOI] [PubMed] [Google Scholar]
- Radiology aco. ACR practice guideline for the performance of computed tomography (CT) colonography in adults online publication 2015.
- Kim DH, Pickhardt PJ, Taylor AJ. Characteristics of advanced adenomas detected at CT colonographic screening: implications for appropriate polyp size thresholds for polypectomy versus surveillance. AJR Am J Roentgenol 2007; 188: 940–944. [DOI] [PubMed] [Google Scholar]
- Force USPST, Bibbins-Domingo K, Grossman DC et al. Screening for colorectal cancer: US preventive services task force recommendation statement. JAMA 2016; 315: 2564–2575. [DOI] [PubMed] [Google Scholar]
- Zauber AG, Lansdorp-Vogelaar I, Knudsen AB et al. Evaluating test strategies for colorectal cancer screening: a decision analysis for the U.S. Preventive Services Task Force. Ann Intern Med 2008; 149: 659–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickhardt PJ, Kim DH, Pooler BD et al. Assessment of volumetric growth rates of small colorectal polyps with CT colonography: a longitudinal study of natural history. Lancet Oncol 2013; 14: 711–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tutein Nolthenius CJ, Boellaard TN, de Haan MC et al. Evolution of screen-detected small (6-9 mm) polyps after a 3-year surveillance interval: assessment of growth with ct colonography compared with histopathology. Am J Gastroenterol 2015; 110: 1682–1690. [DOI] [PubMed] [Google Scholar]
- Hofstad B, Vatn MH, Andersen SN et al. Growth of colorectal polyps: redetection and evaluation of unresected polyps for a period of three years. Gut 1996; 39: 449–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuno K, Suzuki Y, Takeuchi M et al. Natural history of diminutive colorectal polyps: long-term prospective observation by colonoscopy. Dig Endosc 2014; 26: 84–89. [DOI] [PubMed] [Google Scholar]
- Gellad ZF, Voils CI, Lin L et al. Clinical practice variation in the management of diminutive colorectal polyps: results of a national survey of gastroenterologists. Am J Gastroenterol 2013; 108: 873–878. [DOI] [PubMed] [Google Scholar]
- Hashash IH Jana G, Swaytha Ganesh, Nasr John et al. Implementation and Implication of ignoring small polyps at colonoscopy. Arch Clin Gastroenterol 2015; 1: 001–004. [Google Scholar]
- Jung YS, Park JH, Kim HJ et al. Complete biopsy resection of diminutive polyps. Endoscopy 2013; 45: 1024–1029. [DOI] [PubMed] [Google Scholar]
- Ichise Y, Horiuchi A, Nakayama Y et al. Prospective randomized comparison of cold snare polypectomy and conventional polypectomy for small colorectal polyps. Digestion 2011; 84: 78–81. [DOI] [PubMed] [Google Scholar]
- Carpenter S, Petersen BT, Chuttani R et al. Polypectomy devices. Gastrointest Endosc 65: 741–749. [DOI] [PubMed] [Google Scholar]
- Singh N, Harrison M, Rex DK. A survey of colonoscopic polypectomy practices among clinical gastroenterologists. Gastrointest Endosc 60: 414–418. [DOI] [PubMed] [Google Scholar]
- Tan JC, La Nauze R, Roberts SK et al. 714 The efficacy and safety of cold snare polypectomy. Gastrointest Endosc 2015; 81: AB164. [Google Scholar]
- Horiuchi A, Nakayama Y, Kajiyama M et al. Removal of small colorectal polyps in anticoagulated patients: a prospective randomized comparison of cold snare and conventional polypectomy. Gastrointest Endosc 2014; 79: 417–423. [DOI] [PubMed] [Google Scholar]
- Paspatis GA, Tribonias G, Konstantinidis K et al. A prospective randomized comparison of cold vs hot snare polypectomy in the occurrence of postpolypectomy bleeding in small colonic polyps. Colorectal Dis 2011; 13: e345–e348. [DOI] [PubMed] [Google Scholar]
- Horiuchi A, Hosoi K, Kajiyama M et al. Prospective, randomized comparison of 2 methodsof cold snare polypectomy for small colorectal polyps. Gastrointest Endosc 2015; 82: 686–692. [DOI] [PubMed] [Google Scholar]
- Kim JS, Lee BI, Choi H et al. Cold snare polypectomy versus cold forceps polypectomy for diminutive and small colorectal polyps: a randomized controlled trial. Gastrointest Endosc 2015; 81: 741–747. [DOI] [PubMed] [Google Scholar]
- Choksi N, Elmunzer BJ, Stidham RW et al. Cold snare piecemeal resection of colonic and duodenal polyps >/=1 cm. Endosc Int Open 2015; 3: E508–E513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arebi N, Swain D, Suzuki N et al. Endoscopic mucosal resection of 161 cases of large sessile or flat colorectal polyps. Scand J Gastroenterol 2007; 42: 859–866. [DOI] [PubMed] [Google Scholar]
- Conio M, Repici A, Demarquay JF et al. EMR of large sessile colorectal polyps. Gastrointest Endosc 2004; 60: 234–241. [DOI] [PubMed] [Google Scholar]
- Woodward T, Crook JE, Raimondo M et al. Improving complete EMR of colorectal neoplasia: a randomized trial comparing snares and injectate in the resection of large sessile colon polyps. Gastrointest Endosc 2015; 81: 673–681. [DOI] [PubMed] [Google Scholar]
- Belderbos TD, Leenders M, Moons LM et al. Local recurrence after endoscopic mucosal resection of nonpedunculated colorectal lesions: systematic review and meta-analysis. Endoscopy 2014; 46: 388–402. [DOI] [PubMed] [Google Scholar]
- Brooker JC, Saunders BP, Shah SG et al. Treatment with argon plasma coagulation reduces recurrence after piecemeal resection of large sessile colonic polyps: a randomized trial and recommendations. Gastrointest Endosc 2002; 55: 371–375. [DOI] [PubMed] [Google Scholar]
- Swan MP, Bourke MJ, Alexander S et al. Large refractory colonic polyps: is it time to change our practice? A prospective study of the clinical and economic impact of a tertiary referral colonic mucosal resection and polypectomy service (with videos). Gastrointest Endosc 2009; 70: 1128–1136. [DOI] [PubMed] [Google Scholar]
- Briedigkeit A, Sultanie O, Sido B et al. Endoscopic mucosal resection of colorectal adenomas>20 mm: Risk factors for recurrence. World J Gastrointest Endosc 2016; 8: 276–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luigiano C, Consolo P, Scaffidi MG et al. Endoscopic mucosal resection for large and giant sessile and flat colorectal polyps: a single-center experience with long-term follow-up. Endoscopy 2009; 41: 829–835. [DOI] [PubMed] [Google Scholar]
- Liaquat H, Rohn E, Rex DK. Prophylactic clip closure reduced the risk of delayed postpolypectomy hemorrhage: experience in 277 clipped large sessile or flat colorectal lesions and 247 control lesions. Gastrointest Endosc 2013; 77: 401–407. [DOI] [PubMed] [Google Scholar]
- Qumseya BJ, Wolfsen C, Wang Y et al. Factors associated with increased bleeding post-endoscopic mucosal resection. J. Dig Dis 2013; 14: 140–146. [DOI] [PubMed] [Google Scholar]
- Albeniz E, Fraile M, Ibanez B et al. A Scoring System to Determine Risk of Delayed Bleeding After Endoscopic Mucosal Resection of Large Colorectal Lesions. Clin Gastroenterol Hepatol 2016; 14: 1140–1147. [DOI] [PubMed] [Google Scholar]
- Burgess NG, Metz AJ, Williams SJ et al. Risk factors for intraprocedural and clinically significant delayed bleeding after wide-field endoscopic mucosal resection of large colonic lesions. Clin Gastroenterol Hepatol 2014; 12: e1–e3. [DOI] [PubMed] [Google Scholar]
- Zhang QS, Han B, Xu JH et al. Clip closure of defect after endoscopic resection in patients with larger colorectal tumors decreased the adverse events. Gastrointest Endosc 2015; 82: 904–909. [DOI] [PubMed] [Google Scholar]