Abstract
Objectives:
Primary sclerosing cholangitis (PSC) is a chronic inflammatory disease of the bile ducts frequently associated with inflammatory bowel disease (IBD), suggesting an important role for the gut–liver axis. Defensins are small (3.5–4.5 kDa) anti-microbial peptides that contribute to innate immunity at mucosal surfaces and have been implicated in IBD. The aim of this study was to investigate copy number variation of the gene (DEFB4) encoding human β-defensin 2 (HBD2) and protein expression of HBD2 in PSC.
Methods:
US and Italian PSC cases and unaffected controls (US PSC patients n=89, US controls n=87; Italian PSC patients n=46, Italian controls n=84) were used to estimate HBD2 gene copy number by both quantitative real-time PCR and paralog ratio test. Serum levels of HBD2 were measured by enzyme-linked immunosorbent assay and liver expression was analyzed by immunohistochemistry.
Results:
Mean serum levels of HBD2 were significantly greater in PSC (1,086±1,721 ng/μl) compared with primary biliary cholangitis (544±754 ng/μl), ulcerative colitis (417±506 ng/μl), and healthy controls (514±731 ng/μl) (P=0.02). However, no significant differences between the frequencies of high DEFB4 gene copy number, defined by >4 copies, and PSC were found in the US, Italian, or combined cohorts. Importantly, a high number of biliary ducts were found immunopositive in PSC samples compared with controls.
Conclusions:
Our data show that HBD2 serum levels and tissue expression are increased in PSC subjects, suggesting that this arm of innate immunity may be important in the etiopathogenesis of PSC.
Introduction
Primary sclerosing cholangitis (PSC) is a rare chronic cholestatic liver disease, affecting 6 per 100,000 people in the United States, more commonly men. It often affects subjects in the third to fourth decade of life and leads to diffuse stricturing and thickening of intrahepatic and/or extrahepatic ducts.1 Although the etiology of PSC is not fully known, it is recognized to be an immune-mediated liver disease that has autoimmune features including the presence of autoantibodies such as antinuclear antibodies and perinuclear antineutrophil cytoplasmic.2 No effective treatment has been found to slow or alter the progression of PSC, and it remains a leading cause of liver transplantation.3 Median survival from time of diagnosis to liver-related death or liver transplantation is 12–15 years.4 Interestingly, 75% of PSC patients are coaffected with inflammatory bowel disease (IBD)5 and the dysregulation of the key genetic, immunologic, and microbiome compounds of the gut–liver axis are suggested to be the basis for both IBD and PSC.6
Defensins are small (3.5–4.5 kDa) anti-microbial peptides that contribute to innate immunity at mucosal surfaces and have been implicated in IBD. These peptides have bactericidal effects by damaging bacterial cell membranes and/or inhibiting cell wall biosynthesis. Additionally, some defensins possess cytokine-like activities.7, 8 For example, certain defensins use CCR6 to attract dendritic cells.9 There are two classes of defensins in humans: α and β, which are divided, based on the position of three intermolecular disulfide bridges.8, 10 There are two groups of α-defensins: neutrophil α-defensins (HNP-1, -2, and -3) and enteric α-defensins, which are produced by the Paneth cells that reside in the small intestinal crypts (HD-5 and -6).8, 10, 11 Human β-defensins (HBDs) are produced by various epithelial cells such as the skin, respiratory, and gastrointestinal tract8 and have been found distributed on the epithelium of several organs including the liver.12 There are three clusters of β-defensin genes: two on chromosome 20 and one on chromosome 8p23.1.7 Included in a ~260 kb cluster on chromosome 8p23.1 are genes encoding HBDs 2–7, with the gene encoding HBD2 (DEFB4) being the principal focus of this investigation. The copy number of this entire gene cluster is polymorphic and ranges from 2 to 12 copies per individual.7, 13, 14 In terms of expression of specific HBDs, HBD1 is constitutively expressed in epithelial cells, while expression of HBD2 and HBD3 are inducible by inflammatory triggers.13 Variations in β-defensin expression and genomic copy numbers have been linked to several autoimmune and inflammatory diseases15 including celiac disease,16 ulcerative colitis,17 Crohn's disease,13, 18 and psoriasis.14 Based on the high rate of coincidence between PSC and IBD and the key role of the gut–liver axis in PSC pathogenesis, we undertook this study to investigate whether HBD gene copy number variation and/or expression are associated with PSC.
Methods
Subjects
Fresh heparinized peripheral blood samples were obtained from two cohorts of PSC patients and controls located either in the United States or Italy (US PSC patients n=92, US controls n=87; Italian PSC patients n=93, Italian controls n=150). All patients had large duct PSC and had not had a liver transplant at the time of enrollment. Serum was collected from a subset of PSC patients in the US cohort (n=43). Additional serum was collected from healthy (n=37) and disease controls including primary biliary cholangitis (PBC) (n=37) and ulcerative colitis (UC) (n=42). Liver biopsies were performed for diagnostic purposes; healthy tissue was obtained from liver metastasis resection. Table 1 illustrates the clinical characteristics of the PSC patients enrolled. All cases met accepted criteria for the diagnosis of PSC,3 PBC,19 or UC.20 Serum liver function tests were performed using routine laboratory methods. Subjects were excluded from the study if they had malignancies or were using immunosuppressive drugs. Patients and controls were matched for age and sex. The study was approved by the Institutional Review Board of the University of California at Davis (Davis, CA), and all subjects provided written, informed consent before enrollment.
Table 1. Characteristics of the patients with primary sclerosing cholangitis from the two cohorts included in the study.
| Features | US PSC (n=92) | Italian PSC (n=93) |
|---|---|---|
| General | ||
| Male sex, n (%) | 54 (59) | 53 (57) |
| Age (years), median (range) | 47 (15–76) | 48 (18–76) |
| Duration of disease (years), median (range) | 5 (0–25) | 10 (3–35) |
| Colitis, n (%) | 69 (75) | 67 (72) |
PSC, primary sclerosing cholangitis; US, United States.
HBD gene copy number
DNA was extracted from peripheral blood mononuclear cells using standard commercial kits.
Gene copy number (GCN) was estimated by two methods including quantitative RT-PCR (qPCR) and paralog ratio test (PRT) as described previously.18, 21, 22 In principle, both methods rely upon comparing the amplification of target sequences within the cluster of β-defensin genes and single copy genes outside of the cluster. The qPCR method for the measuring GCN has been criticized due to its propensity to produce ambiguous results. Specifically, the results of a test for GCN should result in an integer. However, with qPCR it is common to have fractions of the GCN and it is not clear how best to handle these situations. PRT overcomes this situation and in comparative studies has been shown to more accurate compared with qPCR.21, 22 To assess the HBD gene copy number in our US and Italian cohorts, we used a triplex PRT method previously published in Aldohous et al.21 and Armour et al.22 described in detail in the Supplementary Materials online.
As our “gold standard” control DNA we used six samples from the Human Random Control DNA Panels from European Collection of Cell Cultures purchased from Sigma (catalog number HRC-1 (2 μg)). Similar to our samples, standard control DNA was also diluted to 10 ng/μl. The six samples used from the panel were C0207, C0088, C0849, C0940, C0969, and C0913. The expected copy numbers for β-defensin of the standard control DNA are 5, 4, 6, 4, 5, and 3, respectively.
Serum ELISA of HBD2
To determine levels of HBD2 protein in serum, we used the HBD2 ELISA (enzyme-linked immunosorbent assay) Kit from Peprotech (Rocky Hill, NJ, catalog number 900-K172). We coated ELISA microplates with antigen-affinity-purified goat anti-HBD2+ d-mannitol capture antibody at a concentration of 100 μg/ml overnight. We then performed ELISA on the serum samples diluted 1:2 to 1:10 according to the manufacturer's protocol using biotinylated antigen-affinity-purified goat anti-HBD2+ d-mannitol detection antibody at 100 μg/ml concentration and avidin-HRP conjugate and ABTS liquid substrate. Data for the ELISA plate was obtained on an ELISA plate reader set at 405 nm with the wavelength correction set at 650 nm (Molecular Devices, Sunnyvale, CA). Plates were monitored every 5 min and data were read when the highest standard concentration was at 1.2 U.
Immunohistochemistry
For the immunohistochemistry 2–3-μm-thick sections of each surgical specimen were cut and processed. After deparaffining and rehydration, the sections were immersed in an antigen heat retrieval solution (Diva Decloaker; Biocare Medical, Milan, Italy) and treated by an antigen retrieval procedure by a Decloaking Chamber (Biocare Medical), incubated with 3% H2O2 for 15 min to quench endogenous peroxidase activity, treated with goat anti-HBD2 polyclonal antibody (AbD Serotec, Hercules, CA; AHP849) diluted 1:50 and incubated overnight at 4 °C), and then incubated with the goat HRP-Polymer Detection Kit (Biocare Medical). 3,3′-Diaminobenzidine tetrahydrochloride (Biocare) was used as a chromogen to yield brown reaction products. The nuclei were lightly counterstained with hematoxylin solution. Immunoreactivity was semiquantitatively scored as follows: 0, absence of immunoreactivity; 1, weak; 2, moderate; and 3, intense expression. All images were digitized at x40 objective magnification.
Statistical analysis
Power analysis for the association study was performed using the data of frequency and effect in the Hollox psoriasis GCN analysis.14 Assuming an effect size of 0.37 with an α of 0.05, we would have >0.95 power with a total sample size of 150. Comparison of GCNs was performed using χ2 analysis. A dichotomous variable of “low” and “high” GCN was defined by <4 copies and ≥4 copies. This cutoff was chosen based on the median GCN of 4 and use of this cutoff in prior studies. Continuous variables were compared using the Kruskal–Wallis test.
Results
Association of HBD2 (DEFB4) GCN and PSC
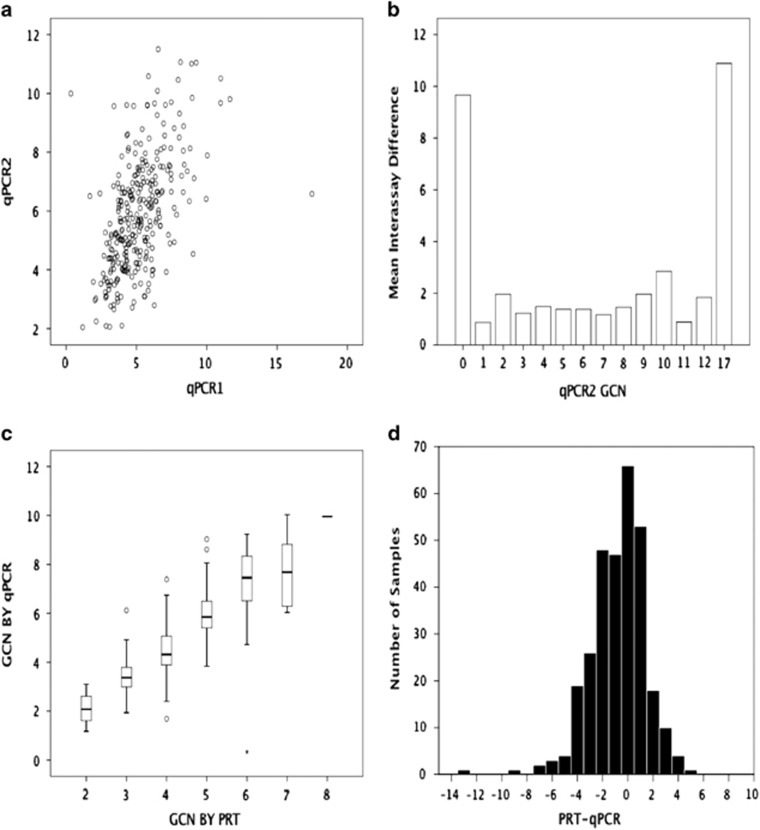
We compared the reproducibility of the qPCR assay and the concordance of results tested by qPCR and PRT on the same samples. Three hundred six samples were tested two times by qPCR (US PSC patients n=89, US controls n=87; Italian PSC patients n=46, Italian controls n=84). The mean GCN difference between samples was 0.7±1.8 (mean±s.d.). The interassay correlation between GCNs estimated by qPCR was relatively poor (Figure 1a, r=0.526, P<0.01), with the greatest variability occurring at the extreme low and high ranges of the assays (Figure 1b). Two hundred and seventy-four samples were tested by qPCR and PRT (US PSC patients n=79, US controls n=85; Italian PSC patients n=33, Italian controls n=77). The mean difference between the GCNs estimated by these methods was 0.7±1.1 (mean±s.d.). Correlation of the estimated GCN by qPCR rounded to the nearest integer and by PRT was good (r=0.772, P<0.001; Figure 1c). Because PRT has been demonstrated to be superior to qPCR,21, 22 further analysis of disease associations were conducted only with the PRT method results.
Figure 1.
Comparison of assays estimating DEFB4 gene copy number. (a) Comparison of gene copy number (GCN) estimates of identical samples tested in two separate quantitative RT-PCR (qPCR) experiments and (b)the differences between qPCR assays according to the rounded GCN of the second assay. (c) Comparison of the rounded GCN in the second qPCR assay vs. GCN estimated in the same samples using the paralog ratio test (PRT) assays. (d) Histogram of the differences in GCN estimated by qPCR and PRT on identical samples.
Comparisons of the mean and median GCNs in the four cohorts are shown in Table 2. No significant differences between the frequencies of high DEFB4 GCN, defined by >4 copies, and PSC were found in the US, Italian, or combined cohorts (Table 3).
Table 2. Comparison of human β-defensin 2 gene copy number in PSC cases and controls.
| Cohort | Mean | S.d. | Median | Range |
|---|---|---|---|---|
| US | ||||
| PSC | 4.43 | 1.04 | 4 | 2–7 |
| Controls | 4.35 | 1.00 | 4 | 2–8 |
| Italy | ||||
| PSC | 4.51 | 0.93 | 4 | 2–7 |
| Controls | 4.30 | 0.99 | 4 | 2–8 |
PSC, primary sclerosing cholangitis; US, United States.
Table 3. Frequency of low (<4) and high (>4) HBD2 gene copies in PSC cases and healthy controls from the US and Italy.
| Cohort |
HBD2
GCN |
Total | χ2 | Pvalue | |
|---|---|---|---|---|---|
| ≤4 | >4 | ||||
| US | |||||
| PSC | 49 | 34 | 83 | ||
| Control | 53 | 32 | 85 | ||
| Total | 102 | 66 | 168 | 0.194 | P=0.660 |
| Italy | |||||
| PSC | 42 | 38 | 80 | ||
| Control | 87 | 57 | 144 | ||
| Total | 129 | 95 | 224 | 1.320 | P=0.251 |
| Total | |||||
| PSC | 91 | 72 | 163 | ||
| Control | 140 | 89 | 229 | ||
| Total | 231 | 161 | 392 | 1.108 | P=0.292 |
GCN, gene copy number; HBD2, human β-defensin 2; PSC, primary sclerosing cholangitis; US, United States.
Moreover, we sought to determine if there was any association between DEFB4 GCN and the presence of IBD by comparing PSC cases with IBD to controls and PSC cases without IBD (Table 4). No significant associations were found, but this analysis was limited to a subset of the US cohort due to missing phenotypic data.
Table 4. Frequency of low (≤4) and high (>4) HBD2 gene copies in PSC cases with and without IBD and healthy controls from the US.
| Cohort |
HBD2
GCN |
Total | χ2 | Pvalue | |
|---|---|---|---|---|---|
| ≤4 | >4 | ||||
| US | |||||
| PSC with IBD | 28 | 20 | 48 | ||
| Control | 53 | 32 | 85 | 0.208 | P=0.648 |
| PSC without IBD | 7 | 8 | 15 | 0.117 | P=0.733 |
GCN, gene copy number; HBD2, human β-defensin 2; IBD, inflammatory bowel disease; PSC, primary sclerosing cholangitis; US, United States.
Serum levels of HBD2 in PSC are greater than in UC, PBC, or healthy controls and correlate with DEFB4 GCN
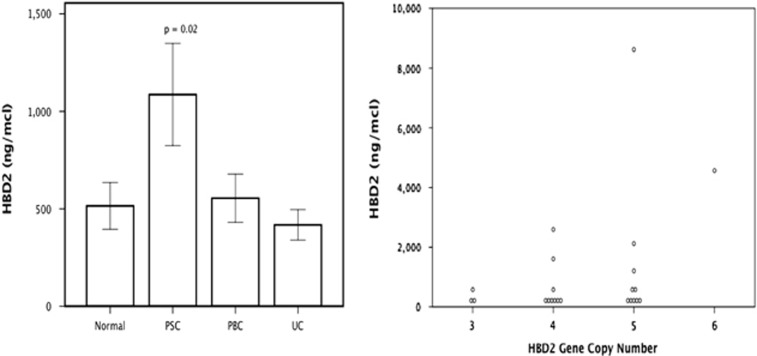
We compared the serum levels of HBD2 from subjects with PSC (n=43), PBC (n=37), or UC (n=42), and healthy controls (n=37). Mean serum levels of HBD2 were significantly greater in PSC (1,086±1,721 ng/μl) compared with PBC (544±754 ng/μl), UC (417±506 ng/μl), and healthy controls (514±731 ng/μl) (P=0.02) (Figure 2a). Within the PSC cohort, 23 subjects had both serum HBD2 levels and GCN measured. There was a significant correlation between the GCN and serum HBD2 levels (Spearman's rank test, r=0.377, P=0.038) (Figure 2b).
Figure 2.
Serum levels of human beta-defensin 2. (Left) Mean serum human β-defensin 2 (HBD2) levels±1 s.e. in healthy controls (n=37), primary sclerosing cholangitis (PSC) (n =43), primary biliary cholangitis (PBC) (n=37), and ulcerative colitis (UC) (n=42). (Right) Serum levels of HBD2 correlate with HBD2 gene copy number in PSC patients (r=0.377, P=0.038).
HBD2 liver expression
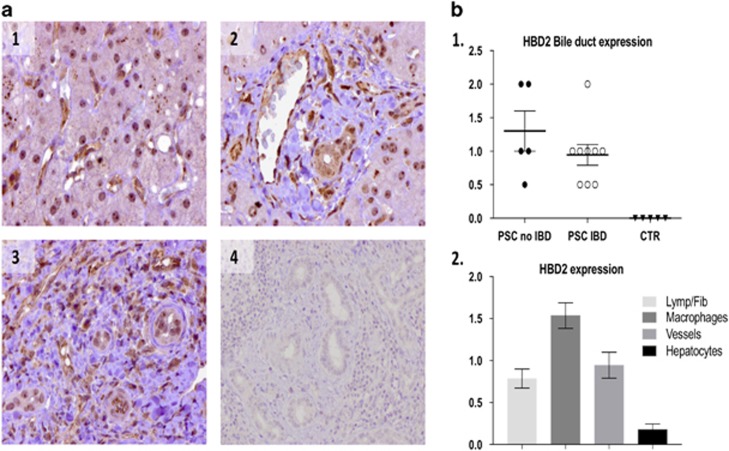
A high number of biliary ducts were immunopositive in PSC samples, while biliary cells from controls appeared to not express the protein (Figure 3). Importantly, in PSC liver HBD2 protein was not observed in hepatocytes, but was noted in resident macrophages (Kupffer cells), vascular structures (of different size), and, in some cases, cells associated with fibrous tissue and inflammatory infiltrates. The images show prototypical examples of HBD2 expression in PSC liver within Kupffer cells (a), bile ducts and vessels (arteries and veins) (b), and in the fibroinflammatory component (c).
Figure 3.
Immunohistochemistry analysis of human β-defensin 2 (HBD2) expression. (a) The images show prototypical examples of HBD2 expression in Kupffer cells (1), bile ducts and vessels (arteries and veins) (2), and in the fibroinflammatory component (3). Normal liver tissue is showed in (4). All the images have been digitized at x40 objective magnification. (b) (1) HBD2 expression in bile ducts is greater in primary sclerosing cholangitis (PSC) patients with inflammatory bowel disease (IBD) compared to normal controls (CTR); (2) HBD2 expression in different cell types in liver tissue of PSC patients are presented. Immunoreactivity was semiquantitatively scored as follows: 0, absence of immunoreactivity; 1, weak; 2, moderate; and 3, intense expression. All the images were digitized at objective magnification x40.
Discussion
HBDs are anti-microbial peptides important in innate immunity that have been implicated in many inflammatory diseases, including IBD.7, 8, 15 Previous studies have demonstrated that, while HBD1 appears to be present in biliary epithelium under physiological conditions, HBD2 is not physiologically expressed but may be induced in large intrahepatic bile ducts, in response to local infection and active inflammation.5, 12 The high rate of coincidence between PSC and IBD and the key role of the gut–liver axis in PSC pathogenesis, led us to investigate whether HBD2 gene (DEFB4) copy number variation and expression were associated with PSC.
We herein show that HBD2 is expressed in biliary epithelial cells from PSC patients and that serum levels are increased compared with controls. Using the most accurate means of estimating GCN in two large cohorts of PSC patients, we did not find any significant differences in DEFB4 GCN in PSC compared with healthy controls. However, serum levels of HBD2 in PSC correlated with the GCN.
Hypotheses surrounding the connection between PSC and IBD generally suggest that molecules from the gastrointestinal tract, which is rich with microbes, translocate to the liver causing inflammation of the bile ducts. One hypothesis is that bile duct epithelial cells are activated by a bacterial pathogen that travels from the gut to the liver leading to chronic inflammation.4, 23 An alternate hypothesis suggests that lymphocytes that are activated in the bowel of IBD patients are aberrantly recruited to extraintestinal sites (i.e., liver) via expression of adhesion molecules and chemokines normally restricted to the gut at sites such as bile duct epithelium.4, 23 Thus, an impaired anti-microbial defense through altered HBD expression at the liver might provide inadequate barrier function leading to a failure to clear pathogens and resulting in a chronic inflammatory response. Moreover, expression of HBD2 is highly inducible in response to many proinflammatory mediators in a range of cell types.13
It has been suggested that HBD2 may contribute to host defense not only through microbial activity but also by recruiting immune cells to sites of inflammation and infection.24 HBD2 attracts T cells, especially Th17 and Th22 cells, and immature dendritic cells through CCR6.9 CCR6 has been implicated in host defense and inflammation at epithelial surfaces and Th17 and Th22 likewise have high inflammatory potential, especially in the skin and gut.25 HBD2 and CCL20 have similar tertiary structures as well as several positively charged residues so that even though they lack sequence similarity both ligands compete for binding to the CCR6 receptor.25 Additionally, HBD2 has been shown to be a potent chemoattractant for neutrophils.24, 25 This suggests an alternate mechanism through which HBD2 may act in a disease state aside from decreased barrier function.
We anticipated that our control samples would have a median of four copies of the gene encoding HBD2 (DEFB4).13, 14, 18 We observed this same median value by both qPCR and PRT approaches. In contrast, conflicting results have been reported in studies on HBD GCN variation in Crohn's disease.13, 18 Fellermann et al.,18 using a qPCR method, showed an association of lower DEFB4 copy number with an increased risk of developing colonic Crohn's disease. They concluded that there was an impaired anti-microbial defense in individuals with <4 copies and this weakened anti-bacterial barrier in the colon led to chronic inflammation and Crohn's disease. On the other hand, Bentley et al.13 also using a qPCR method, showed an association of greater than 4 DEFB4 GCN with a higher risk of developing Crohn's disease. This discrepancy was thoughtfully discussed26 and remains unresolved. Additionally, Bentley et al.13 suggests that the association of HBD2 GCN and HBD2 protein production is not a straightforward gene dosage effect, possibly attributable to DEFB4 being highly inducible by inflammatory stimuli. In psoriasis, a higher genomic copy number for HBD2 genes is significantly associated with disease risk14 and HBD2 serum levels are associated with clinical severity.27 Beta-defensin-2 protein is a serum biomarker for disease activity in psoriasis and reaches biologically relevant concentrations in lesional skin. Based on these findings and those of our current study, serum HBD2 is an attractive candidate as a potential biomarker of disease activity in PSC.
In this study, we used a similar PRT method that is more accurate because it eliminates the possibility of errors due to differential amplification efficiency. Using both qPCR and PRT approaches to estimate DEFB4 GCN, we observed no association of GCN with PSC disease. The lack of an association in this study does not exclude the possibility that an effect too small to be detected by the current study might exist. However, our study was designed to have a total sample size of 150, which would give 0.95 power to identify a genetic association between a GCN >4 and disease with an odds ratio of 1.73. Based on the distribution of GCN from our study, 612 samples would be necessary to have 0.80 power to identify a similar association with an estimated odds ratio of 1.34. Recent development of large cohorts of PSC cases used in genome-wide association studies make such a study feasible.
As suggested before, serum HBD2 protein levels may be the determinative factor of disease activity and not HBD genomic copy number. Our findings suggest that the PSC disease state may not act through a straightforward gene dosage effect. Recent studies have suggested that epigenetics have a critical role in regulating inflammatory responses.28 DNA methylation, histone acetylation, and other epigenetic modifications lead to changes in gene expression. A recent study by Yin and Chung28 in periodontitis, a chronic oral inflammatory disease, showed differential epigenetic regulation of DEFB4 in response to different oral bacteria. Bacteria-induced expression of DEFB4 was regulated through epigenetic mechanisms in gingival epithelial cells. A decreased expression of DNA methyltransferase 1 and histone deacetylase expression was found in response to both an oral pathogen and non-pathogen.28 This may be a possible mechanism that explains our finding of a higher expression levels of HBD2 associated with PSC despite finding no association of GCN with PSC. However, further studies are necessary to establish a significant link between HBD2 and PSC.
In conclusion, our study suggests that HBD2 may have a role in the development of or response to PSC. Future studies should investigate the mechanisms of this association and the potential effects of HBD2 on PSC acting through its anti-microbial activity or chemotactic function.
Study Highlights
Acknowledgments
We are grateful for the help and advice from Edward J. Hollox for on the PRT assays. This work was supported in part by the National Institutes of Health (AI32738, to CLB). We thank Edward J. Hollox for help and advice regarding the PRT assays and the patients who participated in the study.
Footnotes
Supplementary Information accompanies this paper on the Clinical and Translational Gastroenterology website (http://www.nature.com/ctg)
Guarantor of the article: Christopher L. Bowlus, MD.
Specific author contributions: Study design and conduction of the study, laboratory studies, and writing of the manuscript: C.L. Bowlus; study design and supervision, and writing of the manuscript: A. Lleo, P. Invernizzi, and C.L. Bevins; laboratory study and writing of the manuscript: C. Chang; laboratory study: A. Kananurak; histological analysis: F. Grizzi and K. Tsuneyama.
Financial support: The project described was supported by the National Center for Advancing Translational Sciences, National Institutes of Health (UL1 TR001860), and the National Institute of Allergy and Infectious Diseases (AI32738). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Potential competing interests: None.
Supplementary Material
References
- Eaton JE, Talwalkar JA, Lazaridis KN et al. Pathogenesis of primary sclerosing cholangitis and advances in diagnosis and management. Gastroenterology 2013; 145: 521–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angulo P, Peter JB, Gershwin ME et al. Serum autoantibodies in patients with primary sclerosing cholangitis. J Hepatol 2000; 32: 182–187. [DOI] [PubMed] [Google Scholar]
- Chapman R, Fevery J, Kalloo A et al. Diagnosis and management of primary sclerosing cholangitis. Hepatology 2010; 51: 660–678. [DOI] [PubMed] [Google Scholar]
- Aron JH, Bowlus CL. The immunobiology of primary sclerosing cholangitis. Semin Immunopathol 2009; 31: 383–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar S, Bowlus CL. Primary sclerosing cholangitis: multiple phenotypes, multiple approaches. Clin Liver Dis 2016; 20: 67–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eksteen B. The gut–liver axis in primary sclerosing cholangitis. Clin Liver Dis 2016; 20: 1–14. [DOI] [PubMed] [Google Scholar]
- Groth M, Wiegand C, Szafranski K et al. Both copy number and sequence variations affect expression of human DEFB4. Genes Immun 2010; 11: 458–466. [DOI] [PubMed] [Google Scholar]
- Vora P, Youdim A, Thomas LS et al. Beta-defensin-2 expression is regulated by TLR signaling in intestinal epithelial cells. J Immunol 2004; 173: 5398–5405. [DOI] [PubMed] [Google Scholar]
- Yang D, Chertov O, Bykovskaia SN et al. Beta-defensins: linking innate and adaptive immunity through dendritic and T cell CCR6. Science 1999; 286: 525–528. [DOI] [PubMed] [Google Scholar]
- Yamaguchi Y, Ouchi Y. Antimicrobial peptide defensin: identification of novel isoforms and the characterization of their physiological roles and their significance in the pathogenesis of diseases. Proc Jpn Acad Ser B 2012; 88: 152–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linzmeier RM, Ganz T. Human defensin gene copy number polymorphisms: comprehensive analysis of independent variation in alpha- and beta-defensin regions at 8p22-p23. Genomics 2005; 86: 423–430. [DOI] [PubMed] [Google Scholar]
- Harada K, Ohba K, Ozaki S et al. Peptide antibiotic human beta-defensin-1 and -2 contribute to antimicrobial defense of the intrahepatic biliary tree. Hepatology 2004; 40: 925–932. [DOI] [PubMed] [Google Scholar]
- Bentley RW, Pearson J, Gearry RB et al. Association of higher DEFB4 genomic copy number with Crohn's disease. Am J Gastroenterol 2010; 105: 354–359. [DOI] [PubMed] [Google Scholar]
- Hollox EJ, Huffmeier U, Zeeuwen PL et al. Psoriasis is associated with increased beta-defensin genomic copy number. Nat Genet 2008; 40: 23–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Leeuw E, Lu W. Human defensins: turning defense into offense? Infect Disord Drug Targets 2007; 7: 67–70. [DOI] [PubMed] [Google Scholar]
- Fernandez-Jimenez N, Castellanos-Rubio A, Plaza-Izurieta L et al. Analysis of beta-defensin and Toll-like receptor gene copy number variation in celiac disease. Hum Immunol 2010; 71: 833–836. [DOI] [PubMed] [Google Scholar]
- Fahlgren A, Hammarstrom S, Danielsson A et al. Beta-defensin-3 and -4 in intestinal epithelial cells display increased mRNA expression in ulcerative colitis. Clin Exp Immunol 2004; 137: 379–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fellermann K, Stange DE, Schaeffeler E et al. A chromosome 8 gene-cluster polymorphism with low human beta-defensin 2 gene copy number predisposes to Crohn disease of the colon. Am J Hum Genet 2006; 79: 439–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindor KD, Gershwin ME, Poupon R et al. Primary biliary cirrhosis. Hepatology 2009; 50: 291–308. [DOI] [PubMed] [Google Scholar]
- Lichtenstein GR, Abreu MT, Cohen R et al. American Gastroenterological Association Institute medical position statement on corticosteroids, immunomodulators, and infliximab in inflammatory bowel disease. Gastroenterology 2006; 130: 935–939. [DOI] [PubMed] [Google Scholar]
- Aldhous MC, Abu Bakar S, Prescott NJ et al. Measurement methods and accuracy in copy number variation: failure to replicate associations of beta-defensin copy number with Crohn's disease. Hum Mol Genet 2010; 19: 4930–4938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armour JA, Palla R, Zeeuwen PL et al. Accurate, high-throughput typing of copy number variation using paralogue ratios from dispersed repeats. Nucleic Acids Res 2007; 35: e19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Mahony CA, Vierling JM. Etiopathogenesis of primary sclerosing cholangitis. Semin Liver Dis 2006; 26: 3–21. [DOI] [PubMed] [Google Scholar]
- Niyonsaba F, Ogawa H, Nagaoka I. Human beta-defensin-2 functions as a chemotactic agent for tumour necrosis factor-alpha-treated human neutrophils. Immunology 2004; 111: 273–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghannam S, Dejou C, Pedretti N et al. CCL20 and beta-defensin-2 induce arrest of human Th17 cells on inflamed endothelium in vitro under flow conditions. J Immunol 2011; 186: 1411–1420. [DOI] [PubMed] [Google Scholar]
- Hollox EJ. Beta-defensins and Crohn's disease: confusion from counting copies. Am J Gastroenterol 2010; 105: 360–362. [DOI] [PubMed] [Google Scholar]
- Jansen PA, Rodijk-Olthuis D, Hollox EJ et al. Beta-defensin-2 protein is a serum biomarker for disease activity in psoriasis and reaches biologically relevant concentrations in lesional skin. PLoS ONE 2009; 4: e4725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin L, Chung WO. Epigenetic regulation of human beta-defensin 2 and CC chemokine ligand 20 expression in gingival epithelial cells in response to oral bacteria. Mucosal Immunol 2011; 4: 409–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.