Abstract
The mRNA cap-binding protein eukaryotic translation initiation factor 4E (eIF4E) participates in protein synthesis initiation, translational repression of specific mRNAs, and nucleocytoplasmic shuttling. Multiple isoforms of eIF4E are expressed in a variety of organisms, but their specific roles are poorly understood. We investigated one Caenorhabditis elegans isoform, IFE-4, which has homologues in plants and mammals. IFE-4::green fluorescent protein (GFP) was expressed in pharyngeal and tail neurons, body wall muscle, spermatheca, and vulva. Knockout of ife-4 by RNA interference (RNAi) or a null mutation produced a pleiotropic phenotype that included egg-laying defects. Sedimentation analysis demonstrated that IFE-4, but not IFE-1, was present in 48S initiation complexes, indicating that it participates in protein synthesis initiation. mRNAs affected by ife-4 knockout were determined by DNA microarray analysis of polysomal distribution. Polysome shifts, in the absence of total mRNA changes, were observed for only 33 of the 18,967 C. elegans mRNAs tested, of which a disproportionate number were related to egg laying and were expressed in neurons and/or muscle. Translational regulation was confirmed by reduced levels of DAF-12, EGL-15, and KIN-29. The functions of these proteins can explain some phenotypes observed in ife-4 knockout mutants. These results indicate that translation of a limited subset of mRNAs is dependent on a specific isoform of eIF4E.
The most highly regulated phase of protein synthesis is initiation (8, 39). A different class of initiation factors catalyzes each of the individual steps (12). A ternary complex of eukaryotic translation initiation factor 2 (eIF2)-GTP-Met-tRNAi binds to the 40S ribosomal subunit to form the 43S initiation complex. The next step, recruitment of mRNA to the 43S initiation complex to form the 48S initiation complex, is rate limiting for initiation and requires the recognition of the 5′-terminal m7G-containing cap by eIF4E and the 3′-terminal poly(A) tract by the poly(A)-binding protein. The complex of eIF4E, eIF4G, and eIF4A unwinds mRNA secondary structure at the expense of ATP. Global regulation of initiation involves modulation of the canonical initiation factor activities, whereas mRNA-specific regulation is often mediated through proteins that bind cis-regulatory sequences in the 5′- or 3′-untranslated regions (UTRs) (23).
eIF4E has been widely studied in animals, plants, and yeasts (8, 39). At least three regulatory mechanisms govern the levels, activity, and availability of eIF4E for protein synthesis. In mammals, eIF4E is phosphorylated on Ser-209 by the eIF4G-bound protein kinase Mnk1, which is in turn activated by ERK1 and p38 mitogen-activated protein kinases. A study with Drosophila showed that eIF4E phosphorylation is critical for growth (24). Binding of PHAS-I (also called 4E-BP1) to eIF4E prevents its interaction with eIF4G, but phosphorylation of PHAS-I in response to many types of extracellular stimuli releases eIF4E (26, 35). PHAS-I competes with eIF4G for binding to eIF4E, since they both bind to the same site on eIF4E (29). Finally, eIF4E gene transcription is increased by a variety of mitogenic stimuli and is responsive to binding of Myc to an E box in its promoter (19).
Overexpression of eIF4E has a profound effect on cell growth and phenotype, causing accelerated cell division and malignant transformation as well as preventing apoptosis (7). eIF4E overexpression also occurs in most malignancies, including breast, bladder, colon, head-and-neck, prostate, cervix, and lung cancers. Lowering eIF4E levels by expression of antisense RNA causes a decrease in protein synthesis, slower growth, and reversal of malignant phenotype (41, 10). Sequestering eIF4E by introducing PHAS-I (45, 11) also reverses the transformed phenotype. These effects are likely due to enhanced translation of growth-related mRNAs (7).
eIF4E functions in cellular processes in addition to translation initiation. Studies with eIF4E-overexpressing cells suggest that eIF4E could be involved, either directly or indirectly, in the nucleocytoplasmic shuttling of cyclin D1 mRNA (46). The promyelocytic leukemia protein PML, which suppresses oncogenic transformation and growth, binds eIF4E directly and reduces its affinity for the cap (6). This diminishes nucleocytoplasmic transport of cyclin D1 mRNA, protein levels of cyclin D1, and cellular transformation. Some cases of translational suppression by 3′-UTR sequences involve eIF4E as well. In Xenopus, maternal mRNAs such as c-mos are repressed by CPEB binding to the 3′-UTR. CPEB binds Maskin, which in turn binds eIF4E, inactivating the latter for translation initiation (50). In Drosophila embryos, nanos mRNA translation is spatially restricted by translational repression. This is mediated through a 3′-UTR sequence element recognized by the protein Smaug, which in turn interacts with an eIF4E-binding protein, Cup, that blocks the binding of eIF4G to eIF4E (33).
Intriguingly, multiple isoforms of eIF4E have been found in plants, flies, mammals, frogs, nematodes, and fish (reviewed in references 40, 22, and 42). Five eIF4E isoforms, termed IFE-1 through IFE-5, are expressed in Caenorhabditis elegans (17, 22). C. elegans mRNAs contain two types of caps, m7GpppN and m37,2,2GpppN, the latter being generated by trans splicing (27). IFE-3, the most closely related to mammalian eIF4E-1, preferentially binds m7GpppN and is essential for viability. Three closely related isoforms (IFE-1, -2, and -5) bind both m7GpppN and m37,2,2GpppN. At least one of these three isoforms is required for viability. IFE-1 specifically associates with PGL-1, a KH-domain RNA-binding protein resident in P granules, which contain developmentally regulated maternal mRNAs (1). RNA interference (RNAi) depletion of IFE-1 blocks spermatogenesis. IFE-4 preferentially binds m7GpppN, is most closely related to an unusual eIF4E isoform found in plants (nCBP) and mammals (4E-HP), and is not essential for C. elegans viability in any combination of ife RNAi knockout.
Prior studies have attempted to uncover the physiological roles of multiple eIF4E isoforms within a single organism (43, 47, 1, 42, 20, 53). These have included their interactions with caps, eIF4G, and PHAS-I, their activities in supporting in vitro translation, and their ability to complement a yeast mutant lacking eIF4E. However, these experiments have failed to reveal their specific biological roles. In the present study, we performed translational profiling in an ife-4-deficient C. elegans strain to identify potential target mRNAs. We identified a small subset of mRNAs significantly changed in their polysomal distribution but not steady-state levels. We further observed that the ife-4 knockout mutant has a pleiotropic phenotype that includes defects in egg laying (Egl) and low brood size. These phenotypes can be explained by the diminished translation of some of ife-4-sensitive mRNAs.
MATERIALS AND METHODS
Strains.
Worms were grown at 20°C on NGM plates with Escherichia coli strain OP-50 (4). Synchronized L1 larvae (hypochlorite) were grown to the desired larval stage and then harvested. C. elegans strain N2 var. Bristol was used as the wild type.
The initial mutant for the ife-4 gene (ok320) was generated by chemical mutagenesis and obtained from the C. elegans Knockout Consortium (Robert Barstead, Oklahoma Medical Research Foundation). The isolate was backcrossed 10 times to N2 to generate strain KX17 [ife-4(ok320)]. Single-worm genomic PCR (F, 5′-TGAAGCTTTCAATTTTCATTTCCAGGCG-3′; R, 5′-GCAGCATGATCATTTGCAGATAT-3′) was used to monitor the ife-4 deletion and the wild-type alleles. Worms carrying the deletion were homozygosed by self-crossing.
ife-4::GFP was generated by using a PCR-based protocol (15). Briefly, the complete ife-4 coding sequence and 1.5 kb upstream of the 5′-trans-spliced acceptor site (22) were amplified from genomic DNA (F, 5′-TGAAGCTTTCAATTTTCATTTCCAGGCG-3′; R, 5′-AGCTTGCATGCCTGCAGGTCGACTTTTGCAGATATTT-3′). The reverse primer contained a 24-nucleotide (nt) overlap to the GFP sequence. The GFP coding region, preceded by a nuclear localization signal (NLS) and followed by the unc-54 3′-UTR, was amplified from pPD95.67 (32). Nested primers were used to create DNA encoding a fusion protein in a second PCR round. This DNA was microinjected into the gonad of N2 animals with 50 ng of the marker plasmid pRF4 carrying a dominant rol-6(su1006) allele to generate the extrachromosomal array lsEx296[ife-4::NLS::GFP rol-6(su1006)]. A second ife-4::GFP fusion was created as described above except that pPD95.67ΔNLS (the NLS was deleted by KpnI digestion and religation) was used in PCR. For microinjections, the second fusion product was mixed with linearized C. elegans genomic DNA (32) to generate the complex extrachromosomal array lsEx385[ife-4::GFP rol-6(su1006)].
For ife-4(ok320) rescue, lsEx385[ife-4::GFP rol-6(su1006)] hermaphrodites were crossed with male N2 animals to obtain males carrying extrachromosomal ife-4::GFP. Male rollers were then crossed with ife-4(ok320) hermaphrodites, and roller hermaphrodites from the F1 progeny were allowed to self-cross. Sixteen roller hermaphrodites from the F2 progeny were placed on separate plates, and the progeny from each individual were analyzed by PCR for ife-4 deletion (at least 10 nonrollers were picked per reaction). Rescued mutants were identified by PCR.
Other strains used in some experiments were AA120, dhIs26[(daf-12A::GFP lin15(+))I]; CL2070, dvIs70[hsp-16.2::GFP]; PY3018, [Is(kin-29::GFP)IV]; and TU2562, uIs22[(mec-3::GFP)V]; generously donated by Adam Antebi (Max-Planck Institute, Berlin, Germany), Chris Link (University of Colorado, Boulder), Piali Sengupta (Brandeis University, Waltham, Mass.), and Martin Chalfie (Columbia University, New York, N.Y.), respectively.
RNAi.
The SacI fragment (nt 1 to 428) of the ife-4 cDNA (22) was subcloned into plasmid pL4440 (56). ife-4 double-stranded RNA (dsRNA) was expressed in E. coli strain HT-115(DE3) with isopropyl-β-d-thiogalactopyranoside induction, and the bacteria were fed to N2 worms on NGM plates (55). Phenotypes were monitored in the F1 or F2 generation of animals continuously grown in the presence of the appropriate bacteria.
Phenotype characterization.
For fertility tests, three L4 worms were placed on NGM plates seeded with bacteria. Every 24 h the worms were transferred to a fresh plate. The number of progeny was scored every 24 h over 5 days. Three plates were assayed per strain [N2 versus ife-4(ok320)] with five replicates. Egg laying in serotonin (5-hydroxytryptamine [5-HT]) was carried out as described previously (58). Briefly, L4 animals were placed on OP-50-seeded NGM plates 36 h before the assay. Each adult was then placed in a well of 96-well microtiter plates containing 200 μl of M9 or 12.5 mM 5-HT sulfate (Sigma). The number of eggs laid after 90 min was counted for 12 adults per strain in three independent experiments. To assay 5-HT hypersensitivity (locomotion test), 10 animals were each placed in 200 μl of 5-HT solution (0 to 25 mM in M9 buffer) in 96-well microtiter plates. The animals were scored as either active or immobile based on swimming behavior after 5 min. To determine whether ife-4(ok320) egg laying was responsive to food cues, we measured the number of eggs laid by 10 worms in 30 min under three conditions: starved for 2 h, starved for 2 h and then refed, or never starved.
Sucrose gradient analysis of initiation complexes and polysomes.
To separate ribosomal subunits and initiation complexes, 0.5 g of ife-4::GFP(lsEx385) worms were crushed in liquid N2 with 0.5 ml of buffer A (50 mM Tris-HCl [pH 7.4], 140 mM NaCl, 10 mM MgCl2, 1 mM dithiothreitol, 400 U of RNAsin/ml, and Complete EDTA-free protease inhibitor cocktail [Roche]) and centrifuged at 27,000 × g at 4°C for 15 min. A 0.5-ml aliquot of the supernatant was applied to a 10 to 30% sucrose density gradient in buffer B (50 mM Tris-HCl [pH 7.4], 140 mM NaCl, 10 mM MgCl2, 1 mM dithiothreitol) and centrifuged in a Beckman SW41Ti rotor at 38,000 rpm at 4°C for 4 h. Gradients were fractionated with continuous monitoring of absorbance at 260 nm. Proteins from selected fractions (0.5 ml) were precipitated with 10% trichloroacetic acid and analyzed by Western blotting to detect IFE-1, IFE-4, and IFG-1 (see below).
Polysomes and polysomal RNA (63) were obtained from N2 and ife-4(ok320) mixed-stage worms. Frozen pellets were homogenized in four volumes of buffer C (300 mM NaCl, 50 mM Tris-HCl [pH 8.0], 10 mM MgCl2, 1 mM EGTA, 200 mg of heparin/ml, 400 U of RNAsin/ml, 2.5 mM phenylmethylsulfonyl fluoride, and 0.2 mg of cycloheximide/ml) by 40 strokes with a Teflon homogenizer. The lysate was processed as described above, and the supernatant was loaded onto an 11-ml 10 to 45% sucrose gradient in buffer D (140 mM NaCl, 25 mM Tris-HCl [pH 8.0], 10 mM MgCl2). Centrifugation and fraction collection were performed as described above for 10 to 30% sucrose gradients except that the centrifugation time was 2 h and 1-ml fractions were collected.
RNA isolation.
For isolation of RNA from sucrose gradients, fractions were treated with sodium dodecyl sulfate (SDS)-proteinase K, extracted with phenol-chloroform-iso-amyl alcohol (25:24:1) followed by chloroform-isoamyl alcohol (24:1), made 1.5 M in LiCl, and precipitated with an equal volume of isopropanol. For isolation of total RNA from whole N2 and ife-4(ok320) worms, the RNeasy kit (QIAGEN) was used.
Affymetrix microarrays.
RNAs from 10 to 45% sucrose gradients were pooled into fractions representing light (L) (fractions 6 to 8; see Fig. 4) and heavy (H) fractions 9 to 11 polysomes. Total RNA (T) was used in separate samples. Validation of RNA integrity in each sample was performed with an Agilent 2100 bioanalyzer. Amplified biotin-labeled cRNA was produced from RNA (5 μg) according to standard procedures (Affymetrix). After hybridization, microarrays were stained as described in the Affymetrix Expression Analysis Technical Manual. Fluorescent intensity was measured with the Affymetrix GeneChip laser scanner, and the images were processed with the GeneChip software package. Signals from each array were normalized to the array mean signal intensity and analyzed with the software provided by Affymetrix (Microarray Suite MAS 5.0). Each gene was judged either “present,” “absent,” or “marginal.” Comparisons were performed with algorithms from MAS 5.0 with N2 polysomes as the control and ife-4(ok320) polysomes as experimental. Significant changes were scored with a threshold of 2.0-fold. Each experiment was replicated three times with independent worm growths. Statistical significance was calculated with an unpaired t test. For hierarchical clustering, the software HCE 2.0 (http://www.cs.umd.edu/hcil/hce) was used to cluster genes considered present in at least one of the RNA samples (H, L, and T) according to their signal intensity. The GeneSifter.Net microarray data analysis system (VizV Labs LLC, Seattle, Wash.) was used to find significantly changed genes for ife-4(ok320) animals versus N2 animals with a twofold cutoff and P value of <0.05.
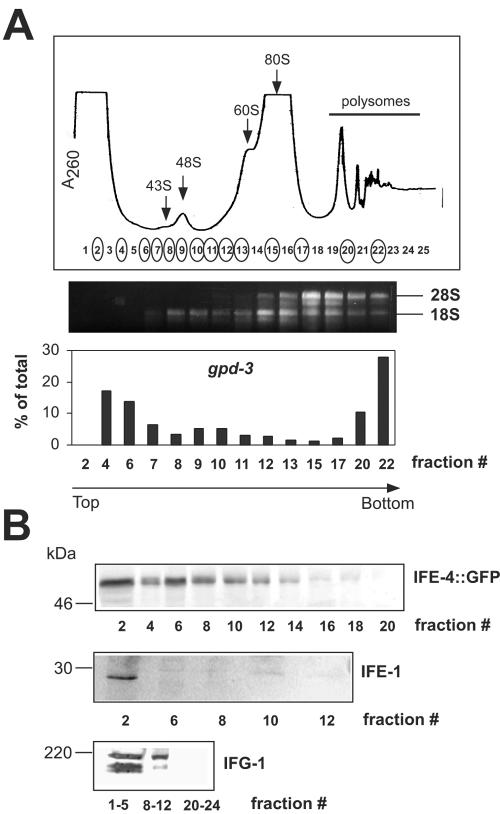
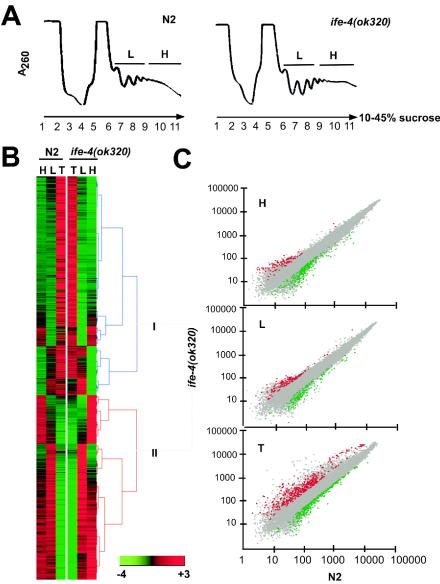
FIG. 4.
IFE-4 distribution in mRNPs and initiation complexes. Extracts from ife-4::GFP(lsEx385) worms were subjected to sedimentation on 10 to 30% sucrose gradients, and 0.5-ml fractions were collected. (A) The A260 profile (top) is shown together with the fractions analyzed for rRNA and gpd-3 mRNA distribution (circled). RNA was extracted as described in Materials and Methods and either subjected to electrophoresis on 1% agarose gels followed by ethidium bromide staining (middle) or assayed for gpd-3 mRNA by real-time PCR (bottom). Arrows indicate ribosomal subunits, initiation complexes, monosomes, and polysomes. (B) Proteins were precipitated with trichloroacetic acid, subjected to SDS-PAGE on 10% gels, and detected by Western blotting with primary antibody against GFP, IFE-1, or IFG-1. This experiment was performed three times with similar results.
Real-time PCR.
Two micrograms of RNA from sucrose gradient fractions or from T RNA were first treated with DNase RQ1 (Promega) and then subjected to reverse transcription with random primers and reverse transcriptase from the GeneAmp RNA PCR kit (Applied Biosystems). When polysome distribution was measured across the entire gradient, individual RNA fractions were processed in this way. When H and L fractions were measured, fractions were pooled as described above before reverse transcription. Quantitative real-time PCR was performed with specific primers designed for each gene with the Beacon Designer tool (Bio-Rad). Amplification and detection were done with the iCycler IQ real-time PCR detection system with IQ SYBRgreen Supermix (Bio-Rad). To calculate polysomal shifts, the threshold cycle (CT) for fraction no. 2 (no product was detected in fraction no. 1) was subtracted from the CT of each fraction (ΔCT). Relative mRNA levels were calculated as 2ΔCT and graphically represented as the percentage of mRNA present in each fraction from the total amount of mRNA (sum of all fractions).
Protein analysis.
Mixed populations or developmentally staged animals were collected in 1 ml of M9, washed three times with M9, and pelleted. Approximately 10 volumes of 1× SDS-polyacrylamide gel electrophoresis (PAGE) sample buffer was added to the pellet. Samples were boiled for 10 min, and 50 μg of protein (20 μl) were separated by SDS-PAGE on 10% gels and transferred to nitrocellulose membranes (Bio-Rad) with a Mini Trans-Blot cell (Bio-Rad). Primary antibodies against the following proteins were used at the indicated dilutions: DAF-12(cE-18) (Santa Cruz Biotech), 1:200; EGL-15(cN-14) (Santa Cruz Biotech), 1:200; actin (Sigma), 1:2,000; GFP (Abcam), 1:3,000; IFE-1 (17), 1:250, or IFG-1 (B. D. Keiper and R. E. Rhoads, unpublished data), 1:500. Incubation with primary antibodies was carried out in 5% milk for 1 h at room temperature. Membranes were washed three times for 10 min with Tris-buffered saline buffer and incubated with secondary antibodies conjugated with peroxidase (Vector Laboratories) at a dilution of 1:2,000 in 5% milk for 1 h at room temperature. Blots were developed with ECL Plus (Amersham) and analyzed with a Storm 860 PhosphorImager (Molecular Dynamics).
Microscopy.
For ife-4::GFP expression analysis, ∼50 animals from different developmental stages were analyzed. For ife-4(RNAi) experiments with ife-4::GFP-, daf-12::GFP-, and kin-29::GFP-expressing strains, 10 animals at the appropriate developmental stage were selected for analysis. Animals were immobilized in 15 mM sodium azide in M9 and examined by fluorescence microscopy with an Olympus AX70 microscope equipped with Nomarski and fluorescence optics. A 41001 filter set (Chroma Technology, Brattleboro, Vt.) was used for GFP imaging. A Roper CoolSnap charge-coupled device coupled to IPLab software was used to register images. The maximum fluorescence in specific small regions of the tissues expressing GFP was measured using the IPlab 3.6 software. The background fluorescence was subtracted from each measurement. Results from three independent experiments including at least five different worm measurements in each were averaged and graphically represented.
RESULTS
IFE-4 is expressed preferentially in neurons, muscle, and vulva.
To gain insight into the physiological roles of IFE-4, we first examined its expression pattern with two transgenic extrachromosomal arrays of ife-4::GFP. One was a simple array containing an NLS, ife-4::NLS::GFP(lsEx296), and the other, a complex array without an NLS, ife-4::GFP(lsEx385). The array with NLS was used to facilitate the identification of ife-4::GFP-expressing cells (Fig. 1A), whereas the array without NLS was expected to produce a subcellular distribution more similar to that of native IFE-4 (32) (Fig. 1B). Both transgenes were expressed in pharyngeal neurons and muscle, ventral nerve cord (VNC), vulval neurons and muscle, tail neurons, and spermatheca sheath. Expression occurred from late embryo to adult, especially in the pharynx. Other tissues, such as vulva and tail, had increased fluorescence during the late L3 and L4 stages. Unlike that of ife-1, ife-3, and ife-5 (1), ife-4::GFP expression was not detected in germ line or somatic gonadal tissues other than spermatheca.
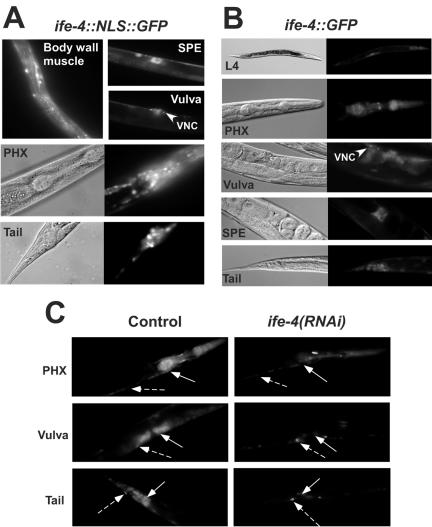
FIG. 1.
Expression of ife-4::GFP in specific tissues of C. elegans (A and B) and reduction of fluorescence by ife-4(RNAi) (C). Transgenic strains were produced by microinjection of an ife-4 genomic fragment containing the complete coding region and ∼1,500 nt upstream fused to the 5′ end of DNA encoding GFP either as a single array with NLS [ife-4::NLS::GFP(lsEx296)] (A) or a complex array without NLS [ife-4::GFP(lsEx385)] (B). Fluorescence is seen in muscle and neurons from pharynx (PHX), neuronal cell bodies, the ventral nerve cord (VNC), vulval muscle and neurons, body wall muscle, and spermatheca (SPE). (C) RNAi was initiated for L4 ife-4::GFP(lsEx385) animals by feeding with bacteria expressing the dsRNA of ife-4 (right). Control animals were fed with bacteria containing empty vector (left). The offspring were compared for fluorescence at L4 after one generation. Solid and broken arrows indicate regions where fluorescence is decreased or not decreased by ife-4(RNAi), respectively.
Previously we used ife-4(RNAi) to test for viability of IFE-4 loss (22). In the present study, we wished to use ife-4(RNAi) to reduce IFE-4 expression in specific tissues. To assess the effectiveness of ife-4(RNAi), we examined the fluorescence of ife-4::GFP(lsEx385) animals fed with E. coli harboring a plasmid that expresses ife-4 dsRNA (Fig. 1C) [ife-4(RNAi)] and compared it to the same strain fed with E. coli harboring the empty vector (Fig. 1C, Control). Fluorescence was substantially decreased by ife-4(RNAi), especially in vulva and many pharyngeal neurons. However, only a modest reduction was observed in other pharyngeal neurons, VNC, and some tail neurons. This suggests that ife-4 is generally sensitive to RNAi but neuronal depletion is variable. Therefore, studying the physiological role of IFE-4 by RNAi might be complicated because of incomplete suppression of ife-4 in some tissues.
Deletion of the ife-4 gene.
Accordingly, we obtained a mutant of ife-4 from the C. elegans Knockout Consortium and backcrossed the initial isolate to generate the homozygous strain KX17[ife-4(ok320)X]. The ok320 mutation is a 1,776-bp deletion including most of the promoter and the first two exons of the gene (Fig. 2A). The deletion removes 81 amino acid residues from the N terminus, including residues that contribute to the cap-binding site (30) and the putative eIF4G-binding site (29). Such a truncated protein should be biologically inactive (34). We therefore consider ife-4(ok320) to be a null mutation.
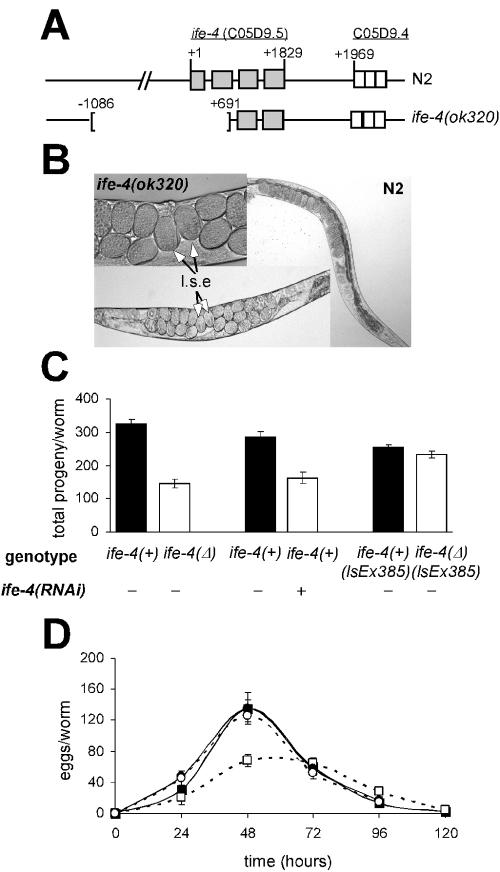
FIG. 2.
Egl phenotype of the ife-4 homozygous deletion mutant. (A) Structure of the wild-type and truncated C. elegans ife-4 gene on the X chromosome. Light gray boxes indicate exons. For ife-4(ok320) animals, 1,776 bp were deleted. (B) Egg retention with ife-4(ok320) animals compared to that with N2 animals. Late-stage embryos (l.s.e) are shown. (C) The progeny laid during the reproductive life is reduced for ife-4(ok320) animals [ife-4(Δ)] and by ife-4(RNAi). Animals carrying the ife-4::GFP extrachromosomal transgene in an ife-4(ok320) background [ife-4(Δ) lsEx385] recover the normal number of progeny. (D) Egg laying for ife-4(Δ) (□) animals is reduced from that for N2 (▪) animals. Time after L4 is shown. ife-4(Δ) lsEx385 (○) animals had an egg-laying pattern similar to that of ife-4(+) lsEx385 (•) animals. The circles overlap in the graph. The results are means from five independent experiments.
ife-4 deletion results in a mixture of phenotypic traits.
ife-4(ok320) homozygotes are viable but reproduce more slowly than the wild type and have an Egl phenotype (Fig. 2B). Egg retention in ife-4(ok320) worms is variable, unlike strong Egl phenotypes (58), and the mutant brood size is half that of N2 (Fig. 2C and D). A similar decrease in brood size was observed for ife-4(RNAi) animals (Fig. 2C). The reduced brood size could be partially related to egg retention. However, a fertility test did not show a delay in ife-4(ok320) egg laying compared to that of wild-type animals (Fig. 2D). One possible explanation is that a decrease in mutant fertility occurs as a consequence of their egg retention. However, we cannot rule out defects in germ cell proliferation or egg fertilization in the spermatheca, especially since ife-4 is expressed in spermatheca. The ife-4 deletion did not affect embryonic viability (data not shown). To test whether the Egl phenotype was due to the ife-4 deletion and not to a closely linked mutation, we crossed the ife-4::GFP(lsEx385) array into the ife-4(ok320) background [ife-4(Δ) lsEx385]. The transgene rescued the Egl phenotype (Fig. 2C and D).
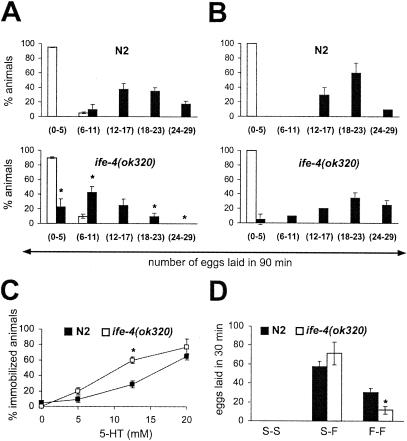
Serotonin (5-HT) mediates egg-laying, locomotion, and pharyngeal pumping (16). Depending on the mutation, an Egl phenotype may or may not be corrected by exogenous 5-HT. We therefore tested the sensitivity of the ife-4(ok320) Egl phenotype to 5-HT. Egg laying was induced by 12.5 mM 5-HT in ife-4(ok320) animals (Fig. 3A, filled bars) but was more strongly induced in N2 animals, even though mutants had more eggs in their uteri (see Fig. 2B). The egg-laying response to 5-HT, although variable, was greater in starved ife-4(ok320) animals than in fed animals [compare Fig. 3B to Fig. 3A for ife-4(ok320) animals]. These results indicate that although ife-4(ok320) egg laying was responsive to exogenous 5-HT, the Egl phenotype was not completely corrected. We used locomotion as another test for 5-HT responsiveness and found that mutants were more sensitive to 5-HT-induced immobilization than wild-type animals (Fig. 3C). About 70% of ife-4(ok320) worms were immobilized after 5 min in the presence of 12.5 mM 5-HT, whereas N2 required a 20 mM concentration for a similar effect. Food induces 5-HT release by the hermaphrodite-specific neurons and modulates egg laying and other behavior (16). We used a food assay to test the egg-laying response of ife-4(ok320) animals (Fig. 3D). Mutants laid fewer eggs than wild-type animals under continuous food presence, but egg laying was similar in both strains for animals previously starved for 2 h. Thus, ife-4(ok320) animals seem unable to process the food signal for egg laying unless first starved.
FIG. 3.
Characterization of the Egl phenotype of ife-4(ok320) animals by serotonin (5-HT) and food assays. (A) ife-4(ok320) worms lay fewer eggs in response to 5-HT stimulation than N2 worms. The number of laid eggs was measured after 90 min of incubation in M9 (□) or 12.5 mM 5-HT (▪) for 20 animals per strain in three independent experiments. (B) The ife-4(ok320) egg-laying response to 5-HT increases after starvation. Conditions were the same as for panel A except that worms were deprived of food for 2 h previous to the 5-HT assay. (C) ife-4(ok320) animals are hypersensitive to 5-HT-induced locomotion arrest. Dose-response curves were generated in three independent experiments with 10 animals per strain per 5-HT concentration. The animals were scored for movement after 5 min in the presence of 5-HT. (D) Egg laying is impaired in fed but not starved ife-4(ok320) animals. The number of laid eggs was scored for 10 animals either starved (S-S), starved for 2 h then placed on food (S-F), or always on food (F-F). The experiment was performed in duplicate four independent times. Significant differences (P < 0.05) between ife-4(ok320) and N2 animals are indicated by an asterisk.
IFE-4 is found in translation initiation complexes.
Because IFE-4 has high amino acid sequence identity to eIF4Es from a variety of species (22), it is likely to function in translation initiation. However, neither IFE-4 nor its homologues in plants (nCBP) or humans (4E-HP) have been shown to bind eIF4G, nor have they been detected in initiation complexes. We therefore examined 48S initiation complexes for the presence of IFE-4.
We used the strain carrying ife-4::GFP(lsEx385) to test for the presence of IFE-4 in the free RNP fraction, initiation complexes, and polysomes by sucrose density gradient centrifugation (Fig. 4A, top). The sedimentation velocity of free 40S and 60S ribosomal subunits was determined in a parallel gradient (data not shown). Fractions 7 to 11 contained only 18S rRNA, indicating the presence of 40S ribosomal subunits, whereas fractions 12 to 22 contained both 18S and 28S rRNA, indicating the presence of both 40S and 60S subunits (middle panel). Glyceraldehyde-3-phosphate dehydrogenase mRNA (gpd-3) was enriched in fractions 4 to 7 (free mRNPs), fractions 8 to 12 (48S initiation complexes), and fractions 20 to 22 (polysomes) but not fractions 13 to 17 (80S monosomes), the latter of which are translationally inactive (bottom panel). The assignment of fractions 8 to 12 as 48S initiation complex was based on the presence of mRNA (48), although it is more common that the peak of optical density at 260 nm is larger for 43S complexes than for 48S complexes.
Western blotting with anti-GFP antibodies showed that IFE-4::GFP was distributed throughout the region of mRNPs and 48S initiation complexes but not 80S monosomes or polysomes (Fig. 4B). IFE-1, on the other hand, was present in the mRNP fraction but not in initiation complexes. As a positive control, we showed by immunoblotting that C. elegans eIF4G (IFG-1) is present in free cytosol and initiation complexes but not polysomes (Fig. 4B, bottom panel). At least two bands were recognized by the C. elegans anti-IFG-1, which may represent protein isoforms or breakdown products, as has been observed for other species (3). Previous analyses of eIF4E in rabbit reticulocyte lysate have demonstrated that eIF4E is predominantly in the supernatant and mRNP fractions, with only a minor portion in 48S initiation complexes (13, 14, 36). The presence of IFE-4::GFP in 48S initiation complexes suggests, but does not prove, that IFE-4 is involved in translation initiation.
ife-4 deletion and RNAi feeding cause changes in the spectrum of translated mRNAs.
To determine the effect of IFE-4 loss on the translation of individual mRNAs, we compared the distribution of all mRNAs on 10 to 45% sucrose density gradients (polysome separation) between N2 and ife-4(ok320) mixed-staged worms. Whole animals and all developmental stages were included in this analysis in an attempt to cover a wide spectrum of the possible IFE-4 targets. During translation, mRNAs that are initiated efficiently are found on larger (heavy) polysomes; a decrease in initiation rate relative to elongation results in a shift from heavy to light polysomes (28). We reasoned that mRNAs requiring IFE-4 for translational initiation would be found on lighter polysomes in ife-4(ok320) animals than in N2 animals.
The sedimentation profiles of polysomes from wild-type and mutant animals were similar, indicating that absence of IFE-4 does not grossly affect overall translation (Fig. 5A). Polysomes were divided into two fractions: light polysomes (L) composed of fractions 6 to 8 (two to four ribosomes bound per mRNA), and heavy polysomes (H) composed of fractions 9 to 11 (five or more ribosomes bound per mRNA). Assignment of ribosomes per mRNA was made on the basis of the optical density profile at 260 nm (18). Total RNA (T) obtained from the same animals was used to measure steady-state mRNA levels. H, L, and T samples were used to obtain labeled cRNA, which was then hybridized to Affymetrix GeneChip microarrays containing probe sets for all known C. elegans genes (∼18,967). According to hybridization signals, 14,726 sequences were judged “present” in at least one of the H, L, or T samples for either N2 or ife-4(ok320) animals or both (data not shown).
FIG. 5.
Polysomal distributions of mRNAs in ife-4(ok320) and N2 animals. (A) Polysomal profiles (A260) of sucrose gradient fractions, subsequently pooled into H and L fractions. RNA was prepared from these fractions, as was total RNA (T) from whole worms. (B) Hierarchical clustering (http://www.cs.umd.edu/hcil/hce) of mRNAs judged present by Affymetrix GeneChip Array analysis in at least one sample (H, L, or T). Clustering was performed with signals normalized by the mean expression signal on the array and averaged over three independent experiments. Group I, mRNAs underrepresented in polysomes; Group II, mRNAs overrepresented in polysomes. (C) Scatter plots for the same signals in H, L, and T from ife-4(ok320) animals (y axis) versus N2 animals (x axis). Plots were made with the Genesifter.Net microarray data analysis system (VizV Labs LLC, Seattle, Wash.). Statistically significant (p < 0.05) differences of ≥2-fold are shown in color. Grey, no change; red, increased in ife-4(ok320) animals; green, decreased in ife-4(ok320) animals.
A hierarchical clustering analysis allowed the visualization of overall relationships of the “present” mRNAs among H, L, and T samples from ife-4(ok320) and N2 animals (Fig. 5B). Green, black, and red colors indicate lower, the same, or higher intensity, respectively, of each mRNA signal relative to the mean signal of the chip. The two main clusters include mRNAs underrepresented (group I) or overrepresented (group II) in polysomal fractions. Further subgroups within groups I and II include mRNAs specifically underrepresented or overrepresented in either the H or L polysomal fraction or both. This clustering indicates a similar overall polysomal distribution of mRNAs for ife-4(ok320) and N2 animals, confirming that loss of IFE-4 does not grossly perturb general translation. However, there were differences for a small number of individual mRNAs. The GeneSifter.Net microarray data analysis system identified specific changes of ≥2-fold (P < 0.05) between ife-4(ok320) and N2 animals [either up-regulated (red) or down-regulated (green) in ife-4(ok320) animals] (Fig. 5C). Such changes ranged from 1 to 3% for comparison of H, L, and T between the two strains.
Because some mRNAs differed in steady-state level (T) between ife-4(ok320) and N2 animals, we conducted a more restricted search to identify mRNAs with changes only in polysomal recruitment. We queried the 14,726 “present” mRNAs for the following behavior. First, there was at least a twofold difference (up or down) in either H, L, or both for ife-4(ok320) versus N2 animals. Second, the difference in T between ife-4(ok320) and N2 animals was less than twofold. Third, for all three independent experiments, there was a significant difference (P < 0.05) between ife-4(ok320) and N2 animals for H, L, or both. This query identified 33 mRNAs (Table 1). Of these, 27 were decreased in H, L, or both for ife-4(ok320) versus N2 animals. By contrast, deletion of the gene for another eIF4E isoform, ife-2, changed a totally different subset of mRNAs (data not shown). Functions are known for ∼60% of the 33 mRNAs in Table 1. Four of these (12%) are related to egg laying: egl-3 (decreased), egl-15 (decreased), kin-29 (decreased), and egl-46 (increased). By contrast, egl genes are quite rare in the C. elegans genome (0.27%). mRNAs affected by IFE-4 are preferentially expressed in pharyngeal or vulval neurons and muscle (Table 1, Tissue), similar to the expression pattern of IFE-4 (Fig. 1).
TABLE 1.
IFE-4 target mRNAs regulated at the level of polysomal recruitment
| Gene or ORFf | Descriptiona | Fold changeb
|
Expressiona
|
|||
|---|---|---|---|---|---|---|
| H | L | T | Tissue | Stage | ||
| K02A2.5 | Nuclear protein | −6.59d | −1.94c | −1.51c | ||
| grl-20 | Ground-like related | −5.34e | −3.22d | −1.37c | HYP, neurons, seam | L2-A |
| F37D6.6 | Transcription regulation | −4.42c | −5.70c | −1.71c | Male | L1, L2, A |
| myo-2 | Myosin head (motor) | −4.07d | −2.72c | −1.59c | PHX, muscle | All |
| peb-1 | Transcription factor | −4.00d | −2.68d | 1.02 | PHX muscle | All |
| xtr-1 | TRA-2 related | −3.94c | −2.01c | 1.32 | L4 | |
| ZK970.7 | OV-17 precursor | −3.73d | −2.15c | 1.15 | Male | L1, A |
| K03B8.7 | Unknown | −3.60c | −3.39e | 1.09 | E, L3, L4, A | |
| C56C10.12 | Unknown | −3.40c | −1.98 | 1.19 | A | |
| C04F12.9 | RNase H, RNA binding | −3.36d | −2.13 | 1.39 | L3-A | |
| glr-4 | Glutamate receptor | −3.26d | −2.33c | −1.86c | Neurons | A |
| myo-1 | Myosin heavy chain | −3.23e | −2.29d | −1.57d | PHX | All |
| daf-12 | Transcription factor | −3.12e | −2.35e | 1.05 | HYP, neurons, PHX | L1-L3 |
| ZC21.3 | Unknown | −3.08c | −2.69c | −1.33c | PHX | L3 |
| F15E6.8 | Unknown | −3.00d | −2.49 | 1.15 | L3-L4 | |
| unknown | Unknown | −2.91c | −1.44 | 1.29 | ||
| egl-15 | FGF receptor | −2.82d | −2.65 | 1.20 | HYP, intestine, vulva | L4-A |
| C01G10.6 | Unknown | −2.82c | −2.01 | 1.19 | L1-A | |
| egl-3 | Protease (Ser) | −2.81c | −2.13 | 1.49 | Neurons, VNC | All |
| kin-29 | Kinase (Ser/Thr) | −2.58e | −2.86d | 1.38 | Neurons, HYP, muscle | |
| lam-3 | Laminin-type, EGF-like | −2.41d | −2.85c | 1.85 | E, L3, A | |
| sol-1 | CUB domain | −2.37d | −1.79c | −1.75d | Male | L1, L2, A |
| C29H12.6 | Unknown | −2.34d | −1.48 | −1.66c | PHX | All |
| srh-2 | Chemoreceptor (7M) | −2.32d | −2.60d | 1.21 | Intestine, muscle | All |
| trp-1 | Ion transporter | −2.29d | −2.85c | −1.62c | Vulva, neurons | L1, L2, A |
| F35C11.4 | Unknown | −2.23e | −2.36c | 1.06 | E, L2, L3 | |
| F55A4.4 | dsRNA binding | −1.88 | −3.02c | 1.18 | Male | L1, L3, A |
| T24B8.5 | Metridin ShK-like toxin | −1.40 | 3.18c | 10.31 | All | |
| hbl-1 | Transcription factor | 2.11c | −2.35 | 1.39 | HYP, PHX | |
| egl-46 | Transcription factor | 2.19d | −2.71d | −1.99c | Neurons | E, L1, L2 |
| C17C3.12 | Acyl-CoA dehydrogenase | 2.93 | 2.39d | 1.06 | E | |
| R08E5.3 | UbiE/COQ5 methyltransferase | 3.02d | 2.27d | 1.02 | All | |
| rpl-27 | Ribosomal L27e protein | 3.45 | 10.31c | 1.07 | All | |
Gene description and expression data were obtained from WormBase (http://www.wormbase.org). E, embryo; A, adult; HYP, hypodermis; PHX, pharynx; VNC, ventral nerve cord.
Fold change for ife-4(ok320) animals versus N2 animals in each RNA sample as calculated from signal log ratios (SLR) provided by Affymetrix MAS 5.0 analysis and averaged for three independent experiments. Fold change is calculated as 2SLR. Comparisons between ife-4(ok320) and N2 animals are shown for heavy polysomes (H), light polysomes (L), and total RNA (T). A decrease in signal comparing ife-4(ok320) to N2 animals is shown as a negative value; an increase is shown as as a positive value.
P ≤ 0.05.
P ≤ 0.01.
P ≤ 0.001.
ORF, open reading frame. Acyl-CoA, acyl coenzyme A.
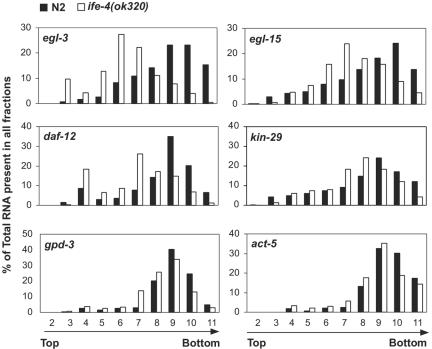
We confirmed the changes in polysomal distribution for several mRNAs selected from Table 1 and the absence of changes for two housekeeping mRNAs. Real-time PCR was used to quantitate the distribution of mRNAs across all of the polysomal fractions rather than just H and L (Fig. 6). As noted above, a decrease in initiation relative to elongation-termination results in a shift from heavier to lighter polysomes. egl-3 mRNA was present on heavy polysomes for N2 animals (fraction 9 to 10) but shifted to light polysomes (fractions 5 to 8) and to the untranslated mRNP pool (fractions 3 to 4) for ife-4(ok320) animals. Similarly, egl-15, daf-12, and kin-29 mRNAs were shifted to lighter polysomes by one to three fractions, as were srh-2 and myo-1 (data not shown). The housekeeping mRNAs act-5 and gpd-3 did not differ in polysomal distribution between ife-4(ok320) and N2 animals (Fig. 6), nor did two other mRNAs arbitrarily selected from those not present in Table 1, egl-21 and myo-3 (data not shown).
FIG. 6.
Polysomal shifts for selected mRNAs from ife-4(ok320) (□) versus N2 (▪) animals. Some of the mRNAs shown in Table 1 that were changed in polysomes (either H, L, or both) but not in T RNA (egl-3, egl-15, daf-12, or kin-29) were further analyzed over the entire polysomal gradient by real-time PCR. gpd-3 and actin (act-5) genes were selected as control mRNAs whose distribution did not change in H, L, or T fractions.
The polysomal shifts observed for selected mRNAs in ife-4(ok320) animals were reproduced by ife-4(RNAi) experiments (data not shown). The translational state (H and L) and T RNA levels for six mRNAs from Table 1 were tested by real-time PCR after ife-4(RNAi). Five of the six mRNAs that were significantly decreased in ife-4(ok320) H polysomes as measured by microarrays or real-time PCR were also greatly reduced in H by ife-4(RNAi) but not changed in T (myo-1, daf-12, egl-3, egl-15, and kin-29). Interestingly, despite the incomplete effectiveness of ife-4(RNAi) observed in some neurons of ife-4::GFP-expressing animals (See Fig. 1C), the neuronally enriched genes daf-12 and kin-29 were significantly affected by ife-4(RNAi). This could imply that reducing the levels of IFE-4 in these tissues is sufficient to affect translation of specific mRNAs. On the contrary, a muscle-enriched mRNA, myo-2, which had been found decreased in ife-4(ok320) polysomes (Table 1), was not altered by ife-4(RNAi), suggesting that it may be indirectly affected by ife-4 deletion (see Discussion). egl-3, which was dramatically decreased in H polysomes of ife-4(ok320) animals by microarrays and real-time PCR, was reduced to a lesser extent by ife-4(RNAi). The mild ife-4(RNAi) effect on this gene may be due to its almost exclusive expression in neurons (21), where RNAi is not completely effective.
ife-4 deletion and RNAi feeding result in the decreased accumulation of DAF-12, EGL-15, and KIN-29.
The foregoing results demonstrating a shift to lighter polysomes of egl-3, egl-15, daf-12, and kin-29 mRNAs by ife-4 mutation (Fig. 6) or ife-4(RNAi) feeding (data not shown) could represent either a decrease in the initiation rate or an increase in the elongation rate. Given that IFE-4 is likely to be an initiation factor (Fig. 4), the former is much more probable. A decrease in the initiation rate would reduce steady-state levels of the encoded protein, all other things being equal, whereas an increase in elongation would increase protein levels. To distinguish between these, we determined the levels of three proteins whose mRNA distributions were changed by ife-4 knockout.
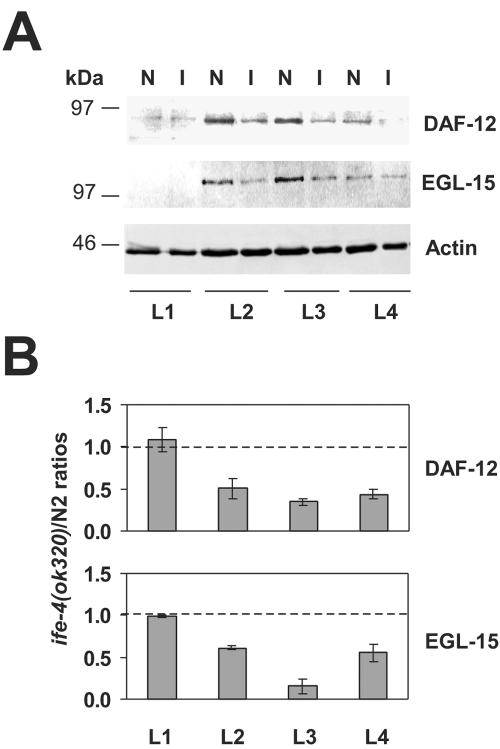
DAF-12 protein levels were measured at several developmental stages by Western blotting of total extracts. We used antibodies raised against a carboxyl-terminal peptide to identify all possible DAF-12 isoforms, since several isoforms differing at the amino terminus are predicted for daf-12 according to the C. elegans genome database. DAF-12 is a nuclear hormone receptor transcription factor involved in dauer formation and the reproductive life of C. elegans (2). According to mutational screens, DAF-12A (∼84 kDa) plays a role in reproductive growth induction, whereas DAF-12B (∼75 kDa) functions in dauer formation. DAF-12 was detected as a single band of ∼82 kDa in Western blots (Fig. 7A), which likely represents the DAF-12A isoform. For N2, this protein was found predominantly at L2 and L3 stages, decreasing in L4 (lanes N). For the ife-4(ok320) strain, this protein had a similar developmental expression pattern but was reduced in the L2 to L4 stages of compared with the pattern for N2 (lanes I). The results with several Western blots are quantitated in Fig. 7B. This indicates that the polysomal shifts of daf-12 mRNA in ife-4(ok320) animals (Fig. 6; Table 1) reflect diminished synthesis of DAF-12A.
FIG. 7.
DAF-12 and EGL-15 protein levels are reduced in ife-4(ok320) animals. (A) Expression of DAF-12 and EGL-15 was detected by Western blotting at the indicated developmental stages (L1 to L4) for N2 (N) and ife-4(ok320) (I) animals. Proteins (50 μg) were resolved by SDS-PAGE on 10% gels. (B) DAF-12 and EGL-15 protein levels were normalized with the actin signal, and the ife-4(ok320)/N2 ratios are graphically represented, with 1.0 shown by the dashed line. The results represent the average for three independent worm populations and immunoblots.
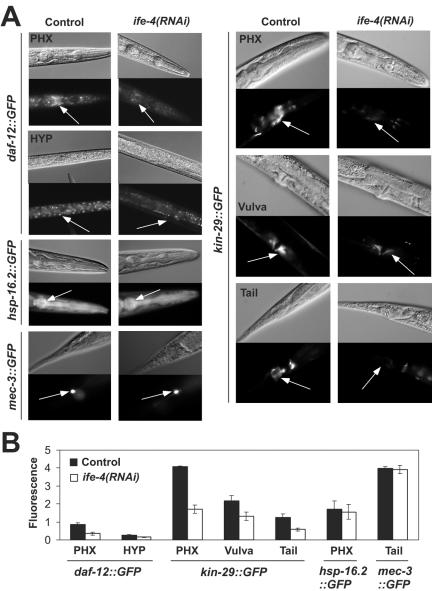
We extended the analysis of daf-12A expression with ife-4(RNAi) in worms expressing an integrated daf-12A::GFP array (2). Animals were scored for fluorescence at the L2/L3 developmental stage (Fig. 8A). Many neurons and neuronal cell bodies around the pharynx, the VNC, and hypodermal cells were fluorescent in controls, as described previously (2). However, ife-4(RNAi) feeding reduced DAF12A::GFP fluorescence in pharyngeal neurons, especially in neuronal cell bodies, and in the hypodermis (Fig. 8A). ife-4(RNAi) had no effect on GFP expressed from other promoters, such as those corresponding to a heat shock protein (hsp-16.2::GFP) or to a touch receptor neuron protein (mec-3::GFP). Thus, both Western blotting for DAF-12A with ife-4(ok320) (Fig. 7) and DAF-12A::GFP fluorescence after ife-4(RNAi) feeding (Fig. 8) demonstrate that ife-4 knockout reduces DAF-12A expression.
FIG. 8.
ife-4(RNAi) feeding reduces the expression of daf-12A::GFP and kin-29::GFP but not of hsp-16.2::GFP or mec-3::GFP in specific tissues. (A) RNAi feeding was performed as described in the legend to Fig. 1. Representative Nomarski (upper) and fluorescence (lower) images are shown for each condition in different tissues. Arrows indicate regions measured for fluorescence quantification. At least five different animals were measured for each condition, and the results were averaged. PHX, pharynx; HYP, hypodermis. (B) The graphical representation indicates the maximum fluorescence for the different strains in various tissues.
We also studied EGL-15 protein levels with antibodies raised against an amino-terminal peptide to detect most isoforms of this protein. EGL-15 is a fibroblast growth factor receptor (52) whose isoforms have been recently shown to perform different specialized functions during development (9). The EGL-15(5A) isoform (∼108 kDa) is required for proper sex myoblast migration during development, and its mutation leads to an Egl phenotype. The EGL-15(5B) isoform (∼120 kDa) performs an essential function, and its loss is lethal. The single band of ∼100 kDa in Fig. 7A most likely represents EGL-15(5A). In N2 animals, this band was first detectable in L2, increased in L3, and then diminished in L4. In ife-4(ok320) animals, the developmental expression pattern was similar, but levels were lower in the L2 to L4 stages than those for N2 animals, the most striking reduction being in L3 (Fig. 7A and B).
The expression of kin-29, a third translationally regulated gene identified by microarray screening (Table 1) and real-time PCR (Fig. 6), was analyzed in vivo with an integrated GFP fusion construct (Fig. 8B). Fluorescence occurred in multiple neurons in the pharynx, VNC, and tail as well as in body wall muscle and vulva, as previously described (25). Interestingly, the kin-29::GFP expression pattern was very similar to that of ife-4::GFP (Fig. 1). The expression of kin-29::GFP was significantly decreased by ife-4(RNAi) feeding, especially in pharyngeal neurons and vulva (Fig. 8), whereas strains carrying GFP fusions of hsp-16.2 or mec-3 were not affected in fluorescence.
DISCUSSION
IFE-4, although not required for viability, is important for specific cellular processes.
eIF4E proteins in a class consisting of IFE-4 in C. elegans (22), nCBP in plants (47), and 4E-HP in mammals (44) lack three of the eight Trp residues conserved in virtually all other eIF4Es and generally diverge in sequence from the canonical proteins. Nonetheless, they contain most of the important amino acid residues required for cap recognition (30, 31), are retained on m7GTP-Sepharose, and bind capped mRNAs. Moreover, nCBP can restore translation in an eIF4E-depleted wheat germ in vitro translation system (47). The predicted three-dimensional structure of 4E-HP suggested that it functions as a canonical eIF4E protein, but interaction with eIF4G failed, at least in vitro (20, 44). Knockout by RNAi injection indicated that ife-4 is completely dispensable for viability, either alone or in combination with knockout of ife-1, -2, or -5 (22). Hence, the biological role of this class of eIF4E isoforms has been a mystery.
In the present study, we examined phenotypic traits of ife-4 knockout beyond embryonic lethality and found complex behavioral defects that result in an Egl phenotype and low brood size. Synthesis and neurotransmission by 5-HT are required for proper egg laying (60, 51). We found that exogenous 5-HT could not fully restore egg laying in ife-4(ok320) animals, especially in continuously fed worms. Therefore, it seems unlikely that defective 5-HT synthesis or release is responsible for the Egl phenotype. The smaller amount of eggs laid by ife-4(ok320) animals compared to N2 animals in the presence of exogenous 5-HT might suggest a diminished sensitivity to 5-HT, but this is contradicted by the hypersensitivity of ife-4(ok320) animals to 5-HT for immobilization. The inhibitory action of 5-HT on movement appears to involve direct inhibition of neurotransmitter release from ventral cord motoneurons. Hermaphrodite-specific neurons and ventral cord motoneurons contain at least three types of neurotransmitters: acetylcholine, serotonin, and neuropeptides, each activating different signaling pathways in the postsynaptic muscle cell or neuron (59). Our results indicate that the response to serotonin of egg laying, but not movement, is partially reduced in ife-4(ok320) animals. Adding complexity to the phenotype of ife-4(ok320) animals is their improper perception of food cues. While N2 animals proceed with normal development and egg laying in the presence of food, most ife-4(ok320) animals do not remain on the bacterial lawn and lay fewer eggs during the fertile period. Some of the putative IFE-4 targets could explain the altered egg-laying, brood size, and other behaviors of ife-4(ok320) animals. For example, egl-3 is required for the egg-laying muscles to become responsive to 5-HT. A curious observation for egl-3 mutants is their hypersensitivity to drug-induced immobilization (21). As noted above, daf-12 and egl-15 participate in reproduction and egg laying, respectively. kin-29 is involved in food sensation; mutants for this gene behave as if starved, even in the presence of food (25). These findings indicate that several components of the egg-laying circuit, especially at the level of perception and neuromuscular signal transmission, are impaired in ife-4(ok320) animals. Though imperfectly understood, this provides the most detailed biological role for a member of the IFE-4 class to date.
ife-4 knockout affects the translational efficiency of only a small subset of mRNAs.
eIF4E is an essential translation factor, and its most important function is the recruitment of mRNAs to the translation machinery (8, 39), although other nontranslational roles have also been described (6, 46). In the present study, we have demonstrated the first linkage between a specific eIF4E isoform and the translation of a discrete subset of mRNAs. In most cases, these mRNAs were found in lighter polysomes or completely released from ribosomes (i.e., decreased in both H and L). These changes argue that IFE-4 directly affects loading of these mRNAs onto polysomes. Among the mRNAs changed in either polysome distribution or total RNA, we did not identify the mRNAs for other known translation factors or eIF4E isoforms, which might have been indirectly responsible for the observed translational changes. Furthermore, ife-4 mRNA was absent in ife-4(ok320) animals, as expected, but there were no significant changes (P > 0.05) for the remaining four ife mRNAs in H, L, and T.
We detected changes in the steady state-levels of some mRNAs by microarray screening. These changes could be due to differences in transcription or mRNA stability and are unlikely to be a direct consequence of ife-4 knockout. In fact, some of the mRNAs affected in polysomal recruitment by ife-4 deletion encode transcription factors (Table 1; F37D6.6, peb-1, daf-12, hbl-1, and egl-46), which are likely to affect the transcriptome profile. One mRNA change detected by microarrays, for myo-2, was not reproduced by ife-4(RNAi) animal feeding, indicating that it is probably an indirect IFE-4 target. Interestingly, peb-1, another of the mRNAs shifted by ife-4 knockout, encodes a transcription factor that enhances myo-2 expression in the pharynx (54).
The loss of selected mRNAs partially explains the complex phenotype of ife-4 knockout.
Genes that are affected by loss of IFE-4 belong to diverse functional categories including transcriptional regulation, signal transduction, growth and development, and metabolism (Table 1). There is no strong preference for any chromosome. However, there is a similarity of tissue expression; considering those genes for which tissue expression information is available, none is expressed in germ line, while 80% are expressed in neurons and/or muscle. The latter observation is particularly significant because differences in germ line cell expression are easily observed; about 70% of an adult is composed of the gonad (38, 37). On the other hand, differences in neurons are more difficult to observe, since these cells represent a much smaller portion of the worm body (62). Therefore, we may still be missing potential targets that are underrepresented in our mixed-stage populations. Three of the genes affected by ife-4 knockout (egl-3, egl-15, and egl-46) are directly involved in egg laying (21, 52, 58, 61), and a fourth (kin-29) is related to food sensation, which in turn affects egg laying (25). egl-3, egl-46, and kin-29 are expressed in neurons, and egl-15 is expressed in vulval muscle, both of which are sites for high ife-4::GFP expression. Thus, reduced translation initiation of these mRNAs is a plausible explanation for the complex Egl phenotype of ife-4(ok320) worms.
The biological relevance of multiple eIF4E isoforms for C. elegans.
Previous studies of multiple eIF4E isoforms within a single organism have attempted to understand their individual physiological roles by examination of tissue distribution (1, 42, 43), cap-binding (5, 17, 22, 42, 44, 47, 49), and binding to other initiation factors (5, 20, 42, 53). These reports have suggested, but not proven, that eIF4E isoforms fulfill specialized functions in the tissues where they are expressed. In the present study, the application of genome-wide translational profiling in C. elegans points to a characteristic subset of mRNAs in neuronal and muscle tissues affected by ife-4 knockout. The effect of IFE-4 loss on the translational efficiency of specific mRNA without a change in intracellular mRNA levels, together with the demonstration that IFE-4 is found on 48S initiation complexes, strongly suggests that it is involved in translation initiation. Interestingly, IFE-1 is not found on 48S initiation complexes, which correlates with the fact that it is found in ribonucleoprotein particles (P granules) in the germ line and may be involved in sequestration of developmentally controlled mRNAs (1). Furthermore, some of the mRNAs affected by IFE-4 loss exhibit a developmental profile of expression that correlates with the expression pattern of ife-4.
However, tissue distribution and developmental stage alone are not sufficient to explain the extremely small number of mRNAs affected exclusively at the translational level by loss of IFE-4 (33 out of 18,976 or ∼0.2% of all mRNAs), given that thousands of mRNAs are being translated in any cell type at any given developmental stage. Further studies are needed to determine the mechanism by which IFE-4 affects such a restricted subset of mRNAs. To date, we have been unable to identify unique features in mRNAs affected by ife-4 loss. It is unlikely that RNA sequence elements are recognized by IFE-4 itself, since tertiary structures of eIF4E suggest molecular contacts with the cap and only the first one or two nucleotide residues of the mRNA (30, 31, 57). Rather, there may be sequence-specific RNA-binding proteins that are uniquely recruited to IFE-4-containing initiation complexes.
Acknowledgments
This work was supported by grant GM20818 from the National Institutes of Health.
We thank the C. elegans Gene Knockout Consortium for providing the ife-4 mutant isolate, Adam Antebi for the daf-12A::GFP strain, Piali Sengupta for the kin-29::GFP strain, Martin Chalfie for the mec-4::GFP strain, and Chris Link for the hsp-16.2:GFP strain. We thank Paula Polk for excellent technical assistance, Kelly Tatchell for the use of his fluorescence microscope, and the LSUHSC-S Research Core Facility for subsidy of PCR and array experiments. We also acknowledge the Feist-Weiller Cancer Institute for support in Affymetrix GeneChip purchase. We are particularly grateful to Marjan Trutschl (Louisiana State University, Shreveport) and Tom Blumenthal (University of Colorado) for helpful discussions.
REFERENCES
- 1.Amiri, A., B. D. Keiper, I. Kawasaki, Y. Fan, Y. Kohara, R. E. Rhoads, and S. Strome. 2001. An isoform of eIF4E is a component of germ granules and is required for spermatogenesis in C. elegans. Development 128:3899-3912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Antebi, A., W.-H. Yeh, D. Tait, E. M. Hedgecock, and D. L. Riddle. 2000. daf-12 encodes a nuclear receptor that regulates the dauer diapause and developmental age in C elegans. Genes Dev. 14:1512-1527. [PMC free article] [PubMed] [Google Scholar]
- 3.Bradley, C. A., J. C. Padovan, T. L. Thompson, C. A. Benoit, B. T. Chait, and R. E. Rhoads. 2002. Mass spectrometric analysis of the N-terminus of translational initiation factor eIF4G-1 reveals novel isoforms. J. Biol. Chem. 277:12559-12571. [DOI] [PubMed] [Google Scholar]
- 4.Brenner, S. 1974. The genetics of Caenorhabditis elegans. Genetics 77:71-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Browning, K. S., C. Webster, J. K. M. Roberts, and J. M. Ravel. 1992. Identification of an isozyme form of protein synthesis initiation factor 4F in plants. J. Biol. Chem. 267:10096-10100. [PubMed] [Google Scholar]
- 6.Cohen, N., M. Sharma, A. Kentsis, J. M. Perez, S. Strudwick, and K. L. B. Borden. 2001. PML RING suppresses oncogenic transformation by reducing the affinity of eIF4E for mRNA. EMBO J. 20:4547-4559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.De Benedetti, A., and J. R. Graff. 2004. eIF-4E expression and its role in malignancies and metastases. Oncogene 23:3189-3199. [DOI] [PubMed] [Google Scholar]
- 8.Gingras, A.-C., B. Raught, and N. Sonenberg. 1999. eIF4 initiation factors: effectors of mRNA recruitment to ribosomes and regulators of translation. Annu. Rev. Biochem. 68:913-963. [DOI] [PubMed] [Google Scholar]
- 9.Goodman, S. J., C. S. Branda, M. K. Robinson, R. D. Burdine, and M. J. Stern. 2003. Alternative splicing affecting a novel domain in the C elegans EGL-15 FGF receptor confers functional specificity. Development 130:3757-3766. [DOI] [PubMed] [Google Scholar]
- 10.Graff, J. R., E. R. Boghaert, A. De Benedetti, S. K. Chan, and S. G. Zimmer. 1995. Reduction of the levels of initiation factor 4E mediates the malignant phenotype of ras-transformed rat fibroblasts. Int. J. Cancer 60:255-263. [DOI] [PubMed] [Google Scholar]
- 11.Herbert, T. P., R. Fåhraeus, A. R. Prescott, D. P. Lane, and C. G. Proud. 2000. Rapid induction of apoptosis by peptides that bind initiation factor eIF4E. Curr. Biol. 10:793-796. [DOI] [PubMed] [Google Scholar]
- 12.Hershey, J. W. B., and W. C. Merrick. 2000. Pathway and mechanism of initiation of protein synthesis, p. 33-88. In N. Sonenberg, J. W. B. Hershey, and M. B. Mathews (ed.), Translational control of gene expression. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 13.Hiremath, L. S., S. T. Hiremath, W. Rychlik, S. Joshi, L. L. Domier, and R. E. Rhoads. 1989. In vitro synthesis, phosphorylation, and localization on 48S initiation complexes of human protein synthesis initiation factor 4E. J. Biol. Chem. 264:1132-1138. [PubMed] [Google Scholar]
- 14.Hiremath, L. S., N. R. Webb, and R. E. Rhoads. 1985. Immunological detection of the messenger RNA cap-binding protein. J. Biol. Chem. 260:7843-7849. [PubMed] [Google Scholar]
- 15.Hobert, O. 2002. PCR fusion-based approach to create reporter gene constructs for expression analysis in transgenic C. elegans. BioTechniques 32:728-730. [DOI] [PubMed] [Google Scholar]
- 16.Horovitz, H. R., M. Chalfie, C. Trent, J. Sulston, and P. Evans. 1982. Serotonin and octopamine in the nematode Caenorhabditis elegans. Science 216:1012-1014. [DOI] [PubMed] [Google Scholar]
- 17.Jankowska-Anyszka, M., B. J. Lamphear, E. J. Aamodt, T. Harrington, E. Darzynkiewicz, R. Stolarski, and R. E. Rhoads. 1998. Multiple isoforms of eukaryotic protein synthesis initiation factor 4E in C. elegans can distinguish between mono- and trimethylated mRNA cap structures. J. Biol. Chem. 273:10538-10542. [DOI] [PubMed] [Google Scholar]
- 18.Johns, P., and D. R. Stanworth. 1976. A simple numerical method for the construction of isokinetic sucrose density gradients, and their application to the characterization of immunoglobulin complexes. J. Immunol. Methods 10:231-252. [DOI] [PubMed] [Google Scholar]
- 19.Jones, R. M., J. Branda, K. A. Johnston, M. Polymenis, M. Gadd, A. Rustgi, L. Callanan, and E. V. Schmidt. 1996. An essential E box in the promoter of the gene encoding the mRNA cap-binding protein (eukaryotic initiation factor 4E) is a target for activation by c-myc. Mol. Cell. Biol. 16:4754-4764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Joshi, B., A. Cameron, and R. Jagus. 2004. Characterization of mammalian eIF4E members. Eur. J. Biochem. 271:2189-2203. [DOI] [PubMed] [Google Scholar]
- 21.Kass, J., T. C. Jacob, P. Kim, and J. M. Kaplan. 2001. The EGL-3 proprotein convertase regulates mechanosensory responses of Caenorhabditis elegans. J. Neurosci. 21:9265-9272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Keiper, B. D., B. J. Lamphear, A. M. Deshpande, M. Jankowska-Anyszka, E. J. Aamodt, T. Blumenthal, and R. E. Rhoads. 2000. Functional characterization of five eIF4E isoforms in Caenorhabditis elegans. J. Biol. Chem. 275:10590-10596. [DOI] [PubMed] [Google Scholar]
- 23.Kuersten, S., and E. B. Goodwin. 2003. The power of the 3′UTR: translational control and development. Nat. Genet. 4:626-637. [DOI] [PubMed] [Google Scholar]
- 24.Lachance, P. E. D., M. Miron, B. Raught, N. Sonenberg, and P. Lasko. 2002. Phosphorylation of eukaryotic translation initiation factor 4E is critical for growth. Mol. Cell. Biol. 22:1656-1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lanjuin, A., and P. Sengupta. 2002. Regulation of chemosensory receptor expression and sensory signaling by the KIN-29 Ser/Thr kinase. Neuron 33:369-381. [DOI] [PubMed] [Google Scholar]
- 26.Lin, T., X. Kong, T. A. J. Haystead, A. Pause, G. Belsham, N. Sonenberg, and J. C. Lawrence. 1994. PHAS-I as a link between mitogen-activated protein kinase and translation initiation. Science 266:653-656. [DOI] [PubMed] [Google Scholar]
- 27.Liou, R. F., and T. Blumenthal. 1990. Trans-spliced Caenorhabditis elegans messenger RNAs retain trimethylguanosine caps. Mol. Cell. Biol. 10:1764-1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lodish, H. F. 1974. Model for the regulation of mRNA translation applied to haemoglobin synthesis. Nature 251:385-388. [DOI] [PubMed] [Google Scholar]
- 29.Marcotrigiano, J., A.-C. Gingras, N. Sonenberg, and S. K. Burley. 1999. Cap-dependent translation initiation in eukaryotes is regulated by a molecular mimic of eIF4G. Mol. Cell 3:707-716. [DOI] [PubMed] [Google Scholar]
- 30.Marcotrigiano, J., A.-C. Gingras, N. Sonenberg, and S. K. Burley. 1997. Cocrystal structure of the messenger RNA 5′ cap-binding protein (eIF4E) bound to 7-methyl-GDP. Cell 89:951-961. [DOI] [PubMed] [Google Scholar]
- 31.Matsuo, H., H. Li, A. M. McGuire, C. M. Fletcher, A.-C. Gingras, N. Sonenberg, and G. Wagner. 1997. Structure of translation factor eIF4E bound to m7GDP and interaction with 4E-binding protein. Nat. Struct. Biol. 4:717-724. [DOI] [PubMed] [Google Scholar]
- 32.Mello, C., and A. Fire. 1995. DNA transformation, p. 451-482. In D. Shakes and H. Epstein (ed.), Methods in cell biology, vol. 48. Academic Press, New York, N.Y. [PubMed]
- 33.Nelson, M. R., A. M. Leidal, and C. A. Smibert. 2004. Drosophila Cup is a eIF4E-binding protein that functions in Smaug-mediated translational repression. EMBO J. 23:150-159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Niedzwiecka, A., J. Marcotrigiano, J. Stepinski, M. Jankowska-Anyszka, A. Wyslouch-Cieszynska, M. Dadlez, A.-C. Gingras, P. Mak, E. Darzynkiewicz, N. Sonenberg, S. K. Burley, and R. Stolarski. 2002. Biophysical studies of eIF4E cap-binding protein: recognition of mRNA 5′ cap structure and synthetic fragments of eIF4G and 4E-BP1 proteins. J. Mol. Biol. 319:615-635. [DOI] [PubMed] [Google Scholar]
- 35.Pause, A., G. J. Belsham, A. Gingras, O. Donze, T. Lin, J. C. Lawrence, and N. Sonenberg. 1994. Insulin-dependent stimulation of protein synthesis by phosphorylation of a regulator of 5′-cap function. Nature 371:762-767. [DOI] [PubMed] [Google Scholar]
- 36.Rau, M., T. Ohlmann, S. J. Morley, and V. M. Pain. 1996. A reevaluation of the cap-binding protein, eIF4E, as a rate-limiting factor for initiation of translation in reticulocyte lysate. J. Biol. Chem. 271:8983-8990. [DOI] [PubMed] [Google Scholar]
- 37.Reinke, V. 2002. Functional exploration of the C. elegans genome using DNA microarrays. Nat. Genet. 32:541-546. [DOI] [PubMed] [Google Scholar]
- 38.Reinke, V., H. E. Smith, J. Nance, J. Wang, C. Van Doren, R. Begley, S. J. Jones, E. B. Davis, S. Scherer, S. Ward, and S. K. Kim. 2000. A global profile of germline gene expression in C. elegans. Mol. Cell 6:605-616. [DOI] [PubMed] [Google Scholar]
- 39.Rhoads, R. E. 1999. Minireview: Signal transduction pathways that regulate eukaryotic protein synthesis. J. Biol. Chem. 274:30337-30340. [DOI] [PubMed] [Google Scholar]
- 40.Rhoads, R. E. 1993. Regulation of eukaryotic protein synthesis by initiation factors. J. Biol. Chem. 268:3017-3020. [PubMed] [Google Scholar]
- 41.Rinker-Schaeffer, C. W., J. R. Graff, A. De Benedetti, S. G. Zimmer, and R. E. Rhoads. 1993. Decreasing the level of translation initiation factor 4E with antisense RNA causes reversal of ras-mediated transformation and tumorigenesis of cloned rat embryo fibroblasts. Int. J. Cancer 55:841-847. [DOI] [PubMed] [Google Scholar]
- 42.Robalino, J., B. Joshi, S. C. Fahrenkrug, and R. Jagus. 2004. Two zebrafish eIF4E family members are differentially expressed and functionally divergent. J. Biol. Chem. 279:10532-10541. [DOI] [PubMed] [Google Scholar]
- 43.Rodriguez, C., M. Freire, C. Camilleri, and C. Robaglia. 1998. The Arabidopsis thaliana cDNAs coding for eIF4E and eIF(iso)4E are not functionally equivalent for yeast complementation and are differentially expressed during plant development. Plant J. 13:465-473. [DOI] [PubMed] [Google Scholar]
- 44.Rom, E., H. C. Kim, A.-C. Gingras, J. Marcotrigiano, D. Favre, H. Olsen, S. K. Burley, and N. Sonenberg. 1998. Cloning and characterization of 4EHP, a novel mammalian eIF4E-related cap-binding protein. J. Biol. Chem. 273:13104-13109. [DOI] [PubMed] [Google Scholar]
- 45.Rousseau, D., A.-C. Gingras, A. Pause, and N. Sonenberg. 1996. The eIF4E-binding proteins 1 and 2 are negative regulators of cell growth. Oncogene 13:2415-2420. [PubMed] [Google Scholar]
- 46.Rousseau, D., R. Kaspar, I. B. Rosenwald, L. Gehrke, and N. Sonenberg. 1996. Translation initiation of ornithine decarboxylase and nucleocytoplasmic transport of cyclin D1 mRNA are increased in cells overexpressing eukaryotic initiation factor 4E. Proc. Natl. Acad. Sci. USA 93:1065-1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ruud, K. A., C. Kuhlow, D. J. Goss, and K. S. Browning. 1998. Identification and characterization of a novel cap-binding protein from Arabidopsis thaliana. J. Biol. Chem. 273:10325-10330. [DOI] [PubMed] [Google Scholar]
- 48.Safer, B., W. Kemper, and R. Jagus. 1978. Identification of a 48 S preinitiation complex in reticulocyte lysate. J. Biol. Chem. 253:3384-3386. [PubMed] [Google Scholar]
- 49.Stachelska, A., Z. Wieczorek, K. Ruszczynska, R. Stolarski, M. Pietrzak, B. J. Lamphear, R. E. Rhoads, E. Darzynkiewicz, and M. Jankowska-Anyszka. 2002. Interaction of three Caenorhabditis elegans isoforms of translation initiation factor eIF4E with mono- and trimethylated mRNA 5′ cap analogues. Acta Biochim. Pol. 49:671-682. [PubMed] [Google Scholar]
- 50.Stebbins-Boaz, B., Q. Cao, C. H. de Moor, R. Mendez, and J. D. Richter. 1999. Maskin is a CPEB-associated factor that transiently interacts with eIF4E. Mol. Cell 4:1017-1027. [DOI] [PubMed] [Google Scholar]
- 51.Sze, J. Y., M. Victor, C. Loer, Y. Shi, and G. Ruvkun. 2000. Food and metabolic signalling defects in a Caenorhabditis elegans serotonin-synthesis mutant. Nature 403:560-564. [DOI] [PubMed] [Google Scholar]
- 52.Szewczyk, N. J., and L. A. Jacobson. 2003. Activated EGL-15 FGF receptor promotes protein degradation in muscles of Caenorhabditis elegans. EMBO J. 22:5058-5067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tee, A. R., J. A. Tee, and J. Blenis. 2004. Characterizing the interaction of the mammalian eIF4E-related protein 4EHP with 4E-BP1. FEBS Lett. 564:58-62. [DOI] [PubMed] [Google Scholar]
- 54.Thatcher, J. D., A. P. Fernandez, L. Beaster-Jones, C. Haun, and P. G. Okkema. 2001. The Caenorhabditis elegans peb-1 gene encodes a novel DNA-binding protein involved in morphogenesis of the pharynx, vulva, and hindgut. Dev. Biol. 229:480-493. [DOI] [PubMed] [Google Scholar]
- 55.Timmons, L., D. L. Court, and A. Fire. 2001. Ingestion of bacterially expressed dsRNAs can produce specific and potent genetic interference in Caenorhabditis elegans. Gene 263:103-112. [DOI] [PubMed] [Google Scholar]
- 56.Timmons, L., and A. Fire. 1998. Specific interference by ingested dsRNA. Nature 395:854. [DOI] [PubMed] [Google Scholar]
- 57.Tomoo, K., X. Shen, K. Okabe, Y. Nozoe, S. Fukuhara, S. Morino, T. Ishida, T. Taniguchi, H. Hasegawa, A. Terashima, M. Sasaki, Y. Katsuya, K. Kitamura, H. Miyoshi, M. Ischikawa, and K. Miura. 2002. Crystal structure of 7-methylguanosine 5′-triphosphate (m7GTP)- and P1-7-methylguanosine-P3-adenosine-5′,5′-triphosphate (m7GpppA)-bound human full-length eukaryotic initiation factor 4E: biological importance of the C-terminal flexible region. Biochem. J. 362:539-544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Trent, C., N. Tsung, and H. R. Horovitz. 1983. Egg-laying defective mutants of the nematode Caenorhabditis elegans. Genetics 104:619-647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Waggoner, L. E., G. T. Zhou, R. W. Schafer, and W. R. Schafer. 1998. Control of alternative behavioral states by serotonin in Caenorhabditis elegans. Neuron 21:203-214. [DOI] [PubMed] [Google Scholar]
- 60.Weinshenker, D., G. Garriga, and J. H. Thomas. 1995. Genetic and pharmacological analysis of neurotransmitters controlling egg laying in C. elegans. J. Neurosci. 15:6975-6985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wu, J. G., A. Duggan, and M. Chalfie. 2001. Inhibition of touch cell fate by egl-44 and egl-46 in C elegans. Genes Dev. 15:789-802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhang, Y., C. Ma, T. Delohery, B. Nasipak, B. C. Foat, A. Bounoutas, H. J. Bussemaker, S. K. Kim, and M. Chalfie. 2002. Identification of genes expressed in C elegans touch receptor neurons. Nature 418:331-335. [DOI] [PubMed] [Google Scholar]
- 63.Zong, Q., M. Schummer, L. Hood, and D. R. Morris. 1999. Messenger RNA translation state: the second dimension of high-throughput expression screening. Proc. Natl. Acad. Sci. USA 96:10632-10636. [DOI] [PMC free article] [PubMed] [Google Scholar]