Abstract
Our studies examining the role of the cell cycle-regulated kinase cyclin A/Cdk2 in progesterone receptor (PR) action have demonstrated that cyclin-dependent kinase activity is required for PR function and that cyclin A/Cdk2 functions as a PR coactivator. Although Cdk2 can phosphorylate PR, elimination of these phosphorylation sites has little effect on the ability of cyclin A/Cdk2 to stimulate PR activity. PR interacts with cyclin A and recruits cyclin A/Cdk2 to progestin-responsive promoters, stimulating transcription. Inhibition of Cdk2 activity abolishes progesterone-dependent activation of PR target genes in part through inhibition of PR-dependent recruitment of steroid receptor coactivator 1 (SRC-1) and subsequent histone H4 acetylation at the target promoter. In vitro studies revealed that the interaction between SRC-1 and PR is dependent upon phosphorylation of SRC-1. This heretofore-unknown mechanism provides a potential means for integrating the regulation of PR activity with cell cycle progression. Moreover, the ability of PR to recruit cyclin A/Cdk2 to target promoters provides locally elevated levels of kinase, which can preferentially facilitate phosphorylation-dependent interactions and enzymatic activities of coactivators at the target promoter.
Numerous studies have implicated cell signaling pathways in modulating the activities of steroid receptors, including the progesterone receptor (PR) (5, 6, 26, 47, 48). Steroid receptors are ligand-activated transcription factors which bind to specific DNA sequences in the promoter region of target genes and recruit coactivators that alter chromatin structure, leading to activation of transcription (30). Human PR is expressed as two isoforms derived from the same gene: PR-B contains 933 amino acids, and PR-A lacks the first 164 amino acids of PR-B (20). The isoforms have distinct activities, and different subsets of genes are induced by the two isoforms (36). Like other steroid receptor family members, PR is a phosphoprotein (7), and previous reports have demonstrated a modulation of PR activity by altered cell signaling (5, 6). Coactivators are also phosphoproteins, whose activities are regulated by phosphorylation (38, 39, 49). Thus, the activity of PR depends not only on the levels of receptor, hormone, and coactivators, but also on cellular kinase and phosphatase activity.
Most of the phosphorylation sites identified in steroid receptors and in coactivators are found in Ser/Thr-Pro motifs, implicating proline-directed kinases such as the cyclin-dependent kinases (Cdks) or the mitogen-activated protein kinases in steroid receptor phosphorylation (11, 21, 27, 37, 38, 46). Consistent with this, the transcriptional activities of two steroid receptors, the androgen receptor and the glucocorticoid receptor (GR), vary as a function of cell cycle, with the highest activity in the S phase (16, 17, 29). In PR, nine phosphorylation sites containing Ser/Thr-Pro motifs have been identified (21). Because our investigators had found that a subset of in vivo sites in PR are phosphorylated in vitro by cyclin A/Cdk2 (21, 53), a kinase active in S phase, we sought to assess the contribution of this kinase to PR activity. We report here that the activity of Cdk2 is required for PR-mediated gene transcription. Surprisingly, rather than acting by direct phosphorylation of any of the sites in the N-terminal domain of PR, cyclin A/Cdk2 is recruited to target gene promoters by PR, facilitating phosphorylation of associated proteins. Among these is steroid receptor coactivator 1 (SRC-1), whose activity and interaction with PR are regulated by Cdk2. Our data support the conclusion that Cdk2 is an obligate component of the PR-dependent transcription pathway. Furthermore, the recruitment of cyclin A/Cdk2 by PR ensures adequate levels of kinase for activation of SRC-1 and perhaps other PR-associated coregulatory proteins. This is the first example of a kinase recruited by a steroid receptor whose activity is required for transcriptional activation of its target genes.
MATERIALS AND METHODS
Materials.
Cell culture reagents were obtained from Invitrogen (Carlsbad, Calif.). Cyclin A, SRC-1, Myc 9E10, TRAP-220, and Cdk2 antibodies were obtained from Santa Cruz Biotechnology, Inc. (Santa Cruz, Calif.). The acetyl lys-5 antibody was from Upstate Biotechnology (Lake Placid, N.Y.). The actin antibody was obtained from Chemicon (Temecula, Calif.). λ phosphatase was obtained from Promega Corporation (Madison, Wis.). The PR antibody 1294 was described earlier (35). Rabbit anti-mouse immunoglobulin G (IgG) was obtained from Zymed Inc. (San Francisco, Calif.). Oligonucleotide-directed mutagenesis reagents were obtained from Stratagene (La Jolla, Calif.). R1881 (methyltrienolone) and R5020 (promegestone) were obtained from NEN Life Science Products (Boston, Mass.). Calcitriol [1,25(OH)2D3] was obtained from Solvay DuPhar, Weesp, The Netherlands. Protein A-Sepharose CL4-B and glutathione-Sepharose beads were obtained from Amersham Pharmacia Biotech (Piscataway, N.J.). Roscovitine was obtained from Calbiochem (La Jolla, Calif.). Purified glutathione S-transferase (GST) protein was obtained from Upstate Biotechnology. All other reagents were analytical grade.
Cell culture.
HeLa, T47D, and COS-1 cells were purchased from the American Type Culture Collection (Manassas, Va.). T47D cells stably transfected with mouse mammary tumor virus (MMTV) chloramphenicol acetyltransferase (CAT) (Cat0 cells) were a kind gift from Andrew Cato (9) and have been used previously to study PR action in chromatin immunoprecipitation (ChIP) assays (28). Cat0 cells were maintained in Dulbecco's modified Eagle medium (DME) plus 10% serum with 200 μg of G418/ml. HeLa and COS cells were maintained in DME plus 10% serum with penicillin-streptomycin. T47D cells were maintained in Roswell Park Memorial Institute medium (RPMI) plus 10% serum with penicillin-streptomycin. HeLa and COS cells were plated in DME plus 10% charcoal-stripped serum with penicillin and streptomycin at 125,000 cells per well in six-well plates and at 1 million cells per dish in 10-cm dishes. T47D cells were plated in RPMI plus 10% charcoal-stripped serum with penicillin and streptomycin at 2.3 million cells per 10-cm dish and at 300,000 cells per well in six-well plates. Cat0 cells were plated in DME plus 10% charcoal-stripped serum with 200 μg of G418/ml at the same density as T47D cells. All cells were maintained at 37°C with 5% CO2 in a tissue culture incubator.
Plasmids and plasmid construction.
Cyclin A, Cdk2, and the empty vector pCDLSRα296 were kind gifts from Michael Garabedian (44). Cyclin A tagged at the N terminus with a Myc epitope and its vector pCMV were also obtained from M. Garabedian (37). Lamin and Cdk2 small interfering RNA (siRNA) plasmids were gifts from Yang Shi (42). The constitutively active luciferase plasmid (cytomegalovirus [CMV] LUC) was a gift from Carolyn Smith, Baylor College of Medicine. The constitutively active Gal-VP16 plasmid expressing both the Gal4 DNA binding domain and the VP16 activation domain was a kind gift from Jiemin Wong, Baylor College of Medicine. Expression and reporter plasmids including pLEN PR-B (45), pLEN PR-A (45), androgen receptor (pCR3.1 AR) (1), pCR3.1 PR-B (1), pCR3.1 SRC-1 (38), VDRE-tk-LUC (a vitamin D receptor [VDR]-responsive promoter) (54), GRE2-E1b-LUC (a PR- and AR-responsive reporter) (32), MMTV LUC (3), pCMV-βGal (4), 17mer luc, pBind SRC-1, pBind CREB binding protein (CBP), pACT SRC-1 (38), the truncated C D/E PR construct (amino acids 545 to 933), pACT N terminus PR-B, pBind PR ligand binding domain (LBD) (43), baculovirus-purified His tag PR-B (21), and baculovirus-expressed SRC-1 (39) have been described previously. The expression vectors for baculovirus-expressed GST-fusion cyclin A and Cdk2 were a gift from Wade Harper (15).
Cdk2 binding mutants of cyclin A and myc-tagged cyclin A were made by mutating arginine 211 (23) to alanine using oligonucleotide-directed mutagenesis reagents. pCR3.1 PR-A was made by subcloning the PR-A fragment digested with BamHI from the pLEN vector backbone into the pCR3.1 vector. The truncated PR-B construct, A/BCD, which contains amino acids 1 to 684, was made by cutting the pCR3.1 vector backbone with NhEI and BamHI and the pCR3.1 PR-B construct with NhEI and BclI and cloning the NhEI/BclI fragment of PR-B into the NhEI/BamH1-cleaved pCR3.1 backbone. Mutations of eight serines and two threonines (Ser190, Ser202, Ser213, Ser294, Ser345, Thr351, Ser400, Thr430, Ser554, and Ser676) to alanines (10 Ala) in PR-A were prepared by oligonucleotide-directed mutagenesis. All of the mutants were sequenced to confirm the incorporation of the mutations at the respective sites.
Transient transfection.
Transient transfections were performed as described earlier using lysine-coupled inactivated adenovirus as noncovalent carriers of the plasmids (33). Twenty-four hours posttransfection, the cells were treated with hormone for an additional 24 h. Due to the growth-inhibitory properties of roscovitine, in experiments utilizing roscovitine the cells were treated with roscovitine for 30 min and then treated with R5020 for only 4 to 6 h prior to harvest. For the CMV LUC study, roscovitine was added to the cells 2 h after the initial transfection and treatment was for 16 h. For immunoprecipitations, the cells were treated with hormone for 60 min at 24 h posttransfection, washed with ice-cold phosphate-buffered saline (PBS), and scraped, and lysates were prepared.
Plasmids for siRNA were transfected into HeLa cells as described above except that the transfection was carried out for 72 h for maximal inhibition of Cdk2 expression. Hormones were added for the final 24 h, cells were harvested, and luciferase assays were performed and normalized to β-galactosidase (β-Gal) activity. The extent of inhibition of Cdk2 expression was determined by Western blotting.
Mammalian two-hybrid interaction assays.
HeLa cells were cotransfected with 0.25 μg of 17-mer LUC and the indicated Gal and VP16 fusion protein expression vectors as described in the figure legends by the adenovirus-mediated method described above. The pBind vector, which contains the Gal4 DNA binding domain, was used to make the Gal4 DNA binding domain fusion proteins of SRC-1 and CBP (38). The cells were treated or not 24 h after transfection, and luciferase assays were performed and normalized to β-Gal activity.
Reporter gene analysis.
The cells were harvested by incubating the cells in TEN (0.15 M NaCl, 0.01 M EDTA, 0.04 M Tris; pH. 8.0) at room temperature for 30 min and pelleting, and protein was extracted with 1× reporter lysis buffer (Promega) containing 0.4 M NaCl for 30 min at room temperature. Luciferase assays were performed using the luciferase assay reagent from Promega Inc. with a Monolight 2010 luminometer (Analytical Luminescence Lab, Ann Arbor, Mich.), and activity was normalized to β-Gal levels. In some experiments, the luciferase levels were normalized to receptor levels measured by Western analysis. Briefly, after the luciferase assay was performed, protein levels were determined in a Bradford assay (Bio-Rad, Hercules, Calif.), and equal amounts of protein were used for Western analysis. The luciferase levels were normalized to receptor levels determined by densitometric analysis of the autoradiogram. The CAT and the β-Gal assays were performed as described earlier (52) using equal amounts of protein.
Immunoprecipitation.
Cell extracts were prepared in homogenization buffer (0.05 M potassium phosphate, 10 mM sodium molybdate, 50 mM sodium fluoride, 2 mM EDTA, 2 mM EGTA, and 0.05% monothioglycerol [pH 7.4] containing 0.4 M NaCl and protease inhibitors [1 mg each of aprotinin, leupeptin, antipain, benzamidine HCl, and pepstatin/ml], 0.2 mM phenylmethylsulfonyl fluoride, and 1 mM sodium vanadate) by three freeze-thaw cycles. Immunoprecipitation was carried out as follows. Briefly, 100 μl of a 1:1 slurry of protein A-Sepharose beads in 1× TE (0.01 M Tris and 0.001 M EDTA) was incubated for 3 h at 4°C with 5 μg of rabbit anti-mouse IgG (for mouse monoclonal primary antibodies). The beads were washed with 1× TE, incubated overnight at 4°C with the primary antibody, washed with 1× TE, and incubated overnight at 4°C with 100 μg of protein extract in 400 μl of the lysis buffer without salt. The beads were then washed for 5 min once each with high-salt buffer (0.1% sodium dodecyl sulfate [SDS], 1% Triton X-100, 2 mM EDTA, 20 mM Tris HCl [pH 8.1], 0.5 M NaCl), low-salt buffer (same as high-salt wash buffer but with 0.15 M NaCl), and 1× TE (10 mM Tris HCl, 1 mM EDTA; pH 8.0). The immunoprecipitated proteins were extracted with 2× Laemmli buffer, separated on an SDS-polyacrylamide gel electrophoresis (SDS-PAGE) gel, and detected by Western blotting.
Western analysis.
For cyclin A and Cdk2 Western analyses, the membranes were blocked with 1% milk in 1× TBST (Tris-buffered saline [TBS] with 10 mM Tris HCl plus 150 mM NaCl plus 0.1% Tween 20; pH 7.5), and Cdk2 Western blot assay mixtures were then incubated with primary antibody (1 μg of Cdk2 antibody/ml) for 2 h in 1% milk in TBST, secondary antibody for 2 h in 1% bovine serum albumin (BSA) in TBST, and horseradish peroxidase-tagged tertiary antibody in TBST for 1 h. For cyclin A Western assays, the membrane was incubated with primary antibody in 1% milk (1 μg of antibody/ml) for 2 h and then with anti-rabbit horseradish peroxidase-tagged antibody in TBST for 1 h. For PR detection, the proteins on the membranes were denatured with 4 M urea for 3 h, washed with TBST, and incubated with 1294 PR antibody (0.5 μg of antibody/ml) in 1% BSA in TBST for 2 h, secondary antibody in 1% BSA for 2 h, and tertiary antibody in TBST for 1 h. All incubations were performed at room temperature. SRC-1 was detected by blocking the blots in 5% milk in TBST overnight at 4°C followed by 1 h of incubation each with the primary (5 μg/ml), secondary, and tertiary antibodies at room temperature. Primary and secondary antibodies were diluted in 1% milk, and tertiary antibody was diluted in TBST. The blots were washed three times with 1× TBS plus 0.1% Tween 20 between antibody incubations and at the end washed once with TBS plus 0.3% Tween 20. All signals were detected by enhanced chemiluminescence (Amersham Pharmacia Biotech).
Purification of GST-cyclin A.
GST-cyclin A was purified from Sf9 cells infected with a baculovirus encoding GST-cyclin A as described earlier (14).
In vitro translation and GST pull-down assay.
pCR3.1 PR-B was in vitro translated according to the manufacturer's protocol using a T7 TNT in vitro translation kit from Promega with [35S]Redivue promix from Amersham Pharmacia. A 1:1 slurry of GST beads in NETN buffer (20 mM Tris [pH 8.0], 50 mM NaCl, 1 mM EDTA [pH 8.0], 0.5% NP-40) was incubated at 4°C with purified GST protein or 1 μg of GST-cyclin A for 3 h. The beads were washed with NETN buffer and then incubated at 4°C overnight with the in vitro-translated PR-B. The beads were washed with NETN buffer, and the complex was extracted with 2× Laemmli buffer and separated on an SDS-6.5% PAGE gel. The proteins were transferred to nitrocellulose and then detected by autoradiography.
In vitro phosphorylation-kinase assay.
In vitro phosphorylation of SRC-1 was performed using baculovirus to express SRC-1 in Sf9 cells as described earlier (39). The Sf9 cell pellets were Dounce homogenized 10 times in a buffer (10 mM Tris [pH 8], 250 mM NaCl, 1 mM EDTA, 1 mM EGTA, 5 mM monothioglycerol, and protease inhibitors [as described for immunoprecipitations]) on ice and purified by immunoprecipitation using an SRC-1 antibody or an AR antibody as control. The SRC-1-bound protein A-Sepharose beads were incubated with Cdk2 kinase reaction buffer (20 mM Tris-HCl [pH 7.5],10 mM MgCl2) and 5 μl of cyclin A/Cdk2 with a final specific activity of 12 μM [γ-32P]ATP of 33,000 dpm of ATP (ICN Biomedicals, Irvine, Calif.)/pmol, in a final reaction volume of 40 μl. The reaction was incubated for 30 min at 30°C and terminated by addition of 4× Laemmli sample buffer followed by electrophoresis on an SDS-6.5% PAGE gel. The phosphorylated SRC-1 was detected by autoradiography.
Lambda phosphatase experiment and in vitro interaction assay.
pCR3.1 SRC-1 was in vitro translated according to the manufacturer's protocol using a T7 TNT in vitro translation kit from Promega with [35S]Redivue promix from Amersham Pharmacia. To dephosphorylate SRC-1, the samples were treated with 600 U of λ phosphatase at 30°C for 30 min, and the reaction was stopped by adding 10 mM sodium vanadate. Controls included incubation of SRC-1 without phosphatase and addition of vanadate prior to incubation with the phosphatase. For the rephosphorylation studies, equal amounts of SRC-1 treated as described above were incubated in the presence or absence of cyclin A/Cdk2 as described for the in vitro phosphorylation assay using radio-inert ATP. For the subsequent interaction studies between SRC-1 and PR, protein A-Sepharose beads were first incubated with 5 μg of rabbit anti-mouse IgG per reaction mixture for 2 h and then with the anti-PR antibody for 6 h. The beads were then incubated with 1 μg of baculovirus-expressed and -purified PR-B overnight. As a control for nonspecific binding of SRC-1, one sample lacked PR-B. The beads were washed with 1× TE and incubated with the treated SRC-1 samples for 6 h at 4°C. The beads were washed, and protein was extracted with 2× Laemmli buffer, run on an SDS-PAGE, transferred to nitrocellulose, and detected by autoradiography.
ChIP assay.
ChIP assays were performed as described earlier (25). Cells plated at 10 million cells per 150-mm dish and maintained in charcoal-stripped serum for 3 days were treated for the indicated times with 10 nM R5020. For roscovitine experiments, the cells were pretreated for 3 h with 30 μM roscovitine prior to R5020 addition. The proteins were cross-linked by incubation with 1% formaldehyde (final concentration) at 37°C for 10 min. The cells were washed with 1× PBS twice, scraped in 1 ml of PBS containing protease inhibitors (described above), pelleted, and resuspended in SDS lysis buffer (1% SDS, 10 mM EDTA, 50 mM Tris-HCl [pH 8.1]). After lysis on ice for 10 min, the cell extract was sonicated (Branson sonifier 250; VWR Scientific, West Chester, Pa.) in a cold room six to eight times for 10 s each at constant duty cycle, with an output of 3 and with incubation on ice after every sonication. The debris was pelleted at 13,000 rpm for 10 min at 4°C, and the supernatant was diluted 10-fold with ChIP dilution buffer (0.01% SDS, 1.1% Triton X-100, 1.2 mM EDTA, 16.7 mM Tris HCl [pH 8.1], 167 mM NaCl). The proteins were precleared with 50 μl of 1:1 protein A-Sepharose beads in TE, 200 μl was reserved as input, and the remaining 800 μl was incubated with 5 μg of antibody and 2 μg of sheared salmon sperm DNA (Stratagene) overnight at 4°C. The protein-DNA-antibody complex (800 μl) was precipitated by incubating with 100 μl of 1:1 protein A-Sepharose beads and 2 μg of salmon sperm DNA at 4°C for 2 h. The beads were pelleted and washed once each with high-salt wash buffer, low-salt wash buffer, and 1× TE. The DNA-protein complex was obtained by extracting the beads with 50 μl of extraction buffer (1% SDS, 0.1 M NaHCO3) three times. The cross-linking of the DNA protein complex was reversed by incubating at 65°C for 6 h to overnight. The DNA was extracted with a QIAquick PCR purification kit (QIAGEN, Valencia, Calif.).
Real-time PCR.
Real-time PCR was performed with an ABI Prism 7700 sequence detector (Applied Biosystems, Foster city, Calif.) using SYBR green dye (Sigma, St. Louis, Mo.) and Taq platinum polymerase (Invitrogen). The primers used (MMTV forward primer, TAT GGT TAC AAA CTG TTC TTA AAA CGA GGA TG, and reverse primer, GCA AGT TTA CTC AAA AAA TCA GCA CTC TTT, with an annealing temperature of 62°C; CAT forward primer, GTG AGC TGG TGA TAT GGG ATA GTG TT, and reverse primer, CAT ATT GGC CAC GTT TAA ATC AAA A, with an annealing temperature of 62°C) were chosen based on a previous report (28) and synthesized by Biosource International (Camarillo, Calif.). The numbers on the y axis of the ChIP assay results were obtained by dividing the arbitrary quantitative PCR numbers obtained for each sample by the input, setting the value at T = 0 (or no hormone) as one and determining the fold change in binding relative to the 0-min time point.
Real-time reverse transcriptase PCR analysis.
Total RNA was isolated from the cells using TRIzol reagent (Invitrogen). Three microliters of the diluted RNA (1:100 for MT2A and 1:1,500 for 18S) were analyzed using real-time PCR (ABI Prism 7700 sequence detector; Applied Biosystems) with TaqMan primers and probe for the metallothionein IIA (MT2A) gene (forward primer, GGC GTC GGA CAA GTG CAG; reverse primer, TTG TGG AAG TCG CGT TCT TTA C; carboxyfluorescein probe, CTG GGA CAG CCC CGC TCC C-tetramethyl carboxyrhodamine; from Biosource International) and with a Taqman primer probe set for 18S rRNA from Applied Biosystems. The reactions were carried out under universal conditions using the one-step rtPCR reaction reagent (Applied Biosystems).
Statistics.
All of the experiments were performed at least three times. The ChIP assay results are averages of a minimum of three separate experiments, whereas the reporter gene assays are representative experiments with each variable performed in triplicate. Metallothionein gene transcription was statistically analyzed by one-way analysis of variance with a Holm-Sidak post hoc test. Statistical analysis was performed using SigmaStat software, and significance was accepted at a P value of <0.05.
RESULTS
Cyclin A stimulates PR and AR but not VDR activity.
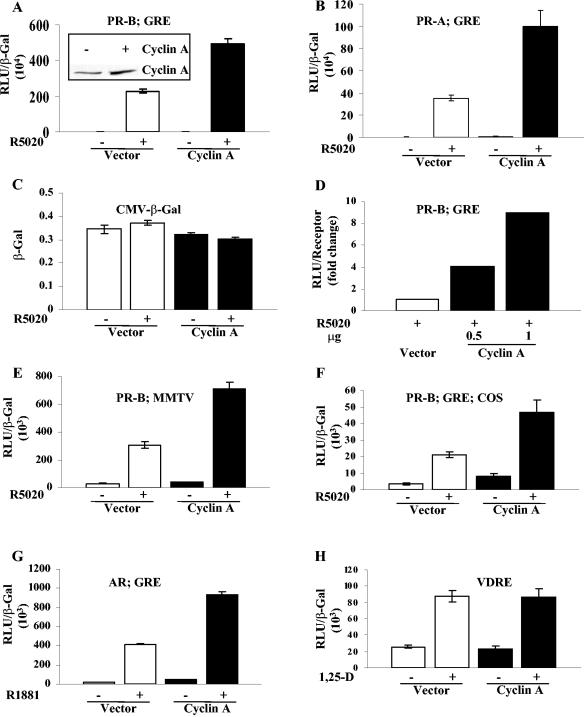
Overexpression of cyclin A in HeLa cells markedly enhanced the hormone-dependent activity of exogenous PR-B or PR-A when measured using the progesterone-responsive luciferase reporter (GRE2-E1b-LUC) (Fig. 1A and B, respectively), while the control β-Gal activity (CMV β-Gal) was unchanged (Fig. 1C). Comparison of the cyclin A expression in vector-transfected and cyclin A-transfected samples showed a two- to threefold increase in the overall expression in cells which transfected with about a 35% transfection efficiency (shown as an inset in Fig. 1A). The stimulation of PR-B activity was not due to increased expression of PR; normalization of activity to PR levels gave comparable stimulation to that obtained by normalizing to β-Gal activity (Fig. 1D, compare the 0.5-μg response with that in A). Similar results were obtained with a progestin-responsive MMTV-LUC reporter (Fig. 1E) and in COS-1 cells (Fig. 1F), showing that the activity was not unique to a single promoter or cell line. The activity of AR (Fig. 1G), but not of VDR (Fig. 1H), was stimulated by cyclin A. Thus, cyclin A acts on a subset of nuclear receptors. In panels E, F, and G the basal activities in the absence of hormone also appear somewhat elevated in the presence of cyclin A, but analysis of the samples in each panel by one-way analysis of variance revealed that the differences were not statistically significant. However, it is possible that cyclin A has a small effect on hormone-independent receptor activity of the receptors under these conditions or on other factors that contribute to the activity of the MMTV promoter.
FIG. 1.
Cyclin A increases the transcriptional activity of PR and AR but not VDR. HeLa cells (A to E, G, and H) or COS cells (F) were transfected as indicated in Materials and Methods with vector (0.5 μg of SRα296) or 0.5 μg of cyclin A, 10 ng of PR-B (A, D, E, and F), PR-A (B), or AR (G), and 0.25 μg of the reporter plasmids indicated in the panels (GRE, GRE2-E1b-luc; MMTV, MMTV-luc; VDRE, VDRE-tkLUC). The cells were treated or not 24 h after transfection with 10 nM concentrations of the indicated hormones. The cells were harvested 24 h after treatment, and luciferase activity was measured and normalized to β-Gal activity in panels A, B, and E to H and to the receptor level in panel D. (C) HeLa cells were cotransfected with 0.5 μg of cyclin A or vector and 0.05 μg of CMV β-Gal, and the β-Gal assay was performed on samples containing equal amounts of protein. The inset of panel A shows that cyclin A expression was increased in cells transfected with cyclin A. A representative Western blot of cyclin A over expression was performed with protein extracts from cells transfected with 0.5 μg of vector or cyclin A. Data shown are the mean and standard error of three independent experiments. RLU, relative light units; 1,25-D, 1,25-dihydroxyvitamin D3.
Cyclin A-dependent stimulation of PR activity is independent of Cdk-dependent phosphorylation of PR.
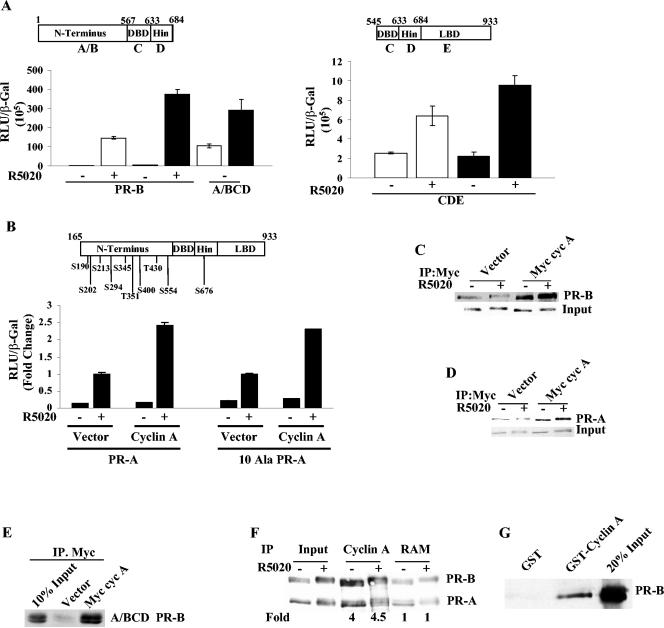
A comparison of the effects of transfected cyclin A on the activity of a PR fragment lacking the LBD (A/BCD) with a PR fragment lacking the N terminus (C D/E) revealed that cyclin A was more effective in enhancing the activity of the A/BCD construct (Fig. 2A) which, interestingly, retains all of the candidate Cdk2 phosphorylation sites. To determine whether cyclin A/Cdk2-dependent PR phosphorylation is required for cyclin A-dependent stimulation of PR activity, all of the possible Ser-Pro and Thr-Pro phosphorylation sites in PR-A were mutated to Ala-Pro (Fig. 2B, top panel), and the ability of cyclin A to stimulate PR activity was measured. Remarkably, these mutations did not influence the stimulation of hormone-dependent PR-mediated transcription by the overexpressed cyclin A (Fig. 2B), implying that the capacity of cyclin A to stimulate PR activity is independent of Cdk2 phosphorylation of PR.
FIG. 2.
Functional and physical interactions between cyclin A and PR. (A) HeLa cells were cotransfected with 10 ng of full-length PR-B or PR-B constructs coding for the N-terminal DNA binding domain (DBD) hinge (left panel) or DBD hinge LBD (right panel), 0.5 μg of vector or cyclin A, 0.25 μg of GRE2-E1b-LUC, and 0.05 μg of β-Gal. The cells were treated or not with 10 nM R5020, and luciferase activity was normalized to β-Gal levels. White bars are vector-transfected samples, and black bars are cyclin A-transfected samples. Also shown are the schematic representations of the PR-B constructs used. (B) HeLa cells were transfected with 0.25 μg of GRE2-E1b-LUC, 10 ng of wild-type PR-A, or a 10 Ala mutant of PR-A (all of the serines and threonines in Ser/Thr-Pro motifs were mutated to alanines), and 0.5 μg of cyclin A or vector. The cells were treated with 10 nM R5020, and luciferase activity was measured and normalized to β-Gal levels. The top panel shows the sites that were mutated to alanine. The hormone-treated vector-transfected samples were set to 1 to facilitate a comparison of fold activation. The actual luciferase and β-Gal values for the hormone-treated wild-type and the 10 Ala mutant-transfected samples were 12.09 and 7.24, respectively. (C and D) HeLa cells were cotransfected with 0.25 μg of PR-B (C) or PR-A (D) and with 2.5 μg of myc-tagged cyclin A or pCMV vector. Twenty-four hours after transfection, the cells were treated with 10 nM R5020 for 60 min and harvested, and 100 μg of extract was incubated with myc 9E10 antibody as indicated in Materials and Methods. The immunoprecipitated protein extracts were run on an SDS-PAGE, and PR was detected with the 1294 antibody. (E) Cyclin A interacts with the N terminus of PR-B. HeLa cells were cotransfected with 0.25 μg of A/BCD PR-B and with 2.5 μg of myc-tagged cyclin A or backbone pCMV; immunoprecipitation and blotting were performed as described for panel C. (F) T47D cells were treated with or without 10 nM R5020 for 60 min, and the cell extracts were immunoprecipitated with cyclin A antibody or a nonspecific rabbit anti-mouse IgG (RAM) as described in Materials and Methods. The immunoprecipitated protein extract was run on an SDS-PAGE gel, and PR was detected using the PR antibody. The films were quantified densitometrically, and the fold change in PR-B immunoprecipitated by cyclin A antibody compared to the RAM-immunoprecipitated samples is given under each lane. The inputs in panels C to E were 10% of the total protein extract used for immunoprecipitation. (G) Cyclin A interacts with PR-B in vitro. One microgram of baculovirus-expressed GST-tagged cyclin A or GST was bound to glutathione-Sepharose beads, and then in vitro-translated [35S]PR-B was added. The beads were washed and eluted, the eluate was run with 20% input on an SDS-PAGE, and radiolabeled PR-B was detected by autoradiography. Myc cyc A, myc-tagged cyclin A; IP, immunoprecipitation; S, serine; T, threonine; DBD, DNA binding domain; Wt, wild type.
Cyclin A interacts with PR in vivo and in vitro.
The finding that cyclin A stimulates PR-A activity independent of its ability to phosphorylate PR suggests either that it is acting as a coactivator through direct interaction with PR or that other receptor-associated cofactors are the targets of cyclin A/Cdk2 kinase activity. Interaction between cyclin A and PR is shown by the coimmunoprecipitation of PR-B (Fig. 2C) or PR-A (Fig. 2D) with Myc-tagged cyclin A from HeLa cells. Consistent with its ability to stimulate activity of the N terminus, immunoprecipitation with the Myc 9E10 antibody also precipitated the A/BCD construct from HeLa cells transfected with Myc-cyclin A, but not the empty vector (Fig. 2E). Immunoprecipitation of endogenous cyclin A from T47D breast cancer cells, which express PR-A and PR-B, coimmunoprecipitated PR (Fig. 2F); fourfold more PR was precipitated by the cyclin A antibody compared to the nonspecific background obtained with rabbit anti-mouse IgG. To test for direct interactions between the proteins, PR-B was in vitro translated with [35S]methionine, and a GST pull-down assay was performed. As shown in Fig. 2G, purified GST-cyclin A interacted with PR-B, whereas the GST control did not.
Cyclin A is recruited to the promoter of a PR-responsive gene.
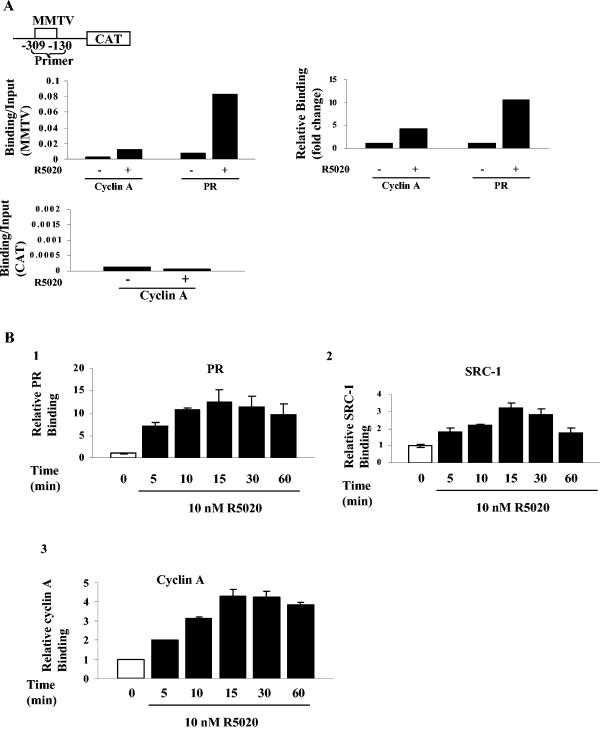
To determine whether the physical interaction between cyclin A and PR leads to the recruitment of cyclin A to progestin-responsive promoters, we performed ChIP assays using T47D cells stably transfected with an MMTV-CAT reporter (Cat0 cells) and detected hormone-dependent recruitment of both cyclin A and PR to the promoter (Fig. 3A). As expected for a specific interaction with a progestin-responsive promoter, cyclin A was not associated with the downstream CAT coding sequence (Fig. 3A). Association of PR and cyclin A with the MMTV promoter peaked by 15 to 30 min and remained at the same level (Fig. 3B). In contrast, the carboxyl-terminal-interacting coactivator, SRC-1, was recruited and released.
FIG. 3.
Cyclin A is rapidly and stably recruited to the MMTV promoter. (A) T47D cells stably transfected with MMTV-CAT (the region of the MMTV promoter probed is shown in the top panel) were treated with 10 nM R5020 for 60 min, protein DNA complexes were cross-linked and then immunoprecipitated with PR or cyclin A antibodies, and ChIP assays were performed as described in Materials and Methods. PCR was also performed with primers generated to the CAT region of the construct. Left panels show the ratios of the signals obtained by PCR amplification of immunoprecipitated MMTV or the CAT region divided by the corresponding input values, and the top right panel shows the fold change in recruitment, with that for the vehicle assigned a value of 1. (B) Cyclin A is recruited to the PR promoter rapidly, and the sites remain occupied for at least 60 min. T47D cells stably transfected with MMTV-CAT were treated with 10 nM R5020 for the indicated times, cross-linked complexes were immunoprecipitated with PR (panel 1), SRC-1 (panel 2), or cyclin A (panel 3) antibodies, and ChIP assays were performed as described in Materials and Methods. Data shown are the mean and standard error of three independent experiments and are shown as the fold change in recruitment, with the time zero value divided by input assigned a value of 1.
Cyclin A requires Cdk2 to activate but not to interact with PR.
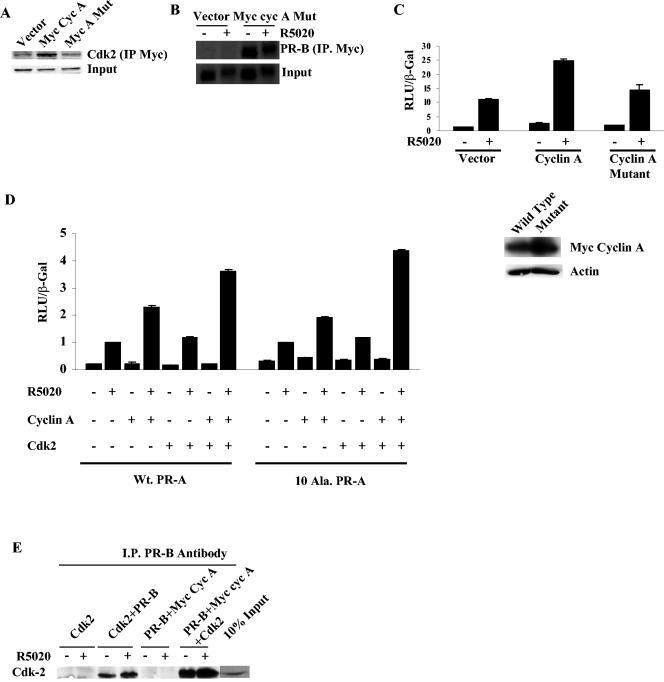
Because all cells contain endogenous levels of the cyclin A kinase partners, Cdk2 and Cdk1, we next asked whether interaction with kinase is required for stimulation of PR activity by eliminating interaction between cyclin A and Cdk and measuring the resulting activity. Arg211, a residue in cyclin A required for the interaction with Cdk (18), was mutated to alanine, and the loss of interaction between cyclin A and Cdk2 was confirmed in a coimmunoprecipitation assay (Fig. 4A). Although the mutant cyclin A interacted with PR (Fig. 4B), it did not stimulate PR activity (Fig. 4C). Thus, the interaction between cyclin A and endogenous kinase is required to stimulate PR activity.
FIG. 4.
Cyclin A requires Cdk2 to stimulate PR activity but not for interaction with PR. (A) Myc-tagged cyclin A wild type or the Cdk2 binding mutant (2.5 μg) was cotransfected with Cdk2 (2.5 μg) into HeLa cells, and the cell extracts were immunoprecipitated with myc 9E10 antibody and blotted for Cdk2. (B) The Cdk2 binding mutant of myc-tagged cyclin A was cotransfected (2.5 μg) with 250 ng of PR-B, immunoprecipitated with myc 9E10 antibody, and blotted for PR. (C) Wild-type, Cdk2 binding mutant of cyclin A or vector backbone was cotransfected (0.5 μg) with 0.25 μg of GRE2-E1b-LUC and 10 ng of PR-B, luciferase activity was measured, and the values were normalized to β-Gal levels. The Western assay in the lower panel shows the expression of the myc cyclin A and the Cdk2 binding mutant of myc cyclin A. (D) HeLa cells were transfected as described in Materials and Methods with 0.25 μg of GRE2-E1b-LUC, 0.5 μg of cyclin A, Cdk2, or combinations of both, and 10 ng of PR-A or the phosphorylation-deficient mutant (10 Ala PR-A). The cells were treated with 10 nM R5020 for 24 h and harvested, and luciferase activity was measured and normalized to the β-Gal levels. The values of hormone-treated vector-transfected samples were set to 1. (E) Cdk2 interacts with PR-B, and the interaction is increased by transfection of cyclin A. HeLa cells were transfected with 0.25 μg of PR-B, 2.5 μg of Cdk2 or myc cyclin A, or a combination of both. After 24 h, the cells were treated with R5020 for 60 min. Protein extracts were immunoprecipitated with PR antibody and then blotted with Cdk2 antibody. I.P., immunoprecipitation; myc cyc A, myc-tagged cyclin A.
Cyclin A and Cdk2 synergize to increase PR activity.
The mutant cyclin A data suggested that additional Cdk2 should further enhance cyclin A's effect on PR. We found that whereas cyclin A alone increased PR-A activity by 2-fold and Cdk2 alone minimally increased PR-A activity, the combination increased PR-A activity 3.5-fold (Fig. 4D). Similar results were obtained with the 10 Ala PR-A mutants. Although immunoprecipitation of PR-B from cells cotransfected with PR-B and Cdk2 brought down some Cdk2, cotransfection of Myc-tagged cyclin A markedly increased the association of Cdk2 with PR (Fig. 4E). Thus, Cdk2 interacts with PR predominantly through cyclin A.
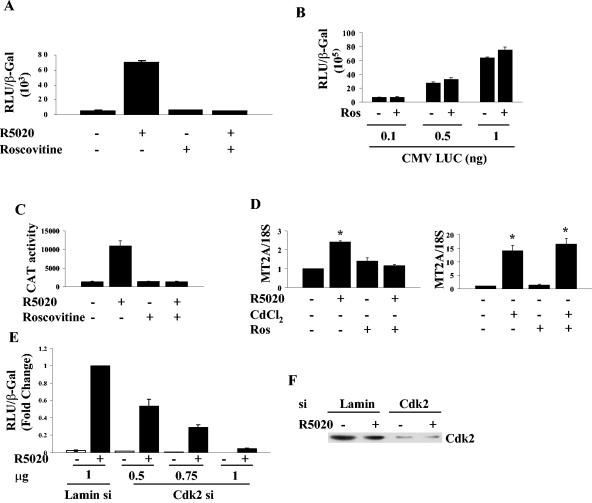
Cdk2 kinase activity is critical for PR transactivation.
To assess the contribution of Cdk2 to the transcriptional activity of PR, HeLa cells expressing PR were treated with roscovitine, a chemical inhibitor of Cdk2, Cdk1, and Cdk5 (10, 12, 31). Remarkably, roscovitine essentially inhibited PR-B-dependent transactivation (Fig. 5A) but did not inhibit the activity of a constitutively active luciferase reporter, showing that there are no nonspecific effects on luciferase activity and that the inhibition is not a result of a general inhibition of transcription (Fig. 5B). Moreover, roscovitine blocked the progesterone-dependent induction of an integrated MMTV-CAT reporter by endogenous PR in T47D cells (Fig. 5C). To further characterize the inhibition of transcription by roscovitine, we examined the expression of MT2A, a gene that can be induced either by hormones or by a heavy metal (19, 41). Roscovitine completely inhibited the R5020-induced transcription but not cadmium chloride-dependent transcription (Fig. 5D).
FIG. 5.
Cdk2 activity is important for PR function. (A) HeLa cells were transfected with 10 ng of PR-B and 0.25 μg of GRE-E1b-LUC and then treated 24 h after transfection with vehicle, 30 μM roscovitine, 10 nM R5020, or a combination of roscovitine and R5020 for 4 h. The cells were harvested, and luciferase activity was measured and normalized to the β-Gal levels. (B) HeLa cells were transfected with increasing concentrations of constitutively active luciferase (CMV LUC) and 0.05 μg of β-Gal and then treated or not immediately after transfection with 30 μM roscovitine for 4 h; luciferase activity was measured and normalized to β-Gal levels. (C) T47D cells stably transfected with MMTV-CAT were treated with vehicle, 30 μM roscovitine, 10 nM R5020, or a combination of roscovitine and R5020 for 4 h. The cells were harvested, and CAT activity was measured and normalized to total cellular protein levels. (D) T47D cells were treated with vehicle, 10 nM R5020, 5 μM CdCl2, or a combination of roscovitine and R5020 or CdCl2 for 6 h. Total cellular RNA was extracted, and then a reverse transcriptase real-time PCR with TaqMan primers and probe was performed for the MT2A gene as described in Materials and Methods. MT2A gene transcription was normalized to the 18S rRNA, and the value for vehicle-treated cells was set at 1. *, significantly different from vehicle-treated samples. (E) HeLa cells were transfected with the indicated amounts of Cdk2 siRNA plasmid, and the concentration of DNA was matched with an siRNA plasmid for lamin. The cells were treated 48 h after transfection with ethanol or 10 nM R5020, harvested 24 h later, and luciferase activity was measured and normalized to β-Gal levels. The activity is expressed relative to activity obtained with 1 μg of lamin siRNA plus R5020. (F) Cdk2 in the samples from panel E was detected by Western blotting using a Cdk2 antibody. Ros, roscovitine; Const. Active Luc, constitutively active luciferase.
Since roscovitine inhibits three kinases, we next investigated whether inhibition of Cdk2 by using siRNA was sufficient to inhibit activity. A vector-based siRNA for Cdk2 was used to decrease Cdk2 expression, and a lamin siRNA vector served as a negative control (42). As shown in Fig. 5E, PR activity in HeLa cells was dose-dependently inhibited by the Cdk2 siRNA vector compared to lamin siRNA vector. Figure 5F shows the reduction in Cdk2 expression.
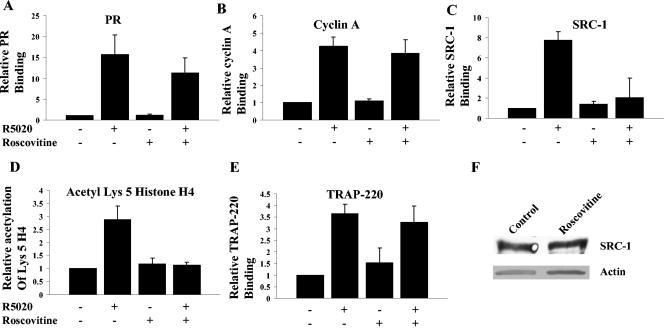
Roscovitine treatment inhibits the acetylation of histone H4 at lysine 5 and recruitment of SRC-1 but not the recruitment of PR, cyclin A, or TRAP-220 to the PR-responsive promoter.
To identify actions dependent on kinase activity, T47D Cat0 cells containing an integrated MMTV promoter, which have been used previously to characterize PR-dependent recruitment of coactivators (28), were treated with roscovitine, R5020, or a combination of both, and a ChIP assay was performed to measure histone acetylation and recruitment of the receptor and coactivators to the MMTV promoter. The recruitment of PR (Fig. 6A) and cyclin A (Fig. 6B) to the MMTV promoter was not affected by roscovitine. However, the recruitment of SRC-1 (Fig. 6C) and the downstream effect of acetylation of histone (Fig. 6D) at lysine 5 on the MMTV promoter were completely inhibited by roscovitine. Recruitment of TRAP-220, a component of the TRAP/mediator complex (40), was not reduced by roscovitine (Fig. 6E). This suggests that the reduced recruitment of SRC-1 and resulting acetylation of histone H4 at lysine 5 is dependent upon Cdk2 activity. This failure to recruit SRC-1 is not due to reduced expression of SRC-1 (Fig. 6F), nor is it due to alteration in the time course of recruitment (data not shown).
FIG. 6.
Roscovitine inhibits recruitment of SRC-1 to the MMTV promoter and the acetylation of histone H4 at lysine 5. T47D cells stably transfected with MMTV-CAT were treated with 10 nM R5020 for 30 min or pretreated with 30 μM roscovitine for 3 h and then treated with R5020 for 30 min, and ChIP assays performed using antibodies to PR (A), cyclin A (B), SRC-1 (C), histone H4 acetyl lysine 5 (D), or TRAP-220 (E). In panels A through E the ratio of signal to input was determined and fold enhancement relative to an assigned value of 1 for vehicle-treated samples is plotted. (F) SRC-1 levels were measured by Western blotting of extracts from cells that were treated or not with roscovitine for 4 h. Also shown in the lower panel is the actin loading control. TRAP-220, thyroid receptor-associated protein; acetyl Lys 5, histone H3 acetyl lysine 5.
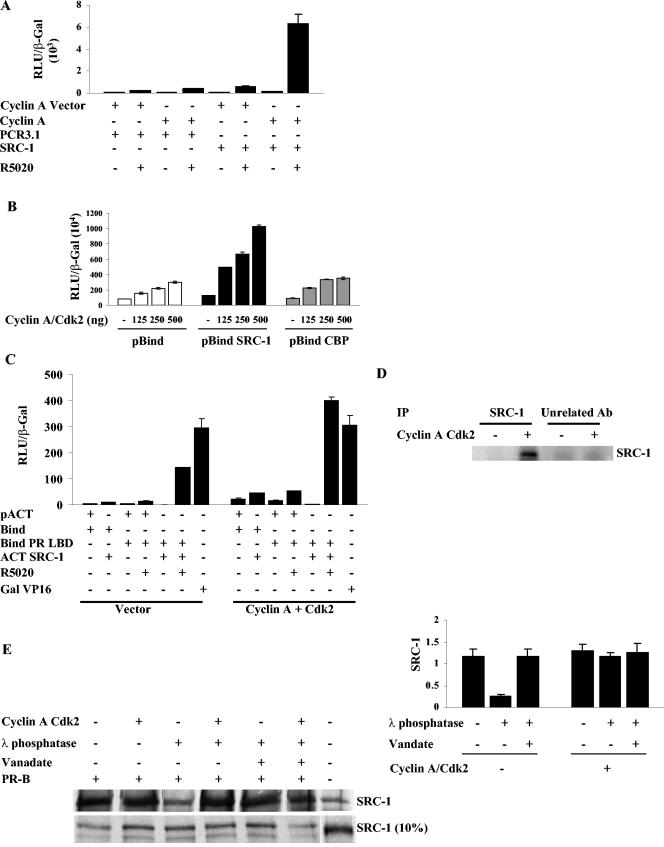
Cyclin A/Cdk2 enhances PR-mediated gene transcription by phosphorylating SRC-1 and increasing activity and interaction of SRC-1 with PR.
The roscovitine studies suggest that coactivation of PR by SRC-1 depends on kinase activity. Consistent with this, cotransfection of both cyclin A and SRC-1 increased PR activity dramatically relative to suboptimal levels of either cyclin A or SRC-1 alone (Fig. 7A). To determine the basis for this synergism, we first asked whether cyclin A enhances the intrinsic activating function of SRC-1 by transfecting HeLa cells with a pBind SRC-1 expression vector, its reporter construct 17-mer Luc, and increasing concentrations of cyclin A and Cdk2. As shown in Fig. 7B, the cyclin A/Cdk2 concentration dependently increased the intrinsic activity of pBind SRC-1 and minimally affected the Gal DNA binding domain (pBind) alone. The effect on the intrinsic activity of pBind CBP, another coactivator that is recruited to PR through binding to SRC-1 (28), was indistinguishable from the effect on pBind alone.
FIG.7.
SRC-1 activity and its interaction with PR is regulated by Cdk2 kinase activity. (A) Cyclin A and SRC-1 synergistically increase PR-B transactivation. HeLa cells were transfected with 0.25 μg of GRE2-E1b-LUC, 0.5 μg of cyclin A, 0.5 μg of SRC-1, vectors for cyclin A or SRC-1, and 10 ng of PR-B. The cells were treated 24 h after transfection with 10 nM R5020 and harvested 48 h after transfection, and luciferase activity was measured and normalized to the β-Gal levels. (B) Cyclin A/Cdk2 increases the intrinsic activity of SRC-1 but not CBP. HeLa cells were cotransfected with 0.25 μg of pBind, pBind SRC-1, or pBind CBP, increasing concentrations of cyclin A/Cdk2, and with the 17-mer LUC reporter. The cells were harvested 48 h after transfection, and luciferase activity was measured and normalized to β-Gal levels. (C) Cyclin A/Cdk2 increases the interaction between PR LBD and SRC-1. HeLa cells were transfected with 0.25 μg of the PR LBD fused to Gal4 DNA binding domain or SRC-1 fused to the VP16 activation domain along with 0.5 μg of vector or 0.25 μg of cyclin A and 0.25 μg of Cdk2. The cells were treated 24 h after transfection and harvested 48 h after transfection, and luciferase activity was measured and normalized to β-Gal levels. As a control, cells were also transfected with Gal VP16 (Gal DNA binding domain fused to the VP16 activation domain) to distinguish between enhanced VP16 activity and authentic enhancement of interactions between PR and SRC-1. (D) Cyclin A/Cdk2 phosphorylates SRC-1 in vitro. Baculovirus-expressed SRC-1 was immunoprecipitated with an SRC-1 antibody or an unrelated control (AR) antibody. The beads were washed and incubated with 5 μl of baculovirus-expressed and purified cyclin A/Cdk2 in an in vitro phosphorylation assay mixture containing [γ32P]ATP. SRC-1 was extracted with 4× Laemmli buffer and run on an SDS-PAGE, and phosphorylated SRC-1 visualized by autoradiography. (E) Interaction between PR and SRC-1 is dependent on phosphorylation of SRC-1. In vitro-translated 35S-labeled SRC-1 was treated with λ phosphatase, and the reaction was stopped with the phosphatase inhibitor sodium vanadate. Samples which received sodium vanadate prior to the addition of phosphatase are indicated as containing sodium vanadate in the figure. Samples containing cyclin A/Cdk2 were incubated with the kinase after the phosphatase treatment. The treated SRC-1 fractions were then incubated with baculovirus-expressed and purified PR-B attached to protein A-Sepharose beads through a PR antibody, and bound SRC-1 was extracted with 2× Laemmli buffer, run on an SDS-PAGE, transferred to nitrocellulose, and detected by autoradiography. SRC-1 incubated with protein A-Sepharose and PR antibody without PR was used as a negative control. The right panel shows the densitometric evaluation of the SRC-1 bands detected by autoradiography. N-Term PRB, N terminus of PR-B; Unrelated Ab, unrelated antibody control.
To test whether overexpression of cyclin A/Cdk2 can increase the interaction between PR and SRC-1, a mammalian two-hybrid assay was performed between PR-LBD fused to Gal4 and SRC-1 fused to the acidic activation domain of VP16 in the presence and absence of overexpressed cyclin A/Cdk2. As shown in Fig. 7C, cyclin A/Cdk2 increased the activity observed when Bind PR LBD and ACT SRC-1 were coexpressed nearly threefold, suggesting that interaction was enhanced. The background activity of Bind with pACT or with pACT SRC-1 was also enhanced somewhat. This is surprising in that it suggests a small degree of interaction between the Gal4 DBD and SRC-1 (note that pACT plus Bind also has lower activity with vector than pACT SRC-1 plus Bind with vector). However, the lack of an effect on the intrinsic activity of a constitutively active Gal VP16 construct demonstrates that the stimulation is not due to enhanced VP16 activity.
Phosphorylation of SRC-1 by cyclin A/Cdk2 regulates its interaction with PR.
Since cyclin A/Cdk2 increased the interaction between PR and SRC-1, we sought to determine whether cyclin A/Cdk2 mediates this effect through phosphorylation of SRC-1. An in vitro kinase assay using baculovirus-expressed SRC-1 revealed that cyclin A/Cdk2 does phosphorylate SRC-1 (Fig. 7D). To determine whether SRC-1 phosphorylation regulates its interaction with PR, in vitro-translated SRC-1 was dephosphorylated using λ phosphatase, and the reaction was stopped by using sodium vanadate. In the control sample, sodium vanadate was added prior to incubation with λ phosphatase. As shown in Fig. 7E, λ phosphatase treatment reduced the interaction between PR and SRC-1 to 25% of the in vitro-translated SRC-1 or sodium vanadate-pretreated samples. To test whether phosphorylating SRC-1 with cyclin A/Cdk2 restored the interaction, we incubated phosphatase-treated SRC-1 with cyclin A/Cdk2 and found that rephosphorylation of SRC-1 restored the interaction between PR and SRC-1 (Fig. 7E, left, representative Western blot, and right, quantification of multiple experiments).
DISCUSSION
Our studies have revealed that cyclin-dependent kinase(s) plays a major role in regulating PR activity and have uncovered a novel means of inducing high local levels of cyclin A/Cdk2 at the promoters of progesterone-responsive genes facilitating phosphorylation of associated proteins. Although cyclin A/Cdk2 stimulates the activity of the estrogen receptor (ER), this stimulation has been attributed to phosphorylation of Ser104/106 in ER, as mutation of these sites inhibits stimulation by cyclin A/Cdk2 (37). In contrast, the candidate Cdk2 sites in PR are not required for the stimulation of PR activity by overexpression of cyclin A, suggesting that cyclin A might instead be functioning as a coactivator. Although it is formally possible that Cdk2 might activate another kinase that phosphorylates PR-A, to our knowledge the only unidentified phosphorylation site(s) in PR is one that is responsible for the hormone-dependent change in mobility on SDS gels. Neither cyclin A nor roscovitine treatment alters mobility on SDS gels (data not shown), making this a very unlikely possibility. PR-A was initially chosen for the mutation study, because the fold stimulation is similar for both PR-B and PR-A and PR-A contains six fewer phosphorylation sites. The only identified in vivo PR-B-specific site that is a substrate for cyclin A/Cdk2 is Ser162 (53). Mutation of this site does not reduce stimulation of PR-B activity by cyclin A (data not shown). Other cyclins have been reported to act as coactivators of steroid receptors. An earlier study revealed that cyclin D1 interacts with the LBD of ER to stimulate transactivation, but cyclin A did not (34). Cyclin E acts as a coactivator of AR (50), while cyclin D1 inhibits AR activity (22). However, in neither case was the cyclin-dependent kinase partner required for stimulation of receptor activity (34, 50). In contrast, although cyclin A interacts with PR, the stimulation of PR activity by cyclin A is dependent on its ability to interact with Cdk2. Overexpression of either cyclin A or Cdk2 stimulated PR activity (presumably due to endogenous protein partners), but the combination was much more effective. Although overexpression of Cdk2 led to some association with PR, likely due to endogenous cyclin A, coexpression of cyclin A substantially increased the amount of Cdk2 associated with PR. Thus, the association between PR and Cdk2 appears to be primarily mediated through cyclin A. Interestingly, the interaction between cyclin A and PR appears to be hormone independent, consistent with our finding that cyclin A interacts with a fragment of PR lacking the LBD (Fig. 2). Thus, it appears that hormones may relocalize a preexisting receptor/cyclin A complex to the promoters of responsive genes. Consistent with a requirement for cyclin A, as previously reported (50), we also have no evidence for stimulation of PR activity by cyclin E, the other Cdk2 cyclin partner (data not shown).
Our studies show that cyclin-dependent kinase activity and, in particular, Cdk2 are required for the activity of PR. This is consistent with the report that the transcriptional activity of exogenous GR expressed in yeast strains lacking components of the cyclin-dependent kinase pathway is reduced compared to wild-type strains (24). However, the basis for the inhibition and the contribution of GR phosphorylation to this change have not been determined. Because cyclin A/Cdk2 participates in cell cycle regulation, it is formally possible that overexpression of cyclin A or treatment with the Cdk2 siRNA could have altered cell cycle distribution in our studies and that there are additional contributors to the alterations in activity. However, expression of cyclin A is only one of many regulators of cell cycle progression, and additional changes in inhibitor and kinase or phosphatase activity are needed for cell cycle progression. Moreover, the companion roscovitine studies were too short term to alter cell cycle distribution, and the in vitro interaction studies support a direct role for Cdk2.
In seeking the molecular basis for the inhibition of PR activity by the Cdk inhibitor roscovitine, we found that neither the expression level of endogenous PR in T47D cells nor the ability of PR to bind to an integrated MMTV promoter was affected by roscovitine. However, there was a striking decrease in the hormone-dependent recruitment of SRC-1 to the MMTV promoter (Fig. 6C) and a corresponding reduction in acetylation of histone H3 at lysine 5 in the presence of roscovitine. Neither the recruitment of cyclin A nor that of TRAP-220, a component of the TRAP/mediator complex (40), was affected by roscovitine treatment. These experiments suggested that SRC-1 might be a target for cyclin A/Cdk2, and subsequent studies revealed that SRC-1 can be phosphorylated by cyclin A/Cdk2 (Fig. 7D). That this phosphorylation is functionally relevant is shown by the interaction studies, which demonstrate that dephosphorylation of SRC-1 reduces the interaction of SRC-1 with purified PR and rephosphorylation restores binding (Fig. 7E).
Interestingly, although GRs also transactivate similar promoters, including the MMTV and MT2A promoters, the response of glucocorticoids to Cdk is quite different. Whereas prolonged exposure to another Cdk2 inhibitor, CVT-313, reduced glucocorticoid-dependent transcription from an integrated MMTV promoter, it did not affect glucocorticoid-dependent induction of MT2A or of a transiently expressed MMTV promoter (8). One key difference between GR- and PR-dependent induction of MMTV activity is that GR recruits TIF2/GRIP1 rather than SRC-1 to the MMTV promoter in T47D Cat0 cells (28). Although GR has been shown to recruit SRC-1 to other promoters in other cells (13), its mode of interaction with SRC-1 may not be not be identical to that of PR. Steroid receptors must compete for a limited pool of coactivators that interact with subsets of transcription factors. Regulated phosphorylation of the transcription factor or the coactivator is a potential determining factor for which complexes are formed (51). Moreover, many of the coactivators have enzymatic activities, such as histone acetyltransferase, ubiquitin ligase, or methyl transferase, and these activities can also be regulated by phosphorylation. Our studies of SRC-1 indicate that phosphorylation plays an important role in the regulation of its activity. Recruitment of cyclin A/Cdk2 to the promoter by PR likely facilitates phosphorylation of a variety of coregulators, increasing overall transcriptional activity. Cdk2 can phosphorylate histone H1, a modification associated with activation (8); in addition, it enhances the histone acetyltransferase activity of CBP (2). Our study shows that phosphorylation of SRC-1 enhances interaction with PR. Moreover, cyclin A/Cdk2 enhances the intrinsic transcriptional activity of SRC-1 (Fig. 7B) independent of its interaction with PR. Thus, the binding of cyclin A/Cdk2 to PR creates a locally high concentration of kinase activity at PR-responsive promoters. The kinase can phosphorylate SRC-1, promoting its interaction with PR as well as stimulating phosphorylation of other associated proteins specifically enhancing their activity on PR target genes. When the cyclin-dependent kinase is inactive or not associated with PR, the interaction site on SRC-1 will not be phosphorylated, thus reducing the ability of PR to recruit SRC-1 and favoring interactions with other transcription factors. The cell cycle-dependent regulation of cyclin-dependent kinase activity coupled with the requirement for this activity in PR action provides a means to regulate progesterone receptor activity during the cell cycle. The ability of PR to recruit cyclin A/Cdk2 to the promoter then ensures that the kinase is locally available to phosphorylate proteins whose interaction with PR or enzymatic activity is phosphorylation dependent.
Acknowledgments
We thank Kurt Christensen and the UC Cancer Center Tissue Culture Core facility for assistance with baculovirus production of recombinant PR, SRC-1, and cyclin A/Cdk2 and Lori Sherman (UCHSC) for the production and purification of 1294 monoclonal antibody. We thank William Edward Bingman III, Jared Gilliam, Judy Roscoe, and Cheryl Parker for assistance with tissue culture and transfection studies.
This work was supported by Public Health Service grant R01 CA-57539 (to N.L.W. and D.P.E.) from the National Cancer Institute and Public Health Service training grant T32 HDO7165 (to R.N.).
REFERENCES
- 1.Agoulnik, I. U., W. C. Krause, W. E. Bingman III, H. T. Rahman, M. Amrikachi, G. E. Ayala, and N. L. Weigel. 2003. Repressors of androgen and progesterone receptor action. J. Biol. Chem. 278:31136-31148. [DOI] [PubMed] [Google Scholar]
- 2.Ait-Si-Ali, S., S. Ramirez, F. X. Barre, F. Dkhissi, L. Magnaghi-Jaulin, J. A. Girault, P. Robin, M. Knibiehler, L. L. Pritchard, B. Ducommun, D. Trouche, and A. Harel-Bellan. 1998. Histone acetyltransferase activity of CBP is controlled by cycle-dependent kinases and oncoprotein E1A. Nature 396:184-186. [DOI] [PubMed] [Google Scholar]
- 3.Archer, T. K., P. Lefebvre, R. G. Wolford, and G. L. Hager. 1992. Transcription factor loading on the MMTV promoter: a bimodal mechanism for promoter activation. Science 255:1573-1576. [DOI] [PubMed] [Google Scholar]
- 4.Bai, W., and N. L. Weigel. 1996. Phosphorylation of Ser211 in the chicken progesterone receptor modulates its transcriptional activity. J. Biol. Chem. 271:12801-12806. [DOI] [PubMed] [Google Scholar]
- 5.Beck, C. A., N. L. Weigel, and D. P. Edwards. 1992. Effects of hormone and cellular modulators of protein phosphorylation on transcriptional activity, DNA binding, and phosphorylation of human progesterone receptors. Mol. Endocrinol. 6:607-620. [DOI] [PubMed] [Google Scholar]
- 6.Beck, C. A., N. L. Weigel, M. L. Moyer, S. K. Nordeen, and D. P. Edwards. 1993. The progesterone antagonist RU486 acquires agonist activity upon stimulation of cAMP signaling pathways. Proc. Natl. Acad. Sci. USA 90:4441-4445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beck, C. A., Y. Zhang, M. Altmann, N. L. Weigel, and D. P. Edwards. 1996. Stoichiometry and site-specific phosphorylation of human progesterone receptor in native target cells and in the baculovirus expression system. J. Biol. Chem. 271:19546-19555. [DOI] [PubMed] [Google Scholar]
- 8.Bhattacharjee, R. N., G. C. Banks, K. W. Trotter, H. L. Lee, and T. K. Archer. 2001. Histone H1 phosphorylation by Cdk2 selectively modulates mouse mammary tumor virus transcription through chromatin remodeling. Mol. Cell. Biol. 21:5417-5425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cato, A. C., D. Henderson, and H. Ponta. 1987. The hormone response element of the mouse mammary tumour virus DNA mediates the progestin and androgen induction of transcription in the proviral long terminal repeat region. EMBO J. 6:363-368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Filgueira de Azevedo, W., Jr., R. T. Gaspar, F. Canduri, J. C. Camera, Jr., and N. J. Freitas da Silveira. 2002. Molecular model of cyclin-dependent kinase 5 complexed with roscovitine. Biochem. Biophys. Res. Commun. 297:1154-1158. [DOI] [PubMed] [Google Scholar]
- 11.Gioeli, D., S. B. Ficarro, J. J. Kwiek, D. Aaronson, M. Hancock, A. D. Catling, F. M. White, R. E. Christian, R. E. Settlage, J. Shabanowitz, D. F. Hunt, and M. J. Weber. 2002. Androgen receptor phosphorylation. Regulation and identification of the phosphorylation sites. J. Biol. Chem. 277:29304-29314. [DOI] [PubMed] [Google Scholar]
- 12.Gray, N., L. Detivaud, C. Doerig, and L. Meijer. 1999. ATP-site directed inhibitors of cyclin-dependent kinases. Curr. Med. Chem. 6:859-875. [PubMed] [Google Scholar]
- 13.Grenier, J., A. Trousson, A. Chauchereau, L. Amazit, A. Lamirand, P. Leclerc, A. Guiochon-Mantel, M. Schumacher, and C. Massaad. 2004. Selective recruitment of p160 coactivators on glucocorticoid-regulated promoters in Schwann cells. Mol. Endocrinol. [Online.] doi:10.1210/me.2004-0241. [DOI] [PubMed]
- 14.Harper, J. W., G. R. Adami, N. Wei, K. Keyomarsi, and S. J. Elledge. 1993. The p21 Cdk-interacting protein Cip1 is a potent inhibitor of G1 cyclin-dependent kinases. Cell 75:805-816. [DOI] [PubMed] [Google Scholar]
- 15.Harper, J. W., S. J. Elledge, K. Keyomarsi, B. Dynlacht, L. H. Tsai, P. Zhang, S. Dobrowolski, C. Bai, L. Connell-Crowley, E. Swindell, et al. 1995. Inhibition of cyclin-dependent kinases by p21. Mol. Biol. Cell 6:387-400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hsu, S. C., and D. B. DeFranco. 1995. Selectivity of cell cycle regulation of glucocorticoid receptor function. J. Biol. Chem. 270:3359-3364. [DOI] [PubMed] [Google Scholar]
- 17.Hu, J., J. Bodwell, and A. Munck. 1994. Cell cycle-dependent glucocorticoid receptor phosphorylation and activity. Mol. Endocrinol. 8:1709-1713. [DOI] [PubMed] [Google Scholar]
- 18.Jackman, M., Y. Kubota, N. den Elzen, A. Hagting, and J. Pines. 2002. Cyclin A- and cyclin E-Cdk complexes shuttle between the nucleus and the cytoplasm. Mol. Biol. Cell 13:1030-1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Karin, M., A. Haslinger, H. Holtgreve, R. I. Richards, P. Krauter, H. M. Westphal, and M. Beato. 1984. Characterization of DNA sequences through which cadmium and glucocorticoid hormones induce human metallothionein-IIA gene. Nature 308:513-519. [DOI] [PubMed] [Google Scholar]
- 20.Kastner, P., A. Krust, B. Turcotte, U. Stropp, L. Tora, H. Gronemeyer, and P. Chambon. 1990. Two distinct estrogen-regulated promoters generate transcripts encoding the two functionally different human progesterone receptor forms A and B. EMBO J. 9:1603-1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Knotts, T. A., R. S. Orkiszewski, R. G. Cook, D. P. Edwards, and N. L. Weigel. 2001. Identification of a phosphorylation site in the hinge region of the human progesterone receptor and additional amino-terminal phosphorylation sites. J. Biol. Chem. 276:8475-8483. [DOI] [PubMed] [Google Scholar]
- 22.Knudsen, K. E., W. K. Cavenee, and K. C. Arden. 1999. D-type cyclins complex with the androgen receptor and inhibit its transcriptional transactivation ability. Cancer Res. 59:2297-2301. [PubMed] [Google Scholar]
- 23.Kobayashi, H., E. Stewart, R. Poon, J. P. Adamczewski, J. Gannon, and T. Hunt. 1992. Identification of the domains in cyclin A required for binding to, and activation of, p34cdc2 and p32cdk2 protein kinase subunits. Mol. Biol. Cell 3:1279-1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Krstic, M., I. Rogatsky, K. Yamamoto, and M. Garabedian. 1997. Mitogen-activated and cyclin-dependent protein kinases selectively and differentially modulate transcriptional enhancement by the glucocorticoid receptor. Mol. Cell. Biol. 17:3947-3954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lambert, J. R., and S. K. Nordeen. 2001. Analysis of steroid hormone-induced histone acetylation by chromatin immunoprecipitation assay. Methods Mol. Biol. 176:273-281. [DOI] [PubMed] [Google Scholar]
- 26.Lange, C. A. 2004. Making sense of cross-talk between steroid hormone receptors and intracellular signaling pathways: who will have the last word? Mol. Endocrinol. 18:269-278. [DOI] [PubMed] [Google Scholar]
- 27.Lange, C. A., T. Shen, and K. B. Horwitz. 2000. Phosphorylation of human progesterone receptors at serine-294 by mitogen-activated protein kinase signals their degradation by the 26S proteasome. Proc. Natl. Acad. Sci. USA 97:1032-1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li, X., J. Wong, S. Y. Tsai, M. J. Tsai, and B. W. O'Malley. 2003. Progesterone and glucocorticoid receptors recruit distinct coactivator complexes and promote distinct patterns of local chromatin modification. Mol. Cell. Biol. 23:3763-3773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Martinez, E. D., and M. Danielsen. 2002. Loss of androgen receptor transcriptional activity at the G1/S transition. J. Biol. Chem. 277:29719-29729. [DOI] [PubMed] [Google Scholar]
- 30.McKenna, N. J., and B. W. O'Malley. 2002. Combinatorial control of gene expression by nuclear receptors and coregulators. Cell 108:465-474. [DOI] [PubMed] [Google Scholar]
- 31.Meijer, L., A. Borgne, O. Mulner, J. P. Chong, J. J. Blow, N. Inagaki, M. Inagaki, J. G. Delcros, and J. P. Moulinoux. 1997. Biochemical and cellular effects of roscovitine, a potent and selective inhibitor of the cyclin-dependent kinases cdc2, cdk2 and cdk5. Eur. J. Biochem. 243:527-536. [DOI] [PubMed] [Google Scholar]
- 32.Nawaz, Z., D. M. Lonard, C. L. Smith, E. Lev-Lehman, S. Y. Tsai, M. J. Tsai, and B. W. O'Malley. 1999. The Angelman syndrome-associated protein, E6-AP, is a coactivator for the nuclear hormone receptor superfamily. Mol. Cell. Biol. 19:1182-1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nazareth, L. V., and N. L. Weigel. 1996. Activation of the human androgen receptor through a protein kinase A signaling pathway. J. Biol. Chem. 271:19900-19907. [DOI] [PubMed] [Google Scholar]
- 34.Neuman, E., M. H. Ladha, N. Lin, T. M. Upton, S. J. Miller, J. DiRenzo, R. G. Pestell, P. W. Hinds, S. F. Dowdy, M. Brown, and M. E. Ewen. 1997. Cyclin D1 stimulation of estrogen receptor transcriptional activity independent of cdk4. Mol. Cell. Biol. 17:5338-5347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Press, M., B. Spaulding, S. Groshen, D. Kaminsky, M. Hagerty, L. Sherman, K. Christensen, and D. P. Edwards. 2002. Comparison of different antibodies for detection of progesterone receptor in breast cancer. Steroids 67:799-813. [DOI] [PubMed] [Google Scholar]
- 36.Richer, J. K., B. M. Jacobsen, N. G. Manning, M. G. Abel, D. M. Wolf, and K. B. Horwitz. 2002. Differential gene regulation by the two progesterone receptor isoforms in human breast cancer cells. J. Biol. Chem. 277:5209-5218. [DOI] [PubMed] [Google Scholar]
- 37.Rogatsky, I., J. M. Trowbridge, and M. J. Garabedian. 1999. Potentiation of human estrogen receptor alpha transcriptional activation through phosphorylation of serines 104 and 106 by the cyclin A-CDK2 complex. J. Biol. Chem. 274:22296-22302. [DOI] [PubMed] [Google Scholar]
- 38.Rowan, B. G., N. Garrison, N. L. Weigel, and B. W. O'Malley. 2000. 8-Bromo-cyclic AMP induces phosphorylation of two sites in SRC-1 that facilitate ligand-independent activation of the chicken progesterone receptor and are critical for functional cooperation between SRC-1 and CREB binding protein. Mol. Cell. Biol. 20:8720-8730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rowan, B. G., N. L. Weigel, and B. W. O'Malley. 2000. Phosphorylation of steroid receptor coactivator-1. Identification of the phosphorylation sites and phosphorylation through the mitogen-activated protein kinase pathway. J. Biol. Chem. 275:4475-4483. [DOI] [PubMed] [Google Scholar]
- 40.Sharma, D., and J. D. Fondell. 2002. Ordered recruitment of histone acetyltransferases and the TRAP/mediator complex to thyroid hormone-responsive promoters in vivo. Proc. Natl. Acad. Sci. USA 99:7934-7939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Slater, E. P., A. C. Cato, M. Karin, J. D. Baxter, and M. Beato. 1988. Progesterone induction of metallothionein-IIA gene expression. Mol. Endocrinol. 2:485-491. [DOI] [PubMed] [Google Scholar]
- 42.Sui, G., C. Soohoo, E. B. Affar, F. Gay, Y. Shi, W. C. Forrester, and Y. Shi. 2002. A DNA vector-based RNAi technology to suppress gene expression in mammalian cells. Proc. Natl. Acad. Sci. USA 99:5515-5520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tetel, M. J., P. H. Giangrande, S. A. Leonhardt, D. P. McDonnell, and D. P. Edwards. 1999. Hormone-dependent interaction between the amino- and carboxyl-terminal domains of progesterone receptor in vitro and in vivo. Mol. Endocrinol. 13:910-924. [DOI] [PubMed] [Google Scholar]
- 44.Trowbridge, J. M., I. Rogatsky, and M. J. Garabedian. 1997. Regulation of estrogen receptor transcriptional enhancement by the cyclin A/Cdk2 complex. Proc. Natl. Acad. Sci. USA 94:10132-10137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vegeto, E., M. M. Shahbaz, D. X. Wen, M. E. Goldman, B. W. O'Malley, and D. P. McDonnell. 1993. Human progesterone receptor A form is a cell- and promoter-specific repressor of human progesterone receptor B function. Mol. Endocrinol. 7:1244-1255. [DOI] [PubMed] [Google Scholar]
- 46.Wang, Z., J. Frederick, and M. J. Garabedian. 2002. Deciphering the phosphorylation “code” of the glucocorticoid receptor in vivo. J. Biol. Chem. 277:26573-26580. [DOI] [PubMed] [Google Scholar]
- 47.Weigel, N. L. 1996. Steroid hormone receptors and their regulation by phosphorylation. Biochem. J. 319:657-667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Weigel, N. L., and Y. Zhang. 1998. Ligand-independent activation of steroid hormone receptors. J. Mol. Med. 76:469-479. [DOI] [PubMed] [Google Scholar]
- 49.Wu, R. C., J. Qin, Y. Hashimoto, J. Wong, J. Xu, S. Y. Tsai, M. J. Tsai, and B. W. O'Malley. 2002. Regulation of SRC-3 (pCIP/ACTR/AIB-1/RAC-3/TRAM-1) coactivator activity by I kappa B kinase. Mol. Cell. Biol. 22:3549-3561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yamamoto, A., Y. Hashimoto, K. Kohri, E. Ogata, S. Kato, K. Ikeda, and M. Nakanishi. 2000. Cyclin E as a coactivator of the androgen receptor. J. Cell Biol. 150:873-880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yang, W., Y. H. Hong, X. Q. Shen, C. Frankowski, H. S. Camp, and T. Leff. 2001. Regulation of transcription by AMP-activated protein kinase: phosphorylation of p300 blocks its interaction with nuclear receptors. J. Biol. Chem. 276:38341-38344. [DOI] [PubMed] [Google Scholar]
- 52.Zhang, Y., W. Bai, V. E. Allgood, and N. L. Weigel. 1994. Multiple signaling pathways activate the chicken progesterone receptor. Mol. Endocrinol. 8:577-584. [DOI] [PubMed] [Google Scholar]
- 53.Zhang, Y., C. A. Beck, A. Poletti, J. P. T. Clement, P. Prendergast, T. T. Yip, T. W. Hutchens, D. P. Edwards, and N. L. Weigel. 1997. Phosphorylation of human progesterone receptor by cyclin-dependent kinase 2 on three sites that are authentic basal phosphorylation sites in vivo. Mol. Endocrinol. 11:823-832. [DOI] [PubMed] [Google Scholar]
- 54.Zou, A., M. G. Elgort, and E. A. Allegretto. 1997. Retinoid X receptor (RXR) ligands activate the human 25-hydroxyvitamin D3-24-hydroxylase promoter via RXR heterodimer binding to two vitamin D-responsive elements and elicit additive effects with 1,25-dihydroxyvitamin D3. J. Biol. Chem. 272:19027-19034. [DOI] [PubMed] [Google Scholar]