Abstract
Objectives. To estimate the survival rate of colorectal cancer (CRC) and determine its predictors among Jordanian patients who were diagnosed in the period of 2005–2010. Methods. This study was based on Jordan cancer registry. All CRC cases that were registered in cancer registry during 2005–2010 were analyzed using the survival analysis. The last date for follow-up was 1st Oct 2016. Results. A total of 3005 patients with CRC were registered during 2005–2010. The overall 5-year and 10-year survival rates for patients with CRC were 58.2% and 51.8%, respectively. The 5-year survival rate decreased significantly from 60.4% for the age <50 years to 49.3% for the age ≥70 years (p < 0.005). The 5-year survival rate was 72.1% for the localized stage, 53.8% for the regional stage, and 22.6% for the distant metastasis. In the multivariate analysis, the only factors that were significantly associated with survival were age, grade, stage, and location of tumor. Conclusions. The overall 5-year and ten-year survival rates for CRC were 58.2% and 51.8%, respectively. Increased age, poor differentiation, advanced cancer stage, and right-sided cancers were associated with lower survival rates. Screening strategies are needed for early detection of colon adenomas and colorectal cancer in Jordan.
1. Introduction
Colorectal cancer (CRC) is the third most common cancer in men and the second in women according to the latest GLOBOCAN worldwide estimation in 2012 [1]. About 55% of the cases are reported in the more developed countries. The highest rates were estimated to be in Australia/New Zealand (age standardized rate (ASR): 44.8 and 32.2 per 100,000 in men and women, resp.) and the lowest in Western Africa (ASR: 4.5 and 3.8 per 100,000) [1].
In 2012, CRC was estimated to cause 694,000 deaths (8.5% of total cancer deaths) with more deaths (52%) occurring in the less developed countries [1]. In the United States, CRC is the third most common cancer and the third leading cause of death due to cancer in both genders [2].
In Jordan, the ASR has increased from 12.6 per 100,000 in 2005 to 17.2 per 100,000 in 2010 [3]. According to the latest comprehensive cancer incidence report in 2012, CRC accounted for 11.3% of all newly diagnosed cases among Jordanians and ranked the second among all cancers in both genders. The overall crude incidence rate was 8.9/100,000 population (8.6 and 9.2/100,000 males and females, resp.). The overall ASR was 16.3/100,000 (15.9 and 16.6/100,000 males and females, resp.) [4]. According to Jordan mortality registry in 2013, neoplasms were the second leading cause of death (16.4% of total deaths), and cancer of small intestine, colon, rectum, and anus accounted for 2% of total deaths [5].
Survival studies have yielded different findings about the survival rate and prognostic factors between countries [6–11]. Different clinical and pathological prognostic factors have been proposed for CRC in the literature, including location of tumor, depth of invasion, tumor size, differentiation of tumor, tumor site, lymph node metastasis, and distant metastasis [9–12]. This study was conducted to estimate the survival rate of CRC and determine its predictors among Jordanian patients who were diagnosed in the period between 2005 and 2010.
2. Methods
This study was based on Jordan cancer registry. All CRC cases among Jordanians who were registered in Jordan cancer registry during the period of 2005–2010, with or without histopathology report, were included in the study and analyzed using the survival analysis. All cases died at the time of diagnosis; non-Jordanian patients and patients with multiple cancers were not included in this study.
For each registered patient, demographic and clinical characteristics were obtained from the Jordan cancer registry files and hospital medical records. Data about the type and stage of cancer were obtained from histopathology reports from governmental and private laboratories in addition to the medical records of hospitals. The histopathology type was categorized according to cancer site. The cancer stage was classified according to Surveillance Epidemiology and End Results staging rules into localized, regional, and distant metastasis and unknown stage. To identify the vital status of these patients, date of the last visit was obtained from the medical records. Besides, the vital status was ascertained from the Civil Registration Department using a unique national identification number. Only cancer related deaths were recorded as “death” in the survival analysis. The few non-cancer-related deaths, as ascertained from the Civil Registration Department, were considered as censored cases.
A period of observation was set for the included patients from the date of diagnosis to the last date of observation if the patient was alive (1st Oct 2016) and to the date of death if the patient died during the observation period. The follow-up end point was death from cancer. Duplication of patients was excluded through verifying national identification number, the use of full four digits' names, and matching the names and the addresses.
The Jordan cancer registry uses forms for data collection to collect data about sociodemographic characteristics including national identification number, name, age, marital status, and address and information related to cancer including histopathology, morphology, stage of cancer, location of tumor, date of diagnosis, date of last visit, and outcome. According to the registry, right-sided colon cancer is defined as cancer of the cecum and the ascending colon up to the hepatic flexure. Left-sided colon cancer comprises cancer of the splenic flexure and cancer in regions distal to the splenic flexure, including the rectum.
Ethical approval was obtained from the Institutional Review Board in Ministry of Health. Data were obtained from Jordan cancer registry through the standard data request form.
Data were analyzed using Statistical Package for Social Sciences Software (SPSS) version (20 IBM). Data were described using means and percentages. The overall survival was estimated using Kaplan-Meier product limit technique. Log-rank test was used to compare survival rates between groups. Cox-regression analysis was used to determine factors associated with the time to death. A p value < 0.05 was considered statistically significant.
3. Results
The total number of patients who were diagnosed with CRC and registered in Jordan cancer registry in the period of 2005–2010 was 3005 patients. The number of patients has increased from 370 in 2005 to 570 in 2010 with an increase of 54% during the five years' period. The median age at diagnosis was 62 years for males and 58 years for females. Male to female ratio was 1.3 : 1. The most commonly affected age group was 60 years and above (52.2%). The demographic and clinical characteristics of patients are shown in Table 1. Of all cases, 26.5% were localized, 23.1% were regional, 17.2% were advanced, and 32.3% were of unknown stage.
Table 1.
The demographic and clinical characteristics of 3005 patients diagnosed with colorectal cancer during the period of 2005–2010 in Jordan.
| n | % | |
|---|---|---|
| Age (year) | ||
| <50 | 748 | 24.9 |
| 50–59.9 | 691 | 23.0 |
| 60–69.9 | 891 | 29.7 |
| 70+ | 675 | 22.5 |
| Sex | ||
| Male | 1,684 | 56.0 |
| Female | 1,321 | 44.0 |
| Year at diagnosis | ||
| 2005 | 370 | 12.3 |
| 2006 | 439 | 14.6 |
| 2007 | 523 | 17.4 |
| 2008 | 549 | 18.3 |
| 2009 | 554 | 18.4 |
| 2010 | 570 | 19.0 |
| Region | ||
| North | 557 | 18.7 |
| Middle | 2,319 | 77.7 |
| South | 109 | 3.7 |
| Smoking | ||
| Nonsmoker | 2155 | 71.7 |
| Smoker | 850 | 28.3 |
| Location | ||
| Anus | 48 | 1.6 |
| Colon | 1970 | 65.6 |
| Rectum | 987 | 32.8 |
| Grade | ||
| Well differentiated | 164 | 5.5 |
| Moderately differentiated | 1,881 | 62.6 |
| Poorly differentiated | 277 | 9.2 |
| Anaplastic | 11 | 0.4 |
| Unknown | 672 | 22.4 |
| Stage | ||
| Localized | 788 | 26.5 |
| Regional | 695 | 23.1 |
| Distant metastasis | 517 | 17.2 |
| Unknown | 972 | 32.3 |
Histopathology of CRC showed that adenocarcinoma was the commonest morphology (85%). The morphology for the rest of patients was mucinous (colloid) adenocarcinoma (8.4%), other carcinomas (4.6%), carcinoids (0.7%), signet ring adenocarcinoma (0.9%), adenocarcinoma in adenomatous polyps (0.2%), and adenocarcinoma in villous adenoma (0.2%).
The patients were followed up to the last date of observation if the patient was alive (1st Oct 2016) or to the date of death if the patient died of cancer. The median follow-up time was 5.2 years. The proportion of patients surviving each time interval and the cumulative survival are shown in Table 2. The overall 5-year and 10-year survival rates for CRC were 58.2% and 51.8%, respectively (Table 2). The 5-year survival rate decreased significantly from 60.4% for the age <50 years to 49.3% for the age ≥70 years (p < 0.005). The survival rate decreased significantly with the advanced stage of the disease (the 5-year survival rate was 72.1% for localized stage, 53.8% for regional stage, and 22.6% for distant metastasis) (Figure 1). Table 3 shows the 5-year survival rate for CRC according to different prognostic variables including sex, age group, and site, morphology, grade, and stage of cancer.
Table 2.
Life table of colorectal cancer cases diagnosed in the period of 2005–2010.
| Interval start time (year) | Number at the beginning of the interval | Number of withdrawal cases | Number of patients exposed to risk | Number of deaths | Survival proportion | Cumulative survival proportion |
|---|---|---|---|---|---|---|
| 1 | 2918 | 0 | 2918.0 | 519 | 82.2% | 82.2% |
| 2 | 2399 | 0 | 2399.0 | 298 | 87.6% | 72.0% |
| 3 | 2101 | 0 | 2101.0 | 174 | 91.7% | 66.0% |
| 4 | 1927 | 0 | 1927.0 | 140 | 92.7% | 61.2% |
| 5 | 1787 | 85 | 1744.5 | 86 | 95.1% | 58.2% |
| 6 | 1616 | 299 | 1466.5 | 53 | 96.4% | 56.1% |
| 7 | 1264 | 283 | 1122.5 | 29 | 97.4% | 54.7% |
| 8 | 952 | 261 | 821.5 | 19 | 97.7% | 53.4% |
| 9 | 672 | 277 | 533.5 | 7 | 98.7% | 52.7% |
| 10 | 388 | 214 | 281.0 | 5 | 98.2% | 51.8% |
Figure 1.
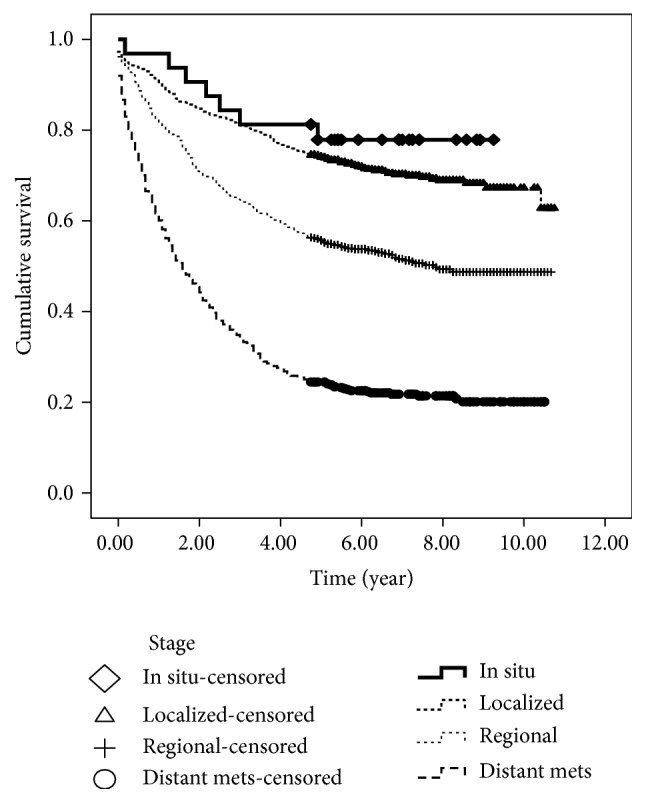
Survival rate for colorectal cancer according to stage.
Table 3.
The 5-year survival rate according to different prognostic factors for 3005 patients diagnosed with colorectal cancer during the period of 2005–2010.
| n | The 5-year survival rate | p value | |
|---|---|---|---|
| Age (year) | <0.005 | ||
| <50 | 748 | 60.4 | |
| 50–59.9 | 691 | 58.2 | |
| 60–69.9 | 891 | 56.4 | |
| 70+ | 675 | 49.3 | |
| Sex | 0.141 | ||
| Male | 1,684 | 54.8 | |
| Female | 1,321 | 58.1 | |
| Region | 0.001 | ||
| North | 557 | 52.8 | |
| Middle | 2,319 | 57.5 | |
| South | 109 | 43.0 | |
| Smoking | 0.634 | ||
| Nonsmoker | 2155 | 56.9 | |
| Smoker | 850 | 55.1 | |
| Location | 0.800 | ||
| Anus | 48 | 53.4 | |
| Colon | 1970 | 57.0 | |
| Rectum | 987 | 54.7 | |
| Grade | <0.005 | ||
| Well differentiated | 164 | 64.9 | |
| Moderately differentiated | 1,881 | 57.9 | |
| Poorly differentiated | 277 | 46.9 | |
| Anaplastic | 11 | 53.7 | |
| Unknown | 672 | 53.7 | |
| Stage | <0.005 | ||
| Localized | 788 | 72.1 | |
| Regional | 695 | 53.8 | |
| Distant metastasis | 517 | 22.6 | |
| Unknown | 972 | 62.7 |
Table 4 shows the multivariate analysis of factors associated with the hazard of death in Cox-regression analysis. The only factors that were significantly associated with death were age and grade, stage, and location of the tumor. The hazard of death increased significantly with increased age being the highest in age ≥70 years. The hazard of death was significantly higher for those with poorly differentiated cancer compared to those with well differentiated cancer (HR = 1.8). The hazard was much higher for patients whose cancer stage was regional (HR = 1.8) and those with distant metastasis (HR = 4.5) compared to those with localized cancer. The patients whose primary tumors originated on the right side of the colon had higher hazard of mortality compared to those whose tumors originated on the left side (HR = 1.3).
Table 4.
The multivariate analysis of factors associated with the hazard of death from colorectal cancer in Cox-regression analysis.
| HR | 95.0% confidence interval for HR | p value | ||
|---|---|---|---|---|
| Sex (female versus male) | 1.1 | 0.9 | 1.2 | 0.706 |
| Age (year) | ||||
| <50 | 1.0 | |||
| 50–59.9 | 1.1 | 1.0 | 1.3 | 0.143 |
| 60–69.9 | 1.4 | 1.1 | 1.7 | 0.001 |
| 70+ | 1.7 | 1.4 | 2.0 | <0.001 |
| Smoking (yes versus no) | 1.1 | 0.9 | 1.2 | 0.253 |
| Location | ||||
| Anus | 1.0 | |||
| Colon | 1.0 | 0.5 | 1.5 | 0.998 |
| Rectum | 1.2 | 0.7 | 1.7 | 0.711 |
| Region | ||||
| North | 1.0 | |||
| Middle | 0.9 | 0.7 | 1.0 | 0.023 |
| South | 1.2 | 0.9 | 1.5 | 0.170 |
| Grade | ||||
| Well differentiated | 1.0 | |||
| Moderately differentiated | 1.2 | 0.9 | 1.5 | 0.238 |
| Poorly differentiated | 1.8 | 1.4 | 2.3 | 0.001 |
| Anaplastic | 1.4 | 0.5 | 2.3 | 0.544 |
| Unknown | 1.3 | 0.9 | 1.8 | 0.051 |
| Stage | ||||
| Localized | 1.0 | |||
| Regional | 1.8 | 1.6 | 3.7 | <0.001 |
| Distant metastasis | 4.5 | 3.7 | 5.1 | <0.001 |
| Unknown | 1.4 | 1.2 | 1.7 | 0.004 |
| Location of tumor | ||||
| Left | 1.0 | |||
| Right | 1.3 | 1.1 | 1.6 | 0.013 |
4. Discussion
Data on the survival analysis of CRC are scant in the Eastern Mediterranean countries including Jordan. Previous studies in other countries have reported variable CRC survival rates. In Asia, the highest survival rates were found in China and the lowest rate was reported in India [13–17]. The 5-year relative survival rate for patients who were diagnosed from 2003 to 2009 in United States was 64.9% [18]. This study showed that the overall 5-year survival rate for patients with CRC was 58.2%. This rate is higher than the reported rates from different countries in the Eastern Mediterranean region [8, 13, 16, 19, 20]. Various research studies from Iran have reported 5-year survival rates of CRC of 47% [16], 41% [8], and 61% [13], respectively. In another retrospective study in Iran, the 5-year survival rate was found to be 27.2% among 284 patients who were diagnosed with CRC between 2003 and 2008 [19]. In Saudi Arabia, the overall 5-year survival rate of CRC was 44.6% using the data from the cancer registry for the period of 1994–2004 [20]. The disparities in CRC survival between Eastern Mediterranean countries may be attributed to several factors including differences in socioeconomic status, stage at diagnosis, treatment, physician characteristics, and hospital factors. The better survival in Jordan compared with other countries in the region might be explained by the fact that cancer care in Jordan is more advanced in comparison to most neighboring countries, and the country hosts many local and western-trained physicians who can deliver various cancer treatment modalities [21]. Currently, the King Hussein Cancer Foundation and Center (KHCC) treats around 60% of cancer cases in Jordan. KHCC is a specialized tertiary hospital that provides all treatment modalities and services to Jordanian patients as well as other patients from neighboring countries. However, further studies are needed to examine the differences in CRC survival between these countries.
There is no significant difference in the survival between males and females in the univariate analysis and multivariate analysis. The lack of gender differences in survival rates was reported in some of the previous studies [13, 14, 16]. However, other studies had reported a lower 5-year survival rate in women [1, 22], that may be due to their increased incidence of right-sided cancer [22]. A systemic review reported that a higher proportion of women presents with right-sided colon cancer than men and the right-sided colon cancer is often at a more advanced stage at diagnosis [22]. In our study, there was no significant difference in the location of the tumor between men and women (p value = 0.098) and this might explain the lack of gender differences in survival rates.
The findings of previous studies concerning the effect of age were diverse. This study showed that the hazard of death increased significantly with increased age being the highest in age ≥70 years. This result was reported in other studies [8, 16] that showed that older patients had a poorer survival rate compared to younger patients. However, other studies [13, 23, 24] reported no difference in survival according to age. The contradictory results of previous studies on age may be due to inclusion of patients from single referral centers and poor adjustment for the effect of possible confounders.
Different clinical and pathological prognostic factors have been proposed for CRC in the literature, including location of the tumor [13, 16, 24, 25], tumor stage [26], differentiation of tumor [13], and surgical and distant metastasis [23]. The multivariate analysis using Cox-regression analysis showed that grade and stage were significant predictors of survival. The hazard of death was significantly higher for those with poorly differentiated cancer compared to those with well differentiated cancer. Moreover, it was much higher for patients whose cancers stage was regional and those with distant metastasis compared to those with localized cancer. A previous review study showed that CRC survival is highly dependent on stage at diagnosis, and the 5-year survival rate varied from 90% for localized stage cancers and 70% for regional cancer to 10% for distant metastatic cancer [25]. The study concluded that the earlier the stage at diagnosis, the higher the chance of survival.
This study showed a higher mortality hazard among patients whose primary tumors originated on the right side of the colon compared to those whose tumors originated on the left side. One study showed that tumors on the left side and right side have different underlying biological characteristic, different macroscopic properties, and different dominant pathways to relapse and hence may explain the differences in survival [27]. The higher hazard of mortality among patients whose primary tumors originated on the right side of the colon might have an implication on the diagnosis of CRC and on research to understand the differences in survival between right- and left-sided cancers.
Our study showed that the 5-year survival rate for CRC was 72.1%, 53.8%, and 22.6% for localized, regional, and distant stage, respectively. This finding is consistent with findings of many other studies [23, 26, 28]. The Thailand study showed that the 5-year stage specific survival rates for stages I, II, III, and IV CRC were 100%, 68%, 44%, and 2%, respectively [29]. In Saudi Arabia, the 5-year survival rate differed significantly according to the stage (63.3% for patients with localized disease, 50.2% for those with regional disease, and 14.7% for patients with metastases) [20]. The differences in the survival according to the stage are explained by the differences in the extent to which the cancer has spread and how many lymph nodes have been affected.
In a retrospective study in Iran on patients diagnosed with CRC from 2003 to 2008, multiple Cox-regression model revealed that survival rate has a significant relationship with other prognostic factors like the primary diagnosis method, income status, history of alcohol use, primary treatment method, and history of metastasis [19]. Data from Jordan cancer registry should be interpreted with caution. As many registries in the region, Jordan cancer registry does not collect information on other possible predictors of mortality such as occupation, level of education, economic status, and comorbidity. Therefore, our estimates of hazards ratio might be biased because of not adjusting for the effect of unmeasured variables.
In conclusion, the overall 5-year and ten-year survival rates for CRC were 58.2% and 51.8%, respectively. Increased age, poor differentiation, advanced cancer stage, and right- sided cancers were associated with lower survival rates. It is well established that CRC is one of those cancers that can largely be prevented by the early detection and removal of adenomatous polyps, and survival is therefore significantly better when colorectal cancer is diagnosed while being still localized. Screening strategies are needed for early detection of colon adenomas and colorectal cancer.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
References
- 1.GLOBOCAN: Estimation of cancer incidence, prevalence and mortality worldwide in 2012, The international Agency for Research on Cancer. World Health Organization, http://globocan.iarc.fr/Pages/fact_sheets_cancer.aspx?cancer=colorectal.
- 2. Colorectal Cancer Facts & Figures 2014–2016, the American Cancer Society, Atlanta, Ga, USA https://www.cancer.org/research/cancer-facts-statistics/colorectal-cancer-facts-figures.html.
- 3.Jordan cancer registry reports (2006–2010) http://www.moh.gov.jo/Pages/viewpage.aspx?pageID=248.
- 4.Annual Incidence of Cancer 2012, JCR, MOH. http://www.moh.gov.jo/Echobusv3.0/SystemAssets/c602eda7-0c36-49cd-bea1-3484e46c0b97.pdf.
- 5.Amman, Jordan: Ministry of Health Jordan; 2013. Annual Mortality Report in 2013. http://www.moh.gov.jo/Echobusv3.0/SystemAssets/3fbfbd39-4fee-4106-8a6a-074d77b9a704.pdf. [Google Scholar]
- 6.Karimi Z., Saadat A., Jalalian H., Esmaeili M. Epidemiology and survival analysis of colorectal cancer, and its related factors. Kowsar Medical Journal. 2011;15(4):239–243. [Google Scholar]
- 7.Yuan Y., Li M. D., Hu H. G., et al. Prognostic and survival analysis of 837 Chinese colorectal cancer patients. World Journal of Gastroenterology. 2013;19(17):2650–2659. doi: 10.3748/wjg.v19.i17.2650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moradi A., Khayamzadeh M., Guya M. M., et al. Survival of colorectal cancer in Iran. Asian Pacific Journal of Cancer Prevention. 2009;10(4):583–586. [PubMed] [Google Scholar]
- 9.Moghimi-Dehkordi B., Safaee A. An overview of colorectal cancer survival rates and prognosis in Asia. World Journal of Gastrointestinal Oncology. 2012;4(4):71–75. doi: 10.4251/wjgo.v4.i4.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huang J., Seow A., Lee H. P. Survival of colorectal cancer patients in singapore by anatomic subsite: a population-based study. Annals of the Academy of Medicine, Singapore. 2000;29(1):79–85. [PubMed] [Google Scholar]
- 11.Lang K., Korn J. R., Lee D. W., Lines L. M., Earle C. C., Menzin J. Factors associated with improved survival among older colorectal cancer patients in the US: a population-based analysis. BMC Cancer. 2009;9, article 227 doi: 10.1186/1471-2407-9-227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Al Nsour M., Brown D. W., Tarawneh M., Haddadin R., Walk H. Breast and cervical cancer screening among women in Jordan: findings from the behavioural risk factor surveillance system - 2007. Open Breast Cancer Journal. 2012;4:1–7. doi: 10.2174/1876817201204010001. [DOI] [Google Scholar]
- 13.Moghimi-Dehkordi B., Safaee A., Zali M. R. Prognostic factors in 1,138 Iranian colorectal cancer patients. International Journal of Colorectal Disease. 2008;23(7):683–688. doi: 10.1007/s00384-008-0463-7. [DOI] [PubMed] [Google Scholar]
- 14.Ghazali A. K., Musa K. I., Naing N. N., Mahmood Z. Prognostic factors in patients with colorectal cancer at hospital universiti sains Malaysia. Asian Journal of Surgery. 2010;33(3):127–133. doi: 10.1016/S1015-9584(10)60022-X. [DOI] [PubMed] [Google Scholar]
- 15.Cai S.-R., Zheng S., Zhang S.-Z. Multivariate analysis of prognostic factors in colorectal cancer patients with different ages. Chinese Journal of Oncology. 2005;27(8):483–485. [PubMed] [Google Scholar]
- 16.Mehrkhani F., Nasiri S., Donboli K., Meysamie A., Hedayat A. Prognostic factors in survival of colorectal cancer patients after surgery. Colorectal Disease. 2009;11(2):157–161. doi: 10.1111/j.1463-1318.2008.01556.x. [DOI] [PubMed] [Google Scholar]
- 17.Oh H., Chung H., Kim H., Choi J. Differences in overall survival when colorectal cancer patients are stratified into new TNM staging strategy. Cancer Research and Treatment. 2007;39(2):61–64. doi: 10.4143/crt.2007.39.2.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Howlader N., Noone A. M., Krapcho M., et al., editors. SEER Cancer Statistics Review, 1975–2013. Bethesda, Md, USA: National Cancer Institute; 2016. [Google Scholar]
- 19.Mehrabani D., Almasi-Hashiani A., Moshfeghi K., Khedmati E. Survival rate and its predictors in colorectal cancer patients, southern Iran. Middle East Journal of Scientific Research. 2012;12(8):1072–1077. [Google Scholar]
- 20.Al-Ahwal M. S., Shafik Y. H., Al-Ahwal H. M. First national survival data for colorectal cancer among Saudis between 1994 and 2004: what's next? BMC Public Health. 2013;13, article 73 doi: 10.1186/1471-2458-13-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Abdel-Razeq H., Attiga F., Mansour A. Cancer care in Jordan. Hematology/ Oncology and Stem Cell Therapy. 2015;8(2):64–70. doi: 10.1016/j.hemonc.2015.02.001. [DOI] [PubMed] [Google Scholar]
- 22.Hansen I. O., Jess P. Possible better long-term survival in left versus right-sided colon cancer—a systematic review. Danish Medical Journal. 2012;59(6, article A4444) [PubMed] [Google Scholar]
- 23.Zhang S., Gao F., Luo J., Yang J. Prognostic factors in survival of colorectal cancer patients with synchronous liver metastasis. Colorectal Disease. 2010;12(8):754–761. doi: 10.1111/j.1463-1318.2009.01911.x. [DOI] [PubMed] [Google Scholar]
- 24.Wang Z., Zhou Z. X., Liang J. W., Bai X. F., Bi J. J. Prognostic factors of colorectal cancer patients with synchronous liver metastasis treated with simultaneous liver and colorectal resection. Zhonghua Zhongliu Zazhi. 2008;30:372–375. [PubMed] [Google Scholar]
- 25.Haggar F. A., Boushey R. P. Colorectal cancer epidemiology: incidence, mortality, survival, and risk factors. Clinics in Colon and Rectal Surgery. 2009;22(4):191–197. doi: 10.1055/s-0029-1242458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Halvorsen T. B., Seim E. Tumour site: A prognostic factor in colorectal cancer?: A multivariate analysis. Scandinavian Journal of Gastroenterology. 1987;22(1):124–128. doi: 10.3109/00365528708991868. [DOI] [PubMed] [Google Scholar]
- 27.Bauer K. M., Hummon A. B., Buechler S. Right-side and left-side colon cancer follow different pathways to relapse. Molecular Carcinogenesis. 2012;51(5):411–421. doi: 10.1002/mc.20804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Newland R. C., Dent O. F., Lyttle M. N. B., Chapuis P. H., Bokey E. L. Pathologic determinants of survival associated with colorectal cancer with lymph node metastases: a multivariate analysis of 579 patients. Cancer. 1994;73(8):2076–2082. doi: 10.1002/1097-0142(19940415)73:8<2076::aid-cncr2820730811>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 29.Laohavinij S., Maneechavakajorn J., Techatanol P. Prognostic factors for survival in colorectal cancer patients. Journal of the Medical Association of Thailand. 2010;93(10):1156–1166. [PubMed] [Google Scholar]


